Abstract
Background
Ankylosing spondylitis (AS) is a chronic inflammatory disease of unknown cause and affects mainly the spine, but can also affect other joints. Disease progression may result in loss of mobility and function. Sulfasalazine is a disease‐modifying antirheumatic drug used in the treatment of AS. However, its efficacy remains unclear. This is an update of a Cochrane review first published in 2005.
Objectives
To evaluate the benefits and harms of sulfasalazine for the treatment of ankylosing spondylitis (AS).
Search methods
We searched for relevant randomized and quasi‐randomized trials in any language, using the following sources: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 11); MEDLINE (2003 to 28 November 2013); EMBASE (2003 to 27 November 2013); CINAHL (2003 to 28 November 2013); Ovid MEDLINE data, World Health Organization International Clinical Trials Registry Platform (28 November 2013); and the reference sections of retrieved articles.
Selection criteria
We evaluated randomized and quasi‐randomized trials examining the benefits and harms of sulfasalazine on AS.
Data collection and analysis
Two review authors independently reviewed unblinded trial reports according to the selection criteria. Disagreements on the inclusion of the studies were resolved, when necessary, by recourse to a third review author. The same authors independently assessed the risk of bias of included trials and entered the data extracted from the included trials. We combined results using mean difference (MD) or standardised mean difference (SMD) for continuous data, and risk ratio (RR) for dichotomous data.
We restructured outcome measures for this update based on recommendations from the editorial group. Major outcomes included: pain, Bath ankylosing spondylitis disease activity index (BASDAI), Bath ankylosing spondylitis function index (BASFI), Bath ankylosing spondylitis metrology index (BASMI), radiographic progression, total number of withdrawals due to adverse events, and serious adverse events.
Main results
We did not add any new studies to this review following the updated search. In the original review, we included 11 studies in the analysis, involving 895 participants in total. All included studies compared sulfasalazine with placebo. We judged most of the studies as low risk of bias or unclear risk of bias in five domains (random sequence generation, allocation concealment, blinding of outcome assessment, selective reporting, and other sources of bias). However, for incomplete outcome data, we only judged one trial at low risk of bias.
None of the included trials assessed BASDAI, BASFI, BASMI or radiographic progression. Different parameters were used to assess pain. The pooled MD for back pain measured on a 0 to 100 mm visual analogue scale was ‐2.96 (95% confidence interval (CI) ‐6.33 to 0.41; absolute risk difference 3%, 95% CI 1% to 6%; 6 trials). Compared to placebo, a significantly higher rate of withdrawals due to adverse effects (RR 1.50, 95% CI 1.04 to 2.15; absolute risk difference 4%, 95% CI 0.4% to 8.8%; 11 trials) was found in the sulfasalazine group. A serious adverse reaction was reported in one patient taking sulfasalazine (Peto odds ratio 7.50, 95% CI 0.15 to 378.16).
Authors' conclusions
There is not enough evidence to support any benefit of sulfasalazine in reducing pain, disease activity, radiographic progression, or improving physical function and spinal mobility in the treatment of AS. A statistically significant benefit in reducing the erythrocyte sedimentation rate and easing spinal stiffness was mentioned in the previous version. However, the effect size was very small and not clinically meaningful. More withdrawals because of side effects occurred with sulfasalazine. Further studies, with larger sample sizes, longer duration, and using validated outcome measures are needed to verify the uncertainty of sulfasalazine in AS.
Keywords: Humans; Antirheumatic Agents; Antirheumatic Agents/therapeutic use; Randomized Controlled Trials as Topic; Spondylitis, Ankylosing; Spondylitis, Ankylosing/drug therapy; Sulfasalazine; Sulfasalazine/therapeutic use
Plain language summary
Sulfasalazine for ankylosing spondylitis
We conducted a review of the effect of sulfasalazine for people with ankylosing spondylitis. After searching for all relevant studies up to November 2013, we found 11 studies involving 895 people. Our findings are summarised below.
The review showed that in people with ankylosing spondylitis:
‐ compared with fake pills, sulfasalazine probably has little or no difference in pain, disease activity, physical function, spinal mobility, patient and physician global assessment;
‐ damage to the spine as seen on x‐ray or magnetic resonance image was not measured and therefore it is not known whether sulfasalazine slows damage;
‐ people had side effects such as stomach upsets, skin reactions/rashes and mouth sores;
‐ more people stopped taking sulfasalazine because of the side effects than when taking fake pills; and
‐ there is not enough evidence to be certain of the benefits and harms of sulfasalazine for ankylosing spondylitis, and more research is needed.
What is ankylosing spondylitis and what is sulfasalazine?
Ankylosing spondylitis is a type of arthritis, usually in the joints and ligaments of the spine. It may also affect the shoulders, hips, or other joints. Pain and stiffness occur and limit movement in the back and in other joints that are affected.
Key results of this review
Pain
‐ People who took sulfasalazine rated their pain to be 3 points lower on a scale of 0 to 100 after 3 to 36 months than those who took placebo (3% absolute improvement).
‐ People who took sulfasalazine rated their pain to be 47 on a scale of 0 to 100 after 3 to 36 months.
‐ People who took placebo rated their pain to be 50 on a scale of 0 to 100 after 3 to 36 months.
Bath ankylosing spondylitis disease activity index (BASDAI)
This outcome was not measured in the studies.
Bath ankylosing spondylitis function index (BASFI)
This outcome was not measured in the studies.
Bath ankylosing spondylitis metrology index (BASMI)
This outcome was not measured in the studies.
Radiographic progress
This outcome was not measured in the studies.
Total number of withdrawals due to adverse events
‐ 23 more people taking sulfasalazine withdrew due to adverse events than those taking placebo.
‐ 13 out of 100 people taking sulfasalazine withdrew due to adverse events.
‐ 9 out of 100 people taking fake pills withdrew due to adverse events.
Serious adverse events
Only one person out of 469 stopped taking sulfasalazine for serious adverse events.
Summary of findings
Summary of findings for the main comparison. Sulfasalazine compared to placebo for ankylosing spondylitis.
| Sulfasalazine compared to placebo for ankylosing spondylitis | ||||||
| Patient or population: Patients with ankylosing spondylitis Settings: Outpatients and inpatients Intervention: Sulfasalazine Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sulfasalazine | |||||
| Back pain (pooled data) 100 mm visual analogue scale, 0 = no pain, 100 = severe Follow‐up: median 26 weeks | The mean back pain (pooled data) in the control groups was 49.5 mm1 | The mean back pain (pooled data) in the intervention groups was 2.96 lower (6.33 lower to 0.41 higher) | 454 (6 studies) | ⊕⊕⊕⊝ Moderate2 | Absolute risk difference 3% lower (95% CI 1% to 6%); Relative percent change = 6% (95% CI 2% to 12%); NNT4 = n/a5 | |
| Mean improvement in Bath ankylosing spondylitis disease activity index (BASDAI) ‐ not reported | See comment | See comment | Not estimable | No data | See comment | Not measured |
| Mean improvement in Bath ankylosing spondylitis function index (BASFI) ‐ not reported | See comment | See comment | Not estimable | No data | See comment | Not measured |
| Mean improvement in Bath ankylosing spondylitis metrology index (BASMI) ‐ not reported | See comment | See comment | Not estimable | No data | See comment | Not measured |
| Radiographic progress ‐ not reported | See comment | See comment | Not estimable | No data | See comment | Not measured |
| Total number of withdrawals due to adverse events Follow‐up: median 26 weeks | 94 per 1000 | 134 per 1000 (98 to 182) | RR 1.43 (1.04 to 1.94) | 895 (11 studies) | ⊕⊕⊕⊝ Moderate2 | Absolute risk difference 4% (95% CI 0.4% to 8.8%); NNTH6 = 25 (95% CI 266 to 12) |
| Serious adverse events Follow‐up: mean 36 weeks | 0 per 1000 | 1 per 1000 (0 to 0) | OR 7.5 (0.15 to 378.16) | 264 (1 study) | ⊕⊕⊕⊝ Moderate3 | Absolute risk difference 750% (95% CI 15% to 37816%) (W); Relative percent change = 205% (95% CI ‐87% to 7309%); NNTH = n/a |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 From Clegg 1996, mean back pain at baseline in placebo = 48.9 (95% CI 3.0 to 94.8). 2 Different baseline value (3 as endpoint value and 3 as change from baseline value). 3 Wide confidence interval.
4 NNT (Number needed to treat). NNT for dichotomous outcomes calculated using Cates NNT calculator (http://www.nntonline.net/visualrx/).
5 n/a means result is not statistically significant.
6NNTH (Number needed to treat to harm).
Background
Description of the condition
Ankylosing spondylitis (AS) is a chronic inflammatory disease of unknown cause and belongs to a group of diseases known as spondyloarthropathies, which includes reactive arthritis, arthritis/spondylitis in inflammatory bowel disease, psoriatic arthritis/spondylitis and undifferentiated spondyloarthropathy. The disease has a relatively early onset, presenting at a mean age of 26 years, and occurs somewhat more frequently in men than in women (Feldtkeller 2003). Depending on the geographical region, the prevalence of AS is about 0.1% to 1.4% of the population (Akkoc 2005). Disease progression may result in loss of mobility and function, and therefore patients can experience a heavy disease burden, with pain and stiffness, loss of physical function, and severe impairment in quality of life (Davis 2005; Zink 2000).
Description of the intervention
Physiotherapy and exercises are the main non‐pharmacological therapies, with modest evidence showing a beneficial effect for AS (Dagfinrud 2008). Non‐steroidal anti‐inflammatory drugs (NSAIDs) have been the corner stone of pharmacological treatment. In 2010, the Assessment of Ankylosing Spondylitis/European League Against Rheumatism suggested that NSAIDs, including Coxibs, were recommended as first‐line drug treatment for AS patients with pain and stiffness. Continuous treatment with NSAIDs is preferred for patients with persistently active, symptomatic disease (Braun 2011a). For patients refractory or intolerant to NSAIDs, disease‐modifying antirheumatic drugs such as methotrexate (Chen 2013) and sulfasalazine have been used as a second‐line approach. Sulfasalazine has been used in rheumatoid arthritis for decades and a clinically and statistically significant benefit in rheumatoid arthritis disease activity has been confirmed in a systematic review (Suarez‐Almazor 1998). It is the best studied disease‐modifying antirheumatic drug used in AS, but its efficacy remains unclear. Although AS is difficult to treat, the treatment options for AS have been broadened since the discovery of TNF‐alpha inhibitors, e.g. adalimumab, etanercept, and infliximab. TNF‐alpha inhibitors have been extensively studied in AS (Zochling 2005). Randomized controlled trials (RCTs) (Braun 2002; Gorman 2002; van den Bosch 2002) showed that they were highly effective in improving disease activity, spinal mobility, function, and pain. However, their expense means that few patients can access them.
How the intervention might work
Sulfasalazine has been described as an anti‐inflammatory drug or immunomodulator (Smedegård 1995). We hypothesized that sulfasalazine had a similar effect on AS. However, the accurate mechanisms of sulfasalazine in AS were unclear.
Why it is important to do this review
There is uncertainty whether sulfasalazine is an effective treatment for AS. We first performed a systematic review in Chen 2005, and this is an update of that original review.
Objectives
To evaluate the benefits and harms of sulfasalazine for the treatment of ankylosing spondylitis (AS).
Methods
Criteria for considering studies for this review
Types of studies
We evaluated randomized controlled trials (RCTs) and quasi‐RCTs in any language, examining the benefits and harms of sulfasalazine on ankylosing spondylitis (AS).
Types of participants
Patients with AS fulfilling any one of the following criteria: 1961 Rome, 1966 New York, modified 1984 New York, Amor or ESSG (European Spondyloarthropathy Study Group) criteria (Olivieri 2002). We included studies with spondyloarthropathies/spondyloarthritis participants if there were available data assessing the outcomes specific to patients with AS. Patients with or without the impairment of peripheral joints were eligible for inclusion.
Types of interventions
Sulfasalazine given orally for at least 12 weeks. We included the following comparisons.
Sulfasalazine versus placebo.
Sulfasalazine versus other medication.
Sulfasalazine versus no medication.
Types of outcome measures
Primary outcomes
According to the Cochrane Musculoskeletal Group (CMSG), the major outcomes included the following.
Pain (visual analogue scale or numerical rating scale).
Bath ankylosing spondylitis disease activity index (BASDAI).
Bath ankylosing spondylitis function index (BASFI).
Bath ankylosing spondylitis metrology index (BASMI).
Radiographic progression.
Total number of withdrawals due to adverse events.
Serious adverse events.
Secondary outcomes
Assessment of Ankylosing Spondylitis responses (e.g. ASAS40, ASAS20, partial remission) (van der Heijde 2002; van der Heijde 2005).
Other disease activity index, e.g. Ankylosing Spondylitis Disease Activity Score.
Physical function.
Spinal mobility and spinal stiffness.
Peripheral joints/entheses (pain, swelling and tenderness).
Patient global assessment and physician global assessment.
Fatigue.
Level of acute phase reactants, including erythrocyte sedimentation rate and C‐reactive protein.
Total number of withdrawals.
Search methods for identification of studies
We searched for relevant randomized controlled trials (RCTs) and quasi‐RCTs in any language, using the following sources: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 11); MEDLINE (2003 to 28 November 2013); EMBASE (2003 to 28 November 2013); CINAHL (2003 to 28 November 2013); Ovid MEDLINE data; World Health Organization International Clinical Trials Registry Platform (28 November 2013); and the reference sections of retrieved articles. Search strategies for the original review are in Appendix 1. Search strategies for the second (current) review are in Appendix 2 and Appendix 2. Search results for the second (current) version are in Appendix 4 and Appendix 3.
Data collection and analysis
Selection of studies
We identified potential trials for inclusion from the search results (see Figure 1). JC and SL independently reviewed unblinded trial reports for this review update (JC and CL for the original review) according to the selection criteria. We resolved disagreements about the inclusion of studies, when necessary, by recourse to a third review author.
1.
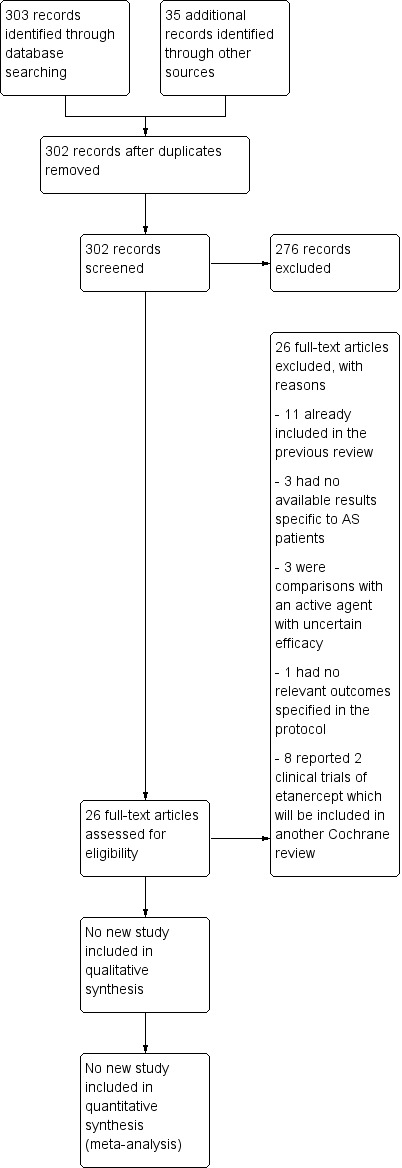
Study flow diagram.
Data extraction and management
JC and SL recorded the extracted data from the included trials onto a prestructured data extraction sheet and entered the data independently into Review Manager 5 (RevMan 2014). We only included outcomes in the review that were specified in the protocol. We entered continuous data (e.g. visual analogue scales of pain, patient's global assessment) as means and standard deviations (SDs), and dichotomous outcomes (e.g. response, improvement) as number of events.
Assessment of risk of bias in included studies
For this review, we assessed risk of bias in all included studies using The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). There were six domains: random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias, as specified in Appendix 4. We categorized risk of bias as 'Low risk', 'High risk', or 'Unclear risk'. We independently addressed the included trials using a data collection form (Appendix 5).
Measures of treatment effect
For continuous data, we used mean difference (MD) or standardised mean difference (SMD) (depending on comparability of scales). For dichotomous data, we used risk ratio (RR).
Unit of analysis issues
In this review we included only RCTs and there were no unit of analysis issues.
Dealing with missing data
For continuous data (e.g. visual analogue scales of pain, patient global assessment), we analyzed only the available data. For dichotomous data of beneficial outcomes (e.g. response, improvement), we used intention‐to‐treat analysis, that is, we included all participants and assumed no expected event occurred in those participants who dropped out.
Assessment of heterogeneity
We explored heterogeneity using the Chi2 test, with significance set at P = 0.10. We measured heterogeneity using I2. We regarded I2≥ 50% as substantial heterogeneity.
Assessment of reporting biases
We planned using a funnel plot to detect publication bias.
Data synthesis
We performed meta‐analysis when the participants and interventions were sufficiently homogeneous, using Review Manager 5 (RevMan 2014). We used a random‐effects model to combine the results where heterogeneity was significant; otherwise, we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned on conducting subgroup analyses based on characteristics of participants (e.g. different AS classification criteria, male or female, with or without peripheral arthritis) and intervention (e.g. different dosage, different duration).
Sensitivity analysis
We performed three sensitivity analyses according to whether:
the allocation to intervention or control groups was truly randomized or quasi‐randomized (in order to explore the potential for selection bias); and
the outcome assessment was blinded (to explore the potential for assessment bias associated with knowledge of the intervention).
If either of the sensitivity analyses affected the results of the review then we would make conservative conclusions.
Summary of findings tables
We graded the evidence using GRADEpro software which was developed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group (www.gradeworkinggroup.org). We included the seven main outcomes (back pain, bath ankylosing spondylitis disease activity index (BASDAI), bath ankylosing spondylitis function index (BASFI), bath ankylosing spondylitis metrology index (BASMI), radiographic progress, total number of withdrawals due to adverse events, and serious adverse events) in Table 1.
Results
Description of studies
Results of the search
We obtained a total of 302 records in the updated search (Figure 1). In the preliminary screening of titles and abstracts, we excluded 276 records. After full‐text assessment, we found 11 records that had already been included in the first version of the review (Clegg 1996; Corkill 1990; Davis 1989; Dougados 1986; Feltelius 1986; Kirwan 1993; Krajnc 1990; Nissila 1988; Schmidt 2002; Taylor 1991; Winkler 1989). We excluded 15 records (nine studies; Braun 2011 included seven records) for the reasons specified in the Characteristics of excluded studies tables. Therefore, we did not add any new studies to this update.
