Abstract
Background
Globally, the subsequent complications that accompany sepsis result in remarkable morbidity and mortality rates. The lung is among the vulnerable organs that incur the sepsis-linked inflammatory storm and frequently culminates into ARDS/ALI. The metformin-prescribed anti-diabetic drug has been revealed with anti-inflammatory effects in sepsis, but the underlying mechanisms remain unclear. This study aimed to ascertain metformin’s effects and functions in a young mouse model of sepsis-induced ALI.
Methods
Mice were randomly divided into 4 groups: sham, sham+ Met, CLP, and CLP+ Met. CLP was established as the sepsis-induced ALI model accompanied by intraperitoneal metformin treatment. At day 7, the survival state of mice was noted, including survival rate, weight, and M-CASS. Lung histological pathology and injury scores were determined by hematoxylin–eosin staining. The pulmonary coefficient was used to evaluate pulmonary edema. Furthermore, IL-1β, CCL3, CXCL11, S100A8, S100A9 and NLRP3 expression in tissues collected from lungs were determined by qPCR, IL-1β, IL-18, TNF-α by ELISA, caspase-1, ASC, NLRP3, P65, p-P65, GSDMD-F, GSDMD-N, IL-1β and S100A8/A9 by Western blot.
Results
The data affirmed that metformin enhanced the survival rate, lessened lung tissue injury, and diminished the expression of inflammatory factors in young mice with sepsis induced by CLP. In contrast to sham mice, the CLP mice were affirmed to manifest ALI-linked pathologies following CLP-induced sepsis. The expressions of pro-inflammatory factors, for instance, IL-1β, IL-18, TNF-α, CXCL11, S100A8, and S100A9 are markedly enhanced by CLP, while metformin abolished this adverse effect. Western blot analyses indicated that metformin inhibited the sepsis-induced activation of GSDMD and the upregulation of S100A8/A9, NLRP3, and ASC.
Conclusion
Metformin could improve the survival rate, lessen lung tissue injury, and minimize the expression of inflammatory factors in young mice with sepsis induced by CLP. Metformin reduced sepsis-induced ALI via inhibiting the NF-κB signaling pathway and inhibiting pyroptosis by the S100A8/A9-NLRP3-IL-1β pathway.
Keywords: sepsis, pediatric, inflammation, pyroptosis, ALI
Introduction
Globally, sepsis stands out as a substantial cause of both morbidity and mortality. Sepsis was defined in 2016 as a life-threatening organ dysfunction condition whose genesis is attributed to impaired host systemic inflammatory as well as immune response to infection,1 modulated via different cytokines produced from innate immune cells. Although over the past decades, a remarkable improvement has been achieved, sepsis syndrome stands out as a major cause of morbidity and mortality in pediatrics in the world,2–4 and a common reason for admitting patients to PICU.5 Severe sepsis in pediatrics is still a cumbersome public health problem. Previous works of literature have affirmed heightened risk for long-term disability, hospital readmission as well as late mortality for sepsis survivors among children.6 At least one-third of these children develop progressive organ dysfunction, whereas approximately 20% of them exhibit new functional disability.7 ALI, which stands out as one of the most susceptible organs to sepsis, exhibits the propensity to progress to ARDS, presenting with acute onset of sepsis, hypotension, severe respiratory distress, and persistent hypoxemia,8 which eventually worsens the dysfunction of multiple organs and as result death remains inevitable.9 During sepsis, activation of inflammatory cells releases various inflammatory mediators from innate immune cells, including TNF, IL-1, IL-6, and oxygen-free radicals, etc., which can severely harm the body’s organs and tissues.10–12 The exact molecular mechanism of lung injury caused by sepsis is still unclear, and the development of novel and effective therapeutic approaches is urgent. New sepsis therapeutics will be increasingly evaluated in children when they are developed.
Metformin, the AMPK activator has been revealed with anti-inflammatory effects in sepsis. Sepsis induces inflammation and oxidative stress leading to multi-organ damage through the production of proinflammatory cytokines and ROS. Metformin has been demonstrated to ameliorate sepsis-induced organ damage and mortality by acting on these mediators.13 In the present adult mouse models of sepsis, researchers observed that metformin can attenuate sepsis-associated brain injury,14 sepsis-related liver injury,15,16 cognitive impairment, and sepsis-induced neuronal injury,17 and endotoxin-induced acute myocarditis via activating AMPK. Increasing evidence implies that metformin exhibits a protective effect against various types of lung injury, including ventilator- and PM2.5-induced lung injuries,18,19 LPS-induced acute lung injury,20–22 sepsis-induced ALI,23 LPS-induced ARDS, and ARDS caused by SARS-CoV-2 infection.24 In COVID-19 cases, clinical trials have found that metformin can decrease COVID-19 severity and mortality via activating AMPK and/or inhibiting the mTOR-mediated signaling pathway primarily by lowering the level of pro-inflammatory signaling and cytokine storm.25 Recent reports of metformin attenuating hyperoxia-induced lung injury by lessening the inflammatory response in neonatal rats26,27 have sparked our thoughts about the potential benefits of metformin in the pediatric sepsis population. Based on the previously documented antioxidant and anti-inflammatory functions of metformin, we hypothesized that metformin would be protective against sepsis-induced lung injury and death in young mice.
The S100 protein family serves as a potent amplifying factor for inflammatory responses. Mrp8 (encoded by Mrp8, also referred to as S100A8) and Mrp14 (encoded by Mrp14, also called S100A9), have a substantial function in sepsis pathogenesis28 and in neutrophils and monocytes, they are the most abundant cytoplasmic proteins.29 As pro-inflammatory molecules, they form a heterodimer and are referred to as calprotectin. Heightened expression of S100A8/A9 enhances the inflammatory response and facilitates neutrophils and macrophages to produce a lot of cytokines, which triggers a vicious cycle that makes the disorder worse. The NLRP3 (NLR family) inflammasome, a cytosolic multiprotein complex, controls the inflammatory cytokines IL-1β and IL-18.30 The pro-inflammatory alarmins S100A8 as well as S100A9 are powerful activators of the NLRP3 inflammasome.31–33 In vivo and ex vivo studies identified32 that the S100A8/A9-TLR4-NLRP3-IL1β/IL-18 signaling circuit played a crucial role in ponatinib-induced excessive inflammation and dysfunction of the heart. Pre-clinical models have affirmed the anti-inflammatory effects of metformin. Additionally, there is a lack of relevant reports on metformin treatment in pediatric sepsis models. Further investigation into the mechanistic role of metformin in septic young mice is warranted. Hence, this study sought to ascertain whether metformin contributes to sepsis-induced ALI by inhibiting the S100A8/A9-TLR4/NLRP3 inflammasome pathway in young mice. As far as we know, this marks the first investigation into the role and mechanism of metformin in sepsis-induced ALI in young mice. Looking at it from a clinical perspective, these findings support the feasibility of immunosuppressive interventions in treating sepsis-induced ALI among pediatric populations.
