Abstract
Purpose
Quality of life (QOL) is among the most important outcomes for women with metastatic breast cancer (MBC), and it predicts survival. QOL is negatively impacted by cognitive impairment, fatigue, and weight gain. We assessed whether a whole food, plant-based (WFPB) diet-promoting weight loss is feasible and might improve QOL.
Methods
Women with MBC on stable systemic treatments were randomized 2:1 to 1) WFPB dietary intervention (n = 21) or 2) usual care (n = 11) for 8 weeks. Participants attended weekly education visits and consumed an ad libitum WFPB diet (3 prepared meals/day provided). Patient-reported outcomes and 3-day food records were assessed at baseline and 8 weeks. The effects of WFPB diet on changes in outcomes were assessed by analysis of covariance model controlling for baseline.
Results
20 intervention and 10 control participants completed the trial. Intervention participants were highly adherent to the WFPB diet (94.3 % total calories on-plan). Intervention group nutrient intakes changed significantly including dietary fat (35.8 % to 20.4 % percent calories from fat, p < 0.001) and fiber content (12.7 to 30.8 g fiber/1000 kcal, p < 0.001). Perceived cognitive function (FACT-Cog total + 16.1; 95 % confidence interval [CI] = 0.8–31.7; p = 0.040) and emotional well-being (FACT-B emotional well-being subscale + 2.3; CI = 0.5–4.1; p = 0.016) improved in the WFPB versus the control group. Fatigue, measured by the BFI, improved within the WFPB group for fatigue severity (M = 4.7 ± 2.5[SD] to 3.7 ± 2.3, p = 0.047) and fatigue at its worst (5.8 ± 2.8 to 4.4 ± 2.4, p = 0.011).
Conclusions
Significant dietary changes in this population are feasible and may improve QOL by improving treatment-related symptoms. Additional study is warranted.
Trial Registration: ClinicalTrials.gov identifier: NCT03045289. Registered 7 February 2017.
Keywords: Metastatic breast cancer, Breast cancer, Obesity, Quality of life, Patient-reported outcomes, Nutrition, Plant-based, Vegan diet
Background
Breast cancer (BC) is the most common cancer other than nonmelanoma skin cancer [1], and increasing numbers of women are living with advanced stage breast cancer [2, 3]. Goals of care include reducing risks of cancer progression and mortality, but also preserving or increasing quality of life (QOL). In fact, QOL is one of the most important outcomes to women with metastatic breast cancer (MBC) [4–6], and recent evidence shows both QOL and patient-reported symptom burden predict survival in MBC patients [7–10].
QOL is strongly affected by treatment-related symptoms [11]: cognitive impairment, fatigue, and weight gain. Self-reported cognitive impairment occurs in 45 % of women receiving systemic therapy [12], and cancer-related fatigue (CRF) adversely impacts QOL by reducing activities of daily living [13]. Obesity, associated with a lower QOL [14], fatigue [15], and persistent cognitive changes [16] and complaints [17], is common among BC patients. The prevalence of obesity in MBC patients mirrors its overall population prevalence, which in U.S. adults is above 42 % [18, 19].
Dietary intervention may affect treatment-related symptoms by affecting body weight and inflammation, which, in turn, are associated with QOL [14, 15]. MBC patients with obesity have been found to have significantly higher levels of inflammatory markers than MBC patients without obesity [14]. Evidence suggests that weight loss [20] and dietary change [21] might reduce systemic inflammation leading to improvements in cognitive function [22, 23] and fatigue [24] and thereby QOL. A whole food, plant-based (WFPB) diet, exclusively comprised of minimally processed plant foods, has been demonstrated to result in significant, clinically meaningful weight loss [25] and significantly lower inflammatory marker levels [26, 27]. While some suggest that weight loss alone can improve QOL [28], the effect of intentional weight loss on cancer-related outcomes in women with MBC remains unexplored [29].
Given the plausible benefits and lack of prior dietary interventions in this population, we designed and conducted a pilot study to explore feasibility and preliminary outcomes. Our intervention resulted in significant improvement of cardiometabolic and hormonal markers and intentional weight loss, described in a separate publication [30]. This paper reports our feasibility results, including dietary adherence, changes in nutritional intake, and patient-reported outcomes (PROs).
Methods
This study was performed in compliance with recognized ethical guidelines, including the U.S. Common Rule. The study protocol was approved by the University of Rochester Research Subject Review Board (ClinicalTrials.gov identifier: NCT03045289; registered 7 February 2017), and written informed consent was obtained from all participants. Women with MBC were recruited from oncology clinics at the University of Rochester Medical Center and via flyers at local support groups. Women age greater than 18 years with stage 4 BC with any ER/PR/HER2 status expected to live at least 6 months by their oncologist and on a stable treatment regimen for ≥ 6 weeks with no expected near future changes were eligible. Exclusions included inability to tolerate a normal diet, active malabsorption syndrome or eating disorder, uncontrolled diarrhea, recent vegan diet, major surgery within 2 months, current insulin, sulfonylurea, or warfarin use, glomerular filtration rate < 30 mL/min/1.73 m2, or serum potassium level > 5.3 mmol/L twice within 90 days, current smoking, illicit drug use, more than 7 alcoholic drinks/week, plant-based food allergies or intolerances, or psychiatric disorder impairing ability to give consent. After consent, additional screening consisted of attendance at an informational session providing an overview of the study, sampling provided study food, and an individual follow-up visit with the study physicians. If a participant consented but did not complete screening procedures or chose not to proceed with participation, she was deemed a screen failure.
At the conclusion of participants’ individual visits, participants were randomized 2:1 to two arms: 1) WFBP diet (n = 21) or 2) usual diet control (n = 11) by computer algorithm with blocks of size 4. Participants in the WFPB arm received 3 prepared meals and one side dish per day for 8 weeks, weekly education with the study physicians (TMC and EKC), and a weekly phone call from one of the study physicians. The ad libitum WFPB diet consisted of fruits, vegetables, whole grains, legumes, nuts, and seeds. Soy foods were allowed as were minimal amounts of added sugars. The diet excluded all animal products, added oils, and solid fats. Participants were encouraged to eat as often and as much as desired to be full. They were allowed to add their own ‘on-plan’ food in addition to, or in place of, the provided food. The provided meals were not designed to provide a specific calorie amount or nutrient intake, but rather to enhance dietary adherence. A daily multivitamin (Centrum Women) was provided to all participants for the 8 week trial duration.