Included studies
We included eleven studies and all of these trials compared sulfasalazine with placebo. They included a total of 895 participants, 469 receiving sulfasalazine and 426 placebo. The sample size ranged from 30 to 264. The duration of treatment ranged from 12 weeks to three years. The dosage of sulfasalazine (or placebo) was 2.0 g/day or up to 3.0 g/day, depending on the efficacy and tolerance. More than 30 outcomes were assessed.
Among the included participants, about 86% were male; Taylor 1991 did not present the information on the sex distribution of participants. Depending on the trial, age and duration of disease was reported as a mean or median value. The age ranged from 26.9 to 45.7 and the duration of disease ranged from 3.8 to 21.9 years. Zero to 68% of participants had peripheral arthritis. All studies claimed that they included participants with active disease, but the definitions of active disease varied. Further details are available in the Characteristics of included studies tables.
Excluded studies
Braun 2006 and Song 2011 are two trials concerning undifferentiated spondyloarthritis and early AS. We excluded them because the data specific to AS patients were unavailable. Three studies were RCTs comparing sulfasalazine with another active agent. These agents were Kunxian capsule (Chinese traditional medicine) (NCT00953979), Bushen Tongdu decoction (Chinese traditional medicine) (Xu 2008), and leflunomide (Zhao 2006). Because the efficacy of these agents was uncertain in AS and comparisons with them did not contribute to understanding the benefits of sulfasalazine, we excluded these three trials. We excluded one study, Deng 2009, because of lack of relevant outcomes specified in the protocol. Braun 2011 and Zhao 2009 were two RCTs comparing etanercept with sulfasalazine. We excluded them because there is another separate Cochrane review addressing biologics in AS (Zochling 2005).
Risk of bias in included studies
The risk of bias of included trials varied greatly and is summarized in Figure 2 and Figure 3.
2.
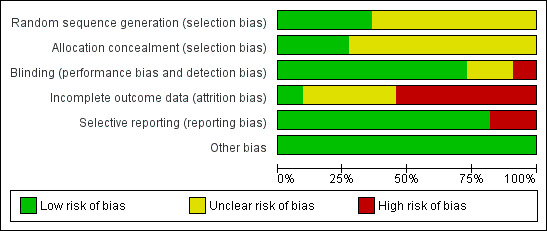
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
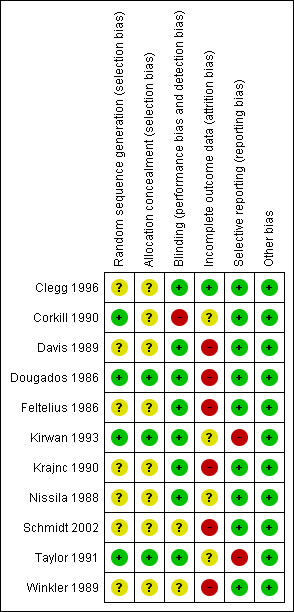
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Seven trials (Clegg 1996; Davis 1989; Feltelius 1986; Krajnc 1990; Nissila 1988; Schmidt 2002; Winkler 1989), were reported as randomized, but details of the methods of randomisation and concealment were not given. Four trials (Corkill 1990; Dougados 1986; Kirwan 1993; Taylor 1991), were reported as double‐blind and randomized. The methods of randomisation were generated by sealed envelopes or random number table. Concealment seemed to be adequate in Dougados 1986, Kirwan 1993 and Taylor 1991.
Blinding
We judged blinding at high risk of bias in Corkill 1990, as blinding was broken during the trial. We judged two trials at unclear risk of bias (Schmidt 2002; Winkler 1989). We judged all other trials at low risk of bias.
Incomplete outcome data
One trial presented complete outcome data (Clegg 1996). Four studies had more than 20% (Dougados 1986; Feltelius 1986; Kirwan 1993; Schmidt 2002), and two had more than 30% of participants drop out (Kirwan 1993; Schmidt 2002). Continuous outcome results were missed in these trials and therefore we judged them at high risk of bias. We judged all other trials at unclear risk of bias.
Selective reporting
Two trials were unable to provide the data of all the outcomes prespecified and we judged them at high risk of bias (Kirwan 1993; Taylor 1991). We judged all other trials at low risk of bias.
Other potential sources of bias
All the included trials appeared to be free of other sources of bias and therefore we judged them at low risk of bias.
Effects of interventions
See: Table 1
Because of multiple sources of heterogeneity, we could not perform meta‐analyses in all outcomes. Instead, we presented a narrative summary of pertinent findings from the individual studies. The main reported findings for the individual trials are also summarised in Additional Table 2 and Table 3.
1. Concise comparison of included trials.
| Study | Methodological quality | Duration/sample size | Disease duration | Peripheral arthritis | Baseline ESR | Intervention | Main results | Drop‐out |
| Clegg 1996 | Mutlicenter RCT Concealment: unclear risk Assessment: blind |
36 weeks/264 | 18.5+/‐11.6 | 29% | SSZ: 24.6+/‐18.0 Placebo: 25.2+/‐22.0 |
SSZ (or placebo) 2.0 g/d | ESR declined more with SSZ than with placebo (P < 0.0001). When comparing SSZ responders with non‐responders, the former had a greater decrease in ESR (P < 0.04) Patients with peripheral arthritis showed improvement that favored SSZ (P < 0.02) No significant difference in other parameters. One patient taking SSZ had severe adverse drug reaction |
19.3% |
| Corkill 1990 | RCT Concealment: unclear risk Assessment: blind |
48 weeks/62 | SSZ: 12.3+/‐8.2 Placebo: 16.1+/‐11.4 |
19% | SSZ: 15+/‐16 Placebo: 24+/‐26 |
SSZ (or placebo) 2.0 g/d | No significant difference between intervention groups | No data |
| Davis 1989 | RCT Concealment: unclear Risk assessment: blind |
3 months/30 | Median SSZ: 8.6 Placebo: 8.4 |
23% | SSZ: 24+/‐7.8(95% confidence limits) Placebo: 26.4+/‐8.6 |
SSZ (or placebo) 2.0 g/d | Claimed effective on the basis of before‐after comparison | 6.7% |
| Dougados 1986 | RCT Concealment: low risk Assessment: blind |
6 months/60 | Median 10 | 0% | SSZ: 13.5(median) Placebo: 11.0 |
SSZ (or placebo) 2.0 g/d | Success in patient assessment was more in SSZ than in placebo group. Function index and NSAIDs dosage were significantly improved in SSZ compared with placebo group. No difference was found in other parameters | 21.7% |
| Feltelius 1986 | RCT Concealment: unclear risk Assessment: blind |
12 weeks/37 | Median SSZ: 12.1 Placebo: 10.4 |
5% | SSZ: 24.3+/‐17.4 Placebo: 28.5+/‐19.5 |
SSZ (or placebo) up to 3.0 g/d | Only graphs (no figures) were presented. Compared with placebo group, morning stiffness and sleep disturbance were significantly improved in SSZ group Analysis of SSZ group showed that the greatest improvement were those with ESR > 20 mm/hr or haptoglobin > 3.8 g/L |
21.6% |
| Kirwan 1993 | RCT Concealment: low risk Assessment: blind |
3 years/89 | SSZ: 19+/‐12 Placebo: 21.9+/‐11.7 |
28% | No data | SSZ (or placebo) 2.0 g/d | Occurence of peripheral joint symptoms was lower in SSZ group: SSZ: 0.298 episodes/yr Placebo: 0.392 episodes/yr, P < 0.05 No difference was found in Schober test, chest expansion and cervical spine lateral flexion. More drop‐outs in SSZ group |
30.3% |
| Krajnc 1990 | RCT Concealment: unclear risk Assessment: blind |
24 weeks/95 SSZ = 71 Placebo = 24 |
No data | 66% | SSZ: 41+/‐19 Placebo: 43+/‐18 |
SSZ (or placebo) up to 3.0 g/d | On the basis of before‐after treatment comparison, duration of morning stiffness, number of painful and swollen joints, and ESR, there was significant improvement in SSZ group Duration of morning stiffness and ESR value were given in the paper and we found no significant difference between the intervention groups |
14.3% |
| Nissila 1988 | RCT Concealment: unclear risk Assessment: blind |
26 weeks/85 | SSZ: 5.4+/‐7.3 Placebo: 3.8+/‐4.3 |
68% | SSZ: 42+/‐20 Placebo: 46+/‐19 |
SSZ (or placebo) up to 3.0 g/d | Significant differences between intervention groups were observed in severity of morning stiffness, chest expansion and ESR. We also found severity of pain significantly improved in SSZ, compared with placebo group | 12.2% |
| Schmidt 2002 | RCT Concealment: unclear risk Assessment: blind? |
26 weeks/70 | SSZ: 16.7+/‐7.2 Placebo: 16.3+/‐7.8 |
36% | SSZ: 23.1+/‐3.2 Placebo 20.4+/‐2.4 |
SSZ (or placebo) 3.0 g/d | No significant difference was found between intervention groups except IgA. There were more drop‐outs in SSZ than in placebo group (18/34 versus 7/36) | 32.9% |
| Taylor 1991 | RCT Concealment: low risk Assessment: blind |
1 year/40 | SSZ: 11+/‐1.6 Placebo: 10.7+/‐1.6 |
15% | SSZ: 27 Placebo: 25 |
SSZ (or placebo) 2.0 g/d | No significant difference was found between intervention groups in all parameters except pain (measured with visual analogue scale). However, the pooled result showed no statistically significant, too | 17.5% |
| Winkler 1989 | RCT Concealment: unclear risk Assessment: blind? |
24 weeks/63 | Median SSZ: 10.8 Placebo: 11.2 |
33% | SSZ: 33.4+/‐20.4 Placebo: 26.9+/‐16.4 |
SSZ (or placebo) 2.0 g/d | The advantage of SSZ over placebo were significant only in the duration of morning stiffness and disturbance of sleep. The same results were found in the patients with axial form (N = 34). In patients with peripheral arthritis (N = 15), articular index showed significant improvement in SSZ over placebo | 22.2% |
ESR ‐ erythrocyte sedimentation rate g/d ‐ grams per day Ig A ‐ immunoglobulin A NSAIDs ‐ non‐steroidal anti‐inflammatory drugs RCT ‐ randomized controlled trials SSZ ‐ sulfasalazine
2. Randomized controlled trials comparing sulphasalazine with placebo.
| Study and Duration | Participants | Outcomes assessed | Results reported | Present analysis | ESR results | Spinal stiffness |
| Clegg 1996, 36 weeks | 264 (SSZ: 31) (Placebo: 133) 29% with PA DD (year): 18.5±11.6 ESR (mm/h): 26.4±18.0 (SSZ) 25.2±22.0 (placebo) |
Primary outcomes included response to treatment, improvement in PhGA, PGA, back pain and morning stiffness. Secondary outcomes included night pain (event), duration of morning stiffness, back pain VAS, spondylitis function index, joint/tenderness score, joint swelling score, dactylitis score, enthesopathy index, spondylitis articular index, chest expansion, Schober's test, occiput‐to‐wall test, fingers‐to‐floor test, ESR and CRP | Drop‐out: 19.3%. Both end point value and change from baseline were presented for all continuous outcomes. No difference was found between treatment groups in all outcomes except ESR, which declined more with SSZ than placebo group (P < 0.0001). When comparing SSZ responders with non‐responders, the former had a greater decrease in ESR (P < 0.04). Subgroup analysis showed that in patients with PA, 55.9% of SSZ group and 30.2% of placebo group got peripheral response (P = 0.023) | All the results reported have been confirmed except subgroup analysis where no information about treatment allocation was available for analysis. MD for ESR (change from baseline) was ‐3.10 mm/h, 95% CI ‐4.85 to ‐1.35 mm/h, favoring SSZ group | Absolute benefit from SSZ: ‐3.6 mm/h Relative difference in change from baseline: ‐14% |
Did not report |
| Corkill 1990, 48 weeks | 62 (SSZ: 32) (Placebo: 30) 19% with PA DD (year): 12.3±8.2 (SSZ) 16.1±11.4 (placebo) ESR (mm/h): 15±16 (SSZ) 24±26 (placebo) |
Spinal pain VAS, spinal stiffness VAS, peripheral joint pain VAS, Schober's test, chest expansion, cervical flexion, cervical rotation, and ESR | Drop‐out: 1.6%. No significant difference was found between treatment groups | Because SDs were not given for all outcomes, these results could not be analyzed | Absolute benefit from SSZ: ‐0.1 mm/h Relative difference in change from baseline: ‐1% |
Absolute benefit from SSZ: ‐9.8 mm on 100 mm VAS Relative difference in change from baseline: ‐25% |
| Davis 1989, 3 months | 30 (SSZ: 15) (Placebo: 15) 23% with PA DD (year): 8.6 (SSZ) 8.4 (placebo) ESR (mm/h): 24±7.8 (SSZ) 26.4±8.6 (placebo) |
Pain VAS, spinal stiffness VAS, sleep disturbance (event), occiput‐to‐wall test, fingers‐to‐floor test, ESR and CRP | Drop‐out: 6.7%. In SSZ group, all clinical outcomes showed significantly improved when initial and 3 months results are compared | Pain VAS, spinal stiffness VAS and CRP could not be analyzed because means and SDs were not given. No significant difference was found in any other outcome | Absolute benefit from SSZ: ‐5.4 mm/h Relatiive difference in change from baseline: ‐20% |
Absolute benefit from SSZ: ‐20 mm on 100 mm VAS Relative difference in change from baseline: ‐40% |
| Dougados 1986, 6 months | 60 (SSZ: 30) (Placebo: 30) None with PA DD (year, median): 10 ESR (mm/h, median): 13.5 (SSZ) 11.0 (placebo) |
PGA, score of daily NSAIDs, pain VAS, joint index, frequency of nocturnal awakening, function index, Schober's test, fingers‐to‐floor test, chest expansion and ESR | Drop‐out: 21.7%. Success in PGA was more in SSZ than in placebo group (15/30 vs 6/30, P < 0.05). SSZ resulted in a significant reduction in score of daily NSAIDs (P < 0.05) and significant improvement of function index (P was not given) compared with placebo. No significant difference was found in other outcomes | All continuous outcomes were presented as median and 95% CI. For RevMan analysis, we assumed that mean is equal to median for each outcome and calculated SD from 95% CI and sample size. Success in PGA was more in SSZ than in placebo group (RR 2.5, 95% CI 1.12 to 5.56). No significant difference was found between treatment groups in other outcomes | Absolute benefit from SSZ: 0 mm/h | Did not report |
| Feltelius 1986, 12 weeks | 37 (SSZ:18) (Placebo: 19) 5% with PA DD (year, median): 12.1 (SSZ) 10.4 (placebo) ESR (mm/h): 24.3±17.4 (SSZ) 28.5±19.5 (placebo) |
Duration of morning stiffness, spinal stiffness VAS, pain VAS, general wellbeing VAS, chest expansion, Schober's test, sleep disturbance (event), sacroiliac pain VAS, ESR | Drop‐out: 21.6%. Spinal stiffness VAS, chest expansion and sleep disturbance were significantly improved in SSZ compared with placebo group | All outcomes were presented as graphs and no data were available for analysis except ESR that showed no significant difference between treatment groups | Absolute benefit from SSZ: 1.3 mm/h Relative difference in change from baseline: 5% |
