Abstract
Infection of adult 129 Sv/Ev mice with consensus Sindbis virus strain TR339 is subclinical due to an inherent restriction in early virus replication and viremic dissemination. By comparing the pathogenesis of TR339 in 129 Sv/Ev mice and alpha/beta interferon receptor null (IFN-α/βR−/−) mice, we have assessed the contribution of IFN-α/β in restricting virus replication and spread and in determining cell and tissue tropism. In adult 129 Sv/Ev mice, subcutaneous inoculation with 100 PFU of TR339 led to extremely low-level virus replication and viremia, with clearance under way by 96 h postinoculation (p.i.). In striking contrast, adult IFN-α/βR−/− mice inoculated subcutaneously with 100 PFU of TR339 succumbed to the infection within 84 h. By 24 h p.i. a high-titer serum viremia had seeded infectious virus systemically, coincident with the systemic induction of the proinflammatory cytokines interleukin-12 (IL-12) p40, IFN-γ, tumor necrosis factor alpha, and IL-6. Replicating virus was located in macrophage-dendritic cell (DC)-like cells at 24 h p.i. in the draining lymph node and in the splenic marginal zone. By 72 h p.i. virus replication was widespread in macrophage-DC-like cells in the spleen, liver, lung, thymus, and kidney and in fibroblast-connective tissue and periosteum, with sporadic neuroinvasion. IFN-α/β-mediated restriction of TR339 infection was mimicked in vitro in peritoneal exudate cells from 129 Sv/Ev versus IFN-α/βR−/− mice. Thus, IFN-α/β protects the normal adult host from viral infection by rapidly conferring an antiviral state on otherwise permissive cell types, both locally and systemically. Ablation of the IFN-α/β system alters the apparent cell and tissue tropism of the virus and renders macrophage-DC-lineage cells permissive to infection.
The prototypic alphavirus, Sindbis virus strain AR339, was isolated by intracerebral (i.c.) inoculation of 3-day-old mice with a mosquito homogenate collected near Sindbis, Egypt (59). The laboratory animal model for natural Sindbis virus infection and pathogenesis is the mouse, inoculated subcutaneously (s.c.) to mimic transmission by the bite of an infected mosquito. In experimental mouse infections, Sindbis virus replicates primarily in skin, fibroblast connective tissue, and muscle, causing a myositis. Virus spreads systemically via a serum viremia and seeds the central nervous system (CNS) (24). However, the severity of Sindbis virus infection under different conditions can range from uniformly fatal to subclinical, with a concurrent decrease in mortality rate and extension of average survival time (AST; reviewed in reference 26). The outcome of infection is heavily dependent upon a number of parameters, including (i) the age of the host when inoculated (e.g., references 18, 20, 25, 47, 59, 60), (ii) the genotype of the virus (e.g., references 29, 32, 44, 45, 54, 61), and (iii) the inoculum dose administered (e.g., reference 34). All Sindbis virus strains are avirulent in adult mice regardless of the dose or route of inoculation, except for those strains that have been neuroadapted by extensive passage in the mouse CNS (32, 62). Although the basis for Sindbis virus virulence has not been fully elucidated, attenuation of virulence typically correlates with reduced virus replication and potential for dissemination, rather than an altered tissue tropism or differential ability of the host to clear virus (18, 29, 60, 61). These data suggest that the innate immune system may play a role in determining the relative permissivity of host tissues to virus infection under different circumstances.
The alpha/beta interferon (IFN-α/β) system is an important component of the host's first line of defense against virus infections (reviewed in references 14 and 25). In vitro, Sindbis virus is particularly sensitive to the antiviral effects of IFN-α/β (41, 43). However, in vivo the extent of IFN-α/β induction parallels the level of virus replication (29, 47, 54, 60, 61, 64). Thus, the amount of IFN-α/β induced in a fatal neonatal infection far exceeds the induction during a subclinical adult infection (60, 64). Consequently, investigation into the role of IFN-α/β in Sindbis virus pathogenesis has been limited to two studies demonstrating that neutralization of IFN-α/β by antiserum increased susceptibility to Sindbis virus infection (61; G. Cole, E. Johnson, A. Schmaljohn, and I. Gresser, Abstr. Fifth Int. Congr. Virol. 1981, abstr. P06/05, p. 97).
In this study, we have reexamined the hypothesis that this innate antiviral defense mechanism is critical in limiting Sindbis virus infection in vivo. The tools for investigating this hypothesis thoroughly have become available relatively recently. First, genetically modified mice have been generated with a deficient IFN-α/β system by interrupting the gene encoding the known receptor subunit (37). These IFN-α/β receptor null (IFN-α/βR−/−) mice are completely unresponsive to extracellular IFN-α/β and are very susceptible to infection with a number of viruses (12, 13, 17, 22, 36, 37, 57), including two other alphaviruses, Semliki Forest virus (SFV) (22, 37) and Venezuelan equine encephalitis virus (VEE) (17). Second, identification of cell culture-adaptive mutations in laboratory Sindbis virus strains (34) has permitted the construction of an infectious clone eliminating known adaptive mutations (28). The virus derived from this clone, designated TR339, reproduces as closely as possible the original AR339 Sindbis virus isolate, does not exhibit cell culture-adaptive heparan sulfate (HS) binding, and is more virulent for neonatal mice than cell culture-adapted, laboratory Sindbis virus strains (28, 29). Comparison of Sindbis virus TR339 pathogenesis in mice with or without a functional IFN-α/β system has allowed us to study several aspects of the interplay between the host's nonspecific immune response and the infecting virus, including (i) the importance of IFN-α/β in the protection of adult mice from fatal Sindbis virus infection, (ii) the role of IFN-α/β protection in attenuation of HS-binding glycoprotein mutants, (iii) the relevance of the IFN-α/β system to age-dependent resistance to Sindbis virus infection, and (iv) the effect of ablating the IFN-α/β system on the apparent cell and tissue tropism of Sindbis virus in vivo.
MATERIALS AND METHODS
Virus.
Baby hamster kidney cells (BHK-21; ATCC CCL-10) were maintained in alpha-minimal essential medium supplemented with 10% donor calf serum (DCS), 2.9 mg of tryptose phosphate per ml, 0.29 mg of l-glutamine per ml, 100 U of penicillin per ml, and 0.05 mg of streptomycin per ml (37°C, 5% CO2). Infectious viral RNA was transcribed in vitro from linearized cDNA templates of the full-length viral genome and transfected into BHK-21 cells by electroporation. Virus particles were harvested from the supernatant 18 to 20 h after electroporation, clarified by centrifugation (1,500 × g, 4°C, 30 min), and stored at −70°C in single-use aliquots. To minimize the accumulation of tissue culture-adaptive mutations in virus stocks, only high-efficiency electroporations causing complete cytopathic effect were used. Virus stocks were not passed further prior to use. Titers of virus stocks were determined by standard BHK-21 cell plaque assay, and titers were expressed as PFU per milliliters. For routine mouse inoculations, a virus stock aliquot was diluted into low-endotoxin phosphate-buffered saline supplemented with 1% DCS (PBS–1% DCS). Single-use aliquots of diluted virus were stored at −70°C, and the titer of one thawed aliquot was confirmed by plaque assay. To concentrate virus for high-dose inoculations, clarified virus preparations were purified by pelleting through 20% sucrose in TNE (0.05 M Tris-HCl, 0.15 M NaCl, 0.001 M EDTA buffer [pH 7.2]; 100,000 × g, 4°C, 5 h). The virus pellet was allowed to swell overnight in PBS–1% DCS before resuspension and then filtered through a 0.2-μm (pore-size) filter. The virus titer was confirmed by plaque assay.
In vitro growth curves.
Peritoneal exudate cells were collected by peritoneal lavage of cervically dislocated mice. A 10-ml portion of RPMI 1640 medium was injected intraperitoneally (i.p.) by using a 10-ml syringe and a 25-gauge needle, and the abdomen of the mouse was agitated. Lavage fluids were collected by using a 10-ml syringe and 18-gauge needle, and cells were pelleted by centrifugation (60 × g, 4°C, 8 min). Cells were resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2.9 mg of tryptose phosphate, 0.29 mg of l-glutamine, 100 U of penicillin, and 0.05 mg of streptomycin per ml and then seeded into 24-well plates (approximately 2 × 104 cells/well). After 24 h of incubation (37°C, 5% CO2) cells were washed with PBS–1% DCS and infected at a multiplicity of infection (MOI) of 10 in a 0.2-ml volume (37°C, 1 h). Cells were washed three times with PBS–1% DCS, and either 1 ml of medium alone or medium supplemented with ca. 8,000 neutralizing U of anti-IFN-α/β antibody (Lee Biomolecular) was added. Virus was titrated from the supernatant over a 48-h time course.
Mice.
Breeder pairs of IFN-α/βR+/+ 129 Sv/Ev and IFN-α/βR−/− mice were kindly provided by Herbert Virgin (Washington University, Saint Louis, Mo.) and Barbara Sherry (North Carolina State University, Raleigh, N.C.), respectively. Mice were bred in the Department of Laboratory Animal Medicine breeding colony facilities at the University of North Carolina at Chapel Hill under specific-pathogen-free conditions. Cross-breeding male 129 Sv/Ev mice with female IFN-α/βR−/− mice generated mice heterozygous for the IFN-α/β receptor (IFN-α/βR+/−). Procedures were carried out in accordance with institutional guidelines for animal care and use.
Mouse procedures.