Education and coaching consisted of one office visit/week for the duration of the 8 week trial, conducted in person (pre-COVID) or via remote teleconferencing (post-COVID) (Zoom Video Communications, Inc., San Jose, CA) with TMC and EKC. In addition to individual assessments and coaching, educational topics included discussions of the food guide, shopping guide, recipes, and label reading, as well as discussing the effects of nutrition on weight loss, cardiovascular health, blood glucose, and behavioral change topics (changing tastes, cravings, and willpower). Between visits, brief weekly telephone calls were made to offer additional coaching and/or helpful resources.
Participants in the usual diet control arm were instructed to continue their usual diets for 8 weeks and received phone calls from a study physician at weeks 2 and 6 to assess for adverse events and treatment changes. As an incentive to maintain participation, control participants received condensed WFPB educational resources and 2 weeks of prepared study meals after completing their final 8-week assessments.
Dietary assessments included two 3 day food records (two weekdays and one weekend day at baseline and week 8) and three unscheduled 24 h recalls. Unscheduled 24 h food recalls were conducted by phone by a dietitian (NW) at approximately 2, 4, and 6 weeks. Three-day food records and 24 h recalls were analyzed using Nutrition Data System for Research (NDSR), version 2017 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Dietary compliance was calculated as the percentage of calories consumed from on-plan foods. On-plan was predefined as foods and meals that did not contain added liquid oils, solid fats, or animal-based products. By our predefined criteria, a participant was adherent if she consumed at least 80 % of her calories from on-plan foods and attended at least 6 of 8 weekly visits.
Participants completed questionnaires at baseline and 8 weeks. Validated questionnaires included the Brief Fatigue Inventory (BFI), European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), Functional Assessment of Cancer Therapy—Breast (FACT-B), Functional Assessment of Cancer Therapy—Cognitive Function (FACT-Cog), and a modified M.D. Anderson Cancer Center Symptom Inventory (https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/md-anderson-symptom-inventory.html). Participants completed a demographic questionnaire at baseline and a feedback questionnaire at 8 weeks.
Statistical analysis
Between arm balance of participants’ clinical and sociodemographic characteristics was evaluated, and relevant descriptive statistics (mean, standard deviation (SD)) were generated. Distribution of outcome variables was assessed at baseline and 8 weeks by study arm graphically and by the descriptive statistics. Within-group change in outcome values from baseline to 8 weeks within each study arm was assessed by paired t test. Analysis of covariance models with arm as the main factor and corresponding baseline levels as the covariate was used to evaluate the effects of the WFPB intervention on PROs at 8 weeks and to estimate the mean between-group difference in change in PRO score from baseline. To account for some deviation from the normality assumption, the results were confirmed in non-parametric sensitivity analysis. Statistical significance was set at two-sided alpha = 0.05 level. Data were analyzed using SAS version 9.4 (SAS Inc, Cary, NC, USA).
Results
Table 1 displays baseline characteristics of participants. The mean BMI of participants was 29.6 kg/m2 with 71 % of participants in either the overweight or obese BMI range. The majority of participants had hormone receptor positive BC. Metastatic disease to the bone was the most common site of metastasis, present in 84 % of participants. The most common treatment regimens were a cyclin-dependent kinase 4/6 inhibitor combined with an aromatase inhibitor.
