Abstract
Provider empathy is a crucial component in establishing therapeutic provider–patient relationships. The benefits of increased perceptions of empathy can support patient psychological adjustment to their cancer as well as patients’ comfort and confidence in disclosing to providers, ultimately promoting patient engagement. Guided by the disclosure decision-making model, this manuscript explores how perceptions of empathy influence patient psychological adjustment and how those variables influence patient disclosure efficacy. The model ultimately predicts patient sharing and withholding of information during the medical interaction. This study tested a mediation model to investigate how current (n = 111) and former (n = 174) breast cancer patients’ psychological adjustment mediates the relationship between patient perceptions of oncologist empathic communication and efficacy to disclose health information to their oncologist and their disclosure enactment in sharing and withholding. Overall, former patients compared to current patients had more positive perceptions of their oncologist’s empathic communication, had better psychological adjustment, felt more self-efficacy to disclose to their oncologist, and shared more and withheld less information from their oncologist (p < .05 in all cases). Structural equation modeling revealed good fit to the data for both current and former patients such that more perceived empathic communication was associated with more efficacy for disclosure, which was associated with more sharing and less withholding. Additionally, there was an indirect relationship from perceptions of empathic communication to disclosure efficacy through patients’ psychological adjustment to the diagnosis. Results reinforce the importance of providers’ empathic communication for cancer patients’ psychological adjustment because patient sharing and withholding of information remain crucially important to achieving holistic care across the cancer trajectory.
Effective patient–provider communication is a crucial component of holistic patient care and is needed to achieve shared goals, create health management strategies, and establish therapeutic partnerships in medical encounters (Hong & Oh, 2020; Kwame & Petrucka, 2021; Mead & Bower, 2000). Patient decisions regarding what information is communicated with or withheld from a provider are particularly important in oncology contexts. After a cancer diagnosis, patients are simultaneously making treatment decisions, processing complex information, and psychologically adjusting to their diagnosis (Nosarti et al., 2002; Sutton et al., 2022). However, patients sometimes withhold information that they deem is not relevant, yet this information is often important for clinicians to support patient care (Venetis et al., 2023).
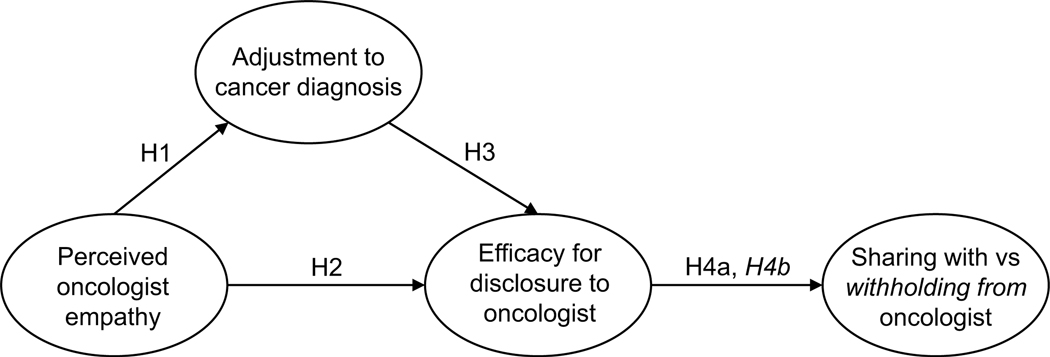
Patient participation in care, including sharing and withholding health information from their healthcare team, is influenced by patients’ psychological adjustment to their cancer diagnosis. For example, patients with cancer experiencing more adaptive coping are often more participative than those experiencing maladaptive coping styles (Venetis et al., 2015). Further, providers’ empathic communication can have a direct influence on patient psychological adjustment to cancer, with more effective communication associated with reduced anxious feelings, increased trust in the oncologist (Zwingmann et al., 2017), and reduced negative feelings about treatment recommendations (Zhou et al., 2021). As such, empathic provider communication may influence both patient psychological adjustment to the diagnosis and, subsequently, participation in care. However, the interaction between these variables (provider empathic communication, psychological adjustment, patient communication) has not been empirically tested and adds value for understanding the healthcare experience of patients with cancer, beyond identifying areas for intervention. Therefore, this study examines how perceptions of empathic provider communication influence (1) patients’ disclosure efficacy, (2) decisions of what is shared with, and (3) decisions of what is withheld from the oncologist. Additionally, this study evaluates the extent to which psychological adjustment mediates the relationship between perceptions of oncologist empathic communication and disclosure efficacy (see Figure 1).
Figure 1.
Hypothesized disclosure models. Latent variables shown without error terms.
Cancer diagnosis and psychological adjustment
The experience of a cancer diagnosis is a stressful event for the patient and close others, and patients are at high-risk to develop psychological comorbidities including depression (Hughes, 1982), anxiety (Mehnert et al., 2014; Nosarti et al., 2002), and general psychological distress (Mehnert et al., 2018; Sutton et al., 2022). Achieving psychological adjustment is the idea that, despite initial psychological distress that comes with a diagnosis of cancer, well-adjusted patients will eventually be able to manage their health and well-being free from significant psychological symptoms (Stanton et al., 2007). Many factors can contribute to an individual’s psychological adjustment after a cancer diagnosis, including socioeconomic factors, levels of social and interpersonal support, as well as coping approaches (Hoyt & Stanton, 2018). Of these factors leading to psychological adjustment, providers can begin to promote positive coping through effective communication within medical appointments (Broadbridge et al., 2023; Dean & Street, 2014).
Provider empathic communication and psychological adjustment
A key component of effective patient–provider communication is empathic language (Mead & Bower, 2000). In the context of cancer care, provider displays of empathy have been associated with distal psychological health outcomes up to a year after diagnosis (Brandão et al., 2017; Butow et al., 1996) and noted as an unaddressed need by early-stage breast cancer patients (Anderson et al., 2020). Patients with breast cancer who perceive their provider’s communication as empathic at the time of diagnosis are less likely to experience the psychological comorbidities that can accompany a cancer diagnosis, underscoring the importance of this aspect of provider communication (Butow et al., 1996). Although empathic communication is expected to benefit patient well-being throughout the cancer trajectory, it is unclear how provider empathy is experienced by patients in the post-treatment phase (“former patients”). Further, the consequences of empathic provider communication and patient psychological adjustment on current and former breast cancer patients’ communication are not well understood. Thus, the following research question was posed:
RQ1: Do patient perceptions of their provider’s empathic communication influence breast cancer patient’s psychological adjustment differently for current versus former patients?
Predicting patient disclosure decisions
Patients’ decisions regarding what they do or do not disclose to their oncologist (i.e., omitting sensitive psychosocial or physical topics from conversation) may limit the quality of cancer health care received. In healthcare contexts outside of oncology, patients have reported holding back from telling their providers about personal history (Friley & Venetis, 2022; Lewis et al., 2011) and hesitate to disclose mental health concerns (Bell et al., 2011). Moreover, a study of current and former cancer patients (breast, digestive, urologic, and others) found that the most commonly endorsed barrier to sharing concerns with their provider was perceptions of a lack of empathy in previous responses and the provider not explicitly asking about concerns (Brandes et al., 2015). However, it is not clear the extent to which these patients held back concerns, only that if they did, it was most likely for the above reasons. Additionally, less is known about whether the same provider behaviors, when effective, could be associated with patients sharing their concerns (versus holding back). Next, we will discuss a model of disclosure that can shed light on this question.
The disclosure decision-making model (DD-MM, Greene, 2009) theorizes about how individuals decide to share or withhold private health information with others through three main constructs: (1) information assessment, the evaluation about features of the information to be shared such as stigma, (2) receiver assessment, the evaluation of features of information recipients such as being supportive, and (3) disclosure efficacy, one’s confidence in sharing the information to achieve the desired goal. Greene (2009) describes that some, but not all, constructs may be relevant in various contexts. Because the contexts of patient–provider communication and patients’ psychological adjustment have not been explored in relation to disclosure efficacy, this study represents an initial investigation into how the DD-MM could be useful in understanding these processes. As such, the current study focuses on receiver assessment and disclosure efficacy as two important constructs expected to be related to provider empathic communication and patients’ psychological adjustment. This investigation conceptualizes receiver assessment as perceived provider empathic communication. According to the DD-MM, perceptions of the patient–provider relationship (receiver assessment) and features of the illness or specific health information (information assessment) both have direct effects on patients’ disclosure efficacy or confidence to share. The DD-MM theorizes that disclosure efficacy influences message enactment by logically predicting specific message features, with higher efficacy resulting in higher levels of disclosure to the provider and lower levels of withholding.
Previous applications of the DD-MM have focused on participant disclosure to close friends, family, or partners (Checton & Greene, 2012; Greene et al., 2012). However, comparatively less work has explored the constructs of the DD-MM within the context of a patient–provider relationship (see Lee & Greene, 2022 for review). One study found that patients with gynecologic cancer hold back particularly sensitive information from their providers, such as fears about their prognosis (Checton et al., 2019). Given that the patient–provider relationship inherently has a power dynamic that is different from relationships with close friends and romantic partners, it is important to clarify how receiver assessment operates specifically in patient–provider interactions. Understanding how receiver assessment (e.g., perceptions of provider empathic communication) influences patients’ disclosure efficacy within oncology care presents an avenue for clinician training interventions as well as providing an extension of the DD-MM model.
Provider empathic communication
Receiver assessment in the DD-MM refers to how individuals evaluate a potential information recipient as someone to whom they could potentially disclose private information. Given the importance of provider displays of empathic communication for cancer patients’ psychological adjustment, this study conceptualized perceived provider empathic communication as a component of receiver assessment, where higher perceptions of provider empathy would be expected to align with perceptions of better relational quality and more positive anticipated responses from the provider. With the relationship between displays of empathic communication and psychological adjustment to cancer described previously, the following hypothesis was proposed for how provider empathy influences psychological adjustment:
H1: Patients who perceive their oncologist as having more empathic communication will report higher levels of psychological adjustment to their cancer diagnosis.
Disclosure efficacy
The DD-MM posits (and research suggests) that a more positive assessment of a provider is associated with higher levels of disclosure efficacy (Greene et al., 2012; Lee & Greene, 2022). For example, better receiver assessments are associated with higher disclosure efficacy when deciding whether to disclose a non-visible illness (Choi et al., 2016). Given the relationship between receiver assessment supported by prior research and the extension of receiver assessment to include providers’ empathic communication discussed above, the following hypothesis is proposed for how perceived provider empathy influences disclosure efficacy:
H2: Patients who perceive their oncologist as having more empathic communication will report higher levels of disclosure efficacy.
Previously we argued that patient psychological adjustment was integral to cancer patients’ experiences both within and following their cancer visits. Although the relationship between disclosure efficacy and psychological adjustment has not previously been tested, we posit that better psychological adjustment will be associated with greater disclosure efficacy. Because psychological adjustment is associated with better overall mental well-being, patients who have better adjustment may feel more empowered to discuss sensitive topics with their provider, independent of their receiver assessment. Thus, the following hypothesis is proposed for how psychological adjustment to the diagnosis relates to disclosure efficacy:
H3: Patients who are better adjusted to their cancer diagnosis will report higher disclosure efficacy.
Disclosure decisions
The DD-MM ultimately posits that disclosure efficacy predicts communication outcomes, including what relevant health information is shared or withheld. Importantly, disclosure efficacy is predicted to be immediately antecedent to message enactment (sharing or withholding). Increased disclosure efficacy has been associated with higher likelihood of disclosing in multiple health contexts (see Lee & Greene, 2022) including, for example, disclosing a nonvisible illness (Choi et al., 2016) and couples’ decisions to disclose infertility (Steuber & Solomon, 2011). Given this relationship between disclosure efficacy and disclosure, the following hypothesis is proposed for how disclosure efficacy influences disclosure decisions:
H4: Patients who report higher disclosure efficacy will report (a) disclosing more and (b) withholding less information from their oncologist.