For routine mouse inoculations, randomized groups of mice were inoculated with 100 PFU of virus in a 50-μl volume (2 × 103 PFU/ml). The inoculum was administered s.c. in the ventral thorax by using a 27-gauge needle and a 1-ml hypodermic syringe. Mock-infected mice received 50 μl of PBS–1% DCS by the same route. For high-dose inoculations, concentrated virus was inoculated s.c. as described above. All surviving mice were challenged i.c. with S.A.AR 86 virus, which causes 100% mortality in naive adult mice when administered by this route (56). Mice were metofane-anesthetized (Schering-Plough Animal Health), and 1,000 PFU of S.A.AR 86 in a 10-μl volume was inoculated i.c. at the suture by using a 27-gauge needle and 100-μl Hamilton syringe. Blood was collected from the tail vein, and serum was separated by centrifugation in microtainer tubes (Becton Dickinson) according to manufacturer's instructions and stored at −70°C prior to use.
Mortality studies.
Virus-infected and mock-infected mice were observed at 6-h intervals and scored for the degree of morbidity, AST, and percent mortality. Serum collected from surviving mice at 3 weeks postinoculation (p.i.) was assayed for the presence of virus by BHK plaque assay and for anti-Sindbis virus antibody by enzyme-linked immunosorbent assay (ELISA) by using mock-infected mice as controls. The surviving mice were then challenged i.c. with S.A.AR 86 virus as described above.
Pathogenesis studies.
The thoracic cavity of each mouse was opened under metofane anesthesia, and blood was collected by cardiac puncture. Serum was separated from whole blood by using microtainer tubes, aliquoted, and stored at −70°C. Each mouse was then perfused with PBS–1% DCS at a rate of 7 ml/min for 15 to 20 min to flush out the blood-associated virus. Tissues were harvested into preweighed Kontes tissue homogenization tubes, and PBS–1% DCS was added to result in 33% (brain) or 10% (all other tissues) suspensions. Tissues were homogenized by one freeze-thaw and mechanical disruption and then clarified by centrifugation. Tissue supernatants and serum were assayed for virus by BHK plaque assay.
Cytokine assays.
Titers of IFN-α/β in serum were determined by standard biological assay on L929 cells by using a commercially prepared IFN-α/β standard (Lee Biomolecular) and encephalomyocarditis virus (EMCV) as the indicator virus as described previously (61). The end point was defined as the dilution of IFN-α/β required to protect 50% of the cells from EMCV-induced cytopathic effect, and the level of IFN-α/β was expressed as IU per milliliters. Serum levels of the proinflammatory cytokines interleukin-12 (IL-12) p40, tumor necrosis factor alpha (TNF-α), IFN-γ, and IL-6 were determined by sandwich ELISA as described previously (39, 40, 50).
Histology.
Under metofane anesthesia, mice were perfused with 4% paraformaldehyde in PBS (PFA, pH 7.4). Whole PFA-perfused mice were fixed in 4% PFA for one additional week and then decalcified in 4% PFA–8% EDTA (pH 6.8, 4°C) for up to 6 weeks. Tissues were paraffin embedded and sectioned at 5-μm thicknesses. Hematoxylin and eosin (H&E)-stained sections were viewed by light microscopy.
ISH.
In situ hybridization (ISH) analyses to detect viral genomic RNA were performed as described previously (16). Radiolabeled riboprobes were generated by in vitro transcription from linearized plasmid DNA in the presence of [α-35S]UTP (Amersham). Riboprobe complementary to a region in the subgenomic viral RNA was generated from plasmid pGSV.SS (61). Riboprobe complementary to a region in the EBER2 protein gene of Epstein-Barr virus and tissue sections from mock-infected mice controlled for nonspecific probe hybridization. Sections were counterstained with hematoxylin and viewed by light microscopy.
IHC.
Immunohistochemistry (IHC) was performed essentially as described previously (33). Mice were sacrificed by cervical dislocation under anesthesia. Dissected tissues were embedded in OCT Compound (Tissue-Tek) and immediately snap-frozen to avoid ischemic changes. Fresh-frozen sections were cut at approximately 10-μm thicknesses and acetone fixed. Prior to IHC staining, sections were washed in PBS to remove OCT Compound and blocked with 10% normal goat serum. Sections were incubated overnight with a 1:4,000 dilution of polyclonal rabbit anti-Sindbis virus serum or normal rabbit serum control. Detection was achieved with a goat anti-rabbit CY2-conjugated secondary antibody. Double staining to colocalize antigens was performed by incubating anti-Sindbis virus stained sections for 1 h with antibody to cell surface markers: DEC-205 (rat anti-mouse NLDC-145, 0.5 μg/ml; American Type Culture Collection [ATCC]), CD11c (hamster anti-mouse N418, 0.3 μg/ml; ATCC), CD11b (rat anti-mouse Mac-1, 3 μg/ml; kindly provided by Herbert Virgin, Washington University, Saint Louis, Mo.), F4/80 (rat anti-mouse, 1 μg/ml; ATCC), or CD45 (biotinylated anti-mouse B220, 0.5 μg/ml; Pharmingen) alongside sections stained with isotype-matched control antibody. Antibody binding was detected with an appropriate Texas red-conjugated secondary antibody. Sections were viewed on a Nikon inverted fluorescence microscope by using fluorescein isothiocyanate (FITC), Texas red, and triple-pass filters.
RESULTS
Virulence of consensus Sindbis virus strain TR339 in adult mice.
Sindbis virus infection of mice is characterized by an age-dependent attenuation of virulence. Neonatal mice succumb to fatal infection with less than 1 PFU of virus, whereas adult mice are uniformly resistant to very high virus doses. As an initial experiment to investigate the role of the IFN-α/β system in protection from Sindbis virus-induced disease, the virulence of TR339 was tested in age-matched, adult (5 to 7 week old) homozygous IFN-α/βR−/− mice, heterozygous IFN-α/βR+/− mice, and congenic background IFN-α/βR+/+ 129 Sv/Ev mice. Mice were inoculated s.c. in the ventral thorax with 100 PFU of TR339 and then scored at 6-h intervals for morbidity (visually and by weight measurement) and mortality. Data from a representative experiment are shown in Table 1. As expected, infection of adult, IFN-α/βR+/+ 129 Sv/Ev mice with TR339 was subclinical, with no morbidity or coincident weight loss during the 3-week observation period. At 3 weeks p.i. TR339-infected 129 Sv/Ev mice had elicited a virus-specific antibody response and were completely protected from i.c. challenge with a related neurovirulent alphavirus, S.A.AR 86, with no morbidity observed (Table 1). S.A.AR 86 is a member of the Sindbis group of alphaviruses, but it is more closely related to Girdwood and Okelbo than to Sindbis AR339 and is neurovirulent in adult mice following i.c. inoculation (56). Anti-Sindbis antibody is cross-protective against S.A.AR 86 infection. TR339 infection of heterozygous IFN-α/βR+/− mice yielded similar results (data not shown). These data indicated that although no clinical signs of infection were evident, TR339 was able to infect both 129 Sv/Ev and heterozygous IFN-α/βR+/− mice, eliciting a high-titer protective serum antibody response. However, in the presence of a functional IFN-α/β system, the infection was resolved with no overt manifestations. In startling contrast, IFN-α/βR−/− mice succumbed to fatal TR339 infection, with an AST of 2.7 ± 0.2 days. TR339 infection appeared to progress rapidly in these animals with morbidity (weight loss and fur ruffling) observed within 36 h p.i. By 48 h p.i., the IFN-α/βR−/− mice had developed severe conjunctivitis and became increasingly ataxic until death.
TABLE 1.
Virulence of TR339 and TRSB-R114 viruses in adult (5- to 7-week-old) IFN-α/βR−/− mice compared with IFN-α/βR+/+ 129 Sv/Ev control micea
| Primary inoculum | Mouse strain | % Morbidityb | % Mortalityc | AST (days) | αTR339 antibody titer | Challenge inoculum | % Mortalityc | AST (days) |
|---|---|---|---|---|---|---|---|---|
| TR339, 100 PFU s.c. | 129 Sv/Ev/ | 0 (0/3) | 0 (0/3) | NAd | >1:40,960 | S.A.AR 86, 103 PFU i.c. | 0 (0/3) | NA |
| IFN-α/βR−/− | 100 (5/5) | 100 (5/5) | 2.7 ± 0.2 | NA | NA | NA | NA | |
| TRSB-R114, 100 PFU s.c. | 129 Sv/Ev | 0 (0/7) | 0 (0/7) | NA | 1:1,783 (±1780) | S.A.AR 86, 103 PFU i.c. | 14.3 (1/7) | 8.0 |
| IFN-α/βR−/− | 21.4 (3/14) | 7.1 (1/14) | 8.0 | >1:40,960 | S.A.AR 86, 103 PFU i.c. | 0 (0/13) | NA | |
| PBS | 129 Sv/Ev | 0 (0/5) | 0 (0/5) | NA | BLDe | S.A.AR 86, 103 PFU i.c. | 100 (5/5) | 7.4 ± 1.9 |
| IFN-α/βR−/− | 0 (0/5) | 0 (0/5) | NA | BLD | S.A.AR 86, 103 PFU i.c. | 100 (5/5) | 2.2 ± 0.4 |
The data presented in this table were collected in one experiment. However, the results are representative of four independent experiments, including a total of 40 TR339-infected IFN-α/βR−/− mice and 38 TR339-infected IFN-α/βR+/+ mice. The ASTs for TR339-infected IFN-α/βR−/− mice were not significantly different between experiments, and no morbidity or mortality was observed in any TR339-infected 129 Sv/Ev mice.
Percent morbidity is defined by weight loss, ruffling of fur, ataxia, paresis, and/or paralysis.
Percent mortality during a 3-week observation period following primary inoculation or challenge.
NA, not applicable.
BLD, below the limit of detection (1:320).
