Abstract
Cell-to-cell spread of tobacco mosaic virus is facilitated by the virus-encoded 30-kDa movement protein (MP). This process involves interaction of viral proteins with host components, including the cytoskeleton and the endoplasmic reticulum (ER). During virus infection, high-molecular-weight forms of MP were detected in tobacco BY-2 protoplasts. Inhibition of the 26S proteasome by MG115 and clasto-lactacystin-β-lactone enhanced the accumulation of high-molecular-weight forms of MP and led to increased stability of the MP. Such treatment also increased the apparent accumulation of polyubiquitinated host proteins. By fusion of MP with the jellyfish green fluorescent protein (GFP), we demonstrated that inhibition of the 26S proteasome led to accumulation of the MP-GFP fusion preferentially on the ER, particularly the perinuclear ER. We suggest that polyubiquitination of MP and subsequent degradation by the 26S proteasome may play a substantial role in regulation of virus spread by reducing the damage caused by the MP on the structure of cortical ER.
To spread infection from cell to cell, plant viruses must cross the rigid cell wall between cells by exploiting cytoplasmic bridges referred to as plasmodesmata. These cytoplasmic channels maintain continuity of the plasma membrane, the cytoplasm, and the endoplasmic reticulum (ER) between neighboring cells (30, 33, 43). Plasmodesmata enable neighboring plant cells to exchange small signal molecules and metabolites but under normal conditions exclude molecules of more than 1 kDa. Thus, plasmodesmata must be modified in order to allow the spread of infection from cell to cell. During replication, plant viruses produce proteins that facilitate cell-cell transport, referred to as movement proteins (MP). MPs have been demonstrated to directly or indirectly modify the size exclusion limit of plasmodesmata to permit viral spread (10, 30, 56).
Tobacco mosaic tobamovirus (TMV), a plus-sense RNA virus, encodes four proteins. The 126-kDa replicase and a 183-kDa replicase readthrough product are involved in genome amplification and are translated from genomic RNA, while the 17.5-kDa coat protein (CP) and the 30-kDa MP are translated from subgenomic RNAs.
Although there have been very detailed investigations of the function of the TMV MP and its intracellular localization, few studies have dealt with the regulation of virus spread and the involvement of the MP in this process. Genetic studies involving transgenic plants expressing functional and nonfunctional MP variants, and studies involving viruses that contain mutations in the MP, established the role of the MP in facilitating cell-cell spread of infection (5, 9, 10, 14, 20, 32). Electron microscopy, fluorescence microscopy, and cell fractionation were used to elucidate the intracellular localization of MP and MP-GFP fusion proteins (1, 18, 19, 31, 38, 45, 46). These studies established that the MP is tightly associated with plasmodesmata in transgenic plants and that during virus infection there is a transient association with several compartments of the cell, including the ER and the cytoskeleton. Dramatic morphological changes of the ER were observed in infected tissue that paralleled the accumulation and degradation of the TMV MP (38), and biochemical studies established that the MP behaves as an integral membrane protein (34, 38).
Several studies have suggested that posttranslational phosphorylation of serine and threonine residues in the MP are involved in regulation of cell-cell spread (2, 7, 16, 52). Radiolabeling of MP and subsequent digestion by trypsin was used to deduce peptides that are phosphorylated (7, 16). In one study a cell wall-associated kinase was reported to be involved in the in vitro phosphorylation of C-terminal MP amino acids (7). It was recently demonstrated that the MP of the tomato mosaic tobamovirus ToMV, a tomato strain of TMV, is phosphorylated in vivo at amino acids 37 and 238. Serine 37, but not serine 238, is essential for function of MP in cell-cell spread and is involved in intracellular localization and stability of the MP (25). During early and mid-stages of virus infection, the MP is produced transiently, while the replicase proteins and the CP are constitutively expressed (50). However, the mechanisms responsible for transient appearance of MP have not been investigated in detail.
Here we describe a posttranslational modification of the MP that is different from phosphorylation. Our data suggest the covalent linkage of multiple copies of ubiquitin to the MP and subsequent degradation of the conjugates by the 26S proteasome. In eukaryotes the sequential addition of ubiquitin monomers to short-lived proteins involves a ubiquitin-activating enzyme (E1) and a ubiquitin-conjugating enzyme (E2); for transfer of the activated ubiquitin from E2 to lysine residues of the target, a ubiquitin-ligase activity (E3) is required (22, 47).
In tobacco protoplasts infected by TMV, we observed low amounts of high-molecular-weight forms of the MP in mid-stages of infection. Here we show that these accumulated to very high levels when infected protoplasts were cultured in the presence of specific proteasome inhibitors. Inhibitor treatment significantly increased the stability of the otherwise transiently expressed MP. In experiments with TMV-MP:GFP, a virus encoding a functional MP-GFP fusion protein, fluorescence levels were increased in treated cells and the MP-GFP fusion accumulated predominantly in aggregates around the nucleus. Taken together, these results suggest that the TMV MP becomes polyubiquitinated during virus infection and subsequently enters the 26S proteasome degradation pathway. We suggest that removal of the MP by proteolysis together with the transcriptional regulation of MP subgenomic RNA levels controls the transient accumulation of the TMV MP during virus infection.
MATERIALS AND METHODS
Constructs.
In vitro transcription was used to prepare infectious transcripts from plasmids pTMV (pU3/12-4 [20]), pTMV-ΔM (35), pTMV-ΔC (18), and pTMV-M:GfusBr (36), subsequently referred to as pTMV-MP:GFP.
Tobacco suspension culture.
Protoplasts were prepared from tobacco BY-2 suspension culture cells and infected with in vitro transcripts by electroporation as described elsewhere (51, 53). For each experiment, protoplasts from the same batch were used and electroporations were performed with single preparations of transcripts. Roughly 2 × 106 protoplasts were used per electroporation and cultured in the absence or presence of inhibitors in batches of 2 × 105 protoplasts. For Western blot analyses, the cells were harvested by centrifugation at 100 × g and resuspended with 2× sodium dodecyl sulfate loading dye. Aliquots that corresponded to 104 protoplasts per lane were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses. Each experiment was repeated at least three times, and individual time points were analyzed by triplicate samples, which were pooled prior to harvesting of the protoplasts.