Method
Sampling and procedures
This study utilized data from a larger cross-sectional online survey of patients with breast cancer and survivors collected between June 2020 and December 2022 that was aimed at characterizing experiences of cancer patients and their communication with the oncology care team. Current breast cancer patients (those who were less than 2 years post-diagnosis and currently undergoing treatment) and former patients (those who were 2 to 5 years post-diagnosis and had completed surgery, chemotherapy, and/or radiation therapy) were recruited through the Love Research Army®. The Love Research Army® is a research registry hosted by the Dr. Susan Love Foundation for Breast Cancer Research, a national advocacy organization for breast cancer patients, survivors, and at-risk family members. Potential participants were emailed a URL directly by the Love Research Army to access an approximately 30-minute online survey. To be eligible to participate, individuals were required to be 18+ years of age, able to read English, have access to a computer or mobile device with Internet access to connect to the survey, able to provide informed consent, and regularly brought a support person with them to their oncology visits (part of a broader study aim). Upon survey completion, participants had the opportunity to enter a drawing for one of the six $50 gift cards. This study was approved by a university Institutional Review Board.
Measures
For each of the following measures, a series of initial analyses were conducted to ensure scale reliability and confirm dimensionality. CFA was performed for each scale using combined data from both current and former patients. Perceptions of provider empathic communication and disclosure efficacy ask participants to rate their general perceptions of each construct. The measure of psychological adjustment focuses specifically on the past month. Overall model fit for CFA was assessed using a combination of metrics including comparative fit (confirmatory fit index, CFI), absolute goodness of fit (root mean square error of approximation, RMSEA; standardized root mean square residual, SRMR), and χ2. The cutoffs for good fit were CFI > .95, RMSEA < .06, and SRMR < .08 (Hu & Bentler, 1999). Adequate fit was considered at CFI > .90, RMSEA < .08, and SRMR < .10. Means and standard deviations are reported in Table 1. Data were analyzed using STATA (version 17.0). Additional detail for each measure is available in the Appendix.
Table 1.
Descriptive statistics for disclosure process variables (N = 285).
| Current patients (n = 111) |
Former patients (n = 174) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Potential range | α | χ2(df)+ | RMSEA | CFI | SRMR | M | SD | M | SD |
|
| ||||||||||
| Perceived empathic communication* | 0–5 | .97 | 14.43(7) | .06 | .997 | .008 | 3.66 | 1.31 | 4.10 | 1.02 |
| Psychological adjustment* | 1–5 | .81 | 5.75(3) | .06 | .995 | .016 | 3.56 | .89 | 3.92 | .72 |
| Disclosure efficacy* | 1–5 | .89 | 5.38(4) | .04 | .998 | .014 | 3.35 | .58 | 3.46 | .64 |
| Disclosure decisions Sharing* | 1–5 | .75 | 19.90(10) | .06 | .853 | .070 | 3.35 | .58 | 3.53 | .67 |
| Withholding* | 0–4 | .89 | 14.56(5) | .08 | .992 | .023 | 1.59 | 1.12 | 1.27 | .97 |
α = Cronbach’s alpha, composite scales for combined current and former patients; M = mean; SD = standard deviation; RMSEA = root mean square error of approximation; CFI = confirmatory fit index.
Current patients’ mean scores significantly differ from former patients’ scores by one-tailed t-test at p < .05 across all measures.
χ2 values significant for perceived empathic communication (p = .04), sharing (p = .03), and withholding (p = .01).
Perception of empathic communication
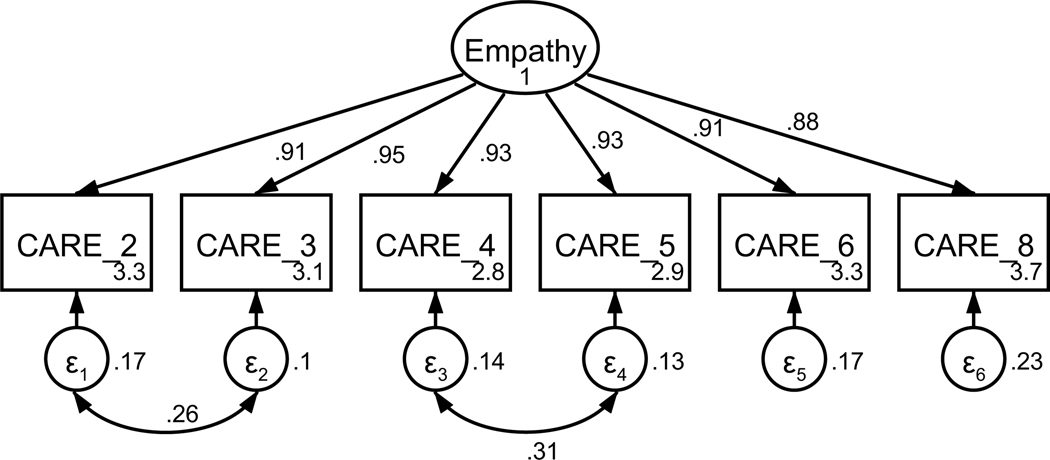
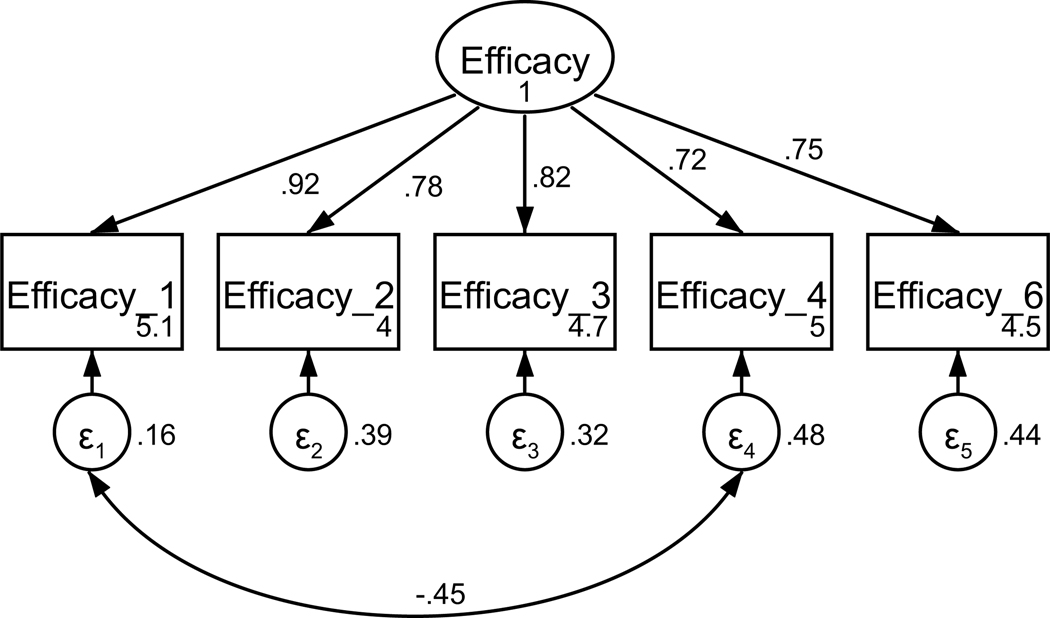
Participants’ perceptions of their oncologist’s empathic communication were captured using the Consultation and Relational Empathy (CARE) questionnaire (Mercer et al., 2004). The CARE questionnaire was adapted to reflect a cancer setting, replacing “doctor” with “oncologist” (see Table A1). The 10-item measure asks participants to rate statements related to their oncologist’s empathic communication on a 6-point scale (really poor to excellent) based on the prompt, “How was the oncologist at …”. Based on factor loadings, model fit, and theoretical relatedness, four items were not retained resulting in a final scale with six items (see Table A1 and Figures A1 and A2). The final unidimensional factor structure supported good model fit (χ2(7) = 14.43, p = .044; RMSEA = .061 (CI .010, .106); CFI = .997; SRMR = .008). Retained items were averaged for a final composite score that could range from 0 to 5 (see Table 1). Higher scores indicate perception of more empathic provider communication. The scale achieved high reliability (α = .97).
Patient adjustment to cancer diagnosis
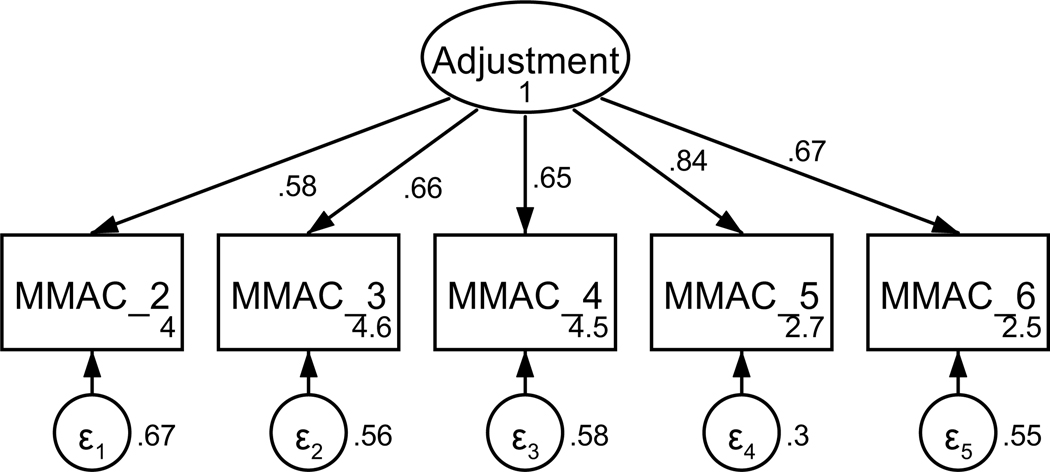
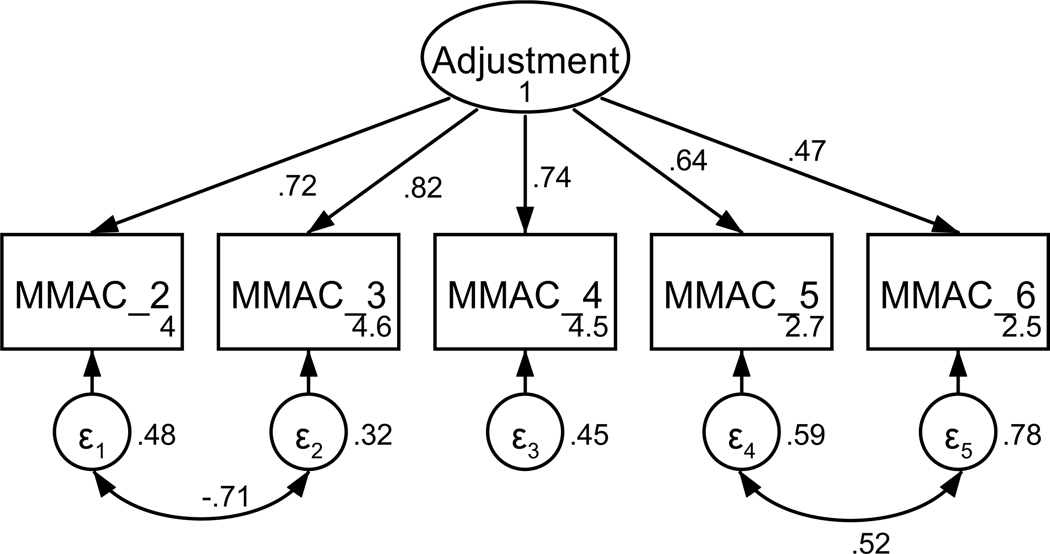
Patients’ psychological adjustment to their cancer diagnosis was measured using a modified form of the Mini-Mental Adjustment to Cancer scale (Watson et al., 1994). Items from four of the five subscales (two items each, fighting spirit, helplessness-hopelessness, anxious preoccupation, and cognitive avoidance) were included based on factor loadings in a previous study in a cancer population and face validity as assessed by the research team (Venetis, 2010). The measure was shortened to reduce participant fatigue. All items were on a 5-point scale from does not apply to me to very strongly applies to me. Based on factor loadings, model fit, and theoretical relatedness, three items were not retained, resulting in a final scale with five items (see Table A2 and Figures A3 and A4). The final unidimensional factor structure supported good model fit (χ2(3) = 5.75, p = .125; RMSEA = .057 (CI < .001, .127); CFI = .995; SRMR = .016). Retained items were averaged for a final composite score that could range from 1 to 5 (see Table 1). Higher scores indicate better psychological adjustment. The scale achieved acceptable reliability (α = .81).