Table 1.
Baseline characteristics
| Characteristics | Mean ± SD | Control (n = 10 *) | Intervention (n = 21) |
|---|---|---|---|
| Age (years) | 64.2 ± 8.9 | 59.1 ± 11.0 | |
| Race | Black, n (%) | 0 (0) | 1 (4.8) |
| White, n (%) | 10 (100.0) | 19 (90.5) | |
| No answer, n (%) | 0 (0) | 1 (4.8) | |
| Ethnicity | Not Hispanic/Latino, n (%) | 10 (100.0) | 20 (95.2) |
| No answer, n (%) | 0 (0) | 1 (4.8) | |
| Marital status | Married, n (%) | 7 (70.0) | 14 (66.7) |
| Divorced, n (%) | 2 (20.0) | 3 (14.3) | |
| Single, n (%) | 1 (10.0) | 3 (14.3) | |
| Widowed, n (%) | 0 (0) | 1 (4.8) | |
| Employment status | Currently employed outside home, n (%) | 3 (30.0) | 6 (28.6) |
| Self-employed, n (%) | 0 (0) | 2 (9.5) | |
| Retired, n (%) | 4 (40.0) | 4 (19.0) | |
| Disability, n (%) | 1 (10.0) | 3 (14.3) | |
| Homemaker, n (%) | 2 (20.0) | 4 (19.0) | |
| Not Working—Other, n (%) | 0 (0) | 2 (9.5) | |
| BMI at study baseline (kg/m2) | Mean ± SD | 28.4 ± 4.4 | 30.2 ± 7.2 |
| Age at first breast cancer diagnosis (years) | Mean ± SD | 52.9 ± 11.7 | 49.4 ± 10.9 |
| Years elapsed since first diagnosis | Mean ± SD | 11.2 ± 7.9 | 9.7 ± 6.4 |
| Years elapsed since diagnosis of metastatic breast cancer | Mean ± SD | 5.3 ± 6.0 | 2.2 ± 1.8 |
| Hormone receptor status | ER +, n (%) | 10 (100.0) | 20 (95.2) |
| PR +, n (%) | 9 (90.0) | 17 (81.0) | |
| HER2 +, n (%) | 3 (30.0) | 6 (28.6) | |
| Location of metastases | Bone, n (%) | 7 (70.0) | 19 (90.5) |
| Lung, n (%) | 4 (40.0) | 8 (38.1) | |
| Brain, n (%) | 1 (10.0) | 3 (14.3) | |
| Liver, n (%) | 2 (20.0) | 1 (4.8) | |
| Other, n (%) | 6 (60.0) | 7 (33.3) | |
| Cancer therapy | Palbociclib, n (%) | 3 (30.0) | 10 (47.6) |
| Abemaciclib, n (%) | 1 (10.0) | 2 (9.5) | |
| Ribociclib, n (%) | 0 (0) | 1 (4.8) | |
| Trastuzumab, n (%) | 2 (20.0) | 5 (23.8) | |
| Pertuzumab, n (%) | 1 (10.0) | 4 (19.0) | |
| Capecitabine, n (%) | 1 (10.0) | 1 (4.8) | |
| Letrozole, n (%) | 3 (30.0) | 13 (61.9) | |
| Anastrozole, n (%) | 3 (30.0) | 1 (4.8) | |
| Fulvestrant, n (%) | 2 (20.0) | 3 (14.3) | |
| Exemestane, n (%) | 1 (10.0) | 2 (9.5) | |
| Denosumab, n (%) | 1 (10.0) | 10 (47.6) | |
| Zoledronic acid, n (%) | 0 (0) | 1 (4.8) | |
| Leuprolide, n (%) | 0 (0) | 2 (9.5) |
* One control subject was lost to follow up immediately after randomization to the control group
Her baseline characteristics were incomplete and not reported here
SD standard deviation, BMI body mass index
Feasibility
Thirty of 32 randomized participants completed their study participation. One participant was lost to follow-up immediately following randomization to the control arm. One intervention participant was withdrawn by investigators in March 2020 shortly after baseline, due to the COVID-19 pandemic shutdown (Fig. 1). All 30 remaining participants completed assessments at 8 weeks. All (100 %) intervention participants attended at least 6 of the 8 weekly visits, our prespecified criterion for study visit adherence.
Fig. 1.
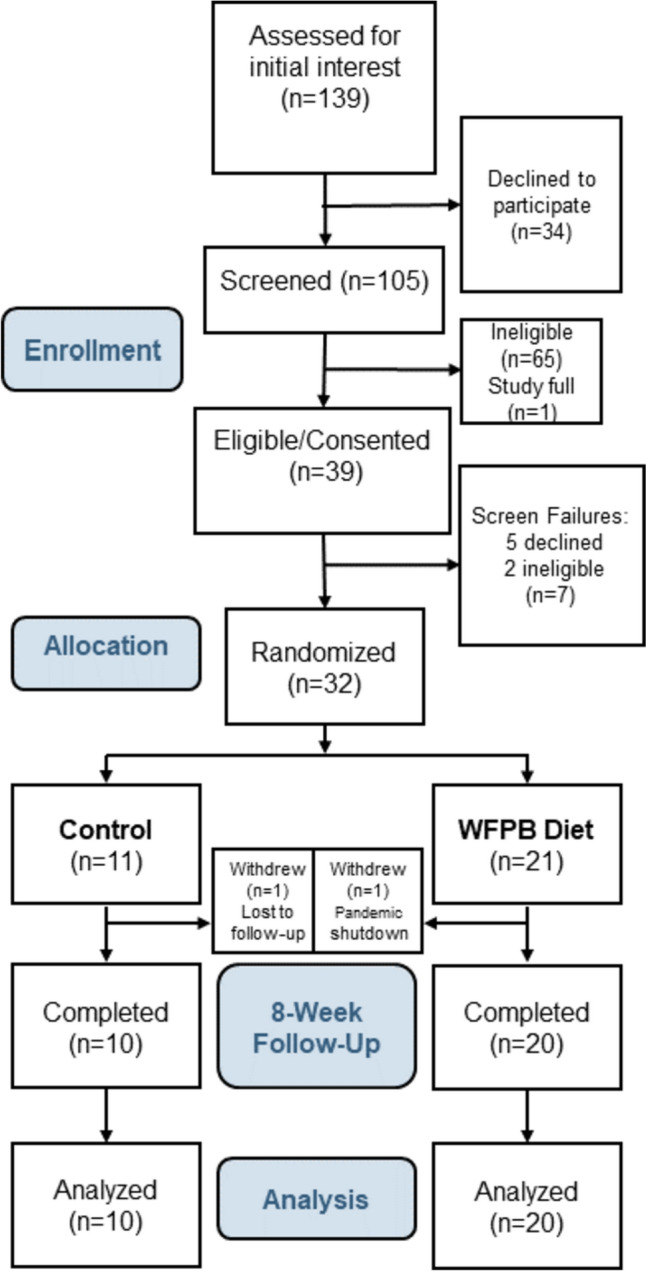
Consort diagram
Intervention participants were highly adherent to the diet. Eighteen of 19 (94.7 %) intervention participants with complete dietary assessments met the study’s prespecified adherence criterion that they derive ≥ 80 % of their kilocalories from on-plan foods. In fact, 94.3 % of total calories consumed by the intervention participants with complete dietary assessments were from on-plan foods (foods without added oils, solid fats, or animal-based ingredients). Changes in dietary intake between baseline and final 3-day food records are shown in Table 2.
Table 2.