Materials and Methods
Animals Grouping
Male C57BL/6J mice (aged 3 weeks with 11 ± 0.7g, the Saike Jingda experimental Animal Co. Ltd, Changsha, Hunan, China) were kept in the Central Laboratory of Hunan Provincial People’s Hospital with 12 h light–dark cycle and regulated humidity and temperature and fed a standard rodent diet as well as water ad libitum. In the first experiment, all the animals (n = 20 per group) were randomized into four groups: sham group (sham), sham + Met group (sham +Met), CLP group (CLP), CLP + Met group (CLP +Met), and weighed every day and followed for 7 days to ascertain the survival rate. In the second experiment, animals were treated with metformin (Selleck Bio, S195013, 1.0), normal saline solution (NS), and randomized in the same four groups (n = 20 per group) to obtain lung tissue after 24h post-operation. The mice in Met groups were administered with metformin through intraperitoneal injection in a volume of 100μL. The CLP group was replaced by the same volume of saline. The befitting and optimal dose of metformin without detectable toxicity to experimental animals is based on previous reports.34,35 After 1 week of acclimatization, the mice were given either 200 mg/kg metformin dissolved in NS or NS alone by intraperitoneal injection for 7 consecutive days.
Animal Model of Sepsis-Induced ALI
As per the previous study, CLP was utilized to induce polymicrobial sepsis in mice.36 Briefly, mice were anesthetized with 2–3% isoflurane (Ringpu Bio, China). The cecum was exposed utilizing a midline surgical excision and ligated by a 4–0 silk suture. Then, a 21G needle punctured the cecum and the abdomen was closed in layers with 4–0 sutures. The cecum was mobilized without the usage of CLP for the sham-operated animals. Resuscitated animals by subcutaneous injection of a prewarmed normal saline (37°C, 50mL/kg) at the end of the surgical procedures, then mice were returned to cages immediately where accessed to water and food in freedom, with a temperature-controlled room (22°C) for 12h light and dark cycles and monitor them every 6h. All animal experiments followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The Ethics Committee of Hunan Provincial People’s Hospital approved this study.
Survival Analysis and M-CASS
A Kaplan-Meier survival curve was produced and analyzed utilizing GraphPad Prism 9.0 software. To ascertain the severity of the disease in a sepsis-induced model, mice were observed after induction of sepsis every 6 h, based on the mice’s facial expressions37 and behavior, then scored the mice per 24 h each day using the M-CASS as a previous study.38
Lung Wet Weight/Body Weight Coefficient
The left wet lung weight(mg) and body weight(g) of mice were obtained after CLP for 24h, then the left lung wet weight/body weight coefficient was developed and chosen to calculate the lung coefficient reported in previous studies39,40 as wet lung weight/body weight.
Measurements of Histological Evidence of ALI
Extraction of the right middle lobes of the lung was executed, and the lobes were fixed using a 4% paraformaldehyde for 24h at 4°C. They were then dehydrated by ethanol, immersed in paraffin, and a microtome (Leica RM2125RT, Leica, Nussloch, Germany) was adopted to section the samples at 5μm thickness, followed by staining with hematoxylin and eosin. The Olympus BX 51 Leica ICC50W light microscope (Leica Microsystems Co., LTD Shanghai, China) recorded the sections. The scores of lung injury were evaluated in a blinded manner for three reviewers as previous study,41 for five independent variables in detail in Table 1. In brief, 20 random high-power fields (400× total magnification) of the sample were viewed at ×400 magnification, and then the resulting injury score between zero and one (inclusive) was normalized to the number of fields examined.
Table 1.
Lung Injury Score
| Parameter | Score Per Field | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| A. Neutrophils in the alveolar space | None | 1~5 | >5 |
| B. Neutrophils in the interstitial space | None | 1~5 | >5 |
| C. Hyaline membranes | None | 1 | >1 |
| D. Proteinaceous debris filling the airspaces | None | 1 | >1 |
| E. Alveolar septal thickening | <2× | 2×~4× | 4× |
Note: Score =[(20 × A) + (14 × B) + (7 × C) + (7 × D) + (2 × E)]/(number of fields × 100).
Quantitative Polymerase Chain Reaction (qPCR)
A total RNA extraction kit (TIANGEN, Beijing, China) was utilized to extract total RNA from mice lung tissues. RNA concentration was quantified spectrophotometrically. The PrimerScript RT reagent kit (Takara, Japan, Code No. RR036A) was employed to reversely transcribe RNA to cDNA as per the manufacturer’s instructions. The level was measured utilizing the TB Green mix (Takara, Japan, Code NO. RR420A) in a quantitative PCR. After that, over 40 cycles of qPCR were run utilizing the StepOne system. The PCR reaction conditions were: 95°C for 30s, 95°C for 5s, and 60°C for 30s. β-actin was as an internal control. The data were analyzed through 2−ΔΔCt. Primers were purchased from Takara and listed in Table 2.
Table 2.
The Sequence of Primers for qPCR
| Gene | Forward (5’->3’) | Reverse (5’->3’) |
|---|---|---|
| β-actin | GCTTCTAGGCGGACTGTTACTGA | CGCCTTCACCGTTCCAGTTTT |
| Arg1 | ACACTCCCCTGACAACCAGC | AGGGTCTACGTCTCGCAAGC |
| CCL3 | GCTCCCAGCCAGGTGTCATT | TCAAGCCCCTGCTCTACACG |
| CXCL11 | GATCTCCAAAGCCCAGGCAGA | GGGCCGATGCAAAGACAGC |
| CD206 | CGGATGGCTCTGGTGTGGAA | CAGCTTGCCCTTGCCTGATG |
| iNOS | GCAACAGGGAGAAAGCGCAA | TGTGGACGGGTCGATGTCAC |
| IL-1β | GGTTCAAGGCATAACAGGCTC | TCTGGACAGCCCAAGTCAAG |
| NLRP3 | GGTGACCTTGTGTGTGCTTG | ATGTCCTGAGCCATGGAAGC |
| S100A8 | AAATCACCATGCCCTCTACAAG | CCCACTTTTATCACCATCGCAA |
| S100A9 | ATACTCTAGGAAGGAAGGACACC | TCCATGATGTCATTTATGAGGGC |
Western Blotting Analysis
After being homogenized, lung tissues and cells were incubated in a lysis buffer that contained cocktail of protease inhibitors. Lung tissues were homogenized in a mixture containing protease inhibitors and phosphatase inhibitors of cracking incubation in the buffer. The tissue proteins were extracted by Radioimmunoprecipitation assay (RIPA) while measured using BCA assay, and at 100°C, the proteins were denatured for 5 min. The proteins (20μg per lane) were identified on a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane. The blots were blocked with 5% nonfat milk at room temperature (RT) for 1h and incubated with primary antibodies for the whole night at 4°C. The primary antibodies against ASC, NLRP3, P65, p-P65, GSDMD, caspase-1 from antibody sampler kit (CST, #98303T), S100A8/A9 (Abcam, Cat NO: ab288715) and β-actin (Zen-Bio, Cat NO: 26441) at a dilution of 1:1000, correspondingly. The membrane was rinsed thrice with TBST for ¼ h before being incubated with a secondary anti-rabbit antibody for 1 h at RT. Again, the membrane was washed with TBST thrice. Ultimately, the Omega Lum C Gel Imaging System (Bio-rad) was utilized to detect and show distinct bands utilizing a chemiluminescent substrate. ImageJ software was utilized to quantify and evaluate protein band intensity.