Disclosure efficacy
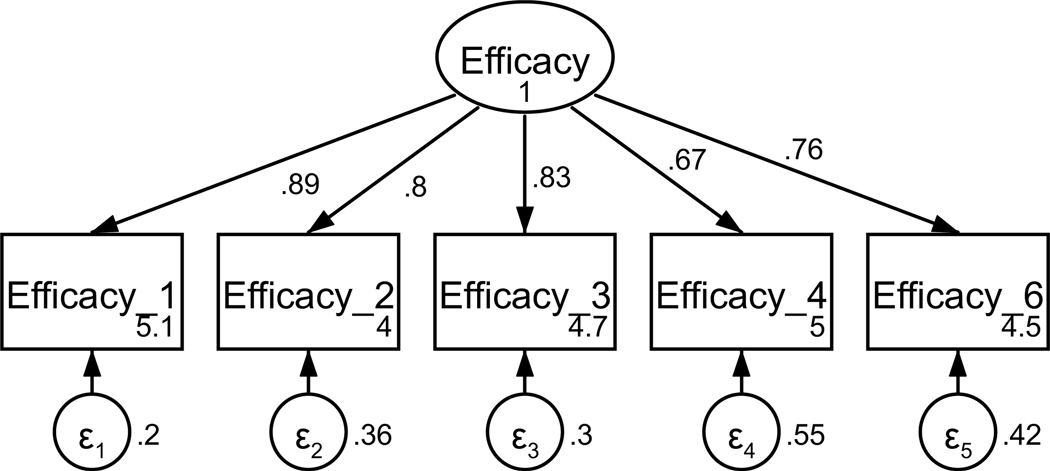
Disclosure efficacy was measured using a modified version of a scale previously used for capturing disclosure efficacy in a cardiac patient population (Checton & Greene, 2012, 2014). Participants were asked to rate the degree to which they agreed with six statements about their efficacy to disclose to their medical team on a 5-point scale (strongly disagree to strongly agree). Based on factor loadings, model fit, and theoretical relatedness, one item was not retained resulting in a final scale with five items (see Table A3 and Figures A5 and A6). The final unidimensional factor structure supported good model fit (χ2(4) = 5.38, p = .250; RMSEA = .035 (CI < .001, .102); CFI = .998; SRMR = .014). Retained items were averaged for a final composite score that could range from 1 to 5 (see Table 1). Higher scores indicate better disclosure efficacy. The scale achieved good reliability (α = .89).
Message enactment
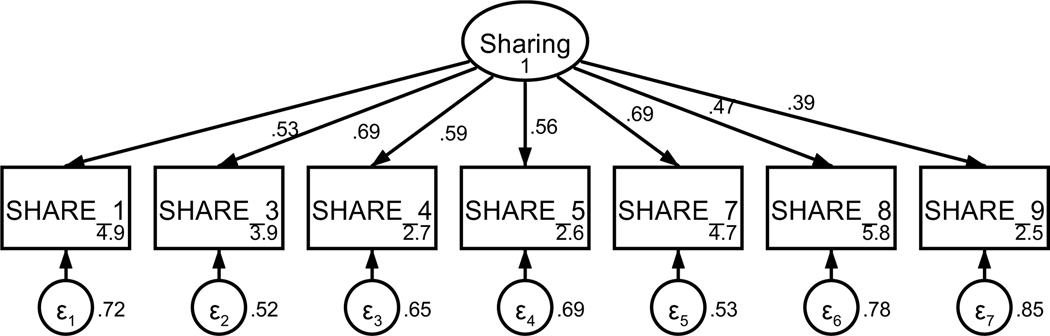
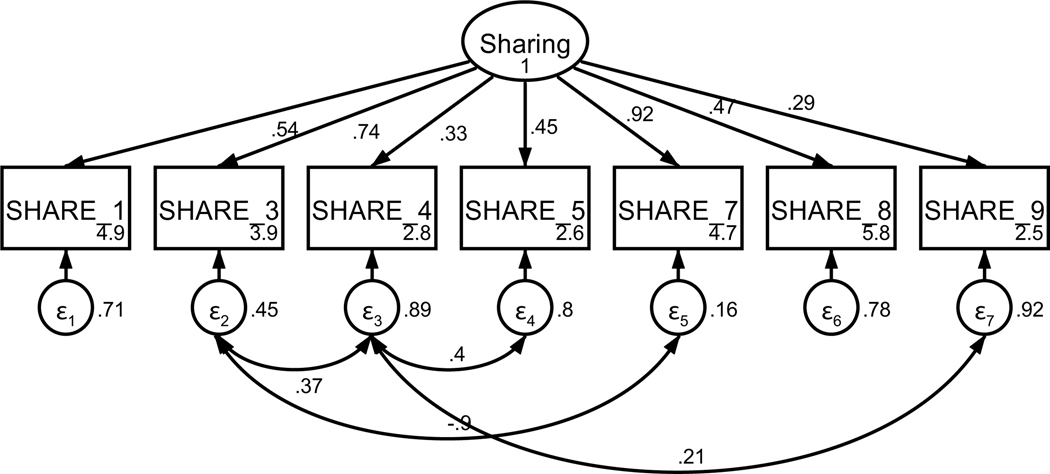
Patient sharing information with the oncologist was measured using an adapted nine-item scale of cancer communication (Kornblith et al., 2006) that assess varying aspects of patient sharing. Based on factor loadings, model fit, and theoretical relatedness, two items were not retained (see Table A4 and Figures A7 and A8) resulting in a final scale with seven items on a 5-point Likert-type scale (strongly disagree to strongly agree). The final unidimensional factor structure supported good model fit (χ2(10) = 19.90, p = .03; RMSEA = .059 (CI .018, .097); CFI = .979; SRMR = .039). Retained items were averaged for a final composite score that could range from 1 to 5 (see Table 1). Higher scores indicate more sharing with the oncologist. The scale achieved acceptable reliability (α = .75).
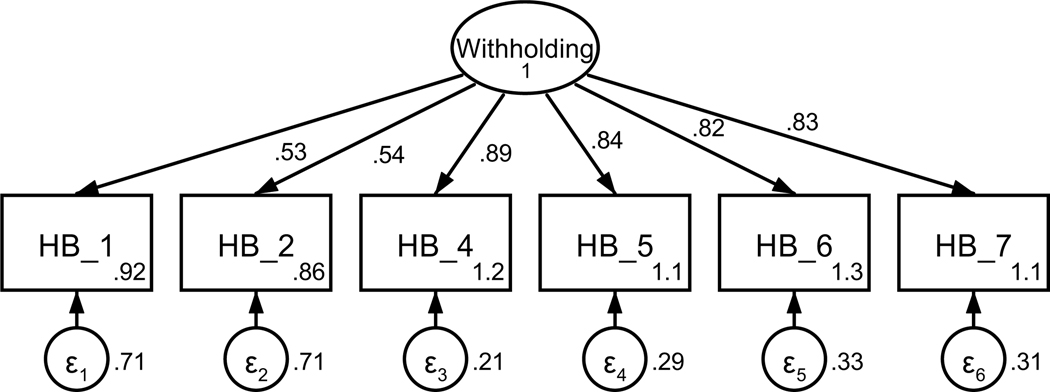
Patient withholding information from the oncologist was captured using 13 items from the Holding Back Scale (Manne et al., 2010; Pistrang & Barker, 1995). Participants were asked to respond to how much they hold back from or actively avoid sharing aspects of their health with their oncologist. Based on theoretical relevance, 11 of the 13 items were included in the CFA (see Table A5). Using a combination of factor loadings and model fit, five additional items were not retained (see Figures A9 and A10) resulting in a final scale with six items on a 5-point scale (never to almost always). The final unidimensional factor structure supported adequate model fit (χ2 (5) = 14.56, p = .012; RMSEA = .082 (CI .035, .133); CFI = .992; SRMR = .023). Retained items were averaged for a final composite score that could range from 0 to 4 (see Table 1). Higher scores indicate more information withheld from the oncologist. The scale achieved good reliability (α = .89).
Analyses
Data were initially cleaned and screened at the univariate level. Mean scale replacement was used for individual items if missing two or fewer items per scale (n = 29 instances). Preliminary analyses included bivariate correlations with Bonferroni-adjusted significance levels (one-tailed) across model variables (see Table 2). Differences between current and former patients were compared on all study variables (demographic data and composite scores) using χ2 and one-tailed t-tests (reported in the Results).
Table 2.
Correlations between variables included in the disclosure enactment model (N = 285).
|
Current patients (n = 111)
|
Former patients (n = 174)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | (1) | (2) | (3) | (4) | (5) | (1) | (2) | (3) | (4) | (5) |
|
| ||||||||||
| (1) Perceived provider empathic communication | 1 | 1 | ||||||||
| (2) Psychological adjustment to diagnosis | .27* | 1 | .29** | 1 | ||||||
| (3) Disclosure efficacy | .26 | .52*** | 1 | .64*** | .43*** | 1 | ||||
| (4) Sharing | .57*** | .39*** | .43*** | 1 | .62*** | .24* | .64*** | 1 | ||
| (5) Withholding | −.24 | −.38*** | −.36*** | −.23 | 1 | −.33*** | −.38*** | −.42*** | −.35*** | 1 |
p < .05
p < .01
p < .001.
Structural equation modeling (SEM) was conducted to assess the overall fit of the proposed model. Four models were tested, one for each disclosure outcome (sharing and withholding from the oncologist, H4a and H4b), repeated separately for current and former patients. Invariance between current and former patients was assessed using Wald tests for each structural path in the sharing and withholding models to explore RQ1. Differences in the stage of cancer experience that were considered as potential influences on the model included former patients having had more time to adjust to the diagnosis, differences in what types of appointments current and former patients participate in (i.e., treatment decision-making vs. watchful waiting), and the immediacy of appointment recall for current patients actively pursuing treatment vs. former patients who may not be interacting with their oncologist as frequently. Overall model fits for SEM were assessed using the same criteria as CFAs. Regarding the study sample as a small N (defined as <200), χ2 was considered adequate if χ2/df < 3 (Bentler & Yuan, 2010; Herzog & Boomsma, 2009).
Results
Sample description
In total, 332 responses were collected. Six participants did not report their cancer type and were not included in analyses. Participants who reported non-breast cancer diagnoses or no diagnosis (n = 12) and male participants (n = 3) were excluded.1 Participants with missing scales were excluded (n = 32), yielding a total of 285 participants for analyses (current patients, n = 111; former patients, n = 174). Average age of respondents was 57 years (range 30–83). Seventy-eight percent of participants reported attaining a bachelor’s degree or higher. Most participants identified as white (92%) and as married/living as married (80%). Results indicated no significant differences between current and former patients on demographic variables of age, education, race, ethnicity, or marital status (see Table 3). The distribution of the stage at diagnosis differed between current and former patients though not skewed in a particular direction (see Table 3).
