Abstract
The role of eculizumab in treating Shiga‐toxin‐producing Escherichia coli (STEC) hemolytic uremic syndrome (HUS) patients with neurological involvement remains unclear. We describe two distinctly different STEC‐HUS patients with neurologic involvement successfully managed with eculizumab, and perform a literature review of all published cases. Both patients had complete resolution of neurological symptoms after initiation of eculizumab. Eighty patients with STEC‐HUS treated with eculizumab were identified in the literature, 68.7% had complete resolution of neurological symptoms. Based on our experience and literature review, three prevailing themes were noted: 1) Early eculizumab administration optimized neurological outcomes, 2) Symptom resolution may not be immediate, neurological symptoms may initially worsen before improvement, and 3) Plasma exchange yielded no benefit. Early administration of eculizumab may reverse neurotoxicity in patients with STEC‐HUS.
Keywords: eculizumab, hemolytic uremic syndrome, neurological involvement, Shiga‐toxin producing Escherichia coli, STEC‐HUS
1. INTRODUCTION
Hemolytic uremic syndrome (HUS) is a form of thrombotic microangiopathy (TMA) that is characterized by a triad of thrombocytopenia, microangiopathic hemolytic anemia, and renal failure [1]. The pathogenesis and etiology of HUS can vary significantly with Shiga‐toxin‐producing Escherichia coli (STEC)‐HUS occurring after gastrointestinal infection of STEC. Secondary or atypical HUS (aHUS) on the other hand, may be triggered by malignant hypertension, drugs (i.e., calcineurin inhibitors), hematopoietic stem cell transplantation (HSCT), or other causes, often, but not exclusively, in the presence of key genetic mutations [1, 2]. Extra‐renal TMA involving the central nervous system (CNS) has been reported in 20%–25% of STEC‐HUS cases and represents a major cause of morbidity and mortality [3]. The key role of complement system dysregulation has been extensively described in atypical HUS (aHUS), for which treatment with eculizumab has demonstrated significant improvements in renal and hematologic outcomes [4]. Its utility, however, has not been extensively described for HUS‐driven neurological impairment.
2. METHODS
A retrospective review identified two adult patients diagnosed with STEC‐HUS who developed neurological involvement and received eculizumab therapy. Stool culture testing for gastrointestinal pathogens and direct antigen testing for Shiga‐Toxin were performed. The diagnosis of HUS was determined based on a composite of numerous factors, which included the presence of TMA symptoms and laboratory abnormalities, such as low or undetectable serum haptoglobin, schistocytes on peripheral smear, and adherence to relevant exclusion criteria, including a negative Direct Coombs test and ADAMTS13 activity > 10%. ADAMTS13 activity testing was performed via quantitative enzyme‐linked immunosorbent assay (ELISA) (ARUP Laboratories). Complement studies for complement components 3 (C3) and 4 (C4), as well as total complement activity (CH50), were quantified by immunoturbidimetry (ARUP Laboratories). Soluble terminal complement complex (sC5b‐9) enzyme immunoassay and serum eculizumab ELISA testing were performed by Cincinnati Children's Laboratory (Clinical Laboratory). Lastly, a comprehensive TMA genetic panel was performed via next‐generation sequencing to test for 20 clinically relevant genetic markers (ADAMTS13, CFB, CFD, CFH, CFHR1‐5, CFI, C2, C3, C3AR1, DGKE, MASP2, MMACHC, MCP/CD46, PLG, THBD, WT1, and C5 polymorphisms) associated with HUS, C3 glomerulopathy, and other hematologic disorders (Machaon Diagnostics, Inc). A literature review was performed to identify all previously published cases of STEC‐HUS with neurological involvement that received eculizumab.
3. RESULTS
3.1. Case presentation 1
A 46‐year‐old male with a recent history of haploidentical HSCT presented with severe abdominal pain and diarrhea. A diagnosis of STEC‐HUS was made based on stool culture findings positive for Shiga‐Toxin‐1 and laboratory abnormalities consistent with TMA (Table 1). The patient was initiated on eculizumab therapy 2 days after onset of encephalopathy. Despite the initial response, neurological status acutely worsened; plasma exchange (PLEX) was initiated, however, no improvement was noted in the patient's condition. After 3 weekly eculizumab infusions, initial soluble terminal complement complex (sC5b‐9) and eculizumab serum monitoring showed levels of 494 ng/mL and 37 mcg/mL, respectively. Eculizumab frequency was increased to every 72 h and by day 23, the patient was alert, oriented, and fully conversant once complement activation was optimally suppressed (Figure 1) [5]. A detailed summary is available in Table S1.