Enzyme-Linked Immunosorbent Assays (ELISAs) of Cytokine
A 2% sodium pentobarbital (50 mg/kg, intraperitoneal) was applied to anesthetize the mice, then lungs were collected and stored at −80°C to furthermore ascertain the level of cytokine level utilizing IL-1β, TNF-α, IL-18 ELISA kits (Invitrogen, Carlsbad, CA, USA) as per manufacturer’s specifications. The results were measured using a microtiter plate reader at 450nm and 570nm, and then we subtracted the values of 570 nm from those of 450 nm and analyzed the data.
Statistical Analysis
Data were expressed as mean ± SEM and were calculated from multiple independent experiments which were triplicated. Statistical comparisons were made by one-way ANOVA utilizing GraphPad Prism 9.0 software. Tukey’s multiple comparisons test and Bonferroni adjustment were involved in correcting for multiple comparisons and executing post hoc analysis, correspondingly. The Student’s t-test and the log-rank (Mantel–Cox) test performed comparisons between the two groups and analyzed the survival rate, respectively. P <0.05 was considered statistically significant. All experiments were repeated at least three times.
Results
Metformin Improves the Survival Rate
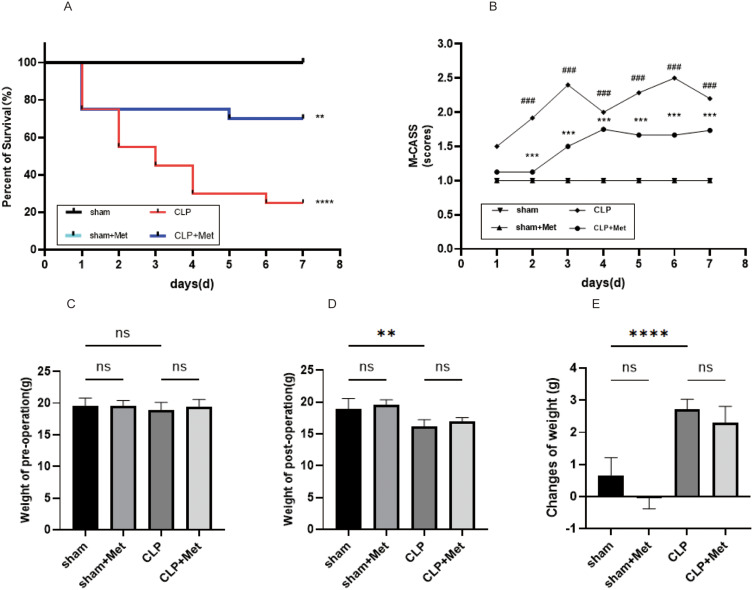
After 12 hours of the CLP, septic mice exhibited signals typical to clinical sepsis, for instance, lethargy, piloerection, and tachypnea, and then progressively to bradypnea, increasingly labored breathing and reduced level of consciousness marked by reduced response to auditory as well as tactile stimuli. Between 2 and 7 days post-CLP induction, metformin enhanced the survival state of septic mice, which decreased M-CASS (Figure 1B, p < 0.001, CLP group vs CLP+ Met group), and the M-CASS of the CLP group was higher in comparison to the sham group (p < 0.001).
Figure 1.
The impact of metformin pre-treatment on survival state. (A) Kaplan–Meier curves for time for 7 days post-operation (n = 20). (B) The M-CASS score changes with time during the CLP-induced sepsis 7-day follow-up (mean ± S.E.M), (C–E). Changes of the weight between pre-operation and post-operation. **p<0.01, ***p<0.001, ****p<0.0001 (CLP+ Met vs CLP); ###p<0.001 (sham vs CLP).
Abbreviation: ns, non-significant.
During the experimental period, the survival rate of the sham group and sham+ Met group was 100%. Metformin treatment is given for 7 days before CLP surgery (Figure 1A) affirmed a survival rate that was 45% greater than the untreated septic animals. The findings as per the Log rank test implied a substantial difference in cumulative survival among the four groups (p < 0.0001).
There were no variations in body weight of all the groups of mice on day 0 of induction of sepsis (Table 3, Figure 1C). Mice in the CLP-induced sepsis groups demonstrated a significant decrease in weight within 24 h of the operation (p < 0.001 vs sham group; Figure 1E), although there was no substantial difference between CLP group and CLP+ Met group in post-operation (Figure 1D and E).
Table 3.
Changes of Weight and Coefficient in Four Groups
| Groups | Pre-Operation Weight (g) | Post-Operation Weight (g) | Left Lung Wet Weight (mg) | Lung Coefficient |
|---|---|---|---|---|
| Sham | 19.54±0.57 | 18.88±0.75 | 44.24±2.19 | 2.25±0.11 |
| Sham + Met | 19.56±0.38 | 19.60±0.34 | 43.9±1.91 | 2.13±0.06 |
| CLP | 18.7±0.42 | 16.03±0.37 | 41.81±0.76 | 2.61±0.11 |
| CLP +Met | 19.21±0.41 | 16.91±0.25 | 38.5±1.73 | 2.27±0.21 |
Metformin Pre-Treatment Attenuated Sepsis-Induced ALI in Young Mice
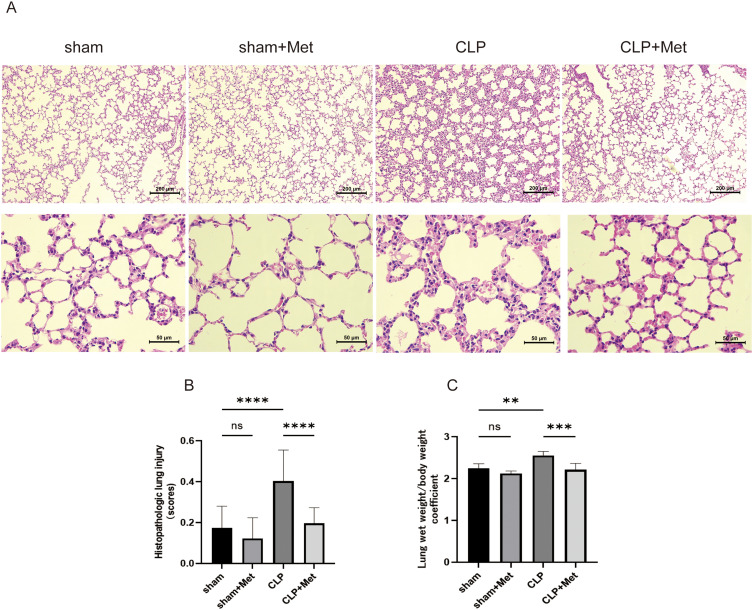
In our research, the model of sepsis-induced ALI was successfully created by CLP operation. Histopathological alterations were evaluated by H&E staining. Our research showed that metformin had no adverse effect on the sham mice, including decreased weight and lung injury (Figure 1C–E). In comparison to the sham group, CLP group demonstrated alveolar septal thickening, inflammatory cell infiltration, hyaline membranes, proteinaceous debris filling the airspaces, which led to higher lung injury scores (Figure 2B, p < 0.0001) and lung wet weight/body weight coefficient (Table 3, Figure 2C, p < 0.001), while metformin reversed these adverse changes induced by CLP (Figure 2A–C). In brief, these results imply that metformin pre-treatment has protective effects on sepsis-induced ALI in young mice.
