Abstract
Background:
The difference in incidence of early onset sepsis (EOS) caused by Group B Streptococcus (GBS) among term neonates whose mothers receive first versus second-line intrapartum prophylaxis is poorly described.
Objective:
To compare the incidence of GBS EOS in term neonates born to mothers who receive first, second-line or no intrapartum antibiotics, and describe the short term and survival outcomes of neonates who developed GBS EOS stratified by maternal antepartum prophylaxis.
Study Design:
This was a retrospective review of electronic medical records. We queried the Pediatrix Medical Group Clinical Data Warehouse to evaluate the outcomes of term neonates born to GBS positive mothers between 2003 – 2020, and compared the incidence and outcomes of neonates with GBS EOS whose mothers received first versus second-line/no intrapartum prophylaxis.
Results:
Among 496,180 neonates, 104,196 (21%) were born to GBS positive mothers. Out of 97,983 GBS positive mothers with adequate prenatal antibiotic documentation, 49,234 (50%), 12,679 (13%) and 36,070 (37%) received first-line, second-line and no intrapartum prophylaxis, respectively. Incidence of GBS EOS among all neonates with maternal GBS carriage was 0.22% (231/104,196). Neonates whose mothers received second-line intrapartum antibiotics and no antibiotics had higher risk of GBS EOS infection compared to first-line intrapartum antibiotics [adjusted odds ratio (aOR) 4.12, 95% confidence interval (CI): 2.66 – 6.38 and aOR 3.80, 95% CI: 2.66 – 5.44, respectively]. No statistically significant difference in the risk of GBS EOS in neonates born to mothers who received second-line versus no antibiotics (aOR 0.92, 95% CI: 0.64 – 1.33) compared to second-line.
Conclusion:
Neonates exposed to second-line maternal GBS prophylaxis had increased risk of GBS EOS compared to those exposed to first-line maternal GBS prophylaxis. There was no statistically significant difference in GBS EOS incidence between second-line versus no antibiotics in mothers with GBS carriage.
Keywords: database, Group B Streptococcus, neonates, neonates, maternal intrapartum antibiotics
Tweetable Statement
Neonates whose mothers are exposed to second-line maternal prophylaxis had increased risk of early-onset sepsis compared to those exposed to first-line maternal prophylaxis. @GroupBStrep @GBSSupport #newborn #GBSaware
Introduction
Group B streptococcus (GBS) is the leading cause of neonatal infection.1–3 The main risk factor for GBS early-onset sepsis (EOS) is maternal colonization of the genitourinary and gastrointestinal tracts. Approximately 30–50% of mothers who are colonized with GBS can transmit the bacteria to their neonates,3,4 via vertical transmission during the intrapartum period. Worldwide, there are currently two approaches used to identify pregnant women that require intrapartum antibiotics to reduce the incidence of GBS EOS: culture-based strategy and risk-based approach.5 The culture-based strategy, which is recommended in the United States, uses vaginal-rectal cultures at 35–37 weeks gestation and implementation of timely appropriate intrapartum antibiotics.5 The risk-based strategy is based on presence of clinical risk factors such as preterm labor, prolonged rupture of membranes, maternal pyrexia, and previous neonate with GBS disease and GBS bacteriuria5 in determining the need for antibiotic prophylaxis.
Epidemiologic data on GBS EOS are mostly limited to studies before and after implementation of appropriate intrapartum GBS prophylaxis with penicillin or ampicillin.1 These studies focused on neonates whose mothers either received or did not receive GBS prophylaxis. Currently, the Center for Disease Control and Prevention (CDC) recommends culture-based screening of all pregnant women at 35 to 37 weeks of gestation and provision of intrapartum antibiotics for GBS-positive women.6 The most widely recommended first-line antibiotics for adequate prophylaxis are ampicillin or penicillin for at least four hours prior to delivery.
However, there is significant practice variability amongst healthcare practitioners for GBS-positive mothers who are unable to receive intrapartum ampicillin or penicillin due to drug allergies.7 In clinical practice, the three most often used second-line therapy for GBS prophylaxis are erythromycin, clindamycin and vancomycin.6,7 In earlier versions of the GBS prophylaxis guidelines, erythromycin and clindamycin were listed as second-line therapy for use in mothers at high risk of anaphylaxis to penicillin.7 However, as GBS resistance to macrolides increased, this is no longer routinely recommended.8 In addition, erythromycin does not cross the placenta, making it a poor choice for intrapartum GBS prophylaxis. As an alternative, some experts have advocated for administration of vancomycin for intrapartum antibiotic prophylaxis for women with penicillin allergy.9 The routine use of vancomycin however has been associated with the emergence of vancomycin-resistant enterococci, which has significant public health implications, hence making it a less attractive choice of antibiotic prophylaxis.9 Currently, there remains a knowledge gap in the role of second-line/no intrapartum antibiotics use in prevention of GBS EOS as opposed to first-line therapy.
