Abstract
The interaction between CD40 on B cells and CD40 ligand (CD40L) on activated T cells is important for B-cell differentiation in T-cell-dependent humoral responses. We have extended our previous murine studies of CD40-CD40L in adenoviral vector-mediated immune responses to rhesus monkeys. Primary immune responses to adenoviral vectors and the ability to readminister vector were studied in rhesus monkeys in the presence or absence of a transient treatment with a humanized anti-CD40 ligand antibody (hu5C8). Adult animals were treated with hu5C8 at the time vector was instilled into the lung. Immunological analyses demonstrated suppression of adenovirus-induced lymphoproliferation and cytokine responses (interleukin-2 [IL-2], gamma interferon, IL-4, and IL-10) in hu5C8-treated animals. Animals treated with hu5C8 secreted adenovirus-specific immunoglobulin M (IgM) levels comparable to control animals, but did not secrete IgA or develop neutralizing antibodies; consequently, the animals could be readministered with adenovirus vector expressing alkaline phosphatase. A second study was designed to examine the long-term effects on immune functions of a short course of hu5C8. Acute hu5C8 treatment resulted in significant and prolonged inhibition of the adenovirus-specific humoral response well beyond the time hu5C8 effects were no longer significant. These studies demonstrate the potential of hu5C8 as an immunomodulatory regimen to enable administration of adenoviral vectors, and they advocate testing this model in humans.
Adenoviral vectors are attractive tools for effectively transducing a wide range of cells (7, 26). The major limitation of adenovirus vectors in gene therapy has been the ensuing immune response to viral proteins and transgene product (8, 24, 29, 30). Extensive studies in mice have demonstrated that a vigorous cell-mediated immune response generated against the late gene products and transgene products eliminate the vector-transduced cells through activation of CD4+ T-cell-dependent, gamma interferon (IFN-γ)-activated, cytotoxic T cells (reviewed in reference 4). Activation of humoral immunity results in the induction of neutralizing antibodies, which prevents readministration. Several studies have now demonstrated that blocking of both T- and B-cell responses results in prolonged transgene expression and effective readministration of adenovirus vector in mice (4, 8, 16, 28). We have established in murine models that blocking antibodies to CD40L abolish adenovirus-vector-specific B-cell functions and severely compromise T-cell responses, allowing for efficient readministration of the vector (32, 33).
The crucial role of the CD40 molecule, expressed on B cells, professional and nonprofessional antigen-presenting cells (APC), endothelial cells, and some epithelial cells for effector cell function, has been clearly established (13, 25). The regulation of T-cell-dependent B-cell functions by CD40-CD40L interactions involves signals transduced through the CD40 molecule. The CD40-mediated signals have been involved in multiple functional responses, e.g., immunoglobulin (Ig) class switching and induction of anti-apoptotic protein BclxL in B cells, upregulation of B7 family proteins on macrophages and dendritic cells, induction of regulatory cytokines and inflammatory cytokines (interleukin-12 [IL-12], IL-1β, IL-6, lymphotoxin-tumor necrosis factor alpha, and IL-8) (15, 23). Thus, the wide distribution of CD40 has implicated its signaling pathway at multiple levels in the regulation of the effector functions of the immune system.
The T-cell counter-receptor for CD40 is the CD40 ligand (CD40L) (gp39, T-BAM, CD154), a type-II integral membrane glycoprotein, transiently expressed on antigen-activated CD4+ T cells. Experiments performed in mice, with in vivo infusion of blocking CD40L monoclonal antibody (MAb) or genetic mutations in its gene, have shown marked dysfunction of humoral immunity as indicated by decreased B-cell proliferation, Ig secretion, and class switching, maintenance of germinal centers, and memory B cells (1). The importance of the CD40-CD40L interactions in the regulation of T cells was implicated in observations of opportunistic infections with Pneumoncystis carinii, Cryptosporidium, and cytomegalovirus in patients with Hyper-IgM syndrome, who have a mutational defect in the CD40L gene (9). Indeed, it is now widely recognized that CD40L-CD40 interactions play pivotal roles in the development of CD4+ T-cell-dependent immune responses. Several mechanisms by which these molecules regulate T-cell functions have been identified, including involvement of interacting costimulatory molecules on APC (e.g., B7-1) and T cells (e.g., CD28, adhesion molecules ICAM-1, CD44, and cytokines) or IL-12 (10, 13).
The central role of the CD40-CD40L interactions in the regulation of immune responses has been exploited in strategies of transplantation immunology of graft acceptance or tolerance (3, 20, 22). The present study was undertaken with the hypothesis that in vivo administration of adenoviral vectors elicits cell-mediated and humoral immune responses, both of which require functional CD4+ T helper cells (28, 31). The in vivo model predicts that inhibition of CD4+ T-cell responses at the time of administration of the vector would interfere with the induction of cellular and humoral responses to adenoviral proteins, resulting in prolonged transgene expression and efficient readministration. In this study, we have analyzed the use of a short course of anti-CD40 ligand antibody (hu5C8) in lung-directed gene therapy in rhesus monkeys.
MATERIALS AND METHODS
Animals and specimen collection.
Wild, caught juvenile rhesus monkeys were purchased from Southwest Foundation for Biomedical Research (San Antonio, Tex.) and underwent full quarantine. The monkeys weighed approximately 3 to 4 kg, and were serologically negative for simian immunodeficiency virus, simian T-cell leukemia virus, other simian retroviruses, and human adenovirus. The protocol was approved by the Infection Control Committee of The Hospital of the University of Pennsylvania, the Environmental Health and Safety Office, the Institutional Biosafety Committee, and The Institutional Animal Care and Use Committee of The University of Pennsylvania. The monkeys are identified in the two protocols as denoted in Fig. 1.