Figure 2.
Metformin alleviated sepsis induced by CLP in mice lung injury. (A) At the end of 24 h following CLP, the histopathological lung injury was evaluated utilizing the HE staining (100x, bar = 200µm; 400x, bar = 50µm). (B) Histopathologic lung injury scores. (C) Lung wet weight/body weight within 24h CLP-induced sepsis. Met + CLP vs CLP, metformin treatment lowered the lung histopathological score in septic mice, and this suggests that this drug can improve the lung tissues with pathological injury. **p<0.01, ***p<0.001, ****p<0.0001.
Abbreviation: ns, non-significant.
Metformin Regulated Sepsis-Induced Inflammatory Cytokine Levels
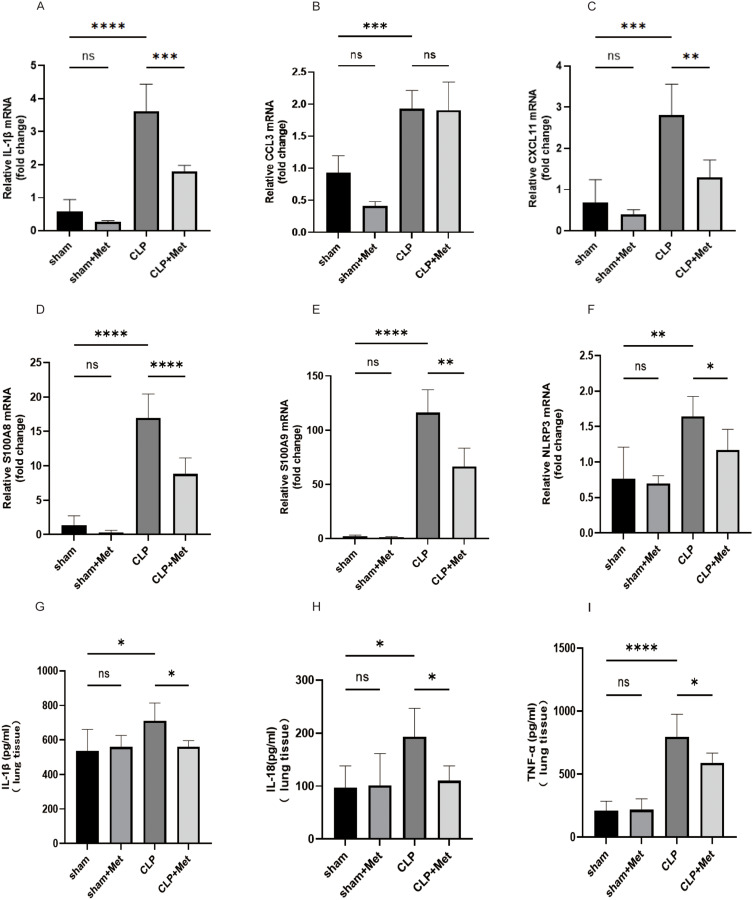
We then delved into examining the fluctuations in inflammatory cytokines in lung 24 h following CLP and their expressions in the lung using qPCR and ELISA. The findings shown in Figure 3. CLP significantly potentiated pro-inflammatory cytokines including IL-1β, CCL3, and CXCL11 mRNA levels (Figure 3A and C), which were reversed by metformin pretreatment (CCL3 excepted). Moreover, ELISA assay revealed that metformin reduces the levels of pro-inflammatory cytokines in lung tissue like IL-1β, IL-18, and TNF-α (Figure 3G–I), respectively. Inflammatory response by the host during sepsis-associated ALI was mediated by pro-inflammatory and anti-inflammatory. The results suggested that metformin augmented the anti-inflammatory responses in CLP-induced ALI and down-regulated pro-inflammatory.
Figure 3.
Metformin pre-treatment regulated inflammatory responses in sepsis-induced mice. (A–F) The levels of pro-inflammatory cytokines (IL-1β, CCL3, CXCL11 S100A8, S100A9), and the inflammasome NLRP3 (G–I) were measured by qPCR assay, (D–F) pro-inflammatory cytokines (IL-1β, TNF-α IL-18) were examined by ELISA assay in the lung tissues. *p<0.05,**p<0.01, ***p<0.001, ****p<0.0001.
Abbreviation: ns, non-significant.
In the CLP group, inflammatory (pro-inflammatory neutrophils and macrophages) infiltrates in lung tissue, up-regulation the expression of pro-inflammatory cytokines S100A8, S100A9, and the inflammasome NLRP3 (Figure 3D–F), while metformin reverses this adverse impact. These findings demonstrate that metformin pre-treatment attenuates the immune response initiated by sepsis in the lung tissues. In a word, our data are suggestive of a potentially remarkable protective effect of metformin.
Metformin Pre-Treatment Inhibited the Expression of S100A8/A9 and Suppressed the NF-κB Signaling Pathway
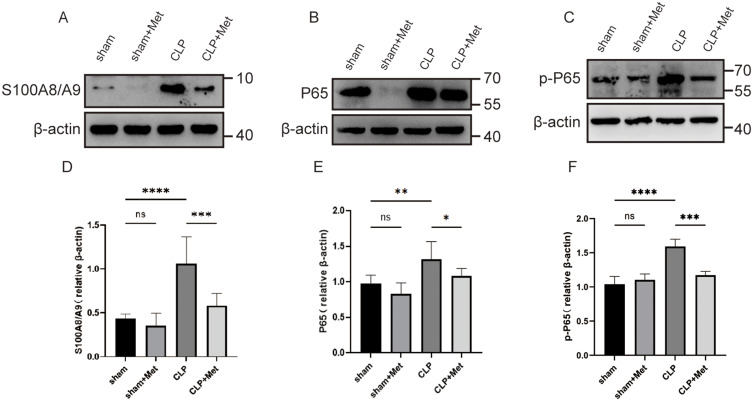
The activation of NLRP3 and production of pro-IL-1β necessitate the activation of the NF-κB signaling pathway.42 Myeloid cells encompassing neutrophils and monocytes are the dominant reservoirs of calcium-binding proteins S100A8/A9, which can be released into extracellular space as DAMPs following an inflammatory stimulus.43 As an endogenous ligand of TLR4, S100A8/A9 induces NF-κB activation via TLR4-MD2 and fosters lethality throughout septic shock.28 Therefore, we examined if metformin hindered S100A8/A9-induced NF-κB activation. Sepsis caused a significant increase (p < 0.05) in lung tissue S100A8/A9, p65, and p-P65 levels measured by Western blot when compared with sham-operated mice. The metformin pre-treatment significantly reduced S100A8/A9 (p < 0.001), p65 (p < 0.05), and p-P65 (p < 0.001) levels in comparison to the CLP group (Figure 4A–F). Briefly, our data confirmed our hypothesis that metformin inhibits S100A8/A9-induced NF-κB activation.