Nutrient intake of intervention and control groups at baseline and 8 weeks
| Intervention group (n = 19)*** | Control group (n = 10) | Between group (adjusted for baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 8 Final | Baseline | Week 8 Final | Difference in change | p- value | |
| Energy (kcal) | 1782.3 ± 330.4 | 1321.3 ± 262.4 ** | 1590.2 ± 442.7 | 1431.2 ± 401.0 * | − 301.9 ± 285.7 | < 0.001 |
| Fat (% of total kcal) | 35.8 ± 6.2 | 20.4 ± 5.8 ** | 35.0 ± 5.8 | 34.8 ± 11.9 | − 15.2 ± 8.9 | 0.012 |
| Carbohydrate (% of total kcal) | 48.4 ± 7.3 | 66.2 ± 8.0 ** | 44.8 ± 10.0 | 45.4 ± 17.7 | 17.3 ± 11.4 | < 0.001 |
| Protein (% of total kcal) | 14.6 ± 2.5 | 12.6 ± 2.9 * | 19.3 ± 9.7 | 18.8 ± 7.8 | − 1.5 ± 4.6 | 0.490 |
| % total protein provided by plant sources | 46.6 ± 11.3 | 95.7 ± 11.8 ** | 45.5 ± 25.9 | 43.0 ± 21.9 | 51.6 ± 14.7 | < 0.001 |
| Dietary Cholesterol (mg) | 214.2 ± 104.6 | 7.5 ± 18.3 ** | 217.1 ± 139.5 | 188.2 ± 119.6 | − 177.8 ± 133.3 | 0.002 |
| Dietary Fiber (g/1000 kcal) | 12.7 ± 5.7 | 30.8 ± 5.6 ** | 16.5 ± 6.0 | 14.9 ± 3.9 | 19.7 ± 6.4 | < 0.001 |
*** One intervention participant was missing the final 3 day food record
*p < 0.05 for within-group change
**p < 0.01 for within-group change
Within the intervention group, dietary intake changed significantly. Intervention participants consumed 25.9 % fewer calories (p < 0.001). Fat as a percentage of total kilocalories and dietary cholesterol intake were significantly reduced by 43.0 % (p < 0.001) and 96.5 % (p < 0.001), respectively. Carbohydrates as a percentage of total kilocalories increased 36.8 % (p < 0.001) and dietary fiber per 1000 kcal increased 242.5 % (p < 0.001). Protein as a percentage of total kilocalories was modestly reduced (− 13.7 %, p < 0.017). The proportion of total protein provided by plant sources increased 105.4 % (p < 0.001).
The composition of the control group’s diet was largely unchanged despite knowing that we were studying a WFPB diet. Within the control group, participants reduced their total calories (− 10.0 %, p < 0.034). There were no statistically significant changes to the percent of total calories from any of the macronutrients, dietary cholesterol, the proportion of protein provided by plant sources, or dietary fiber per 1000 kcal.
The groups differed significantly in their mean nutrient changes from baseline to week 8 in all nutrients displayed in Table 2 except for change in percent of calories from protein.
Of note, there was a significant between-group effect (p < 0.01); intervention group participants lost a mean of 6.6 % of their body weight, whereas control group participants lost a mean of 0.7 % of their body weight. When adjusted for baseline weight, intervention participants lost 9.0 pounds more than control participants (p < 0.001). Changes in BMI, cardiometabolic outcomes, and hormonal markers are described in a separate publication [30].
Adverse events
Adverse events during the trial were infrequent and mild. Grade 2 hypotension occurred in three intervention participants. In each case, symptoms were mild and resolved after referral to their routine medical providers for medication adjustments. One control participant reported lightheadedness following a blood draw. Other adverse events included aphthous ulcer, transient, mild hyponatremia, and mild, transient neutropenia, all deemed medication related. One participant in each arm had her primary cancer treatment dose reduced by her oncologist due to adverse events typical of that medication.
Patient-reported outcomes
Table 3 shows the effect of the intervention arms on various patient-reported outcomes. The total FACT-B total score (+8.0, p < 0.001), as well as physical (+1.5, p = 0.016) and emotional well-being (+1.8, p = 0.012), and BC-specific symptoms (+3.2, p = 0.002) subscale scores improved significantly within the intervention group; surpassing the clinically important difference (CID) for each of these measures established in prior studies [31–33]. The change in emotional well-being score from baseline to 8 weeks was significantly different between the groups (+2.3, CID = 0.9 [31]; p = 0.016, ES = 0.54). The between-group difference in the FACT-B score approached significance (+5.1, CID = 6.0 [32, 33]; p = 0.067, ES = 0.29).
Table 3.
Patient-reported outcomes
| Intervention Group (n = 20) | Control Group (n = 10) | Between-Group Difference Mean ± SE | Between Group Effect Sizea | p-value (between-group)b | |||||
|---|---|---|---|---|---|---|---|---|---|
| Questionnaire | Better Score | Baseline Mean ± SD | Final Mean ± SD | Baseline Mean ± SD | Final Mean ± SD | ||||
| FACT-Cog total | 140.8 ± 32.0 | 156.6 ± 29.6 ** | 146.4 ± 40.6 | 145.1 ± 41.8 | 16.1 ± 7.4 | 0.46 | 0.040 | ||
| Perceived cognitive impairment | Higher | 81.3 ± 21.6 | 90.8 ± 19.0 ** | 84.3 ± 24.2 | 84.7 ± 24.8 | 8.4 ± 4.6 | 0.37 | 0.076 | |
| Perceived cognitive abilities | Higher | 24.9 ± 9.6 | 27.7 ± 6.7 | 28.7 ± 7.9 | 29.1 ± 7.9 | 0.7 ± 1.9 | 0.08 | 0.701 | |
| Comments from others | Higher | 10.2 ± 2.1 | 11.2 ± 1.3 * | 10.5 ± 2.1 | 9.8 ± 3.0 | 1.6 ± 0.6 | 0.79 | 0.006 | |
