Highlights
-
•
The etiology of most encephalitis cases remained unknown.
-
•
Chikungunya virus was the predominant virus associated with encephalitis cases.
-
•
Chikungunya virus is a significant cause of encephalitis in Brazil.
Keywords: Encephalitis, Arbovirus, Chikungunya, Brazil
Abstract
Objectives
Encephalitis is a severe neurological syndrome for which herpesvirus and enteroviruses are the most common etiological agents. Arboviruses, a wildly diverse group of pathogens, are also critical epidemiological agents associated with encephalitis. In Brazil, little is known about the causative agents of encephalitis.
Methods
We conducted a hospital surveillance for encephalitis between 2020 and 2022. Molecular (RT-PCR and qPCR) and serological (virus-specific IgM and viral antigens) techniques were performed in cerebrospinal fluid and serum samples obtained from study participants.
Results
In the 43 participants evaluated, the etiologic agent or the presence of IgM was detected in 16 (37.2%). Nine (20.9%) cases were positive for chikungunya virus (CHIKV), three (7.0%) for dengue virus, two (4.7%) for human adenovirus, one (2.3%) for varicella-zoster virus, and one (2.3%) for enterovirus. Whole-genome sequencing revealed that the CHIKV identified belongs to the East/Central/South African lineage.
Conclusion
Herein, CHIKV is a common pathogen identified in encephalitis cases. Our results reinforce previous evidence that chikungunya represents a significant cause of encephalitis during CHIKV outbreaks and epidemics and add to existing information on the epidemiology of encephalitis in Brazil.
Introduction
Viral encephalitis is mainly characterized by fever, headache, and decreased consciousness. Although, in most cases, the underlying cause of encephalitis is unknown, viruses of the Herpesviridae family are the most common, accounting for 50%–75% of identified agents [1]. In the Americas, the most common arboviruses related to encephalitis are West Nile virus and St. Louis encephalitis virus (SLEV) [1]. On the other hand, encephalitis has uncommonly been associated with dengue (DENV), chikungunya (CHIKV), and zika (ZIKV) viral infections.
In Brazil, few studies have investigated the etiological agents responsible for viral encephalitis, with relatively low success in pathogen identification [[2], [3], [4]]. The present study analyzed a series of encephalitis cases in northeastern Brazil, a region with a high incidence of arboviruses.
Methodology
Participants and ethical aspects
A cross-sectional study was performed from March 2020 to August 2022 at 12 hospitals in three different cities in Bahia, northeastern Brazil. Patients admitted to any of the participating hospitals who met the clinical criteria for acute encephalitis (patients with altered mental status lasting ≥24 hours with no alternative cause identified and two or more of the following conditions: documented fever, seizures, new onset focal neurological findings, cerebrospinal fluid (CSF) leukocyte count >5/mm3, abnormality on neuroimaging or EEG compatible with encephalitis [5], were enrolled. Patients were excluded if psychiatric disorders, substance abuse, cancer, toxins, autoimmune diseases affecting the nervous system, and non-infectious inflammatory conditions were diagnosed. Clinical and epidemiological data were obtained through the review of medical records. Data management was performed using REDCap 9.3.1-2021 (Vanderbilt University, USA). Biological samples (serum and CSF) were collected by health professionals and transported to the Gonçalo Moniz Institute (IGM)—Fiocruz. This study was approved by the IGM-Fiocruz IRB (CAAE 30400320.5.0000.0040). Written informed consent was obtained from all participants.
Serology
Serum samples were screened for CHIKV, DENV, and ZIKV IgM antibodies and NS1 antigen using commercial kits (Euroimmun, Lübeck, Germany).
Molecular detection
All samples were submitted to nucleic acid extraction using a commercial PureLink Mini RNA and DNA Purification Kit (Invitrogen). The Multi Neuro 9 (XGEN, Mobius Life Science) kit was performed in CSF samples to detect human adenovirus (HadV), cytomegalovirus, Epstein–Barr virus (EBV), herpes simplex virus (HSV-1 and 2), varicella-zoster virus (VZV), human parechovirus, erythrovirus B19, human herpes virus types 6 (HHV6) and 7 (HHV7) and members of the enterovirus genus (EV). A Multiplex ZIKV, DENV, and CHIKV RT-qPCR kit (ZDC, Bio-Manguinhos) was performed in serum and CSF samples. All amplifications were performed on an Applied Biosystems 7500 Real-Time PCR Systems analyzer.
CHIKV genomic sequencing and phylogenetic analysis
Whole-genome sequencing was performed on PCR-positive CHIKV samples. Samples were subjected to nucleic acid extraction and purification using the Magmax Pathogen RNA/DNA kit and the KingFisher Plex Purification System (Thermo Fisher). Amplicon sequencing was performed on a MinION device (Oxford Nanopore Technologies), while CHIKV whole-genome multiplex PCR and library preparation were performed as previously described [6]. Genome assembly and final consensus sequences were obtained using Genome Detective software. The genomes generated herein are available at GenBank under accessions OR453562, OR453563, and OR453564.
