Abstract
Purpose
To investigate the prognostic significance of lymphovascular invasion in invasive breast cancer and the value of using specific vascular endothelial markers to further classify lymphovascular invasion.
Methods
We collected 2124 patients with invasive breast cancer who were hospitalized at the First Hospital of Dalian Medical University from 2012 to 2020. Statistical methods were used to investigate the relationship between lymphovascular invasion and clinicopathological characteristics of breast cancer, and the correlation between lymphovascular invasion on overall survival (OS) and disease-free survival (DFS) of various categories of breast cancers. Immunohistochemical staining of breast cancer samples containing lymphovascular invasion using specific vascular endothelial markers D2-40 and CD34 was used to classify lymphovascular invasion and to investigate the relationship between lymphovascular invasion and breast cancer progression.
Results
There was a high correlation between lymphovascular invasion and T stage, N stage and nerve invasion. Survival analyses showed that patients with lymphovascular invasion, especially luminal B, triple-negative, and Her-2 overexpression breast cancer patients, had poorer OS and DFS prognosis, and that lymphovascular invasion was an independent prognostic factor affecting OS and DFS in breast cancer. The immunohistochemical staining results showed that positive D2-40 staining of lymphovascular invasion was linked to the N stage and localized recurrence of breast cancer.
Conclusion
Lymphovascular invasion is associated with aggressive clinicopathological features and is an independent poor prognostic factor in invasive breast cancer. Breast cancer localized recurrence rate and lymph node metastases are influenced by lymphatic vessel invasion. Immunohistochemical techniques should be added to the routine diagnosis of lymphovascular invasion.
Keywords: Breast cancer, Lymphovascular invasion, Prognosis, Specific vascular endothelial marker
Introduction
Based on the worldwide cancer data released by CA Cancer J Clin 2021[1], breast cancer is the most prevalent malignant tumor worldwide at the moment. According to the data, breast cancer accounts for 15.5 percent of cancer mortality in women, which is higher than other types of cancers such as lung, colorectal, and cervical cancer. The diagnosis and treatment of breast cancer have evolved as we approach the era of precision therapy. An exact disease risk assessment is essential to creating a tailored breast cancer therapy regimen. However, conventional predictive risk markers like TNM staging and tumor histological grading have not demonstrated high clinical utility in cases of early-stage breast cancer. Therefore, they are no longer the sole criteria for prognostic assessment and therapeutic targets for breast cancer [2].
Lymphovascular invasion (LVI), an important component of the tumor microenvironment, has previously been limited by the limitations of detection techniques. The most common method for testing for LVI in prior research has been hematoxylin–eosin (H&E) staining. However, as immunohistochemistry (IHC) technology has advanced, the objective detection rate of LVI in breast cancer has been further enhanced by the combination of IHC detection with H&E staining [3], which has advanced LVI research. There is disagreement concerning the findings of earlier research on LVI. Lee et al. [4] concluded that LVI is an independent factor for poor prognosis in patients with early-stage breast cancer, regardless of lymph node status and molecular subtype. A study by Houvenaeghel et al. [5] showed that the presence of LVI had an independent negative impact on the prognosis of patients with early-stage breast cancer, except for ER-positive grade 3 tumors and luminal A tumors treated with adjuvant chemotherapy. However, Bent et al. [6] concluded that LVI could not be used as an independent risk factor for breast cancer and was insufficient to move patients from a low to a high risk of recurrence. Munzone et al. [7] discovered that in patients with lymph node-positive breast cancer, LVI had no effect on the overall prognosis but did increase the probability of localized recurrence. Meanwhile, other researchers have focused their research on the influence of LVI on the selection of breast cancer therapy strategies. Zhong et al. [8] demonstrated that patients with early-stage LVI positive breast cancer who undergo breast-conserving surgery have a worse prognosis than patients with LVI negative breast cancer. They concluded that these patients should receive extensive systemic therapy in addition to a mastectomy as part of their treatment. The American Joint Committee on Cancer (AJCC), a reputable prognostic rating method, does not include LVI based on the outcomes of currently available research. Consequently, additional research is still required to confirm the predictive significance of LVI for breast cancer.
Pathologists have employed endothelial cells with various molecular characteristics in recent years to further objectively and standardly define LVI using IHC technology and specific vascular endothelial markers. The growing recognition of the significance of lymphatic endothelial cells in tumor progression and metastasis has been made possible by the development of markers for these cells [9]. On the other hand, since the principle of anti-angiogenic therapy for solid tumors was proposed in 1972 [10], targeted anti-angiogenic drugs combined with conventional chemotherapy and radiotherapy have positively influenced the treatment of tumors as an anti-tumor therapeutic strategy and great breakthroughs have been made in the study of breast cancer-related angiogenesis. Many vascular endothelial markers specific to breast cancer have emerged, and some of these markers have been gradually employed to help diagnose breast cancer-related LVI, improving upon the limitations of conventional H&E staining in this regard. Therefore, researching cancer-related lymphatic and blood vessels using endothelial markers and IHC technology can help to further explore the role that LVI plays in the progression of breast cancer.
In this study, to determine if LVI can be utilized in addition to conventional clinicopathological criteria to help determine the prognosis of breast cancer, we analyzed the association between LVI and clinicopathological characteristics and prognosis in invasive breast cancer. Simultaneously, we labeled LVI in breast cancer using specific vascular endothelial markers to investigate the mechanism of LVI development. The objectives of our research were to investigate the predictive significance of LVI in breast cancer and to offer a theoretical framework for the development of appropriate therapeutic options.