Relative susceptibilities of 129 Sv/Ev and IFN-α/βR−/− mice to TR339 infection.
In an attempt to overcome the resistance of IFN-α/βR+/+ adult mice to fatal TR339 infection, eight background 129 Sv/Ev mice were inoculated s.c. with approximately 108 PFU of TR339. However, no morbidity or mortality was recorded among these mice over the 3-week observation period. Therefore, the 50% lethal dose (LD50) for TR339 in 129 Sv/Ev mice was >108 PFU, while the LD50 in IFN-α/βR−/− mice was <100 PFU, a difference in susceptibility of at least 106-fold. These data suggest that a functional IFN-α/β system is critical for protection of mice from fatal TR339 infection, since removal of this nonspecific immune mechanism renders the mice extremely susceptible to infection even in animals with an otherwise intact adaptive immune system.
Attenuation of glycoprotein mutant TRSB-R114 in IFN-α/βR−/− mice.
TRSB-R114 is a cell culture-adapted Sindbis virus mutant with two mutations in the E2 envelope glycoprotein that facilitate HS-mediated cell surface attachment (28). TRSB-R114 is attenuated in neonatal mice compared with TR339 and replicates to significantly lower titer in all tissues (28, 61). To investigate the contribution of IFN-α/β to the attenuated phenotype of TRSB-R114, age-matched, adult IFN-α/βR−/− mice and background IFN-α/βR+/+ 129 Sv/Ev mice were inoculated s.c. with 100 PFU of TRSB-R114 and then scored for morbidity and/or mortality at 6-h intervals (Table 1). No morbidity or mortality was observed in TRSB-R114-infected 129 Sv/Ev mice, and these mice exhibited relatively low anti-Sindbis virus antibody titers (1:1,783 ± 1780) at 3 weeks p.i., suggesting that replication of TRSB-R114 was reduced compared to that of TR339. After S.A.AR 86 challenge, 100% morbidity and 14.3% mortality were observed (Table 1). TRSB-R114 was also significantly attenuated in IFN-α/βR−/− mice, causing mild paresis with neurological signs in 3 of 14 mice (21.4%) and death in only 1 (7.1%) at 8 days p.i. The surviving IFN-α/βR−/− mice responded with high-titer antibody to Sindbis virus by 3 weeks p.i. (>1:40,960) and were protected from s.c. challenge with S.A.AR 86. These data indicated that the glycoprotein mutant TRSB-R114 was highly attenuated compared to TR339 even in the absence of a functioning IFN-α/β response and that the induction of anti-Sindbis virus serum antibody correlated with the extent of virus replication.
Disseminated replication of TR339 virus in IFN-α/βR−/− mice.
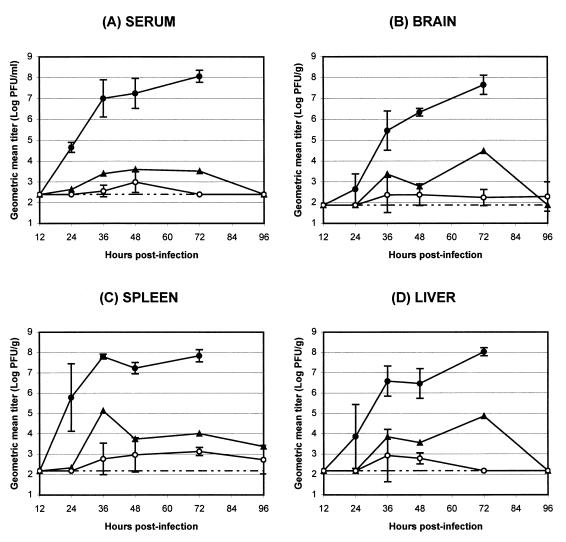
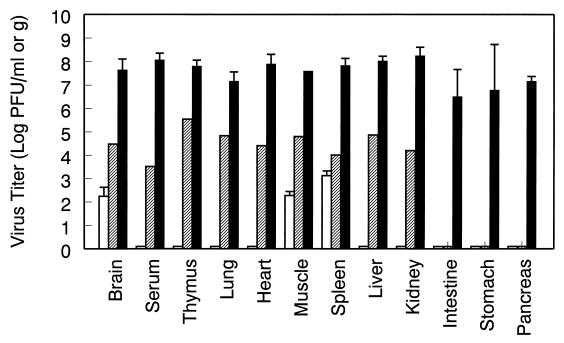
Given the well-characterized role of IFN-α/β in control of virus replication and dissemination, it was anticipated that the dramatically increased susceptibility of mice to TR339 infection in the absence of the IFN-α/β system would reflect an increase in the replication of virus in tissues normally susceptible to virus infection. Therefore, the ability of TR339 to replicate and disseminate after s.c. inoculation was assessed by titration of infectious virus from mouse tissues (Fig. 1 and 2). Randomized groups of 5- to 7-week-old 129 Sv/Ev, IFN-α/βR+/−, and IFN-α/βR−/− mice were inoculated s.c. with 100 PFU of TR339 or PBS–1% DCS. At 12, 24, 36, 48, 72, and 96 h p.i., two (IFN-α/βR+/−) or three (129 Sv/Ev and IFN-α/βR−/−) mice per group were perfused with PBS–1% DCS to minimize the contamination of tissues with blood-associated virus. The titers of infectious virus were determined from serum and the homogenates of multiple tissues. Replication of TR339 in 129 Sv/Ev mice was restricted, not exceeding 104 PFU/ml or g in any tissue at any time point p.i. Although virus was detectable 96 h p.i. in the muscle, brain, or spleen of all three 129 Sv/Ev mice, the trend indicated that virus clearance was underway (Fig. 1 and data not shown). In IFN-α/βR−/− mice, however, TR339 was able to disseminate rapidly from the site of inoculation and replicate to a high titer in all of the tissues tested (Fig. 1 and 2). At between 12 and 24 h p.i., infectious virus became detectable in serum and most tissues. Between 24 and 72 h p.i., the virus titer continued to increase in serum and all tissues with no evidence of clearance. Therefore, not only were virus titers in IFN-α/βR−/− mice much higher in tissues associated with Sindbis virus replication in normal adult mice, but high-level virus replication was evident in all of the tissues examined.
FIG. 1.
Effects of IFN-α/β on the replication and dissemination of Sindbis virus TR339 in vivo. 129 Sv/Ev IFN-α/βR+/+ mice (○), IFN-α/βR+/− heterozygous mice (▴), and IFN-α/βR−/− mice (●) were inoculated s.c. with 100 PFU of TR339 and sacrificed at various times p.i., and virus titers from serum (A), brain (B), spleen (C), and liver (D) were determined. Values represent the geometric mean virus titer (log10 PFU/ml or g) for two (IFN-α/βR+/−) or three (IFN-α/βR−/− and 129 Sv/Ev) mice as determined on BHK cells. Datum points are shown ± the standard deviation (SD), where n = 3. The lower limit of detection is indicated (broken line).
FIG. 2.
Comparison of TR339 virus titers from multiple tissues at 72 h p.i. 129 Sv/Ev IFN-α/βR+/+ mice (open bars), IFN-α/βR+/− heterozygous mice (hatched bars), and IFN-α/βR−/− mice (solid bars) were inoculated, and tissues were harvested as described in Fig. 1. The values represent the geometric mean virus titer (log10 PFU/ml or g) for two (IFN-α/βR+/−) or three (IFN-α/βR−/− and 129 Sv/Ev) mice. Datum points are shown ± the SD, where n = 3. A 0.5 log10 PFU/ml bar denotes a value below the limit of detection, i.e., <1.88 log10 PFU/g for brain, <2.40 log10 PFU/ml for serum, and <2.18 log10 PFU/g for all other tissues.
Interestingly, in heterozygous IFN-α/βR+/− mice intermediate virus titers were observed, suggesting a dose dependence for expression of this subunit of the IFN-α/β receptor. These titers were still below the threshold level for clinical signs of disease in the heterozygous mice, however, and clearance of the virus was not impeded significantly. The significance of increased replication in some tissues of the IFN-α/βR+/− mice (e.g., serum, thymus, lung, heart, liver, and kidney) but not others (e.g., intestine, stomach, and pancreas) is not yet understood.
Restricted replication of TRSB-R114 virus in IFN-α/βR−/− mice.
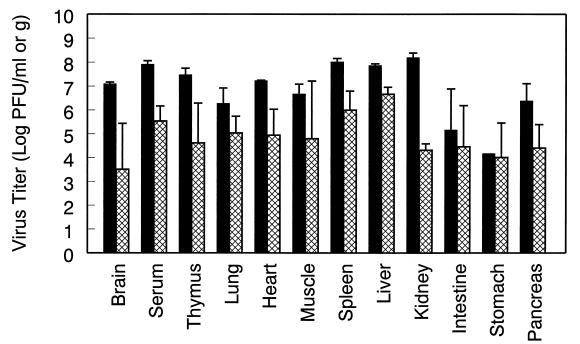
The replication of virulent TR339 and the attenuated glycoprotein mutant TRSB-R114 were compared in IFN-α/βR−/− mice. Three mice per treatment were sacrificed at 72 h p.i., shortly before TR339-infected mice would have succumbed to viral infection, and tissues were processed for virus titration as described previously. Figure 3 demonstrates that the titers of infectious TRSB-R114 in IFN-α/βR−/− mice were reduced in most tissues compared with TR339 titers.
FIG. 3.
Replication of TR339 and glycoprotein mutant TRSB-R114 compared in IFN-α/βR−/− mice. Comparison of TR339 (solid bars) and TRSB-R114 (cross-hatched bars) virus titers in multiple tissues from IFN-α/βR−/− mice at 72 h p.i. The values represent the geometric mean virus titer (log10 PFU/ml or g) ± the SD for three mice.