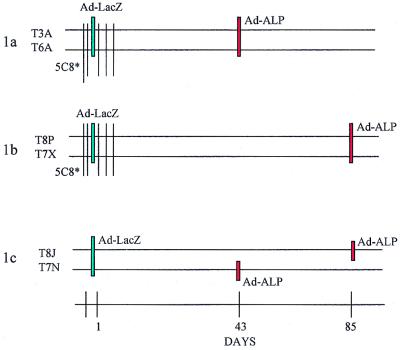
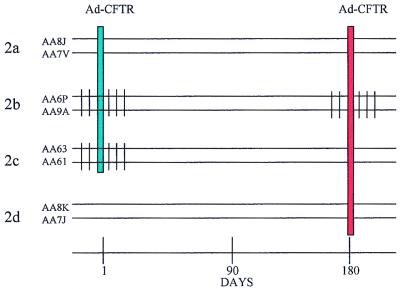
FIG. 1.
Schematic representation of the study designs. (Top) Experimental protocol 1 comprised six animals (in three groups: 1a, 1b, and 1c), four of which received hu5C8 antibody (T3A, T6A, T8P, and T7X) in a six-injection regimen of 5 mg/kg/dose intravenously on days −2, −1, 1, 3, 5, and 7 while two served as controls (T8J and T7N). All the animals were intratracheally instilled with Ad-LacZ in the left lung on day 1. Ad-ALP vector was instilled in the right lung in animals T7N, T3A, and T6A on day 43, and in animals T8J, T7X, and T8P on day 85. Peripheral blood and BAL were taken at various time points. Animals were necropsied for analyses of transgene expression in the lung on either day 46 (T7N, T3A, and T6A) or day 88 (T8J, T7X, and T8P). (Bottom) Experimental protocol 2 comprised eight animals in four groups (2a, 2b, 2c, and 2d). hu5C8 was administered intravenously in a six-injection regimen of 5 mg/kg/dose on days −2, −1, 1, 3, 5, and 7 (in group 2b and 2c) and again in group 2b on days 178, 179, 180, 183, 185, and 187. Animals were intratracheally instilled with Ad-CFTR on days 1 and 180. Group 2a, animals received Ad-CFTR on day 1 and readministered Ad-CFTR on day 180; Group 2b, animals were administered hu5C8, both during first and second vector instillation; Group 2c, animals were administered hu5C8 during the first, but not the second vector instillation; Group 2d, animals served as controls and received only Ad-CFTR on day 180. Peripheral blood and BAL were drawn at various time points. All animals were necropsied on day 210 for analysis of toxicity and immune responses.
Antibody.
The anti-CD154 MAb hu5C8 was prepared as previously described (18) and humanized (Biogen, Inc., Cambridge, Mass.). hu5C8 dosing is as specified in the legend to Fig. 1.
Vectors.
The construction and production of H5.010CBLacZ (henceforth called Ad-LacZ) and H5.100CBALP (henceforth called Ad-ALP), the E1-deleted recombinant adenovirus vectors expressing LacZ and alkaline phosphatase (ALP), respectively, have been described previously (11, 12). Virus was titered at a particle-to-PFU ratio of 100. For the study protocol 2, H5.020CBCFTR (henceforth called Ad-CFTR) was used, which expresses the human cystic fibrosis transmembrane receptor gene. This vector has been used in a Phase I clinical trial and has been previously described (5).
Vector administration.
Monkeys were anesthetized with ketamine-atropine. Physical examination was performed, and a 22-gauge intravenous needle was inserted for emergency medications. After chest X ray and blood draws, the monkey was brought to the operating room suite. Pulse oximetry was applied, and the monkey was placed supine with the head in the sniffing position. Using the laryngoscope, the vocal cords were visualized and sprayed with Cetacaine. The bronchoscope was passed through the vocal cords, and the membranous trachea and carina were identified. By using these landmarks, we identified the right mainstem bronchus and entered under direct vision. Sterile saline (10 ml) was injected into a peripheral branch and aspirated into a mucus trap (for bronchoalveolar lavage [BAL]). For administration, 1 ml of the virus (5 × 1012 particles) was instilled into the mainstem bronchus through the biopsy port of the bronchoscope. The bronchoscope was withdrawn under direct vision, and the monkeys were allowed to emerge from anesthesia and were returned to the colony in stable condition.
ALP histochemistry for ALP expression.
Cryostat sections (6 μm) of lung tissue were fixed in 0.5% glutaraldehyde and were rinsed with 1 mM MgCl2 in phosphate-buffered saline (PBS) (all reagents were obtained from Sigma Chemical Co., St. Louis, Mo.). Slides were incubated in ALP substrate consisting of 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (NBT) in PBS for 30 min in the dark at 37°C to detect the presence of ALP enzyme activity. Sections were counterstained with neutral red and mounted.
Lymphoproliferative (LPR) responses.
Peripheral blood mononuclear cells (PBMC) from the various time points of the study were separated by Ficoll-Hypaque density gradient centrifugation. In some cases, cells were cryopreserved in liquid nitrogen. At the time of the assay, PBMC were thawed by standard protocols. Triplicate cultures of 106 PBMC per ml (100 μl) were cultured with either 10 multiplicity of infection (MOI) of inactivated Ad-LacZ, 10 μg of phytohemagglutinin (PHA) per ml (Difco, Franklin Lakes, N.J.), 100 ng of Staphylococcus enterotoxin B (SEB) per ml (Toxin Technologies, Sarasota, Fla.) or medium alone. PHA-stimulated cultures were harvested on day 3, and SEB- and antigen-stimulated cultures were harvested on day 6. In some cases, anti-CD28 (1 μg/ml) (Coulter Immunotech, Miami, Fla.) was added to the cultures. Proliferation was measured by a 16-h [3H]thymidine (1 μCi/well) pulse. Results were presented as a stimulation index, which represents a ratio of counts per minute of adenovirus-stimulated cultures to counts per minute of cultures with medium alone.
Cytokine release assays.