Figure 4.
Metformin exerts inhibitory effects on the NF -κB pathway by repressing the release of S00A8/A9 in inflammatory cells. (A–F) S00A8/A9, p65, and p-P65 protein quantification was performed through Western blot and was measured by densitometry. *p<0.05,**p<0.01, ***p<0.001, ****p<0.0001.
Abbreviation: ns, non-significant.
Metformin Pre-Treatment Inhibited Pyroptosis in the Young Mice Lung Tissue
Pyroptosis denotes a lytic type of programmed cell death, necessitating the participation of membrane-damaging GSDMD, and is initiated by inflammatory caspases. These caspases are triggered within complex inflammasomes, particularly NLRP3, formed in reaction to pathogens as well as endogenous danger signals. NLRP3 inflammasome, comprising a sensor component such as NLRP3, an adaptor ASC, and the effector pro-caspase-1, regulates activation of caspase-1, which can mediate the cleavage of pro-IL-1β, pro-IL-1844 and GSDMD which induces pyroptotic cell death by pore formation.45,46
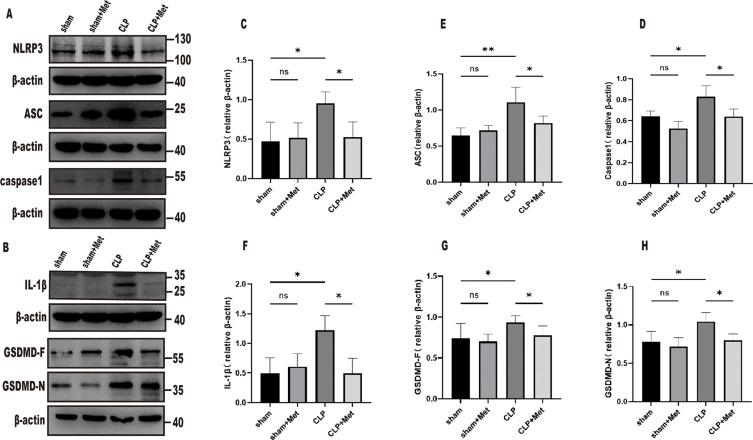
To investigate further the impact of metformin on pyroptosis in septic ALI, we ascertained the expression of NLRP3 inflammasome-linked proteins NLRP3, ASC, Pro-Caspase1, GSDMD-F, Pro-IL-1β, and GSDMD-N in the tissues of septic young mice lungs. Compared with the sham group, NLRP3, ASC, pro-caspase1, GSDMD-F, Pro-IL-1β, and GSDMD-N, in the CLP group were increased, while the expressions of the metformin pre-treatment group were decreased (Figure 5). These results suggest that metformin inhibited pyroptosis by suppressing NLRP3 inflammasome activation.
Figure 5.
Metformin inhibits pyroptosis via suppressing NLRP3 inflammasome activation and suppressing the cleavage of GSDMD. (A–H) NLRP3, ASC, caspase-1, GSDMD-F, GSDMD-N, and IL-1β, protein quantification was performed through Western blot and was measured by densitometry. *p<0.05,**p<0.01.
Abbreviation: ns, non-significant.
Discussion
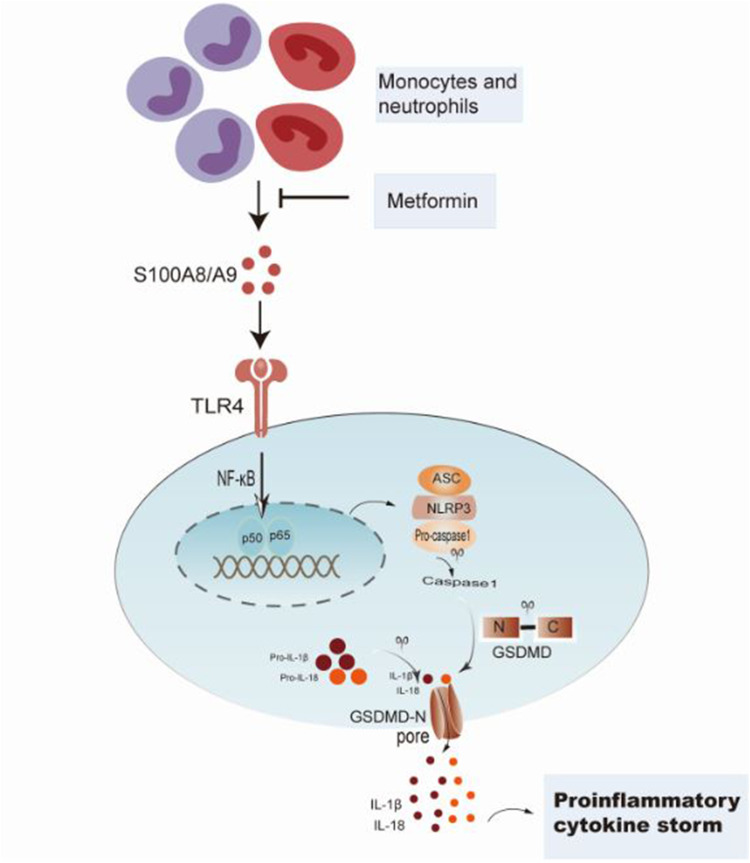
Our research highlighted the crucial function of pyroptosis in the pathogenesis of sepsis-induced ALI young mice. We identified the vital role of the S100A8/A9-TLR4-NLRP3-IL-1β signaling circuit in sepsis-mediated inflammation and ALI in young mice. As presented in the schematic figure (Figure 6), sepsis triggers S100A8/A9 synthesis, which primes the NLRP3 inflammasome and excites the production of proinflammatory IL-1β. The antidiabetic therapeutic agent, metformin, manifested protective effects towards sepsis-induced ALI via inhibiting the expression of S100A8/A9.
Figure 6.
Presentation of metformin protecting sepsis-induced ALI in young mice by repressing S100A8/A9-NLRP3-IL-1β signaling pathway.