Materials and methods
Data collection and processing
We collected a total of 2479 invasive breast cancer cases hospitalized in the Department of Breast Surgery of the First Hospital of Dalian Medical University from January 2012 to December 2020. These patients were subsequently screened using the following inclusion and exclusion criteria. Inclusion criteria: (1) patients who were hospitalized for the first time for radical breast cancer surgery with postoperative pathological confirmation of invasive breast cancer; (2) complete clinicopathological and follow-up data. Exclusion criteria: (1) patients with adjuvant treatment such as neoadjuvant chemotherapy before surgery; (2) special types of breast cancer such as inflammatory breast cancer and Paget's disease of the breast; (3) Stage IV breast cancer; (4) bilateral invasive breast cancer; (5) patients who could not undergo radical mastectomy for breast cancer due to poorer physical status, etc.; (6) presence of a history of breast tumor or other types of tumors; and (7) missing clinicopathological and follow-up data. Finally, 2124 eligible cases were screened, of which 397 cases showed the presence of LVI by H&E staining. The clinicopathological characteristics of the enrolled cases are shown in Table 1.
Table 1.
Baseline information table for breast cancer patients
| Characteristics | Levels | Amount(n = 2124) | Percentage(%) |
|---|---|---|---|
| LVI | Positive | 397 | 18.7 |
| Negative | 1727 | 81.3 | |
| Age | < 40 | 194 | 9.1 |
| ≥ 40 | 1930 | 90.9 | |
| Anatomic neoplasm subdivisions | Left | 1099 | 51.7 |
| Right | 1025 | 48.3 | |
| Menopause status | Premenopausal | 800 | 37.7 |
| Postmenopausal | 1291 | 60.8 | |
| Hysterectomy | 33 | 1.5 | |
| Histological type | Infiltrating ductal carcinoma | 1969 | 92.7 |
| Infiltrating lobular carcinoma | 60 | 2.8 | |
| Other types | 95 | 4.5 | |
| Histological grade | I | 49 | 2.3 |
| II | 1030 | 48.5 | |
| III | 917 | 43.2 | |
| Not available | 128 | 6.0 | |
| T stage | T1 | 1182 | 55.6 |
| T2 | 858 | 40.4 | |
| T3 | 61 | 2.9 | |
| T4 | 23 | 1.1 | |
| N stage | N0 | 1253 | 59.0 |
| N1 | 560 | 26.4 | |
| N2 | 176 | 8.3 | |
| N3 | 135 | 6.3 | |
| PAM50 | Luminal A | 404 | 19.0 |
| Luminal B | 1289 | 60.7 | |
| Triple-negative | 261 | 12.3 | |
| Her-2 overexpressing | 170 | 8.0 | |
| ER status | Negative | 471 | 22.2 |
| Weakly positive | 74 | 3.5 | |
| Positive | 1579 | 74.3 | |
| PR status | Negative | 593 | 27.9 |
| Weakly positive | 186 | 8.8 | |
| Positive | 1345 | 63.3 | |
| Ki67 status | ≤ 14% | 496 | 23.4 |
| > 14% | 1628 | 76.6 | |
| HER-2 status | Negative | 1673 | 78.8 |
| Positive | 451 | 21.2 | |
| Pathologic stage | Stage I | 841 | 39.6 |
| Stage II | 948 | 44.6 | |
| Stage III | 335 | 15.8 | |
| Neurological invasion | Positive | 212 | 10.0 |
| Negative | 1912 | 90.0 | |
| Soft tissue invasion | Positive | 73 | 3.4 |
| Negative | 2051 | 96.6 |
Assessment of LVI
According to the Chinese guidelines for the diagnosis and treatment of breast cancer, the presence or absence of LVI in the lesions of breast cancer patients should be routinely included in the postoperative pathology reports after they have undergone the appropriate radical mastectomy for breast cancer. The final diagnosis of LVI in all the cases in this study was based on the official pathology reports published by the Department of Pathology of the First Affiliated Hospital of Dalian Medical University. The pathological evaluation of LVI in breast cancer is as follows: using H&E staining, LVI is diagnosed if there are clusters of tumor cells in the lymphovascular lumen (including lymphatic lumen or vascular lumen) within 1 mm around the tumor, and it should be noted that due to the fixation of tissues, H&E staining is unable to differentiate between LVI and contractile space. Therefore, in the few cases where it is not possible to confirm whether the lumen surrounding the tumor is a lymphovascular lumen, additional immunohistochemical staining should be performed to assist in the diagnosis of this indistinguishable pathological tissue.
Pathological sample collection
245 paraffin-embedded breast cancer tissues from January 2016 to December 2020 were collected by the Department of Pathology of the First Hospital of Dalian Medical University. Under H&E staining, these tissues previously showed the existence of LVI.
Immunohistochemistry
Immunohistochemical staining using the Envision [11] technique as a guide. The following are the precise steps: Breast cancer tissues were fixed in 10% formalin, paraffin-embedded, and sectioned to 4-6 μm on slides. After dewaxing, hydration, and microwave antigen repair of the slides, an endogenous peroxidase blocker (3% hydrogen peroxide) was added. Slides were incubated overnight at 4 °C with the D2-40/CD34 antibody. Subsequently, the slides were incubated with the secondary antibody for 20 min at room temperature. DAB chromogenic stain, then hematoxylin re-staining, hydrochloric acid alcohol differentiation, and return to blue. Finally, the sections were dehydrated, transparent, and sealed. The stained sections were observed and the results were interpreted under a light microscope. The discriminatory criterion for D2-40/CD34 positivity is to specify the presence of positive staining (brown-yellow staining) of vascular endothelial cells. Under the microscope, vascular endothelial cells in the lumen of a small vessel (lymphatic lumen or vascular lumen) surrounding the LVI tissue are positive for D2-40/CD34 if they show brown-yellow staining, and negative for D2-40/CD34 if they do not show brown-yellow staining. All the experimental reagents involved in the immunohistochemical staining process were purchased from ZSGB-BIO.