Table 3.
Demographic information and descriptive statistics (N = 285).
| Total | Current patients (n = 111) | Former patients (n = 174) | p-value | |
|---|---|---|---|---|
|
| ||||
| Age – mean (SD) | 57.0 (11.9) | 56.9 (12.3) | 57.0 (11.7) | .93a |
| Stage at diagnosis – frequency | <.01b | |||
| Stage 0 | 27 | 12 | 15 | |
| Stage I | 113 | 52 | 61 | |
| Stage II | 79 | 25 | 54 | |
| Stage III | 36 | 7 | 29 | |
| Stage IV | 14 | 11 | 3 | |
| Unknown/unsure | 9 | 3 | 6 | |
| Education – frequency | .93b | |||
| Less than high school diploma | 0 | 0 | 0 | |
| High school graduate | 2 | 1 | 1 | |
| Vocational, technical, business, or trade school certificate or diploma | 14 | 5 | 9 | |
| Some college | 46 | 19 | 27 | |
| Bachelor’s degree | 95 | 39 | 56 | |
| Master’s, professional, or doctoral degree | 125 | 45 | 80 | |
| Race – frequency | .43b | |||
| White/Caucasian | 235 | 96 | 139 | |
| Black/African American | 10 | 3 | 7 | |
| American Indian/Alaska Native | 1 | 0 | 1 | |
| Asian | 4 | 0 | 4 | |
| Multiracial | 7 | 3 | 4 | |
| Ethnicity – frequency | .64b | |||
| Hispanic/Latino | 33 | 14 | 19 | |
| Marital status | .61b | |||
| Single | 20 | 8 | 12 | |
| Married/living as | 207 | 86 | 121 | |
| Divorced | 17 | 5 | 12 | |
| Widowed | 8 | 2 | 6 | |
| Separated | 2 | 1 | 1 | |
| Dating | 3 | 0 | 3 | |
Indicates one-tailed t-tests. Inicates χ2 tests.
Descriptive statistics
Across model variables, former patients consistently reported more favorable ratings than current cancer patients. Former patients perceived more empathic communication from their oncologist, had better psychological adjustment, reported higher disclosure efficacy, shared more, and withheld less from their oncologist (see Table 2). These differences supported the notion that former patients experienced these model factors more positively than current patients. SEM models were tested separately to test for replicability among these two groups of patients at different points in their cancer experience.
Structural equation models of sharing with oncologist
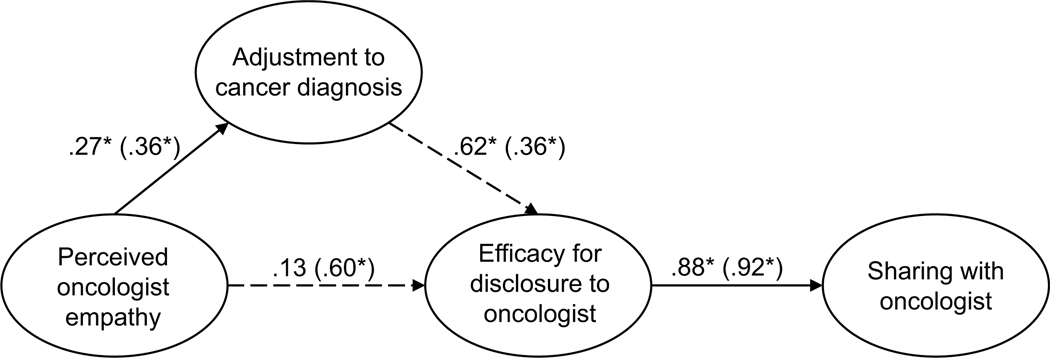
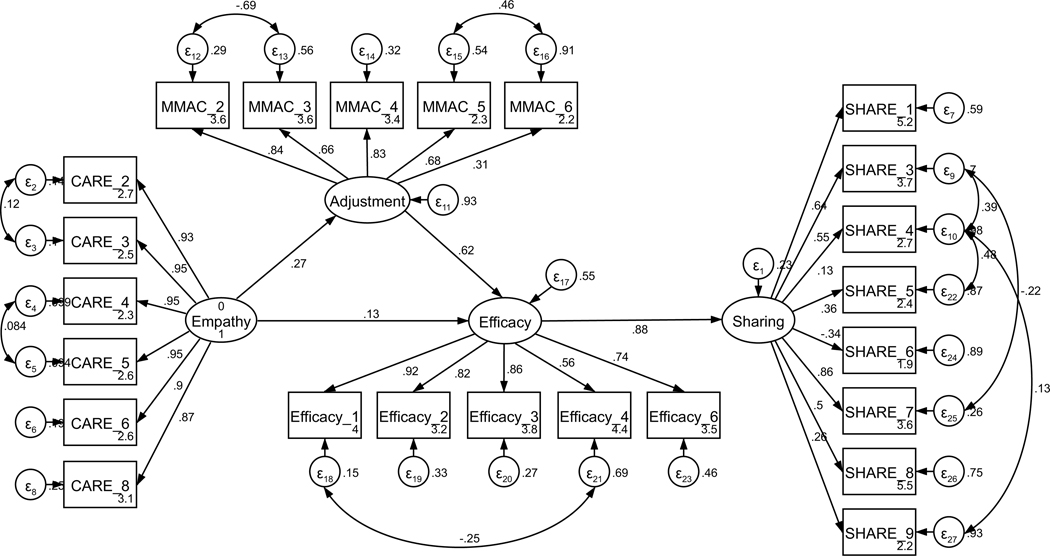
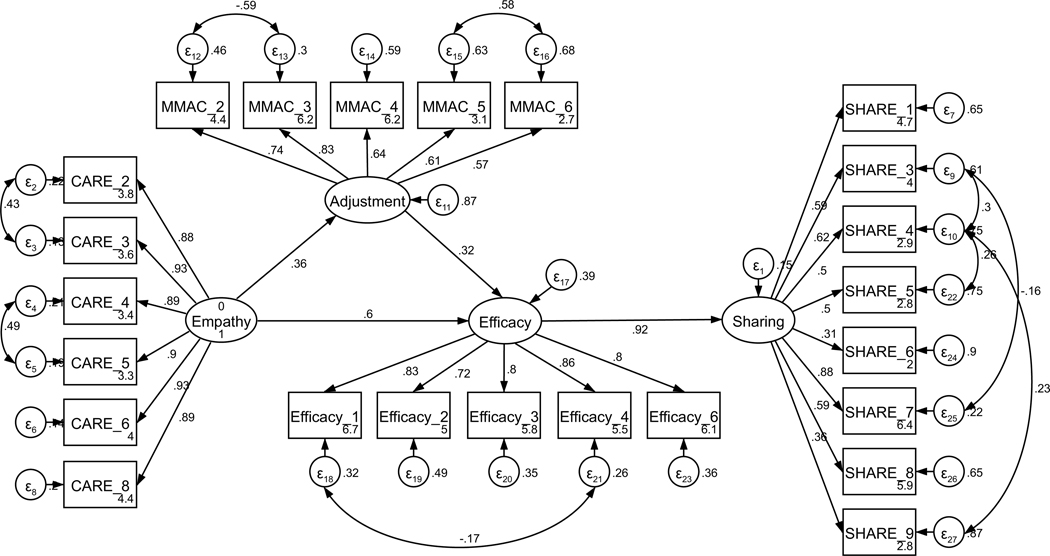
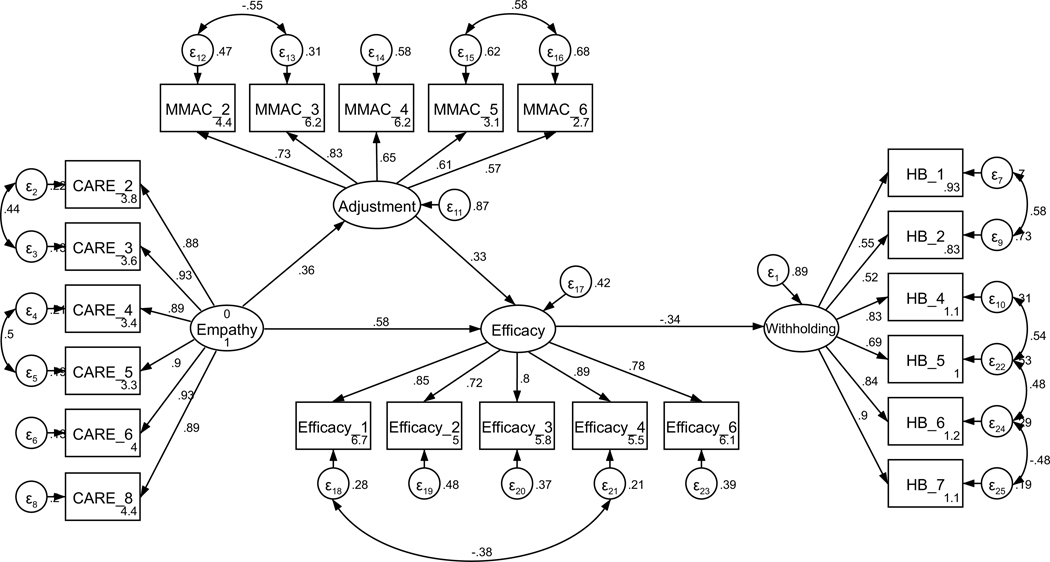
Parallel SEM analyses were conducted to test the theoretical model of sharing, repeated separately for current and former patients (see Figure 1). The model was assembled using the measurement structures established through CFA (see Figures A11 and A12). No theoretically meaningful modification indices were indicated; thus, the model was not further adjusted. All hypothesized paths were retained in both current and former patient models. The model of sharing for current patients was supported only to a limited degree by the data (χ2 (239) = 485.18, p < .001; RMSEA = .097 (CI .084, .109); CFI = .878; SRMR = .127). The direct path from perceived empathic communication to disclosure efficacy was not significant (H2, p = .121); exploratory removal of this path did not contribute to a better model fit. All other hypothesized structural paths were supported (see Figure 2 for the final models). In contrast to current patients, the model of sharing for former patients was adequately supported by the data (χ2 (239) = 444.15, p < .001; RMSEA = .070 (CI .060, .081); CFI = .932; SRMR = .069), and all hypothesized structural paths were supported.
Figure 2.
Parameter estimates are standardized. Model fit indices for current cancer patients (paths outside of parentheses) were c2(239) = 485.18, p < .001; RMSEA = .097 (CI .084, .109); CFI = .878; SRMR = .127. Model fit indices for former cancer patients (paths inside of parentheses) were χ2(239) = 444.15, p < .001; RMSEA = .070 (CI .060, .081); CFI = .932; SRMR = .069. Wald test p-values (difference between model paths between current and former patients) are reflected as solid or dashed paths. Solid lines represent paths that are not significantly different between current and former patients by Wald test (p > .05). Dashed lines represent significantly different paths between current and former patients (p < .05). Note: *p < .01 for path weights.
Unconstrained testing for invariance between current and former patients revealed two significantly different paths between the two structural models (RQ1). These differences included the paths between (1) perceived oncologist empathy and disclosure efficacy (p < .001) and (2) adjustment to the diagnosis and disclosure efficacy (p = .033). For current patients, the strength of the relationship from perceived oncologist empathy to efficacy for disclosure was weaker than for former patients. By contrast, the strength of the relationship between adjustment to the diagnosis and disclosure efficacy was stronger for current patients than for former patients. The final structural models are presented in Figure 2.