The most severe form of ALI, known as ARDS, is an unfavorable clinical complication secondary to bacterial sepsis, with a mortality rate of 40 to 60%.47 ALI/ARDS, marked by heightened lung inflammatory response, including inflammatory cells such as neutrophils and macrophages infiltrated, causes a vicious cycle that further potentiates the pooling of the aforementioned cells.48 Alveolar macrophages play crucial functions in the progression of ALI/ARDS through the production and release of numerous inflammatory mediators.49 Remarkable studies affirm that pyroptosis of alveolar macrophages may lead to the onset and advancement of ALI by mediating inflammation.50 Recent evidence indicates that reduced NLRP3 inflammasome suppresses pyroptosis as well as inflammatory cytokines, including IL-1β and IL-18, in alveolar macrophages, thereby improving lung injury.51,52 Metformin has been affirmed to exert cardiovascular protective effects via lessened NLRP3 protein expression and NLRP3 inflammasome activation.53 Previous research indicated that metformin is instrumental in abrogating pulmonary inflammation and NLRP3 inflammasome activation.20,24 In their study,32 Sultan Tousif et al elucidate that heightened expression of S100A8/A9 can trigger the inflammatory cascade via TLR4 activation, resulting in the excessive release of IL-1β from NLRP3 inflammasomes, consequently instigating myocardial and systemic inflammation. As anticipated, we documented that metformin suppressed NLRP3 inflammasome activation and NLRP3-stimulated pyroptosis induction, as evidenced by reduced levels of N-terminal fragment of GSDMD, pro-caspase-1, and protein contents of IL-1β in lung tissues of young mice treated with CLP.
S100A8 as well as S100A9 have already been verified to exhibit a decisive function in inflammation development. They are members of the S100 family, which was derived first from the neural proteins of the bovine brain in 1965. Neutrophils and monocytes secrete S100A8 and S100A9, which form a stable homodimer or heterodimer both in vitro and in vivo. The secretion of S100A8/A9 is primarily fueled by infection-induced inflammation. When a bacterial infection occurs, neutrophils, macrophages, and monocytes express markedly and produce S100A8/A9 to control inflammatory processes with the entry of ROS, inflammatory cytokines, and nitric oxide (NO).54 As DAMPs, alarmin S100A8/A9 are strong inflammasome priming agents, subsequently interacting with TLR4, prime the NLRP3 inflammasome, and stimulate the production of IL-1β, leading to myocardial infarction.55 The S100A8/A9-TLR4 axis could mediate coronavirus SARS-CoV-2 infection-induced disorder of antiviral innate immunity, subsequently leading to ARDS.56 A previous study indicated that S100A8/A9 in the plasma of patients following ALI brought about by sepsis or pneumonia significantly elevated as compared with healthy controls.57 S100A8/A9 has been established to be one of the most distinctive DAMPs in sepsis,58,59 which interacts with cell surface receptors on various immune cells, platelets, endothelial cells, and intracellular PRRs, exerting detrimental impacts on sepsis pathogenesis.43 Specifically, activation of the S100A8/A9 complex through binding with TLR4 can induce pro-inflammatory cytokine production and promote not only recruitment but also activation of immune cells, ultimately leading to tissue damage and dysfunctions of organs in sepsis. Due to its function in sepsis, S100A8/A9 has attracted our interest in its mediation of sepsis-induced ALI/ARDS. To ascertain if S100A8/A9 is also heightened in the sepsis-induced young mice, we evaluated S100A8/A9 in the tissue of young mice lung post 24h CLP by qPCR and Western blot. Sepsis-induced ALI revealed remarkably elevated lung tissue S100A8/A9 levels, while metformin decreased the expression of S100A8/A9 as compared with the sham group.
Increasing evidence suggests that the immune response is also initiated by endogenous ligands, also known as DAMPs or alarmins.58 DAMPs are intracellular molecules that predominantly engage in cell homeostasis. However, they can additionally serve as extracellular danger signals when they are produced by activated or damaged cells. During the progression of sepsis, S100A8/A9 has been considered a DAMP that results in constant immune stimulation by modulating various signaling pathways in distinct cells. Levels were also heightened in septic patients and exhibited an inverse correlation with survival.60 During the activation of phagocytes, S100A8/S100A9 complexes are released and mediate their actions via TLR4, resulting in the synthesis of TNF-α and other cytokines.28 Apart from SARS-CoV-2-infected animal models, inappropriate activation of the S100A8/A9-TLR4 signaling pathway was markedly induced in also in patients with COVID-19, repressing the buildup of aberrant neutrophils and rescued mice from ARDS.56 However, the specific mechanisms require further investigation and development. Research showed that blocking S100A9 could effectively decrease neutrophil infiltration and activation and shield against the development of edema and destruction of tissues during sepsis.61 Taken together, our results indicate that metformin blocking S100A8/A9-TLR4 reduces macrophage infiltration in addition to activation and protects against ALI in septic young mice. Thus, our study affirms the function of S100A8/A9 in septic lung damage and propounds that S100A8/A9 could exhibit a substantial function as a target to alleviate tissue damage and lung inflammation in abdominal sepsis in youth.
The NF-κB pathway encodes various transcriptional genes, leading to the production of pro-inflammatory cytokines. Additionally, it tightly controls the expression of NLRP3.62 NF-κB activation requires the breakdown of its distinct inhibitors, the inhibitor of NF-κB (IκB) proteins, after their phosphorylation by the IκB kinase (IKK) complex. The general types of NF-κB signaling pathways are classical and alternative pathways. The aforementioned two pathways are also denoted as canonical and noncanonical pathways, correspondingly.63,64 The triggers in the classical pathway are genotoxic stress and pro-inflammatory stimuli. DAMPs can promote the classical pathway and also exert a weaker activation on the alternative pathway in response to inflammatory stimuli,64 subsequently releasing cytokines including IL-1, IL-6, and TNF into extracellular space. In a word, as a sensor for PAMPs, TLR4 can activate the NF-κB signaling pathway, inducing the production of chemokines and cytokine precursors and releasing mature pro-inflammatory factors under the influence of NLRP3 inflammasomes.65 In the present research, we have revealed that NF-κB dimers (p65/p50) with a key function in the induction of genes taking part in inflammation were liberated during metformin pre-treatment. In addition, the present results demonstrated that NF-κB is elevated following CLP treatment, while metformin pre-treatment down-regulated the level of NF-κB. Prior research66 found that stimulation with S100A8/9 lead to increase the expression level of TLR4. Zheng et al showed that67 S100A8/A9 activated the NF-kB signaling pathway via toll-like receptor-4, precipitating intervertebral disc degeneration and inflammation-associated pain in rats. Briefly, S100A8/9 can activate the NF-kB signaling pathway through TLR4. Therefore, our data showed that the metformin pre-treatment may inactivated the NF-κB pathway via S100A8/9-TLR4 in the young mice.