We aimed to fill this knowledge gap by studying the role of second-line or no intrapartum antibiotics (i.e., macrolides, clindamycin or vancomycin) in preventing GBS EOS as opposed to mothers who received first-line intrapartum antibiotics (i.e., ampicillin or penicillin). The primary objective of this study was to compare the incidence of GBS EOS in term neonates born to mothers who receive either first, second-line or no intrapartum antibiotics. neonates A secondary aim was to describe the short term and survival outcomes of neonates born in the groups who developed GBS EOS. We hypothesized that routine second-line prophylaxis is not associated with improved clinical outcomes in these term neonates compared to intrapartum ampicillin/penicillin.
Materials and Methods
Study Population
This is a retrospective cohort study. Electronic medical records (EMR) linked to a Clinical Data Warehouse (CDW) that prospectively captures information from neonates cared for by the Pediatrix Medical Group across 446 neonatal intensive care units (NICUs) in North America were used.10 Data on multiple aspects of care were entered into a shared EMR to generate admission and daily progress notes, and discharge summaries. Information regarding maternal history, demographics, medications, laboratory results, diagnoses, and procedures were then transferred to the de-identified Pediatrix CDW for quality improvement and research purposes.10 Institutional IRB waiver of consent was obtained (Pro00113571).
We included term neonates who were subsequently discharged from a Pediatrix NICU between 2003 and 2020. We excluded neonates with missing information with unspecified discharge status and those with major congenital anomalies. We extracted data on all days of hospitalization in the NICU. Baseline data collected included duration of antibiotics administered to the neonates, maternal, antenatal and neonatal clinical and laboratory findings.
Definitions
All GBS positive mothers (i.e., mothers who were colonized with GBS or had a urogenital GBS infection during her pregnancy) were included. Maternal GBS status was defined as a mother being tested as positive for GBS during pregnancy. Neonates born at 37 or more completed weeks’ gestation were categorized as being born full term.11 In the infant, GBS EOS was defined as isolation of GBS from the blood or cerebrospinal fluid (CSF) before the first 7 days of life (i.e., days 0 through 6 inclusive). First-line therapy was defined as maternal treatment with penicillin, ampicillin, or ampicillin/sulbactam. Second-line therapy was defined as other prescribed antibiotics. Mothers who received both a first-line and second-line antibiotic were considered as treated by first-line therapy. Mothers who received no documented antibiotic therapy were analyzed as the no antibiotic group. Those who were documented as receiving antibiotics without a specific antibiotic listed were excluded from the treatment-based outcome analysis. Major congenital anomaly was defined as an anomaly present at birth that was lethal, life-shortening, life-threatening, requiring major surgery, or affecting the neonate’s quality of life in a significant way. Invasive ventilator support was defined as either conventional mechanical ventilation or high-frequency ventilation. Days of invasive ventilation or supplemental oxygen included all days during the hospitalization a neonate received either invasive ventilation or supplemental oxygen respectively. Percent days with invasive ventilation or supplemental oxygen was calculated as days of support divided by total hospital days. Supplemental oxygen at discharge was considered present if the infant was discharged alive and received supplemental oxygen on either the day before or the day of discharge. We defined vasopressor treatment as use of any of the following drugs: dobutamine, dopamine, epinephrine, milrinone, norepinephrine, or vasopressin. We assessed for invasive ventilation and vasopressor use within first week of life (i.e., days 0 through 6 inclusive).
The use of instrumentation included forceps and vacuum delivery. Use of tracheostomy at discharge and incidence of ventriculitis, septic arthritis, cellulitis, and hydrocephalus were included if documented by the treating clinician. Failed hearing screen was based on the results of the last hearing test prior to discharge with either a fail or defer considered a fail.
Outcomes
Our primary outcome of interest was incidence of GBS EOS in term neonates born to all mothers with GBS. Our secondary outcomes of interest were type of antibiotic used for GBS positive mothers, as well as in-patient mortality, duration of NICU stay, and morbidities such as septic arthritis, and sensorineural hearing loss for neonates with GBS EOS. We assessed these outcomes across three groups defined by maternal GBS treatment (first-line; second-line; and no antibiotic treatment).
Statistical analyses
Data were analyzed using descriptive statistics to calculate proportions. Data were summarized as count (percentage) and median [interquartile range] for categorical and continuous variables, respectively. Between group differences across single variables were assessed with chi square and Kruskal-Wallis tests. Multivariable logistic regression adjusting for maternal and infant factors used in univariate analysis was performed to calculate the adjusted odds ratio (aOR) of GBS EOS based on intrapartum prophylaxis received. We assessed for site adjustment as a random effect and planned to include a site adjustment if the coefficient was significantly different than 0 or otherwise use a pooled regression for the final analysis. We performed an additional sensitivity analysis, repeating the adjusted odds ratios and excluding all neonates born by Caesarean section. A p-value of less than 0.05 was considered statistically significant. Data management and statistical analyses were performed using Stata, version 16.1 (StataCorp, College Station, Texas, USA).