Did not report |
| Kirwan 1993, 3 years | 89 (SSZ: 44) (Placebo: 45) 28% with PA DD (year): 19±12 (SSZ) 21.9±11.7 (placebo) ESR not given |
Primary outcomes included Schober's test, chest expansion, and lateral cervical flexion. Secondary outcomes included function (HAQ), back pain VAS, consumption of anti‐inflammatory drugs, sleep disturbance VAS, PGA, episodes of peripheral arthritis, episodes of heel pain, flares in general AS symptoms, episodes of arthritis | Drop‐out: 30.3%. No significant difference was found between treatment groups in all outcomes except occurrence of peripheral joint symptoms. The episodes of PA were 0.289 episodes/year in SSZ and 0.392 episodes/year in placebo group, respectively (P < 0.05) | There were significantly more drop‐outs for any reason in SSZ than in placebo group. RR was 2.43 (95% CI 1.19 to 4.96). No data were available for analysis in any other outcome | Did not report | Did not report |
| Krajnc 1990, 24 weeks | 95 (SSZ: 71) (Placebo: 24) 66% with PA DD not given ESR (mm/h): 41±19 (SSZ) 43±18 (placebo) |
Duration of morning stiffness, Schober's test, chest expansion, fingers‐to‐floor test, number of painful/swollen joints and ESR | Drop‐out: 14.3%. In SSZ group, duration of morning stiffness, chest expansion, number of painful/swollen joints and ESR showed significantly improved when initial and 24 weeks results are compared | No significant difference was found between treatment groups in all outcomes except ESR (MD ‐17.00 mm/h, 95% CI ‐26.99 to ‐7.01mm/h, favoring SSZ) | Absolute benefit from SSZ: 15 mm/h Relatiive difference in change from baseline: ‐35% |
Absoluete benefit from SSZ: ‐4 mm on 100 mm VAS Relative difference in change from baseline: ‐8% |
| Nissila 1988, 26 weeks | 85 (SSZ: 43 (Placebo: 42) 68% with PA DD (year): 5.4±7.3 (SSZ) 3.8±4.3 (placebo) ESR (mm/h): 42±20 (SSZ) 46±19 (placebo) |
Duration of morning stiffness, spinal stiffness VAS, chest expansion, Schober's test, fingers‐to‐floor test, occiput‐to‐wall test, number of painful joints, number of swollen joints, general wellbeing VAS, ESR and CRP | Drop‐out: 12.2%. Significant differences between treatment groups were found in morning stiffness VAS (P = 0.02), chest expansion (P = 0.03) and ESR (P = 0.02), favoring SSZ. No significant difference was found in other outcomes. Note: we suspected that results of chest expansion were errors because they were impossible to be about 40 to 50 cm. So we divided them by 10 for analysis |
Significant differences were found in morning stiffness VAS 100 mm (0 = no stiffness, 100 = severe, MD ‐14.00, 95% CI ‐23.78 to ‐4.22), chest expansion (MD 1.00 cm, 95% CI 0.10 to 1.90 cm), occiput‐to‐wall test (MD ‐0.80 cm, 95% CI ‐1.55 to 0.05 cm), ESR (MD ‐19.00 mm/h, 95% CI ‐29.65 to ‐8.35 mm/h), and general wellbeing VAS 100 mm (0 = the best, 100 = the worst, MD ‐11.00, 95% CI ‐19.84 to ‐2.16), favoring SSZ. No significant difference was found in other outcomes | Absolute benefit from SSZ: ‐15 mm/h Relatiive difference in change from baseline: ‐33% |
Absolute benefit from SSZ: ‐6 mm on 100 mm VAS Relative difference in change from baseline: ‐15% |
| Schmidt 2002, 26 weeks | 70 (SSZ: 34) (Placebo: 36) 36% with PA DD (year): 16.7±7.2 (SSZ) 16.3±7.8 (placebo) ESR (mm/h): 23.1±3.2 (SSZ) 20.4±2.4 (placebo) |
Back pain VAS, nocturnal awakening (event), pain/tenderness score, duration of morning stiffness, number of painful joints, number of swollen joints, spondylitis function index, PGA, PhGA, Schober's test, fingers‐to‐floor test, chin sternum distance, chest expansion, ESR and CRP | Drop‐out: 36%. No significant difference was reported between treatment groups. There were more drop‐outs in SSZ than in placebo (38% vs 11%) | All continuous outcomes were analyzed as change from baseline. Significant differences were found between treatment groups in back pain VAS 100 mm (0 = no pain, 100 = severe pain, MD ‐2.30, 95% CI ‐4.44 to ‐0.16), chest expansion (MD 0.30 cm, 95% CI 0.16 to 0.44 cm), Schober's test (MD 0.50 cm, 95 CI 0.44 to 0.56 cm), duration of morning stiffness (MD ‐0.39 h, 95% CI ‐0.48 to ‐0.30 h), ESR (MD ‐3.10 mm/h, 95% CI ‐4.85 to ‐1.35 mm/h) and CRP (MD ‐2.50 µg/ml, 95% CI ‐4.70 to ‐0.30 µg/ml), favoring SSZ group. But in occiput‐to‐wall test, the difference (MD 0.70 cm, 95% CI 0.32 to 1.08 cm) favored placebo over SSZ. No significant difference was found in other outcomes. There were significantly more withdrawals for side effects and drop‐outs for any reason in SSZ than in placebo group. RRs were 3.44 (95% CI 1.24 to 9.52) and 2.42 (95% CI 1.14 to 5.15), respectively | Absolute benefit from SSZ: ‐3.1 mm/h Relatiive difference in change from baseline: ‐15% |
Did not report |
| Taylor 1991, 1 year | 40 (SSZ: 20) (Placebo: 20) 15% with PA DD (year): 11±1.6 (SSZ) 10.7±1.6 (placebo) ESR (mm/h, mean): 27 (SSZ) 25 (placebo) |
Back pain VAS, fingers‐to‐floor test, chest expansion, sleep disturbance (event), forced vital volume, occiput‐to‐wall test, Schober's test, spinal stiffness VAS, reduction or stop of NSAIDs (event) | Drop‐out: 17.5%. No significant difference was found between treatment groups in all outcomes except pain VAS (P < 0.05, favoring SSZ) | No significant difference was found between treatment groups in all outcomes including pain VAS | Did not report | Absolute benefit from SSZ: ‐13.5 mm on 100 mm VAS Relative difference in change from baseline: ‐42% |
| Winkler 1989, 24 weeks | 63 (SSZ: 31) (Placebo: 32) 33% with PA DD (year, median): 10.8 (SSZ) 11.2 (placebo) ESR (mm/h): 33.4±20.4 (SSZ) 26.9±16.4 (placebo) |
ESR, duration of morning stiffness, back pain VAS, score of sleep disturbance, chest expansion, Schober's test, fingers‐to‐floor test, disease severity in PGA | Drop‐out: 22.2%. The advantage of SSZ over placebo was significant only in the duration of morning stiffness (P < 0.05) and score of sleep disturbance (P< 0.05). In subgroup analysis, the same results were found in patients with axial form (N = 34). In patients with peripheral arthritis (N = 15), articular index showed significant improvement in SSZ over placebo (P < 0.05) | No significant difference was found between treatment groups in all outcomes. In subgroup analysis of patients with axial form, we found significant difference favoring SSZ over placebo in back pain VAS 100 mm (0 = no pain, 100 = severe pain). MD was ‐9.20 and 95% CI ‐17.81 to 0.59 | Absolute benefit from SSZ: ‐2.7 mm/h Relatiive difference in change from baseline: ‐10% |
Did not report |
CI ‐ confidence interval CRP ‐ C‐reactive protein DD ‐ duration of disease ESR ‐ erythrocyte sedimentation rate HAQ ‐ health assessment questionnaire MD ‐ mean difference mm/hr ‐ millimetre per hour NSAIDs ‐ non‐steroidal anti‐inflammatory drugs PA ‐ peripheral arthritis PGA ‐ patient global assessment PhGA ‐ physician global assessment RR ‐ relative risk SD ‐ standard deviation SSZ ‐ sulphasalazine VAS ‐ visual analogue scale
Primary outcomes
Different parameters were used to assess pain in the included trials. They included improvement in back pain (Analysis 1.3), back pain measured with 100 mm visual analogue scale (Analysis 1.4; Analysis 1.5), night pain (% no pain) (Analysis 1.6), score of sleep disturbance (score 0 to 4) (Analysis 1.7), frequency of nocturnal awaking (Analysis 1.8), score of daily non‐steroidal anti‐inflammatory drugs (NSAIDs) (change from baseline, usual dosage as 10) (Analysis 1.9), and reducing and stopping NSAIDs (Analysis 1.10). Among these parameters, back pain was the most used one which we chose to include in Table 1. Six trials assessed back pain measured with visual analogue scale (0 mm to 100 mm) (Clegg 1996; Dougados 1986; Nissila 1988; Schmidt 2002; Taylor 1991; Winkler 1989). Two trials found significant lower back pain measured with visual analogue scale in sulfasalazine compared with the placebo group (Nissila 1988; Schmidt 2002). However, pooled MD was ‐2.96 (95% CI ‐6.33 to 0.41) and the difference was not statistically significant (Analysis 1.4). We did not find significant heterogeneity among the trials.
1.3. Analysis.
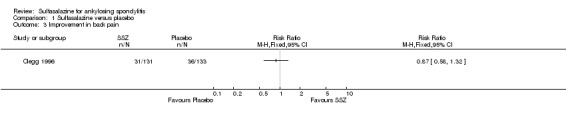
Comparison 1 Sulfasalazine versus placebo, Outcome 3 Improvement in back pain.
1.4. Analysis.
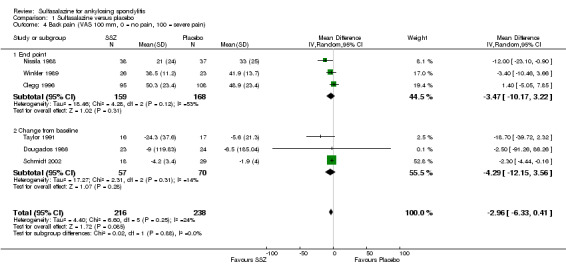
Comparison 1 Sulfasalazine versus placebo, Outcome 4 Back pain (VAS 100 mm, 0 = no pain, 100 = severe pain).
1.5. Analysis.
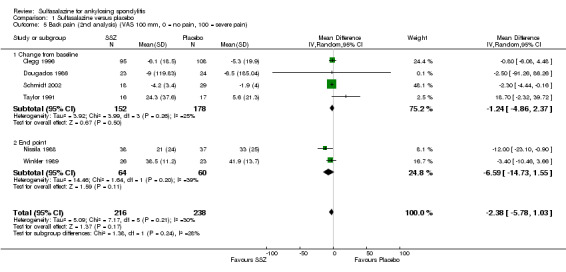
Comparison 1 Sulfasalazine versus placebo, Outcome 5 Back pain (2nd analysis) (VAS 100 mm, 0 = no pain, 100 = severe pain).
1.6. Analysis.
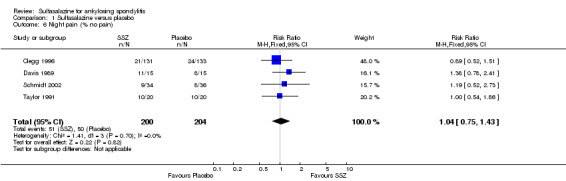
Comparison 1 Sulfasalazine versus placebo, Outcome 6 Night pain (% no pain).
1.7. Analysis.
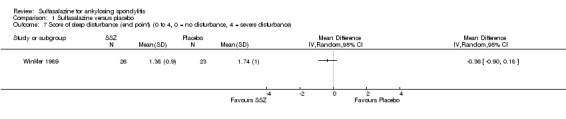
Comparison 1 Sulfasalazine versus placebo, Outcome 7 Score of sleep disturbance (end point) (0 to 4, 0 = no disturbance, 4 = severe disturbance).
1.8. Analysis.
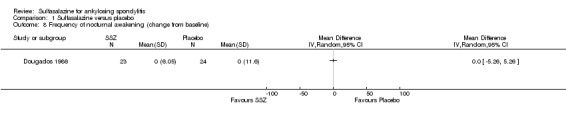
Comparison 1 Sulfasalazine versus placebo, Outcome 8 Frequency of nocturnal awakening (change from baseline).
1.9. Analysis.
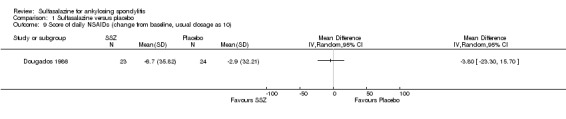
Comparison 1 Sulfasalazine versus placebo, Outcome 9 Score of daily NSAIDs (change from baseline, usual dosage as 10).
1.10. Analysis.
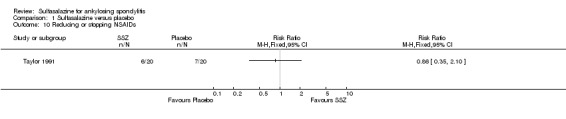
Comparison 1 Sulfasalazine versus placebo, Outcome 10 Reducing or stopping NSAIDs.
No data were available in these trials for bath ankylosing spondylitis disease activity index (BASDAI), bath ankylosing spondylitis function index (BASFI), bath ankylosing spondylitis metrology index (BASMI), or radiographic progression.
For safety outcomes, significantly higher rates of withdrawal due to side effects were found in the sulfasalazine group compared with the placebo group. Pooled RR was 1.50 (95% CI 1.04 to 2.15) (Analysis 1.44). Among 469 participants receiving sulfasalazine, a serious adverse reaction was reported in one patient who developed a generalized, erythematous, raised, pruritic eruption which was associated with nausea, anorexia and insomnia (Clegg 1996).
1.44. Analysis.
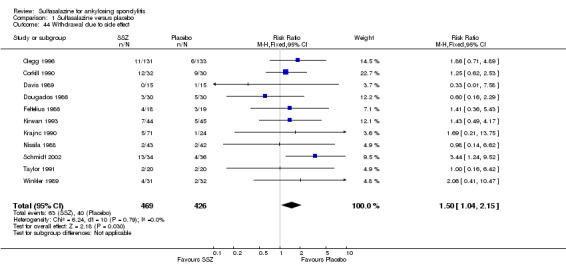
Comparison 1 Sulfasalazine versus placebo, Outcome 44 Withdrawal due to side effect.
Secondary outcomes
The included trials did not report Assessment of Ankylosing Spondylitis response, other disease activity index, or fatigue.
Three trials assessed physical function index and none of them found a statistically significant difference between the sulfasalazine and placebo groups in the analysis of either end point data or change from baseline data (Clegg 1996; Dougados 1986; Schmidt 2002). Pooled analysis showed a similar result and no significant heterogeneity among these trials (Analysis 1.1; Analysis 1.2).
1.1. Analysis.
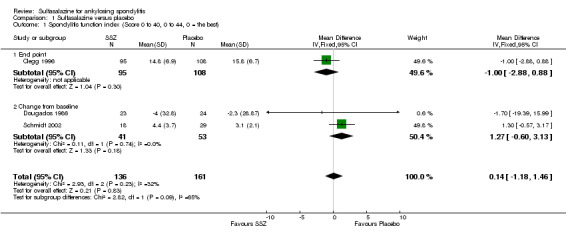
Comparison 1 Sulfasalazine versus placebo, Outcome 1 Spondylitis function index (Score 0 to 40, 0 to 44, 0 = the best).
1.2. Analysis.
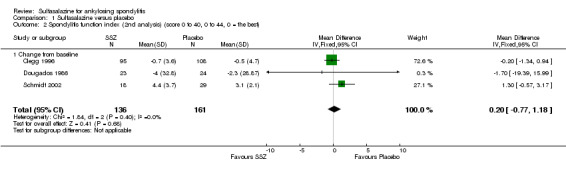
Comparison 1 Sulfasalazine versus placebo, Outcome 2 Spondylitis function index (2nd analysis) (score 0 to 40, 0 to 44, 0 = the best).
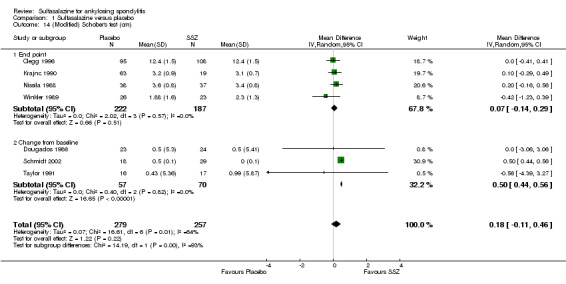
Spinal mobility outcomes included chest expansion (Analysis 1.11; Analysis 1.12), forced vital volume (Analysis 1.13), modified Schober's test (Analysis 1.14; Analysis 1.15), occiput‐to‐wall test (Analysis 1.16; Analysis 1.17), fingers‐to‐floor test (Analysis 1.18; Analysis 1.19), and chin sternum distance (Analysis 1.20). Two trials showed better chest expansion in the sulfasalazine compared with placebo group (Nissila 1988; Schmidt 2002) (Analysis 1.11; Analysis 1.12). This statistically significant difference was confirmed in pooled analysis with weighted MD as 0.30 cm (Analysis 1.11) and 0.31 cm (Analysis 1.12). There was significant heterogeneity among the trials (P < 0.0001) (Analysis 1.12). Schmidt 2002, a trial with more than 30% of drop‐outs, contributed more than 80% weight. When excluded from the analysis, the difference and heterogeneity were insignificant. No statistically significant difference was found between intervention groups in other outcomes of spinal mobility. However, significant heterogeneity was also found among the included studies in Schober's test (Analysis 1.15), occiput‐to‐wall test (Analysis 1.16), and improvement in patient global assessment (Analysis 1.31), and physician global assessment (Analysis 1.34). Again, when the Schmidt 2002 trial was excluded, heterogeneity became insignificant.
1.11. Analysis.
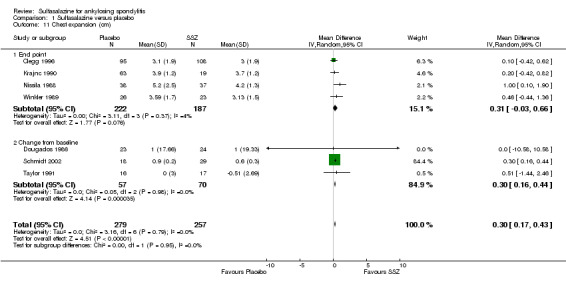
Comparison 1 Sulfasalazine versus placebo, Outcome 11 Chest expansion (cm).
1.12. Analysis.
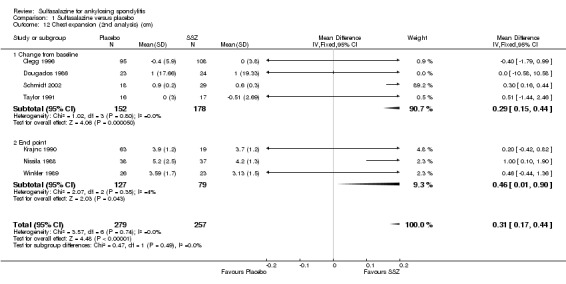
Comparison 1 Sulfasalazine versus placebo, Outcome 12 Chest expansion (2nd analysis) (cm).
1.13. Analysis.
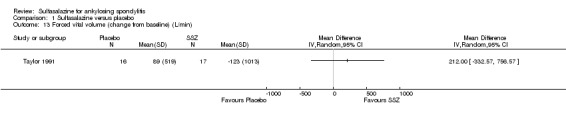
Comparison 1 Sulfasalazine versus placebo, Outcome 13 Forced vital volume (change from baseline) (L/min).
1.14. Analysis.
Comparison 1 Sulfasalazine versus placebo, Outcome 14 (Modified) Schober's test (cm).
1.15. Analysis.
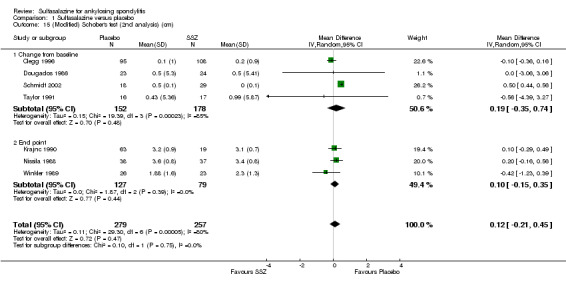
Comparison 1 Sulfasalazine versus placebo, Outcome 15 (Modified) Schober's test (2nd analysis) (cm).
1.16. Analysis.
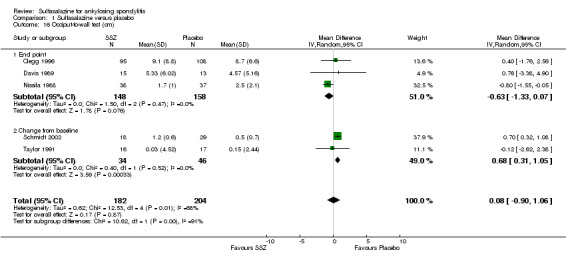
Comparison 1 Sulfasalazine versus placebo, Outcome 16 Occiput‐to‐wall test (cm).
1.17. Analysis.
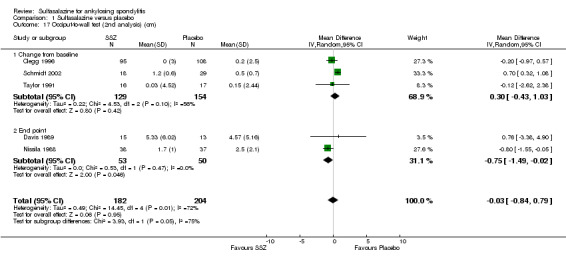
Comparison 1 Sulfasalazine versus placebo, Outcome 17 Occiput‐to‐wall test (2nd analysis) (cm).