Structural equation models of withholding from oncologist
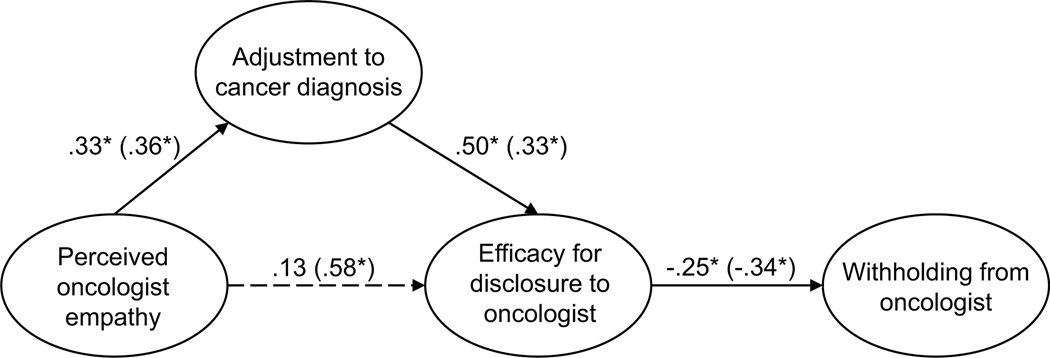
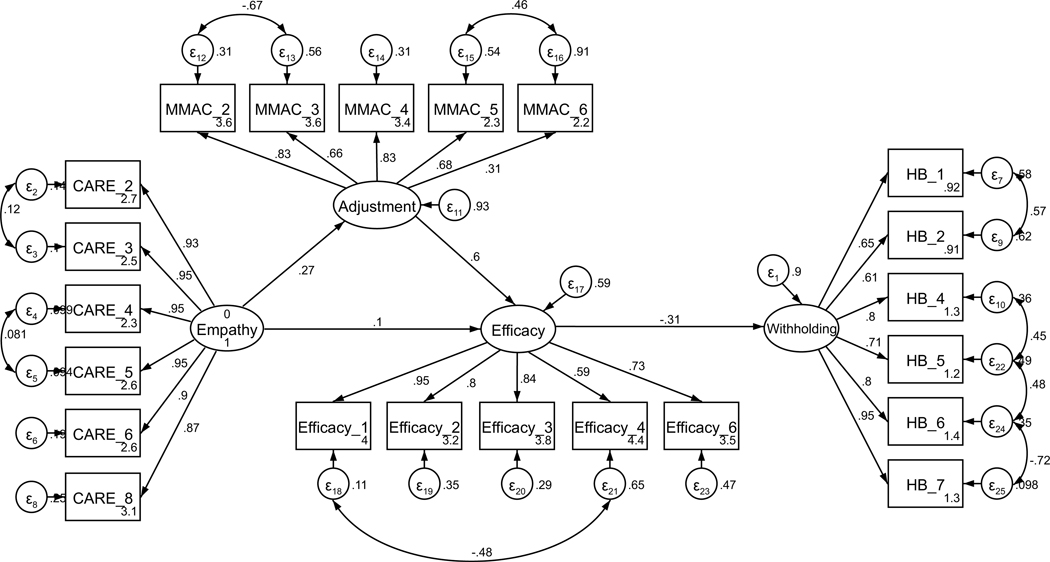
Parallel SEM analyses were conducted to test the theoretical model of withholding, repeated separately for current and former patients. As above, the model was assembled using the measurement structures established through CFA (see Figures A13 and A14). No theoretically meaningful modification indices were indicated; thus, the model was not further adjusted. Additionally, all hypothesized paths were retained in both current and former patient models. The model of withholding for current patients was adequately supported by the data (χ2(196) = 329.90, p < .001; RMSEA = .079 (CI .064, .093); CFI = .936; SRMR = .107). As with the sharing model, the direct path from perceived empathic communication to disclosure efficacy was not significant (H2, p = .207). Exploratory removal of this path did not contribute to a better model fit. All other hypothesized structural paths of the model were supported (see Figure 3 for the final models). Likewise, the model of withholding for former patients was supported by the data (χ2(196) = 335.63, p < .001; RMSEA = .064 (CI .052, .076); CFI = .955; SRMR = .087), and all hypothesized structural paths of the model were supported.
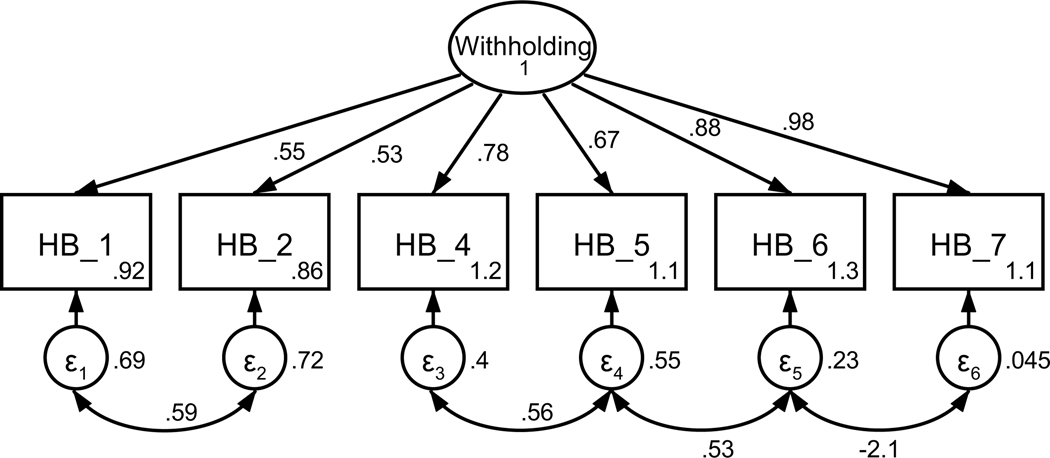
Figure 3.
Parameter estimates are standardized. Model fit indices for current cancer patients (paths outside of parentheses) were χ2(196) = 329.90, p < .001; RMSEA = .079 (CI .064, .093); CFI = .936; SRMR = .107. Model fit indices for former cancer patients (paths inside of parentheses) were χ2(196) = 335.63, p < .001; RMSEA = .064 (CI .052, .076); CFI = .955; SRMR = .087. Wald test p-values (difference between model paths between current and former patients) are reflected as solid or dashed paths. Solid lines represent paths that are not significantly different between current and former patients by Wald test (p > .05). Dashed lines represent significantly different paths between current and former patients (p < .05). Note: *p < .01 for path weights.
Unconstrained testing for invariance between current and former patients revealed one significantly different path between structural models (RQ1). This difference was between the path from perceived oncologist empathy and disclosure efficacy (p < .001). Specifically, for current patients, the strength of the relationship from perceived oncologist empathy to efficacy for disclosure was weaker than for former patients. The final models are presented in Figure 3.
Discussion
This study explored how current and former breast cancer patients’ perceptions of their oncologists’ empathic communication is associated with their psychological adjustment to the cancer diagnosis and their disclosure efficacy and how these variables are associated with sharing and withholding information from oncologists. The hypothesized relationships between model variables were supported with remarkable similarity between current and former patients (see Figures 2 and 3). We found that, for both current and former cancer patients, oncology provider empathic communication was positively associated with better psychological adjustment to cancer (H1) and disclosure efficacy (H2). Better psychological adjustment was also associated with higher disclosure efficacy (H3), and higher disclosure efficacy was associated with more sharing (H4a) and less withholding (H4b) to oncologists. We also found that, although model fit overall were supported (at least in part) by both current and former patient responses, the strength of particular relationships differed between patient groups (RQ1). The relationship between perceived oncologist empathy and disclosure efficacy (H2) was more positive for former patients in both models. In the models of withholding, the relationship between psychological adjustment and disclosure efficacy (H3) was more positive for former patients, though the relationship between efficacy and withholding was not significantly different.
A potential explanation for the one difference in path coefficient significance (the non-significant relationship between empathy and disclosure efficacy for current patients across all models) and the different relationship strengths between current and former cancer patient models is that the immediacy of dealing with uncertainty and treatment decisions early in diagnosis may change the way that empathy drives the processes represented in the model tested. Previous research has demonstrated that both current and former patients endorse previous provider displays of empathy as a reason to hold back from sharing concerns, though differences across the cancer trajectory were not reported (Brandes et al., 2015). Additionally, knowing the salience of provider communication at the time of diagnosis and the influence on downstream psychological adjustment (Butow et al., 1996), it is possible that the effects of oncologist empathy that were revealed for current patients (e.g., those closer to the time of diagnosis) more strongly influenced the model than was the case in the model for former patients. This is an area where additional research utilizing designs that incorporate disease stage, time since diagnosis, and/or tracking longitudinally can further our understanding of these key processes that influence important patient outcomes.
Theoretical implications
Overall, the results of this study align with previous research on sharing health information and topic avoidance as well as literature on empathic communication. Results support that higher disclosure efficacy for patients throughout the cancer experience indeed results in more sharing and less withholding of health information with their oncologist, consistent with the expected message enactment outcomes of the DD-MM (Greene, 2009; Lee & Greene, 2022). This finding underscores the critical importance of bolstering patient disclosure efficacy through both clinician and patient training. Additionally, the strong relationship between perceived oncologist empathy and psychological adjustment supports the notion that empathic communication is critical for both proximal goals as well as more distal psychological outcomes (Roberts et al., 1994), and this relationship persists even beyond initial diagnosis and treatment phases. This finding is consistent with prior conceptualizations of patient-centered communication and the influence of provider communication on health outcomes (Street et al., 2009; Zwingmann et al., 2017). In this way, patients’ perceptions of provider communication matter for patient psychological health and continued focus on provider training in empathic communication is needed to meet the needs of these populations.
From a theoretical perspective, this study adds to understanding and application of the DD-MM (Greene, 2009) and its constructs in several key ways. First, the reconceptualization of receiver assessment as empathic communication in the patient–provider relationship setting expands our understanding of some of the components of patients’ appraisals of their providers when they decide whether to share or withhold health information in this context. Perceived empathic communication in this study replicated the relationship in previous research between receiver assessment and disclosure efficacy (Choi et al., 2016; Steuber & Solomon, 2011), with positive assessments of oncologist empathy associated with more disclosure efficacy. Additionally, this conceptualization of receiver assessment provides a unique integration of research on patient-centered communication and the DD-MM. This integration both expands the scope of the DD-MM and provides models for two potential patient communication outcomes that can be further refined and tested in other patient-centered communication contexts. Finally, the inclusion of psychological adjustment as a mediator between perceived empathic communication and disclosure efficacy introduces a way to capture the psychological effects of patients’ disease experiences within the disclosure decision-making process, a variable not previously explored in connection to this model and other information management literature. More broadly, prior research on information management has often been limited to exploring information sharing/disclosure and information withholding/topic avoidance without investigating the dialectical tensions between these message enactment variables and their predictors. This study contributes to filling this gap in how patients manage these complex information management decisions in the healthcare interaction.
Clinical implications
The findings of this study present a model of patient–provider communication for breast cancer patients and oncologists and provide opportunities for development of communicative interventions for healthcare providers and potentially interventions for patients. First, these data underscore the importance of healthcare providers displaying empathic communication both at the time of diagnosis and throughout the cancer care experience. Both current and former patient data supported a direct effect from perceptions of oncologist empathic communication to psychological adjustment to cancer – a critically important process in the cancer journey. Perceived empathic communication in this study included provider behaviors such as helping the patient feel fully listened to, allowing the patient to tell their story, and displaying interest in the patient as a whole person. These empathic communication behaviors are aligned with the goals and processes of patient-centered communication, and training in empathic communication is an area of ongoing research and provider training (LaNoue & Roter, 2018; Pehrson et al., 2016). Thus, the results from this study provide additional justification for provider awareness of the importance of empathic communication and continued provider training emphasizing empathic communication skills.