PBMC were cultured with or without antigen (i.e., inactivated Ad-LacZ at a particle-to-cell ratio of 10) for 48 h in a 24-well plate. Cell-free supernatants were collected and analyzed for the presence of IL-2, IL-4, IFN-γ, and IL-10 by commercial enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Camarrillo, Calif.) by following manufacturers' protocols.
Adenovirus-specific Igs.
Serum (diluted 1:200) and BAL (diluted 1:20) samples from animals were analyzed for adenovirus-specific isotype-specific Igs (IgM, IgG, and IgA) by ELISA. For the ELISA, 96-well flat-bottomed, high-binding Immulon-IV plates were coated with 100 μl of Ad-LacZ antigen (5 × 109 particles/ml) in PBS overnight at 4°C, were washed four times in PBS–0.05% Tween, and were blocked in PBS–1% bovine serum albumin for 1 h at 37°C. Appropriately diluted samples were added to antigen-coated wells and were incubated for 4 h at 37°C. Plates were washed four times in PBS–0.05% Tween and were incubated with peroxidase-conjugated goat anti-human IgM, IgG, or IgA (1:2,000 dilution) (Sigma Chemical Co.) for 2 h at 37°C. Plates were washed as described above, and ABTS substrate (Kirkegaard and Perry, Gaithersburg, Md.) was added. Optical densities were read at 405 nm on a microplate reader (Dynatech Laboratories, Chantilly, Va.).
Anti-adenovirus neutralizing antibodies.
Neutralizing antibody (NAB) titers were analyzed by determining the ability of serum or BAL antibody to inhibit transduction of reporter virus, Ad-LacZ, into HeLa cells. Various dilutions of antibodies (twofold dilutions, 1:20 to 1:2560) preincubated with the reporter virus (100 MOI) for 1 h at 37°C were added to 90% confluent HeLa cell cultures (104 cells/well in a 96-well plate). Cells were incubated for 16 h at 37°C in a humidified CO2 incubator, and expression of β-galactosidase was analyzed by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining. The neutralizing titer of antibody was calculated by the highest dilution with which 50% of the cells turned blue.
RESULTS AND DISCUSSION
In vivo treatment of rhesus monkeys with hu5C8 markedly inhibits initiation of the adenovirus-specific immune responses.
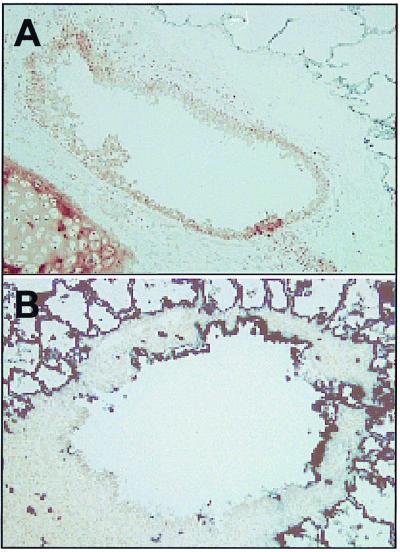
Experimental protocol 1 (Fig. 1) comprised six animals. Group 1a animals (T3A and TGA) received Ad-LacZ with hu5C8 on day 1 and were subsequently administered Ad-ALP on day 43. Group 1b animals (T8P and T7X) were treated the same as those of group 1a except that the Ad-ALP was administered on day 85. Group 1c was a control with both animals receiving Ad-LacZ without antibody on day 1 and Ad-ALP on either day 43 (animal T7N) or day 85 (animal T8J). In experimental protocol 1, cell-mediated and humoral immune responses were analyzed in animals administered Ad-LacZ (Fig. 2). Intratracheal administration of Ad-LacZ in the absence of hu5C8 (group 1c) resulted in generation of a brisk LPR response, measured on day 14. Analyses of humoral immune responses showed that these animals generated strong neutralizing antibodies and adenovirus-specific IgA in BAL. The presence of a neutralizing antibody response was confirmed by the inability to readminister Ad-ALP in bronchoepithelial cells of these animals (T7N and T8J) either on day 43 or 85 (Fig. 3 and Table 1).
FIG. 2.
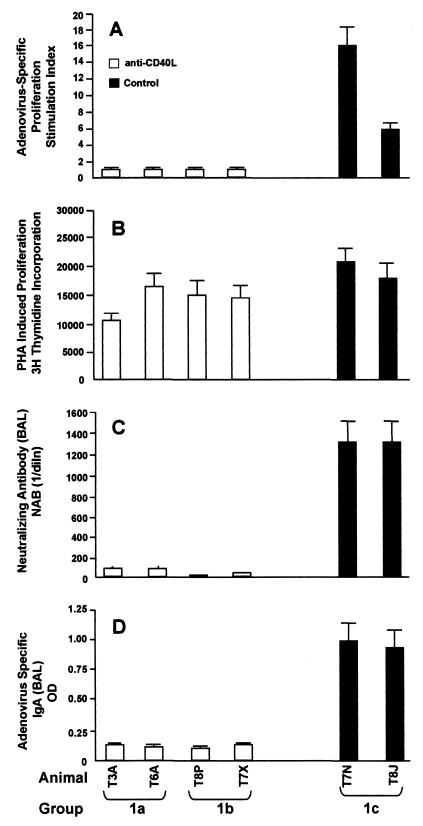
In vivo treatment of rhesus monkeys with hu5C8 antibody inhibits adenovirus-vector-mediated, cell-mediated, and humoral immune responses. Rhesus monkeys were treated with Ad-LacZ with (groups 1a and 1b) or without (group 1c) hu5C8 as described in the legend of Fig. 1 top. (A and B) PBMC were isolated from heparinized blood, drawn on day 14 post-vector administration from experimental protocol 1. Cells were cultured with inactivated adenovirus antigen or PHA. Lymphoproliferative responses were measured by [3H]thymidine incorporation as described in Materials and Methods. Results are denoted as a stimulation index (counts per minute of adenovirus stimulated cells/counts per minute of cells in medium alone) or counts per minute of [3H]thymidine incorporation. All the 5C8-treated animals had a significant decrease in induction of adenovirus-induced LPR responses (P < 0.05, Student's t test); PHA responses were not significantly different from controls. (C and D) BAL samples taken on day 28 were analyzed for the presence of adenovirus-specific neutralizing antibodies and IgA. The NAB titer was calculated from the highest dilution of the sera required to block 50% of the cells turning blue and was denoted as reciprocal dilution. IgA in BAL was measured by ELISA.
