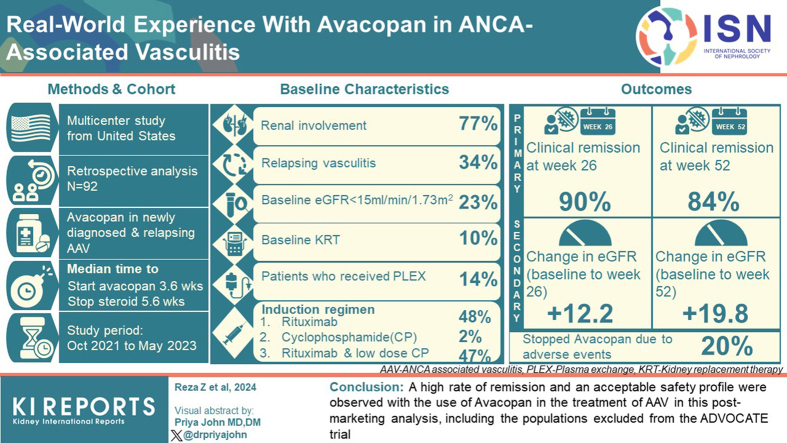
Abstract
Introduction
Postmarketing data on outcomes of avacopan use in antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) are lacking.
Methods
We performed a multicenter retrospective analysis of 92 patients with newly diagnosed or relapsing AAV who received therapy with avacopan. The coprimary outcome measures were clinical remission at 26 and 52 weeks. We use descriptive statistics and univariate logistic regression to assess outcomes and predictors of remission, respectively.
Results
Of the 92 patients, 23% (n = 21) had a baseline estimated glomerular filtration rate (eGFR) < 15 ml/min per 1.73 m2 and 10% on kidney replacement therapy at baseline. Among those with kidney involvement, mean (SD) enrollment eGFR was 33 (27) ml/min per 1.73 m2 with a mean (SD) change of +12 (25) and +20 (23) ml/min per 1.73 m2 at weeks 26 and 52, respectively. In addition to avacopan, 47% of patients received combination therapy of rituximab and low-dose cyclophosphamide, and 14% of patients received plasma exchange (PLEX). After induction, the median (interquartile range [IQR]) time to start avacopan was 3.6 (2.1–7.7) weeks, and the median time to discontinue prednisone after starting avacopan was 5.6 (3.3–9.5) weeks. Clinical remission was achieved in 90% of patients at week 26 and 84% of patients at week 52. Of the patients, 20% stopped avacopan due to adverse events, with the most common being elevated serum aminotransferases (4.3%).
Conclusion
A high rate of remission and an acceptable safety profile were observed with the use of avacopan in the treatment of AAV in this postmarketing analysis, including the populations excluded from the ADVOCATE trial.
Keywords: avacopan, ANCA-associated vasculitis, complement, remission, kidney recovery
Graphical abstract
AAV is a predominantly small vessel vasculitis with a predilection for the kidneys and respiratory tract.1 It is a potentially life threatening disease that can present with rapidly progressive glomerulonephritis and pulmonary hemorrhage. Prompt initiation of immunosuppressive therapy is paramount to halt tissue injury and prevent accumulation of organ damage, including ESKD. Glucocorticoids (GCs) have been a cornerstone in the treatment of AAV largely due to their immediate anti-inflammatory effects. However, their benefits are accompanied by toxicities, including infection, diabetes, mood instability, osteoporosis, skin thinning, vascular fragility, edema, and hypertension, among others, which has prompted the search for GC-sparing agents.2
One such agent is avacopan, an oral complement component 5a receptor blocker approved by the US Food and Drug Administration in October 2021 for adjunctive treatment of adult patients with severe, active AAV (granulomatosis with polyangiitis or microscopic polyangiitis) in combination with standard therapy. By blocking chemoattraction of neutrophils, avacopan reduces inflammatory injury by ANCA at the target tissue. Its efficacy was demonstrated in the ADVOCATE study, a phase 3 randomized controlled trial, where sustained remission at week 52 was observed at a higher rate in the avacopan group than in the prednisone group (65.7% vs. 54.9%, respectively).3 Second, there was a higher least squares mean increase in eGFR from baseline to week 52 in avacopan compared to prednisone (7.3 ml/min per 1.73 m2 vs. 4.1 ml/min per 1.73 m2, respectively) with even more pronounced improvements in eGFR in patients with a low baseline eGFR in the avacopan arm.4 Finally, avacopan resulted in far less use of GC than in the prednisone group (mean cumulative dose 1676 mg vs. 3847 mg, respectively). However, despite the promising results, avacopan is a first in class drug and postmarketing data on the use of avacopan in the treatment of AAV are lacking.