In addition to evidence supporting continued focus on empathic communication training, this study supports the addition of training skills for bolstering patients’ disclosure efficacy – a skillset currently under or unaddressed in clinician training literature. Given the positive association of disclosure efficacy with sharing health information and negative association with withholding in our models, bolstering patients’ disclosure efficacy may have the potential to enhance patient–provider communication, particularly when patients are more hesitant to share (i.e., earlier in the cancer diagnosis, as was seen in this study). Promoting disclosure efficacy might include providers asking open-ended questions to facilitate an expectation of willingness to listen and valuing patients’ input. This empathic behavior could also include specific communication behaviors such as asking patients whether there is anything they are hesitant or unsure about asking. Motivational interviewing techniques developed as a brief intervention to increase disclosure among persons living with HIV have been successful in promoting disclosure efficacy (Greene et al., 2013) and could be an area of future research in the cancer context. Further, these results support the need for patient-focused training such as interventions to promote disclosure efficacy, bring awareness to patients’ own psychosocial needs, and/or develop patient strategies for sharing stressful or sensitive topics with their oncologist.
The findings of this study also support the continued assessment of patients’ psychological adjustment throughout the cancer care journey, even after the initial treatment phase is completed. For both current and former patients, better psychological adjustment was positively associated with more disclosure efficacy, more sharing, and less withholding. Based on our model, it is possible that bolstering psychological adjustment alone may lead to increased disclosure efficacy. Conversely, poor psychological adjustment correlates with lower efficacy. In other words, current and former cancer patients with poor psychological adjustment may feel less empowered to communicate well with their oncologist. This underscores the need for oncology providers to engage in continued psychological assessment (and appropriate referrals), even post-treatment.
Limitations
Although this study provides new insight into the ways in which provider empathic communication is associated with downstream communication behaviors, there are several limitations to this cross-sectional survey worth noting. Participants were a self-selected population motivated to participate in research and reflected a majority white, married, highly educated, and digitally literate breast cancer population, which may not represent how other populations of breast cancer patients, other cancer patients, or patients with other health conditions make decisions about what to share with or withhold from their providers. Additionally, because this survey was conducted online and recruitment was through an online database, this population had a degree of digital literacy that may not represent all patients with breast cancer. For example, a retrospective study of cancer patients’ electronic health record use and presence of an e-mail address on file found that patients who had no e-mail address on file had significantly worse overall survival (Heudel et al., 2022).
Future research should explore whether the results found in this study area consistent within other populations, particularly within marginalized communities and in cancer contexts that equally affect both men and women. Previous research has found that concordance between patients and providers across social demographics of gender, race, age, and educational attainment can result in better patient affect and satisfaction with care (Thornton et al., 2011), and this was not addressed in the present study. Just over 30% of oncologists in the United States are women and only 2% identify as African American (Towle, 2016). Although we did not collect data about the oncologists’ social demographics, profiles of the available oncology workforce indicate it is likely that many of the participants in this study experienced some social concordance across multiple demographic variables (i.e., race and educational attainment but perhaps not gender) and therefore may have a more positive view of their oncology and healthcare experience than individuals with minoritized identities.
Prospective and longitudinal studies measuring perceived empathic communication, psychological adjustment, disclosure efficacy, and message enactment early in the diagnosis as well as across the treatment trajectory are needed to better understand the differences in these processes over time as well as how recall affects these patients’ self-reports. Future research should investigate more nuanced differences across time (i.e., using cancer stage versus pre- and post-treatment phases). Interactional coding data from video recorded patient–provider interactions could be also paired with these measures to better understand the actual communication behaviors of the patient and oncologist – including but not limited to empathic behaviors – perhaps even to compare actual provider behaviors with patient perceptions. Future research should also investigate how the information assessment construct of the DD-MM functions with the new conceptualization of receiver assessment and inclusion of psychological adjustment in the model.
Conclusions
Results from this study reinforce previous research on the importance of providers’ empathic patient-centered communication for the psychological adjustment of patients with breast cancer and sharing or withholding information from the provider. Additionally, the final models for both current and former breast cancer patients support hypotheses of disclosure theorizing that have not previously been explored in this context. Specifically, the association of disclosure efficacy with communication enactment of sharing or withholding of health information from the oncologist adds to current literature on health information management and is an area for continued research to focus on improving patient outcomes.
Acknowledgements
The authors would like to thank the participants who spent their time and energy to provide these data. Recruitment facilitated by Dr. Susan Love Research Foundation’s Love Research Army®. We would like to acknowledge the Rutgers Cancer Institute of New Jersey’s (CINJ) Cancer Survivorship & Outcomes Center (CSOC).
Funding
This study was partially funded by Rutgers School of Communication and Information’s Small Grants for Individual Faculty Research (GFIR) and the Rutgers Cancer Institute of New Jersey (CINJ) and RWJBarnabas Health Mission Support, Cancer Survivorship and Outcomes Center (CSOC) Award.
Appendix
This appendix provides a detailed overview of how each variable measured in this study was treated. For each measure, the appendix includes (1) the wording of all items, (2) factor loadings, (3) item retention decisions, (4) means and standard deviations of each item, (5) initial confirmatory factor analysis results and goodness-of-fit parameters, (6) final factor structures, including the modification indices that supported any covaried error terms. The measured variables are:
A.1 Perception of Empathic Communication
A.2 Adjustment to the Cancer Diagnosis
A.3 Disclosure Efficacy
A.4 Message Enactment – Sharing
A.5 Message Enactment – Withholding
In addition, section A.6 provides the initial and final full structural equation models for current and former patients, including goodness-of-fit measures and relevant modification indices.
A.1. Perception of empathic communication
Table A1.
Means, standard deviations, and items of the Consultation and Relational Empathy (CARE) questionnaire (N = 285).
| Current patients (n = 111) |
Former patients (n = 174) |
||||||
|---|---|---|---|---|---|---|---|
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| Instructions: Please focus on your main oncologist or doctor responsible for treating your cancer. How was this person at… | |||||||
| CARE_1 | Making me feel at ease? | .913 | N | 3.75 | 1.36 | 4.23 | 1.05 |
| CARE_2 | Letting me tell me story? | .920 | Y | 3.68 | 1.36 | 4.11 | 1.07 |
| CARE_3 | Really listening to me? | .937 | Y | 3.65 | 1.45 | 4.12 | 1.14 |
| CARE_4 | Being interested in me as a whole person? | .920 | Y | 3.45 | 1.49 | 3.96 | 1.17 |
| CARE_5 | Fully understanding my concerns? | .924 | Y | 3.57 | 1.39 | 3.94 | 1.21 |
| CARE_6 | Showing care and compassion? | .907 | Y | 3.74 | 1.43 | 4.20 | 1.05 |
| CARE_7 | Remaining hopeful? | .786 | N | 4.07 | 1.13 | 4.38 | 0.90 |
| CARE_8 | Explaining things clearly? | .902 | Y | 3.87 | 1.26 | 4.28 | 0.98 |
| CARE_9 | Helping me to take control? | .913 | N | 3.50 | 1.54 | 3.98 | 1.11 |
| CARE_10 | Making a plan of action with me? | .807 | N | 3.61 | 1.48 | 3.91 | 1.28 |
| Mean composite score | 3.69 | 1.26 | 4.11 | .98 | |||
All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. Items that were more global statements about how the provider made them feel (CARE_1) or planning for the future (CARE_9 and CARE_10) were not retained, despite high factor loading, to focus on specific provider behaviors. Items with loadings < .80 (CARE_7) were not retained.
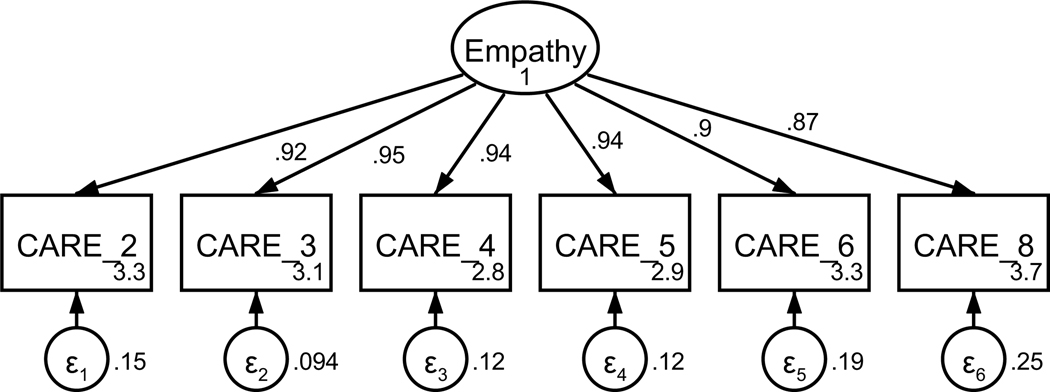
Figure A1. CFA of retained items in the Consultation and Relational Empathy (CARE) questionnaire without covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(9) = 47.55, p < .001; RMSEA = .123 (CI .090, .158); CFI = .983; SRMR = .013.
Figure A2. CFA of retained items in the Consultation and Relational Empathy (CARE) questionnaire with covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(7) = 14.43, p = .04; RMSEA = .061 (CI .010, .106); CFI = .997; SRMR = .008. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 26.48 Δχ2 improvement of fit for the covariation of items four and five (χ2(8) = 23.12, p = .003; RMSEA = .082 (CI .044, .121); CFI = .993; SRMR = .010). Modification indices next supported a 9.46 Δχ2 improvement of fit for the covariation of items two and three, resulting in the above model.
A.2. Adjustment to the cancer diagnosis
Table A2.
Means, standard deviations, and items of the Mini-Mental Adjustment to Cancer scale (N = 285).
|
Current patients (n = 111) |
Former patient (n = 174) |
||||||
|---|---|---|---|---|---|---|---|
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| Instructions: Please think about the following statements on a scale of 1–5 in terms of how you felt in the past month about having cancer. | |||||||
| MMAC_1 | I am determined to do everything I can to beat this disease. | .146 | N | 4.40 | 0.82 | 4.27 | 0.85 |
| MMAC_2 | I am very optimistic. | .615 | Y | 3.97 | 1.11 | 3.93 | 0.90 |
| MMAC_3 | I feel completely at a loss about what to do. (Reverse coded) | .684 | Y | 4.10 | 1.16 | 4.49 | 0.73 |
| MMAC_4 | I feel there is nothing I can do to help myself. (Reverse coded) | .726 | Y | 4.11 | 1.21 | 4.55 | 0.74 |
| MMAC_5 | I suffer great anxiety about having cancer. (Reverse coded) | .778 | Y | 3.01 | 1.31 | 3.66 | 1.20 |
| MMAC_6 | I am apprehensive about my cancer progressing. (Reverse coded) | .619 | Y | 2.60 | 1.18 | 2.99 | 1.10 |
| MMAC_7 | I make a positive effort not to think about my cancer. | .029 | N | 3.67 | 1.19 | 3.17 | 1.21 |
| MMAC_8 | I distract myself when thoughts about my cancer come into my head. | −.030 | N | 3.14 | 1.14 | 2.85 | 1.20 |
| Mean composite score | 3.63 | 0.69 | 3.74 | 0.55 | |||
All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. Items with loadings <.60 were removed from the scale one at a time, with the lowest loadings removed first. Loadings were reassessed after each item was removed.
Figure A3. CFA of retained items in the modified mini-mental adjustment to cancer scale without covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(5) = 98.76, p < .001; RMSEA = .257 (CI .214, .302); CFI = .820; SRMR = .072.
Figure A4. CFA of retained items in the modified mini-mental adjustment to cancer scale with covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(3) = 5.75, p = .13; RMSEA = .057 (CI < .001, .127); CFI = .995; SRMR = .016. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 60.37 Δχ2 improvement of fit for the covariation of items five and six (χ2(4) = 39.27, p < .001; RMSEA = .176 (CI .239, .228); CFI = .932; SRMR = .054). Modification indices next supported a 27.65 Δχ2 improvement of fit for the covariation of items two and three, resulting in the above model.