Inhibitor studies.
Stock solutions of inhibitors used in these studies were prepared in either water (E-64 and lactacystin) or dimethyl sulfoxide (all others). Inhibitors were used at final concentrations of 50 μM E-64 ([l-3-trans-carboxyoxiran-2-carbonyl]-l-leucyl-agmatin; Peptides International, Louisville, Ky.), 25 μM ALLM (N-acetyl-l-leucyl-l-leucyl-l-methioninal; Sigma), 50 μM MG115 (Z-leucyl-leucyl-norvaline-H; Peptides International), 20 μM lactacystin (Calbiochem, San Diego, Calif.), and 20 μM clasto-lactacystin-β-lactone (Calbiochem). Final concentration of dimethyl sulfoxide (DMSO) in the protoplast culture medium was 0.1%. Western blot analysis was performed as described elsewhere (38). Mouse antiubiquitin monoclonal antibody 1510 (Chemicon International, Temecula, Calif.) was used at 1:1,000 dilution. Affinity-purified anti-MP antibody (24) was used at 1:1,000 dilution. Antireplicase antiserum 5 (H. Padgett, unpublished data) was used at 1:10,000. Anti-CP antiserum was used at 1:5,000 dilution. All primary antibodies were incubated overnight at 4°C. Secondary antibodies (ImmunoPure goat anti-rabbit/anti-mouse immunoglobulin G [heavy plus light chain], peroxidase conjugated; Pierce, Rockford, Ill.) were used at 1:100,000 dilution for 90 min at room temperature. Quantification of the Western blots was performed using a phosphorimaging system (Molecular Imager System GS-525; Bio-Rad, Hercules, Calif.) with screens for analysis of chemiluminescence. Imaging data were analyzed using Multi-Analyst software (version 1.0.2; Bio-Rad).
Fluorescence microscopy.
The microscopic studies were performed as described elsewhere (24). Shortly before microscopy, aliquots of the cultured protoplasts were centrifuged at approximately 100 × g, and the protoplast pellet was carefully resuspended in a small volume of culture medium. Aliquots of 6.5 μl of protoplast solution were covered by 19- by 19-mm cover slips and immediately used for conventional fluorescence microscopy. Pictures were processed and digitized as described elsewhere (38). Protoplasts used for Fig. 5 and 6 originated from the same protoplast preparation.
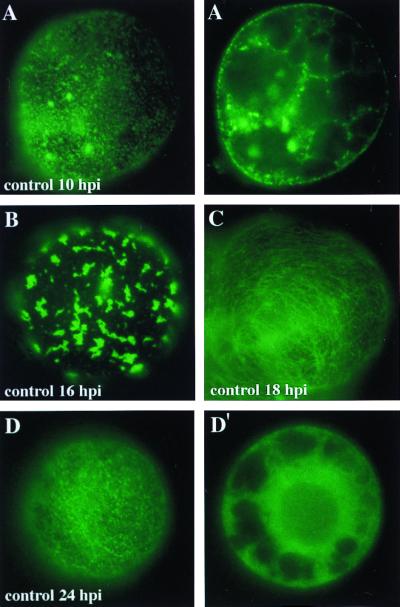
FIG. 5.
Intracellular localization of MP-GFP during TMV-MP:GFP infection. Tobacco BY-2 protoplasts were cultured in the absence of protease or proteasome inhibitors, and aliquots were prepared for conventional fluorescence microscopy of living cells at 10, 16, 18, and 24 hpi. Panels A, A′, D, and D′ represent peripheral views (A and D) and nuclear sections (A′ and D′) of single protoplasts.
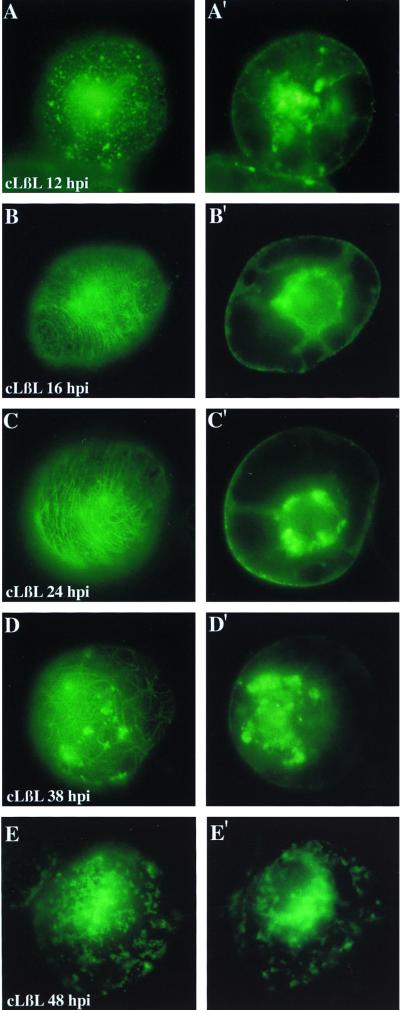
FIG. 6.
Effects of the inhibition of the 26S proteasome on the intracellular localization and accumulation of a fusion protein of MP and GFP. Tobacco BY-2 protoplasts were cultured in the presence of the proteasome inhibitor clasto-lactacystin-β-lactone (cLβL) and aliquots were prepared for conventional fluorescence microscopy of living cells at 12, 16, 24, 38, and 48 hpi. (A to E) Views of the protoplast periphery; (A′ to E′) optical planes sectioning the nucleus. Individual pictures were taken with a 35-mm camera on Kodak 400 ASA slide film. Slides were scanned with a Nikon slide scanner and assembled using Adobe Photoshop 4.0.