We conducted a multicenter retrospective cohort analysis of 92 patients who received avacopan for the treatment of new or relapsing AAV. Our objective was to describe the real-world experience and outcomes with avacopan in the United States since its approval. To our knowledge, this is the largest postmarketing study of avacopan published to date.
Methods
Study Design and Patient Cohort
We performed a multicenter retrospective analysis of 92 patients with active AAV who received treatment with avacopan at 12 academic medical centers across the United States from October 2021 to May 2023. The inclusion criteria included patients aged ≥18 years; newly diagnosed or relapsing AAV as defined by the 2012 Chapel Hill Consensus Conference definitions5; and having received at least 2 weeks of avacopan. AAV was deemed active if a Birmingham vasculitis activity score (BVAS) version 3 was ≥ 3 (at least 1 major or 3 non-major items).6 The exclusion criteria included antiglomerular basement membrane disease. We also excluded patients with missing values necessary to determine clinical remission, cumulative GC dosing, eGFR, and other study outcomes. All data were abstracted from electronic medical records. The study was approved by the institutional review boards at each participating site. The requirement for informed consent was waived due to the retrospective nature of the study.
Treatment Regimen
Patients were prescribed avacopan 30 mg orally twice daily with intention for 1 year duration of therapy plus standard remission induction therapy followed by maintenance therapy. When rituximab was used for standard induction therapy, it was administered i.v. as 2 doses of 1000 mg given 2 weeks apart or as 375 mg/m2/wk for 4 weeks. When cyclophosphamide was used alone, it was given as standard i.v. or oral induction dosing.7 In case of dual therapy with cyclophosphamide and rituximab, the dosing and duration was at the discretion of the treating physician. Similarly, use of PLEX, GC (i.e., dose, duration, and pulse therapy), and choice or timing of remission maintenance therapy was at the discretion of the treating physician. Remission-maintenance therapy comprised either azathioprine (target dose of 2 mg/kg/d), methotrexate, or rituximab (500 mg or 1000 mg i.v. dose every 4–6 months). Prophylaxis to pneumocystis jirovecci, osteoporosis, and gastrointestinal ulceration was used at the discretion of the treating physician.
Outcomes and Follow-Up
The primary outcome measure was the proportion of patients in clinical remission at week 26, and in sustained clinical remission at week 52. Clinical remission at week 26 and week 52 was defined by the treating physician as both no signs or symptoms of vasculitis activity and a prednisone dose ≤5 mg/d. Secondary outcome measures included prednisone dose at week 12 and 52, cumulative GC dose, liberation from kidney replacement therapy, change in eGFR at week 26 and 52, reduction in proteinuria at week 26 and 52, nadir proteinuria, resolution of hematuria, disease relapse, infection requiring hospitalization, as well as kidney and patient survival. eGFR was calculated using the race-free 2021 chronic kidney disease epidemiology collaboration equation.8 Assessment of outcomes on changes in eGFR, proteinuria, and hematuria were limited to patients with kidney involvement. Disease relapse was defined as AAV disease recurrence requiring escalation of immunosuppression at any phase during treatment. Further analysis was performed comparing patients with entry eGFR < 15 ml/min per 1.73 m2 versus ≥ 15 ml/min per 1.73 m2; avacopan initiation within 30 days of induction therapy start (vs. ≥ 30 days), PLEX versus no PLEX use, and in those who continued versus stopped steroids. All patients in our series had at least 4 weeks of follow-up evaluation at the time of data analysis, and no patients were excluded because of loss to follow-up, early discontinuation of treatment, an adverse event, or death.