FIG. 3.
Micrographs of readministration of ALP staining in the broncholes of rhesus monkeys. Rhesus monkeys were administered Ad-ALP in the right lower lobe as depicted in Fig. 1 top. Animals were necropsied, and the right lower lobe was sectioned for ALP expression, as described in Materials and Methods. Alkaline phosphatase expression in the broncholes of animals T7N (A) and T8P (B) were analyzed by staining with buffer containing BCIP and NBT, as described in Materials and Methods. There was significant alveolar endogenous ALP staining in all the tissue. However, extensive analyses of lungs of rhesus monkeys not administered ALP-expressing adenovirus showed that none of the broncholar epithelial cells had endogenous ALP staining. Panel A, animal T7N; panel B, animal T8P; magnification, ×186.
TABLE 1.
Morphometric analyses of recipient lungs for efficiency of recombinant gene expression in experimental protocol 1a
| Animal | Group | Level of transgene expression (%)
|
||
|---|---|---|---|---|
| 0–5 | 5–25 | >25 | ||
| T3A | 1a | 71.43 | 14.29 | 14.29 |
| T6A | 1a | 75.00 | 10.71 | 14.29 |
| T8P | 1b | 57.14 | 17.14 | 25.71 |
| T7X | 1b | 40.00 | 20.00 | 40.00 |
| T8J | 1c | 100.00 | 0.00 | 0.00 |
| T7N | 1c | 94.12 | 5.88 | 0.00 |
Rhesus monkeys were administered Ad-ALP in the right lower lobe as depicted in Fig. 1 top, and animals were necropsied according to the schedule described in the legend to Fig. 1 top. The right lower lobe was sectioned for ALP expression, as described in Materials and Methods. ALP transgene expression in 30 broncholes from multiple blocks in the right lower lobe of each animal was quantitated by the Phase III image software. For this purpose, a tight gate was drawn around each bronchole, and the percentage of blue pixels was calculated electronically. The transgene expression in bronchoepithelial cells was evaluated according to the following criteria: minimal transgene expression (0 to 5%), transgene expression in 5 to 25% of epithelial cells per bronchole, and transgene expression in >25% of epithelial cells. The numbers represent the percentage of ALP expression from 30 individual bronchi in each animal.
Treatment of animals with hu5C8 resulted in marked inhibition of adenovirus-specific LPR responses. PHA-induced responses were not significantly affected, indicating that hu5C8 did not result in the depletion of T-cell populations (Fig. 2B). Animals treated with hu5C8 had markedly suppressed adenovirus-specific neutralizing antibodies and IgA responses, as compared to untreated animals (Fig. 2C and D). Animals could be efficiently readministered Ad-ALP on day 43 (animals T3A and T6A) (Table 1) and day 85 (animals T8P and T7X) (Table 1 and Fig. 3), demonstrating that the transient antibody treatment resulted in prolonged inhibition of the vector-specific B-cell responses, sufficient to readminister Ad-ALP. In the hu5C8-treated animals, readministration of Ad-ALP on day 43 (T3A and T6A) induced a strong NAB response (data not shown), demonstrating that the hu5C8 treatment does not induce tolerance to adenovirus vector, and only affects initiation of a primary immune response.
Effect of treatment of rhesus monkeys with hu5C8 MAb results in long-term inhibition of adenovirus-specific cell-mediated and humoral immune responses.
Experimental protocol 2 (Fig. 1 bottom) was designed to analyze the impact of transient treatment with hu5C8 treatment on suppression of adenovirus vector-specific cell-mediated and humoral immune responses up to 180 days. This study also addressed whether treatment of animals with hu5C8, along with readministration of vector 2, suppressed subsequent immune responses.
Experimental protocol 2 comprised eight animals in four groups (2a, 2b, 2c, and 2d) as follows: Group 2a, Ad vector on days 1 and 180; Group 2b, Ad vector on days 1 and 180 with hu5C8 administered with each vector; Group 2c, Ad vector on days 1 and 180 with hu5C8 administered with the first vector; and Group 2d, only vector on day 180.
Intratracheal administration of Ad-CFTR into the lungs of rhesus monkeys resulted in a strong inflammatory response, as measured by IL-8 secretion (Fig. 4). Antigen-specific LPR responses to adenoviral antigens were determined at various time points during the study (Fig. 5). Intratracheal instillation of Ad-CFTR to rhesus monkeys in the absence of hu5C8 treatment (animals AA8J and AA7V) induced an LPR response which peaked on day 90 and diminished by day 136. Antigen specificity was confirmed by the lack of LPR responses in control animals, which did not receive Ad-CFTR on day 1 (Group 2d). The qualitative nature of the response was determined by analyses of cytokine secretion profiles on day 36. PBMC from animals treated with vector alone secreted IL-2, IFN-γ, IL-4, and IL-10 in response to in vitro adenovirus stimulation (Fig. 6). Humoral immune responses were measured by analyzing NABs in serum and BAL fluids. Table 2 shows that none of the animals had preexisting NABs to adenovirus on day −2. NAB was observed in serum samples of animals treated with vector alone by day 14, which peaked at day 29 and diminished through day 180. The mucosal humoral immune response was analyzed by measuring NAB in BAL fluid. BAL from control animal Group 2a (i.e., vector but no hu5C8) developed NAB which peaked on day 29. Interestingly, the NAB in BAL in these animals (AA8J and AA7V) diminished to baseline titers by day 90.