Follow-up visit
Two follow-up visits were conducted in this study, and all cases were followed up by telephone or outpatient review. The first follow-up: November 2021. Follow-up target: all cases meeting the enrollment criteria. Second follow-up: November 2023. Follow-up target: 245 breast cancer patients corresponding to the collected pathological samples. In this study, overall survival (OS) and disease-free survival (DFS) were used as indicators to assess the prognosis of breast cancer patients.
Survival analysis
Survival analyses were performed using the Kaplan–Meier method and log-rank test, with the cut-off value set in the presence of LVI. Univariate and multivariate Cox regression analyses were used to assess the effect of clinical variables on patients' OS and DFS. Prognostic variables with P < 0.05 in the univariate Cox regression analysis were included in the multivariate Cox regression analysis. Forest plots were visualized using the R package ggplot2 [12].
Statistical analysis
The xiantao platform (https://www.xiantao.love), built on R, was used to perform the statistical analyses. All statistical analyses were performed using R (version 4.2.1). The correlation between LVI and clinicopathological factors was analyzed using the chi-square test, and factors satisfying P < 0.05 in the chi-square test were included in the multivariate logistic regression. The correlations between D2-40 staining of LVI and N stage, localized recurrence of breast cancer, as well as the correlation between CD34 staining and organ metastasis of breast cancer, were examined using the chi-square test. All tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Correlation between LVI and clinicopathological factors
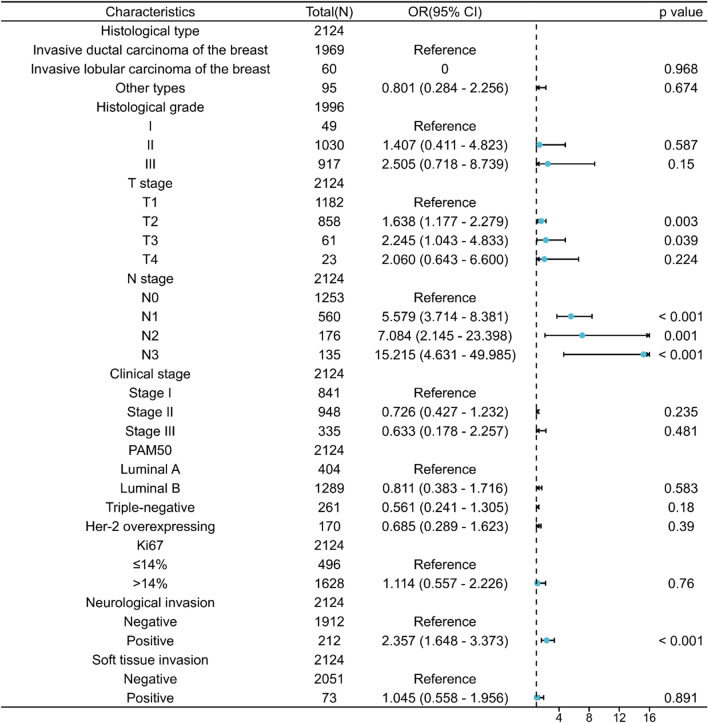
Univariate analysis showed that LVI in breast cancer was associated with T stage, N stage, histological type, histological grade, pathological molecular type, Ki-67 status, pathologic stage, neurological invasion, and soft tissue invasion (P < 0.05). LVI was not associated with anatomic neoplasm subdivisions, menopause status, age, ER status, PR status, and Her-2 status (Table 2). A multivariate logistic regression model and forest map based on the association of LVI showed that LVI was strongly associated with T stage, N stage, and neurological invasion (P < 0.05) (Fig. 1).
Table 2.
Clinicopathological characteristics of LVI positive and LVI negative expression groups
| Characteristics | Levels | LVI positive | LVI negative | P value |
|---|---|---|---|---|
| Anatomic neoplasm subdivisions, n (%) | Right | 205(9.7%) | 820(38.6%) | 0.135 |
| Left | 192(9%) | 907(42.7%) | ||
| Age, n (%) | ≥ 40 | 357(16.8%) | 1573(74.1%) | 0.470 |
| < 40 | 40(1.9%) | 154(7.3%) | ||
| Menopause status, n (%) | Postmenopausal | 244(11.5%) | 1047(49.3%) | 0.849 |
| Premenopausal | 148(7%) | 652(30.7%) | ||
| Hysterectomy | 5(0.2%) | 28(1.3%) | ||
| Histological type, n (%) | Infiltrating ductal carcinoma | 377(17.7%) | 1592(75%) | 0.003 |
| Infiltrating lobular carcinoma | 1(0%) | 59(2.8%) | ||
| Other types | 19(0.9%) | 76(3.6%) | ||
| Histological grade, n (%) | I | 3(0.2%) | 46(2.3%) | < 0.001 |
| II | 153(7.7%) | 877(43.9%) | ||
| III | 223(11.2%) | 694(34.8%) | ||
| T stage, n (%) | T1 | 148(7%) | 1034(48.7%) | < 0.001 |
| T2 | 212(10%) | 646(30.4%) | ||
| T3 | 26(1.2%) | 35(1.6%) | ||
| T4 | 11(0.5%) | 12(0.6%) | ||
| N stage, n (%) | N0 | 92(4.3%) | 1161(54.7%) | < 0.001 |
| N1 | 167(7.9%) | 393(18.5%) | ||
| N2 | 62(2.9%) | 114(5.4%) | ||
| N3 | 76(3.6%) | 59(2.8%) | ||
| Pathologic stage, n (%) | Stage I | 60(2.8%) | 781(36.8%) | < 0.001 |
| Stage II | 189(8.9%) | 759(35.7%) | ||
| Stage III | 148(7%) | 187(8.8%) | ||
| PAM50, n (%) | Luminal A | 52(2.4%) | 352(16.6%) | 0.005 |
| Luminal B | 263(12.4%) | 1026(48.3%) | ||
| Triple-negative | 45(2.1%) | 216(10.2%) | ||
| Her-2 overexpressing | 37(1.7%) | 133(6.3%) | ||
| ER status, n (%) | Positive | 288(13.6%) | 1291(60.8%) | 0.442 |
| Negative | 97(4.6%) | 374(17.6%) | ||
| Weakly positive | 12(0.6%) | 62(2.9%) | ||
| PR status, n (%) | Positive | 246(11.6%) | 1099(51.7%) | 0.823 |
| Negative | 115(5.4%) | 478(22.5%) | ||
| Weakly positive | 36(1.7%) | 150(7.1%) | ||
| Ki67 status, n (%) | ≤ 14% | 66(3.1%) | 430(20.2%) | < 0.001 |
| > 14% | 331(15.6%) | 1297(61.1%) | ||
| HER-2 status, n (%) | Positive | 98(4.6%) | 353(16.6%) | 0.062 |
| Negative | 299(14.1%) | 1374(64.7%) | ||
| Neurological invasion, n (%) | Positive | 78(3.7%) | 134(6.3%) | < 0.001 |
| Negative | 319(15%) | 1593(75%) | ||
| Soft tissue invasion, n (%) | Positive | 30(1.4%) | 43(2%) | < 0.001 |
| Negative | 367(17.3%) | 1684(79.3%) |
Fig. 1.