Results
Among 496,180 term neonates across 419 sites, 104,196 (21%) were born to GBS positive mothers. 97,983 GBS positive mothers had adequate prenatal antibiotic documentation: 49,234 (50%) and 12,679 (13%) mothers received first-line and second-line intrapartum prophylaxis, respectively, and 36,070 (37%) mothers received no antibiotic therapy (Figure 1).
Figure 1:
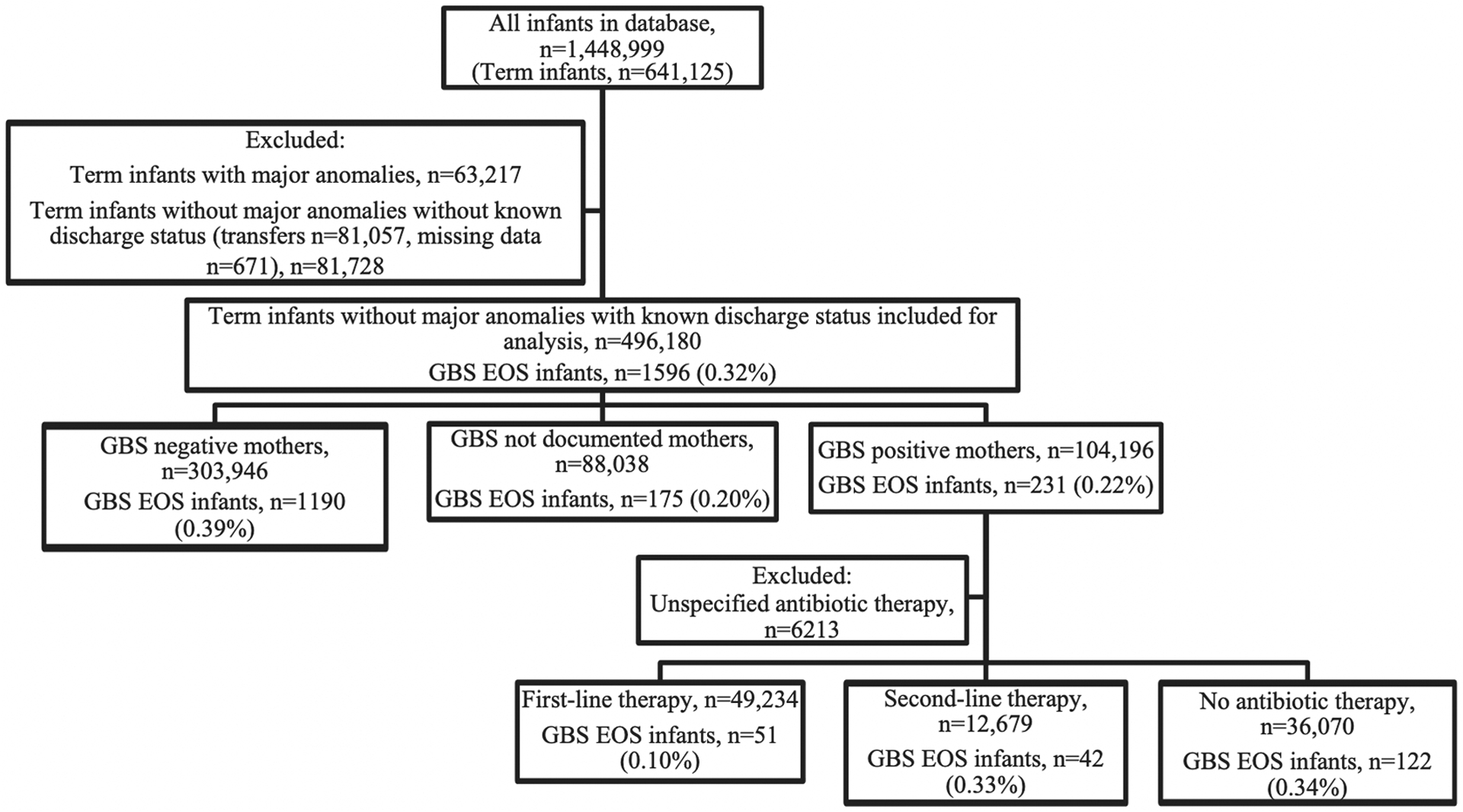
Flow diagram of GBS EOS incidence by maternal GBS status and maternal antibiotic treatment type for neonates from 2003 to 2020. Numbers given as count (percentage). All infants were born at ≥ 37 weeks’ gestation (full term). EOS, early onset sepsis; GBS, group B streptococcus
Maternal antibiotic usage for first-line prophylaxis were 54%, 45%, and 1% for penicillin, ampicillin, and ampicillin/sulbactam, respectively. Commonly used antibiotics for second-line prophylaxis were cefazolin (46%), clindamycin (28%), and vancomycin (9%). A detailed breakdown of the usage of other second-line antibiotics is detailed in Supplementary Table 1.