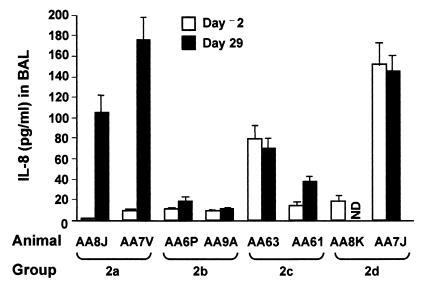
FIG. 4.
hu5C8 treatment inhibits generation on inflammatory cytokine IL-8 in the lung. Rhesus monkeys were instilled with Ad-CFTR on day 1 with or without hu5C8 as described in the legend to Fig. 1 bottom. BAL obtained on day −2 (prior to vector instillation) and 29 were analyzed for the presence of IL-8 by ELISA. Treatments of the animals in the various groups are described in the legend to Fig. 1 bottom. All of the 5C8-treated animals had a significant decrease in induction of IL-8 (P < 0.05, Wilcoxon's test) as compared to those who received vector but were not administered 5C8. Since the rhesus monkeys were caught in the wild, the prevector inflammatory status of the lung could not be controlled. All the monkeys underwent a 3-month quarantine period, when they were tested for a variety of infectious agents; all tests were negative.
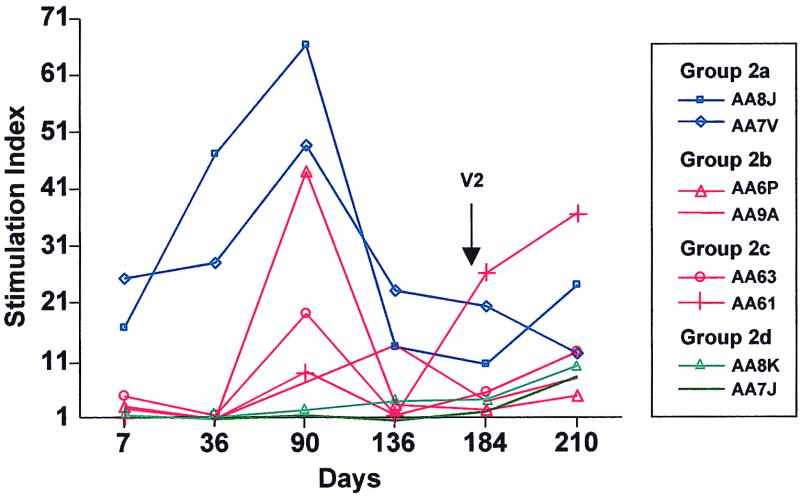
FIG. 5.
Adenovirus-vector-mediated LPR responses in rhesus monkeys in experimental protocol 2. Rhesus monkeys were instilled with Ad-CFTR on days 1 and 180 with or without hu5C8 as described in the legend to Fig. 1 bottom. PBMC isolated from heparinized blood were cultured with inactivated adenovirus vector. Lymphoproliferative responses, measured by [3H]thymidine incorporation, are presented as a stimulation index, which is the ratio of the counts per minute of PBMC cultured with adenovirus vector and counts per minute of PBMC cultured with medium alone. Adenovirus-specific responses induced following the first intratracheal vector instillation were analyzed on various days of the experimental protocol. Blue lines represent animals in Group 2a, red lines represent animals treated with hu5C8 (Groups 2b and 2c), and green lines represent animals in Group 2d.
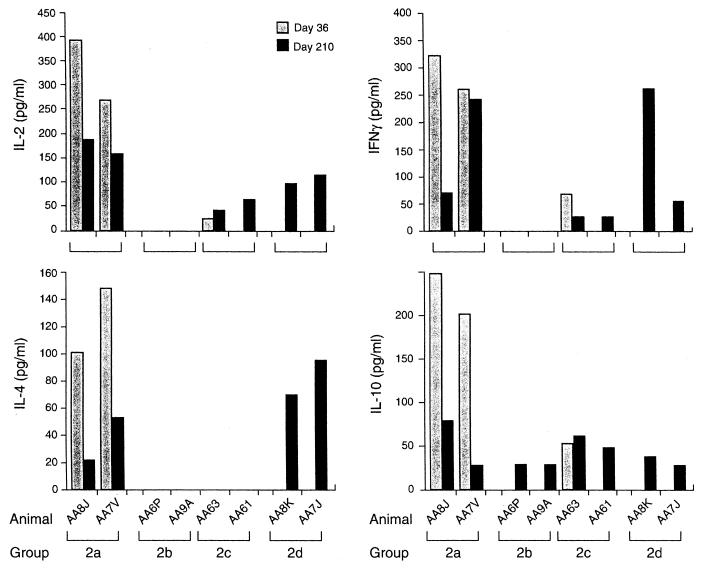
FIG. 6.
Cytokine secretion profiles of animals in experimental protocol 2. Rhesus monkeys were instilled with Ad-CFTR on days 1 and 180 with or without hu5C8 as described in the legend to Fig. 1 bottom. IFN-γ, IL-2, IL-4, and IL-10 cytokines secreted by PBMC were measured on day 36 after vector-1 instillation and on day 210 (i.e., 30 days after vector-2 instillation). PBMC were cultured in the presence or absence of inactivated Ad-LacZ for 48 h, and culture supernatants were analyzed for the indicated cytokines by commercial ELISA kits. None of the culture supernatants from PBMC cultured in the absence of adenovirus vector antigen induced cytokines; only adenovirus-vector-induced cytokines are shown in the figure.
TABLE 2.