RESULTS
Standard Western blot analyses of TMV-infected tissues often reveals high-molecular-weight bands that react with the anti-MP antibody. These forms accumulate during the course of virus infection and are most prominent in mid-stages of infection (Fig. 1). The fact that the TMV MP is only transiently expressed during virus infection (50), and that a strong pattern of degradation products of the MP is observed by Western blot analysis (24), led us to investigate the effects of several protease inhibitors on the accumulation of the degradation products as well as on the high-molecular-weight forms.
FIG. 1.
Time course experiment of TMV infection in BY-2 protoplasts. Samples were collected at 2, 4, 8, 10, 20, and 24 hpi and subjected to Western blot analysis with anti-MP antibodies. The transient accumulation of MP and of MP degradation products is demonstrated.
Effects of protease and proteasome inhibitors.
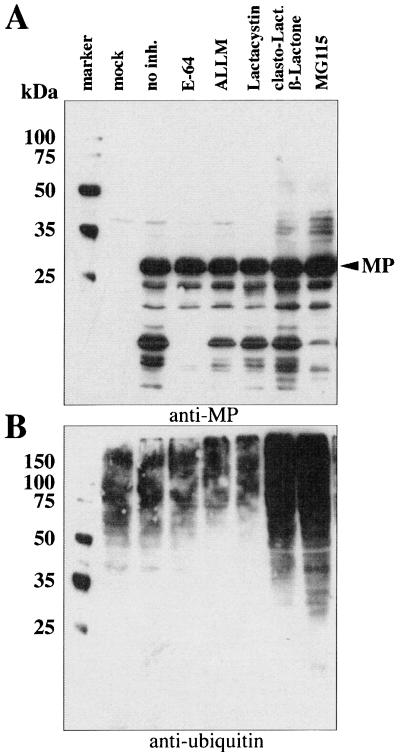
To test the effects of protease and proteasome inhibitors on the accumulation of degradation products and high-molecular-weight forms of the MP, we infected tobacco BY-2 protoplasts with TMV transcripts. The protoplasts were subsequently cultured in the absence or presence of inhibitors of lysosomal proteases (E-64 and ALLM) or inhibitors of the 26S proteasome degradation pathway (lactacystin, clasto-lactacystin-β-lactone, and MG115). A sample of nontreated, mock-inoculated protoplasts was processed in parallel in each experiment. Ten hours after infection, the protoplasts were harvested and subjected to Western blot analysis with anti-MP and antiubiquitin antibodies (Fig. 2). Mock-inoculated protoplasts revealed only a weakly cross-reacting band at approximately 40 kDa, which was also visible in most of the other samples probed with anti-MP antibody (Fig. 2A). Cells infected with TMV but not treated with any inhibitor showed a strong band of MP at about 30 kDa and a complex pattern of bands with higher mobility; the latter are presumed to be degradation products of the MP. In protoplasts treated with inhibitors of lysosomal proteases (E-64 and ALLM), the pattern of degradation products was less pronounced than for nontreated cells, in particular for cells treated with E-64.
FIG. 2.
Effects of protease and proteasome inhibitors on the accumulation of MP during TMV infection of tobacco BY-2 protoplasts. Protoplasts were harvested after the indicated time and subjected to Western blot analysis with anti-MP and antiubiquitin antibodies. (A) Protoplasts cultured in the absence or presence of inhibitors were analyzed with anti-MP antibody. (B) Aliquots of the same samples were analyzed in parallel with antiubiquitin antibodies.
Samples treated with proteasome inhibitors clasto-lactacystin-β-lactone and MG115 accumulated high-molecular-weight forms of MP, indicating that inhibition of this degradation pathway has a strong effect on the accumulation of MP. It was surprising that lactacystin, a well-established inhibitor of the proteasome pathway, did not have a measurable effect on the accumulation of MP. However, since the actual active species of lactacystin, clasto-lactacystin-β-lactone (11), led to accumulation of high-molecular-weight products, it is likely that lactacystin either was not taken up by these particular cells or is inactive in plant cells. The overall patterns of accumulation of high-molecular-weight bands were very similar for clasto-lactacystin-β-lactone and MG115, supporting the conclusion that both inhibit the same pathway.
To further test the function of the proteasome inhibitors, Western blot analysis of aliquots of these samples was performed with antiubiquitin antibody. The antiubiquitin antibody recognized a complex pattern of bands that ranged in apparent molecular mass from 25 to more than 150 kDa (Fig. 2B). The intensity of the signal was significantly increased in the samples treated with clasto-lactacystin-β-lactone and MG115, the two proteasome inhibitors which induced the accumulation of high-molecular-weight forms of the MP. The accumulation of ubiquitinated host proteins demonstrated the strong activity of these inhibitors (Fig. 2B). Again, lactacystin had no measurable effect.
Increased stability of MP.
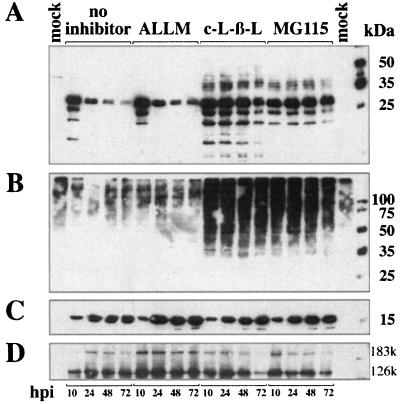
To determine whether the proteasome inhibitors alter the stability of MP, we examined their effect in a time course experiment. Infected protoplasts were incubated either in the absence of inhibitor or with ALLM, clasto-lactacystin-β-lactone, or MG115. Samples were harvested after 10, 24, 48, and 72 h and subjected to Western blot analyses. The transient nature of accumulation of MP during virus infection is clearly demonstrated in the nontreated samples and the samples treated with the protease inhibitor ALLM (Fig. 3). Quantification of the band for the native (30-kDa) MP was performed using a phosphorimaging device. By arbitrarily setting the amount of MP at 10 h postinfection (hpi) to 100%, the amount of MP decreased at 24 hpi to approximately 30 to 40% and dropped to less than 10% at the end of the experiment (Table 1). This is in agreement with the results of previous studies (19, 37, 50).