1.18. Analysis.
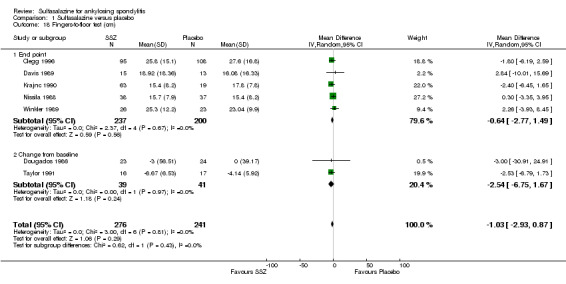
Comparison 1 Sulfasalazine versus placebo, Outcome 18 Fingers‐to‐floor test (cm).
1.19. Analysis.
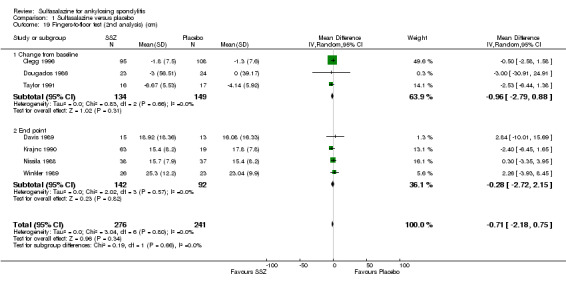
Comparison 1 Sulfasalazine versus placebo, Outcome 19 Fingers‐to‐floor test (2nd analysis) (cm).
1.20. Analysis.
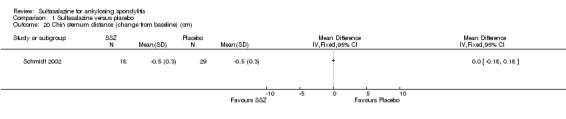
Comparison 1 Sulfasalazine versus placebo, Outcome 20 Chin sternum distance (change from baseline) (cm).
1.31. Analysis.
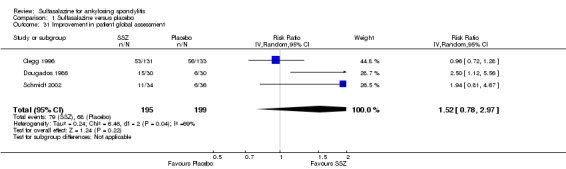
Comparison 1 Sulfasalazine versus placebo, Outcome 31 Improvement in patient global assessment.
1.34. Analysis.
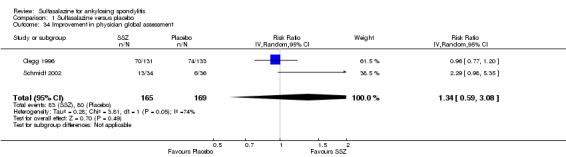
Comparison 1 Sulfasalazine versus placebo, Outcome 34 Improvement in physician global assessment.
Peripheral joints/entheses were assessed in several studies (Clegg 1996; Dougados 1986; Kirwan 1993; Nissila 1988; Schmidt 2002). Outcomes included joint pain/tenderness score (0 to 198) or number (Analysis 1.21; Analysis 1.22), joint swelling score (0 to 198) or number (Analysis 1.23; Analysis 1.24), dactylitis score (0 to 3) (Analysis 1.25; Analysis 1.26), enthesopathy index (0 to 90) (Analysis 1.27; Analysis 1.28) and spondylitis articular index (0 to 90) (Analysis 1.29; Analysis 1.30). There was no statistically significant difference between intervention groups in either individual study or pooled data except for the Kirwan 1993 study, which found that occurrence of peripheral joint symptoms were lower in the sulfasalazine group (0.298 episodes/year) than in the placebo group (0.392 episodes/year) (P < 0.05). This difference was not shown in our analysis because of lack of available data.
1.21. Analysis.
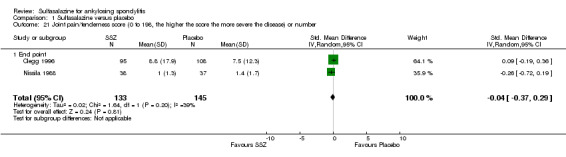
Comparison 1 Sulfasalazine versus placebo, Outcome 21 Joint pain/tenderness score (0 to 198, the higher the score the more severe the disease) or number.
1.22. Analysis.
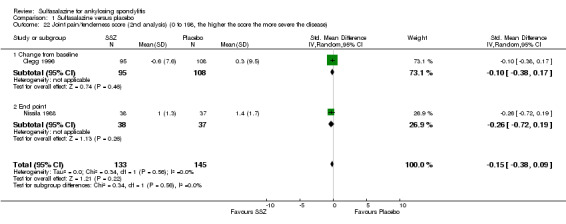
Comparison 1 Sulfasalazine versus placebo, Outcome 22 Joint pain/tenderness score (2nd analysis) (0 to 198, the higher the score the more severe the disease).
1.23. Analysis.
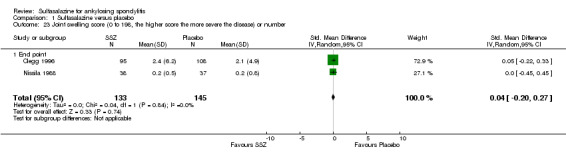
Comparison 1 Sulfasalazine versus placebo, Outcome 23 Joint swelling score (0 to 198, the higher score the more severe the disease) or number.
1.24. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 24 Joint swelling score (2nd analysis) (0 to 198, the higher the score the more severe the disease).
1.25. Analysis.
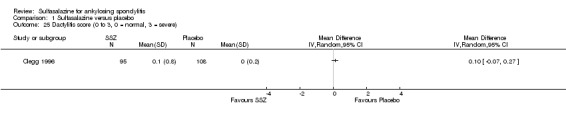
Comparison 1 Sulfasalazine versus placebo, Outcome 25 Dactylitis score (0 to 3, 0 = normal, 3 = severe).
1.26. Analysis.
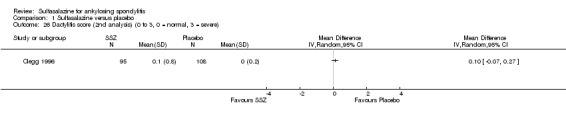
Comparison 1 Sulfasalazine versus placebo, Outcome 26 Dactylitis score (2nd analysis) (0 to 3, 0 = normal, 3 = severe).
1.27. Analysis.
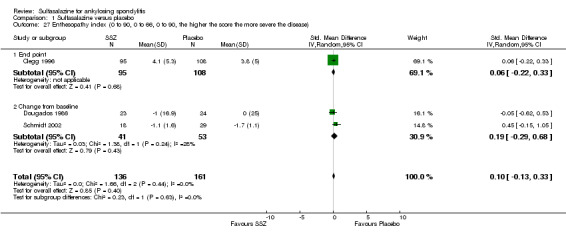
Comparison 1 Sulfasalazine versus placebo, Outcome 27 Enthesopathy index (0 to 90, 0 to 66, 0 to 90, the higher the score the more severe the disease).
1.28. Analysis.
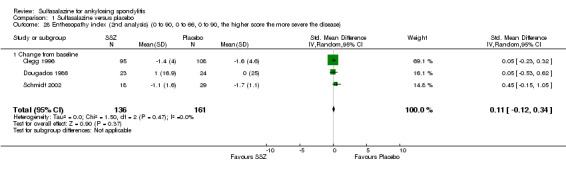
Comparison 1 Sulfasalazine versus placebo, Outcome 28 Enthesopathy index (2nd analysis) (0 to 90, 0 to 66, 0 to 90, the higher score the more severe the disease).
1.29. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 29 Spondylitis articular index (0 to 90, the higher score the more severe the disease).
1.30. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 30 Spondylitis articular index (2nd analysis) (0 to 90, the higher score the more severe the disease).
Dougados 1986 found more participants improved in terms of patient global assessment in the sulfasalazine group than placebo group, but two other trials, Clegg 1996 and Schmidt 2002, did not, and neither did our pooled analysis (Analysis 1.31). One trial, Winkler 1989, compared patient assessment of disease severity (100 mm visual analogue scale) between the sulfasalazine and placebo groups and found no difference (Analysis 1.32).
1.32. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 32 Patient assessment of disease severity (end point) (VAS 100 mm, 0 = very good, 100 = very poor).
One trial, Nissila 1988, assessed general wellbeing (100 mm visual analogue scale) and found the sulfasalazine group was better than the placebo group (MD ‐11.00, 95% CI ‐19.84 to ‐2.16) (Analysis 1.33). Two trials, Clegg 1996 and Schmidt 2002, analyzed improvement in physician global assessment and found no difference between the sulfasalazine and placebo groups, neither did our pooled result (Analysis 1.34). One trial, Clegg 1996, assessed response to treatment which was based on both patient and physician global assessment and found no difference between the sulfasalazine and placebo groups (Analysis 1.35).
1.33. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 33 General well‐being (end point) (VAS 100 mm, 0 = very good, 100 = very poor).
1.35. Analysis.
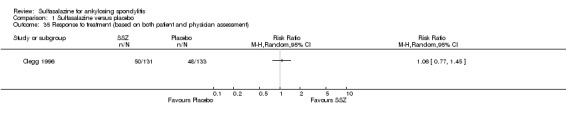
Comparison 1 Sulfasalazine versus placebo, Outcome 35 Response to treatment (based on both patient and physician assessment).
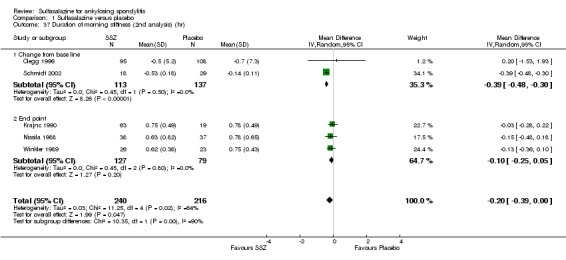
Five trials assessed duration of morning stiffness (Clegg 1996; Krajnc 1990; Nissila 1988; Schmidt 2002; Winkler 1989). Schmidt 2002, found a statistically significant difference between intervention groups, favoring sulfasalazine over placebo. But the difference of pooled results was statistically insignificant (Analysis 1.36; Analysis 1.37). Significant heterogeneity existed among the studies. When we excluded the Schmidt 2002 trial, heterogeneity became insignificant. Two trials assessed morning stiffness with a 100 mm visual analogue scale (where 0 = no stiffness, 100 = severe) (Nissila 1988; Taylor 1991). One study showed a statistically significant difference favoring sulfasalazine over placebo (Nissila 1988). Pooled data showed a similar result (MD ‐13.89, 95% CI ‐22.54 to ‐5.24 (Analysis 1.38).
1.36. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 36 Duration of morning stiffness (hr).
1.37. Analysis.
Comparison 1 Sulfasalazine versus placebo, Outcome 37 Duration of morning stiffness (2nd analysis) (hr).
1.38. Analysis.
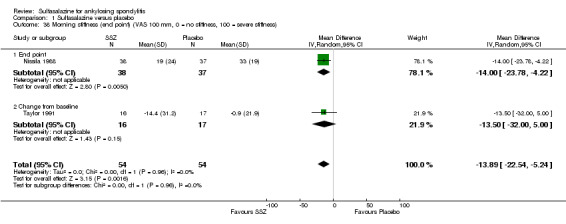
Comparison 1 Sulfasalazine versus placebo, Outcome 38 Morning stiffness (end point) (VAS 100 mm, 0 = no stiffness, 100 = severe stiffness).
For erythrocyte sedimentation rate, four trials found statistically significant differences between intervention groups, favoring sulfasalazine over placebo (Clegg 1996; Krajnc 1990; Nissila 1988; Schmidt 2002). A similar result was found in the pooled data (Analysis 1.40; Analysis 1.41). MD of change from baseline was ‐3.11 mm/hr, 95% CI ‐4.62 to ‐1.60 mm/hr. MD of end point was ‐7.07 mm/hr, 95% CI ‐14.39 to 0.25 (not statistically significant). Significant heterogeneity existed among the trials (P = 0.02). This could be due to the large difference of erythrocyte sedimentation rate at baseline levels among the studies. Three trials, Clegg 1996, Nissila 1988 and Schmidt 2002, assessed C‐reactive protein and only one, Schmidt 2002, showed a statistically significant difference between intervention groups, favoring sulfasalazine over placebo. The pooled result showed no statistically significant difference (Analysis 1.42; Analysis 1.43).
1.40. Analysis.
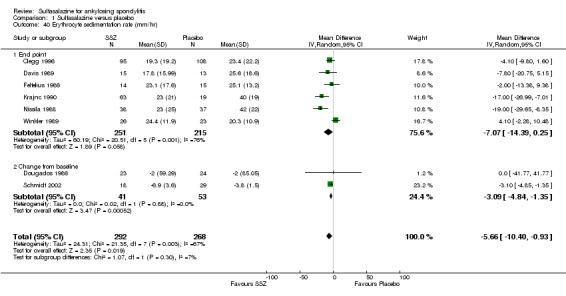
Comparison 1 Sulfasalazine versus placebo, Outcome 40 Erythrocyte sedimentation rate (mm/hr).
1.41. Analysis.
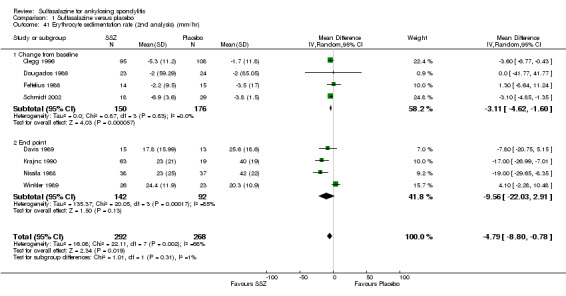
Comparison 1 Sulfasalazine versus placebo, Outcome 41 Erythrocyte sedimentation rate (2nd analysis) (mm/hr).
1.42. Analysis.

Comparison 1 Sulfasalazine versus placebo, Outcome 42 C‐reactive protein (ug/ml).
1.43. Analysis.
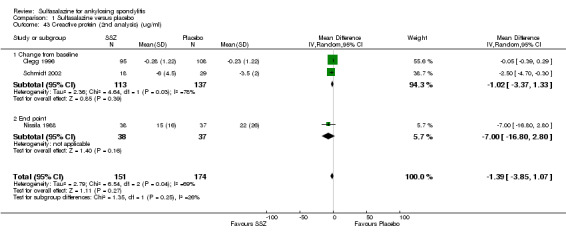
Comparison 1 Sulfasalazine versus placebo, Outcome 43 C‐reactive protein (2nd analysis) (ug/ml).
Ten trials reported the total number of withdrawals (Clegg 1996; Davis 1989; Dougados 1986; Feltelius 1986; Kirwan 1993; Krajnc 1990; Nissila 1988; Schmidt 2002; Taylor 1991; Winkler 1989). A significantly higher rate of withdrawals due to any reason was found in the sulfasalazine groups compared with the placebo groups (pooled RR 1.33, 95% CI 1.03 to 1.73) (Analysis 1.46).
1.46. Analysis.
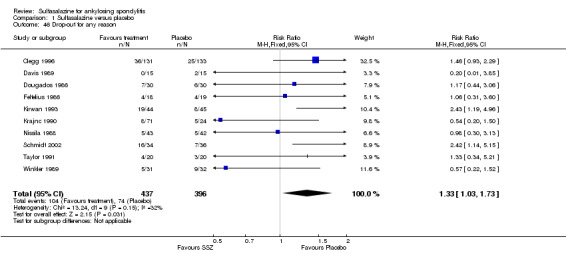
Comparison 1 Sulfasalazine versus placebo, Outcome 46 Drop‐out for any reason.
Sensitivity analysis
We did not perform a sensitivity analysis against concealment because we did not rank any included trials at high risk of bias. Blinding was reported in all studies, however, three participants broke the blinding during the trial of Corkill 1990. Continuous outcome data were unavailable in this trial. For the dichotomous outcome, withdrawals due to side effect, exclusion of this trial from the analysis showed a similar result (Analysis 1.44). Since continuous data included only the participants who completed the trials, we excluded the trials with more than 30% drop‐outs (Kirwan 1993; Schmidt 2002), to see if a high percentage of drop‐outs affected the results. After exclusion of Schmidt 2002 (no continuous data was available in Kirwan 1993), we found that the MD of chest expansion and MD of duration of morning stiffness (change from baseline) became statistically insignificant, which have been mentioned above. Other outcomes remained similar. Only the median and CI values were available in Dougados 1986. We assumed that mean was equal to median for each outcome and calculated the SD from the CI and sample size. Therefore, the mean and SD were estimated in this trial. Sensitivity analysis, however, found no obvious difference between inclusion and exclusion of this trial in the pooled result. The Davis 1989 and Feltelius 1986 trials had the least number of participants and the shortest periods. When excluded, the pooled difference of erythrocyte sedimentation rate (end point) became statistically significant (weighted MD ‐6.13 mm/hr, 95% CI ‐11.89 to ‐0.37 mm/hr), while other results remained similar.
Subgroup data
We could not group studies of sulfasalazine versus placebo according to characteristics of interventions and participants. Only one study (Winkler 1989) presented data of subgroups (participants with and without peripheral arthritis). In participants with peripheral arthritis (N = 15), we did not find a statistically significant difference between intervention groups in back pain, score of sleep disturbance, chest expansion, Schober's test, fingers‐to‐floor test, articular index, degree of joint swelling, patient assessment of disease severity, duration of morning stiffness, or erythrocyte sedimentation rate. For participants without peripheral arthritis (N = 34), we did not find a statistically significant difference in these outcomes (but articular index and degree of joint swelling were not assessed) except back pain measured with a 100 mm visual analogue scale (where 0 = no pain, 100 = severe), which was shown to significantly favor sulfasalazine over placebo (MD ‐9.20, 95% CI ‐17.81 to ‐0.59) (Analysis 3.1). Another study separately analyzed the results of participants with peripheral arthritis (N = 77) and found more peripheral responses in the sulfasalazine than placebo group (55.9% versus 30.2%, P = 0.023) (Clegg 1996). Here, peripheral response was a composition of four parameters, e.g. patient self assessment, physician assessment, joint pain/tenderness score, and joint swelling score. Peripheral response was defined as improvement in at least two parameters, with no worsening in other parameters. We did not analyze these parameters in Review Manager 5 because the information on treatment allocation was not given (RevMan 2014).
3.1. Analysis.
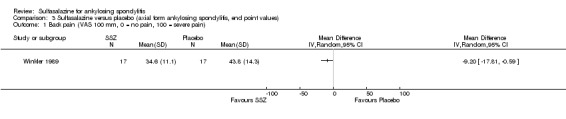
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 1 Back pain (VAS 100 mm, 0 = no pain, 100 = severe pain).