A.3. Disclosure efficacy
Table A3.
Means, standard deviations, and items of disclosure efficacy scale (N = 285).
|
Current patients (n = 111) |
Former patients (n = 174) |
||||||
|---|---|---|---|---|---|---|---|
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| Instructions: These questions ask about sharing information about your cancer with your medical team. | |||||||
| Efficacy_1 | I am confident that I can share information about my cancer with my medical team when I want to. | .851 | Y | 4.25 | 1.07 | 4.57 | 0.68 |
| Efficacy_2 | I have difficulty sharing information about my cancer with my medical team. (Reverse coded) | .778 | Y | 4.01 | 1.25 | 4.41 | 0.88 |
| Efficacy_3 | I know how to share information with my medical team about my cancer. | .805 | Y | 4.05 | 1.08 | 4.40 | 0.76 |
| Efficacy_4 | I do not know what to say when I try to share information with my medical team about my cancer. (Reverse coded) | .728 | Y | 4.20 | 0.95 | 4.39 | 0.80 |
| Efficacy_5 | I ordinarily feel very tense and nervous when having a conversation with my medical team about my cancer. (Reverse coded) | .491 | N | 3.77 | 1.27 | 4.06 | 1.08 |
| Efficacy_6 | While participating in a conversation with my medical team about my cancer, I am afraid to speak up. (Reverse coded) | .779 | Y | 4.11 | 1.20 | 4.48 | 0.74 |
| Mean composite score | 4.07 | 0.85 | 4.39 | 0.67 | |||
All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. Loadings were reassessed after each item was removed.
Figure A5. CFA of retained items in the disclosure efficacy scale without covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(5) = 24.14, p < .001; RMSEA = .116 (CI .072, .164); CFI = .977; SRMR = .027.
Figure A6. CFA of retained items in the disclosure efficacy with covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(4) = 5.38, p = .25; RMSEA = .035 (CI < .001, .102); CFI = .998; SRMR = .014. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 16.59 Δχ2 improvement of fit for the covariation of items one and four, resulting in the above model.
A.4. Message enactment – Sharing
Table A4.
Means, standard deviations, and items of the sharing scale (N = 285).
|
Current patients (n = 111) |
Former patients (n = 174) |
||||||
|---|---|---|---|---|---|---|---|
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| Instructions: People talk about some topics but not others with their medical team. Please indicate your level of agreement with the following statements. | |||||||
| SHARE_1 | We discuss what treatment I should have. | .517 | Y | 4.30 | 0.84 | 4.22 | 0.90 |
| SHARE_2 | I share with my friends more than my medical team about my cancer experience. (Reverse coded) | .396 | N | 3.28 | 1.22 | 3.78 | 1.18 |
| SHARE_3 | My medical team understands what it was like for me to be treated for cancer. | .675 | Y | 3.68 | 1.00 | 3.90 | 0.97 |
| SHARE_4 | My medical team and I talk about our worries about whether my cancer treatment worked. | .605 | Y | 3.07 | 1.19 | 3.39 | 1.19 |
| SHARE_5 | I talk with my medical team about what to do if my condition should get significantly worse. | .587 | Y | 2.92 | 1.24 | 3.36 | 1.22 |
| SHARE_6 | I talk with my medical team about how cancer affects me sexually. | .176 | N | 2.31 | 1.24 | 2.42 | 1.24 |
| SHARE_7 | I can talk about cancer with my medical team. | .696 | Y | 4.11 | 1.15 | 4.44 | 0.70 |
| SHARE_8 | When it comes to cancer, I only tell my medical team what they want to hear. (Reverse coded) | .450 | Y | 4.38 | 0.80 | 4.41 | 0.75 |
| SHARE_9 | I tell my medical team how scared I am about having cancer. | .409 | Y | 2.85 | 1.29 | 3.06 | 1.11 |
| Mean composite score | 3.43 | 0.55 | 3.66 | 0.63 | |||
All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. Loadings were reassessed after each item was removed.
Figure A7. CFA of retained items in the sharing scale without covaried error terms.
Parameter estimates are standardized. Model fit indices were χ22(14) = 111.03, p < .001; RMSEA = .156 (CI .130, .184); CFI = .790; SRMR = .080.
Figure A8. CFA of retained items in the sharing scale with covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(10) = 19.90, p = .03; RMSEA = .059 (CI .018, .097); CFI = .979; SRMR = .039. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 30.85 Δχ2 improvement of fit for the covariation of items four and five (χ2(13) = 79.72, p < .001; RMSEA = .134 (CI .107, .164); CFI = .853; SRMR = .070). Modification indices next supported a 30.71 Δχ2 improvement of fit for the covariation of items three and four (χ2(12) = 48.38, p < .001; RMSEA = .103 (CI .074, .135); CFI = .921; SRMR = .062). Modification indices next supported a 13.02 Δχ2 improvement of fit for the covariation of items four and nine (χ2(11) = 35.07, p < .001; RMSEA = .088 (CI .056, .121); CFI = .948; SRMR = .048). Finally, modification indices supported a 12.82 Δχ2 improvement of fit for the covariation of items three and seven, resulting in the above model.
A.5. Message enactment – Withholding
Table A5.
Means, standard deviations, and items of the holding back scale (N = 285).
|
Current patients (n = 111) |
Former patients (n = 174) |
||||||
|---|---|---|---|---|---|---|---|
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| Instructions: For each statement, please respond to how much you hold back from or actively avoid sharing the concern in the past month with your medical team. | |||||||
| HB_1 | Concerns about my physical symptoms (e.g., pain, fatigue, breathing, swallowing, speaking) | .455 | Y | 1.07 | 1.17 | 1.04 | 1.12 |
| HB_2 | Concerns about my cancer treatment (e.g., medical or surgical treatments, medicines, interactions with doctors and nurses, being in the hospital) | .477 | Y | 1.17 | 1.29 | .885 | 1.07 |
| HB_3 | Concerns about my ability to function sexually | .683 | N | 1.63 | 1.67 | 1.48 | 1.52 |
| HB_4 | Emotions such as fear, worry, or sadness | .838 | Y | 1.84 | 1.42 | 1.42 | 1.28 |
| HB_5 | Fear of death or that I might die from this disease | .836 | Y | 1.84 | 1.58 | 1.45 | 1.41 |
| HB_6 | Fear of disease progressing or coming back | .746 | Y | 1.82 | 1.32 | 1.48 | 1.23 |
| HB_7 | Concerns about my well-being | .795 | Y | 1.81 | 1.40 | 1.34 | 1.26 |
| HB_8 | Concerns about [my support person’s] well-being | .835 | N | 1.80 | 1.55 | 1.26 | 1.48 |
| HB_9 | Concerns about my relationship with [my support person] | .831 | N | 1.57 | 1.65 | 1.19 | 1.50 |
| HB_10 | Dissatisfaction or embarrassment about my body image or appearance | .796 | N | 1.82 | 1.48 | 1.34 | 1.40 |
| HB_11 | Concerns about your relationship with others (e.g., children, other family members, friends) | .878 | N | 1.60 | 1.56 | 1.14 | 1.40 |
| HB_12 | Financial concerns (including insurance, household costs, and medical bills) | .775 | N | 1.36 | 1.60 | 1.04 | 1.39 |
| HB_13 | Job-related concerns | .774 | N | 1.24 | 1.64 | 0.95 | 1.37 |
| Mean composite score | 1.58 | 1.18 | 1.23 | 1.00 | |||
All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. The two items related to financial concerns (HB_12, HB_13) were not included based on the focus of this study. Additionally, items related to other people’s well-being (HB_8, HB_9, HB_10, HB_11) were not included based on the focus of this study despite high factor loadings. Items with low loadings after the removal of the above items were not retained for further analysis (HB_3). Loadings were reassessed after each item was removed.
Figure A9. CFA of retained items in the sharing scale without covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2(9) = 232.45, p < .001; RMSEA = .296 (CI .263, .329); CFI = .805; SRMR = .110.
Figure A10. CFA of retained items in the sharing scale with covaried error terms.
Parameter estimates are standardized. Model fit indices were χ2 (5) = 14.56, p = .01; RMSEA = .082 (CI .035, .133); CFI = .992; SRMR = .023. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 110.22 Δχ2 improvement of fit for the covariation of items one and two (χ2(8) = 99.88, p < .001; RMSEA = .201 (CI .167, .237); CFI = .920; SRMR = .060). Modification indices next supported a 33.64 Δχ2 improvement of fit for the covariation of items five and six (χ2(7) = 70.72, p < .001; RMSEA = .179 (CI .143, .218); CFI = .944; SRMR = .053). Modification indices next supported a 36.83 Δχ2 improvement of fit for the covariation of items four and five (χ2(6) = 38.27, p < .001; RMSEA = .138 (CI .098, .181); CFI = .972; SRMR = .041). Finally, modification indices supported a 21.10 Δχ2 improvement of fit for the covariation of items six and seven, resulting in the above model.
A.5. Full structural equation models
Figure A11. SEM predicting sharing with current patients (n = 111).
Parameter estimates are standardized. Model fit indices were χ2(239) = 485.18, p < .001; RMSEA = .097 (CI .084, .109); CFI = .878; SRMR = .127.
Figure A12. SEM predicting sharing with former patients (n = 174).
Parameter estimates are standardized. Model fit indices were χ2(239) = 444.15, p < .001; RMSEA = .070 (CI .060, .081); CFI = .932; SRMR = .069.
Figure A13. SEM predicting withholding with current patients (n = 111).
Parameter estimates are standardized. Model fit indices were χ2(196) = 329.90, p < .001; RMSEA = .079 (CI .064, .093); CFI = .936; SRMR = .107.
Figure A14. SEM predicting withholding with former patients (n = 174).
Parameter estimates are standardized. Model fit indices were χ2(196) = 335.63, p < .001; RMSEA = .064 (CI .052, .076); CFI = .955; SRMR = .087.
Note: Parameter estimates are standardized. Model fit indices were χ2(196) = 335.63, p < .001; RMSEA = .064 (CI .052, .076); CFI = .955; SRMR = .087.
Footnotes
Male patients with breast cancer were excluded because there were an insufficient number of participants to draw meaningful inferences.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics approval
This study was approved by the Rutgers University Institutional Review Board (protocol and approval # E17–664).
Data availability statement
The data that support the findings of this study are available from the corresponding author.