FIG. 3.
Effects of protease and proteasome inhibitors on the accumulation of MP in BY-2 protoplasts in a time course experiment. Protoplasts were cultured with or without inhibitors and harvested at the indicated times. Aliquots of the samples were subjected to Western blot analyses with anti-MP (A), antiubiquitin (B), anti-CP antibodies (C), and antireplicase (D) antibodies. The two forms of the TMV replicase are indicated by 183k and 126k. c-L-β-L, = clasto-lactacystin-β-lactone.
TABLE 1.
Quantification by phosphorimaging of levels of monomeric MP determined by Western blotting
| Inhibitor | % of level at 10 hpi
|
||
|---|---|---|---|
| 24 hpi | 48 hpi | 72 hpi | |
| None | 40.5 | 8.2 | 4.0 |
| ALLM | 27.5 | 5.9 | 8.7 |
| clasto-Lactacystin-β-lactone | 96.0 | 76.1 | 34.3 |
| MG115 | 78.8 | 46.8 | 30.5 |
In contrast, the samples treated with the proteasome inhibitors showed a remarkable increase in stability of the MP. All samples treated with clasto-lactacystin-β-lactone or MG115 showed a complex pattern of high-molecular-weight forms of the MP. The stability of the protein was remarkably increased in the presence of these inhibitors, and even after 72 h at least 30% of the 30-kDa band representing the MP monomer remained in the cells (Fig. 3; Table 1). This is in strong contrast to the nontreated samples, supporting the conclusion that the 26S proteasome is involved in the degradation of the MP.
Samples of the experiment described above, which included noninfected and infected cells, were also probed with antiubiquitin antibody following treatment with inhibitor. As in the previous experiments, clasto-lactacystin-β-lactone and MG115 led to increased accumulation of high-molecular-weight proteins (Fig. 3). These are presumed to be polyubiquitinated host proteins that accumulate due to inhibition of the 26S proteasome.
To examine the possibility that clasto-lactacystin-β-lactone and MG115 have a general, nonspecific effect on virus replication per se, and thus on the accumulation of other viral proteins, we probed Western blot membranes prepared from aliquots of the same experiment with anti-CP and antireplicase serum. Both proteins accumulate during virus infection and do not share the transient nature of accumulation with the MP (50). It is apparent that the CP accumulated during the course of the experiment and reached its highest level toward the end of the time course (Fig. 3). The antireplicase serum recognizes two forms of the TMV replicase that are essential for TMV replication—the 126- and 183-kDa forms. Both proteins accumulated during the experiment, and only at very late stages of infection was a decrease of the two proteins observed in samples treated with proteasome inhibitors (Fig. 3, 72 hpi). We therefore concluded that inhibition of the 26S proteasome pathway specifically affects the transient nature of MP synthesis and degradation and has no significant influence on the accumulation of the other viral proteins.
Specificity of modification of the MP.
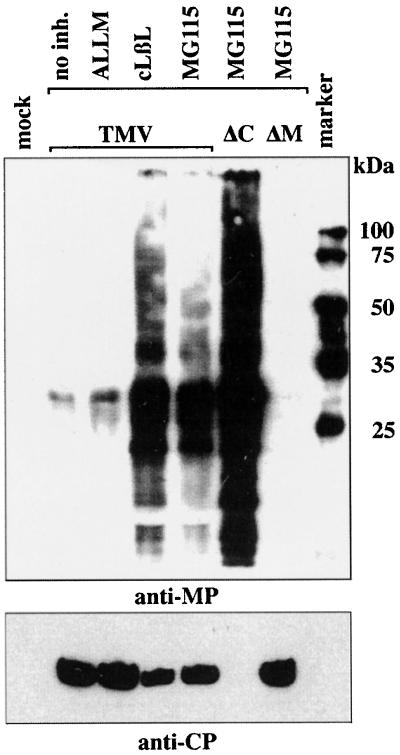
To further test whether the accumulation of high-molecular-weight proteins is derived from MP, we examined cells infected with a mutant virus that lacked a functional MP gene (TMV-ΔM). Cells were infected with either wild-type TMV, TMV-ΔC, a virus construct lacking the coat protein, or with TMV-ΔM. Samples were harvested at 72 hpi and subjected to Western blot analyses using anti-MP antibody (Fig. 4). In the lanes which contain nontreated samples or samples treated with ALLM, low levels of MP were detected at 72 hpi, consistent with the results presented in Fig. 3 and Table 1. In samples treated with clasto-lactacystin-β-lactone and MG115, a strong band of the 30-kDa native MP and high-molecular-weight forms of MP were detected. Protoplasts infected with TMV-ΔC and treated with MG115 showed a pattern similar to that for cells infected with wild-type TMV, although significantly more MP was observed in cells infected with TMV-ΔC than in cells infected with wild-type TMV. It was previously shown that TMV lacking the CP produces more MP than does wild-type virus (8). When cells infected with a virus lacking a functional MP were treated with MG115, no MP was detected. Furthermore, there was no accumulation of high-molecular-weight bands, confirming that bands detected in cells infected with wild-type TMV (or TMV-ΔC) correspond to forms of the MP, presumably polyubiquitinated forms. The same samples were also probed with anti-CP antiserum to confirm that virus accumulation in these experiments was normal and that in the case of the cells infected with TMV-ΔM, virus infection had occurred.
FIG. 4.