Forest map based on logistic models associated with LVI
Prognostic value of LVI in breast cancer
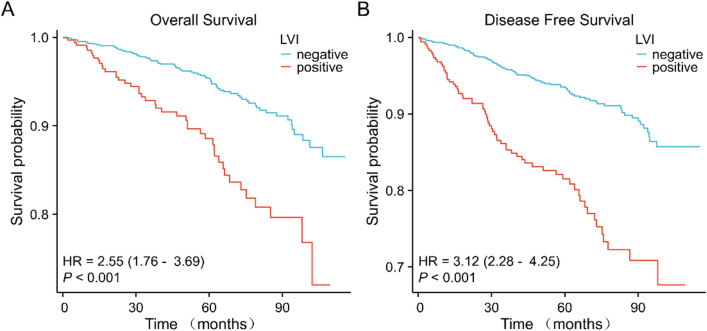
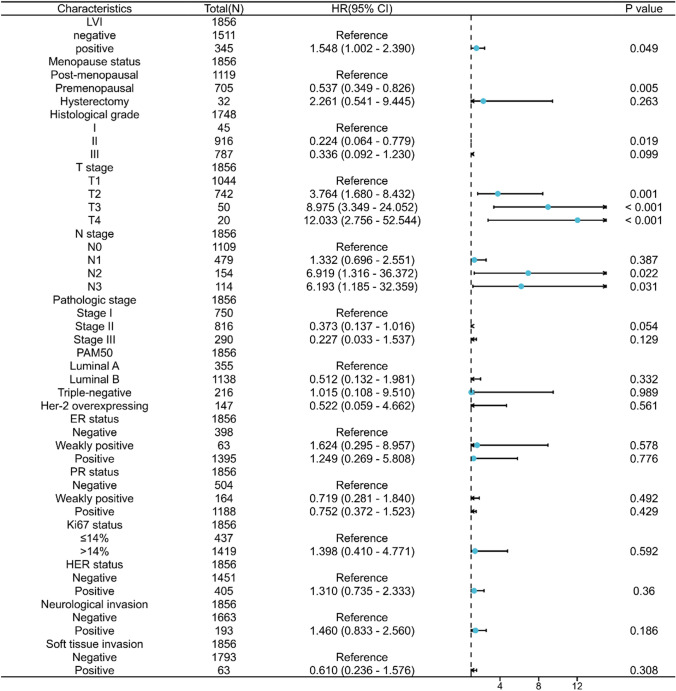
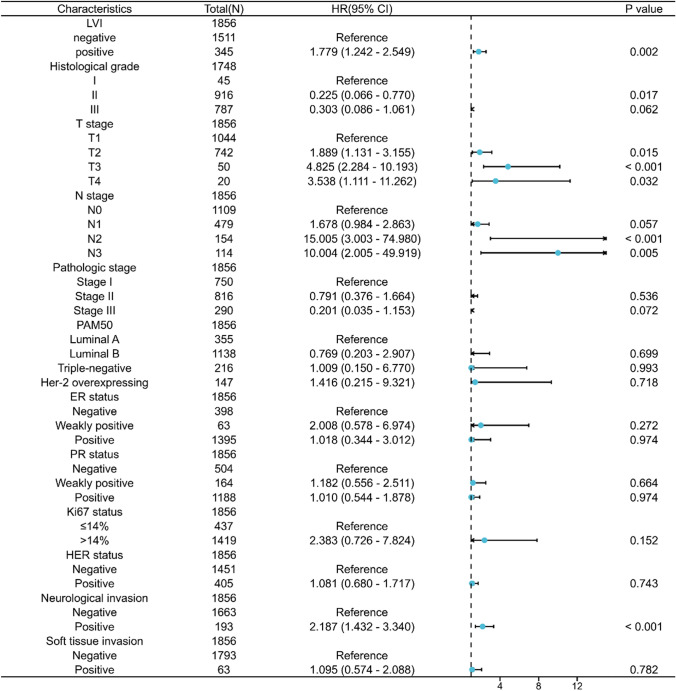
Survival analyses showed that patients with LVI had a worse prognosis for both OS and DFS compared with patients without LVI (OS: hazard ratio [HR] = 2.55, 95%CI = 1.76–3.69, P < 0.001; DFS: HR = 3.12, 95% CI = 2.28–4.25, P < 0.001) (Fig. 2A, B). The results of multivariate Cox regression analysis showed that LVI was an independent prognostic factor affecting OS and DFS in invasive breast cancer (OS: adjusted HR = 1.548, 95% CI = 1.002–2.390, P = 0.049; DFS: adjusted HR = 1.779, 95% CI = 1.242–2.549, P = 0.002) (Fig. 3, Fig. 4).
