Abstract
Some cultured cell lines undergo typical apoptosis upon infection with influenza virus. However, the release of replicated virus into the culture medium does not change when apoptosis is inhibited. Since apoptotic cells are heterophagically eliminated at early stages of the apoptosis pathway, we anticipated that the coexistence of phagocytic cells with virus-infected cells affects the extent of virus growth. When influenza A virus-infected HeLa cells were mixed with activated mouse peritoneal macrophages, efficient phagocytosis, which was abrogated in the presence of a caspase inhibitor, occurred. At the same time, the release of virus into the culture medium was completely inhibited, and this required direct contact between virus-infected cells and macrophages. Furthermore, an immunoelectron microscopic analysis detected influenza virus particles associated with phagosome-like structures within macrophages. These results indicate that apoptosis-dependent phagocytosis of virus-infected cells may lead to direct elimination of the pathogen.
A variety of viruses induce apoptosis in host cells upon infection, but the physiological role of this phenomenon remains uncertain (9, 21). Influenza virus causes apoptotic death of cultured cell lines (6, 11, 20) as well as that of tissues in infected animals (12). Although it has been postulated that the death of virus-infected cells should lead to the spread of virus progeny to neighboring cells (21), inhibition of apoptosis of influenza A virus-infected cells by a caspase inhibitor does not affect the amount of replicated virus released into the culture medium (19).
Since cells undergoing apoptosis are eventually engulfed and digested by phagocytic cells (4, 22), elucidation of the mechanism and consequences of phagocytosis of apoptotic virus-infected cells may be required for understanding the physiological role of the induction of apoptosis. Albert et al. recently reported that influenza virus-infected monocytes are phagocytosed by dendritic cells in an apoptosis-dependent manner and that this reaction leads to antigen presentation resulting in stimulation of class I-restricted CD8+ cytotoxic T lymphocytes (1). They further showed that the same cells were phagocytosed by macrophages without antigen presentation (2). The consequences of phagocytosis of influenza virus-infected cells by macrophages have thus remained uncertain. Here we examined the effect of macrophages on virus growth.
Infection and growth of influenza virus in HeLa cells.
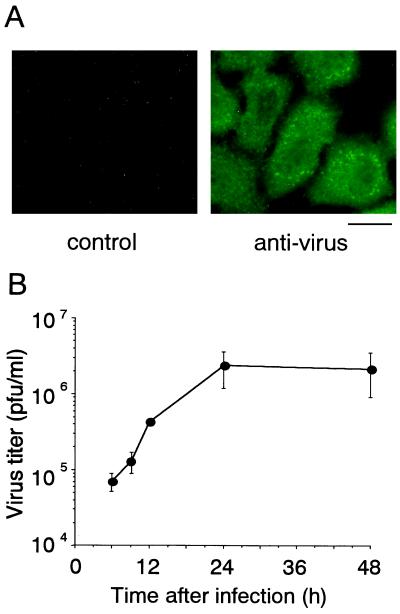
HeLa S3 cells were maintained in Eagle's minimal essential medium–10% fetal bovine serum at 37°C with 5% CO2 in air. The cells were infected with a wild-type strain of influenza A/Udorn/72(H3N2) virus, SP626, at a multiplicity of infection of 3 as described previously (10). Influenza virus-infected HeLa cells were maintained in poly-Lys-coated culture containers for 9 h, fixed with 2% paraformaldehyde–0.1% glutaraldehyde–0.05% Triton X-100, rinsed in methanol, washed with phosphate-buffered saline (PBS), and treated with an anti-influenza A virus antiserum which was raised against purified virions and recognizes nucleocapsid protein, M1, hemagglutinin, and neuraminidase (16). The cells were then washed with 0.2% Triton X-100-containing PBS, treated with a biotin-labeled anti-rabbit immunoglobulin G (IgG) antibody (Vector), and washed as described above. The samples were finally treated with fluorescein isothiocyanate-conjugated avidin (Vector) and examined with a fluorescence and phase-contrast microscope (BX50; Olympus). Nearly 100% of the cells showed positive signals (Fig. 1A) while the same antibody did not react to mock-infected cells (data not shown). The virus titer, determined by a plaque assay using MDCK cells and expressed as PFU as described previously (20), in the culture medium increased, reaching a maximum level at 24 h of infection (Fig. 1B). On the other hand, completion of apoptosis as determined by various biochemical assays required almost 48 h (data not shown), as reported previously (7, 20). This suggests that viral replication proceeds before host cell macromolecular synthesis becomes nonfunctional due to apoptosis.
FIG. 1.