Comparison of the effects of proteasome inhibition on the accumulation of viral proteins during infection with TMV, TMV-ΔC (ΔC), and TMV-ΔM (ΔM). BY-2 protoplasts were cultured in the absence (lane 1, mock) or presence of protease or proteasome inhibitors and harvested at 72 hpi. Lane 2, no inhibitor; lane 3, treated with ALLM; lane 4, treated with clasto-lactacystin-β-lactone (cLβL); lanes 5 to 7, treated with MG115. Aliquots of samples were subjected to Western blot analyses with either anti-MP antibody (top) or anti-CP serum (bottom).
Effect of proteasome inhibition on accumulation of MP-GFP.
To investigate whether inhibition of the 26S proteasome degradation pathway had a significant effect on the intracellular localization of the MP, protoplasts were infected with the virus construct TMV-MP:GFP. This construct produces MP fused to the jellyfish GFP. In this experiment, the protoplasts were cultured either in the absence or presence of the proteasome inhibitor clasto-lactacystin-β-lactone. Samples of the cultured cells were harvested 10, 12, 16, 18, 24, 38, and 48 hpi and subjected to conventional fluorescence microscopy. Two optical planes were photographed for each cell, one corresponding to the cell surface and the other through the nucleus. The protoplasts were not fixed prior to microscopy, and thus the experiments represent examples of live cells. Protoplasts not treated with inhibitors are presented in Fig. 5 at 10, 16, 18, and 24 hpi to illustrate the normal phenotypes during infection by TMV-MP:GFP. Detailed descriptions of protoplasts infected with TMV-MP:GFP or transiently expressing MP-GFP, are provided in other recent publications (18, 19, 29, 31, 37). The protease inhibitors ALLM and E-64 did not significantly alter the distribution of MP-GFP (not shown). In nontreated cells, MP-GFP accumulated early in infection at peripheral, punctate sites (Fig. 5A). At the same time a few small, fluorescent aggregates were observed throughout the cytoplasm and in the nuclear periphery (Fig. 5A′). At 16 hpi, aggregates of MP-GFP appeared to have coalesced to a low number of large aggregates (Fig. 5B) (29). Later, MP-GFP fluorescence at the cell periphery was observed as filamentous structures, which have been demonstrated to result from colocalization of MP-GFP with microtubules and microfilaments (Fig. 5C) (18, 19, 31). Fluorescence levels typically decreased 24 h after infection, at which time fluorescence at the cell periphery was localized to punctate sites (Fig. 5D), while fluorescence in the cytoplasm was mostly nonlocalized and presumably resulted from partially degraded MP-GFP (Fig. 5D′) (19, 29). At 36 and 48 hpi very little if any fluorescence remained, and in the few fluorescent cells, fluorescence was not localized; i.e., it was visible only as a fluorescent haze (not shown) (19, 29).
In contrast, in cells treated with the proteasome inhibitor clasto-lactacystin-β-lactone, significantly increased levels of fluorescence were observed during mid-stages and up to very late stages of infection, reflecting significantly increased stability of the protein. On the periphery of the cells, MP-GFP was mainly organized as small cortical aggregates and punctate fluorescence in early stages of infection (Fig. 6A). In later stages of infection, we observed filamentous structures that are presumed to represent colocalization of MP-GFP with elements of the cytoskeleton based upon previous studies (Fig. 6B to D) (18, 31). At 38 and 48 hpi there were also larger aggregates that accumulated near the cell surface (Fig. 6D and E). Even at very late stages of infection, MP-GFP was localized to irregularly shaped bodies on the cell periphery (Fig. 6E). Throughout the experiment large aggregates of MP-GFP were visualized around the nucleus. Although similar structures were previously described as accumulating early in infection (19, 29) (Fig. 5A′), these were the predominant structures found in cells treated with proteasome inhibitors and were observed even at very late stages of infection (Fig. 6A′ to E′).
Similar results were obtained with infected protoplasts treated with the proteasome inhibitor MG115 (not shown). However, in contrast to cells treated with clasto-lactacystin-β-lactone, in the experiments with MG115 the fluorescence level decreased more rapidly. This may be explained by the fact that MG115 is a reversible inhibitor, while clasto-lactacystin-β-lactone was reported to irreversibly inhibit the proteasome (11, 39). The phenotypes described in Fig. 5 and 6 were observed in repeated experiments. Treatment of protoplasts with the inhibitors significantly increased the stability of the MP-GFP and accumulation of MP-GFP occurred predominantly around the nucleus, presumably on the ER.
DISCUSSION
In this study we investigated the factors that control the degradation of the TMV MP. These studies demonstrate that the proteasome inhibitors MG115 and clasto-lactacystin-β-lactone, but not lactacystin, effectively block MP degradation. It was previously demonstrated that clasto-lactacystin-β-lactone specifically and irreversibly interacts with the active site threonine residues of the proteasome β subunits, while lactacystin is probably a precursor of the active molecule. These compounds restrict proteasome activity but have no measurable effects on other cellular proteases (11, 27). Lactacystin apparently is either not taken up by BY-2 protoplasts or not effectively converted to clasto-lactacystin-β-lactone.
We concluded from the results of this study that high-molecular-weight proteins in samples of infected cells treated with the inhibitors are polyubiquitinated forms of the MP that in untreated cells are degraded by the 26S proteasome. Specific inhibition of this degradation pathway increased the stability of MP and resulted in accumulation of large aggregates of MP-GFP around the nucleus, presumably on the ER.
The role of the MP of TMV, and other plant viruses, in virus cell-cell movement has been the subject of intense investigation. Although much is known about the intracellular localization of the MP in transgenic plants and during virus infection, regulation of the transient expression of MP has not been studied in detail. Watanabe et al. (50) demonstrated in studies with TMV-infected BY-2 protoplasts that the subgenomic RNA that encodes the MP is synthesized from 2 to 9 hpi and that MP synthesis takes place during the same period of time. Together with the data presented here, the studies demonstrate a complex regulatory mechanism of the accumulation of MP that involves transcriptional, translational, and posttranslational control of the synthesis and degradation of the MP mRNA and the MP.