Maternal and infant characteristics based on type of antibiotics received for GBS positive mothers who delivered term babies are presented in Tables 1 and 2, respectively. Mothers who received second-line antibiotics compared to first-line antibiotics and no antibiotics were older (median [IQR]: 29 [24, 33] vs. 27 [22, 32] vs. 28 [24, 33] years old; p <.001), were more likely to be from White ethnicity (57% vs. 45% vs. 51%; p <.001), were more likely to be smokers (2% vs. 1% vs. 1%; p <.001), and more likely to have maternal gestational diabetes mellitus (18% vs. 11% vs. 15%; p <.001). They also had less instrumentation during labor (3% vs. 6% vs. 3%; p <.001). Term neonates across all 3 groups had similar baseline characteristics in terms of gestational age, birth anthropometric measures, gender distribution, and 5-minute and 10-minute APGAR scores. Term neonates whose mothers received second-line peripartum antibiotics, however, were more likely to be delivered via Caesarean section (64% vs. 30% vs. 55%; p <.001).
Table 1.
Maternal characteristics based on type of antibiotics received for GBS positive mothers of full-term neonates.
| All mothers (n = 97,983) |
First-line antibiotics (n = 49,234) |
Second-line antibiotics (n = 12,679) |
No antibiotics (n=36,070) | p-value | |
|---|---|---|---|---|---|
| Maternal age, median [IQR], years | 28 [23, 32] | 27 [22, 32] | 29 [24, 33] | 28 [24, 33] | <.001 |
| Ethnicity | <.001 | ||||
| White | 44,462 (45.4) | 20,792 (42.2) | 6,693 (52.8) | 16,977 (47.1) | |
| Black | 18,800 (19.2) | 9,970 (20.3) | 2,327 (18.4) | 6,503 (18.0) | |
| Hispanic | 22,112 (22.6) | 12,159 (24.7) | 2,177 (17.2) | 7,776 (21.6) | |
| Other | 5,842 (6.0) | 3,220 (6.5) | 638 (5.0) | 1,984 (5.5) | |
| Maternal GDM | 13,099 (13.4) | 5,388 (10.9) | 2,241 (17.7) | 5,470 (15.2) | <.001 |
| Maternal smoking | 732 (0.7) | 288 (0.6) | 160 (1.3) | 284 (0.8) | <.001 |
| Use of instrumentation | 4,610 (4.7) | 3,086 (6.3) | 431 (3.4) | 1,093 (3.0) | <.001 |
| Prolonged rupture of membranes for >18 hours | 6,457 (6.6) | 4,558 (9.3) | 711 (5.6) | 1,188 (3.3) | <.001 |
Data are presented as n (%) unless otherwise specified.
GBS, Group B Streptococcus; GDM, Gestational Diabetes Mellitus; IQR, Interquartile Range.
Chi square and Kruskal-Wallis tests were used to assess for differences across groups by type of antibiotic received for each variable.
Table 2.
Neonatal characteristics based on prenatal antibiotic received for GBS positive mothers of full-term neonates.
| All neonates (n = 97,983) |
First-line antibiotics (n = 49,234) |
Second-line antibiotics (n = 12,679) |
No antibiotics (n= 36,070) |
p-value | |
|---|---|---|---|---|---|
| Gestational age, median [IQR], weeks | 39 [38, 40] | 39 [38, 40] | 39 [38, 39] | 39 [38, 39] | <.001 |
| Birth weight, median [IQR], kg | 3.33 [2.98, 3.70] | 3.33 [2.99, 3.68] | 3.36 [2.98, 3.76] | 3.32 [2.95, 3.71] | <.001 |
| Length, median [IQR], cm | 50.8 [48.5, 52] | 50.8 [48.7, 52.1] | 50.5 [48.3, 52] | 50.2 [48.3, 52] | <.001 |
| Head circumference, median [IQR], cm | 34 [33, 35.5] | 34 [33, 35] | 34.5 [33, 35.5] | 34.3 [33, 35.5] | <.001 |
| Female | 41,297 (42.1) | 20,912 (42.5) | 5,287 (41.7) | 15,098 (41.9) | .11 |
| Singleton | 96,400 (98.4) | 48,807 (99.1) | 12,370 (97.6) | 35,223 (97.7) | <.001 |
| Mode of Delivery | <.001 | ||||
| Vaginal | 54,514 (55.6) | 34,277 (69.6) | 4,450 (35.1) | 15,787 (43.8) | |
| Caesarean-section | 41,885 (42.7) | 14,358 (29.2) | 8,041 (63.4) | 19,486 (54.0) | |
| 5-minute APGAR, median [IQR] | 9 [8, 9] | 9 [8, 9] | 9 [8, 9] | 9 [8, 9] | <.001 |
| 10-minute APGAR, median [IQR] | 8 [7, 9] | 8 [7, 9] | 8 [7, 9] | 8 [7, 9] | <.001 |
Data are presented as n (%) unless otherwise specified.
GBS, Group B Streptococcus; IQR, Interquartile Range.
Chi square and Kruskal-Wallis tests were used to assess for differences across groups by type of antibiotic received for each variable.