| Impact of perceived cognitive impairments on QOL | Higher | 22.0 ± 7.4 | 25.1 ± 6.5 * | 22.9 ± 9.0 | 21.5 ± 8.6 | 4.1 ± 2.0 | 0.52 | 0.049 | |
| Symptom Inventory | |||||||||
| Problems remembering things | Lower | 3.2 ± 2.6 | 2.0 ± 1.4 ** | 1.6 ± 2.0 | 2.6 ± 3.3 | − 1.6 ± 0.7 | − 0.67 | 0.024 | |
| Problems concentrating | Lower | 2.75 ± 2.5 | 1.9 ± 1.6 * | 1.8 ± 2.7 | 2.6 ± 3.1 | − 1.3 ± 0.6 | − 0.50 | 0.039 | |
| Problems paying attention | Lower | 2.3 ± 2.3 | 1.5 ± 1.8 | 1.8 ± 2.7 | 2.5 ± 3.2 | − 1.3 ± 0.7 | − 0.54 | 0.056 | |
| Problems multitasking | Lower | 2.0 ± 2.2 | 1.6 ± 1.7 | 1.9 ± 2.0 | 3.1 ± 3.5 | − 1.5 ± 0.7 | − 0.73 | 0.029 | |
| Fatigue | Lower | 4.6 ± 2.1 | 3.6 ± 2.4 | 3.8 ± 3.1 | 3.9 ± 2.4 | − 0.8 ± 0.8 | − 0.31 | 0.334 | |
| Shortness of breath | Lower | 1.8 ± 2.4 | 1.1 ± 2.3 * | 1.5 ± 2.4 | 1.2 ± 1.8 | − 0.3 ± 0.5 | − 0.12 | 0.560 | |
| Diarrhea | Lower | 1.5 ± 2.8 | 0.75 ± 1.4 | 0.5 ± 1.6 | 2.2 ± 3.4 | − 1.8 ± 0.8 | − 0.72 | 0.044 | |
| Skin problems | Lower | 0.8 ± 2.1 | 0.5 ± 1.7 | 1.0 ± 2.5 | 1.3 ± 2.3 | − 0.6 ± 0.2 | − 0.28 | 0.007 | |
| Symptoms interfered with general physical activity | Lower | 2.6 ± 2.6 | 1.8 ± 2.2 | 2.6 ± 2.3 | 2.5 ± 3.0 | − 0.7 ± 0.6 | − 0.29 | 0.259 | |
| Symptoms interfered with walking | Lower | 2.1 ± 2.5 | 1.2 ± 2.1 * | 1.9 ± 2.9 | 1.3 ± 2.4 | − 0.2 ± 0.5 | − 0.09 | 0.627 | |
| Symptoms interfered with QOL | Lower | 2.7 ± 2.6 | 1.7 ± 2.2 | 2.0 ± 2.4 | 2.3 ± 2.7 | − 1.1 ± 0.7 | − 0.41 | 0.126 | |
| FACT-B total | Higher | 103.1 ± 14.8 | 111.0 ± 14.0 * | 109.7 ± 21.8 | 111.2 ± 17.1 | 5.1 ± 2.7 | 0.29 | 0.067 | |
| Physical well-being | Higher | 21.4 ± 4.3 | 22.8 ± 4.5 * | 22.6 ± 5.8 | 22.1 ± 5.1 | 1.7 ± 1.0 | 0.36 | 0.093 | |
| Social/family well-being | Higher | 21.8 ± 4.8 | 22.6 ± 4.5 | 22.9 ± 4.3 | 24.1 ± 4.3 | − 0.6 ± 1.2 | − 0.14 | 0.611 | |
| Emotional well-being | Higher | 16.2 ± 4.2 | 18.0 ± 3.3 * | 18.1 ± 4.1 | 16.8 ± 3.2 | 2.3 ± 0.9 | 0.54 | 0.016 | |
| Functional well-being | Higher | 18.0 ± 5.2 | 18.7 ± 5.0 | 19.1 ± 7.0 | 20.7 ± 5.3 | − 1.2 ± 1.1 | − 0.20 | 0.283 | |
| Breast cancer subscale | Higher | 25.8 ± 4.0 | 28.9 ± 3.7 ** | 27.0 ± 5.0 | 27.5 ± 4.5 | 2.1 ± 1.3 | 0.49 | 0.102 | |
| BFI global fatigue | Lower | 3.4 ± 2.2 | 2.6 ± 2.1 | 2.6 ± 2.8 | 2.3 ± 2.0 | − 0.2 ± 0.6 | − 0.07 | 0.779 | |
| Fatigue severity | Lower | 4.7 ± 2.5 | 3.7 ± 2.3 * | 3.5 ± 3.1 | 3.2 ± 1.6 | 0.01 ± 0.7 | 0.01 | 0.984 | |
| Fatigue at its worst | Lower | 5.8 ± 2.8 | 4.4 ± 2.4 * | 4.3 ± 3.4 | 3.7 ± 2.0 | − 0.01 ± 0.7 | − 0.004 | 0.986 | |
| EORTC QLQ-C30 | |||||||||
| Global health status/QOL | Higher | 67.9 ± 22.0 | 71.3 ± 23.5 | 75 ± 16.1 | 79.6 ± 11.9 | − 3.1 ± 5.8 | − 0.15 | 0.600 | |
| Physical functioning | Higher | 77.2 ± 20.8 | 78.3 ± 22.8 | 78.5 ± 27.0 | 73.3 ± 24.5 * | 6.1 ± 5.0 | 0.27 | 0.234 | |
| Role functioning | Higher | 80.0 ± 27.9 | 81.7 ± 24.7 | 68.3 ± 30.9 | 75.0 ± 30.7 | − 2.1 ± 6.4 | − 0.07 | 0.744 | |
| Emotional functioning | Higher | 67.9 ± 19.4 | 74.2 ± 20.0 | 74.2 ± 28.7 | 79.2 ± 19.7 | − 1.8 ± 6.4 | − 0.08 | 0.786 | |
| Cognitive functioning | Higher | 73.3 ± 19.8 | 84.2 ± 15.7 ** | 76.7 ± 31.6 | 75.0 ± 29.7 | 11.3 ± 5.8 | 0.47 | 0.061 | |
| Social functioning | Higher | 71.1 ± 24.1 | 80.7 ± 21.7 * | 75.0 ± 25.2 | 83.3 ± 22.2 | − 0.14 ± 6.2 | − 0.01 | 0.983 | |
| Fatigue | Lower | 43.9 ± 23.5 | 34.4 ± 22.8 * | 37.8 ± 28.3 | 35.6 ± 26.1 | − 5.6 ± 6.1 | − 0.22 | 0.370 | |
| Insomnia | Lower | 35.0 ± 27.5 | 21.7 ± 27.1 | 20.0 ± 32.2 | 26.7 ± 34.2 | − 11.7 ± 10.8 | − 0.40 | 0.289 | |
*p < 0.05 for within-group change
**p < 0.01 for within-group change
Bold rowsare questionnaires or subsets of questionnaires with p<0.05 for between-group differences
aEffect size was calculated as difference relative to baseline standard deviation
bp value is assessing the between-group difference in change from the baseline to 8 weeks in analysis of covariance model
SD standard deviation, SE standard error, FACT-Cog Functional Assessment of Cancer Therapy—Cognitive Function, Symptom Inventory M.D. Anderson Cancer Center Symptom Inventory, FACT-B Functional Assessment of Cancer Therapy—Breast, BFI Brief Fatigue Inventory, EORTC QLQ-C30 European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire
Perceived cognitive function, as measured by the FACT-Cog questionnaire, showed clinically significant improvements within the intervention group as well as between the groups. (Table 3). Between groups, there were statistically and clinically significant improvements on the overall FACT-Cog score (+ 16.1, CID = 9.6 [34, 35]; p = 0.04, ES = 0.46) as well as on the comments from others subscale (+ 1.6, CID = 0.4 [35]; p = 0.006, ES = 0.79) and the impact of perceived cognitive impairments on QOL subscale (+ 4.1, CID = 0.9 [35]; p = 0.049, ES = 0.52) in the intervention group compared to the control. Perceived cognitive impairment improved in the intervention group (p = 0.007) and the between-group difference in change from the baseline to 8 weeks approached significance (+ 8.4, CID = 5.5 [34]; p = 0.076, ES = 0.37). Improvement in the perceived cognitive ability subscale approached significance within the intervention group (p = 0.056).