Discussion
Summary of main results
We identified 11 RCTs evaluating the benefits and harms of sulfasalazine in ankylosing spondylitis (AS). These trials enrolled 895 participants in total. All these trials compared sulfasalazine with placebo. More than 30 outcomes were assessed. Most of the pooled data in the present review included only a few trials (less than five) and few participants (less than 400). Seven major outcomes, e.g. pain, bath ankylosing spondylitis disease activity index (BASDAI), bath ankylosing spondylitis function index (BASFI), bath ankylosing spondylitis metrology index (BASMI), radiographic progression, total number of withdrawals, and total number of withdrawals due to adverse events, were included in the present review. None of the included studies assessed BASDAI, BASFI, BASMI or radiographic progression. Different parameters were used to assess pain. We chose the most used one, e.g. back pain, to be included in Table 1. We did not find any statistically significant difference in either individual trial data or pooled analysis results, except that back pain measured with visual analogue scale (0 mm to 100 mm) was found to be statistically significantly lower in the sulfasalazine group compared with the placebo group in two trials (Nissila 1988; Schmidt 2002). However, the pooled mean difference was not statistically significant.
With regard to the safety of sulfasalazine, the total number of adverse events was higher in the sulfasalazine group than in the placebo group; a serious adverse reaction occurred in one patient taking sulfasalazine. Statistically significantly higher rates of withdrawals due to side effects, and for any reason were found in the sulfasalazine group compared to the placebo group. The most frequent side effects with sulfasalazine were nausea, heartburn, epigastric distress, skin reactions, lightheadedness, and headache.
In addition, the pooled data showed that sulfasalazine did significantly ease morning stiffness and reduce erythrocyte sedimentation rate, even though the slight difference does not have much clinical significance. For other outcomes, it was not possible to pool the data.
Among the included trials, only two (Clegg 1996; Winkler 1989), analyzed the effect of sulfasalazine on peripheral arthritis. One trial presented data of the subgroup with peripheral arthritis (N = 15) and found no statistically significant difference between the sulfasalazine and placebo group (Winkler 1989). The other study separately analyzed the results of participants with peripheral arthritis (N = 77) and found more peripheral responses in the sulfasalazine group than in the placebo group (Clegg 1996). There is not enough evidence suggesting sulfasalazine is better for peripheral joints than for the spine.
Overall completeness and applicability of evidence
The included studies did not report most of the major, validated outcomes such as BASDAI, BASFI, BASMI or radiographic progression. In addition, the study population in the included trials involved 895 AS participants who fulfilled New York criteria (Olivieri 2002) which required radiographic change. The majority of these participants were adults, and considered to have active disease, although the definition of active disease differed among the trials. The duration of disease ranged from 3.8 to 21.9 years. Therefore, the evidence was not applicable to juvenile patients and those patients with early disease onset.
Quality of the evidence
The present review shows limited benefit of sulfasalazine in AS. The strength of evidence in this review should be ranked as moderate level for several reasons. First, the potential benefit of sulfasalazine might be overlooked because most of the pooled data in the present review included only a few trials (less than five) and few participants (less than 400). Moreover, obvious methodological drawbacks existed as specified in the Risk of bias in included studies section. Finally, the included participants were all patients at an advanced stage of AS who might not respond well to sulfasalazine.
Potential biases in the review process
We conducted a thorough search in several databases and updated the search strategies during the process of updating this review. We included all trials that met our inclusion criteria and applied no language restrictions. Two review authors independently reviewed the trial reports according to the selection criteria. When necessary, we contacted the trial authors to clarify any information regarding their trials. We resolved any disagreements regarding the inclusion of studies by discussion.
Agreements and disagreements with other studies or reviews
Ferraz 1990 conducted a meta‐analysis of five RCTs and concluded that sulfasalazine significantly relieved pain and morning stiffness, compared with placebo (Davis 1989; Dougados 1986; Feltelius 1986; Nissila 1988; Winkler 1989). Six RCTs have been published since then (Clegg 1996; Corkill 1990; Kirwan 1993; Krajnc 1990; Schmidt 2002; Taylor 1991). Our previous review (Chen 2005), and the current update showed limited benefit of sulfasalazine in reducing erythrocyte sedimentation rate and easing spinal stiffness, as the effect size was small and not clinically significant. We did not find any other published systematic review, meta‐analysis or RCT comparing sulfasalazine with placebo in our updated search. For peripheral arthritis of AS, the present review did not support any benefit of sulfasalazine, in accordance with the experts' recommendation of using TNF‐alpha inhibitors (such as etanercept), rather than sulfasalazine, if available (van der Heijde 2011). A randomized double‐blind study comparing etanercept with sulfasalazine found that etanercept was more effective in all joint assessments, regardless of swollen joint involvement (Braun 2011). Therefore, etanercept might be a better choice for management of patients with AS and peripheral joint involvement.
Authors' conclusions
Implications for practice.
In this review, there was no evidence to support any benefit of sulfasalazine in reducing pain, disease activity, radiographic progression, or improving physical function and spinal mobility. Meanwhile, adverse events were common and there were more withdrawals because of side effects among participants taking sulfasalazine than placebo, although serious adverse events were rare.
Implications for research.
RCTs of larger sample sizes and with longer duration are needed. Assessment of Ankylosing Spondylitis response (van der Heijde 2002; van der Heijde 2005), and the core set for the evaluation of disease controlling anti‐rheumatic treatment (van der Heijde 1999; van der Heijde 2002), should be used to assess outcomes. It is also important to subgroup appropriately for peripheral joint involvement, disease duration and gender.
What's new
| Date | Event | Description |
|---|---|---|
| 28 November 2013 | New search has been performed | New search but no new studies included |
| 14 September 2012 | New citation required but conclusions have not changed | Change in authorship |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. CMSG ID: C079‐R |
| 24 February 2005 | Amended | Review first published |
| 9 November 2004 | Amended | Protocol first published |
Acknowledgements
The review authors would like to thank the editorial team of the Cochrane Musculoskeletal Group (CMSG) for their helpful comments and Sally Green, Steve McDonald, Janet Piehl, Denise O'Connor, Elmer Villanueva and the staff of the Australasian Cochrane Centre for their supervision and technical support. The review authors would also like to thank M Dougados, J Kirwan, I Krajnc, J Braun, and J Heather for offering extra information about their studies; and I Krajnc, D Schuenemann and S Herter for language translation.
Appendices
Appendix 1. Search strategies for the original review
| The search strategy for MEDLINE (Ovid) was: 1 Spondylitis, Ankylosing/ 2 (bechtere$ disease$ or marie‐struempell disease$ or rheumatoid spondylitis or spondylarthritis ankylopoietica or ankylo$ spondyl$ or Spin$ Ankylosis or Vertebral Ankylosis.tw. 3 1 or 2 4 (Salazosulfapyridine or sulfasalazine or Sulfosalazine or Sulfasal#zine or Salazopyridin$ or asulfidine or azulf#dine).tw. 5 sulfasalazine/ 6 Sulfasalazine.rn. 7 or/4‐6 8 3 and 7 9 from 8 keep 1‐132 | The search strategy for EMBASE (Ovid) was: 1 Spondylitis, Ankylosing/ 2 (bechtere$ disease$ or marie‐struempell disease$ or rheumatoid spondylitis or spondylarthritis ankylopoietica or ankylo$ spondyl$ or Spin$ Ankylosis or Vertebral Ankylosis).tw. 3 1 or 2 4 Salazosulfapyridine/ 5 (Salazosulfapyridine or sulfasalazine or Sulfosalazine or Sulfasal#zine or Salazopyridin$ or asulfidine or azulf#dine).tw. 6 4 or 5 7 3 and 6 8 from 7 keep 1‐307 |
Appendix 2. Search strategies for the second (current) review (28 November, 2013)
The Cochrane Library Issue 11 of 12, November 2013
1. MeSH descriptor: [Spondylarthropathies] explode all trees
2. MeSH descriptor: [Spondylitis, Ankylosing] explode all trees
3. ankylosing or spondyl.ti,ab
4. bekhterev or bechterew.ti,ab.
5. bechtere* disease* or marie‐struempell disease or rheumatoid spondylitis or spondylarthritis ankylopoietica or Spin* Ankylosis or Vertebral Ankylosis
6. MeSH descriptor: [Sulfasalazine] explode all trees
7. Salazosulfapyridine or asulfidine
8. Salazosulfapyridine or sulfasalazine or Sulfosalazine or Sulfasal*zine or Salazopyridin* or asulfidine or azulf*dine
9. #1 or #2 or #3 or #4 or #5
10. #6 or #7 or #8
11. #9 and #10
EMBASE Classic + EMBASE 1947 to Nov 27 2013
1. Spondylitis, Ankylosing/
2. (bechtere$ disease$ or marie‐struempell disease$ or rheumatoid spondylitis or spondylarthritis ankylopoietica or ankylo$ spondyl$ or Spin$ Ankylosis or Vertebral Ankylosis).tw.
3. 1 or 2
4. (Salazosulfapyridine or sulfasalazine or Sulfosalazine or Sulfasal#zine or Salazopyridin$ or asulfidine or azulf#dine).tw.
5. sulfasalazine/
6. 4 or 5
7. 3 and 6
8. random$.tw.
9. factorial$.tw
10. crossover$.tw.
11. cross over.tw.
12. cross‐over.tw.
13. placebo$.tw.
14. (doubl$ adj blind$).tw.
15. (singl$ adj blind$).tw.
16. assign$.tw.
17. allocat$.tw.
18. volunteer$.tw.
19. crossover procedure/
20. double blind procedure/
21. randomized controlled trial/
22. single blind procedure/
23. or/8‐22
24. 7 and 23
Database: Ovid MEDLINE(R) In‐Process Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Nov 27 2013>
1. Spondylitis, Ankylosing/
2. (bechtere$ disease$ or marie‐struempell disease$ or rheumatoid spondylitis or spondylarthritis ankylopoietica or ankylo$ spondyl$ or Spin$ Ankylosis or Vertebral Ankylosis).tw.
3. 1 or 2
4. (Salazosulfapyridine or sulfasalazine or Sulfosalazine or Sulfasal#zine or Salazopyridin$ or asulfidine or azulf#dine).tw.
5. sulfasalazine/
6. Sulfasalazine.rn.
7. or/4‐6
8. 3 and 7
CINAHL via Ebscohost
| S16 | S10 and S15 |
| S15 | S11 or S12 |
| S14 | "asulfidine" |
| S13 | "Sulfosalazine" |
| S12 | "Salazosulfapyridine" |
| S11 | (MM "Sulfasalazine") |
| S10 | S1 or S6 or S8 or S9 |
| S9 | (MH "Spondylosis") |
| S8 | "Vertebral Ankylosis" |
| S7 | "spondylarthritis ankylopoietica" |
| S6 | (MH "Spondylarthritis") |
| S5 | "rheumatoid spondylitis" |
| S4 | ""marie‐struempell"" |
| S3 | "marie‐struempell" |
| S2 | "bechtere disease" |
| S1 | (MH "Spondylitis, Ankylosing") |
ClinicalTrials.gov
Search Strategy: (advanced screen)
Intervention: Salazosulfapyridine or sulfasalazine or asulfidine
Appendix 3. Search result for the second version (28 November, 2013)
| Database, platform, issue | Search date | Results | Results after deduplicate |
| MEDLINE‐OVID (1946 to present) 2012‐2013 | November 28, 2013 | 24 | 23 |
| EMBASE‐OVID (1974 to Nov 26 2013) 2012‐2013 | November 28, 2013 | 21 | 21 |
| (CDSR) part of The Cochrane Library 2012‐2013 www.thecochranelibrary.com |
November 28, 2013 | 4 | 4 |
| CENTRAL, part of The Cochrane Library 2012‐2013 www.thecochranelibrary.com |
November 28, 2013 | 0 | 0 |
| DARE, part of The Cochrane Library 2012‐2013 www.thecochranelibrary.com |
November 28, 2013 | 0 | 0 |
| HTA Database, part of The Cochrane Library 2012‐2013 www.thecochranelibrary.com |
November 28, 2013 | 0 | 0 |
| NHS EED, part of The Cochrane Library 2012‐2013 www.thecochranelibrary.com | November 28, 2013 | 0 | 0 |
| CINAHL | November 28, 2013 | 5 | 5 |
| TOTALS | 101 | 100 |
Appendix 4. Risk of bias assessment criteria
|
SEQUENCE GENERATION Was the allocation sequence adequately generated? [Short form: Adequate sequence generation?] | |
| Criteria for a judgment of 'YES' (i.e. low risk of bias) | The investigators describe a random component in the sequence generation process such as:
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random |
| Criteria for the judgment of 'NO' (i.e. high risk of bias) | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgment or some method of non‐random categorization of participants, for example:
|
| Criteria for the judgment of 'UNCLEAR' (uncertain risk of bias) | Insufficient information about the sequence generation process to permit judgment of 'Yes' or 'No' |
|
ALLOCATION CONCEALMENT Was allocation adequately concealed? [Short form: Allocation concealment?] | |
| Criteria for a judgment of 'YES' (i.e. low risk of bias) | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgment of 'NO' (i.e. high risk of bias) | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgment of 'UNCLEAR' (uncertain risk of bias) | Insufficient information to permit judgment of 'Yes' or 'No'. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgment ¨C for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
|
BLINDING OF PARTICIPANTS, PERSONNEL AND OUTCOME ASSESSORS Was knowledge of the allocated interventions adequately prevented during the study? [Short form: Blinding?] | |
| Criteria for a judgment of 'YES' (i.e. low risk of bias) | Any one of the following:
|
| Criteria for the judgment of 'NO' (i.e. high risk of bias) | Any one of the following:
|
| Criteria for the judgment of 'UNCLEAR' (uncertain risk of bias) | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA Were incomplete outcome data adequately addressed? [Short form: Incomplete outcome data addressed?] | |
| Criteria for a judgment of 'YES' (i.e. low risk of bias) |
Any one of the following:
|
| Criteria for the judgment of 'NO' (i.e. high risk of bias) | Any one of the following:
|
| Criteria for the judgment of 'UNCLEAR' (uncertain risk of bias) | Any one of the following:
|
|
SELECTIVE OUTCOME REPORTING Are reports of the study free of suggestion of selective outcome reporting? [Short form: Free of selective reporting?] | |
| Criteria for a judgment of 'YES' (i.e. low risk of bias) | Any of the following:
|
| Criteria for the judgment of 'NO' (i.e. high risk of bias) | Any one of the following:
|
| Criteria for the judgment of 'UNCLEAR' (uncertain risk of bias) | Insufficient information to permit judgment of 'Yes' or 'No'. It is likely that the majority of studies will fall into this category |
|
OTHER POTENTIAL THREATS TO VALIDITY Was the study apparently free of other problems that could put it at a risk of bias? [Short form: Free of other bias?] | |
| Criteria for a judgment of 'YES' (i.e. low risk of bias) | The study appears to be free of other sources of bias |
| Criteria for the judgment of 'NO' (i.e. high risk of bias) | There is at least one important risk of bias. For example, the study:
|
| Criteria for the judgment of 'UNCLEAR' (uncertain risk of bias) | There may be a risk of bias, but there is either:
|
Appendix 5. Sample data collection form for assessment of risk of bias
| DOMAIN | JUDGMENT (YES, NO, UNCLEAR) | REASON FOR JUDGMENT (copy and paste directly from text of trial) |
| Was the allocation sequence adequately generated? | ||
| Was allocation adequately concealed? | ||
| Was knowledge of the allocated interventions adequately prevented during the study? | ||
| Blinding of personnel | ||
| Blinding of participants | ||
| Blinding of outcome assessors | ||
| Were incomplete outcome data adequately addressed? | ||
| Are reports of the study free of suggestion of selective outcome reporting | ||
| Was the study apparently free of other problems that could put it at a risk of bias? |
Data and analyses
Comparison 1. Sulfasalazine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spondylitis function index (Score 0 to 40, 0 to 44, 0 = the best) | 3 | 297 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.18, 1.46] |
| 1.1 End point | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.88, 0.88] |
| 1.2 Change from baseline | 2 | 94 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [‐0.60, 3.13] |
| 2 Spondylitis function index (2nd analysis) (score 0 to 40, 0 to 44, 0 = the best) | 3 | 297 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.77, 1.18] |
| 2.1 Change from baseline | 3 | 297 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.77, 1.18] |
| 3 Improvement in back pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Back pain (VAS 100 mm, 0 = no pain, 100 = severe pain) | 6 | 454 | Mean Difference (IV, Random, 95% CI) | ‐2.96 [‐6.33, 0.41] |
| 4.1 End point | 3 | 327 | Mean Difference (IV, Random, 95% CI) | ‐3.47 [‐10.17, 3.22] |
| 4.2 Change from baseline | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐4.29 [‐12.15, 3.56] |
| 5 Back pain (2nd analysis) (VAS 100 mm, 0 = no pain, 100 = severe pain) | 6 | 454 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐5.78, 1.03] |
| 5.1 Change from baseline | 4 | 330 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐4.86, 2.37] |
| 5.2 End point | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐6.59 [‐14.73, 1.55] |
| 6 Night pain (% no pain) | 4 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.75, 1.43] |
| 7 Score of sleep disturbance (end point) (0 to 4, 0 = no disturbance, 4 = severe disturbance) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Frequency of nocturnal awakening (change from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Score of daily NSAIDs (change from baseline, usual dosage as 10) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Reducing or stopping NSAIDs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Chest expansion (cm) | 7 | 536 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.17, 0.43] |
| 11.1 End point | 4 | 409 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.03, 0.66] |
| 11.2 Change from baseline | 3 | 127 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.16, 0.44] |
| 12 Chest expansion (2nd analysis) (cm) | 7 | 536 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.17, 0.44] |
| 12.1 Change from baseline | 4 | 330 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.15, 0.44] |
| 12.2 End point | 3 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [0.01, 0.90] |
| 13 Forced vital volume (change from baseline) (L/min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 (Modified) Schober's test (cm) | 7 | 536 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.11, 0.46] |
| 14.1 End point | 4 | 409 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.14, 0.29] |
| 14.2 Change from baseline | 3 | 127 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.44, 0.56] |
| 15 (Modified) Schober's test (2nd analysis) (cm) | 7 | 536 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.21, 0.45] |
| 15.1 Change from baseline | 4 | 330 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.35, 0.74] |
| 15.2 End point | 3 | 206 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.15, 0.35] |
| 16 Occiput‐to‐wall test (cm) | 5 | 386 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.90, 1.06] |
| 16.1 End point | 3 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.33, 0.07] |
| 16.2 Change from baseline | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.31, 1.05] |
| 17 Occiput‐to‐wall test (2nd analysis) (cm) | 5 | 386 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.84, 0.79] |
| 17.1 Change from baseline | 3 | 283 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.43, 1.03] |
| 17.2 End point | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.49, ‐0.02] |
| 18 Fingers‐to‐floor test (cm) | 7 | 517 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.93, 0.87] |
| 18.1 End point | 5 | 437 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐2.77, 1.49] |
| 18.2 Change from baseline | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐6.75, 1.67] |
| 19 Fingers‐to‐floor test (2nd analysis) (cm) | 7 | 517 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.18, 0.75] |
| 19.1 Change from baseline | 3 | 283 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐2.79, 0.88] |
| 19.2 End point | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐2.72, 2.15] |
| 20 Chin sternum distance (change from baseline) (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21 Joint pain/tenderness score (0 to 198, the higher the score the more severe the disease) or number | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.37, 0.29] |
| 21.1 End point | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.37, 0.29] |
| 22 Joint pain/tenderness score (2nd analysis) (0 to 198, the higher the score the more severe the disease) | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.38, 0.09] |
| 22.1 Change from baseline | 1 | 203 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.38, 0.17] |
| 22.2 End point | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.72, 0.19] |
| 23 Joint swelling score (0 to 198, the higher score the more severe the disease) or number | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.20, 0.27] |
| 23.1 End point | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.20, 0.27] |
| 24 Joint swelling score (2nd analysis) (0 to 198, the higher the score the more severe the disease) | 2 | 278 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.29, 0.29] |
| 24.1 Change from baseline | 1 | 203 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.97, 0.97] |
| 24.2 End point | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.30, 0.30] |
| 25 Dactylitis score (0 to 3, 0 = normal, 3 = severe) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 26 Dactylitis score (2nd analysis) (0 to 3, 0 = normal, 3 = severe) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27 Enthesopathy index (0 to 90, 0 to 66, 0 to 90, the higher the score the more severe the disease) | 3 | 297 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.13, 0.33] |
| 27.1 End point | 1 | 203 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.33] |
| 27.2 Change from baseline | 2 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.29, 0.68] |
| 28 Enthesopathy index (2nd analysis) (0 to 90, 0 to 66, 0 to 90, the higher score the more severe the disease) | 3 | 297 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.12, 0.34] |
| 28.1 Change from baseline | 3 | 297 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.12, 0.34] |
| 29 Spondylitis articular index (0 to 90, the higher score the more severe the disease) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30 Spondylitis articular index (2nd analysis) (0 to 90, the higher score the more severe the disease) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31 Improvement in patient global assessment | 3 | 394 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.78, 2.97] |
| 32 Patient assessment of disease severity (end point) (VAS 100 mm, 0 = very good, 100 = very poor) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 33 General well‐being (end point) (VAS 100 mm, 0 = very good, 100 = very poor) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 34 Improvement in physician global assessment | 2 | 334 | Risk Ratio (IV, Random, 95% CI) | 1.34 [0.59, 3.08] |
| 35 Response to treatment (based on both patient and physician assessment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 36 Duration of morning stiffness (hr) | 5 | 456 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 36.1 End point | 4 | 409 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 36.2 Change from baseline | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.48, ‐0.30] |
| 37 Duration of morning stiffness (2nd analysis) (hr) | 5 | 456 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.39, ‐0.00] |
| 37.1 Change from base line | 2 | 250 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.48, ‐0.30] |
| 37.2 End point | 3 | 206 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 38 Morning stiffness (end point) (VAS 100 mm, 0 = no stiffness, 100 = severe stiffness) | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐13.89 [‐22.54, ‐5.24] |
| 38.1 End point | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐12.00 [‐23.78, ‐4.22] |
| 38.2 Change from baseline | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐13.5 [‐30.00, 5.00] |
| 39 Improvement in morning stiffness | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 40 Erythrocyte sedimentation rate (mm/hr) | 8 | 560 | Mean Difference (IV, Random, 95% CI) | ‐5.66 [‐10.40, ‐0.93] |
| 40.1 End point | 6 | 466 | Mean Difference (IV, Random, 95% CI) | ‐7.07 [‐14.39, 0.25] |
| 40.2 Change from baseline | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐3.09 [‐4.84, ‐1.35] |
| 41 Erythrocyte sedimentation rate (2nd analysis) (mm/hr) | 8 | 560 | Mean Difference (IV, Random, 95% CI) | ‐4.79 [‐8.80, ‐0.78] |
| 41.1 Change from baseline | 4 | 326 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.62, ‐1.60] |
| 41.2 End point | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐9.56 [‐22.03, 2.91] |
| 42 C‐reactive protein (ug/ml) | 3 | 325 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐3.76, 0.92] |
| 42.1 End pointSub‐category | 2 | 278 | Mean Difference (IV, Random, 95% CI) | ‐1.76 [‐7.42, 3.90] |
| 42.2 Change from baseline | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐4.70, ‐0.30] |
| 43 C‐reactive protein (2nd analysis) (ug/ml) | 3 | 325 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.85, 1.07] |
| 43.1 Change from baseline | 2 | 250 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐3.37, 1.33] |
| 43.2 End point | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐16.80, 2.80] |
| 44 Withdrawal due to side effect | 11 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.04, 2.15] |
| 45 Withdrawal due to ineffectiveness | 10 | 833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.42] |
| 46 Drop‐out for any reason | 10 | 833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.03, 1.73] |
| 47 Serious adverse events | 1 | 264 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.50 [0.15, 378.16] |