Induction of proinflammatory cytokines during TR339 infection.
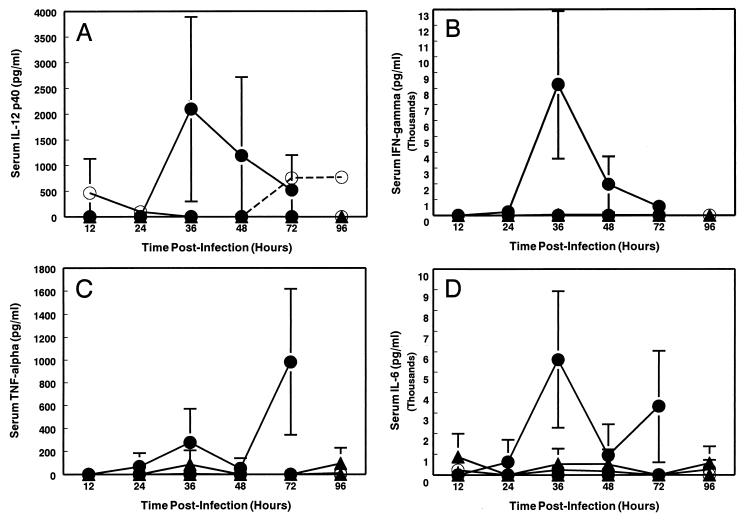
We have previously observed a strong correlation between enhanced in vivo replicative potential of TR339, induction of a systemic inflammatory response syndrome (SIRS), and virulence defined as decreased AST and increased mortality rate (29). It is likely that the SIRS contributes significantly to the proximal cause of death from virulent Sindbis virus infection. These observations, coupled with the very rapid disease progression observed in the TR339-infected IFN-α/βR−/− mice in this study, prompted the comparison of proinflammatory cytokine levels in the serum of the IFN-α/βR−/−, IFN-α/βR+/−, and 129 Sv/Ev mice (Fig. 4). No significant induction of proinflammatory cytokines was detectable in 129 Sv/Ev mice. In contrast, IFN-α/βR−/− mice exhibited elevated IL-12 p40, IFN-γ, TNF-α, and IL-6 in a pattern consistent with SIRS (2). IL-12 p40 levels peaked at 36 h p.i. at 2,094.6 ± 1,795.9 pg/ml (Fig. 4A). A burst of IFN-γ was produced in response to virus infection; this was first detectable 24 h p.i. (222.8 ± 386.0 pg/ml), peaking 6 h later at 8,245.2 ± 4,645.1 pg/ml and then declining through 72 h p.i. (Fig. 4B). TNF-α was first detectable at 24 h p.i. (67.5 ± 116.9 pg/ml) and continued to rise through 72 h p.i. (980.9 ± 637.3 pg/ml) (Fig. 4C). IL-6 was produced with similar kinetics, peaking at 36 h p.i. at 5,607.2 ± 3,322.2 pg/ml (Fig. 4D). Although TNF-α and IL-6 levels had fallen by 48 h p.i., by 72 h p.i., i.e., immediately prior to the death of these mice, the levels were significantly elevated again. Induction of TNF-α and IL-6 was sporadically detectable in heterozygous IFN-α/βR+/− mice, a finding correlating with slightly increased virus titers in these animals compared with 129 Sv/Ev controls.
FIG. 4.
Cytokine levels in serum from TR339-infected and mock-infected mice. 129 Sv/Ev IFN-α/βR+/+ mice (○), IFN-α/βR+/− heterozygous mice (▴), and IFN-α/βR−/− mice (●) were inoculated s.c. with 100 PFU of TR339 (solid line) or with PBS (dashed line), and the serum was harvested at various times p.i. The serum levels of proinflammatory cytokines were determined by ELISA, as follows: (A) IL-12 p40 (confidence limit of detection, 223.2 pg/ml), (B) IFN-γ (confidence limit of detection, 48.83 pg/ml), (C) TNF-α (confidence limit of detection, 97.66 pg/ml), and (D) IL-6 (confidence limit of detection, 195.31 pg/ml). The values represent the mean cytokine level for two (IFN-α/βR+/−) or three (IFN-α/βR−/− and 129 Sv/Ev) mice. Datum points are shown ± the SD, where n = 3. The cytokine levels in PBS-inoculated controls were below the limit of detection except for Fig. 4A, where IL-12 p40 was detectable in 129 Sv/Ev mice at 72 and 96 h p.i.
Tissue tropism of TR339 virus in IFN-α/βR−/− mice.
The presence of high virus titers in widespread tissues of IFN-α/βR−/− mice indicated that the IFN-α/β system imposes a strong restriction on replication and dissemination of TR339 in the adult 129 Sv/Ev mouse. We wished to establish whether the dramatically increased viral loads observed in the adult IFN-α/βR−/− mouse were due to (i) generalized replication throughout the tissues of these mice or (ii) replication in a limited subset of cells in which the IFN-α/β system normally suppresses virus growth. ISH was utilized initially to identify the sites of virus replication definitively. No virus-specific ISH signal was observed in any tissue examined from TR339-infected 129 Sv/Ev mice.
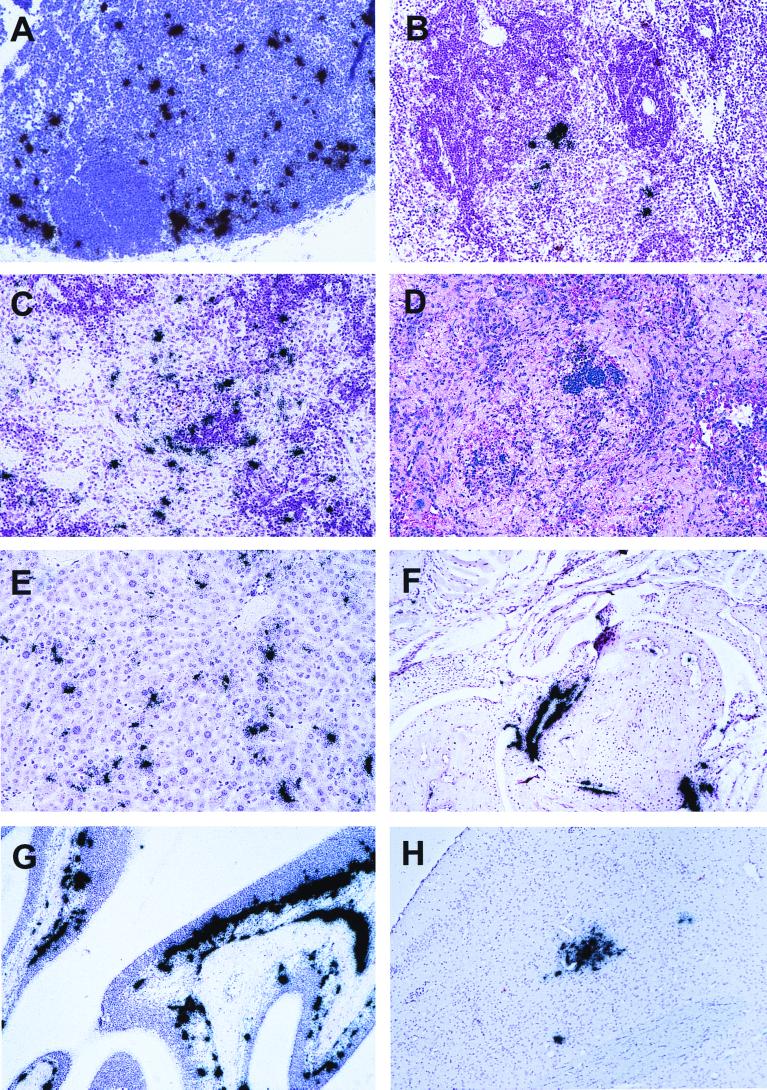
Between 12 and 24 h p.i. a specific virus ISH signal became detectable in the draining lymph nodes (DLNs) and spleens of IFN-α/βR−/− mice (Fig. 5A and B). The axial and brachial LNs were identified as the LNs draining the inoculation site by the injection of India ink. By ISH, replicating virus was observed, particularly in the subcapsular region of the DLN, with sporadic signal found in the cells surrounding the follicle. In the spleen, the virus appeared to be replicating in large discrete cells located in the marginal zone, surrounding the periarteriole lymphocytic sheath (PALS). The location and morphology of these cells allowed their provisional classification to the macrophage-dendritic-cell (DC) lineage. No infected cells were detectable at the site of inoculation or in the lung, liver, kidney, heart, thymus, head, or hindlimb sections at 24 h p.i. Examination of H&E-stained sections did not reveal any tissue pathology at this time.
FIG. 5.
Histopathological and ISH analyses of tissue sections from TR339-infected IFN-α/βR−/− mice. Magnification, ×200. ISH analyses were counterstained with hematoxylin. (A) ISH showing extensive virus replication in large cells of the DLN at 24 h p.i. The B-cell follicle stained more intensely with hematoxylin. (B) ISH showing virus replication in the spleen at 24 h p.i. Again, the more intensely stained region is the follicle with central arteriole. (C) ISH analysis of the spleen at 72 h p.i. showing a more widespread virus infection. (D) H&E stain of the same spleen section at 72 h p.i. revealing a severe loss of splenic architecture and reduced cellularity; the more intensely staining triangular region in the center is the B-cell follicle. (E) ISH showing virus replication in large discrete cells of the liver at 72 h p.i., some of which have an elongated appearance, suggesting that they may be sinusoid lining cells. (F) ISH analysis of a sagittal section of the right forelimb joint at 72 h p.i. Extensive virus replication is evident in the periosteum of the bone. (G) ISH showing virus replication at 72 h p.i. in the nasal turbinates, both in the lamina propria and extending into the darker-staining neuroepithelial layer. Virus signal is also seen in the periosteum of the bone. (H) ISH showing an isolated viral lesion in the olfactory bulb of one mouse at 72 h p.i.