The CHIKV consensus sequences were combined with globally available complete genome sequences from West-African (n = 14), Asian-Caribbean (n = 386), and East-Central-South African (ECSA) (n = 647) CHIKV genotypes retrieved from NCBI. All sequences were aligned using MAFFT and manually edited using Aliview. A maximum likelihood phylogenetic tree was estimated using IQ-TREE2 software, and tree topology robustness was determined using 1000 bootstrap replicates [6].
Results
Of the 43 enrolled patients, the majority were male (55.8%) and ≥60 years old (37.2%). The symptoms mostly reported were fever (37.2%), arthralgia (20.9%) and headache (16.3%). Mental confusion (32.5%), somnolence (30.2%), psychomotor agitation (25.6%), and epileptic seizures (25.6%) were the predominant neurological symptoms observed upon admission. Twenty-four patients (55.8%) reported having at least one comorbidity. The most reported comorbidity was Systemic Arterial Hypertension (34.9%) and Diabetes Mellitus (13.9%). No HIV infection or other immunodeficiency were identified (Supplementary Table 1).
Anti-CHIKV IgM serology was performed in 39 serum samples, with positivity detected in nine (23.1%). IgM anti-DENV serology performed in 33 samples returned one (3%) positive test, while NS1 DENV antigen yielded two (6.1%) positive results. Anti-ZIKV IgM serology was negative in all samples (Table 1).
Table 1.
Results of molecular and serological virus testing in 43 patients hospitalized due to symptomatology compatible with encephalitis (Bahia-Brazil).
| N positive/N tested (% positive) |
|||
|---|---|---|---|
| Target | CSF samples | Serum samples | |
| Molecular | EBV | 3/40 (7.5) | |
| HAdV | 2/40 (5.0) | ||
| EV | 1/40 (2.5) | ||
| VZV | 1/40 (2.5) | ||
| HSV1/HSV2 CMV |
0/40 (0) 0/40 (0) |
||
| HpeV | 0/40 (0) | ||
| HHV6 | 0/40 (0) | ||
| HHV7 | 0/40 (0) | ||
| B19 | 0/40 (0) | ||
| CHIKV | 1/43 (2.3) | 2/35 (5.7) | |
| DENV | 0/43 (0) | 0/35 (0) | |
| ZIKV | 0/43 (0) | 0/35 (0) | |
| flavivirus genus | 0/43 (0) | 0/29 (0) | |
| SARS-CoV-2 | 0/34 (0) | ||
| Serology | IgM anti-CHIKV | 9/39 (23.1) | |
| IgM anti-DENV | 1/33 (3) | ||
| IgM anti-ZIKV | 0/33 (0) | ||
| Anti-DENV NS1 | 2/33 (6.1) | ||
B19, erythrovirus B19; CHIKV, chikungunya virus; CMV, cytomegalovirus; DENV, dengue virus; EBV, Epstein–Barr virus; EV, enterovirus genus; HadV, human adenovirus; HHV6, human herpes virus types 6; HHV7, human herpes virus types 7; HpeV, human parechovirus; HSV, herpes simplex virus; VZV, varicella-zoster virus; ZIKV, zika virus.
The Multi Neuro 9 test was performed in 40 CSF samples with two positive results for HAdV (5%), one for EV (2.5%), and one for VZV (2.5%) (Table 1). Three CSF samples tested positive for EBV but were interpreted as indicative of viral reactivation. ZDC RTq-PCR was performed on all 43 CSF samples and 35 serum samples. All samples tested negative for ZIKV and DENV, while CHIKV was detected in one (2.3%) CSF sample and two (5.7%) serum samples from two patients. Patient ID2 was PCR positive for CHIKV both in serum and CSF samples and IgM positive in serum sample, while patient ID7 was PCR positive for CHIKV in serum.
In total, the etiologic agent, or the presence of IgM, was identified in 16 (37.2%) individuals, with 12 (27.9%) of them positive for arboviruses: nine (20.9%) for CHIKV and three (7.0%) for DENV.
Considering recently confirmed CHIKV infections reported in Bahia, seven out of nine acute encephalitis patients who tested positive for CHIKV (by IgM or PCR) were sampled during the 2020 outbreak. Notably, June 2020 was the month with the highest number of encephalitis cases included in our study, just 1 month after the peak of the 2020 CHIKV outbreak in Bahia (Supplementary Figure 1).