1.39. Analysis.
Comparison 1 Sulfasalazine versus placebo, Outcome 39 Improvement in morning stiffness.
1.45. Analysis.
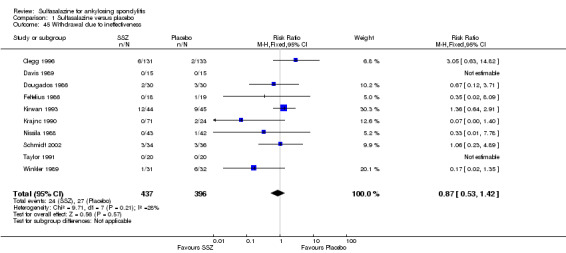
Comparison 1 Sulfasalazine versus placebo, Outcome 45 Withdrawal due to ineffectiveness.
1.47. Analysis.
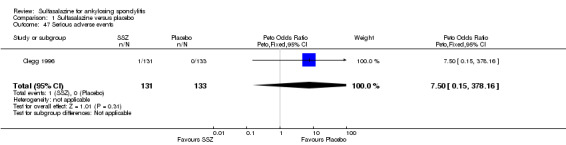
Comparison 1 Sulfasalazine versus placebo, Outcome 47 Serious adverse events.
Comparison 2. Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Back pain (VAS 100 mm, 0 = no pain, 100 = severe pain) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Score of sleep disturbance (0 to 4, 0 = no disturbance, 4 = severe disturbance) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Chest expansion (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Schober's test (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Fingers‐to‐floor test (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Articular index (0 to 66, the higher the score the more severe the disease) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Degree of joint swelling (0 to 66, the higher the score the more severe the disease) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Patient assessment of disease severity (VAS 100 mm, 0 = very good, 100 = very poor) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Duration of morning stiffness (hr) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Erythrocyte sedimentation rate (mm/hr) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
2.1. Analysis.
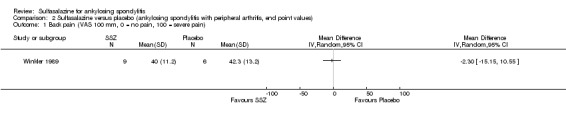
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 1 Back pain (VAS 100 mm, 0 = no pain, 100 = severe pain).
2.2. Analysis.

Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 2 Score of sleep disturbance (0 to 4, 0 = no disturbance, 4 = severe disturbance).
2.3. Analysis.
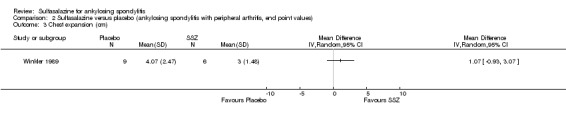
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 3 Chest expansion (cm).
2.4. Analysis.
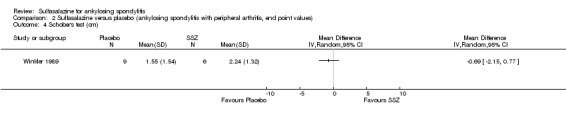
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 4 Schober's test (cm).
2.5. Analysis.
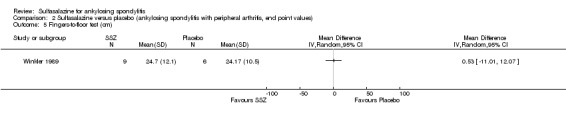
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 5 Fingers‐to‐floor test (cm).
2.6. Analysis.
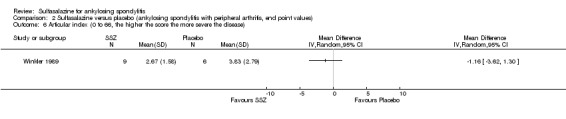
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 6 Articular index (0 to 66, the higher the score the more severe the disease).
2.7. Analysis.
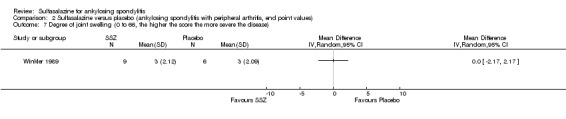
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 7 Degree of joint swelling (0 to 66, the higher the score the more severe the disease).
2.8. Analysis.

Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 8 Patient assessment of disease severity (VAS 100 mm, 0 = very good, 100 = very poor).
2.9. Analysis.
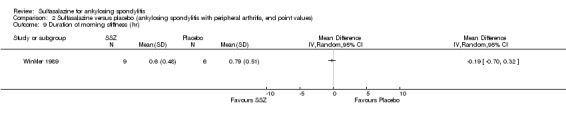
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 9 Duration of morning stiffness (hr).
2.10. Analysis.
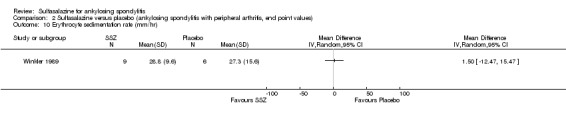
Comparison 2 Sulfasalazine versus placebo (ankylosing spondylitis with peripheral arthritis, end point values), Outcome 10 Erythrocyte sedimentation rate (mm/hr).
Comparison 3. Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Back pain (VAS 100 mm, 0 = no pain, 100 = severe pain) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Score of sleep disturbance (0 to 4, 0 = no disturbance, 4 = severe disturbance) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Chest expansion (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Schober's test (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Fingers‐to‐floor test (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Patient assessment of disease severity (VAS 100 mm, 0 = very good, 100 = very poor) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Duration of morning stiffness (hr) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Erythrocyte sedimentation rate (mm/hr) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.2. Analysis.
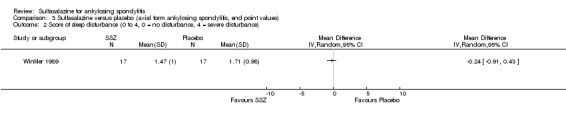
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 2 Score of sleep disturbance (0 to 4, 0 = no disturbance, 4 = severe disturbance).
3.3. Analysis.
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 3 Chest expansion (cm).
3.4. Analysis.
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 4 Schober's test (cm).
3.5. Analysis.
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 5 Fingers‐to‐floor test (cm).
3.6. Analysis.
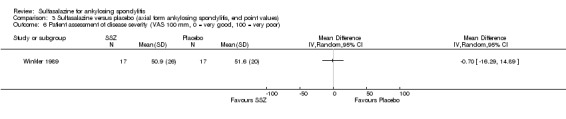
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 6 Patient assessment of disease severity (VAS 100 mm, 0 = very good, 100 = very poor).
3.7. Analysis.
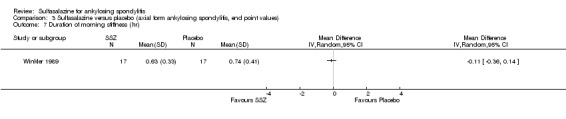
Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 7 Duration of morning stiffness (hr).
3.8. Analysis.

Comparison 3 Sulfasalazine versus placebo (axial form ankylosing spondylitis, end point values), Outcome 8 Erythrocyte sedimentation rate (mm/hr).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clegg 1996.
| Methods | Multicenter Randomized allocation Double‐blind allocation and assessment Parallel design Duration: 36 weeks Sample size at entry: 264 SSZ: 131 Placebo: 133 Clear description of withdrawal and drop‐outs Primary outcomes were analyzed according to intention‐to‐treat, secondary outcomes included only those who completed the trial | |
| Participants | Participants fulfilling the modified New York criteria for definite AS Other inclusion criteria: 1. active spondylitis, defined as morning stiffness at least 45 min, inflammatory pain, patient and physician global assessment of disease activity of "moderate" or higher, failure to respond to a trial of aspirin or another NSAID 2. maintained on a stable dose of aspirin or another NSAID for at least 4 weeks Exclusion criteria: 1. evidence of complete ankylosis of the entire spine 2. other known causes of sacroiliitis 3. positive rheumatoid factor or anti‐nuclear antibody (> 1:80) 4. history of inflammatory bowel diseases or other rheumatic diseases 5. previously treated with SSZ 6. history of sensitivity to salicylates, sulfa‐containing drugs or tartrazine 7. with chronic diseases (according to the investigator) Age: 44.6 +/‐ 12.6 Male: 95% Duration of disease: 18.5 +/‐ 11.6 HLA B27: 81% With peripheral arthritis: 29% |
|
| Interventions | SSZ 1.0 g orally, twice a day Placebo 1.0 g orally, twice a day | |
| Outcomes | Primary:
1. Response to treatment (event)
2. Improvement in physician global assessment (event)
3. Improvement in patient global assessment (event)
4. Improvement in morning stiffness (event)
5. Improvement in back pain (event) Secondary: 1. Night pain (no bother, event) 2. Duration of morning stiffness (hr) 3. Back pain (100 mm visual analogue scale) 4. Spondylitis function index (score 0 to 40) 5. Joint pain/tenderness score (0 to 198) 6. Joint swelling score (0 to 198) 7. Dactylitis score (0 to 3) 8. Enthesopathy index (0 to 90) 9. Spondylitis articular index (0 to 30) 10. Chest expansion (cm) 11. Modified Schober's test (cm) 12. Occiput‐to‐ wall test (cm) 13. Fingers‐to‐ floor test (cm) 14. ESR (mm/hr) 15. CRP (ug/mL) 16. Withdrawal due to side effect 17. Withdrawal due to ineffectiveness 17. Drop out for any reason |
|
| Notes | This trial was funded by Department of Veterans Affairs and Medical Research Service All continuous outcomes were presented as both end point and change from baseline Subgroup analysis: In participants without peripheral disease (N = 187), 40.2% of SSZ group and 43.3% of placebo group showed axial response In participants with peripheral diseases (N = 77), 32.4% of SSZ group and 20.9% of placebo group showed axial response, while 55.9% of SSZ group and 30.2% of placebo group showed peripheral response No description about the number under each intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients received either 500 mg of enteric‐coated SSZ tablets or an identical placebo". "The following physical assessment was made by a clinician" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Withdrawals from the study were fewer than was anticipated by the Planning Committee". 36 patients from the SSZ group and 25 patients from the placebo group, the reasons for withdrawal were similar in both groups. The main study analysis based on intention‐to‐treat principles |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Corkill 1990.
| Methods | Randomized allocation Double‐blind and assessment blind Parallel design Duration: 48 weeks Sample size at entry:62 SSZ: 32 Placebo: 30 Clear description of withdrawal due to side effect Unclear about which intervention group the 6 drop‐outs belonged to Intention‐to‐treat analysis: unclear | |
| Participants | Participants fulfilling the New York criteria for AS and requiring daily NSAIDs or analgesics Exclusion criteria: 1. psoriasis or acknowledged inflammatory bowel diseases 2. previously treated with SSZ 3. currently treated with corticosteroid or immunosuppressive drugs Age in SSZ group: 37.4 +/‐ 8.5 Age in placebo group: 28.2 +/‐ 11.4 Male: 87% Duration of symptom in SSZ group: 12.3 +/‐ 8.2 yr Duration of symptom in placebo group: 16.1 +/‐ 11.4 yr With peripheral arthritis: 19% |
|
| Interventions | SSZ 1.0 g orally, twice a day Placebo 1.0 g orally, twice a day | |
| Outcomes | 1. Spinal pain (100 mm visual analogue scale) 2. Spinal stiffness (100 mm visual analogue scale) 3. Peripheral joint pain (100 mm visual analogue scale) 4. Modified Schober's test (cm) 5. Chest expansion (cm) 6. Cervical flexion (degree) 7. Cervical rotation (degree) 8. ESR (mm/h) 9. Withdrawal due to side effect 10. Withdrawal due to ineffectiveness 11. Drop out for any reason | |
| Notes | The active and placebo tablets were offered by Pharmacia Company and the author was supported by the Arthritis Foundation of New Zealand All continuous outcomes were presented as change from baseline Outcomes were also assessed at 4, 8, 12, 24, 36, weeks after the trial began Results were presented as means for each intervention group and 95% CI for difference between them SDs for each group were not given No significant difference was found between the intervention groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to receive SASP or placebo using a randomisation protocol constructed from a table of random numbers in block four" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial is double‐blind placebo‐controlled. Although three participants broke the blinding, and two participants became aware of their therapy, the observers remained blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mention how many dropped out from either active group or placebo group |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Davis 1989.
| Methods | Randomized allocation Assessment blind Parallel design Duration: 3 months Sample size at entry: 28 SSZ: 15 Placebo: 13 Clear description of drop‐outs Outcomes included only those who completed the trial | |
| Participants | Participants fulfilling the New York criteria for AS and with active disease defined as:
1. low back morning stiffness > 10 min or sleep disturbance due to pain or stiffness
2. ESR > 30 mm/hr or CRP > 20 ug/mL or IgA > 272 IU/mL Exclusion criteria: 1. history of inflammatory bowel diseases, Reiter's disease or psoriasis 2. fused sacroiliac joints or more than three syndesmophytes in the lumber spine 3. positive rheumatoid factor Age (median and range) SSZ group: 35 (23 to 49) Placebo group: 40 (21 to 57) Male: 25/28 Duration of disease (median and range) SSZ group: 8.6 (1 to 30) Placebo group: 8.4 (1 to 25) With peripheral arthritis: 23% |
|
| Interventions | SSZ 2.0 g orally daily Placebo 2.0 g orally daily | |
| Outcomes | 1. Pain (100 mm visual analogue scale) 2. Spinal stiffness (100 mm visual analogue scale) 3. Sleep disturbance (event) 4. Occiput‐to‐wall test (cm) 5. Fingers‐to‐floor test (cm) 6. ESR (mm/hr) 7. CRP (ug/mL) 8. Withdrawal due to side effect 9. Withdrawal due to ineffectiveness 10. Drop out for any reason | |
| Notes | The active and placebo tablets were offered by Pharmacia Company All continuous outcomes were presented as end point values We changed sleep disturbance into night pain (no bother) Participants who dropped out were counted as night pain (bother) The following were presented as median (interquartile range): Pain: SSZ group 20 (0 to 60) Placebo group 30 (20 to 70) Spinal stiffness: SSZ group 20 (0 to 50) Placebo group 20 (0 to 60) CRP: SSZ group 11 (6 to 23) Placebo group 28 (6 to 34) Other outcomes were presented as mean +/‐ 95% CI, from which we calculated SD We changed the unit from cm to mm, inch to cm and mg/L to ug/mL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | The study did not address allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This is placebo‐controlled trial and the observer blinding was stressed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are 30 participants recruited at the beginning of the trial, but only the participants who completed 3 months' treatment were analysed |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dougados 1986.