Posttranslational modifications, especially phosphorylation and dephosphorylation of proteins, play an important role in the regulation of enzyme activity. Phosphorylation of several serine and threonine residues of the TMV MP has been described (7, 16, 52), but the role of phosphorylation in MP function is not yet clear. Kawakami and colleagues recently demonstrated that phosphorylation of the ToMV MP at amino acids 37 and 238 occurs in vivo and that phosphorylation at amino acid 37 was essential for MP function and influenced the intracellular localization of the MP (25). Thus, a role of phosphorylation per se in the regulation of MP function and/or stability is quite obvious.
Protein degradation by the 26S proteasome has been shown to regulate key steps in the cell cycle (reviewed in reference 21) and is involved in the regulation of gene transcription (reviewed in reference 44). There are several examples of viral pathogens that exploit this cellular pathway to promote their own successful amplification. The human immunodeficiency virus type 1 protein Vpu mediates proteasome degradation of CD4, the major cellular receptor for the virus, and thus surveillance by the host defense machinery is prevented (13, 28, 41, 42, 55). The tumor suppressor factor p53 is degraded in papillomavirus-infected cells. Interaction of p53 with the viral oncoprotein E6 and with the cellular E6-associated protein targets p53 for degradation in the proteasome (40). In another example, the human cytomegalovirus protein US11 mediates the degradation of major histocompatibility complex class I molecules by the proteasome, thereby escaping recognition by the host immune system (54).
In recent years many components of the 26S proteasome degradation pathway have been identified in plants and shown to have remarkable homology to yeast and mammalian systems (48, 49). Interestingly, ubiquitin was implicated in plant-pathogen interactions (49), and ubiquitination of some viral coat proteins has been demonstrated (12, 17). A very small proportion of the TMV CP was found to be monoubiquitinated (approximately one event per virion), although a function of this modification has not been determined (12).
Although we did not detect a significant effect of the proteasome inhibitors on accumulation of CP and replicase proteins, at very late stages of infection a drop of replicase protein amounts was sometimes observed (Fig. 3, 72 hpi). Inhibition of the 26S proteasome, which can lead to cell cycle arrest in BY-2 cells (15), may in some undetermined way be indirectly responsible for the observed decrease of replicase in late stages of infection. However, since this was observed only during very late stages of infection, we believe that there is no direct effect of proteasome inhibition on any of the other viral proteins.
In other studies, an altered response of tobacco plants to TMV infection and severe growth alterations due to the perturbation of the ubiquitin system were described (3, 4). Interestingly, plants with lowered levels of functional ubiquitin, due either to cosuppression or to competition with a ubiquitin variant, showed elevated resistance to TMV infection and reduced virus replication (4). These results imply that ubiquitin-sensitive processes play an important role in TMV infection, replication, or movement.
It is reasonable to assume that targeted degradation of the MP by proteasomes, as suggested in our experiments, plays an important regulatory role in the TMV life cycle. It is known that the MP is tightly associated with ER during infection and behaves as an integral ER membrane protein (38). Recent studies have shown that in the absence of MP, virus replication is restricted to small, ER-rich sites. When MP is present, replication sites are combined to form a few, very large “virus factories” (29). Our observations suggest that the MP is later degraded by the cytoplasmic 26S proteasome and imply that the MP is dislocated from the ER during the course of infection and targeted to the proteasome for degradation. Other examples of ER resident proteins and integral ER membrane proteins that follow the same route of degradation have been described (reviewed in references 6 and 26).
Expression of MP in transgenic plants leads to an increase of the size exclusion limit of plasmodesmata (10, 56). Such an increase during virus infection, however, is transient and occurs early in infection (36). It was also shown that the MP colocalizes with the cytoskeleton (18, 31) and with ER (19, 38). Early in infection extensive, transient disruption of the ER occurs in parallel with accumulation of large amounts of MP. Furthermore, the ER reverts to its preinfection state only after the MP is depleted from these cells (38). Constitutive high-level expression of MP during virus infection would presumably have dramatic, negative influences on normal plant development. Removal of the MP by the 26S proteasome degradation pathway may thus be necessary to allow the plant to recover from deleterious effects of infection and regain normal cellular functions.
It is interesting that recent experiments suggest that the MP serves as an anchor of the viral genomic RNA to the ER during mid- and late stages of infection (29). Colocalization of the viral genomic RNA with replicase and MP on sites of the nuclear periphery and in cytoplasmic bodies (29) thus may be mediated by the MP. In addition, the MP probably plays an important role in intracellular transport of replication complexes as suggested in earlier studies (19, 38). These results indicate a pivotal role of the MP, which seems to orchestrate many of the viral activities. This makes the MP an important target for host defense responses. Ultimately, however, removal of the MP from the cell is also in the interest of the virus, as it allows a return to normal cellular function and thus survival of the host organism.
A recent report describes the intracellular accumulation of misfolded, ubiquitinated proteins into inclusion bodies that the authors refer to as aggresomes (23). These inclusion bodies are commonly found in many neurodegenerative diseases, despite the ability of the cell to recognize and degrade the misfolded proteins. The authors suggest that formation of aggresomes is a response to exceeding the capacity of the cell to degrade the misfolded proteins. Aggresomes accumulate in close vicinity of the nucleus, similar to accumulations of MP-GFP observed during TMV infection, especially after inhibition of the 26S proteasome (Fig. 6).
It will be important to investigate the precise role of the 26S proteasome in TMV and other virus infections. Polyubiquitination and removal of the MP from infected cells may primarily be a stress response by the cell. Alternatively, it may be another example of exploiting a host pathway to ensure effective virus invasion. Last, it will be important to determine the role, if any, that phosphorylation of the MP plays in the degradation of the MP by the 26S proteasome.