References
- Anderson JN, Graff JC, Krukowski RA, Schwartzberg L, Vidal GA, Waters TM, Paladino AJ, Jones TN, Blue R, Kocak M, & Graetz I. (2020). “Nobody will tell you. You’ve got to ask!”: An examination of patient-provider communication needs and preferences among black and white women with early-stage breast cancer. Health Communication, 36(11), 1331–1342. 10.1080/10410236.2020.1751383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Franks P, Duberstein PR, Epstein RM, Feldman MD, Garcia EF, & Kravitz RL (2011). Suffering in silence: Reasons for not disclosing depression in primary care. The Annals of Family Medicine, 9(5), 439–446. 10.1370/afm.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM, & Yuan KH (2010). Structural equation modeling with small samples: Test statistics. Multivariate Behavioral Research, 34(2), 181–197. 10.1207/S15327906Mb340203 [DOI] [PubMed] [Google Scholar]
- Brandão T, Schulz MS, & Matos PM (2017). Psychological adjustment after breast cancer: A systematic review of longitudinal studies. Psycho-Oncology, 26(7), 917–926. 10.1002/pon.4230 [DOI] [PubMed] [Google Scholar]
- Brandes K, Linn AJ, Smit EG, & van Weert JCM (2015). Patients’ reports of barriers to expressing concerns during cancer consultations. Patient Education and Counseling, 98(3), 317–322. 10.1016/j.pec.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Broadbridge E, Greene K, Venetis MK, Lee LE, Banerjee SC, Saraiya B, & Devine KA (2023). Facilitating psychological adjustment for breast cancer patients through empathic communication and uncertainty reduction. Patient Education and Counseling. Advance online publication. 10.1016/j.pec.2023.107791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butow PN, Kazemi JN, Beeney LJ, Griffin A-M, Dunn SM, & Tattersall MHN (1996). When the diagnosis is cancer: Patient communication experiences and preferences. Cancer, 77(12), 2630–2637. [DOI] [PubMed] [Google Scholar]
- Checton MG, & Greene K. (2012). Beyond initial disclosure: The role of prognosis and symptom uncertainty in patterns of disclosure in relationships. Health Communication, 27(2), 145–157. 10.1080/10410236.2011.571755 [DOI] [PubMed] [Google Scholar]
- Checton MG, & Greene K. (2014). Elderly patients’ heart-related conditions: Disclosing health information differs by target. Psychology, Health & Medicine, 20(5), 594–604. 10.1080/13548506.2014.986141 [DOI] [PubMed] [Google Scholar]
- Checton MG, Venetis MK, Catona D, Bontempo AC, Greene K, de Meritens AB, & Devine KA (2019). Reports of sharing and withholding cancer-related information by patients with gynecologic cancer and their supporters. Oncology Nursing Forum, 46(6), 676–685. 10.1188/19.ONF.676-685 [DOI] [PubMed] [Google Scholar]
- Choi SY, Venetis MK, Greene K, Magsamen-Conrad K, Checton MG, & Banerjee SC (2016). Planning a stigmatized nonvisible illness disclosure: Applying the disclosure decision-making model. The Journal of Psychology, 150(8), 1004–1025. 10.1080/00223980.2016.1226742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, & Street RL (2014). A 3-stage model of patient-centered communication for addressing cancer patients’ emotional distress. Patient Education and Counseling, 94(2), 143–148. 10.1016/J.PEC.2013.09.025 [DOI] [PubMed] [Google Scholar]
- Friley BL, & Venetis MK (2022). Decision-making criteria when contemplating disclosure of transgender identity to medical providers. Health Communication, 37(8), 1031–1040. 10.1080/10410236.2021.1885774 [DOI] [PubMed] [Google Scholar]
- Greene K. (2009). An integrated model of health disclosure decision-making. In Afifi TD & Afifi WA (Eds.), Uncertainty, information management, and disclosure decisions: Theories and applications (pp. 226–253). Routledge/Taylor & Francis Group. [Google Scholar]
- Greene K, Carpenter A, Catona D, & Magsamen-Conrad K. (2013). The brief disclosure intervention (BDI): Facilitating African Americans’ disclosure of HIV. Journal of Communication, 63(1), 138–158. 10.1111/JCOM.12010 [DOI] [Google Scholar]
- Greene K, Magsamen-Conrad K, Venetis MK, Checton MG, Bagdasarov Z, & Banerjee SC (2012). Assessing health diagnosis disclosure decisions in relationships: Testing the disclosure decision-making model. Health Communication, 27(4), 356–368. 10.1080/10410236.2011.586988 [DOI] [PubMed] [Google Scholar]
- Herzog W, & Boomsma A. (2009). Small-sample robust estimators of noncentrality-based and incremental model fit. Structural Equation Modeling: A Multidisciplinary Journal, 16(1), 1–27. 10.1080/10705510802561279 [DOI] [Google Scholar]
- Heudel PE, Delrieu L, Dumas E, Crochet H, Hodroj K, Charrier I, Chvetzoff G, Durand T, & Blay J-Y (2022). Impact of limited e-health literacy on the overall survival of patients with cancer. JCO Clinical Cancer Informatics, 6(6), 1–8. 10.1200/CCI.21.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, & Oh HJ (2020). The effects of patient-centered communication: Exploring the mediating role of trust in healthcare providers. Health Communication, 35(4), 502–511. 10.1080/10410236.2019.1570427 [DOI] [PubMed] [Google Scholar]
- Hoyt MA, & Stanton AL (2018). Adjustment to chronic illness. In Baum A. & Revenson TA (Eds.), Handbook of health psychology (pp. 179–194). Taylor & Francis. [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6 (1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Hughes J. (1982). Emotional reactions to the diagnosis and treatment of early breast cancer. Journal of Psychosomatic Research, 26(2), 277–283. 10.1016/0022-3999(82)90047-2 [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Regan MM, Kim Y, Greer G, Parker B, Bennett S, & Winer E. (2006). Cancer-related communication between female patients and male partners scale: A pilot study. Psycho-Oncology, 15(9), 780–794. 10.1002/pon.1004 [DOI] [PubMed] [Google Scholar]
- Kwame A, & Petrucka PM (2021). A literature-based study of patient-centered care and communication in nurse-patient interactions: Barriers, facilitators, and the way forward. BMC Nursing, 20(1), 1–10. 10.1186/s12912-021-00684-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNoue MD, & Roter DL (2018). Exploring patient-centeredness: The relationship between self-reported empathy and patient-centered communication in medical trainees. Patient Education and Counseling, 101(6), 1143–1146. 10.1016/j.pec.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Lee LE, & Greene K. (2022). Disclosure decision-making model. In Ho E, Bylund C, van Weert J, Basnyat I, Bol N. & Dean M. (Eds.), The international encyclopedia of health communication (pp. 1–8). Wiley. 10.1002/9781119678816.iehc0715 [DOI] [Google Scholar]
- Lewis CC, Matheson DH, & Elizabeth BCA (2011). Factors influencing patient disclosure to physicians in birth control clinics: An application of the communication privacy management theory. Health Communication, 26(6), 502–511. 10.1080/10410236.2011.556081 [DOI] [PubMed] [Google Scholar]
- Manne S, Badr H, Zaider T, Nelson C, & Kissane D. (2010). Cancer-related communication, relationship intimacy, and psychological distress among couples coping with localized prostate cancer. Journal of Cancer Survivorship, 4(1), 74–85. 10.1007/s11764-009-0109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead N, & Bower P. (2000). Patient-centredness: A conceptual framework and review of the empirical literature. Social Science & Medicine, 51(7), 1087–1110. 10.1016/S0277-9536(00)00098-8 [DOI] [PubMed] [Google Scholar]
- Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, Wegscheider K, Weis J, Boehncke A, Hund B, Reuter K, Richard M, Sehner S, Sommerfeldt S, Szalai C, Wittchen H-U, & Koch U. (2014). Four-week prevalence of mental disorders in patients with cancer across major tumor entities. Journal of Clinical Oncology, 32 (31), 3540–3546. 10.1200/JCO.2014.56.0086 [DOI] [PubMed] [Google Scholar]
- Mehnert A, Hartung TJ, Friedrich M, Vehling S, Brähler E, Härter M, Hubshman M, Schulz H, Wegscheider K, Weis J, Koch U, & Faller H. (2018). One in two cancer patients is significantly distressed: Prevalence and indicators of distress. Psycho-Oncology, 27(1), 75–82. 10.1002/pon.4464 [DOI] [PubMed] [Google Scholar]
- Mercer SW, Maxwell M, Heaney D, & Watt GCM (2004). The consultation and relational empathy (CARE) measure: Development and preliminary validation and reliability of an empathy-based consultation process measure. Family Practice, 21(6), 699–705. 10.1093/FAMPRA/CMH621 [DOI] [PubMed] [Google Scholar]
- Nosarti C, Roberts JV, Crayford T, McKenzie K, & David AS (2002). Early psychological adjustment in breast cancer patients: A prospective study. Journal of Psychosomatic Research, 53(6), 1123–1130. 10.1016/S0022-3999(02)00350-1 [DOI] [PubMed] [Google Scholar]
- Pehrson C, Banerjee SC, Manna R, Shen MJ, Hammonds S, Coyle N, Krueger CA, Maloney E, Zaider T, & Bylund CL (2016). Responding empathically to patients: Development, implementation, and evaluation of a communication skills training module for oncology nurses. Patient Education and Counseling, 99(4), 610–616. 10.1016/j.pec.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistrang N, & Barker C. (1995). The partner relationship in psychological response to breast cancer. Social Science & Medicine, 40(6), 789–797. 10.1016/0277-9536(94)00136-H [DOI] [PubMed] [Google Scholar]
- Roberts CS, Cox CE, Reintgen DS, Baile WF, & Gibertini M. (1994). Influence of physician communication on newly diagnosed breast patients’ psychologic adjustment and decision-making. Cancer, 74(S1), 336–341. 10.1002/CNCR.2820741319 [DOI] [PubMed] [Google Scholar]
- Stanton AL, Revenson TA, & Tennen H. (2007). Health psychology: Psychological adjustment to chronic disease. Annual Review of Psychology, 58(1), 565–592. 10.1146/annurev.psych.58.110405.085615 [DOI] [PubMed] [Google Scholar]
- Steuber KR, & Solomon DH (2011). Factors that predict married partners’ disclosure about infertility to social network members. Journal of Applied Communication Research, 39(3), 250–270. 10.1080/00909882.2011.585401 [DOI] [Google Scholar]
- Street RL, Makoul G, Arora NK, & Epstein RM (2009). How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Education and Counseling, 74(3), 295–301. 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Sutton TL, Koprowski MA, Grossblatt-Wait A, Brown S, McCarthy G, Liu B, Gross A, Macuiba C, Hedlund S, Brody JR, & Sheppard BC (2022). Psychosocial distress is dynamic across the spectrum of cancer care and requires longitudinal screening for patient-centered care. Supportive Care in Cancer, 30(5), 4255–4264. 10.1007/s00520-022-06814-z [DOI] [PubMed] [Google Scholar]
- Thornton RLJ, Powe NR, Roter DL, & Cooper LA (2011). Patient–physician social concordance, medical visit communication and patients’ perceptions of health care quality. Patient Education and Counseling, 85(3), e201–e208. 10.1016/j.pec.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle E. (2016). Demographics of the US oncology workforce. Journal of Oncology Practice, 12(2), 99. 10.1200/JOP.2015.010124 [DOI] [PubMed] [Google Scholar]
- Venetis MK (2010). Communication-participation behavior during the delivery of breast-cancer care [Doctoral dissertation]. Rutgers University. 10.7282/T3K9377N [DOI] [Google Scholar]
- Venetis MK, Bontempo AC, Catona D, Buckley de Meritens A, Devine KA, & Greene K. (2023). Dilemmas and strategy when companion participation during appointments differs from patient and companion expectations. Health Communication. Advance online publication. 10.1080/10410236.2023.2190244 [DOI] [PubMed] [Google Scholar]
- Venetis MK, Robinson JD, & Kearney T. (2015). Breast-cancer patients’ participation behavior and coping during presurgical consultations: A pilot study. Health Communication, 30(1), 19–25. 10.1080/10410236.2014.943633 [DOI] [PubMed] [Google Scholar]
- Watson M, Law MG, Santos MD, Greer S, Baruch J, & Bliss J. (1994). The mini-MAC: Further development of the mental adjustment to cancer scale. Journal of Psychosocial Oncology, 12(3), 33–46. 10.1300/J077V12N03_03 [DOI] [Google Scholar]
- Zhou Y, Acevedo Callejas ML, Li Y, & MacGeorge EL (2021). What does patient-centered communication look like? Linguistic markers of provider compassionate care and shared decision-making and their impacts on patient outcomes. Health Communication, 38(5), 1003–1013. 10.1080/10410236.2021.1989139 [DOI] [PubMed] [Google Scholar]
- Zwingmann J, Baile WF, Schmier JW, Bernhard J, & Keller M. (2017). Effects of patient-centered communication on anxiety, negative affect, and trust in the physician in delivering a cancer diagnosis: A randomized, experimental study. Cancer, 123(16), 3167–3175. 10.1002/cncr.30694 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.