The overall incidence of GBS EOS was 0.32% (1,596/496,180) amongst all term neonates. The incidence of GBS EOS in neonates who were born to mothers with positive GBS carriage was 0.22% (231/104,196) (Figure 1). For neonates born to GBS positive mothers, the incidence of GBS EOS was 0.10% (51/49,234) for those who received first-line antibiotics, compared to 0.33% (42/12,679) and 0.34% (122/36,070) for those who received second-line or no antibiotics, respectively. The unadjusted odds ratio of GBS EOS incidence by treatment type for term neonates whose mothers received second-line intrapartum antibiotics and no antibiotics were OR 3.21, 95% CI: 2.13 – 4.82 and OR 3.27, 95% CI: 2.36 – 4.54, respectively, compared to first-line intrapartum antibiotics. A site effect was assessed for, but the coefficient was not significantly different from 0, so a pooled regression was used for the final analysis. On adjusted analysis using multivariable pooled regression, term neonates whose mothers received second-line intrapartum antibiotics and no antibiotics had higher risk of GBS EOS compared to first-line intrapartum antibiotics (aOR 4.12, 95% CI: 2.66 – 6.38 and aOR 3.80, 95% CI: 2.66 – 5.44, respectively). There was no statistically significant difference in the risk of GBS EOS in term neonates born to mothers who received second-line and versus no antibiotics (aOR 0.92, 95% CI: 0.64 – 1.33) compared to second-line. In a sensitivity analysis excluding all Caesarean section births, adjusted odds of GBS EOS remained higher in term neonates whose mothers received either second line (aOR 6.20 [3.47 – 11.1]) or no antibiotics (aOR 5.23 [3.28 – 8.33]) compared to first line antibiotics (Supplemental Table 2),
The outcomes of GBS EOS based on peripartum antibiotic received for full term neonates with GBS positive mothers are described in Tables 4 and 5. Of note, there were no significant difference in neonatal mortality rate or either pressor or invasive ventilator use in first week of life amongst all groups. With regards to respiratory support, we did not observe any significant difference between days of invasive ventilation or use of supplemental oxygen amongst all groups. Of the term neonates who developed GBS EOS, there were no significant differences in long term morbidities amongst all groups with regards to tracheostomy, rates of ventriculitis, septic arthritis, cellulitis, hydrocephalus or failed hearing screen.
Table 4.
Outcomes of GBS EOS episodes based on peripartum antibiotic received for full term neonates with GBS positive mothers.
| First-line antibiotics (n = 51) |
Second-line antibiotics (n = 42) |
No antibiotics (n = 122) |
p-value | |
|---|---|---|---|---|
| Postnatal age at positive GBS culture, median [IQR], days | 0 [0, 0] | 0 [0, 0] | 0 [0, 1] | .004 |
| Pressor use in first week of life | 5 (9.8) | 4 (9.5) | 14 (11.5) | .91 |
| Invasive ventilation in first week of life | 9 (17.6) | 9 (21.4) | 24 (19.7) | .90 |
| Days of invasive ventilation, median [IQR] | 0 [0, 0] | 0 (0, 0) | 0 [0, 0] | .94 |
| Percent days with invasive ventilation, median [IQR] | 0 [0, 0] | 0 (0, 0) | 0 [0, 0] | .87 |
| Days on supplemental oxygen, median [IQR] | 1 [0, 3] | 0 [0, 2] | 1 [0, 3] | .36 |
| Percent days with supplemental oxygen, median [IQR] | 9 [0, 26] | 0 [0, 18] | 8 [0, 20] | .37 |
| Length of stay (days), median [IQR] | 11 [10, 12] | 11.5 [11, 14] | 12 [11, 15] | 0.06 |
Data are presented as n (%) unless otherwise specified.
GBS, Group B Streptococcus; EOS, Early-Onset Sepsis.
Chi square and Kruskal-Wallis tests were used to assess for differences across groups by type of antibiotic received for each variable.
Table 5.
Complications and short-term morbidities of GBS EOS episodes based on type of prenatal antibiotic received for full term neonates with GBS positive mothers.
| First-line antibiotics (n = 51) |
Second-line antibiotics (n = 42) |
No antibiotics (n = 122) |
p-value | |
|---|---|---|---|---|
| Mortality | 1 (2.0) | 1 (2.4) | 6 (4.9) | .70 |
| Tracheostomy at discharge | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Supplemental oxygen at discharge | 3 (5.9) | 0 (0.0) | 0 (0.0) | .02 |
| Ventriculitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Septic Arthritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Cellulitis | 0 (0.0) | 0 (0.0) | 1 (0.8) | 1 |
| Hydrocephalus | 0 (0.0) | 0 (0.0) | 0 (0) | - |
| Failed hearing | 3 (5.9) | 0 (0.0) | 2 (1.6) | .16 |
Data are presented as n (%) unless otherwise specified.
GBS, Group B Streptococcus; EOS, Early-Onset Sepsis.