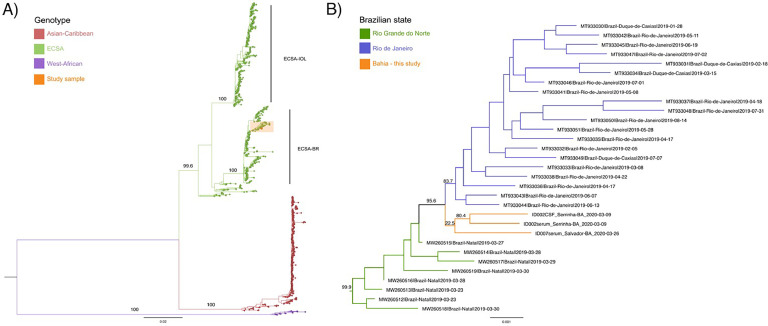
Whole genome sequencing was performed on the three CHIKV PCR-positive samples obtained from two participants: ID2 (serum and CSF) and ID7 (serum). Phylogenetic analysis revealed that all three sequences belonged to the ECSA genotype. The sequences were consistent with the viral diversity reported in Brazil, clustering within a highly supported (99.9 bootstrap value) clade containing sequences isolated in the states of Rio Grande do Norte and Rio de Janeiro in 2019 (Figure 1). The three newly generated sequences grouped with low support (bootstrap value <70); in turn, the CSF and serum samples from patient ID2 clustered with high support (80.4 bootstrap value). Of note, high genetic diversity was observed between these two samples, with eight synonymous mutations and 18 non-synonymous mutations affecting 13 codons detected throughout the viral genome (Supplementary Table 2).
Figure 1.
Phylogenetic analysis of the CHIKV genomes generated in this study. (a) Maximum likelihood (ML) tree representing CHIKV global diversity; ECSA-IOL (East, Central, South African genotype—Indian Ocean Lineage), ECSA-BR (ECSA—Brazilian lineage). (b) ML tree showing the clade formed by newly generated sequences from Bahia highlighted in orange. Bootstrap support shown in key branches.
Discussion
The present study attempted to reveal novel insights into the epidemiology of encephalitis in Brazil. Herein, the etiological agent of encephalitis was identified in 37.2% of the cases, a higher frequency than in other Brazilian studies. Considering only the PCR-positive cases (13.9%), our results are similar to previous studies, which detected the etiological agent in 5.2%–29.7% of encephalitis cases [[2], [3], [4]].
In this study, the rates of EVs at 2.3% were consistent with previous findings in Brazil, which ranged from 3.0% to 18.1% of encephalitis cases attributed to EVs [3,4,7]. Similarly, viruses belonging to the Herpesviridae family were detected in 10% of the samples, aligning with other Brazilian studies reporting herpesvirus prevalence in CSF samples ranging from 3.8% to 20.6% [3,4,7]. However, after excluding the three cases of EBV considered to be reactivation, only one (2.3%) case of VZV was observed. Surprisingly, no cases of HSV-1 or HSV-2 were detected. Consistently, previous Brazilian studies also reported low detection rates of HSV-1/HSV-2, ranging from 0.4% to 5% [[2], [3], [4]]. HAdV, identified in two (4.7%) cases herein, has not been reported in other Brazilian studies.
Few Brazilian studies have investigated the presence of arboviruses in patients with encephalitis. A previous study identified 2.7% ZIKV and 0.8% DENV [8], and another study reported 0.8% DENV and 0.4% SLEV [3]. Thus, the present study's findings reveal a more significant role played by arboviruses as the etiological agent in cases of encephalitis.
Evidence indicating CHIKV as a neuroinvasive agent was reported during a 2005–2006 epidemic on the island of La Réunion, resulting in a 20-fold increase in encephalitis cases [9]. In Brazil, reports of CHIKV detection have occurred in CNS viral infections [10]. Among 68 CHIKV fatal cases, 39 presented neurological signs, and CHIKV RNA was found in the CSF of 92.3% of them [11].
CHIKV strains identified herein belonged to the ECSA genotype, which is responsible for the recent chikungunya fever outbreak in Brazil [6]. High genetic diversity was observed between the CHIKV genomes generated from CSF and serum samples from participant ID2, suggesting the compartmentalized evolution of the virus in different host tissues. Similar results were reported in a study investigating paired samples of serum and CSF or pairs of mothers and newborns infected with CHIKV [12].
We identified CHIKV as the most common etiological agent in encephalitis cases in our region. The majority of CHIKV-positive samples were collected between March and July 2020, coinciding with a significant outbreak of this arbovirus in Bahia. Unfortunately, we were unable to conduct anti-CHIKV IgM testing on CSF samples, and we acknowledge that serum IgM positivity for CHIKV does not necessarily imply a causal relationship between CHIKV infection and encephalitis. However, the two positive PCR results confirming the presence of CHIKV in CSF and/or serum provide further support for CHIKV as a causative agent for encephalitis in endemic regions.