Fig. 2.
Prognostic values of LVI in patients with breast cancer evaluated by the Kaplan–Meier method. Overall survival (A) and disease-specific survival (B) for breast cancer patients with or without LVI
Fig. 3.
Forest map based on multivariate Cox analysis for overall survival
Fig. 4.
Forest map based on multivariate Cox analysis for disease-free survival
Prognostic value of LVI in different subgroups of breast cancer
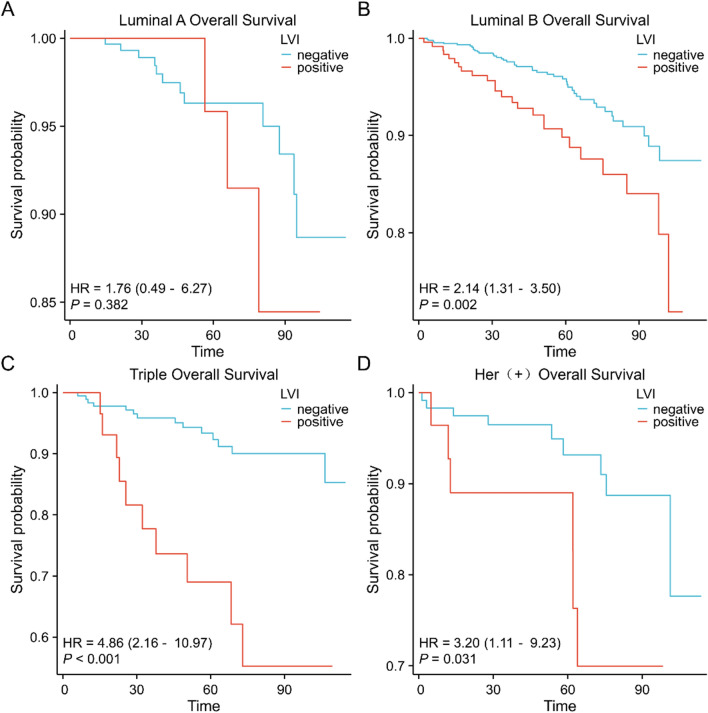
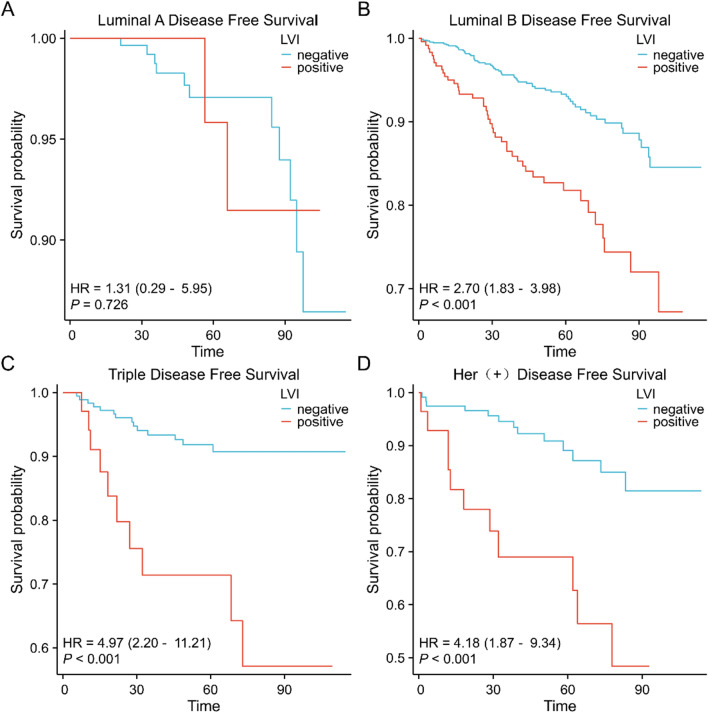
Survival analyses in different subgroups of breast cancer showed that patients with LVI had a worse OS and DFS prognosis in luminal B, triple-negative, and Her-2 overexpressing breast cancers. In luminal A breast cancer, the difference in OS and DFS prognosis between patients with LVI and controls was not statistically significant (Figs. 5A–D, 6A–D).
Fig. 5.
Prognostic value of LVI on overall survival in different subgroups of breast cancer evaluated by the Kaplan–Meier method A. luminal A breast cancer B. luminal B breast cancer C. triple-negative breast cancer D. Her-2 overexpressing breast cancer
Fig. 6.
Prognostic value of LVI on disease-free survival in different subgroups of breast cancer evaluated by the Kaplan–Meier method A. luminal A breast cancer B. luminal B breast cancer C. triple-negative breast cancer D. Her-2 overexpressing breast cancer
The value of specific vascular endothelial markers
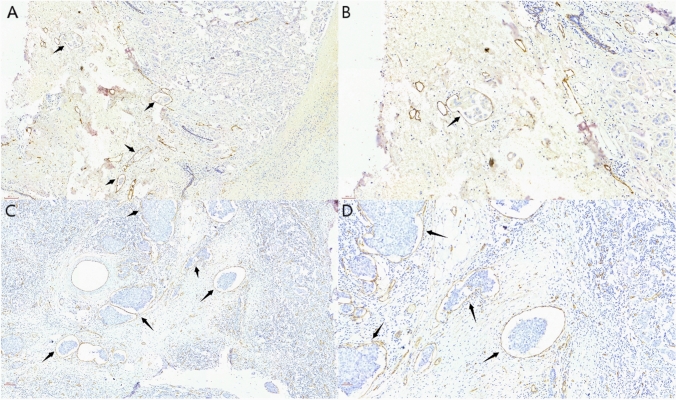
We used D2-40 versus CD34 for immunohistochemical labeling of each of the 245 paraffin-embedded breast cancer tissues. Of these tissues, 68 had positive staining for D2-40, while 50 had positive staining for CD34. Representative images are shown in Fig. 7 (Fig. 7A–D). Chi-square analysis results indicated that positive D2-40 staining of LVI was linked to both N stage and localized recurrence of breast cancer. On the other hand, there was no correlation found between positive CD34 staining of LVI and organ metastases of breast cancer (Table 3, 4).