Infection and growth of influenza virus in HeLa cells. (A) Immunofluorescence analysis of virus-infected HeLa cells with an anti-influenza virus antiserum. The cells at 9 h after infection were stained with control rabbit IgG (left) or the anti-influenza virus antibody (right) and examined by fluorescence microscopy. Scale bar = 25 μm. (B) Time course of virus growth. The amount of virus released into the culture medium was determined by a plaque assay. The means and standard deviations of a typical example from three independent experiments are presented.
Phagocytosis of influenza virus-infected cells by macrophages.
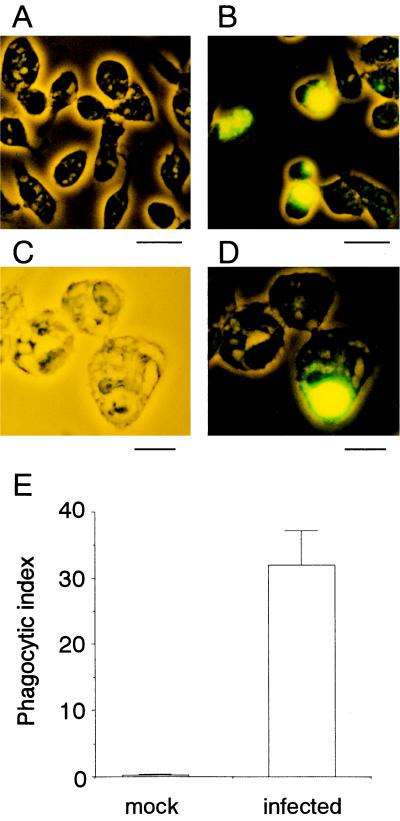
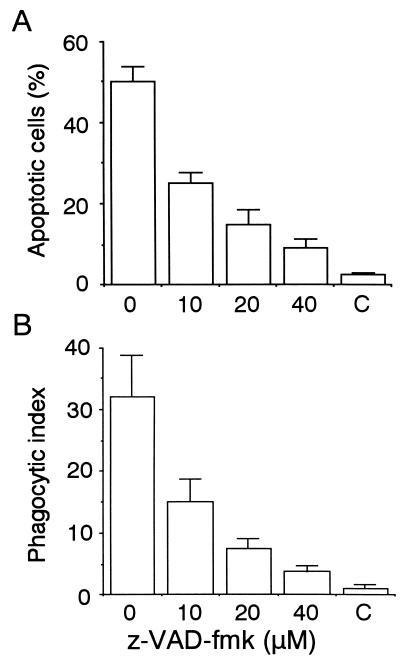
Since cells treated with a variety of proapoptotic stimuli are engulfed by phagocytic cells at early stages of the apoptotic pathway (4, 5, 13–15, 22), we anticipated that influenza virus-infected cells might be susceptible to phagocytosis before production of infectious virus begins. The phagocytosis assay with mouse peritoneal macrophages was conducted as described previously (17, 18). In brief, macrophages were isolated from peritoneal fluids of thioglycollate-treated BDF1 mice and maintained in RPMI 1640–10% fetal bovine serum. HeLa cells were collected at 9 h after infection, a time at which the amount of virus released into the culture medium is only 1/20 of the maximum level (see Fig. 1B), labeled with biotin (NHS-LC-biotin; Pierce), and added to the macrophage culture at a ratio of one HeLa cell to two macrophages. The mixed culture was incubated for 30 min at 37°C, washed extensively, fixed and permeabilized, supplemented with fluorescein isothiocyanate-labeled avidin, and examined with a fluorescence and phase-contrast microscope (Fig. 2A to D). The number of macrophages that contained fluorescent signals was determined and expressed relative to the total number of macrophages as the phagocytic index (Fig. 2E). More than 30% of the macrophages appeared to incorporate virus-infected cells, whereas they did not react with mock-infected cells. These results suggested that macrophages selectively recognized and phagocytosed influenza virus-infected cells. We next examined whether this phagocytosis requires the occurrence of apoptosis in the virus-infected cells. To test this, z-VAD-fmk, a caspase inhibitor, was added to the culture from the beginning of infection. The drug reduced apoptosis, as determined by chromatin condensation, to 1/4 of the level in the control (Fig. 3A) as described previously (19), and at the same time, phagocytosis of the z-VAD-fmk-treated cells decreased to a similar extent (Fig. 3B). This indicated that macrophages phagocytosed virus-infected cells that were undergoing apoptosis.
FIG. 2.