TABLE 1.
Baseline demographics and relevant laboratory findings for the included cases.
| Patient characteristics | Patient #1 | Patient #2 |
|---|---|---|
| Past medical history | 46 year‐old‐male with a history of severe aplastic anemia who received a haploidentical hematopoietic stem cell transplantation | A 43‐year‐old female with no significant past medical history |
| Eculizumab treatment course |
|
|
| Stool culture tests | Positive for Shiga‐toxin‐1 by DAT; Positive for non‐O157 E‐Coli |
Positive for Shiga‐toxin‐2 by DAT; Positive for O157‐H7 E‐Coli |
| TMA complete genetic testing | No disease‐associated variants identified | Heterozygous missense variant (c.1157G > A, p.Arg386Gln) in exon 10 of the PLG gene |
| Laboratory values at baseline and during the acute phase | ||
| Serum creatinine (mg/dL) |
1.4 [Highest: 4.6] (Reference: 0.7–1.3 mg/dL) |
3.1 [Highest: 10.0] (Reference: 0.6–1.2 mg/dL) |
| LDH (U/L) |
320 [Highest: 1329] (Reference: 96–199 U/L) |
2,087 [Highest: 2514] (Reference: 96–199 U/L) |
| White blood cells (cells × 109/L) |
0.9 [Lowest: 0.8] (Reference: 4.0–10.5 × 109/L) |
22.7 [Lowest: 37.0] (Reference: 4.0–10.5 × 109/L) |
| Absolute neutrophil count (cells × 109/L) |
0.7 [Lowest: 0.4] (Reference: 2.0–8.1 × 109/L) |
13.9 [Lowest: 2.0] (Reference: 2.0–8.1 × 109/L) |
| Hemoglobin (g/dL) |
8.1 [Lowest: 5.7] (Reference: 13.5–16.9 g/dL) |
16.9 [Lowest: 5.6] (Reference: 11.5–15.0 g/dL) |
| Platelet (cells × 109/L) |
14 [Lowest: 10] (Reference: 150–400 × 109/L) |
52 [Lowest: 27] (Reference: 150–400 × 109/L) |
| Haptoglobin (mg/dL) |
< 30 [Highest: 164] (Reference: 44–215 mg/dL) |
< 30 [Highest: 60] (Reference: 44–215 mg/dL) |
| Schistocytes (%) |
1%–5% (Reference: Normal < 1%) |
1%–5% (Reference: Normal < 1%) |
| Broad spectrum coombs serum | Negative | Negative |
| Reticulocyte count (%) |
0.2% (Reference: 0.9%–2.5%) |
2.4% (Reference: 0.9%–2.5%) |
| ADAMTS13 Activity (%) |
41% (Reference: Normal > 61%) |
29% (Reference: Normal > 61%) |
| C3 (mg/dL) |
96 (Reference: 65–175 mg/dL) |
78 b (Reference: 65–175 mg/dL) |
| C4 (mg/dL) |
33 (Reference: 13–39 mg/dL) |
34 b (Reference: 13–39 mg/dL) |
| CH50 (U/mL) |
16.5 (Reference: 38.7–89.9 U/mL) |
13.2 b (Reference: 38.7–89.9 U/mL) |
| sC5b‐9 (ng/mL) |
494 a (Reference: Normal < 244 ng/mL) |
Not collected |
| Serum eculizumab level (mcg/mL) |
37 a (Reference: Therapeutic > 99 mcg/mL) |
Not collected |
Abbreviations: C3, complement component 3; C4, complement component 4; CH50, total complement activity; DAT, direct antigen testing; LDH, lactate dehydrogenase; sC5b‐9, soluble terminal complement complex; TMA, thrombotic microangiopathy.
Collected on day 20 of eculizumab therapy.
Collected on day 27 of eculizumab therapy.
FIGURE 1.
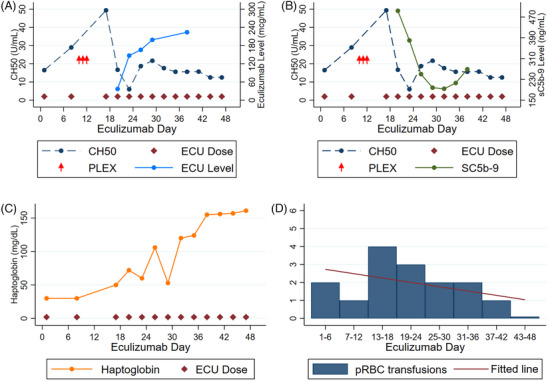
Temporal association for Case #1 between eculizumab dosing with (A) total complement (CH50) and eculizumab level, (B) CH50 and terminal complement (sC5b‐9), (C) haptoglobin, and (D) packed red blood cell transfusion requirement. ECU, eculizumab; PLEX, plasma exchange; pRBC, packed red blood cell.