Statistical Analysis
Baseline summary statistics were calculated using median (IQR) and mean (SD). Given the exploratory nature of this study, we stratified outcomes by those who started avacopan within 30 days of induction, those who did not receive PLEX, and those who discontinued oral steroids. Data across categories were compared using chi-square test, fisher exact test, t test, and Wilcoxon signed rank test, as indicated. Individuals on dialysis (at each time point) were considered to have an eGFR of 5 ml/min per 1.73 m2. There was 1 patient with extrarenal vasculitis who was already on chronic dialysis at study entry, therefore this patient was excluded from eGFR, proteinuria, hematuria and dialysis related analyses (i.e., change in eGFR and ESKD at follow-up).
We looked at univariate logistic regression across key clinical demographics; multivariable logistic regression was not performed due to the low number of events. We created 3 figures. First, we plotted the mean eGFR ± SD over follow-up for all participants with kidney involvement at baseline. Second, we plotted the difference in eGFR over 1 year for patients with complete follow-up data stratified by avacopan initiation timing. Finally, using the survminer package in R (R Foundation for Statistical Computing, Vienna, Austria), we plotted the Kaplan-Meier estimate for steroid discontinuation in patients on steroids ≥30 days (with follow-up starting at day 30) with patients stratified by avacopan use at day 30.
Significant test results were determined by 2-tailed P-value ≤ 0.05. Analyses were carried out using R Core Team, Version 4.2.2.
Results
Study Population
This study included 92 patients across 12 academic medical centers in the United States. The median (IQR) follow-up time of the study (from start of induction therapy to last follow-up) was 6.0 (3.8–10.6) months. The mean (SD) age at induction therapy was 59 years (17), 64% were female, and mean BVAS was 14.7 Among the 92 patients, 66 (72%) were MPO-ANCA, 25 (27%) were PR3-ANCA, and 1 (1%) was ANCA-negative. Thirty-one patients (34%) had relapsing disease. Mean (SD) eGFR for the 92 patients at induction was 43.5 (34.2) ml/min per 1.73 m2, with 21 patients having eGFR < 15 ml/min per 1.73 m2, and 10 patients on kidney replacement therapy including 1 patient with ESKD with an extrarenal flare. Median (IQR) proteinuria was 1.7 (0.4–2.7) g. Baseline characteristics are presented in Table 1. Seventy-one patients (77%) had kidney involvement (defined by individual study site investigators) with 49 (69%) having a kidney biopsy at study entry. The mean (SD) baseline eGFR in those with kidney involvement was 32.9 (26.7) ml/min per 1.73 m2.
Table 1.
Baseline population characteristics
| Number of patients | N = 92 |
|---|---|
| Age, yr, mean (SD) | 59 (17) |
| Female, n (%) | 59 (64%) |
| Caucasian, n (%) | 75 (82%) |
| BMI (kg/m2), mean (SD) | 28.2 (7.1) |
| ANCA serotype, n (%) | |
| PR3 | 25 (27%) |
| MPO | 66 (72%) |
| Negative | 1 (1%) |
| Organ involvement, n (%) | |
| Kidney | 71/92 (77%) |
| eGFR at diagnosis, ml/min per 1.73 m2, mean (SD) | 32.9 (26.7) |
| eGFR < 15 ml/min per 1.73 m2 | 21/92 (23%) |
| Kidney replacement therapya | 9/92 (10%) |
| Hematuria | 61/71 (87%) |
| Proteinuria, median (IQR) | 1693 mg/g Cr (571–2675) |
| Biopsy data | |
| Focal class | 10/48 (21%) |
| Crescentic class | 18/48 (37%) |
| Mixed class | 11/48 (23%) |
| Sclerotic class | 9/48 (19%) |
| Pulmonary | 48/92 (52%) |
| Constitutional | 46/92 (50%) |
| ENT | 27/92 (29%) |
| Cutaneous | 14/92 (15%) |
| Eye | 10/92 (11%) |
| Nervous system | 8/92 (9%) |
| Heart | 5/92 (5%) |
| BVAS, mean (SD) | 14 (7) |
| Relapsing disease | 31/92 (34%) |
ANCA, antineutrophil cytoplasmic autoantibody; BMI, body-mass index; BVAS, Birmingham vasculitis activity score; eGFR, estimated glomerular filtration rate; ENT, ear, nose and throat; ESKD, end-stage kidney disease; IQR, interquartile range; MPO, myeloperoxidase; PR3, proteinase 3.
eGFR and proteinuria calculations are among patients with kidney involvement.
One patient with ESKD prior to study enrollment was excluded.