Treatment of rhesus monkeys with 5C8 inhibits NAB responsesa
| NAB source | Animal | Group | Treatment | Titer on day:
|
Secondary treatment | Titer on day:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | 1 | 7 | 14 | 29 | 36 | 60 | 90 | 104 | 136 | 178 | 180 | 184 | 191 | 210 | |||||
| Serum | AA8J | 2a | V1 | 20 | 20 | 20 | 160 | 1,280 | 1,280 | 2,860 | 1,280 | 1,280 | 1,280 | 80 | 80 | V2 | 320 | 5,120 | 5,120 |
| AA7V | 2a | V1 | 20 | 20 | 20 | 640 | 2,560 | 1,280 | 640 | 1,280 | 1,280 | 640 | 320 | 320 | V2 | 1,280 | 5,120 | 5,120 | |
| AA6P | 2b | 5C8 + V1 | 20 | 20 | 20 | 20 | 80 | 40 | 80 | 40 | 80 | 80 | 80 | 80 | 5C8 + V2 | 320 | 1,280 | 5,120 | |
| AA9A | 2b | 5C8 + V1 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 40 | 160 | 80 | 80 | 80 | 5C8 + V2 | 160 | 2,560 | 5,120 | |
| AA63 | 2c | 5C8 + V1 | 20 | 20 | 20 | 40 | 40 | 40 | 160 | 320 | 320 | 40 | 20 | 20 | V2 | 40 | 1,280 | 5,120 | |
| AA61 | 2c | 5C8 + V1 | 20 | 20 | 20 | 40 | 40 | 20 | 40 | 40 | 80 | 40 | 40 | 40 | V2 | 160 | 2,560 | 5,120 | |
| AA8K | 2d | Control | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 40 | V2 | 20 | 1,280 | 1,280 | |
| AA7J | 2d | Control | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | V2 | 20 | 640 | 320 | |
| BAL | AA8J | 2a | V1 | 20 | 1,280 | 20 | 20 | V2 | 640 | ||||||||||
| AA7V | 2a | V1 | 20 | 1,280 | 80 | 20 | V2 | 640 | |||||||||||
| AA6P | 2b | 5C8 + V1 | 20 | 40 | 20 | 20 | 5C8 + V2 | 320 | |||||||||||
| AA9A | 2b | 5C8 + V1 | 20 | 80 | 20 | 20 | 5C8 + V2 | 640 | |||||||||||
| AA63 | 2c | 5C8 + V1 | 20 | 20 | 20 | 20 | V2 | 40 | |||||||||||
| AA61 | 2c | 5C8 + V1 | 20 | 40 | 20 | 20 | V2 | 640 | |||||||||||
| AA8K | 2d | Control | 20 | 20 | 20 | 20 | V2 | 20 | |||||||||||
| AA7J | 2d | Control | 20 | 20 | 20 | 20 | V2 | 40 | |||||||||||
Rhesus monkeys were instilled with Ad-CFTR on days 1 and 180 with or without hu5C8, as described in the legend to Fig. 1 top. NAB in serum and BAL obtained from the animals in experimental protocol 2 were analyzed by the ability of serum or BAL to block Ad-LacZ virus infection of HeLa cells in vitro. The NAB titer was calculated from the highest dilution of the sera required to block 50% of the cells turning blue and is denoted as reciprocal dilution. Days −2 to 180 represent NAB responses following vector 1 instillation; days 184, 191, and 210 represent days 4, 11, and 30 post-vector-2 administration. hu5C8 treatment of the animals was done as described in the legend to Fig. 1 bottom and in Materials and Methods.
CD40-CD40L interactions have been recently implicated in induction of inflammatory responses in the lung. In this study, animals administered vector alone generated a brisk local inflammatory response, as measured by secretion of IL-8. hu5C8-treated animals elicited a markedly blunted IL-8 response (Fig. 4). Since IL-8 is predominantly secreted by inflammatory cells, our results suggest that hu5C8 treatment inhibits recruitment of inflammatory cells into the lung or activation of those recruited cells. These observations are consistent with several studies, which have implicated CD40-CD40L interactions in inflammatory responses (13).
The effect of hu5C8 treatment on adenovirus-specific LPR responses at various time points of the study is shown in Fig. 5. None of the four hu5C8-treated animals elicited an adenovirus LPR response through at least day 36. By day 90, animals administered hu5C8 elicited modest LPR responses, which were less than those of control animals, and which remained relatively low through day 136. During the course of the study, hu5C8 treatment did not affect SEB-induced LPR responses (data not shown).
Additional experiments were performed to determine whether lack of T-cell priming in the absence of CD40L was due to inhibition of T-cell activation, anergy induction, or deletion of antigen-specific T cells. PBMC were treated in vitro with anti-CD28 MAb and were stimulated with vector-derived antigens. Costimulation of PBMC from hu5C8-treated animals in vitro with adenovirus antigen and anti-CD28 MAb completely restored lymphoproliferative responses and IL-2 secretion (Table 3), suggesting that hu5C8 treatment inhibited antigen-specific T-cell functions, and did not delete them. Concanavalin-A-induced LPR responses were within normal ranges in all animals. Furthermore, extensive phenotype analyses showed no significant changes in CD4, CD8, and CD19 cells (data not shown). These studies demonstrate that inhibition of CD40L-CD40 interactions lead to a marked inhibition of induction of T-cell responses without cell depletion.
TABLE 3.