Above all, our current study suggested that metformin may confer protection against sepsis-induced lung injury by downregulating inflammatory genes and inhibiting the expression of pyroptosis-related proteins. The regulatory role of metformin may also involve other receptors or signaling molecules, necessitating further experimentation.
Certainly, our study also has certain limitations. Firstly, our experimental data are derived from experiments conducted on rodents, and there are species differences between rodents and humans, which will require further research to validate its effectiveness. Secondly, the administration of metformin for 7 days in our study serves as pretreatment, and future administration post-sepsis occurrence may better approximate clinical usage. Lastly, the limited sample size in this study encompasses only single types, overlooking the distinct pathological characteristics among clinical patients. Improving sample diversity is crucial to effectively evaluate clinical applicability.
Conclusion
In the present research, we investigated for the first time and unearthed the function of metformin against sepsis-induced mortality and its pulmonary protective effects in young mice. Overall, our findings discovered that metformin may deactivate the S100A8/A9-NLRP3-IL-1β pathway as well as NLRP3 inflammasome to lessen the damage to lung tissue in septic young mice. As a result, metformin may help treat sepsis-induced ALI in pediatrics as per our findings.
Funding Statement
This project was financially supported by the Natural Science Foundation of Hunan Province (2023JJ60101).
Data Sharing Statement
The datasets of the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The Ethics Committee of Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University) approved this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no potential conflicts of interest in this work.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–2417. doi: 10.1097/CCM.0000000000000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S3–S5. doi: 10.1097/01.PCC.0000161289.22464.C3 [DOI] [PubMed] [Google Scholar]
- 4.Tan B, Wong JJ, Sultana R, et al. Global case-fatality rates in pediatric severe sepsis and septic shock: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(4):352–362. doi: 10.1001/jamapediatrics.2018.4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindell RB, Nishisaki A, Weiss SL, Traynor DM, Fitzgerald JC. Risk of mortality in immunocompromised children with severe sepsis and septic shock. Crit Care Med. 2020;48(7):1026–1033. doi: 10.1097/CCM.0000000000004329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman JJ, Banks R, Berg RA, et al. Trajectory of mortality and health-related quality of life morbidity following community-acquired pediatric septic shock. Crit Care Med. 2020;48(3):329–337. doi: 10.1097/CCM.0000000000004123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–1157. doi: 10.1164/rccm.201412-2323OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Hou Y, Li X, Pan R, Zhao D. Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-kappaB pathway and NLRP3 inflammasome. Int Immunopharmacol. 2021;96:107623. doi: 10.1016/j.intimp.2021.107623 [DOI] [PubMed] [Google Scholar]
- 9.Shi X, Li T, Liu Y, et al. HSF1 protects sepsis-induced acute lung injury by inhibiting NLRP3 inflammasome activation. Front Immunol. 2022;13:781003. doi: 10.3389/fimmu.2022.781003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E-P, Lin M-J, Wu H-P. Time-serial expression of toll-like receptor 4 signaling during polymicrobial sepsis in rats. Int J Immunopathol Pharmacol. 2022;36:3946320221090021. doi: 10.1177/03946320221090021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8 [DOI] [PubMed] [Google Scholar]
- 12.Ding R, Meng Y, Ma X. The central role of the inflammatory response in understanding the heterogeneity of Sepsis-3. Biomed Res Int. 2018;2018:5086516. doi: 10.1155/2018/5086516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail hassan F, Didari T, Khan F, Niaz K, Mojtahedzadeh M, Abdollahi M. A review on the protective effects of metformin in sepsis-induced organ failure. Cell J. 2020;21(4):363–370. doi: 10.22074/cellj.2020.6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail Hassan F, Didari T, Baeeri M, et al. Metformin attenuates brain injury by inhibiting inflammation and regulating tight junction proteins in septic rats. Cell J. 2020;22(Suppl 1):29–37. doi: 10.22074/cellj.2020.7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang H, Song H, Zhang X, et al. Metformin attenuated sepsis-related liver injury by modulating gut microbiota. Emerg Microbes Infect. 2022;11(1):815–828. doi: 10.1080/22221751.2022.2045876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H, Zhang X, Zhai R, et al. Metformin attenuated sepsis-associated liver injury and inflammatory response in aged mice. Bioengineered. 2022;13(2):4598–4609. doi: 10.1080/21655979.2022.2036305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Z, Zhou C, Xiao X, Guo C. Metformin attenuates sepsis-induced neuronal injury and cognitive impairment. BMC Neurosci. 2021;22(1):78. doi: 10.1186/s12868-021-00683-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsaknis G, Siempos II, Kopterides P, et al. Metformin attenuates ventilator-induced lung injury. Crit Care. 2012;16(4):R134. doi: 10.1186/cc11439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J, Yuan J, Wang Q, et al. Metformin protects against PM2.5-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase alpha2. Redox Biol. 2020;28:101345. doi: 10.1016/j.redox.2019.101345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang H, Li S, Huang K, Jiang L, Wang Y. Metformin alleviates LPS-induced acute lung injury by regulating the SIRT1/NF-kappaB/NLRP3 pathway and inhibiting endothelial cell pyroptosis. Front Pharmacol. 2022;13:801337. doi: 10.3389/fphar.2022.801337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaez H, Najafi M, Toutounchi NS, Barar J, Barzegari A, Garjani A. Metformin alleviates lipopolysaccharide-induced acute lung injury through suppressing toll-like receptor 4 signaling. Iran J Allergy Asthma Immunol. 2016;15(6):498–507. [PubMed] [Google Scholar]
- 22.Wu K, Tian R, Huang J, et al. Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem Biol Interact. 2018;291:1–6. doi: 10.1016/j.cbi.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 23.Wan Y, Wang S, Niu Y, et al. Effect of metformin on sepsis-associated acute lung injury and gut microbiota in aged rats with sepsis. Front Cell Infect Microbiol. 2023;13. doi: 10.3389/fcimb.2023.1139436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xian H, Liu Y, Rundberg Nilsson A, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54(7):1463–1477 e11. doi: 10.1016/j.immuni.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamyshnyi O, Matskevych V, Lenchuk T, Strilbytska O, Storey K, Lushchak O. Metformin to decrease COVID-19 severity and mortality: molecular mechanisms and therapeutic potential. Biomed Pharmacother. 2021;144:112230. doi: 10.1016/j.biopha.2021.112230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Liu Y, Han D, Zhong J, Yang C, Chen X. Dose-dependent immunomodulatory effects of metformin on human neonatal monocyte-derived macrophages. Cell Immunol. 2022;377:104557. doi: 10.1016/j.cellimm.2022.104557 [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Walther FJ, Sengers RM, et al. Metformin attenuates hyperoxia-induced lung injury in neonatal rats by reducing the inflammatory response. Am J Physiol Lung Cell Mol Physiol. 2015;309(3):L262–70. doi: 10.1152/ajplung.00389.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 29.Edgeworth J, Freemont P, Hogg N. Ionomycin-regulated phosphorylation of the myeloid calcium-binding protein p14. Nature. 1989;342(6246):189–192. doi: 10.1038/342189a0 [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–1217. doi: 10.1002/eji.201141550 [DOI] [PubMed] [Google Scholar]