Treatment Regimens and GC Use
All patients received avacopan. The median (IQR) time to start avacopan was 3.6 (2.1–7.7) weeks after start of induction of remission therapy. The median (IQR) time to terminally taper prednisone after starting avacopan was 5.6 (3.3–9.5) weeks. Treatment regimens are presented in Table 2. Induction regimens involved i.v. pulse of methylprednisolone in 64% (n = 59), and PLEX in 14% (n = 13). The induction regimen was rituximab in 48% (n = 44), combination of rituximab and low-dose cyclophosphamide in 47% (n = 43), and other therapies in 4% (n = 4), including standard-dosing cyclophosphamide (n = 2), methotrexate (n = 1), and high dose prednisone monotherapy (n = 1). After induction of remission, 71% of patients (n = 65), started maintenance of remission therapy. Rituximab was used in 98% (n = 64), and azathioprine was used in 5% (n = 3), with 2 of the 65 patients having received both rituximab and azathioprine for remission maintenance. Mean (SD) cumulative oral prednisone dose at weeks 12 and 52 were 1797 (1104) mg, and 2212 (1550) mg, respectively.
Table 2.
Induction of remission therapy and glucocorticoid use
| Induction regimen | |
| Combination therapy (RTX + low-dose CYC)a | 43 (47%) |
| RTX | 44 (48%) |
| CYC (standard dosing) | 2 (2%) |
| Other | 2 (2%) |
| Plasma exchange | 13 (14%) |
| Methylprednisolone | |
| Received i.v. pulse | 59 (64%) |
| Cumulative dose, mg | 1340 (1319) |
| Prednisone, mg | |
| Dose at wk 26 | 1.8 (3.7) |
| Dose at wk 52 | 0.6 (2.5) |
| Cumulative dose at wk 12 | 1797 (1104) |
| Cumulative dose at wk 52 | 2212 (1550) |
| Off prednisone (as of last follow-up) | 64 (72%) |
| Time to start avacopan (from start of induction), wk | 3.6 (2.1–7.7) |
| Time to stop prednisone (from start of avacopan), wk | 5.6 (3.3–9.5) |
CYC, cyclophosphamide; RTX, rituximab.
Values are presented as either number (%), median (IQR), or mean ± (SD).
Typically, 8 weeks of daily oral CYC treatment, beginning with the first dose of RTX. Dosing was typically 2.5 mg/kg/d for the first week and 1.5 mg/kg/d for 7 weeks, with adjustments made for kidney function.
Outcomes
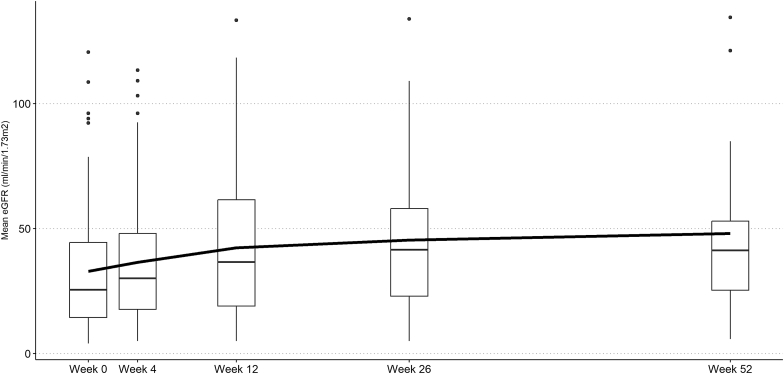
Clinical remission at week 26 and week 52 were achieved in 61 of 68 patients (90%) and 32 of 38 patients (84%), respectively. In those with kidney involvement and follow-up at week 26 or week 52, the mean (SD) eGFR change from baseline to week 26 and week 52 was +12.2 (25.4) and +19.8 (23.1) ml/min per 1.73 m2, respectively (Table 3 and Figure 1). At initiation, 86% (n = 61) had hematuria, and the median (IQR) duration until resolution was 15 (9–20) weeks. The median (IQR) nadir proteinuria was 331 (117–852) mg/g. The median (IQR) time to nadir proteinuria was 15 (7–29) weeks. At most recent follow-up, 5 of 9 patients (56%) were liberated from kidney replacement therapy, and 2 additional patients initiated dialysis. A clinical relapse occurred in 3%, infections requiring hospitalizations occurred in 13%, and death occurred in 4% of the patients (Table 3). Death was caused by ovarian cancer (n = 1) and severe coronavirus disease 19 (n = 3). In univariable logistic regression analysis, age, early avacopan initiation, PLEX, pulse methylprednisolone, rituximab monotherapy, and combination therapy were not associated with a higher odds of clinical remission at 26 weeks (Table 4).