On the symptom inventory, there were statistically significant between group differences favoring the intervention group in improvements in problems remembering things (p = 0.024, ES = − 0.67), problems concentrating (p = 0.039, ES = − 0.5), and problems multitasking (p = 0.029, ES = − 0.73). Reported problems paying attention trended towards improvement in the intervention group compared to the control group in which the score worsened (p = 0.056, ES = − 0.54). Diarrhea (p = 0.044) and skin problems (p = 0.007) improved in the intervention group compared to the control. Shortness of breath (p = 0.039) and interference of symptoms with physical activity (p = 0.057), walking (p = 0.020), and QOL (p = 0.053) improved within the intervention group. Fatigue trended towards improvement within the intervention group (p = 0.054).
Mirroring results of the FACT-Cog questionnaire, there was a significant improvement in perceived cognitive functioning in the intervention group (p = 0.004) as assessed by the EORTC QLQ-C30. This difference approached significance between the groups (p = 0.061, ES = 0.47). Social functioning (p = 0.023) and fatigue (p = 0.013) improved significantly in the intervention group, but not between groups. There was a trend towards improved insomnia in the intervention group (p = 0.072).
Fatigue, as measured by the BFI, improved in the intervention group. There were significant within-group improvements in fatigue severity (p = 0.047) and fatigue at its worst (p = 0.011) and a trend towards improvement in global fatigue (p = 0.058) for the WFPB group.
Acceptability
Intervention participants had positive perceptions of the intervention and provided food. When asked “Do you feel that your health has benefited from the study intervention?”, 19 of 20 intervention participants answered “Yes” (95.0 %, 1 did not give an answer). As shown in Table 4, participants strongly recommended the intervention, reported minimal hunger, and positively reviewed both the amount and taste of provided meals.
Table 4.
Participant perception of intervention (n = 20)
| Mean ± SD | |
|---|---|
| “On a scale from 1 to 10, how strongly would you recommend that other cancer patients be given this type of nutrition and support intervention if they were able and willing to participate?” (1 = “Would not recommend; 10 = “Highly recommend”) | 9.5 ± 1.2 |
| “On a scale of 1 to 10, please rate the taste of the provided food” (1 = “It was hardly edible”; 10 = “Exceptional taste”) | 7.8 ± 1.6 |
| “On a scale of 1 to 10, please rate your overall hunger during the course of this study” (1= “Way too hungry most of the time”; 10= “Satisfied and full most of the time”) | 8.6 ± 2.2 |
| “On the following scale (of 1 to 5), please rate the amount of provided food” (1 = “It was never, enough”; 3 = “Just right”; 5 = “Far too much”) | 4.1 ± 0.7 |
Discussion
The findings of this RCT demonstrate that our WFPB dietary intervention in women being treated for MBC is acceptable and feasible, resulting in significant, large changes in nutrient intakes. Clinically and statistically significant improvements were noted for QOL and treatment-related symptoms including perceived cognitive function, physical and emotional well-being, and fatigue. To our knowledge, this is one of the first dietary intervention RCTs that improved QOL and treatment-related symptoms in women currently receiving anti-neoplastic therapy, as the majority of studies have focused on cancer survivors who completed primary therapy.
Feedback from women in the study was overwhelmingly positive: participants highly recommended the intervention, felt that it had improved their health, and based on participant ratings, taste and hunger were not adherence barriers. Intervention group adherence was excellent for both WFPB diet and weekly visit attendance. The intervention was safe and well tolerated.
The changes in nutrient intake achieved in this intervention are large compared to other dietary interventions in BC survivors. Our study achieved a 25.9 % decrease in energy intake, a 43.0 % decrease in energy from fat, and an 84.6 % increase in dietary fiber grams per day. In the Women’s Healthy Eating and Living (WHEL) randomized trial, the largest dietary changes achieved were a 5.8 % decrease in energy (kilocalories) intake, a 25.6 % decrease in energy from fat, and a 46.4 % increase in dietary fiber from baseline [36]. The Women’s Intervention Nutrition Study (WINS) demonstrated a 13.5 % decrease in energy intake, a 31.4 % decrease in energy from fat, and a 6 % increase in dietary fiber at 12 months [37]. We hypothesize that providing food enhanced dietary adherence and facilitated large nutrient intake changes.