Fisher’s exact test was used to compare for difference across groups by type of antibiotic received for each variable. p-values could not be calculated due to too few observations.
Comment
Principal Findings
Using one of the largest repositories of neonatal data in the world, we demonstrated that neonates exposed to second-line maternal GBS prophylaxis had an increased risk of GBS EOS compared to those exposed to first-line maternal GBS prophylaxis. In addition, there was no statistical significant difference in GBS EOS incidence between second-line versus no antibiotics in mothers with GBS carriage.
Our data offers insights into the type of second-line antibiotic prophylaxis of choice by clinicians and the efficacy of the various intrapartum antibiotics amongst GBS positive mothers who did not receive first-line antibiotics. While cefazolin was the most common second-line antibiotic prophylaxis used, clindamycin and vancomycin were the second and third antibiotic of choice amongst clinicians, respectively. Many physicians continue to use antibiotics which are not routinely recommended, (e.g., gentamicin, azithromycin and oral amoxicillin).
Furthermore, our data reveals that 37% of mothers received no antibiotics at the time of delivery after they were screened to be GBS positive, indicating a large portion of neonates in our cohort received suboptimal antibiotic coverage during labor.
Results in the context of what is known
Our study findings reinforce that the current practice of using penicillin or ampicillin as first-line antibiotic prophylaxis is effective in reducing the risk of GBS EOS in term neonates, in line with the American College of Obstetrics and Gynaecology (ACOG) guidelines.12,13 In addition, since 2019, the ACOG has continued to endorse the universal antenatal culture-based approach to identify women who would receive intrapartum antibiotic prophylaxis to prevent GBS EOS.12 From a mechanistic point of view, penicillin or intravenous ampicillin would be the preferred priority agent for intrapartum GBS prophylaxis because of its more targeted antimicrobial activity and a lower likelihood of inducing resistance in other vaginal organisms.14
Additionally, our findings support the Centers for Disease Control and Prevention (CDC) guidelines whereby the use of clindamycin should only be considered as an appropriate alternative for GBS intrapartum prophylaxis if the GBS isolate is known to be susceptible to clindamycin.1 Our data also question the utility of offering routine second-line antibiotics based on physician preference to mothers who are unable to receive penicillin. There has been increasing resistance to second-line antibiotics, such as erythromycin and clindamycin, amongst GBS, with several countries noting increased resistance rates in recent years.15 Moreover, resistance to other antibiotic classes, such as fluoroquinolones and aminoglycosides, continues to rise.15 To compound the problem, current compliance to recommendations for outpatient GBS susceptibility testing for penicillin-allergic patients is suboptimal, and may occur in only 65% of patients when indicated.16 This may potentially explain the relatively high rates of vancomycin use in our study.
Our findings mirror that of other studies which showed that despite clear guidelines for GBS prophylaxis, both undertreatment (no antibiotics, underuse or overuse) and overtreatment were common,17 whereby nearly 1 in 6 GBS positive, penicillin-allergic mothers did not receive any form of intrapartum antibiotic.14 Contributing factors to inappropriate use of antibiotics include the lack of detailed allergy history and lack of GBS susceptibility testing.1,18
Clinical Implications
Inappropriate use of antibiotics for GBS prophylaxis potentially has dire consequences for neonatal early onset infections and may indicate that hospitals need improvement in adopting guideline compliant GBS prophylaxis practices.
Strengths and Limitations
Strengths of this study include the use of a comprehensive database which allowed for an unbiased extraction of information from an entire neonatal population cared for in a large number of NICUs. Our dataset is highly representative of the scope of practice in the United States as it represents the practice of newborn medicine ranging from small community intensive care units to some of the largest NICUs in the United States. However, this study is not without limitations. First, although the data was prospectively collected, we do not know the specific indication for intrapartum antibiotics therapy and were unable to decipher the reasons for physicians’ choice of intrapartum antibiotics and the antimicrobial susceptibility patterns which could influence the choice of intrapartum antibiotics. Second, we do not have access to the antibiotic dosing regimen, including dose or frequency of administration. It is possible that participating centres may have mis-dosed therapies in either the first- or second-line group which could result in inappropriate underestimation (for first-line mis-dosing) or overestimation (for second-line mis-dosing) of the differences between both groups. Third, we only included mother-neonate dyads where the term neonates were admitted to neonatal intensive care units and this would limit generalizability of our findings to all maternal GBS carriers. Fourth, there were several potential confounders we were unable to adjust for as they were not collected variables in our dataset: the presence of active labor prior to Caesarean section, if a Caesarean section was elective, the gestational age at membrane rupture for neonates with PROM, presence of maternal GBS bacteriuria, and presence of chorioamnionitis. We attempted to partially account for these missing variables related to Caesarean sections with the sensitivity analysis excluding all neonates born by Caesarean section which showed similar adjusted odds of GBS EOS by treatment group. Finally, there was inadequate documentation to assess why women in the no antibiotic group did not receive therapy. This may potentially be due to physicians’ concerns for maternal allergy or precipitous delivery.