Table 3.
Primary and secondary outcomes
| Primary outcome | |
| Clinical remission at wk 26 | 61/68 (90%) |
| Clinical remission at wk 52 | 32/38 (84%) |
| Secondary outcomes | |
| Change in eGFR (baseline to wk 26 (n = 48) | +12.2 (25.4) |
| Change in eGFR (baseline to wk 52) (n = 22) | +19.8 (23.1) |
| Duration of hematuria, wk | 14.4 (9.1–20.6) |
| Resolution of hematuria | 42 (68%) |
| Proteinuria at wk 26, mg/g Cr | 454 (154–1163) |
| Proteinuria at wk 52, mg/g Cr | 290 (143–742) |
| Proteinuria, nadir, mg/g Cr | 397 (150–896) |
| Time to nadir proteinuria, wk | 15.4 (8.6–29.2) |
| Clinical relapse | 3 (3%) |
| Infections requiring hospitalization | 12 (13%) |
| Dialysis dependencea | 6 (9%) |
| Death | 4 (4%) |
Cr, creatinine; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease.
eGFR values presented as ml/min per 1.73 m2.
Nine patients were newly on dialysis at study initiation. At last follow-up, 5 patients had kidney recovery off dialysis and 2 patients newly initiated dialysis. One patient with ESKD at study initiation was excluded from this count. Values are presented as either number (%), median (IQR), or mean ± SD.
Figure 1.
eGFR during follow-up among patients with kidney involvement (n = 71 at week 0 which decreased to n = 22 by week 52). We plot the boxplot at each time point and the mean eGFR (dark black line). Black dots represent data points >1.5× the interquartile range of each respective follow-up. Individuals on dialysis were given an eGFR of 5 ml/min per 1.73m2. eGFR, estimated glomerular filtration rate.
Table 4.
Predictors of clinical remission at 26 weeks
| Predictors | OR (95% CI) |
|---|---|
| Age (per/yr) | 1.04 (0.99–1.11) |
| Early avacopan initiation | 1.79 (0.37–9.8) |
| PLEX | 0.20 (0.04–1.17) |
| Pulse methylprednisolone (per 100 mg) | 1.01 (0.95–1.07) |
| RTX therapy (no CYC) | 0.64 (0.11–3.11) |
| Combination therapy (RTX + low-dose CYC) | 2.42 (0.48–17.8) |
| CYC therapy (no RTX) | N/A |
CI, confidence interval; CYC, cyclophosphamide; N/A, not applicable; OR, odds ratio; PLEX, plasma exchange; RTX, rituximab.
Odds ratios were calculated using univariate logistic regression. Early avacopan initiation is defined as within 30 days of start of induction therapy. Unable to estimate OR for CYC therapy due to low numbers.
Comparison of Patients With Entry eGFR < 15 and ≥ 15 ml/min per 1.73 m2
Excluding the 1 patient on chronic hemodialysis prior to study entry who was treated for a disease flare for ophthalmologic, cardiac, and nerve involvement, 77% (n = 70) had entry eGFR ≥ 15 ml/min per 1.73 m2 and 23% (n = 21) had entry eGFR < 15 ml/min per 1.73 m2. Significant findings in the group with entry eGFR < 15 ml/min per 1.732 more frequent PLEX use in 9 patients (43%) (P < 0.01); delayed use of avacopan at a median (IQR) time of 47 (21–58) days compared to 21 (14–49) days (P = 0.02); and higher risk for ESKD, 6 patients (30%) compared to 0 (P < 0.01). At week 26, the mean (SD) increase in eGFR from baseline was 19.4 (22.7) ml/min per 1.73 m2 in the group with eGFR < 15 ml/min per 1.73 m2 compared to 5.0 (25.0) ml/min per 1.73 m2 in the group with eGFR ≥ 15 ml/min per 1.73 m2 (P = 0.06). At week 52, the mean (SD) increase in eGFR from baseline was 25.1 (15.6) ml/min per 1.73 m2 in the group with eGFR < 15 ml/min per 1.73 m2 compared to 9.4 (25.6) ml/min per 1.73 m2 in the group with eGFR ≥ 15 ml/min per 1.73 m2 (P = 0.48). There were no differences in other induction therapies, cumulative prednisone dose at week 12 and 52, resolution of hematuria, nadir proteinuria, time to nadir proteinuria, clinical remission at week 26 and week 52, disease relapse, infections requiring hospitalizations, and death.