At 72 h p.i., multiple ISH-positive cells of the morphology described above were found in the spleen (Fig. 5C). Examination of H&E-stained spleen sections revealed that the clearly defined follicular architecture normally observed had disintegrated significantly by this time, with a notable reduction in cellularity and morphological evidence of apoptosis at the level of the light microscope (Fig. 5D). The PALS and B-cell follicle regions appeared to be most severely affected. The severe pathology in the spleen was disproportionate to the number of infected cells, suggesting that some tissue damage may be due to a cytokine-mediated bystander effect. This speculation is supported by the presence of high-level IFN-γ at this time point, which previously has been found to contribute to spleen pathology (58). Similar large discrete cells were infected at 72 h p.i. in the liver (Fig. 5E), kidney, lung, and thymus (data not shown). In the liver, a significant proportion of the infected cells appeared to be sinusoid-lining cells, indicating that they might be Küpffer cells and/or endothelial cells and not hepatocytes. In addition, at 72 h p.i. extensive virus-specific signal with associated tissue damage was located in the periosteum and endosteum of the bones, occasionally extending into tendon and fibroblast connective tissue (Fig. 5F). Extensive virus-specific signal was also associated with the nasal turbinates. In addition to infected periosteum of the skull, virus-infected cells also were found lining the lamina propria of the respiratory and neuroepithelia (Fig. 5G). These cells were tentatively identified as macrophage-DC-lineage cells based on location and morphology. Other cells, which appeared to span the neuroepithelium, may be olfactory neurons, as has been suggested in VEE infection (6). Virus-specific signal was observed in the meninges of all three IFN-α/βR−/− mice by 72 h p.i. (data not shown). Thus, replication in the meningeal membranes may account for the infectious virus titered from CNS tissue, since ISH analyses indicated that neuroinvasion had occurred in only one of three mice. In this mouse, an isolated viral lesion was found in the olfactory bulb, suggesting that neuroinvasion may occur via the olfactory nerve from the nasal epithelium (Fig. 5H), as described previously for VEE (6).
Identification of infected cell type(s).
Instead of a generalized infection of all tissues in the IFN-α/βR−/− mice, ISH revealed a preferential infection of cells identified by morphology and distribution as resident tissue macrophages and/or DCs. We have used IHC to identify cells in which Sindbis virus antigens and surface molecules specific for particular cell types were colocalized. Markers included were for macrophages (Mac-1 and F4/80), DCs (NLDC145, N418, and Mac-1), and B cells (B220). Overall, IHC appeared to be considerably more sensitive in detecting virus than was ISH. Since ISH detects only replicating virus, whereas IHC detects viral antigen, this difference in sensitivity may be due to the ability of IHC to detect cells with replicating virus, cells in which replication has ceased (dead or dying cells) and possibly also trapped or phagocytosed virus and antigen presented on the cell surface. However, comparison of IHC and ISH analyses suggested that a notable subset of Sindbis virus antigen-positive cells were actively infected.
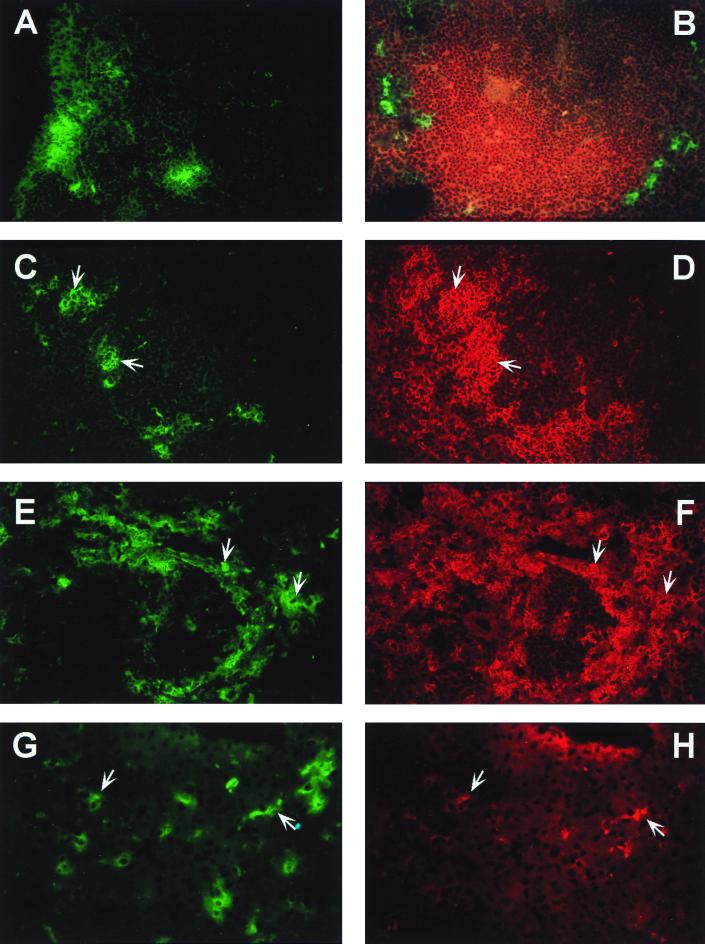
At 24 h p.i., TR339 antigen could be detected in the DLN, spleen, and sporadically in the liver of IFN-α/βR−/− mice. In the DLN, large cells with dendritic processes were detected mostly in the subcapsular region but also occasionally extending between follicles into the medullary region (Fig. 6A). Colocalization of viral antigen was observed in N418-, NLDC145-, and/or Mac-1-positive cells (data not shown), confirming the preliminary placement of infected cells in the macrophage-DC lineage. In the spleen at 24 h p.i., large macrophage-DC-like infected cells were detected in circular patterns. Double staining with a pan-B-cell marker (B220) revealed that these cells were located in the marginal zone surrounding the PALS, concentrated on the outer aspect of the B-cell follicle (Fig. 6B). Marginal zone macrophages have previously been found to stain with Mac-1 but not F4/80, while F4/80 was abundant on macrophages in the splenic red pulp (65). N418-positive DCs are typically found dividing the marginal zone macrophage layer (1). More recent reports have identified an N418 (bright), Mac-1 (bright) DC population in the splenic marginal zone (31, 46). Cells positive for Sindbis virus antigen were identified in the same region of the splenic marginal zone as cells positive for Mac-1 (Fig. 6C and D) and N418 (data not shown), suggesting that TR339 may be able to infect these antigen-presenting cells in IFN-α/βR−/− mice. Limited colocalization with F4/80 was also observed in the red pulp region (data not shown). NLDC145 stained a limited number of cells in the PALS of the spleen but did not colocalize with anti-Sindbis virus staining (data not shown). No anti-Sindbis virus staining was found in any of these tissues from 129 Sv/Ev mice.
FIG. 6.
IHC analyses of tissue sections from TR339-infected IFN-α/βR−/− mice. Magnification, ×400. (A) Distribution of Sindbis virus antigen in the DLN at 24 h p.i. (detected by using CY2-conjugated secondary antibody and visualized by using an FITC filter). (B) Spleen at 24 h p.i. costained for Sindbis virus antigen (CY2) and B-cell marker, B220 (Texas red). Micrographs were taken with FITC and Texas red filters superimposed to demonstrate localization of Sindbis virus antigen in the splenic marginal zone surrounding B-cell follicle. Panels C and D represent TR339-infected spleen, at 24 h p.i., costained for Sindbis virus antigen (CY2) and macrophage marker, Mac-1 (Texas red), respectively. Cells on which virus antigen and Mac-1 colocalize are indicated with paired arrows. Panels E and F show anti-Sindbis (CY2) and Mac-1 (Texas red) costaining, respectively, in the spleen at 72 h p.i. Extensive colocalization of signal is evident, as indicated by paired arrows, and the tissue appears to be severely damaged in keeping with ISH and H&E data. Panels G and H show colocalization of Sindbis virus antigen (CY2) and the Küpffer cell marker, F4/80 (Texas red), in virus-infected liver at 72 h p.i.
By 72 h p.i., anti-Sindbis virus staining was considerably more extensive in both the spleen and the liver of IFN-α/βR−/− mice. By this time there appeared to be more dead and dying cells, in keeping with H&E and ISH analyses. Extensive colocalization of virus antigen with Mac-1 (Fig. 6E and F) and/or N418 (data not shown) was observed once again in spleen, with a more limited F4/80 colocalization outside the marginal zone in the red pulp (data not shown). Compared with spleens from 129 Sv/Ev and mock-infected IFN-α/βR−/− mice, there appears to have been an enormous increase in N418-positive and Mac-1-positive cells; presumably, this was due to either upregulation of the cell surface marker or infiltration of cells into the infected area. In the liver, viral antigen colocalization appeared to be with F4/80 (Fig. 6G and H), although the expression of this marker seemed to have been downregulated compared with controls (possibly due to the shutoff of host protein synthesis by the virus infection). Once again, an increase in Mac-1-positive cells was observed. In 129 Sv/Ev mice IHC analyses of the same tissues from three mice revealed only two Sindbis virus-positive cells, which were detected in the spleen of one animal (data not shown).
In vitro replication of TR339 in macrophage-DC-like cells.