| Methods | Randomized allocation Double‐blind and assessment blind Parallel design Duration: 6 months Sample size at the entry: 60 SSZ: 30 Placebo: 30 Clear description of withdrawal and drop out Treatment failure and drop out were analyzed according to intention‐to‐treat | |
| Participants | Participants fulfilling the New York criteria for AS and requiring daily NSAIDs 8 had family history and/or minimal localised lesions of cutaneous psoriasis None suffered from peripheral arthritis None had symptoms suggestive of inflammatory bowel diseases Age (median and confidence interval): SSZ group: 38.5 (13.1 to 65.3) Placebo group: 37.0 (19.0 to 59.0) Male: 77% Duration of disease (median and confidence interval): SSZ group: 10 (‐8.8 to 33.2) Placebo group: 10 (‐5.0 to 29.2) HLA B27: 85% With peripheral arthritis: 0 |
|
| Interventions | SSZ 2.0 g orally daily Placebo 2.0 g orally daily | |
| Outcomes | 1. Treatment failure of overall patient assessment (event, including any withdrawal) 2. Score of daily NSAIDs (usual dosage as 10) 3. Pain (100 mm visual analogue scale) 4. Joint index (0 to 66) 5. Frequency of nocturnal awakening 6. Function index (0 to 40) 7. Schober's test (cm) 8. Fingers‐to‐floor test (cm) 9. Chest expansion (cm) 10. ESR (mm/hr) 11. Withdrawal due to side effect 12. Withdrawal due to ineffectiveness 13. Drop out for any reason | |
| Notes | There was no financial interest to report All continuous outcomes were presented as change from baseline and as median (95% CI) We assumed that mean was equal to median for each outcome and calculated SD from CI and sample size We changed 'treatment failure of overall patient assessment' into 'improvement in patient global assessment' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was in blocks of six |
| Allocation concealment (selection bias) | Low risk | The study supplied the active drug in numerical order |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This is a double‐blind (participants and investigators), placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For continuous outcome data, the analysis was made on those participants who completed the trials (47/60). The only one dichotomous outcome (treatment success or failure) was analyzed as intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Feltelius 1986.
| Methods | Randomized allocation Blind outcome assessment: unclear Parallel design Duration: 12 weeks Sample size at entry: 37 SSZ: 18 Placebo: 19 Clear description of withdrawal and drop out Outcomes included only those who completed the trial | |
| Participants | Participants fulfilling AS criteria of the American Rheumatism Association (1966) Other inclusion criteria: 1. HLA B27 (+) 2. ESR > 30 mm/hr or haptoglobin >= 2.0 g/L or orosomucoid >= 1.2 g/L 3. morning stiffness > 30 min or disturbed sleep due to pain or stiffness Exclusion criteria: 1. history of inflammatory bowel diseases, Reiter's disease or psoriasis 2. with chronic infection, malignancy or other concomitant illness which might interfere the trial 3. Known allergy or intolerance to sulphonamide or salicylates 4. significant renal, hepatic, or hematological disease Age (median and actual range): SSZ group: 41.3 (25 to 57) Placebo group: 36.5 (19 to 57) Male: 76% Duration of disease (median and actual range): SSZ group: 12.1 (2 to 30) Placebo group: 10.4 (2 to 20) HLA B27: 100% With peripheral arthritis: 5% |
|
| Interventions | SSZ initially 1.0 g/d orally, increased 0.5 to 1.0 g weekly until 3.0 g/d or the highest dose the patient could tolerate Placebo: the same as SSZ | |
| Outcomes | 1. Duration of morning stiffness (hr) 2. Spinal stiffness (100 mm visual analogue scale, change from baseline) 3. Pain (100 mm visual analogue scale) 4. Genral wellbeing (100 mm visual analogue scale) 5. Chest expansion (cm). Schober's test (cm) 7. Sleep disturbance (not specify event or degree) 8. Sacroiliac pain (100 mm visual analogue scale) 9. ESR (mm/hr, end point and change from baseline) 10. Withdrawal for side effect 11. Withdrawal for ineffectiveness 12. Drop out for any reason | |
| Notes | There was no financial interest to report Most outcomes were presented as graph which could not be transformed into figure Among continuous outcomes, only ESR was available for our analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This is double‐blind, placebo‐controlled trial. Although there is no information about who were blinded, it appeared to not affect the result much because only one outcome (ESR) was available for the present review |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only those who completed the trial were included in the analysis |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kirwan 1993.
| Methods | Randomized allocation Double‐blind Outcome assessment blind: unclear Parallel design Duration: 3 years Sample size at entry: 89 SSZ: 44 Placebo: 45 Clear description of withdrawal and drop‐outs Intention‐to‐treat analysis | |
| Participants | Participants fulfilling the New York criteria for AS Those who clinically had very little or no spinal mobility were excluded Age SSZ group: 44.1 +/‐ 13.3 Placebo group: 45.7 +/‐ 12.2 Male: 85% Duration of disease : SSZ group: 19.0 +/‐12.0 Placebo group: 21.9 +/‐ 11.7 HLA B27: 98% With peripheral arthritis: 28% |
|
| Interventions | SSZ 1.0 g orally twice a day Placebo 1.0 g orally twice a day | |
| Outcomes | Primary:
1. Modified Schober's test (cm)
2. Chest expansion (cm)
3. Lateral cervical flexion (degree) Secondary 4. Function (Health assessment questionnaire) 5. Back pain (visual analogue scale) 6. Early morning back stiffness (visual analogue scale) 7. Consumption of anti‐inflammatory drugs 8. Sleep disturbance (visual analogue scale) 9. Patient assessment of response (4 point scale) 10. Episodes of peripheral arthritis 11. Episodes of heel pain 12. Flares in general AS symptoms 13. Episodes of iritis 14. Withdrawal for side effect 15. Withdrawal for ineffectiveness 16. Drop out for any reason |
|
| Notes | There was no financial interest to report Primary outcomes were presented only as graph where no significant difference was reported between two groups For secondary outcomes, no figure was given except for episodes of peripheral arthritis which showed significant difference (0.298 episodes/yr in SSZ group vs 0.392 episodes/yr in placebo group) (P<0.05) Extra information about randomisation, concealment and withdrawal was offered by the author 35 (39%) participants withdrew from treatment. Few (5 placebo, 8 SSZ) were precipitated by specific adverse reactions. The majority withdrew because they preferred to stop taking tablets regularly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was randomised using computer generated random numbers sealed in envelops and opened sequentially as each patient was entered into the study |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacy was not revealed to the participants or assessors |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Indistinguishable placebo tablets were used in the study. Analysis of primary outcome measures was performed by the same assessor, and the results of statistics were disclosed to the investigators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the Methods section, the authors stated that the participants were assessed even if they discontinued the trial medicine. However, in the results section, they did not provide the sample size of each outcome |
| Selective reporting (reporting bias) | High risk | Some of the prespecified primary outcomes have not been reported, such as function, consumption of NSAIDs, patients assessment of response |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Krajnc 1990.
| Methods | Randomized allocation Double‐blind Blind outcome assessment: unclear Parallel design Duration: 24 weeks Sample size at the entry: 95 SSZ: 71 Placebo: 24 Clear description of withdrawal and drop out Intention‐to‐treat analysis? | |
| Participants | Participants fulfilling the New York criteria for AS, with ESR > 25 mm/hr, morning stiffness > 50 min Exclusion criteria: 1. history of intestinal tract inflammation, Reiter's syndrome, psoriasis, malignant neoplasm 2. allergic to sulphasalazine and salicylates 3. pathologic tests of liver, kidney and hematology Age SSZ group: 38.25 +/‐ 6.3 Placebo group: 37.6 +/‐ 9.1 Male: 79% Duration of disease: not given With peripheral arthritis: 66% |
|
| Interventions | SSZ initially 1.0 g/d orally, increased 0.5 to 1.0 g weekly until 3.0 g/d or depending on the efficacy and tolerance Placebo: the same as SSZ | |
| Outcomes | 1. Duration of morning stiffness (hr) 2. Schober's test (cm) 3. Chest expansion (cm) 4. Fingers‐to‐floor test (cm) 5. ESR (mm/hr) 6. Withdrawal due to side effect 7. Withdrawal due to ineffectiveness 8. Drop out for any reason | |
| Notes | There was no financial interest to report All continuous outcomes were presented as end point values The author offered the full‐text paper and English translation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Neither the physician nor the patients were acquainted about the sort of medicine they actually use" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Number of patients interrupting therapy because of side effects, SSZ group 8/71 (2 skin rash, 3 nausea, 3 did not want to continue therapy), placebo group 5/24 (1 skin rash, 2 inefficiency, 2 did not want to continue)". Did not use intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nissila 1988.
| Methods | Randomized allocation Double‐blind Blind outcome assessment: unclear Parallel design Duration: 26 weeks Sample size at the entry: 85 SSZ: 43 Placebo: 42 Clear description of withdrawal and drop out Intention‐to‐treat analysis: unclear | |
| Participants | Participants fulfilling the New York criteria for AS Other inclusion criteria: 1. ESR >=30 mm/hr or CRP >= 20 mg/L 2. morning stiffness > 30 min 3. seronegative Exclusion criteria: 1. history or presence of intestinal disease, Reiter's disease, psoriasis, chronic infection, malignancy and other disease which could interfere with the trial 2. allergic to sulfonamide or salicylates 3. with renal, hepatic and hematologic disease 4. advanced cases of ankylosed sacroiliac joints Age: SSZ group: 36.5 +/‐ 9.3 placebo group: 39.1 +/‐ 8.0 Male: 79% Duration of disease: SSZ group: 5.4 +/‐ 7.3 Placebo group: 3.8 +/‐ 4.3 With peripheral arthritis: 68% |
|
| Interventions | SSZ initially 1.0 g/d orally, increased 0.5 to 1.0 g weekly until 3.0 g/d or depending on the efficacy and tolerance Placebo: the same as SSZ | |
| Outcomes | 1. Duration of morning stiffness (hr) 2. Spinal stiffness (100 mm visual analogue scale) 3. Back pain (100 mm visual analogue scale) 4. Chest expansion (cm) 5. Schober's test (cm) 6. Fingers‐to‐floor test (cm) 7. Occiput‐to‐wall test (cm) 8. Number of painful joints 9. Number of swollen joints 10. General wellbeing (100 mm visual analogue scale) 11. ESR (mm/hr) 12. CRP (ug/mL) 13. Withdrawal due to side effect 14. Withdrawal due to ineffectiveness 15. Drop out for any reason | |
| Notes | There was no financial interest to report All continuous outcomes were presented as end point values We suspected that results of 'chest expansion' were errors because they were impossible to be about 40 to 50 cm so we divided them by 10 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The doctors and patients were not informed of the drug codes" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 2 drop‐outs in each group but no information about missing outcome results |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Schmidt 2002.
| Methods | Randomized allocation Double‐blind Outcome assessment blind: unclear Parallel design Duration: 26 weeks Sample size at the entry: 70 SSZ: 34 Placebo: 36 Clear description of withdrawal and drop out Intention‐to‐treat analysis: unclear | |
| Participants | Participants fulfilling the modified New York criteria for AS Other inclusion criteria: 1. rheumatoid factor negative 2. morning stiffness >= 20 min 3. pain in the field of the axial skeleton >= 25 mm (100 mm visual analogue scale) Exclusion criteria: 1. known allergic to sulfonamide, salicylates and tartrazin 2. hematological diseases including thrombocytopenia (PLT < 140 G/L) and leukocytopenia (WBC < 4.0 G/L) 3. Severe liver diseases including GOT or GPT values > double of the upper norm limits 4. known renal diseases or increased creatinine 5. known G6PD deficiency, acute porphyria, asthma bronchial, pregnancy or urgent desire of an own baby, chronic inflammatory intestine diseases, RA, psoriasis, reactive arthritis, SLE, gout 6. corticosteroid treatment within the last month 7. severe diseases which were dangerous to participate in the trial Age: 27.5 +/‐ 8.3 SSZ group: 26.9 +/‐ 7.8 Placebo group: 28.0 +/‐ 8.8 Male: 87% Duration of disease: 16.7 +/‐ 7.2 SSZ group: 16.3 +/‐ 7.8 Placebo group: 17.1 +/‐ 6.6 With peripheral arthritis: 36% |
|
| Interventions | SSZ 1.0 g orally, 3 times a day Placebo: the same as SSZ | |
| Outcomes | 1. Back pain (100 mm visual analogue scale) 2. Nocturnal awakening (event) 3. Enthesopathy index (0 to 90) 4. Duration of morning stiffness (hr) 5. Number of painful joints 6. Number of swollen joints 7. Spondylitis function index (0 to 44) 8. Effectiveness in patient assessment (event) 9. Effectiveness in physician assessment (event) 10. Schober's test 11. Fingers‐to‐floor test (cm) 12. Occiput‐to‐wall test (cm) 13. Chin sternum distance (cm) 14. Chest expansion (cm) 15. ESR (mm/hr) 16. CRP (ug/mL) 17. Withdrawal due to side effect 18. Withdrawal due to ineffectiveness 19. Drop out for any reason | |
| Notes | There was no financial interest to report All continuous outcomes were presented as change from baseline We changed 'effectiveness in patient assessment' to 'improvement in patient global assessment' We changed 'effectiveness in physician assessment' to 'improvement in physician global assessment' Those dropping out were counted as not improved We changed 'nocturnal awakening' to 'night pain ('no bother' participants who dropped out were counted as night pain (bother) For 'number of painful joints' and 'number of swollen joints', no SD was given For 'fingers‐to‐floor test' (cm), SD of SSZ group was missed German full‐text was translated into English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "It treat of a prospective, randomized, double blind, placebo‐controlled study with intention‐to‐treat analyse of the results". But no information about which two were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 16/34 and 7/36 participants withdrew from the trial in SZZ and placebo, respectively. No information about the missing data |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Taylor 1991.
| Methods | Randomized allocation Double‐blind Blind outcome assessment Parallel design Duration: 1 year Sample size at entry: 40 SSZ: 20 Placebo: 20 Clear description of withdrawal and drop out Intention‐to‐treat analysis: unclear | |
| Participants | Participants fulfilling the New York criteria for AS Other inclusion criteria: 1. morning stiffness > 10 min or sleep disturbance due to pain 2. ESR > 30 mm/hr or PCR > 20 mg/L or IgA > 272 IU/mL 3. require regular NSAIDs or analgesics Exclusion criteria: 1. complete fusion of both sacroiliac joints or the presence of 3 bridged syndesmophytes on the lateral lumbar spine X‐ray 2. Reiter's disease, IBD or psoriasis Age: SSZ group: 34.7 +/‐1.8 Placebo group: 39.4 +/‐ 2.2 Duration of disease: SSZ group: 11 +/‐ 1.6 Placebo group: 10.7 +/‐ 1.9 With peripheral arthritis: 15% |
|
| Interventions | SSZ orally, maximum tolerated dose or 2.0 g/d Placebo: the same as SSZ | |
| Outcomes | 1. Back pain (100 mm visual analogue scale) 2. Fingers‐to‐floor test (cm) 3. Chest expansion (cm) 4. Sleep disturbance (%) 5. Forced vital volume (L/min) 6. Occiput‐to‐wall test (cm) 7. Schober's test (cm) 8. Spinal stiffness (100 mm visual analogue scale) 9. Reduction or stop NSAIDs (event) 10. Withdrawal due to side effect 11. Withdrawal due to ineffectiveness 12. Drop out for any reason | |
| Notes | This study was supported by the Haywood Rheumatism Research and Development Foundation and Pharmacia All continuous outcomes were presented as change from baseline We changed 'sleep disturbance (%)' to 'night pain (no bother)' We changed visual analogue scale 10 cm to visual analogue scale 100 mm and inch to cm Sacroiliac joints and lumbar spine radiograph score were assessed but no figures given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed within groups of 4 by Pharmacia |
| Allocation concealment (selection bias) | Low risk | The Department Pharmacia was not revealed to the participants or assessors |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All observers were blinded to which therapy the patients were taking". "The clinical measurements were performed by a single observer". "The erosion count was confirmed for each patients by 2 observers blinded to treat" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address the issue of missing data of those drop‐outs and did not use principle of intention‐to‐treat |
| Selective reporting (reporting bias) | High risk | The study did not report the scores of patients' general wellbeing |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Winkler 1989.