3.2. Case presentation 2
A 43‐year‐old female with no significant past medical history presented with vomiting, epigastric pain, and bloody diarrhea. A diagnosis of STEC‐HUS was made based on stool culture findings positive for Shiga‐Toxin‐2 and laboratory abnormalities consistent with TMA (Table 1). A TMA genetic panel also revealed a heterozygous missense variant (c.1157G > A, p.Arg386Gln) in exon 10 of the plasminogen or PLG gene which plays a functional role in the plasma fibrinolytic system. The PLG gene is one of a few genetic mutations beyond the complement system that has been implicated in complement‐mediated HUS [6]. Eculizumab therapy was started on day 8 of hospitalization, 2 days after onset of acute encephalopathy. Despite repeated seizures, 2 days later, the patient showed dramatic improvement and became alert, and oriented, and was able to follow commands 4 days after starting eculizumab. A detailed summary is available in Table S1.
3.3. Literature review
A review of the literature identified 80 patients with STEC‐HUS treated with eculizumab (including our two cases) of whom nine (11.3%) died from complications. The majority (n = 76; 95%) were pediatric patients while 5% (n = 4) were adults. Fifty‐five patients (68.7%) had complete resolution of neurologic symptoms, while 16 (20%) had some long‐term neurologic sequelae (compiled in Table S2).
4. DISCUSSION
The two cases reported herein highlight several important findings. Case 1 represents a report of successful eculizumab use to treat STEC‐HUS in an adult allogeneic HSCT patient. This is a rare clinical occurrence, with only one other case post‐HSCT described in the literature [7]. In contrast to data in other TMAs, an intensified high‐dose eculizumab regimen was required [5]. Elevated sC5b‐9 levels have been described with severe multi‐visceral TMA, which can affect blood vessels of the lungs, CNS, or intestines, and it is possible that higher sC5b‐9 levels may also be evident in STEC‐HUS patients with neurological involvement, although future studies evaluating this as a potential biomarker are warranted [8]. Case 2 was in a patient harboring a heterozygous missense variant in the PLG gene, which may have increased her risk for complement activation and development of neurological symptoms. Despite the nuanced differences between our two cases, early and tailored eculizumab dosing resulted in the resolution of neurological symptoms, due to complete complement blockade in both patients.
The complement system is activated in patients with severe STEC‐HUS by Shiga‐toxin, which has been suggested to incite an inflammatory and thrombogenic effect within the circulatory system [9]. Endothelial damage of the renal microvasculature as a result, is a well‐understood mechanism of HUS pathology, leading up to AKI. Shiga‐toxin binding on globotriaosylceramide (Gb3) receptors throughout the cerebral endothelium and neuronal cells represents a main cause of neurological complications in 20–25% of STEC‐HUS patients [10]. Supportive care measures represent the standard approach in STEC‐HUS treatment as early studies have failed to show the impact of adjunct therapies. In a large STEC outbreak, no short‐term benefit of eculizumab on mortality and renal outcomes was reported. In these retrospective reviews, 193 adult and 13 pediatric patients received eculizumab of which 69% and 76.9%, respectively, had neurological complications [11, 12]. More patients receiving eculizumab and PLEX in the study by Kielstein et al. had baseline neurological symptoms compared to the PLEX (89% vs. 59%) or best supportive care (45%) arms, and no difference in clinical outcomes was reported across the three cohorts, although improvement in neurological status was not explicitly evaluated. In a recently published trial, investigators found no benefit with eculizumab on renal outcomes in STEC‐HUS. Patients with neurological involvement, however, were excluded from randomization [13]. In contrast, our review of the literature suggests positive outcomes utilizing eculizumab for STEC‐HUS with neurological symptoms and has revealed three emerging points.
First, early eculizumab administration is essential to maximize efficacy and reduce long‐term neurologic sequelae. In both our cases, eculizumab was initiated within 2 days of neurological symptoms. This is consistent with reports of successful eculizumab utilization where treatment was started within 6 days of neurological symptom onset (when reported) except for three cases (Table S2). This may also explain the lack of benefit reported by Kielstein et al. and Loos et al. where eculizumab initiation was delayed with a mean of 11 days in the adult and a median of 22 days in the pediatric cohorts [11, 12]. Three of five patient deaths reported in Table S2 also described delays from symptom onset to eculizumab initiation. Of the five patients with descriptive time frames and long‐term neurologic sequelae, three had delays in receiving eculizumab (10 days from symptom onset in two and > 72 h after extensive PLEX [unknown time] in one). Early treatment may allow for complement inhibition prior to the onset of severe and/or permanent neurological damage.