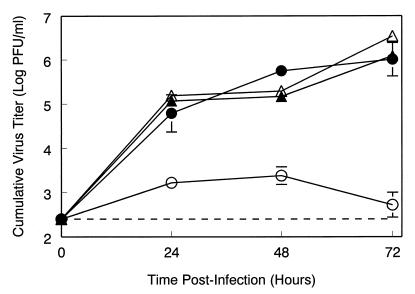
Peritoneal exudate cells from 129 Sv/Ev or IFN-α/βR−/− mice, cultured in vitro, were infected at a high multiplicity (MOI = 10) with TR339 virus in the presence or absence of anti-IFN-α/β antibody. When not elicited in vivo with thioglycollate, this peritoneal cell population includes both immature macrophages and DC progenitors (48). Progeny virus harvested from cell supernatants was barely above the inoculum titer in 129 Sv/Ev cells, whereas more than 106 PFU/ml were produced from IFN-α/βR−/− cells (Fig. 7). However, when anti-IFN-α/β antibody was added to neutralize IFN-α/β released into the supernatant, TR339 was able to replicate to similar titers in 129 Sv/Ev cells. Thus, exogenous IFN-α/β, released from cultured peritoneal cells in response to the virus, suppresses Sindbis virus replication.
FIG. 7.
Effects of IFN-α/β on TR339 replication in primary cell cultures. Primary peritoneal macrophage cultures were infected with TR339 virus (MOI = 10), and titers of progeny virus released into the supernatant were determined. Virus growth curves were determined in cells harvested from IFN-α/βR−/− mice (●, n = 3) and 129 Sv/Ev IFN-α/βR+/+ mice (○, n = 3). Parallel growth curves were determined in the presence of 8,000 neutralizing U of anti-IFN-α/β antiserum in both IFN-α/βR−/− mice (▴, n = 2) and 129 Sv/Ev IFN-α/βR+/+ mice (▵, n = 2). Values represent the geometric mean virus titer (log10 PFU/ml). Data points are shown ± the SD, where n = 3.
DISCUSSION
Viral pathogenesis is the result of a complex interaction between two dynamic systems, the virus and the host, both of which change in multiple ways in response to the other. During the course of a single infection, a virus may change antigenically to avoid the immune system or, in the longer term, it may evolve specific mechanisms to thwart the host's immune surveillance. The host responds to the virus with an impressive array of nonspecific and adaptive immune systems designed to slow the infection and eventually clear the virus. The results presented here attest to the remarkable speed with which nonspecific host defenses are deployed and to the profound effect of these systems on the apparent cell and tissue tropism of the virus.
This study of Sindbis virus infection of mice lacking functional receptors for IFN-α/β suggests (i) that the IFN-α/β host defense mechanism protects adult Sindbis virus-infected animals from fatal disease, (ii) that priming by low-level endogenous IFN-α/β or early induction of IFN-α/β rapidly induces an antiviral state in a variety of otherwise-permissive cells and tissues such that they appear refractory to Sindbis virus infection, and (iii) that IFN-α/β plays an essential role in the protection of sentinel macrophage-DC-like cells from Sindbis virus infection, cells critical for the development of innate and specific immunity.
IFN-α/β is the primary host defense mechanism against fatal TR339 infection.
Wild-type (nonneuroadapted) Sindbis virus is limited to a subclinical infection in adult mice, regardless of the mouse strain, inoculum dose, or route of administration. Following the observation made by Taylor et al. (59) that the mouse virulence of the original Sindbis virus AR339 isolate was host age dependent, many investigators have attempted to better characterize this phenomenon and thereby understand the attenuation of Sindbis virus in adult mice. It has been suggested that the increased resistance of adult mice to Sindbis virus infection might be due to reduced receptor abundance (25, 63), complement-mediated inactivation (21), macrophage clearance (20), and/or resistance of infected neurons to apoptosis (e.g., reference 19). In this study, we have characterized an essential role for the IFN-α/β system in the protection of adult mice from fatal Sindbis virus infection.
Mortality studies comparing adult IFN-α/βR+/+ 129 Sv/Ev mice with IFN-α/βR−/− mice, which are genetically unable to respond to IFN-α/β, demonstrated the importance of IFN-α/β in controlling TR339 infection. IFN-α/βR−/− mice were more than 106-fold more susceptible to fatal TR339 infection than 129 Sv/Ev mice, with s.c. LD50 values of <100 and >108 PFU, respectively. Other studies with either IFN-α/β-defective mice or anti-IFN-α/β antibody have demonstrated a protective role for IFN-α/β in infections with other viruses, including Theiler's virus (12), vesicular stomatitis virus (VSV) (57), influenza virus (13), measles virus (36), VEE (15, 17), and SFV (22, 37). However, few viruses have shown such a complete dependence on this nonspecific immune mechanism for host survival. This is in keeping with the especially acute sensitivity of Sindbis virus to the effects of IFN-α/β in vitro (41, 43; W. B. Klimstra and R. E. Johnston, unpublished observations). Whereas TR339 replication and dissemination were severely restricted in 129 Sv/Ev mice, in IFN-α/βR−/− mice this virus rapidly disseminated beyond the site of inoculation, established a high-titer serum viremia, and seeded a systemic infection in distal tissues. The enormous differential in early virus replication created by ablating the IFN-α/β receptor suggests that IFN-α/β normally restricts early virus replication and spread from the inoculation site very effectively through an autocrine and/or paracrine mechanism. Tissues beyond the site of inoculation may be primed for a rapid antiviral response by low-level endogenous IFN-α/β or may be inherently permissive for virus replication but become refractory through the systemic action of IFN-α/β. Alternatively, permissive distal tissues may remain largely unavailable to the virus under conditions of low or absent viremia.
In IFN-α/βR−/− mice inoculated s.c. with TR339, the virus presumably spreads locally by infecting permissive cells at the site of inoculation, while simultaneously being carried to the DLN either as free virus or in migratory Langerhans' cells or macrophages that have phagocytosed virus and/or become infected by 24 h p.i. Several alphaviruses have previously been shown to replicate within regional LNs following peripheral inoculation, thereby amplifying the serum viremia, including SFV (52), Ross River virus (42, 53), Getah virus (30), and VEE (16). It has recently been demonstrated that replication of VEE in the DLN occurs primarily in cells of the DC lineage (33). A similar tropism has not previously been demonstrated in Sindbis virus-infected mice. However, in the absence of an IFN-α/β response, TR339 spread from the site of inoculation to the DLN by 24 h p.i. and replicated in cells of macrophage-DC lineage. This suggested that Sindbis virus may infect such cells in normal mice but that replication is either prevented or rapidly suppressed by the IFN-α/β response.
A primary viremia, established by between 12 and 24 h p.i., facilitates the rapid systemic dissemination of virus to other permissive tissues by 24 to 36 h p.i. The majority of viruses entering the circulation will be trafficked via the splenic artery to the spleen, where particulate antigens are trapped in macrophages and DCs of the marginal zone and red pulp. In the IFN-α/βR−/− mice exposure of these cells to virus apparently results in infection. Virus from the spleen likely enters the liver via the hepatic portal vein and again appears to infect cells designed to capture antigen: sinusoid-lining Küpffer cells and/or interstitial macrophage-DC-like cells. Infected cells in other tissues, including the kidney, thymus, lung, and respiratory and neuroepithelium, also appear to be macrophage-DC-like by morphological and locational criteria.
Role of IFN-α/β in attenuation of fatal Sindbis virus infection with increasing host age or virus mutation.
In common with some other members of the Alphavirus genus, the outcome of infection with Sindbis virus is strongly dependent on the age and developmental stage of the host (59). In a short time-span between 5 and 11 days of age, mice abruptly acquire resistance to fatal Sindbis virus infection. The mortality rate falls to zero and, unless the virus has been experimentally neuroadapted, Sindbis virus is avirulent in adult mice regardless of virus strain, dose, or route of inoculation (reviewed in reference 26). In neonatal mice, s.c. inoculation of TR339 is rapidly fatal, causing ataxia, paresis, and death by 72 h p.i. (29). The infection is characterized by widespread and extensive virus replication, most notably in the dermis of the skin, fibroblast connective tissue, and skeletal muscle, disseminating to the CNS shortly before death. Simultaneously, induction of an SIRS occurs with significantly elevated levels of proinflammatory cytokines IFN-α/β, IFN-γ, TNF-α, and IL-6.
The development of an effective IFN-α/β system is likely to be an important factor in the acquisition of age-related resistance to fatal Sindbis virus infection, as suggested by several observations. First, the lack of an effective IFN-α/β response in adult animals is a major factor in determining adult resistance to Sindbis infection. Second, the replication of Sindbis virus to a high titer in neonatal mice in the face of an IFN-α/β response on the order of 106 IU/ml of serum (29), combined with the exquisite sensitivity of Sindbis virus to an IFN-α/β-induced antiviral state in vitro, suggests that the IFN-α/β system may be ineffective in the neonate. Third, there are at least superficial similarities between the pathogenesis of TR339 in adult IFN-α/βR−/− and neonatal outbred mice, including extensive virus replication, induction of an SIRS-like cytokine profile, and rapid mortality. However, an age-dependent reduction in the permissivity of certain key tissues (including muscle, fibroblast connective tissue, skin, and CNS) clearly occurs in adults even in the absence of an effective IFN-α/β response. Conversely, in neonatal animals TR339 infection of the liver, kidney, and spleen was limited to occasional ISH-positive cells (29). The ability of Sindbis virus to infect cells in the DLN of neonatal mice has not been investigated. Thus, although potentiation of the IFN-α/β system may play an important role in the acquisition of age-dependent resistance to infection, as suggested above, it is not likely to be the sole determinant of age-dependent attenuation. We conclude from these observations that additional factors, which remain to be identified, are acting in concert with a more effective IFN-α/β response to restrict the replicative potential of TR339 (and other Sindbis virus strains) with increasing host age.