ACKNOWLEDGMENTS
We thank Andreas Bachmair (University of Vienna, Vienna, Austria) for technical help and the antiubiquitin serum used in preliminary studies. We also thank Hal S. Padgett (Biosource Technologies Inc., Vacaville, Calif.), Bret Cooper (TSRI, La Jolla, Calif.), and Yuichiro Watanabe (University of Tokyo, Tokyo, Japan) for helpful discussions. We are grateful to Paloma Más (TSRI) for critical reading of the manuscript and to Bernadette Kurtz (Danforth Center, St. Louis, Mo.) for help with preparing the manuscript.
C.R. was supported by fellowship Re 1222/1-1 from the Deutsche Forschungsgemeinschaft. Other support was provided by a grant from the National Science Foundation (MCB 9631124) to R.N.B.
REFERENCES
- 1.Atkins D, Hull R, Wells B, Roberts K, Moore P, Beachy R N. The tobacco mosaic virus 30K movement protein in transgenic tobacco plants is localized to plasmodesmata. J Gen Virol. 1991;72:209–212. doi: 10.1099/0022-1317-72-1-209. [DOI] [PubMed] [Google Scholar]
- 2.Atkins D, Roberts K, Hull R, Prehaud C, Bishop D H L. Expression of the tobacco mosaic virus movement protein using a baculovirus expression vector. J Gen Virol. 1991;72:2831–2836. doi: 10.1099/0022-1317-72-11-2831. [DOI] [PubMed] [Google Scholar]
- 3.Bachmair A, Becker F, Masterson R V, Schell J. Perturbation of the ubiquitin system causes leaf curling, vascular tissue alterations and necrotic lesions in a higher plant. EMBO J. 1990;9:4543–4550. doi: 10.1002/j.1460-2075.1990.tb07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker F, Buschfeld E, Schell J, Bachmair A. Altered response to viral infection by tobacco plants perturbed in ubiquitin system. Plant J. 1993;3:875–881. [Google Scholar]
- 5.Berna A, Gafny R, Wolf S, Lucas W J, Holt C A, Beachy R N. The TMV movement protein: role of the carboxyl-terminal 73 amino acids in subcellular localization and function. Virology. 1991;182:682–689. doi: 10.1016/0042-6822(91)90609-f. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky J L, McCracken A A. ER-associated and proteasome-mediated protein degradation: how two topologically restricted events came together. Trends Cell Biol. 1997;7:151–156. doi: 10.1016/S0962-8924(97)01020-9. [DOI] [PubMed] [Google Scholar]
- 7.Citovsky V, McLean B G, Zupan J R, Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993;7:904–910. doi: 10.1101/gad.7.5.904. [DOI] [PubMed] [Google Scholar]
- 8.Culver J N, Lehto K, Close S M, Hilf M E, Dawson W O. Genomic position affects the expression of tobacco mosaic virus movement and coat protein genes. Proc Natl Acad Sci USA. 1993;90:2055–2059. doi: 10.1073/pnas.90.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deom C M, Oliver M J, Beachy R N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987;237:389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- 10.Deom C M, Schubert K R, Wolf S, Holt C A, Lucas W J, Beachy R N. Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc Natl Acad Sci USA. 1990;87:3284–3288. doi: 10.1073/pnas.87.9.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick L R, Cruikshank A A, Grenier L, Melandri F D, Nunes S L, Stein R L. Mechanistic studies on the inactivation of the proteasome by lactacystin: a central role for clasto-lactacystin beta-lactone. J Biol Chem. 1996;271:7273–7276. doi: 10.1074/jbc.271.13.7273. [DOI] [PubMed] [Google Scholar]
- 12.Dunigan D D, Dietzgen R G, Schoelz J E, Zaitlin M. Tobacco mosaic virus particles contain ubiquitinated coat protein subunits. Virology. 1988;165:310–312. doi: 10.1016/0042-6822(88)90691-5. [DOI] [PubMed] [Google Scholar]
- 13.Fujita K, Omura S, Silver J. Rapid degradation of CD4 in cells expressing human immunodeficiency virus type 1 Env and Vpu is blocked by proteasome inhibitors. J Gen Virol. 1997;78:619–625. doi: 10.1099/0022-1317-78-3-619. [DOI] [PubMed] [Google Scholar]
- 14.Gafny R, Lapidot M, Berna A, Holt C A, Deom C M, Beachy R N. Effects of terminal deletion mutations on function of the movement protein of tobacco mosaic virus. Virology. 1992;187:499–507. doi: 10.1016/0042-6822(92)90452-u. [DOI] [PubMed] [Google Scholar]
- 15.Genschik P, Criqui M C, Parmentier Y, Derevier A, Fleck J. Cell cycle-dependent proteolysis in plants: identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor MG132. Plant Cell. 1998;10:2063–2075. doi: 10.1105/tpc.10.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haley A, Hunter T, Kiberstis P, Zimmern D. Multiple serine phosphorylation sites on the 30 kDa TMV cell-to-cell movement protein synthesized in tobacco protoplasts. Plant J. 1995;8:715–724. doi: 10.1046/j.1365-313x.1995.08050715.x. [DOI] [PubMed] [Google Scholar]
- 17.Hazelwood D, Zaitlin M. Ubiquitinated conjugates are found in preparations of several plant viruses. Virology. 1990;177:352–356. doi: 10.1016/0042-6822(90)90490-i. [DOI] [PubMed] [Google Scholar]
- 18.Heinlein M, Epel B L, Padgett H S, Beachy R N. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- 19.Heinlein M, Padgett H S, Gens J S, Pickard B G, Casper S J, Epel B L, Beachy R N. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell. 1998;10:1107–1120. doi: 10.1105/tpc.10.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt C A, Beachy R N. In vivo complementation of infectious transcripts from mutant tobacco mosaic virus cDNAs in transgenic plants. Virology. 1991;181:109–117. doi: 10.1016/0042-6822(91)90475-q. [DOI] [PubMed] [Google Scholar]
- 21.Hoyt M A. Eliminating all obstacles: regulated proteolysis in the eukaryotic cell cycle. Cell. 1997;91:149–151. doi: 10.1016/s0092-8674(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 22.Jentsch S, Ulrich H D. Ubiquitous déjà vu. Nature. 1998;395:321–323. doi: 10.1038/26335. [DOI] [PubMed] [Google Scholar]