Declarations of competing interest
The authors declare no conflicts of interest.
Acknowledgments
Author contributions
MPS: performed molecular diagnosis and wrote the first draft of the manuscript. MSR: supervision of recruitment and data collection. LCM, TLAF, PAPJ, DSF, MSS, AJPG, FOC, MCB, CS, JRLPC, JMMT, MML, BGGC, GAV: recruitment of participants and data collection. HF, JX, MG, ALSM, FMP, and VF: performed whole-genome sequencing and phylogenetic analysis. GSC, IABL, YSFM, MVOF, FMLC, MBRS, and RK: contributed with molecular biology experiments. ICS and TG: conceived, designed the study, analyzed the data, and critically revised the manuscript. All authors agreed to be accountable for all aspects of the work and approved the final version to be published.
Funding
This study was supported by the Instituto Gonçalo Moniz—Fundação Oswaldo Cruz (Excellence in Research Program 077/2020), the Serrapilheira Institute (grant number Serra—1912-31772), and through National Institutes of Health USA grant U01AI151698 for the United World Arbovirus Research Network (UWARN). ICS was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 316456/2021-7).
Acknowledgments
The authors are grateful to the health professionals involved in the patient's clinical treatment and would like to thank Andris K. Walter for critical analysis, English language revision, and manuscript copyediting assistance. MG is supported in part by the CRP-ICGEB RESEARCH GRANT 2020 Project CRP/BRA20-03, Contract CRP/20/03.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2024.107090.
Appendix. Supplementary materials
References
- 1.Tyler K.L. Acute viral encephalitis. N Engl J Med. 2018;379:557–566. doi: 10.1056/NEJMra1708714. [DOI] [PubMed] [Google Scholar]
- 2.Bastos M.S., Lessa N., Naveca F.G., Monte R.L., Braga W.S., Figueiredo L.T.M., et al. Detection of herpesvirus, enterovirus, and arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J Med Virol. 2014;86:1522–1527. doi: 10.1002/jmv.23953. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira J.E., Ferreira S.C., Almeida-Neto C., Nishiya A.S., Alencar C.S., Gouveia G.R., et al. Molecular characterization of viruses associated with encephalitis in São Paulo, Brazil. PLoS One. 2019;14:1–10. doi: 10.1371/journal.pone.0209993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza L.P., Bronzoni R.V., de M., Takayanagui O.M., Aquino V.H., Moraes Figueiredo L.T. Viral infections of the central nervous system in Brazil. J Infect. 2007;54:589–596. doi: 10.1016/j.jinf.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xavier J., Alcantara L.C.J., Fonseca V., Lima M., Castro E., Fritsch H., et al. Increased interregional virus exchange and nucleotide diversity outline the expansion of chikungunya virus in Brazil. Nat Commun. 2023;14:4413. doi: 10.1038/s41467-023-40099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle D.A.D., Santos M.L.S.F., Giamberardino H.I.G., Raboni S.M., Scola R.H. Acute childhood viral encephalitis in Southern Brazil. Pediatr Infect Dis J. 2020;39:894–898. doi: 10.1097/INF.0000000000002709. [DOI] [PubMed] [Google Scholar]
- 8.Milhim B.H.G.A., da Rocha L.C., Terzian A.C.B, Mazaro C.C.P, Augusto M.T, Luchs A, et al. Arboviral infections in neurological disorders in hospitalized patients in São José do Rio Preto, São Paulo, Brazil. Viruses. 2022;14:1488. doi: 10.3390/V14071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gérardin P., Couderc T., Bintner M., Tournebize P., Renouil M., Lémant J., et al. Chikungunya virus-associated encephalitis: a cohort study on La Réunion Island, 2005-2009. Neurology. 2016;86:94–102. doi: 10.1212/WNL.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 10.Mello C., da S., Cabral-Castro M.J., Silva de Faria L.C., Peralta J.M., Puccioni-Sohler M. Dengue and chikungunya infection in neurologic disorders from endemic areas in Brazil. Neurol Clin Pract. 2020;10:497–502. doi: 10.1212/CPJ.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lima S.T.S., de Souza W.M., Cavalcante J.W., da Silva Candido D., Fumagalli M.J., Carrera J.P., et al. Fatal outcome of chikungunya virus infection in Brazil. Clin Infect Dis. 2020;73:e2436–e2443. doi: 10.1093/cid/ciaa1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres M.C., Di Maio F., Brown D., Spyer M., Nastouli E., Brasil P., et al. In depth viral diversity analysis in atypical neurological and neonatal chikungunya infections in Rio de Janeiro, Brazil. Viruses. 2022;14:2006. doi: 10.3390/v14092006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.