The attenuated glycoprotein mutant, TRSB-R114, was adapted to growth in cell culture by selection for rapid penetration on BHK cells. Unlike TR339, TRSB-R114 attaches efficiently to cells by a cell surface HS-dependent binding interaction (28). We believe that the attenuated phenotype displayed by TRSB-R114 in neonatal mice compared with TR339 results, at least in part, from the nonproductive binding of this virus to glycosaminoglycans in the extracellular matrix, blocking infection and inhibiting dissemination (28). Although this virus was significantly more virulent in adult IFN-α/βR−/− mice than in 129 Sv/Ev controls, it remained attenuated relative to TR339 and replicated much less efficiently. Thus, there appears to be a replication threshold for virulence. Provided that replication remains below this threshold when the specific immune response begins to clear the virus, the mice survive. Any mechanism which restricts replication or spread of the virus in adult animals is likely to act together with a fully functional IFN-α/β system to efficiently localize and clear the infection.
Role of IFN-α/β in determining apparent tissue tropism.
The results of this study and others (13, 36) suggest that the apparent virus tissue and cell tropism may be IFN-α/β dependent. Within hours, if not minutes, of virus infection, the host is physiologically changed from its state at the time of infection, and the primary mediators of these changes are cytokines such as IFN-α/β induced in virus-infected cells. Such mediators can act locally at the site of infection to limit virus replication and hence spread to other tissues. Alternatively, they can act systemically to confer an antiviral refractory state on distant tissues. In either case, failure of a given tissue to evidence virus replication is not necessarily a function of its innate permissiveness or nonpermissiveness for the virus.
Recent studies have demonstrated that, although many cell types produce IFN-α/β upon stimulation with various agents in vitro, the main IFN-α/β-producing cells during a virus infection in vivo are cells of the macrophage-DC lineage, including leukocytes in the DLN (49), marginal metallophilic and marginal zone macrophages in the spleen (10, 11), and DC precursors in the blood (55). The presence of double-stranded RNA replicative intermediates of influenza virus induces the IFN-α/β-mediated maturation, activation, and protection of DC in vitro (5). These reports are in keeping with our finding that the IFN-α/β system appears to govern the susceptibility of cells of the macrophage-DC lineage to productive infection by Sindbis virus. In contrast to the cell tropism evident in normal animals, the absence of a functional IFN-α/β response was associated with infection of cells of the macrophage-DC lineage throughout the mouse. The presence of large numbers of infected splenic marginal zone cells, liver Küpffer cells, and interstitial DCs in IFN-α/βR−/− mice suggests that IFN-α/β is normally responsible for protecting these cells from infection. Therefore, these results have highlighted an important protective function for IFN-α/β, since the location of macrophage-DC lineage cells and their ability to take up particulate antigens predisposes these critical cells of the immune system as primary targets for virus infection (reviewed in reference 27). Interestingly, a similar pattern of infection and uptake in the marginal zone of the spleen has been shown for a number of other viruses, including dengue (4), lymphocytic choriomeningitis virus (51), VSV (7), Pichinde (38), and VEE viruses (16).
There is considerable evidence that even though the virus has access to cells of the macrophage-DC lineage in normal mice, replication of the virus is severely restricted in these cell types. Previous Sindbis virus studies identified viral antigens in adherent cells of the DLN, without evidence of virus replication (18). Hackbarth et al. (20) showed that radiolabeled Sindbis virus particles introduced intracardially are cleared from the circulation by antigen-presenting cells of the splenic marginal zone and reticuloendothelial cells of the liver, either via virus-mediated entry or by phagocytosis. By ISH, we detected virus replication sporadically in the spleen and liver of normal outbred neonatal mice (29) and not at all in the spleen or liver of normal adult mouse. When 129 Sv/Ev mice were inoculated s.c. with 100 PFU of TR339, the virus was not detectable in the DLN 24 h p.i., and preliminary experiments simulating a serum viremia in 129 Sv/Ev mice by intravenous inoculation of 108 PFU of TR339 revealed no detectable infection of splenic marginal zone cells. Nevertheless, we propose that these cells in fact are infected in normal animals but that a paracrine and/or autocrine IFN-α/β response suppresses virus replication and renders such infections abortive. Peritoneal macrophages cultured from 129 Sv/Ev mice appeared to be nonpermissive for TR339, whereas these same cells were clearly permissive in the presence of antibody which neutralizes extracellular IFN-α/β or in analogous cells isolated from IFN-α/βR−/− mice.
Proinflammatory cytokines IL-12, IFN-γ, TNF-α, and IL-6 are typically induced during gram-negative bacterial sepsis or in response to bacterial endotoxin in an SIRS. Relatively little is known about the function and control of these cytokines in viral infection (3). During the early local inflammatory response to virus infection, IL-12 is produced primarily from activated macrophages and DCs, inducing migration and maturation (5, 8, 9, 35). IL-12 induces the production of IFN-γ and TNF-α, as well as activating macrophages, T cells, and NK cells. However, the induction of IL-12, as well as resultant IFN-γ, is controlled during virus infection by IFN-α/β (8). Thus, IL-12 induction observed in TR339 infection of IFN-α/βR−/− mice (and, secondary to that, induction of the other proinflammatory cytokines IFN-γ, TNF-α, and IL-6) may be the direct result of viral replication in cells of this lineage in the absence of IFN-α/β regulation.
The results of these studies of Sindbis infection in IFN-α/βR−/− mice have provided several novel insights into the early control of virus replication. First, even in the presence of a completely functional adaptive immune response, it is the IFN-α/β system which is primarily responsible for protection of adult mice from fatal Sindbis virus infection. Second, it is possible that the effectiveness of the IFN-α/β system increases with the age of the animal and that this is at least partially responsible for age-related resistance to Sindbis virus infections. Finally, the similarities observed between the pathogenesis of TR339 infection in adult IFN-α/βR−/− mice and VEE infection of normal mice (6, 16) suggest that the apparent tissue tropism differences observed between these alphaviruses are, at least in part, determined by differential sensitivity to IFN-α/β and not entirely or even predominantly by differences in cell-specific targeting directed by the virus envelope glycoproteins. Sequence differences in the noncoding regions and nonstructural proteins may confer the relative resistance of VEE to the effects IFN-α/β, allowing this virus to exhibit a lymphotropic phase in adult mice. Therefore, IFN-α/β may be influencing the tissue tropism of Sindbis virus by limiting its access to potentially permissive cells and making otherwise permissive cells nonpermissive for virus replication. Consequently, notions of viral tissue tropism may require revision to account for the early effects of IFN-α/β: rapid establishment of an antiviral state in local and distant cells prior to dissemination as well as the suppression of viral replication in already-infected cells.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI22186 and CA41268 from the NIH. W.B.K. was supported by an NIH Predoctoral Traineeship (T32 AI07419) and by the U.S. Army Research Office (DAAH04-95-1-0224), and K.B.N. was supported by a training grant (T32-ES07272).
We thank Kristen Bernard and Gene MacDonald for assistance with the interpretation of histopathology and the IHC staining techniques, respectively, and Jacque Bailey for excellent technical assistance.