Fig. 7.
Immunohistochemical staining of D2-40 and CD34 of LVI in breast cancer (arrowed) A. D2-40 staining of LVI at 10× B. D2-40 staining of LVI at 20× C. CD34 staining of LVI at 10× D. CD34 staining of LVI at 20x
Table 3.
Correlations of D2-40 staining of LVI with N stage and localized recurrence of breast cancer
| Characteristics | Negative expression of D2-40 | Positive expression of D2-40 | P value |
|---|---|---|---|
| N stage, n (%) | 0.013 | ||
| N0 | 45 (18.4%) | 11 (4.5%) | |
| N1 | 79 (32.2%) | 28 (11.4%) | |
| N2 | 31 (12.7%) | 9 (3.7%) | |
| N3 | 22 (9%) | 20 (8.2%) | |
| Localized recurrence, n (%) | 0.034 | ||
| Normal | 166 (67.8%) | 58 (23.7%) | |
| Recurrence | 11 (4.5%) | 10 (4.1%) |
Table 4.
Correlation of CD34 staining of LVI with organ metastasis of breast cancer
| Characteristics | Negative expression of CD34 | Positive expression of CD34 | P value |
|---|---|---|---|
| Tumor organ metastases, n (%) | 0.234 | ||
| Normal | 167 (68.2%) | 46 (18.8%) | |
| Metastasis | 28 (11.4%) | 4 (1.6%) |
Discussion
Clinicopathological characteristics that predict the prognostic risk of cancer are responsible for the improved survival results that breast cancer patients experience under the present therapy paradigm. Through effective targeting, clinicians are provided with appropriate therapeutic approaches for patients with breast cancer in various risk groups. Precision medicine is now the standard of care for breast cancer patients, and creating effective treatment regimens requires accurate risk classification of the illness. Conventional prognostic risk indicators, like TNM staging and histological grading, are well known and used in reputable prognostic evaluation systems for breast cancer, like the AJCC [13]. However, these factors did not show high prognostic value in specific categories of breast cancer. This suggests that current staging systems are still insufficient to accurately predict prognosis and accurately reflect the biological heterogeneity of breast cancer [14, 15].
The essential condition for the systematic spread of breast cancer cells to other regions is that they must first penetrate and spread throughout the lymphovascular system [16]. Tumor cells in the endothelial lining space of lymphatic vessels or blood vessels surrounding the primary tumor might enter adjacent lymphatic vessels or blood vessels and bind to one another, forming a tumor embolism known as LVI [17]. The College of American Pathologists recommends assessing and reporting LVI in all cancer protocols, which is the gold standard for cancer reporting [18]. Though its predictive utility in breast cancer is still debatable, LVI certainly plays a role in treatment selection for breast cancer. For patients with LVI, the St. Gallen International Consensus Guidelines recommend whole-breast irradiation as opposed to localized irradiation [19]. According to recent investigations, the 21-gene recurrence score can be reliably determined by detecting LVI [20]. Consequently, knowing how LVI affects breast cancer patient survival and the progression of the disease may lead to new therapeutic and diagnostic approaches that will enhance the prognosis of breast cancer patients.
In this study, we collected data from approximately two thousand invasive breast cancer patients who had therapy at our medical center during the previous nine years. Consistent with earlier studies [21], statistical analysis revealed that LVI was linked to detrimental clinicopathological characteristics, such as T stage, N stage, and neurological invasion. Tumor size and lymph node metastasis of breast cancer are intuitive bases for predicting cancer recurrence and metastasis, both of which are closely related to the degree of differentiation of the tumor cells and the invasive ability of the cancer. Our study's findings indicate that even in the absence of lymph node metastases, breast cancer patients with LVI are at risk for recurrence, so it's important to evaluate carefully whether postoperative adjuvant radiation therapy should be used in these cases following the recommended course of treatment for lymph node metastases.
Key indicators for evaluating the prognosis of patients are the overall and disease-free survival rates for breast cancer. In our study, breast cancer patients with LVI had worse OS and DFS prognosis. LVI predicts a poor prognosis in all subtypes of breast cancer, except luminal A. LVI, T stage, N stage, and menstrual status were independent predictive biomarkers of poor OS and DFS in patients with invasive breast cancer, according to survival analysis. Our research indicates that, when it comes to determining the predictive risk of breast cancer, LVI is not less useful than conventional clinicopathological factors such as tumor size and lymph node metastatic status. As a result, when formulating treatment plans, physicians shouldn't ignore the prognostic significance of LVI in breast cancer. In the meanwhile, patients having LVI for breast cancer may have an increased chance of developing recurring metastases. These individuals ought to be categorized as having a medium or high risk of developing new cancer metastases, and they ought to be the target of appropriate adjuvant therapy plans.