Second, the benefit of eculizumab is not immediate as sC5b‐9 normalization takes time after treatment initiation. In the 17 cases reporting time to events (including this current report), neurological status improvement was seen at a median of 4 days (range, 1–42) after eculizumab initiation, of which 57.1% of cases reporting clinical courses (n = 8/14) described initial worsening or new onset of symptoms in addition to one describing a persistent pyramidal syndrome and coma for 6 weeks. This lag time in symptom improvement can be explained by elevated sC5b‐9 levels, which have been described to occur with neurologic changes in severe multi‐visceral TMA [8, 14]. When higher than normal sC5b‐9 levels are present, control of complement and normalization of sC5b‐9 levels are delayed [15]; thus, treating providers should be deterred from early termination of complement‐inhibiting therapies without adequate evidence indicating treatment failure. Time to neurological symptom improvement was 23 and 4 days, respectively, in our two cases, both of whom had initial worsening of symptoms.
Finally, the benefit of PLEX in STEC‐HUS is poorly defined, with little reported benefit [16, 17]. While PLEX has been rationalized in removing circulating toxins and inflammatory mediators involved in the complement pathway, evidence is largely drawn from aHUS patients. Furthermore, PLEX should be used with caution in those who may initiate eculizumab or those already receiving treatment. PLEX significantly reduces the efficacy of eculizumab by rapidly increasing the clearance of serum eculizumab, decreasing its half‐life to an estimated 1.26 h, and further limiting its activity [18]. In order to ensure that adequate exposure to eculizumab is achieved, supplemental doses of eculizumab are warranted after PLEX or prior to infusion of any fresh frozen plasma. This could further explain the poor outcomes reported by Kielstein et al., in which all patients receiving eculizumab also underwent PLEX and only 61% received supplemental eculizumab doses after PLEX [9]. Impact on treatment prioritization and delay in eculizumab initiation as the result of PLEX was documented in two patients who died and two who developed irreversible neurological sequelae, further highlighting the consequences of delaying eculizumab initiation for PLEX, potentially explaining the lack of efficacy in these patients.
In conclusion, prompt initiation of eculizumab for the treatment of STEC‐HUS in adult patients with neurological involvement may be an effective therapeutic modality, particularly in those already at a higher risk of TMA. Based on the different eculizumab dosing regimens required to achieve terminal complement blockade in our two patients, future studies should focus on complement activation biomarker levels in STEC‐HUS patients to optimize eculizumab dosing for these patients.
AUTHOR CONTRIBUTIONS
Benjamin J. Lee conceptualized and designed the study as well as collected the data. Benjamin J. Lee and Zhaohui Arter wrote the initial draft of the manuscript. Benjamin J. Lee; Zhaohui Arter; Jean Doh; Shawn P. Griffin; Minh‐Ha Tran; Piyanuch Kongtim; and Stefan O. Ciurea cared for the patient. Jean Doh; Shawn P. Griffin; Pongthep Vittayawacharin; Steven Atallah; Kevin R. Shieh; Minh‐Ha Tran; Sonata Jodele; Piyanuch Kongtim; and Stefan O. Ciurea interpreted and analyzed the clinical data. All authors critically reviewed; made major edits and approved the final version of the manuscript.
Zhaohui Arter, Jean Doh, Shawn P. Griffin, Pongthep Vittayawacharin, Steven Atallah, Kevin R. Shieh, Minh‐Ha Tran, Sonata Jodele, Piyanuch Kongtim, Stefan O. Ciurea
CONFLICT OF INTEREST STATEMENT
Sonata Jodele has received consulting fees, payments, or honoraria from Omeros, Sobi, and Alexion Pharmaceuticals. Sonata Jodele has also received grant funding from Alexion Pharmaceuticals. The remaining authors declare no conflict of interest.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
ETHICS STATEMENT
The submitted manuscript was designated as exempt status from Institutional Review by the University of California Irvine Office of Research, protocol #2858.
PATIENT CONSENT STATEMENT
Informed consent was waived in accordance with the University of California Irvine Office of Research policies as no patient‐identifiable images or data have been included in this manuscript. A Waiver of Informed Consent to publish was granted by the University of California Irvine Office of Research Institutional Review Board as a common practice in the United States for retrospective study.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors have nothing to report.