Research Implications
Further study needs to be done to understand the reasons underlying the non-receipt of antibiotic therapy in GBS positive mothers, and also how to optimise care for this cohort of patients.
Conclusions
In summary, our main findings were twofold. First, there appears to be increased risk of GBS EOS with use of second-line compared to first-line maternal GBS prophylaxis. Second, there was no statistical significant difference in GBS EOS infection incidence between second-line versus no antibiotics in mothers with GBS carriage. Penicillin allergy patch testing should be more widespread to ensure that women get the appropriate antibiotics when needed. Future large-scale randomized controlled trials need to be carried out to delineate the utility and cost effectiveness of second-line antibiotics in GBS prophylaxis for mothers who are unable to receive first line antibiotics.
Supplementary Material
Table 3.
Unadjusted and adjusted odds ratios of GBS EOS infection incidence by treatment type with pooled logistic regression.
| Unadjusted odds ratios of GBS EOS infection incidence by treatment type | ||
|---|---|---|
| Treatment group | Unadjusted OR (95% CI) | p-value |
| First-line | (reference) | - |
| Second-line | 3.21 (2.13 – 4.82) | <.001 |
| No antibiotics | 3.27 (2.36 – 4.54) | <.001 |
| Adjusted odds ratios of GBS EOS infection incidence by treatment type with pooled multivariable logistic regression | ||
| Treatment group | Adjusted odds ratio, aOR (95% CI) |
p-value |
| First-line | (reference) | - |
| Second-line | 4.12 (2.66 – 6.38) | <.001 |
| No antibiotics | 3.80 (2.66 – 5.44) | <.001 |
| Second-line | (reference) | - |
| First-line | 0.24 (0.16 – 0.38) | <.001 |
| No antibiotics | 0.92 (0.64 – 1.33) | .66 |
| Covariates | ||
| Maternal age | 0.96 (0.94 – 0.99) | .005 |
| Race | ||
| White | (reference) | - |
| Black | 1.01 (0.70 – 1.46) | .96 |
| Hispanic | 0.72 (0.48 – 1.09) | .12 |
| Other | 1.62 (0.98 – 2.70) | .06 |
| Use of instrumentation | 0.65 (0.30 – 1.40) | .27 |
| Prolonged rupture of membranes | 1.05 (0.58 – 1.89) | .88 |
| Gestational age | 1.19 (1.05 – 1.35) | .007 |
| Birth weight | 0.92 (0.71 – 1.20) | .54 |
| Male gender | 0.72 (0.54 – 0.97) | .029 |
| Vaginal birth | 1.33 (0.98 – 1.81) | .07 |
| 5-minute APGAR | 0.80 (0.75 – 0.86) | <.001 |
GBS, Group B Streptococcus; EOS, Early-Onset Sepsis. The aOR for vaginal birth in compared to the reference group of Caesarean section births.
AJOG at a Glance.
- Why was this study conducted?
- To understand the role of second-line or no intrapartum antibiotics in preventing GBS EOS (Group B streptococcus early-onset sepsis) as opposed to mothers who received first-line intrapartum antibiotics.
- What are the key findings?
- 50% and 13% of GBS positive mothers received first-line and second-line intrapartum antibiotic prophylaxis, respectively, while 37% received no antibiotic therapy.
- Compared to neonates whose mothers received first-line intrapartum antibiotics, neonates whose mothers received second-line or nil intrapartum antibiotics were 4.1x and 3.8x more likely to develop GBS EOS, respectively.
- What does this study add to what is already known?
- There appears to be an increased risk of GBS EOS with use of second-line compared to first-line maternal GBS prophylaxis, but no statistical significant difference in risk between second-line versus no maternal GBS prophylaxis.
- Large-scale randomized trials would be useful to investigate the utility and cost-effectiveness of second-line antibiotics in GBS prophylaxis.
Acknowledgements.
The authors would like to thank Ms Sheena Nishanti Ramasamy (Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore) for her assistance with editing, formatting and submission of the manuscript.
Competing interests.
R.G.G. and C.P.H. received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/). The other authors report no conflicts of interests.
Role of funding source.
J.M.L. is supported by the National University Health System Clinician Scientist Program 2.0 (Award number: NCSP2.0/2023/PVO/LJM). J.H.L.’s institution received research funding from National Medical Research Council, Singapore and Thrasher Research Fund, United States. H.P.F. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Award number: T32HD094671). None of the funders were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication
Footnotes
Ethics, Consent and Permission. Institutional Review Board waiver of consent was obtained (Pro00113571).
Availability of data and materials.