REFERENCES
- 1.Agger R, Crowley M T, Witmer-Pack M D. The surface of dendritic cells in the mouse as studied with monoclonal antibodies. Int Rev Immunol. 1990;6:89–101. doi: 10.3109/08830189009056621. [DOI] [PubMed] [Google Scholar]
- 2.Billiau A, Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur J Clin Investig. 1991;21:559–573. doi: 10.1111/j.1365-2362.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 3.Biron C A. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 4.Boonpucknavig S, Vuttiviroj O, Boonpucknavig V. Infection of young adult mice with dengue virus type 2. Trans R Soc Trop Med Hyg. 1981;75:647–653. doi: 10.1016/0035-9203(81)90142-5. [DOI] [PubMed] [Google Scholar]
- 5.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles P C, Walters E, Margolis F, Johnston R E. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 7.Ciavarra R P, Buhrer K, Van Rooijen N, Tedeschi B. T cell priming against vesicular stomatitis virus analyzed in situ: red pulp macrophages, but neither marginal metallophilic nor marginal zone macrophages, are required for priming CD4+ and CD8+ T cells. J Immunol. 1997;158:1749–1755. [PubMed] [Google Scholar]
- 8.Cousens L P, Orange J S, Su H C, Biron C A. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cousens L P, Peterson R, Hsu S, Dorner A, Altman J D, Ahmed R, Biron C A. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eloranta M L, Alm G V. Splenic marginal metallophilic macrophages and marginal zone macrophages are the major interferon-alpha/beta producers in mice upon intravenous challenge with herpes simplex virus. Scand J Immunol. 1999;49:391–394. doi: 10.1046/j.1365-3083.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 11.Eloranta M L, Sandberg K, Alm G V. The interferon-alpha/beta responses of mice to herpes simplex virus studied at the blood and tissue level in vitro and in vivo. Scand J Immunol. 1996;43:356–360. doi: 10.1046/j.1365-3083.1996.d01-62.x. [DOI] [PubMed] [Google Scholar]
- 12.Fiette L, Aubert C, Muller U, Huang S, Aguet M, Brahic M, Bureau J F. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Sastre A, Durbin R K, Zheng H, Palese P, Gertner R, Levy D E, Durbin J E. The role of interferon in influenza virus tissue tropism. J Virol. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gresser I. Wherefore interferon? J Leukoc Biol. 1997;61:567–574. doi: 10.1002/jlb.61.5.567. [DOI] [PubMed] [Google Scholar]
- 15.Grieder F B, Davis B K, Zhou X D, Chen S J, Finkelman F D, Gause W C. Kinetics of cytokine expression and regulation of host protection following infection with molecularly cloned Venezuelan equine encephalitis virus. Virology. 1997;233:302–312. doi: 10.1006/viro.1997.8617. [DOI] [PubMed] [Google Scholar]
- 16.Grieder F B, Davis N L, Aronson J F, Charles P C, Sellon D C, Suzuki K, Johnston R E. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 17.Grieder F B, Vogel S N. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257:106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- 18.Griffin D E. Role of the immune response in age-dependent resistance of mice to encephalitis due to Sindbis virus. J Infect Dis. 1976;133:456–464. doi: 10.1093/infdis/133.4.456. [DOI] [PubMed] [Google Scholar]
- 19.Griffin D E, Levine B, Tyor W R, Tucker P C, Hardwick J M. Age-dependent susceptibility to fatal encephalitis: alphavirus infection of neurons. Arch Virol Suppl. 1994;9:31–39. doi: 10.1007/978-3-7091-9326-6_4. [DOI] [PubMed] [Google Scholar]
- 20.Hackbarth S A, Reinarz A B G, Sagik B P. Age-dependent resistance of mice to Sindbis virus infection: reticuloendothelial role. J Reticuloendothel Soc. 1973;14:405–425. [PubMed] [Google Scholar]
- 21.Hirsch R L, Griffin D E, Winkelstein J A. The effect of complement depletion on the course of Sindbis virus infection in mice. J Immunol. 1978;121:1276–1278. [PubMed] [Google Scholar]
- 22.Hwang S Y, Hertzog P J, Holland K A, Sumarsono S H, Tymms M J, Hamilton J A, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs B L, Langland J O. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R T. Virus invasion of the central nervous system. A study of Sindbis virus infection in the mouse using fluorescent antibody. Am J Pathol. 1965;46:929–943. [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson R T, McFarland H F, Levy S E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972;125:257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- 26.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 843–898. [Google Scholar]
- 27.Klagge I M, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–833. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 28.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimstra W B, Ryman K D, Nguyen K B, Biron C A, Johnston R E. Infection of neonatal mice with Sindbis virus results in a systemic inflammatory response syndrome. J Virol. 1999;73:10387–10398. doi: 10.1128/jvi.73.12.10387-10398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumanomido T, Wada R, Kanemaru T, Kamada M, Hirasawa K, Akiyama Y. Clinical and virological observations on swine experimentally infected with Getah virus. Vet Microbiol. 1988;16:295–301. doi: 10.1016/0378-1135(88)90033-8. [DOI] [PubMed] [Google Scholar]
- 31.Leenen P J, Radosevic K, Voerman J S, Salomon B, Van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J Immunol. 1998;160:2166–2173. [PubMed] [Google Scholar]
- 32.Lustig S, Jackson A C, Hahn C S, Griffin D E, Strauss E G, Strauss J H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988;62:2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald G, Johnston R E. Dendritic cell targeting and its role in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKnight K L, Simpson D A, Lin S-C, Knott T A, Polo J M, Pence D F, Johannsen D B, Heidner H W, Davis N L, Johnston R E. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol. 1996;70:1981–1989. doi: 10.1128/jvi.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morel A S, Quaratino S, Douek D C, Londei M. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cells: definition of a maturative step. Eur J Immunol. 1997;27:26–34. doi: 10.1002/eji.1830270105. [DOI] [PubMed] [Google Scholar]
- 36.Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz C J, Hourcade D, Atkinson J P, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 38.Murphy F A, Buchmeier M J, Rawls W E. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab Investig. 1977;37:502–515. [PubMed] [Google Scholar]
- 39.Nguyen K B, Biron C A. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J Immunol. 1999;162:5238–5246. [PubMed] [Google Scholar]
- 40.Orange J S, Salazar-Mather T P, Opal S M, Spencer R L, Miller A H, McEwen B S, Biron C A. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overall J C, Jr, Yeh T J, Kern E R. Sensitivity of herpes simplex virus types 1 and 2 to three preparations of human interferon. J Infect Dis. 1980;142:943. doi: 10.1093/infdis/142.6.943. [DOI] [PubMed] [Google Scholar]
- 42.Pearson L D, Doherty P C, Hapel A, Marshall I D. The responses of the popliteal lymph node of the sheep to Ross River and Kunjin viruses. Aust J Exp Biol Med Sci. 1976;54:371–379. doi: 10.1038/icb.1976.37. [DOI] [PubMed] [Google Scholar]
- 43.Petrillo-Peixoto M L, Ferreira P C, Mezencio J M, Golgher R R. Sensitivity of group C arboviruses (bunyaviridae) to human amnion interferon. Intervirology. 1980;14:16–20. doi: 10.1159/000149157. [DOI] [PubMed] [Google Scholar]
- 44.Polo J M, Davis N L, Rice C M, Huang H V, Johnston R E. Molecular analysis of Sindbis virus pathogenesis in neonatal mice by using virus recombinants constructed in vitro. J Virol. 1988;62:2124–2133. doi: 10.1128/jvi.62.6.2124-2133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polo J M, Johnston R E. Attenuating mutations in glycoproteins E1 and E2 of Sindbis virus produce a highly attenuated strain when combined in vitro. J Virol. 1990;64:4438–4444. doi: 10.1128/jvi.64.9.4438-4444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulendran B, Lingappa J, Kennedy M K, Smith J, Teepe M, Rudensky A, Maliszewski C R, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 47.Reinarz A B G, Broome M G, Sagik B P. Age-dependent resistance of mice to Sindbis virus infection: viral replication as a function of host age. Infect Immun. 1971;3:268–273. doi: 10.1128/iai.3.2.268-273.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rezzani R, Rodella L, Zauli G, Caimi L, Vitale M. Mouse peritoneal cells as a reservoir of late dendritic cell progenitors. Br J Haematol. 1999;104:111–118. doi: 10.1046/j.1365-2141.1999.01138.x. [DOI] [PubMed] [Google Scholar]
- 49.Riffault S, Eloranta M L, Carrat C, Sandberg K, Charley B, Alm G. Herpes simplex virus induces appearance of interferon-alpha/beta-producing cells and partially interferon-alpha/beta-dependent accumulation of leukocytes in murine regional lymph nodes. J Interferon Cytokine Res. 1996;16:1007–1014. doi: 10.1089/jir.1996.16.1007. [DOI] [PubMed] [Google Scholar]
- 50.Ruzek M C, Miller A H, Opal S M, Pearce B D, Biron C A. Characterization of early cytokine responses and an IL-6-depandent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandberg K, Kemper P, Stalder A, Zhang J, Hobbs M V, Whitton J L, Campbell I L. Altered tissue distribution of viral replication and T cell spreading is pivotal in the protection against fatal lymphocytic choriomeningitis in mice after neutralization of IFN-alpha/beta. J Immunol. 1994;153:220–231. [PubMed] [Google Scholar]
- 52.Seamer J, Randles W J, Fitzgeorge R. The course of Semliki Forest virus in mice. Br J Exp Pathol. 1967;48:395–402. [PMC free article] [PubMed] [Google Scholar]
- 53.Seay A R, Griffin D E, Johnson R T. Experimental viral polymyositis: age dependency and immune responses to Ross River virus infection in mice. Neurology. 1981;31:656–660. doi: 10.1212/wnl.31.6.656. [DOI] [PubMed] [Google Scholar]
- 54.Sherman L A, Griffin D E. Pathogenesis of encephalitis induced in newborn mice by virulent and avirulent strains of Sindbis virus. J Virol. 1990;64:2041–2046. doi: 10.1128/jvi.64.5.2041-2046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegal F P, Kadowaki N, Shodell M, Fitzgerald-Bocarsly P A, Shah K, Ho S, Antonenko S, Liu Y J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 56.Simpson D A, Davis N L, Seh-Ching L, Russell D, Johnston R E. Complete nucleotide sequence and full-length cDNA clone of S.A.AR86, a South African alphavirus related to Sindbis. Virology. 1996;222:464–469. doi: 10.1006/viro.1996.0445. [DOI] [PubMed] [Google Scholar]
- 57.Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel R M. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steiniger B, van der Meide P H. High-dose interferon-gamma alters the distribution of B lymphocytes and macrophages in rat spleen and lymph nodes. Immunology. 1993;78:461–467. [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor R M, Hurlbut H S, Work T H, Kingston J R, Frothingham T E. Sindbis virus: a newly recognized arthropod-transmitted virus. Am J Trop Med Hyg. 1953;4:844–862. doi: 10.4269/ajtmh.1955.4.844. [DOI] [PubMed] [Google Scholar]
- 60.Trgovcich J, Aronson J F, Eldridge J C, Johnston R E. TNF-alpha, interferon and stress response induction as a function of age-related susceptibility to fatal Sindbis virus infection of mice. Virology. 1999;263:339–348. doi: 10.1006/viro.1999.9913. [DOI] [PubMed] [Google Scholar]
- 61.Trgovcich J, Aronson J F, Johnston R E. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology. 1996;224:73–83. doi: 10.1006/viro.1996.0508. [DOI] [PubMed] [Google Scholar]
- 62.Tucker P C, Griffin D E. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 glycoprotein. J Virol. 1991;65:1551–1557. doi: 10.1128/jvi.65.3.1551-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ubol S, Griffin D E. Identification of a putative alphavirus receptor on mouse neural cells. J Virol. 1991;65:6913–6921. doi: 10.1128/jvi.65.12.6913-6921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vilcek J. Production of interferon by newborn and adult mice infected with Sindbis virus. Virology. 1964;22:651–652. doi: 10.1016/0042-6822(64)90091-1. [DOI] [PubMed] [Google Scholar]
- 65.Witmer M D, Steinman R M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984;170:465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]