Lee BJ, Arter Z, Doh J, Griffin SP, Vittayawacharin P, Atallah S, et al. Eculizumab for Shiga‐toxin‐induced hemolytic uremic syndrome in adults with neurological involvement. eJHaem. 2024;5:548–553. 10.1002/jha2.902
Benjamin J. Lee and Zhaohui Arter equally contributed to the manuscript as first authors.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this are included in this published article.
REFERENCES
- 1. Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(4):1035–1050. [DOI] [PubMed] [Google Scholar]
- 2. Tomazos I, Garlo K, Wang Y, Chen P, Laurence J. Triggers in patients with atypical hemolytic uremic syndrome: an observational cohort study using a US claims database. Blood. 2020;136(Supplement 1):30–31. [Google Scholar]
- 3. Nathanson S, Kwon T, Elmaleh M, Charbit M, Launay EA, Harambat J, et al. Acute neurological involvement in diarrhea‐associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5(7):1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal complement inhibitor eculizumab in atypical hemolytic‐uremic syndrome. N Engl J Med. 2013;368(23):2169–2181. [DOI] [PubMed] [Google Scholar]
- 5. Mizuno K, Dandoy CE, Teusink‐Cross A, Davies SM, Vinks AA, Jodele S. Eculizumab precision‐dosing algorithm for thrombotic microangiopathy in children and young adults undergoing HSCT. Blood Adv. 2022;6(5):1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasco M, Guillén E, Quintana LF, Garcia‐Herrera A, Piñeiro G, Poch E, et al. Thrombotic microangiopathies assessment: mind the complement. Clin Kidney J. 2020;14(4):1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vera‐Aguilera J, Duma N, Gast K, Alkhateeb H, Tande A, Leung N, et al. Hemolytic uremic syndrome associated with Escherichia coli O157 infection in an allogenic stem cell transplant recipient. Mayo Clin Proc Innov Qual Outcomes. 2018;2(4):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jodele S, Dandoy CE, Lane A, Laskin BL, Teusink‐Cross A, Myers KC, et al. Complement blockade for TA‐TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ståhl A‐L, Sartz L, Karpman D. Complement activation on platelet‐leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli‐induced hemolytic uremic syndrome. Blood. 2011;117(20):5503–5513. [DOI] [PubMed] [Google Scholar]
- 10. Obata F, Tohyama K, Bonev AD, Kolling GL, Keepers TR, Gross LK, et al. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J Infect Dis. 2008;198(9):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kielstein JT, Beutel G, Fleig S, Steinhoff J, Meyer TN, Hafer C, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga‐toxin‐producing E. coli O104:H4 induced haemolytic‐uraemic syndrome: an analysis of the German STEC‐HUS registry. Nephrol Dial Transplant. 2012;27(10):3807–3815. [DOI] [PubMed] [Google Scholar]
- 12. Loos S, Ahlenstiel T, Kranz B, Staude H, Pape L, Hartel C, et al. An outbreak of Shiga toxin‐producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short‐tern outcome in children. Clin Infect Dis. 2012;55(6):753–759. [DOI] [PubMed] [Google Scholar]
- 13. Garnier A, Brochard K, Kwon T, Sellier‐Leclerc A‐L, Lahoche A, Launay EA, et al. Efficacy and safety of eculizumab in pediatric patients affected by Shiga toxin‐related hemolytic and uremic syndrome: a randomized, placebo‐controlled trial. J Am Soc Nephrol. 2023;34(9):1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jodele S, Laskin BL, Dandoy CE, Myers KC, El‐Bietar J, Davies SM, et al. A new paradigm: diagnosis and management of HSCT‐associated thrombotic microangiopathy as multi‐system endothelial injury. Blood Rev. 2015;29(3):191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, et al. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(2):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padmanabhan A, Connelly‐Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354. [DOI] [PubMed] [Google Scholar]
- 17. Keenswijk W, Raes A, De Clerck M, Vande Walle J. Is plasma exchange efficacious in Shiga toxin‐associated hemolytic uremic syndrome? A narrative review of current evidence. Ther Apher Dial. 2019;23(2):118–125. [DOI] [PubMed] [Google Scholar]
- 18. Wijnsma KL, Ter Heine R, Moes DJAR, Langemeijer S, Schols SEM, Volokhina EB, et al. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin Pharmacokinet. 2019;58(7):859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
All data generated or analyzed during this are included in this published article.