PROs improved across multiple instruments and outcome types, with consistent improvements in overall QOL, physical and emotional well-being, cognitive function, and fatigue. The change in the total FACT-B score within the intervention group surpassed the CID [32, 33], and the between-group difference approached significance. The FACT-B emotional well-being subscale score improved significantly between the groups, surpassing the CID [31]. Between groups, perceived cognitive function improved across three different instruments (FACT-Cog, Symptom Inventory, and EORTC QLQ-C30). Improvements on the total FACT-Cog score surpassed the CID [34, 35]. Improvements in fatigue in the intervention group reached or approached significance on the Symptom Inventory, BFI, and EORTC QLQ-C30.
The large changes to nutrient intakes and subsequent intentional weight loss that occurred over the course of our trial may have positively affected PRO measures and thereby QOL. Participants in the intervention group had lost 6.6 % of their body weight at 8 weeks. Evidence suggests that excess weight negatively affects QOL and that weight loss can improve QOL and cognitive function. Obesity is associated with a lower QOL in both clinically stable ER + MBC patients [14] and general adult populations [38]. In patients undergoing weight loss interventions with obesity but without cancer, improved QOL has been demonstrated status post bariatric surgery but more variably after non-surgical weight loss, possibly due to degree of weight loss [38]. Cognitive function also appears to improve with weight loss in overweight and obese patients without cancer [23]. While weight loss appears to be an important factor, dietary composition itself likely plays a role: despite no significant change in BMI, a pilot study of an isocaloric fatigue reduction diet, rich in produce, whole grains, and omega-3 fatty acid rich foods resulted in a 44 % reduction in fatigue [39]. It is possible that our WFPB dietary intervention improved QOL by facilitating clinically meaningful weight loss in the context of significant changes to dietary composition.
Limitations of this study include its small size, particularly the smaller control group, short duration, and lack of a time and attention control. Given the study limitations, it is not possible to elucidate the exact mechanisms or specific intervention components that produced change, but rather to demonstrate initial feasibility and preliminary results. Strengths include the intensity of the intervention and multiple dietary assessments. Provision of meals and intensive education and follow-up likely enhanced adherence, resulting in large changes in dietary intake.
Conclusion
This is one of the first dietary RCTs to improve disease-specific QOL and treatment-related symptoms in participants receiving treatment for metastatic disease. Our WFPB dietary intervention is both feasible and acceptable; it resulted in large changes in nutrient intake and clinically significant improvements in QOL and symptoms. Given the growing population of patients with MBC and the negative impact of both cancer and ongoing treatment on QOL, these findings are promising. Further study with trials of longer duration and follow-up is warranted to determine sustainability and durability of these benefits.
Acknowledgements
The authors would like to acknowledge Kelly-Jo Koch for her work coordinating a portion of this study and Laurie Taillie for her role in provision of study meals. This work was supported by the Highland Hospital Foundation, with donations from the Ladybug Foundation, T. Colin Campbell Center for Nutrition Studies, and multiple individuals. Support was also provided by US National Institutes of Health (UG1-CA189961).
Abbreviations
- QOL
Quality of life
- MBC
Metastatic breast cancer
- WFPB
Whole food, plant-based
- CI
95 % Confidence interval
- BC
Breast cancer
- CRF
Cancer-related fatigue
- PRO
Patient-reported outcome
- BMI
Body mass index
- SD
Standard deviation
- SE
Standard error
- FACT-Cog
Functional Assessment of Cancer Therapy-Cognitive Function
- Symptom Inventory
M.D. Anderson Cancer Center Symptom Inventory
- FACT-B
Functional Assessment Cancer Therapy-Breast
- BFI
Brief Fatigue Inventory
- EORTC QLQ- C30
European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire
- RCT
Randomized controlled trial
Author contributions
EKC (Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft); TMC (Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing); EC (Data curation; Formal analysis; Methodology; Resources; Writing—review & editing); LMB (Data curation; Formal analysis; Investigation; Project administration; Writing—review & editing); NW (Data curation; Investigation; Methodology; Project administration; Resources; Writing—review & editing); JJG (Data curation; Formal analysis; Writing—review & editing); JF (Conceptualization; Methodology; Writing—review & editing); AH (Resources; Writing—review & editing); MS (Resources; Writing—review & editing); MCJ (Resources; Writing—review & editing); KMM (Funding acquisition; Resources; Writing—review & editing); RGM (Resources; Writing—review & editing); LJP (Conceptualization; Methodology; Project administration; Resources; Supervision; Visualization; Writing—review & editing). All authors read and approved the final manuscript.
Funding
This work was supported by the Highland Hospital Foundation, with donations from the Ladybug Foundation, T. Colin Campbell Center for Nutrition Studies, and multiple individuals. Support was also provided by US National Institutes of Health (UG1-CA189961). Funders had no role in study design; collection, analysis, and interpretation of data, or writing of the report and there were no restrictions regarding the submission of the report for publication. Angle, PLC provided Parsortix testing kits at no charge as well as services related to analysis of Parasortix results.
Data availability
The data underlying this article are available by request at https://gitlab-public.circ.rochester.edu/WFPB-breast-cancer/biomarkers. Email Thomas_campbell@urmc.rochester.edu for access to data and/or materials.
Declarations
Conflict of interest
TMC: Royalties from general interest books about plant-based nutrition (Benbella Books, Penguin Random House) and income from a lifestyle medicine practice, Thomas M. Campbell, MD PLLC; EKC: Conflicts of spouse (TMC); AH: MJH Healthcare Holdings (OncLive), Mediflix (Skipta/Informa); RGM: Consultant for Fujirebio Diagnostics and research funding from Angle, PLC. The rest of the authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the U.S. Common Rule. The study protocol was approved by the University of Rochester Research Subject Review Board (ClinicalTrials.gov identifier: NCT03045289; registration 7 February 2017).