Phagocytosis of influenza virus-infected cells by macrophages. HeLa cells infected with influenza virus for 9 h (B to D) or control mock-infected cells (A) were subjected to a phagocytosis assay with mouse peritoneal macrophages. Fluorescence and phase-contrast views (A, B, and D) and a phase-contrast view (C) are shown. Scale bars: A and B, 25 μm; C and D, 10 μm. (E) The phagocytic index means and standard deviations of a typical example from three independent experiments are presented.
FIG. 3.
Effect of a caspase inhibitor on apoptosis and phagocytosis of influenza virus-infected cells. HeLa cells were infected with influenza virus for 15 h in the presence of caspase inhibitor z-VAD-fmk (Peptide Institute), and the extents of apoptosis (A) and phagocytosis by macrophages (B) were determined. C, results with mock-infected cells.
Inhibition of virus growth in the presence of macrophages.
We then examined whether virus growth is affected in the presence of macrophages. HeLa cells were infected with influenza virus and added to a macrophage culture after 9 h. The coculture was further maintained, and the virus titer in the culture medium was determined at 24 and 48 h after infection. The virus titer in the medium of the cocultures showed no increase and instead gradually decreased, while that in a control culture without macrophages increased as the culturing time increased (Fig. 4A). These results indicated that release of influenza virus into the culture medium was completely inhibited when virus-treated cells were cultured in the presence of macrophages and strongly suggested that this was due to phagocytosis of infected cells by macrophages. However, the possibility remained that soluble factors secreted from macrophages caused decreased replication and/or release of virus. Virus-treated HeLa cells and macrophages were therefore placed on opposite sides of a permeable membrane, and virus growth was monitored. The results clearly showed that direct association between virus-infected cells and macrophages is required for inhibition of virus growth (Fig. 4B).
FIG. 4.
Inhibition of influenza virus growth in the presence of macrophages. (A) HeLa cells infected with influenza virus for 9 h were further cultured in the absence (open circles) or presence (closed circles) of macrophages, and the virus titer in the culture medium was determined. The means and standard deviations of a typical example from three independent experiments are presented. (B) HeLa cells infected with virus for 9 h and macrophages were placed on opposite sides of a permeable membrane (0.4-μm pores, Millicell-CM; Millipore), and the virus titer in the culture medium was determined after 15 h. The means and standard deviations of a typical example from three independent experiments are presented.
Association of influenza virus with phagosome-like structures in macrophages.
We next used electron microscopy to directly detect virus particles in macrophages that engulfed virus-infected cells (Fig. 5). A triple fixation method (3) was adopted to detect influenza virus particles. HeLa cells were fixed with 0.1 M phosphate buffer (pH 7.2) containing 2% glutaraldehyde for 2 h at 4°C, washed, and treated with 1% OsO4 for 1 h at 4°C. The cells were then washed with 0.1 M sodium acetate, further fixed with 0.5% uranyl acetate for 20 min at 4°C, and washed again with 0.1 M sodium acetate. The fixed cells were scraped off culture containers, mixed with 2% agarose, and pelleted by centrifugation. After the agarose solidified, a piece of it was dehydrated with an ethanol series and embedded in Quetol 653, an epon resin (Nisshin EM), and ultrathin sections were prepared with a diamond knife. The sections were treated successively with 4% uranyl acetate and lead citrate and examined with an electron microscope (JEM-1200EXII; JEOL). When HeLa cells infected with virus for 9 h were examined, electron-dense particles with a size and shape similar to those of influenza virus were visible along the periphery (indicated by arrows in Fig. 5A) while they were undetectable in mock-infected cells (Fig. 5B). To examine if those particles were influenza virus, immune electron microscopic analyses were conducted with the anti-influenza virus antiserum. Ultrathin sections prepared as described above were mounted on a nickel grid and treated with 1% hydrogen peroxide and then with PBS containing 0.1% Tween 20–1% bovine serum albumin–0.1% sodium azide. The samples were then treated with the antiserum for 1 h at room temperature and washed. They were supplemented with 10-nm gold particle-conjugated anti-rabbit IgG (British Biocell), incubated for 1 h at room temperature, washed, and examined by electron microscopy. Many particles were positive for gold particles when treated with the anti-influenza virus antibody (Fig. 5C) but not when treated with a control antibody (Fig. 5D). Examination of the cells after the phagocytosis reaction revealed macrophages that interact with apoptotic virus-infected cells using pseudopodia (Fig. 5E and F). Some macrophages contained materials that were high in electron density and surrounded by membranes (Fig. 5G and H), suggesting that they were apoptotic cells within phagosomes. Moreover, the engulfed materials were associated with influenza virus particles (indicated by arrows in Fig. 5G and H). These results indicated that macrophages phagocytosed influenza virus together with infected host cells.
FIG. 5.