Addition of anti-CD28 in vitro restores adenovirus-specific LPR responsesa
| Animal | Group | Treatment | Lymphoproliferative responses induced by:
|
|||
|---|---|---|---|---|---|---|
| Medium | Adenovirus | Ad + CD28 | Concanavalin A | |||
| AA8J | 2a | V1 | 161 | 2,161 | 8,954 | 18,412 |
| AA7V | 2a | V1 | 103 | 2,382 | 5,542 | 20,151 |
| AA6P | 2b | 5C8 + V1 | 102 | 310 | 6,570 | 6,894 |
| AA9A | 2b | 5C8 + V1 | 139 | 188 | 2,084 | 11,189 |
| AA63 | 2c | 5C8 + V1 | 140 | 181 | 6,771 | 37,859 |
| AA61 | 2c | 5C8 + V1 | 201 | 249 | 3,985 | 21,015 |
| AA8K | 2d | Control | 263 | 194 | 442 | 15,789 |
| AA7J | 2d | Control | 320 | 78 | 401 | 11,214 |
PBMC isolated from heparinized blood were cultured with inactivated adenovirus vector (as described in Materials and Methods) in the presence or absence of 1 μg of anti-CD28 MAb per ml. Cultures were harvested, and radioactivity was measured by liquid scintillation. Lymphoproliferative responses, measured by [3H]thymidine incorporation, are presented as counts per minute.
Treatment of animals with hu5C8 antibody resulted in diminished serum NAB, compared to untreated controls, up to day 36 of the study, after which a modest increase in NAB titer was observed. These NAB responses were maintained at low titer throughout the 180-day period and in some cases returned to baseline. Analyses of the mucosal response in BAL showed that hu5C8-treated animals did not elicit a NAB response on day 29 and remained below the level of detection through day 180.
Ad-CFTR was readministered to all the animals in the study on day 180 (Fig. 1 bottom). LPR responses following vector administration were analyzed on days 184 and 210 (4 and 30 days after vector readministration). Figure 5 shows that all the animals generated LPR responses, irrespective of hu5C8 treatment status, indicating that acute hu5C8 treatment did not induce tolerance to the adenovirus vector.
Cytokine secretion profile was analyzed 30 days post-second-vector instillation (day 210 of the study) (Fig. 6). Animals which received vector alone (i.e., no hu5C8) on days 1 and 180 (Group 2a) or only on day 180 (Group 2d) induced secretion of all cytokines tested. Interestingly, animals in Group 2c (which received vector 2 without hu5C8 treatment) generated a modest IL-2, IFN-γ, and IL-10 response, while animals in Group 2b (which received vector 2 along with a second course of hu5C8) were suppressed in their ability to secrete IL-2, IFN-γ, and IL-4, but not IL-10.
The development of NAB to adenovirus was not inhibited after second-vector instillation, irrespective of whether the animals were retreated with hu5C8. Thus, following readministration of the vector on day 180, all animals (either untreated or treated with hu5C8) developed high titers of NAB both in serum and BAL by day 210.
Interaction of CD40L on activated T cells with CD40 on APC and B cells plays a central role in activation of B- and T-cell responses (13, 25). As we have demonstrated in mice (19, 32), intratracheal administration of adenoviral vectors in rhesus monkeys generated strong cell-mediated immune responses, comprised of both TH1-type and TH2-type responses, as evidenced by secretion of IFN-γ and IL-4. Blocking of CD40L-CD40 interaction with hu5C8 treatment in vivo abrogated induction of lymphoproliferative responses and cytokine secretion, through day 36. Longitudinal analyses of lymphoproliferative responses showed that LPR responses emerged at low levels over time as the circulating levels of hu5C8 were diminished (unpublished observations). The immunomodulatory effects of hu5C8 are consistent with various models of in vivo primary immune responses, and confirm in a large animal model that CD40-CD40L interactions are critical for induction of immune responses.
Several studies have demonstrated that administration of adenovirus vectors induce NABs (for a review, see reference 4). CD4 cell depletion studies in mice have established that the humoral response is CD4+ T-cell-dependent. Consistent with the observations in mice (32, 33), treatment of monkeys with hu5C8 antibody markedly impaired development of anti-adenovirus-specific IgG antibodies in serum (data not shown) and anti-IgA antibodies in BAL. The lack of isotype switching correlated with the inability of these antibodies to neutralize Ad-LacZ infection of HeLa cells in vitro. The absence of neutralizing antibodies in BAL was confirmed in vivo when animals instilled with Ad-ALP on day 85 showed impressive transgene expression in the epithelial cells of the bronchoalveolar cells, compared to untreated animals. In the second experimental protocol, hu5C8-treated animals elicited markedly decreased NABs, compared to untreated animals, until day 36 post-vector administration. Following this time point, there was an emergence of modest anti-adenovirus-specific LPR and NAB responses, correlating with a decrease in circulating hu5C8 concentrations. These decreased hu5C8 levels correlated with the appearance of low titers of antibodies to hu5C8 (unpublished results). These studies further demonstrate that although acute treatment of rhesus monkeys with a humanized anti-CD40L MAb abrogates development of a primary adenovirus-neutralizing response and limits the response to itself, hu5C8 does not prevent the elicitation of a virus-specific antibody response upon secondary challenge with vector. The diminished effectiveness of the second sequential immune blockade could be due to a number of mechanisms such as insufficient initial CD40L-CD40 inhibition, persistence of antigen beyond the duration of CD40L-CD40 inhibition, the requirement of inhibiting an alternate pathway, or incomplete blockade of CD40 signaling due to antibodies that interfere with hu5C8 function.
The duration of systemic and mucosal humoral immune responses (in serum and BAL, respectively) clearly diverged. Animals administered adenovirus vector in the absence of hu5C8 in experimental protocol 2 elicited strong NABs in serum and BAL on day 29. While the NAB in serum persisted until at least day 180, antibodies in BAL reverted to baseline levels by day 90. It has been postulated that most of the antibody present in the serum comes from plasma cells in the bone marrow, whereas mucosal antibody levels are induced by plasma cells in the mucosal sites (21). It is likely that possible differences in the life spans of plasma cells in the two anatomical locations may contribute to the short-lived antibody levels in the BAL. These observations may have important implications for the readministration of viral vectors in the lung.