Consent to participant
Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, et al. Cancer statistics. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Caswell-Jin JL, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. 2018 doi: 10.1093/jncics/pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallicchio L, et al. Estimation of the numbers of individuals living with metastatic cancer in the United States. J Natl Cancer Inst. 2022 doi: 10.1093/jnci/djac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Mortensen G, et al. Quality of life and care needs in women with estrogen positive metastatic breast cancer: a qualitative study. Acta Oncol. 2018;57(1):146–151. doi: 10.1080/0284186x.2017.1406141. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins V, et al. Do drugs offering only PFS maintain quality of life sufficiently from a patient's perspective? Results from AVALPROFS (assessing the 'VALue' to patients of progression free survival) study. Supp Care Cancer. 2018;26(11):3941–3949. doi: 10.1007/s00520-018-4273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertz S, et al. Progression-free survival and quality of life in metastatic breast cancer: the patient perspective. Breast. 2022;65:84–90. doi: 10.1016/j.breast.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butow PN, Coates AS, Dunn SM. Psychosocial predictors of survival: metastatic breast cancer. Annal Oncol. 2000;11(4):469–474. doi: 10.1023/A:1008396330433. [DOI] [PubMed] [Google Scholar]
- 8.Kypriotakis G, et al. The longitudinal relationship between quality of life and survival in advanced stage cancer. Psychooncology. 2016;25(2):225–231. doi: 10.1002/pon.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batra A, et al. Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Supp Care Cancer. 2021;29(3):1423–1431. doi: 10.1007/s00520-020-05623-6. [DOI] [PubMed] [Google Scholar]
- 10.Badaoui S, et al. Patient-reported outcomes predict progression-free survival of patients with advanced breast cancer treated with Abemaciclib. Oncologist. 2021;26(7):562–568. doi: 10.1002/onco.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrans CE, et al. Conceptual model of health-related quality of life. J Nurs Scholarsh. 2005;37(4):336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 12.Janelsins MC, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter prospective longitudinal study. J Clin Oncol. 2017;35(5):506–514. doi: 10.1200/jco.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiensch AE, et al. Design of a multinational randomized controlled trial to assess the effects of structured and individualized exercise in patients with metastatic breast cancer on fatigue and quality of life: the EFFECT study. Trials. 2022;23(1):610. doi: 10.1186/s13063-022-06556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheean P, et al. Body composition, serum biomarkers of inflammation and quality of life in clinically stable women with estrogen receptor positive metastatic breast cancer. Nutr Cancer. 2019;71(6):981–991. doi: 10.1080/01635581.2019.1595053. [DOI] [PubMed] [Google Scholar]
- 15.Inglis JE, et al. Excess body weight and cancer-related fatigue, systemic inflammation, and serum lipids in breast cancer survivors. Nutr Cancer. 2021;73(9):1676–1686. doi: 10.1080/01635581.2020.1807574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z, et al. Aging, obesity, and post-therapy cognitive recovery in breast cancer survivors. Oncotarget. 2017;8(7):12364–12373. doi: 10.18632/oncotarget.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemp JR, et al. Cognitive functioning and quality of life following chemotherapy in pre- and peri-menopausal women with breast cancer. Supp Care Cancer. 2018;26(2):575–583. doi: 10.1007/s00520-017-3869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hales CM, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 19.Ligibel JA, et al. Physical activity, weight, and outcomes in patients receiving chemotherapy for metastatic breast cancer (C40502/Alliance) JNCI Cancer Spect. 2021 doi: 10.1093/jncics/pkab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21(2):117–133. doi: 10.1017/s0954422408138732. [DOI] [PubMed] [Google Scholar]
- 21.Eichelmann F, et al. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. 2016;17(11):1067–1079. doi: 10.1111/obr.12439. [DOI] [PubMed] [Google Scholar]
- 22.Onzi GR, et al. Chemobrain in breast cancer: mechanisms, clinical manifestations, and potential interventions. Drug Saf. 2022;45(6):601–621. doi: 10.1007/s40264-022-01182-3. [DOI] [PubMed] [Google Scholar]
- 23.Veronese N, et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;72:87–94. doi: 10.1016/j.neubiorev.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Thong MSY, et al. Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol. 2020;21(2):17. doi: 10.1007/s11864-020-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright N, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. doi: 10.1038/nutd.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnard ND, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89(5):1588s–1596s. doi: 10.3945/ajcn.2009.26736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dod HS, et al. Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am J Cardiol. 2010;105(3):362–367. doi: 10.1016/j.amjcard.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Kroes M, et al. Impact of weight change on quality of life in adults with overweight/obesity in the United States: a systematic review. Curr Med Res Opin. 2016;32(3):485–508. doi: 10.1185/03007995.2015.1128403. [DOI] [PubMed] [Google Scholar]
- 29.Sheean P, et al. Exploring diet, physical activity, and quality of life in females with metastatic breast cancer: a pilot study to support future intervention. J Acad Nutr Diet. 2015;115(10):1690–1698. doi: 10.1016/j.jand.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell TM, et al. A whole-food, plant-based randomized controlled trial in metastatic breast cancer: weight, cardiometabolic, and hormonal outcome. Breast Cancer Res Treat. 2024 doi: 10.1007/s10549-024-07266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11(3):207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. J Pain Symp Manage. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 33.Eton DT, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Bell ML, et al. Important differences and meaningful changes for the functional assessment of cancer therapy-cognitive function (FACT-Cog) J Patient-Rep Outcomes. 2018;2(1):48. doi: 10.1186/s41687-018-0071-4. [DOI] [Google Scholar]
- 35.Cheung YT, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67(7):811–820. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Pierce JP, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the women's healthy eating and living (WHEL) randomized trial. JAMA J Am Med Assoc. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chlebowski RT, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the women's intervention nutrition study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 38.Ul-Haq Z, et al. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity (Silver Spring) 2013;21(3):E322–E327. doi: 10.1002/oby.20107. [DOI] [PubMed] [Google Scholar]
- 39.Zick SM, et al. Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat. 2017;161(2):299–310. doi: 10.1007/s10549-016-4070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available by request at https://gitlab-public.circ.rochester.edu/WFPB-breast-cancer/biomarkers. Email Thomas_campbell@urmc.rochester.edu for access to data and/or materials.