Presence of influenza virus within phagosome-like structures in macrophages. Influenza virus-infected HeLa cells (at 9 h) and macrophages after phagocytosis reactions were examined by electron microscopy. Shown are the periphery of a virus-infected (A) or mock-infected (B) HeLa cell (particles detectable only with virus-infected cells are indicated with arrows); an immunoelectron microscopic analysis of virus-infected HeLa cells with anti-influenza virus (C) or control (D) antibody; (E) a virus-infected HeLa cell (left) and a macrophage (right); (F) a higher magnification view of panel E (the arrows point to virus particles); and (G and H) a phagosome-like structure within a macrophage (the arrows point to virus particles). Scale bars: A and B, 0.5 μm; C and D, 0.1 μm; E, 2 μm; F and G, 0.5 μm; H, 0.1 μm.
Taken together, the present findings showed that influenza virus-infected HeLa cells are phagocytosed by macrophages and that the phagocytosis depends on the occurrence of apoptosis. This resulted in the inhibition of virus growth, suggesting that the virus, together with its host cells, was digested in macrophages. These findings suggest that apoptosis of influenza virus-infected cells induces inhibition of virus growth and ultimately protects the organism from viral diseases. Alveolar macrophages and polymorphonuclear leukocytes were shown to be responsible for inhibition of influenza virus growth in the lungs of virus-infected mice, although it is uncertain that virus clearance was caused by phagocytosis of virus-infected cells (8). It is important to expand this proposed system under the in vivo situation. Since phagocytosis of influenza virus-infected cells by dendritic cells leads to antigen presentation toward lymphocytes (1, 2), induction of apoptosis upon virus infection would allow the organism to escape from the pathogen via two pathways.
Acknowledgments
This study was supported by grants from the Organized Research Combination System of the Science and Technology Agency of Japan and from the Hokuriku Industrial Advancement Center.
We thank A. Shiratsuchi for advice about macrophage preparation and phagocytosis assays; K. Hirai, S. Iseki, and Y. Hosaka for help and comments about electron microscopy; and K. Shimizu for the anti-influenza virus antiserum.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Albert M L, Pearce S F A, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando T, Fujimoto K, Mayahara H, Miyajima H, Ogawa K. A new one-step method for the histochemistry and cytochemistry of Ca2+-ATPase activity. Acta Histochem Cytochem. 1981;14:705–726. [Google Scholar]
- 4.Ellis R E, Yuan J, Horvitz H R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 5.Fadok V A, Bratton D L, Frasch S C, Warner M L, Henson P M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 6.Fasq H, Bacher M, Nain M, Gemsa D. Programmed cells death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 1994;190:175–182. doi: 10.1016/S0171-2985(11)80292-5. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto I, Takizawa T, Ohba Y, Nakanishi Y. Co-expression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ. 1998;5:426–431. doi: 10.1038/sj.cdd.4400362. [DOI] [PubMed] [Google Scholar]
- 8.Fujisawa H, Tsuru S, Taniguchi M, Zinnaka Y, Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol. 1987;68:425–432. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- 9.Granville D J, Carthy C M, Yang D, Hunt D W C, McManus B M. Interaction of viral proteins with host cell death machinery. Cell Death Differ. 1998;5:653–659. doi: 10.1038/sj.cdd.4400388. [DOI] [PubMed] [Google Scholar]
- 10.Hatada E, Takizawa T, Fukuda R. Specific binding of influenza A virus NS1 protein to the virus minus-sense RNA in vivo. J Gen Virol. 1992;73:17–25. doi: 10.1099/0022-1317-73-1-17. [DOI] [PubMed] [Google Scholar]
- 11.Hinshaw V G, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76:2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 13.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998;5:563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 14.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 15.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Mukaigawa J, Oguro M, Ono Y, Nakajima K, Kida H. Inhibition of transcriptase activity of influenza A virus in vitro by anti-haemagglutinin antibodies. Vaccine. 1985;3:207–210. doi: 10.1016/0264-410x(85)90107-0. [DOI] [PubMed] [Google Scholar]
- 17.Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. J Biol Chem. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- 18.Shiratsuchi A, Osada S, Kanazawa S, Nakanishi Y. Essential role of phosphatidylserine externalization in apoptosing cell phagocytosis by macrophages. Biochem Biophys Res Commun. 1998;246:549–555. doi: 10.1006/bbrc.1998.8663. [DOI] [PubMed] [Google Scholar]
- 19.Takizawa T, Tatematsu C, Ohashi K, Nakanishi Y. Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol Immunol. 1999;43:245–252. doi: 10.1111/j.1348-0421.1999.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 21.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]