Malignant tumor cells extrude the junction of lymphatic endothelial cells (LEC) and move along the LEC to the subsequent stop lymph nodes and organs during lymphatic metastasis of breast cancer [9]. Tumor cells use LEC to facilitate malignancy invasion within the tumor microenvironment [22]. Kahn et al. [23] identified the monoclonal antibody D2-40 as a lymphatic vessel endothelial marker in 2002. When it comes to assessing lymphatic vessel invasion, D2-40 outperforms H&E staining in two ways: first, it can identify tumor emboli that are obstructing the lymphatic vessel lumen; second, it can distinguish between tumor aggregates because of tissue contraction during fixation and retraction artifacts that separate tumor emboli in the lymphatic vessel space. D2-40 enhanced the detection of lymphatic vessel invasion in primary tumors, according to Kahn's study. Debald et al. [24] recommended routine D2-40 immunohistochemical staining to improve recognition of lymphatic vessel invasion. In this study, we used D2-40 immunohistochemical staining to mark breast cancer tissue showing LVI under H&E staining. According to the findings, patients with positive D2-40 staining predicted a higher risk of N-stage and localized recurrence of the tumor. Since positive D2-40 staining suggests the presence of lymphatic vessel invasion, it is reasonable to assume that lymphatic vessel invasion, rather than blood vessel invasion (BVI), is a relevant risk factor for promoting lymph node metastasis and localized recurrence of breast cancer. This offers novel perspectives on how lymphatic vessel invasion affects the progression of breast cancer development. Therefore, we believe that D2-40 could be added to the diagnostic procedures for breast cancer LVI as a complement to H&E staining.
Tumor-associated angiogenesis is one of the mechanisms that contribute to the high degree of invasiveness of breast cancer as it advances. According to Modi et al. [25], there is an important connection between inflammatory breast cancer and the dermal vasculature. They also proposed that the extent of dermal vascular involvement be clarified. Lin et al. [26] concluded that BVI is an independent predictor of poor prognosis in operable breast cancer and is associated with aggressive clinicopathological features. CD34 is a currently recognized sensitive marker of tumor neovascular endothelium, which is present in the endothelial cells of microvessels [27]. In this study, we attempted to utilize CD34 IHC staining to identify LVI in breast cancer. The results of this research, however, did not support the hypothesis that the presence of tumor cells in the bloodstream causes organ metastases in breast cancer. The low sample size of the research and the restriction on the BVI detection rate by CD34 are potential causes of this. Consequently, more research is still required to confirm the correlation between vascular invasion and breast cancer.
Although our study provides novel insights into the predictive significance of LVI in breast cancer, there are limitations that must be taken into account. First off, selection bias could have occurred because all of the cases in this study originated from only one therapy center. Second, even though all study participants received the recommended course of treatments under the guidance and supervision of medical professionals and in accordance with breast cancer management guidelines, we cannot completely rule out the possibility that some patients' failure to comply with the recommended course of treatment due to the side effects of adjuvant therapy may have influenced the study's findings. Finally, the irregular structure of LVI led to a false-negative rate when we used D2-40 and CD34 for specific labeling of LVI in breast cancer. This was because it was impossible to ensure complete labeling of each LVI category during the sampling and staining process. Therefore, deeper investigations may be required to demonstrate the effect of LVI on the prognosis of breast cancer and the significance of specific vascular endothelial markers in research.
Conclusions
According to this study, LVI is an independent poor prognostic factor in invasive breast cancer and is associated with aggressive clinicopathological characteristics. Furthermore, the metastasis of lymph nodes and the localized recurrence rate of breast cancer are influenced by lymphatic vessel invasion. Immunohistochemical staining for specific vascular endothelial markers can help to classify LVI and advance further research on LVI in breast cancer. According to our findings, LVI is an effective biomarker for predicting the prognosis of breast cancer. Nevertheless, further study is required to clarify and evaluate the mechanisms via which LVI regulates the development and progression of breast cancer.
Acknowledgements
We acknowledge ZSGB-BIO for providing the experimental reagents and instruments. We acknowledge the website of the xiantao platform (https://www.xiantao.love) for the help in data analysis.
Abbreviations
- LVI
Lymphovascular invasion
- H&E
Hematoxylin–eosin
- IHC
Immunohistochemistry
- OS
Overall survival
- DFS
Disease-free survival
- AJCC
American Joint Committee on Cancer
- OR
Odds ratio
- HR
Hazard ratio
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- LEC
Lymphatic endothelial cells
- BVI
Blood vessel invasion
Author contributions
Yuyang Zhang: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing—Original Draft; Huali Wang: Conceptualization, Data Curation, Formal Analysis, Validation, Visualization, Writing—Original Draft; Huahui Zhao: Formal Analysis, Investigation; Xueming He: Data Curation, Investigation; Ya Wang: Conceptualization, Project Administration, Resources, Supervision, Validation, Writing—Review & Editing; Hongjiang Wang: Conceptualization, Funding Acquisition, Project Administration, Resources, Supervision, Validation, Writing—Review & Editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81572582).
Data availability
All data generated or analyzed in this study are included in the article and further inquiries can be directed to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare that they have no competing financial interests or personal nature that could be perceived to influence the work reported in this paper.
Ethics approval
Studies involving human participants, human material, or human data were conducted under the relevant guidelines and provisions of the Declaration of Helsinki. The study was approved by the Medical Ethics Committee of the First Hospital of Dalian Medical University on 12 January 2023 (approval number: PJ-KS-KY-2023–28).
Consent to participation
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuyang Zhang and Huali Wang are co-first authors, and contributed equally to this work.
Contributor Information
Ya Wang, Email: 13478616227@163.com.