Induction of TH1 or TH2 responses was evaluated following vector 2 administration by analyzing cytokine secretion profiles on day 210. Animals treated with repeat doses of hu5C8 (Group 2b) were inhibited in their ability to secrete IL-2, IFN-γ, or IL-4, but secreted moderate levels of IL-10. It is possible that monocyte/macrophages contributed to the IL-10 response. Alternatively, hu5C8 treatment affected TH1-type responses more effectively than IL-10 responses during secondary immune responses. To this effect, a previous report has shown that CD40L blockade results in inhibition of TH1, but not TH2 responses (20). Further studies need to be done to clarify the role of CD40-CD40L interactions in regulation of TH1 and TH2 responses in this animal model.
In summary, these studies have investigated the effect of blocking CD40L-CD40 interactions in adenovirus vector-mediated somatic gene transfer. Administration of hu5C8 MAb resulted in a marked inhibition of induction of antigen-specific T- and B-cell responses. Analyses of secondary immune responses showed that generation of secondary B-cell responses was not markedly affected. Another unexpected observation was that during secondary responses, TH1 cytokine responses were more affected than TH2 responses. These studies demonstrate the potential of hu5C8 to prolong transgene expression in immune-suppressive regimens and permit vector readministration. Further studies using a combination of blocking agents (e.g., TRANCE, CTLA4-Ig, and anti-CD4 MAb) (2, 6, 14, 17, 27) along with anti-CD40L MAbs may provide a more complete abrogation of T- and B-cell immune responses.
ACKNOWLEDGMENTS
We thank the members of the Wilson laboratory for helpful discussions. We thank Eric Wheeldon for performing the experiments on the rhesus monkeys. Support from Cell Morphology Core, Immunology Core (Ruth Qian, George Qian, and Parag Dhagat), and Animal Services Unit (Ernest Glover and Lisa Stephens) of the Institute for Human Gene Therapy is greatly appreciated.
This work was funded by grants from the NIH (P30 DK47757-05 and R01 HL49040-08), Cystic Fibrosis Foundation, and Genovo, Inc., a biotechnology company J. M. Wilson founded and has equity in.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Maraskovsky E, Billingsley W L, Dougall W C, Tometsko M E, Roux E R, Teepe M C, BuBose R F, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 3.Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt H O, Sarvetnick N. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–4627. [PubMed] [Google Scholar]
- 4.Bromberg J S, Debruyne L A, Qin L. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv Immunol. 1998;69:353–409. [PubMed] [Google Scholar]
- 5.Chirmule N, Hughes J V, Gao G-P, Raper S E, Wilson J M. Role of E4 in eliciting CD4 T-cell and B-cell responses to adenovirus vectors delivered to murine and nonhuman primate lungs. J Virol. 1998;72:6138–6145. doi: 10.1128/jvi.72.7.6138-6145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirmule N, Truneh A, Haecker S A, Tazelaar J T, Gao G-P, Raper S E, Hughes J V, Wilson J M. Repeated administration of adenoviral vectors in lungs of human CD4 transgenic mice treated with a non-depleting CD4 antibody. J Immunol. 1999;163:448–455. [PubMed] [Google Scholar]
- 7.Crystal R. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Schwartz E M, Gu D, Zhang W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiSanto J, Bonnefoy J, Gauchel J, Fisher A, deSainte Basile G. CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 10.Durie F H, Foy T M, Masters S R, Laman J D, Noelle R J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994;15:406–411. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt J F, Simon R H, Yang Y, Zepeda M, Pendleton S W, Doranz B, Grossman M, Wilson J M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: biological efficacy study. Hum Gene Ther. 1993;4:759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M J, Litzky L, Engelhardt J F, Wilson J M. Transfer of the CFTR gene to the lung of nonhuman primates with E1 deleted, E2a defective recombinant adenoviruses: a preclinical toxicology study. Hum Gene Ther. 1995;6:839–851. doi: 10.1089/hum.1995.6.7-839. [DOI] [PubMed] [Google Scholar]
- 13.Grewal I S, Flavell R A. CD40 and CD154 in cell mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 14.Guibinga G-H, Lockmuller H, Massie B, Nalbantoglu J, Karpati G, Petrof B J. Combinatorial blockade of calcineurin and CD28 signaling facilitates primary and secondary therapeutic gene transfer by adenovirus vectors in dystrophic (mdx) mouse muscles. J Virol. 1998;72:4601–4609. doi: 10.1128/jvi.72.6.4601-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henn V, Slupsky J R, Grafe M, Anagnostopoulos I, Foster R, Muller-Berghaus G, Kroczek R A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 16.Jooss K, Yang Y, Wilson J M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 17.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederman S, Yellin M J, Krichevsky A, Belko J, Lee J J, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1997;175:1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClane S, Chirmule N, Burke C, Raper S E. Characterization of the immune response after local delivery of recombinant adenovirus in murine pancreas and successful strategies for readministration. Hum Gene Ther. 1997;8:2207–2216. doi: 10.1089/hum.1997.8.18-2207. [DOI] [PubMed] [Google Scholar]
- 20.Samoilova E, Horton J, Zhang H, Chen Y. CD40L blockade prevents autoimmune encephalomyelitis and hampers TH1 but not TH2 pathway of T cell differentiation. J Mol Med. 1997;75:603–608. doi: 10.1007/s001090050145. [DOI] [PubMed] [Google Scholar]
- 21.Slifka M K, Antia R, Whitmore J K, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 22.Soong L, Xu J C, Grewal I S, Kima P, Sun J, Longley B J, Jr, Ruddle N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 23.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 24.Tripathy S K, Goldwasser B H, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 25.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 26.Wilson J M. Adenoviruses as gene delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 27.Wong B R, Josien R, Lee S Y, Vologodskaia A, Steinmann R M, Choi Y. The TRAF family of signal transducers mediates NFkB activation by the TRANCE receptor. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Greenough K, Wilson J M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 29.Yang Y, Jooss K, Su Q, Ertl H C J, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1995;3:137–144. [PubMed] [Google Scholar]
- 30.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]