- 23.Johnston J A, Ward C L, Kopito R R. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn T W, Lapidot M, Heinlein M, Reichel C, Cooper B, Gafny R, Beachy R N. Domains of the TMV movement protein involved in subcellular localization. Plant J. 1998;15:15–25. doi: 10.1046/j.1365-313x.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami S, Padgett H S, Hosokawa D, Okada Y, Beachy R N, Watanabe Y. Phosphorylation and/or presence of serine 37 in the movement protein of tomato mosaic tobamovirus is essential for intracellular localization and stability in vivo. J Virol. 1999;73:6831–6840. doi: 10.1128/jvi.73.8.6831-6840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopito R R. ER quality control: the cytoplasmic connection. Cell. 1997;88:427–430. doi: 10.1016/s0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee D H, Goldberg A L. Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J Biol Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 28.Margottin F, Bour S P, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta-TrCP, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 29.Más, P., and R. N. Beachy. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J. Cell Biol. 147:945–958. [DOI] [PMC free article] [PubMed]
- 30.McLean B G, Hempel F D, Zambryski P C. Plant intercellular communication via plasmodesmata. Plant Cell. 1997;9:1043–1054. doi: 10.1105/tpc.9.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean B G, Zupan J, Zambryski P C. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995;7:2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meshi T, Watanabe Y, Saito T, Sugimoto A, Maeda T, Okada Y. Function of the 30 kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987;6:2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mezitt L A, Lucas W J. Plasmodesmal cell-to-cell transport of proteins and nucleic acids. Plant Mol Biol. 1996;32:251–273. doi: 10.1007/BF00039385. [DOI] [PubMed] [Google Scholar]
- 34.Moore P J, Fenczik C A, Deom C M, Beachy R N. Developmental changes in plasmodesmata in transgenic tobacco expressing the movement protein of tobacco mosaic virus. Protoplasma. 1992;170:115–127. [Google Scholar]
- 35.Nejidat A, Cellier F, Holt C A, Gafny R, Eggenberger A L, Beachy R N. Transfer of the movement protein gene between two tobamoviruses: influence on local lesion development. Virology. 1991;180:318–326. doi: 10.1016/0042-6822(91)90036-b. [DOI] [PubMed] [Google Scholar]
- 36.Oparka K J, Prior D A M, Santa Cruz S, Padgett H S, Beachy R N. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV) Plant J. 1997;12:781–789. doi: 10.1046/j.1365-313x.1997.12040781.x. [DOI] [PubMed] [Google Scholar]
- 37.Padgett H S, Epel B L, Kahn T W, Heinlein M, Watanabe Y, Beachy R N. Distribution of tobamovirus movement protein in infected cells and implications for cell-to-cell spread of infection. Plant J. 1996;10:1079–1088. doi: 10.1046/j.1365-313x.1996.10061079.x. [DOI] [PubMed] [Google Scholar]
- 38.Reichel C, Beachy R N. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc Natl Acad Sci USA. 1998;95:11169–11174. doi: 10.1073/pnas.95.19.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 40.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 41.Schubert U, Anton L C, Bacik I, Cox J H, Bour S, Bennink J R, Orlowski M, Strebel K, Yewdell J W. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J Virol. 1998;72:2280–2288. doi: 10.1128/jvi.72.3.2280-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert U, Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J Virol. 1994;68:2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staehelin L A. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J. 1997;11:1151–1165. doi: 10.1046/j.1365-313x.1997.11061151.x. [DOI] [PubMed] [Google Scholar]
- 44.Stancovski I, Baltimore D. NF-kappa-B activation: the I-kappa-B kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 45.Susi P. Detection of tobacco mosaic virus movement protein in association with tobacco nuclei isolated from intact and detached leaves. J Phytopathol. 1998;146:27–30. [Google Scholar]
- 46.Tomenius K, Clapham D, Meshi T. Localization of immunogold cytochemistry of the virus-coded 30k protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology. 1987;160:363–371. doi: 10.1016/0042-6822(87)90007-9. [DOI] [PubMed] [Google Scholar]
- 47.Varshavsky A. The ubiquitin system. Trends Biochem Sci. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- 48.Vierstra R D. Proteolysis in plants: mechanisms and functions. Plant Mol Biol. 1996;32:275–302. doi: 10.1007/BF00039386. [DOI] [PubMed] [Google Scholar]
- 49.Von Kampen J, Wettern M, Schulz M. The ubiquitin system in plants. Physiol Plant. 1996;97:618–624. [Google Scholar]
- 50.Watanabe Y, Emori Y, Ooshika I, Meshi T, Ohno T, Okada Y. Synthesis of TMV-specific RNAs and proteins at the early stage of infection in tobacco protoplasts: transient expression of the 30k protein and its mRNA. Virology. 1984;133:18–24. doi: 10.1016/0042-6822(84)90421-5. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe Y, Meshi T, Okada Y. Infection of tobacco protoplasts with in-vitro transcribed tobacco mosaic virus RNA using an improved electroporation method. FEBS Lett. 1987;219:65–69. [Google Scholar]
- 52.Watanabe Y, Ogawa T, Okada Y. In-vivo phosphorylation of the 30-Kda protein of tobacco mosaic virus. FEBS Lett. 1992;313:181–184. doi: 10.1016/0014-5793(92)81440-w. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe Y, Ohno T, Okada Y. Virus multiplication in tobacco protoplasts inoculated with tobacco mosaic virus RNA encapsulated in large unilamellar vesicle liposomes. Virology. 1982;120:478–480. doi: 10.1016/0042-6822(82)90048-4. [DOI] [PubMed] [Google Scholar]
- 54.Wiertz E J H J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 55.Willey R L, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of Cd4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf S, Deom C M, Beachy R N, Lucas W J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]