Hongjiang Wang, Email: wanghj2001@outlook.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clinicians. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Katerji H, Zhang H, Hicks DG, Turner BM. The evolution of prognostic and predictive markers in breast cancer. Chin J New Clin Med. 2021;14:1169–1181. doi: 10.3969/j.issn.1674-3806.2021.12.03. [DOI] [Google Scholar]
- 3.Gujam FJA, Going JJ, Edwards J, Mohammed ZMA, Mcmillan DC. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol. 2014;89:231–241. doi: 10.1016/j.critrevonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Go J, Ahn B, Ahn J, Kim J, Park H, Kim S, Park B, Park S. Lymphovascular invasion is an independent prognostic factor in breast cancer irrespective of axillary node metastasis and molecular subtypes. Front Oncol. 2023;13:1269971. doi: 10.3389/fonc.2023.1269971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houvenaeghel G, Cohen M, Classe JM, Reyal F, Mazouni C, Chopin N, Martinez A, Daraï E, Coutant C, Colombo PE, Gimbergues P, Chauvet MP, Azuar AS, Rouzier R. ESMO Open. 2021;6:100316. doi: 10.1016/j.esmoop.2021.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bent E, Maj-Britt J, Fritz R, Rasmussen BB, Peer C, Niels K, Kvistgaard ME, Marie O, Toftdahl DB, Mouridsen HT. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst. 2010 doi: 10.1093/jnci/djp090. [DOI] [PubMed] [Google Scholar]
- 7.Munzone E, Bagnardi V, Rotmensz N, Sporchia A, Mazza M, Pruneri G, Intra M, Sciandivasci A, Gentilini O, Luini A. Prognostic relevance of peritumoral vascular invasion in immunohistochemically defined subtypes of node-positive breast cancer. Breast Cancer Res Treat. 2014;146:573–582. doi: 10.1007/s10549-014-3043-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Y, Tong F, Shen J. Lympho-vascular invasion impacts the prognosis in breast-conserving surgery: a systematic review and meta-analysis. BMC Cancer. 2022;22:102. doi: 10.1186/s12885-022-09193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He M, He Q, Cai X, Chen Z, Lao S, Deng H, Liu X, Zheng Y, Liu X, Liu J, Xie Z, Yao M, Liang W, He J. Role of lymphatic endothelial cells in the tumor microenvironment-a narrative review of recent advances. Trans Lung Cancer Res. 2021;10:2252–2277. doi: 10.21037/tlcr-21-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Abd A, Alamoudi A, Abdel-Naim A, Neamatallah T, Ashour O. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies—a review. J Adv Res. 2017;8:591–605. doi: 10.1016/j.jare.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G, Pileri S. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickham H. Ggplot2: elegant graphics for data analysis. Cham: Springer Publishing Company; 2009. [Google Scholar]
- 13.Teichgraeber D, Guirguis M, Whitman G. AJCC cancer staging manualbreast cancer staging: updates in the, 8th edition, and current challenges for radiologists, from the special series on cancer staging. AJR Am J Roentgenol. 2021;217:278–290. doi: 10.2214/AJR.20.25223. [DOI] [PubMed] [Google Scholar]
- 14.Deng M, Xiong C, He Z, Bin Q, Song J, Li W, Qin J. MCTS1 as a novel prognostic biomarker and its correlation with immune infiltrates in breast cancer. Front Genet. 2022;13:825901. doi: 10.3389/fgene.2022.825901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andre F, Ismaila N, Allison K, Barlow W, Collyar D, Damodaran S, Henry N, Jhaveri K, Kalinsky K, Kuderer N, Litvak A, Mayer E, Pusztai L, Raab R, Wolff A, Stearns V. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022;40:1816–1837. doi: 10.1200/JCO.22.00069. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn E, Gambini D, Despini L, Asnaghi D, Runza L, Ferrero S. Updates on lymphovascular invasion in breast cancer. Biomedicines. 2023 doi: 10.3390/biomedicines11030968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gujam FJ, Going JJ, Mohammed ZM, Orange C, Edwards J, McMillan DC. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer. 2014;14:676. doi: 10.1186/1471-2407-14-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torous V, Simpson R, Balani J, Baras A, Berman M, Birdsong G, Giannico G, Paner G, Pettus J, Sessions Z, Sirintrapun S, Srigley J, Spencer S. College of American pathologists cancer protocols: from optimizing cancer patient care to facilitating interoperable reporting and downstream data use. JCO Clinic Cancer Inform. 2021;5:47–55. doi: 10.1200/CCI.20.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstein H, Curigliano G, Thürlimann B, Weber W, Poortmans P, Regan M, Senn H, Winer E, Gnant M. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen international consensus guidelines for treatment of early breast cancer 2021. Annals Oncol. 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makower D, Lin J, Xue X, Sparano J. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. NPJ breast cancer. 2021;7:20. doi: 10.1038/s41523-021-00231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakha E, Martin S, Lee A, Morgan D, Pharoah P, Hodi Z, Macmillan D, Ellis I. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X. Lymphatic vasculature in tumor metastasis and immunobiology. J Zhejiang Univ Sci B. 2019;21:3–11. doi: 10.1631/jzus.B1800633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn HJ, Marks A. A New Monoclonal Antibody, D2–40, for Detection of Lymphatic Invasion in Primary Tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 24.Debald M, Pölcher M, Flucke U, Walgenbach-Brünagel G, Walgenbach KJ, Höller T, Wolfgarten M, Rudlowski C, Büttner R, Schild H. Increased detection of lymphatic vessel invasion by D2–40 (podoplanin) in early breast cancer: possible influence on patient selection for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;77:1128–1133. doi: 10.1016/j.ijrobp.2009.06.088. [DOI] [PubMed] [Google Scholar]
- 25.Modi A, Nguyen J, Wang J, Ahn J, Libling W, Klein J, Mazumder P, Barsky S. Geometric tumor embolic budding characterizes inflammatory breast cancer. Breast Cancer Res Treat. 2023;197:461–478. doi: 10.1007/s10549-022-06819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Zhang Y, Fang H, Hu Q, Duan H, Zhang L, Pang D. Survival and clinicopathological significance of blood vessel invasion in operable breast cancer: a systematic review and meta-analysis. Jpn J Clin Oncol. 2023;53:35–45. doi: 10.1093/jjco/hyac149. [DOI] [PubMed] [Google Scholar]
- 27.Moore T, Lee AH. Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast. Histopathology. 2001;38:62–67. doi: 10.1046/j.1365-2559.2001.01053.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in the article and further inquiries can be directed to the corresponding author.