| Methods | Randomized allocation Single‐blind Outcome assessment blind: unclear Parallel design Duration: 24 weeks Sample size at the entry: 63 SSZ: 31 Placebo: 32 Clear description of withdrawal and drop out Outcomes included only those who completed the trial | |
| Participants | Participants fulfilling the New York criteria for AS Other inclusion criteria: 1. Duration of disease > 1 yr 2. active disease, e.g. severe pain, sleep disturbed and morning stiffness, did not respond to NSAIDs Exclusion criteria: 1. history of sulphonamide allergy or intolerance 2. psoriasis, reactive arthritis, inflammatory bowel diseases, chronic infection, malignancy, liver, renal or hematological diseases Age (median and range) SSZ group: 40.5 (25 to 66) placebo group: 40.2 (27 to 60) Male: 83% Duration of disease (median and range): SSZ group: 11.8 (1 to 30) Placebo group: 10.2 (2 to 28) With peripheral arthritis: 33% |
|
| Interventions | SSZ 2.0 g/d orally Placebo: the same as SSZ | |
| Outcomes | 1. ESR (mm/hr) 2. Duration of morning stiffness (hr) 3. Back pain (100 mm visual analogue scale) 4. Score of sleep disturbance (0 to 4) 5. Chest expansion (cm) 6. Schober's test (cm) 7. Fingers‐to‐floor test (cm) 8. Patient assessment of disease severity (100 mm visual analogue scale) 9. Articular index (score 0 to 36) 10. Degree of joint swelling (0 to 36) 11. Withdrawal for side effect 12. Withdrawal for ineffectiveness 13. Drop out for any reason | |
| Notes | There was no financial interest to report All continuous outcomes were presented as end point values We changed min to hr |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of Yes or No |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this item |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The result were analyzed with intention‐to‐treat principle, 5 participants from active group and 9 participants from placebo group dropped out, SSZ treatment was withdrawn due to adverse side‐effects, placebo treatment because of its effectiveness. Reason for missing outcome data likely to be related to true outcome, with imbalance in reasons for missing data across intervention groups |
| Selective reporting (reporting bias) | Low risk | They reported the expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
AS ‐ ankylosing spondylitis CI ‐ confidence interval CRP ‐ C‐reactive protein ESR ‐ erythrocyte sedimentation rate GOT ‐ glutamic‐oxaloacetic transaminase GPT ‐ glutamic‐pyruvic transaminase G6PD ‐ glucose‐6‐phosphate dehydrogenease g/d ‐ grams per day HLA B27 ‐ human leukocyte antigen B27 Ig A ‐ immunoglobulin A mm/hr ‐ millimetre per hour NSAID ‐ non‐steroidal anti‐inflammatory drug RA ‐ rheumatoid arthritis SAPA ‐ sulfasalazine SD ‐ standard deviation SLE ‐ systemic lupus erythematosus SSZ ‐ sulfasalazineug ‐ microgram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benitez‐Del‐Castillo | No relevant outcomes |
| Braun 2006 | This was a multicenter, double‐blind, placebo controlled RCT. The participants were 242 patients with inflammatory back pain due to undifferentiated spondyloarthritis and early AS, including 12 AS patients. We were unable to get the results for AS patients alone |
| Braun 2011 | This was a multicenter, double‐blind, placebo controlled RCT comparing etanercept with SSZ in patients with AS. We excluded it because there is another separate Cochrane review addressing biologics in AS |
| Burgos‐Vargas 2002 | The participants were 33 patients with juvenile onset spondyloarthropathy, including 13 AS patients. No outcome specific to AS patients was presented |
| Deng 2009 | No relevant outcomes |
| NCT00953979 | This is a protocol; attempts to contact the authors to access full‐text have been unsuccessful so far |
| Song 2011 | The participants were 76 patients with axial spondyloarthritis; no outcome specific to AS patients were presented |
| Xu 2008 | This was a RCT, comparing the effects of Bushen Tongdu Decoction (Chinese traditional medicine) and SSZ on serum tumour necrosis factor‐alpha and transforming growth factor beta1 in patients with AS. Because the efficacy of Bushen Tongdu Decoction is unclear in AS, we excluded this study |
| Zhao 2006 | This was a RCT about long‐term effectiveness of leflunomide compared with SSZ in treatment of AS. The efficacy of leflunomide in AS is unclear and therefore we decided to exclude this trial after a discussion |
| Zhao 2009 | This was a RCT, comparing the effects of etanercept and SSZ. We excluded it because there was another separate Cochrane review addressing biologics in AS |
AS ‐ ankylosing spondylitis RCT ‐ randomized controlled trials SSZ ‐ sulfasalazine
Differences between protocol and review
In this updated review, we used The Cochrane Collaboration's tool (Higgins 2011) for assessing risk of bias. We used GRADEpro software and included a 'Summary of findings' table to present the main results. We modified the primary and secondary outcomes to reflect the recommendation by the CMSG editors.
Contributions of authors
JC: Registered the title; developed the protocol; searched for relevant studies; selected the studies and assessed their risk of bias; extracted and synthesized the data; and wrote the systematic review.
SL (for the update), CL (for the original review): Selected studies, assessed their risk of bias and extracted data from them; and wrote the systematic review, independently from JC.
Sources of support
Internal sources
The First Affiliated Hospital of Fujian Medical University, China.
External sources
Science development fund, Fujian Medical University, China.
Declarations of interest
JC ‐ none SL ‐ none CL ‐ none
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Clegg 1996 {published data only}
- Clegg DO, Reda DJ, Adbellaitf M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondyloarthropathies: a Department of Veterans Affairs cooperative study. Arthritis and Rheumatism 1999;42(11):2325‐9. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Weisman MH, Blackburn WD, Cush JJ, Cannon GW, et al. Comparison of sulfasalazine and placebo in the treatment of ankylosing spondylitis: a Department of Veterans Affairs Cooperative Group. Arthritis and Rheumatism 1996;39:2004‐12. [DOI] [PubMed] [Google Scholar]
- Reda D, Anderson R, Abdellatif M, Williams D, Clegg D. Longitudinal analysis of binary data in the V.A. Cooperative Study of sulfasalazine for the treatment of seronegative spondyloarthropathies. Controlled Clinical Trials 1995;16(3 Suppl):90s‐91s. [Google Scholar]
Corkill 1990 {published data only}
- Corkill MM, Jobanputra P, Gibson T, Macfarlane DG. A controlled trial of sulphasalazine treatment of chronic ankylosing spondylitis: failure to demonstrate a clinical effect. British Journal of Rheumatology 1990;29(1):41‐5. [DOI] [PubMed] [Google Scholar]
Davis 1989 {published data only}
- Davis MJ, Dawes PT, Beswick E, Lewin IV, Stanworth DR. Sulphasalazine therapy in ankylosing spondylitis: its effect on disease activity, immunoglobulin A and the complex immunoglobulin A‐alpha‐1‐antitrypsin. British Journal of Rheumatology 1989;28(5):410‐3. [DOI] [PubMed] [Google Scholar]
Dougados 1986 {published data only}
- Dougados M, Boumier P, Amor B. Sulphasalazine in ankylosing spondylitis: A double blind controlled study in 60 patients. British Medical Journal 1986;293(6552):911‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougados M, Boumier P, Amor B. Treatment of ankylosing spondylarthritis with salazosulfapyridin. A double‐blind, controlled study in 60 patients [French]. Revue du Rhumatisme et des Maladies Osteo Articulaires 1987;54(3):255‐60. [PubMed] [Google Scholar]
- Dougados M, Nguyen M, Mijiyawa M, Amor B. Sulfasalazine in Spondylarthropathies. Zeitschrift fur Rheumatologie 1990;49(Suppl 1):80. [Google Scholar]
Feltelius 1986 {published data only}
- Feltelius N, Hallgren R. Sulphasalazine in ankylosing spondylitis. Annals of the Rheumatic Diseases 1986;45(5):396‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwan 1993 {published data only}
- Kirwan J, Edwards A, Huitfeldt B, Thompson P, Currey H. The course of established ankylosing spondylitis and the effects of sulphasalazine over 3 years. British Journal of Rheumatology 1993;32(8):729‐33. [DOI] [PubMed] [Google Scholar]
Krajnc 1990 {published data only}
- Krajnc I. Sulphasalazine in the treatment of ankylosing spondylitis [Serbocroatian]. Lijecnicki Vjesnik 1990;112(5‐6):171‐4. [PubMed] [Google Scholar]
Nissila 1988 {published data only}
- Nissila M, Lahesmaa R, Leirisalo‐Repo M, Lehtinen K, Toivanen P, Granfors K. Antibodies to Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in ankylosing spondylitis: effect of sulfasalazine treatment. Journal of Rheumatology 1994;21(11):2082‐7. [PubMed] [Google Scholar]
- Nissila M, Lehtinen K, Leirisalo‐Repo M, Luukkainen R, Mutru O, Yli‐Kerttula U. Sulfasalazine in the treatment of ankylosing spondylitis. A twenty‐six‐week, placebo‐controlled clinical trial. Arthritis and Rheumatism 1988;31(9):1111‐6. [DOI] [PubMed] [Google Scholar]
Schmidt 2002 {published data only}
- Schmidt WA, Wierth S, Milleck D, Droste U, Gromnica‐Ihle E. Sulfasalazine in ankylosing spondylitis: a prospective, randomized, double‐blind placebo‐controlled study and comparison with other controlled studies [German]. Zeitschrift fur Rheumatologie 2002;61(2):159‐67. [DOI] [PubMed] [Google Scholar]
- Schmidt WA, Wierth S, Milleck D, Droste U, Gromnica‐Ihle E. Sulphasalazine in ankylosing spondylitis: a prospective, randomized, double‐blind, placebo‐controlled study and meta‐analysis of other controlled studies. Zeitschrift fur Rheumatologie 2009;59(Suppl 3):69. [DOI] [PubMed] [Google Scholar]
Taylor 1991 {published data only}
- Taylor HG, Beswick EJ, Dawes PT. Sulphasalazine in ankylosing spondylitis. A radiological, clinical and laboratory assessment. Clinical Rheumatology 1991;10(1):43‐8. [DOI] [PubMed] [Google Scholar]
Winkler 1989 {published data only}
- Winkler V. Sulphasalazine treatment in ankylosing spondylitis: A comparison of sulphasalazine with placebo. Magyar Reumatologia 1989;30(Suppl):29‐37. [Google Scholar]
References to studies excluded from this review
Benitez‐Del‐Castillo {published data only}
- Benitez‐Del‐Castillo JM, Garcia‐Sanchez J, Iradier T, Banares A. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye 2000;14(3A):340‐3. [DOI] [PubMed] [Google Scholar]
Braun 2006 {published data only}
- Braun J, Zochling J, Baraliakos X, Alten R, Burmester G, Grasedyck K, et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Annals of the Rheumatic Diseases 2006;65(9):1147‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2011 {published data only}
- Braun J, Pavelka K, Ramos‐Remus C, Dimic A, Vlahos B, Freundlich B, et al. Clinical efficacy of etanercept versus sulfasalazine in ankylosing spondylitis subjects with peripheral joint involvement. Journal of Rheumatology 2012;39(4):836‐40. [DOI] [PubMed] [Google Scholar]
- Braun J, Horst‐Bruinsma IE, Huang F, Burgos‐Vargas R, Vlahos B, Koenig AS, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: A randomized, double‐blind trial. Arthritis and Rheumatism 2011;63(6):1543‐51. [DOI] [PubMed] [Google Scholar]
- Freundlich B, Braun J, Huang F, Burgos‐Vargas R, Horst‐Bruinsma IE, Vlahos B, et al. Assessment of clinical efficacy in a randomized, double‐blind study of etanercept and sulphasalazine in patients with ankylosing spondylitis. Internal Medicine Journal 2009;39:A55. [Google Scholar]
- Guzman R, Burgos‐Vargas R, Braun J, Freundlich B, Vlahos B, Koenig AS. Etanercept is significantly more effective than sulfasalazine in patients with ankylosing spondylitis. Journal of Clinical Rheumatology 2010;16:S73. [Google Scholar]
- NCT00247962. Study Evaluating Etanercept and Sulphasalazine in Ankylosing Spondylitis. clinicaltrials.gov/ct2/show/record/NCT00247962 2008.
- Heijde D, Braun J, Sieper J, Wishneski C, Vlahos B, Szumski A, et al. The ankylosing spondylitis disease activity score in subjects treated with etanercept (ETN) or sulfasalazine: Comparison with standard efficacy measures. Rheumatology 2010;49:i55. [Google Scholar]
- Heijde DM, Braun J, Dougados M, Szumski A, Pedersen R, Vlahos B, et al. Clinical improvement with Etanercept versus sulfasalazine treatment in patients with ankylosing spondylitis: Comparative performance of various efficacy measurements (ASCEND). Arthritis and rheumatism 2010;62:1927. [Google Scholar]
Burgos‐Vargas 2002 {published data only}
- Burgos‐Vargas R, Pacheco‐Tena C, Vazquez‐Mellado J. A short‐term follow‐up of enthesitis and arthritis in the active phase of juvenile onset spondyloarthropathies. Clinical and Experimental Rheumatology 2002;20(5):727‐31. [PubMed] [Google Scholar]
- Burgos‐Vargas R, Vazquez‐Mellado J, Pacheco‐Tena C, Hernandez‐Garduno A, Goycochea‐Robles MV. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Annals of the Rheumatic Diseases 2002;61(10):941‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deng 2009 {published data only}
- Deng X, Huang F, Zhang J. Thalidomide delays the rate of relapse in ankylosing spondylitis after discontinuing etanercept treatment [停用依那西普后应用沙立度胺可延缓强直性脊柱炎的复发]. Arthritis and Rheumatism 2009;60:1776. [Google Scholar]
NCT00953979 {published data only}
- NCT00953979. Efficacy and safety of Kunxian capsule in treatment of patients with early ankylosing spondylitis [昆仙胶囊治疗强直性脊柱炎的疗效及安全性]. clinicaltrials.gov/ct2/show/record/NCT00953979.
Song 2011 {published data only}
- Song I‐H, Althoff CE, Haibel H, Hermann K‐GA, Poddubnyy D, Listing J, et al. Frequency and duration of drug‐free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Annals of the Rheumatic Diseases 2012;71(7):1212‐5. [DOI] [PubMed] [Google Scholar]
- Song IH, Hermann KG, Haibel H, Althoff CE, Listing J, Burmester GR, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole‐body MRI (ESTHER): A 48‐week randomised controlled trial. Annals of the Rheumatic Diseases 2011;70(4):590‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2008 {published data only}
- Xu JR, Zhang CY, Li WM. Effects of bushen tongdu decoction on serum tumour necrosis factor‐alpha and transforming growth factor beta1, in patients with ankylosing spondylitis [补肾通督方对强直性脊柱炎血清TNF‐α及TGF‐β_1水平的影响]. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi/Chinese Journal of Integrated Traditional and Western Medicine 2008;28(12):1093‐5. [PubMed] [Google Scholar]
Zhao 2006 {published data only}
- Zhao FT, Zhao H, Guan JL, Han XH. Clinical study on long‐term effectiveness of leflunomide compared with sulfasalazine in treatment of ankylosing spondylitis [Chinese]. Pharmaceutical Care and Research 2006;6(6):430‐2. [Google Scholar]
Zhao 2009 {published data only}
- Zhao FT, Zhao F, Wang YL. Efficacy of etanercept on ankylosing spondylitis [依那西普治疗强直性脊柱炎的疗效分析]. Journal of Shanghai Jiaotong University (Medical Science) 2009;29(12):1506‐8. [Google Scholar]
Additional references
Akkoc 2005
- Akkoc N, Khan MA. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: comment on the article by Braun et al. Arthritis and Rheumatism 2005;52(12):4048‐9. [DOI] [PubMed] [Google Scholar]
Braun 2002
- Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicenter trial. Lancet 2002;359(9313):1187‐93. [DOI] [PubMed] [Google Scholar]
Braun 2011a
- Braun J, Berg R, Baraliakos X, Boehm H, Burgos‐Vargas R, Collantes‐Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Annals of the Rheumatic Diseases 2011;70(6):896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013
- Chen J, Veras MM, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004524.pub4] [DOI] [PubMed] [Google Scholar]
Dagfinrud 2008
- Dagfinrud H, Hagen KB, Kvien TK. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002822.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2005
- Davis JC, Heijde D, Dougados M, Woolley JM. Reductions in health‐related quality of life in patients with ankylosing spondylitis and improvements with etanercept therapy. Arthritis and Rheumatism 2005;53(4):494‐501. [DOI] [PubMed] [Google Scholar]
Feldtkeller 2003
- Feldtkeller E, Khan MA, Heijde D, Linden S, Braun J. Age at disease onset and diagnosis delay in HLA‐B27 negative vs. positive patients with ankylosing spondylitis. Rheumatology International 2003;23(2):61‐6. [DOI] [PubMed] [Google Scholar]
Ferraz 1990
- Ferraz MB, Tugwell P, Goldsmith CH, Atra E. Meta‐analysis of sulfasalazine in ankylosing spondylitis. The Journal of Rheumatology 1990;17(11):1482‐6. [PubMed] [Google Scholar]
Gorman 2002
- Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. New England Journal of Medicine 2002;346(18):1349‐56. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Olivieri 2002
- Olivieri I, Tubergen A, Salvarani C, Linden S. Seronegative spondyloarthritides. Best Practice and Research Clinical Rheumatology 2002;16(5):723‐39. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smedegård 1995
- Smedegård G, Björk J. Sulphasalazine: mechanism of action in rheumatoid arthritis. British Journal of Rheumatology 1995;34 Suppl 2:7‐15. [PubMed] [Google Scholar]
Suarez‐Almazor 1998
- Suarez‐Almazor ME, Belseck E, Shea B, Tugwell P, Wells GA. Sulfasalazine for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000958] [DOI] [Google Scholar]
van den Bosch 2002
- Bosch F, Kruithof E, Baeten D, Herssens A, keyser F, Mielants H, et al. Randomized double‐blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis and Rheumatism 2002;46(3):755‐65. [DOI] [PubMed] [Google Scholar]
van der Heijde 1999
- Heijde D, Linden S, Bellamy N, Calin A, Dougados M, Khan MA. Which domains should be included in a core set for endpoints in ankylosing spondylitis? Introduction to the ankylosing spondylitis module of OMERACT IV. The Journal of Rheumatology 1999;26(4):945‐7. [PubMed] [Google Scholar]
van der Heijde 2002
- Heijde D, Braun J, McGonagle D, Siegel J. Treatment trials in ankylosing spondylitis: current and future considerations. Annals of the Rheumatic Diseases 2002;61 Suppl 3:iii24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heijde 2005
- Heijde D, Dougados M, Davis J, Weisman MH, Maksymowych W, Braun J, et al. Assessment in Ankylosing Spondylitis International Working Group/Spondylitis Association of America recommendations for conducting clinical trials in ankylosing spondylitis. Arthritis and Rheumatism 2005;52(2):386‐94. [DOI] [PubMed] [Google Scholar]
van der Heijde 2011
- Heijde D, Sieper J, Maksymowych WP, Dougados M, Burgos‐Vargas R, Landewé R, et al. Assessment of SpondyloArthritis International Society. 2010 Update of the international ASAS recommendations for the use of anti‐TNF agents in patients with axial spondyloarthritis. Annals of the Rheumatic Diseases 2011;70(6):905. [DOI] [PubMed] [Google Scholar]
Zink 2000
- Zink A, Braun J, Listing J, Wollenhaupt J. Disability and handicapin in rheumatoid arthritis and ankylosing spondylitis ‐ results from the German rheumatological database. German Collaborative Arthritis Centers. Journal of Rheumatology 2007;27(3):613‐22. [PubMed] [Google Scholar]
Zochling 2005
- Zochling J, Maxwell L, Beardmore J, Boonen A. TNF‐alpha inhibitors for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005468] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2005
- Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004800.pub2] [DOI] [PubMed] [Google Scholar]