- 31.Simard JC, Cesaro A, Chapeton-Montes J, et al. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-kappaB(1). PLoS One. 2013;8(8):e72138. doi: 10.1371/journal.pone.0072138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tousif S, Singh AP, Umbarkar P, et al. Ponatinib drives cardiotoxicity by S100A8/A9-NLRP3-IL-1beta mediated inflammation. Circ Res. 2023;132(3):267–289. doi: 10.1161/CIRCRESAHA.122.321504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan X, Zheng X, Huang Z, Lin J, Xie C, Lin Y. Involvement of S100A8/A9-TLR4-NLRP3 inflammasome pathway in contrast-induced acute kidney injury. Cell Physiol Biochem. 2017;43(1):209–222. doi: 10.1159/000480340 [DOI] [PubMed] [Google Scholar]
- 34.Quaile MP, Melich DH, Jordan HL, et al. Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol. 2010;243(3):340–347. doi: 10.1016/j.taap.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 35.Jin L, Jin F, Guo S, et al. Metformin Inhibits NLR family pyrin domain containing 3 (NLRP)-relevant neuroinflammation via an Adenosine-5’-Monophosphate-Activated Protein Kinase (AMPK)-dependent pathway to alleviate early brain injury after subarachnoid hemorrhage in mice. Front Pharmacol. 2022;13:796616. doi: 10.3389/fphar.2022.796616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7(6):447–449. doi: 10.1038/nmeth.1455 [DOI] [PubMed] [Google Scholar]
- 38.Huet O, Ramsey D, Miljavec S, et al. Ensuring animal welfare while meeting scientific aims using a murine pneumonia model of septic shock. Shock. 2013;39(6):488–494. doi: 10.1097/SHK.0b013e3182939831 [DOI] [PubMed] [Google Scholar]
- 39.Cao Z, Qin H, Huang Y, et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model. Bioengineered. 2022;13(3):4810–4820. doi: 10.1080/21655979.2022.2033381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Cao Z, Liang H, Zhao C, Gong B, Hu J. Effect of low-dose ethanol on NLRP3 inflammasome in diabetes-induced lung injury. Exp Anim. 2021;70(3):364–371. doi: 10.1538/expanim.20-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Lu B, Fu J, Zhu X, Song E, Song Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J Hazard Mater. 2021;404(Pt B):124050. doi: 10.1016/j.jhazmat.2020.124050 [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Long G, Luo H, et al. S100A8/A9: an emerging player in sepsis and sepsis-induced organ injury. Biomed Pharmacother. 2023;168:115674. doi: 10.1016/j.biopha.2023.115674 [DOI] [PubMed] [Google Scholar]
- 44.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–831. doi: 10.1016/j.cell.2007.12.040 [DOI] [PubMed] [Google Scholar]
- 45.Sborgi L, Ruhl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi: 10.15252/embj.201694696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 47.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/nejm200005043421806 [DOI] [PubMed] [Google Scholar]
- 48.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chimenti L, Camprubi-Rimblas M, Guillamat-Prats R, et al. Nebulized heparin attenuates pulmonary coagulopathy and inflammation through alveolar macrophages in a rat model of acute lung injury. Thromb Haemost. 2017;117(11):2125–2134. doi: 10.1160/TH17-05-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Y, Li M, Yangzhong X, et al. Pyroptosis in inflammation-related respiratory disease. J Physiol Biochem. 2022;78(4):721–737. doi: 10.1007/s13105-022-00909-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ying Y, Mao Y, Yao M. NLRP3 inflammasome activation by MicroRNA-495 promoter methylation may contribute to the progression of acute lung injury. Mol Ther Nucleic Acids. 2019;18:801–814. doi: 10.1016/j.omtn.2019.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, Wang Z, He R, et al. Buformin alleviates sepsis-induced acute lung injury via inhibiting NLRP3-mediated pyroptosis through an AMPK-dependent pathway. Clin Sci. 2022;136(4):273–289. doi: 10.1042/CS20211156 [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Lu L, Zhong X, et al. Metformin reduced NLRP3 inflammasome activity in Ox-LDL stimulated macrophages through adenosine monophosphate activated protein kinase and protein phosphatase 2A. Eur J Pharmacol. 2019;852:99–106. doi: 10.1016/j.ejphar.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreejit G, Abdel-Latif A, Athmanathan B, et al. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation. 2020;141(13):1080–1094. doi: 10.1161/circulationaha.119.043833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo Q, Zhao Y, Li J, et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe. 2021;29(2):222–235 e4. doi: 10.1016/j.chom.2020.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denstaedt SJ, Bustamante AC, Newstead MW, et al. Long-term survivors of murine sepsis are predisposed to enhanced LPS-induced lung injury and proinflammatory immune reprogramming. Am J Physiol Lung Cell Mol Physiol. 2021;321(2):L451–L465. doi: 10.1152/ajplung.00123.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122(8):2711–2719. doi: 10.1172/JCI62423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakobsson G, Papareddy P, Andersson H, et al. Therapeutic S100A8/A9 blockade inhibits myocardial and systemic inflammation and mitigates sepsis-induced myocardial dysfunction. Crit Care. 2023;27(1):374. doi: 10.1186/s13054-023-04652-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Payen D, Lukaszewicz AC, Belikova I, et al. Gene profiling in human blood leucocytes during recovery from septic shock. Intensive Care Med. 2008;34(8):1371–1376. doi: 10.1007/s00134-008-1048-1 [DOI] [PubMed] [Google Scholar]
- 61.Ding Z, Du F, Averitt VR, et al. Targeting S100A9 reduces neutrophil recruitment, inflammation and lung damage in abdominal sepsis. Int J Mol Sci. 2021;22(23):12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703 [DOI] [PubMed] [Google Scholar]
- 64.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324. doi: 10.1038/nri.2017.142 [DOI] [PubMed] [Google Scholar]
- 65.Yang X, An X, Wang C, et al. Protective effect of oxytocin on ventilator-induced lung injury through NLRP3-mediated pathways. Front Pharmacol. 2021;12. doi: 10.3389/fphar.2021.722907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M, Im M, Lee JS, et al. Effect of S100A8 and S100A9 on expressions of cytokine and skin barrier protein in human keratinocytes. Mol Med Rep. 2019. doi: 10.3892/mmr.2019.10454 [DOI] [PubMed] [Google Scholar]
- 67.Zheng J, Wang J, Liu H, et al. Alarmins S100A8/A9 promote intervertebral disc degeneration and inflammation-related pain in a rat model through toll-like receptor-4 and activation of the NF-kappaB signaling pathway. Osteoarthritis Cartilage. 2022;30(7):998–1011. doi: 10.1016/j.joca.2022.03.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author upon reasonable request.