The computer code for analysis as well as the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797 [published correction appears in Obstet Gynecol. 2020 Apr;135(4):978–979]. Obstet Gynecol. 2020;135(2):e51–e72. doi: 10.1097/AOG.0000000000003668 [DOI] [PubMed] [Google Scholar]
- 2.Muller-Pebody B, Johnson AP, Heath PT, et al. Empirical treatment of neonatal sepsis: are the current guidelines adequate?. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F4–F8. doi: 10.1136/adc.2009.178483 [DOI] [PubMed] [Google Scholar]
- 3.Seale AC, Bianchi-Jassir F, Russell NJ, et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin Infect Dis. 2017;65(suppl_2):S200–S219. doi: 10.1093/cid/cix664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yow MD, Leeds LJ, Thompson PK, Mason EO Jr, Clark DJ, Beachler CW. The natural history of group B streptococcal colonization in the pregnant woman and her offspring. I. Colonization studies. Am J Obstet Gynecol. 1980;137(1):34–38. doi: 10.1016/0002-9378(80)90382-8 [DOI] [PubMed] [Google Scholar]
- 5.Le Doare K, O’Driscoll M, Turner K, et al. Intrapartum Antibiotic Chemoprophylaxis Policies for the Prevention of Group B Streptococcal Disease Worldwide: Systematic Review. Clin Infect Dis. 2017;65(suppl_2):S143–S151. doi: 10.1093/cid/cix654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puopolo KM, Lynfield R, Cummings JJ; COMMITTEE ON FETUS AND NEWBORN; COMMITTEE ON INFECTIOUS DISEASES. Management of Infants at Risk for Group B Streptococcal Disease [published correction appears in Pediatrics. 2019 Oct;144(4):]. Pediatrics. 2019;144(2):e20191881. doi: 10.1542/peds.2019-1881 [DOI] [PubMed] [Google Scholar]
- 7.Nanduri SA, Petit S, Smelser C, et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance [published correction appears in JAMA Pediatr. 2019 Mar 1;173(3):296] [published correction appears in JAMA Pediatr. 2019 May 1;173(5):502]. JAMA Pediatr. 2019;173(3):224–233. doi: 10.1001/jamapediatrics.2018.4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice LB. Emergence of vancomycin-resistant enterococci. Emerg Infect Dis. 2001;7(2):183–187. doi: 10.3201/eid0702.010205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang HY, Perencevich EN, Nair R, et al. Incidence and Outcomes Associated With Infections Caused by Vancomycin-Resistant Enterococci in the United States: Systematic Literature Review and Meta-Analysis. Infect Control Hosp Epidemiol. 2017;38(2):203–215. doi: 10.1017/ice.2016.254 [DOI] [PubMed] [Google Scholar]
- 10.Spitzer AR, Ellsbury D, Clark RH. The Pediatrix BabySteps® Data Warehouse--a unique national resource for improving outcomes for neonates [published correction appears in Indian J Pediatr. 2015 Jul;82(7):669]. Indian J Pediatr. 2015;82(1):71–79. doi: 10.1007/s12098-014-1585-2 [DOI] [PubMed] [Google Scholar]
- 11.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445–2446. doi: 10.1001/jama.2013.6235 [DOI] [PubMed] [Google Scholar]
- 12.The American College of Obstetricians and Gynecologists (ACOG). Prevention of Group B Streptococcal Early-Onset Disease in Newborns ACOG: ACOG; 2020. [cited 2023 March 28]. Available from: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns?utm_source=vanity&utm_medium=web&utm_campaign=clinical [Google Scholar]
- 13.Wang X, Chan PHY, Lau HYS, Tsoi K, Lam HS. Epidemiologic Changes of Neonatal Early-onset Sepsis After the Implementation of Universal Maternal Screening for Group B Streptococcus in Hong Kong [published online ahead of print, 2023 Jul 3]. Pediatr Infect Dis J. 2023;10.1097/INF.0000000000004022. doi: 10.1097/INF.0000000000004022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Lin XZ. Updates in prevention policies of early-onset group B streptococcal infection in newborns. Pediatr Neonatol. 2021;62(5):465–475. doi: 10.1016/j.pedneo.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 15.Hayes K, O’Halloran F, Cotter L. A review of antibiotic resistance in Group B Streptococcus: the story so far. Crit Rev Microbiol. 2020;46(3):253–269. doi: 10.1080/1040841X.2020.1758626 [DOI] [PubMed] [Google Scholar]
- 16.Paccione KA, Wiesenfeld HC. Guideline adherence for intrapartum group B streptococci prophylaxis in penicillin-allergic patients. Infect Dis Obstet Gynecol. 2013;2013:917304. doi: 10.1155/2013/917304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pineles BL, Goodman KE, Pineles L, Harris AD. Appropriate Antibiotic Use for Group B Streptococcus Prophylaxis Among Penicillin-Allergic Patients in Academic and Nonacademic Hospitals. Open Forum Infect Dis. 2022;9(10):ofac514. Published 2022 Oct 5. doi: 10.1093/ofid/ofac514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cate JJM, Burn M, Kwah J, et al. Survey of Obstetric Providers to Assess the Knowledge and Management of a Reported Penicillin Allergy in Pregnant Women. Am J Perinatol. 2023;40(1):1–8. doi: 10.1055/a-1877-9970 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The computer code for analysis as well as the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