Comparison of Patients Starting Avacopan Within 30 Days (vs. ≥ 30 Days) of Induction
Avacopan was started within 30 days of induction treatment in 60% (n = 55), and it was started later in the remainder (40%, n = 37) of patients. Patients who received avacopan within 30 days of induction therapy were more likely to have higher BVAS at initiation (8.3 vs. 4.4) (P < 0.01) and less likely to require PLEX at study entry (7% vs. 24%) (P = 0.05). There was also a trend toward more rituximab (56% vs. 35%) and less pulse dose steroids (56% vs. 76%) in those who received avacopan within 30 days compared to those who received it ≥30 days, respectively. There was no significant difference in mean (SD) eGFR at baseline in those who received avacopan within 30 days compared to those who received it ≥30 days (42.5 [39.6] ml/min per 1.73 vs. 44.2 [30.5]) ml/min per 1.73 (P = 0.82), respectively.
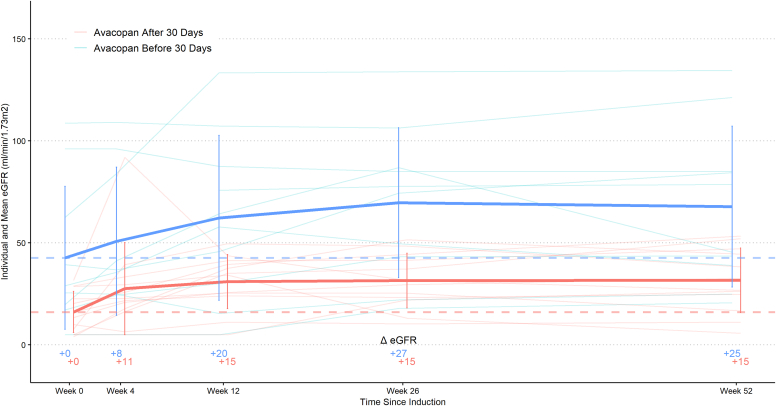
Patients who initiated avacopan ≥ 30 days from induction treatment, compared to <30 days, had a higher cumulative mean (SD) prednisone dose at week 12 (2158 (996) mg versus 1501 (1141) mg (P = 0.03), respectively). They also had a higher cumulative mean (SD) prednisone dose at week 52 (2723 (1398) mg versus 1787 (1610) mg (P = 0.03), respectively). In patients with kidney involvement (n = 71), those who started avacopan ≥ 30 days after start of induction treatment had longer median (IQR) time to achieve nadir proteinuria: 173 (100–260) days versus 84 (43–142) days (P = 0.01). There was a numerically higher but not statistically significant increase in mean (SD) eGFR from baseline at weeks 26 and 52 in patients who started avacopan within 30 days of induction therapy compared to patients who started avacopan later (Figure 2). There were no differences in clinical remission at weeks 26 and 52, disease relapse, infections requiring hospitalizations, ESKD, and death.
Figure 2.
eGFR stratified by timing of avacopan initiation among those with kidney involvement and 52 weeks of follow-up (n = 22). We plot the difference in mean eGFR between those who started avacopan within and after 30 days of induction date. eGFR, estimated glomerular filtration rate.
Comparison of Patients Receiving Avacopan With and Without PLEX
PLEX was administered to 14% (n = 13) of patients. There was a higher proportion of patients requiring dialysis at entry in the PLEX group, 39%, compared to 6% in the no PLEX group (P < 0.01). In addition, the mean (SD) cumulative methylprednisolone dose was higher in the PLEX group compared to the no PLEX group: 2.1 (1.1) g versus 1.2 (1.3) g, respectively (P = 0.02). There were no differences in the cumulative prednisone dose at weeks 12 and 52, clinical remission at weeks 26 and 52, disease relapse, infections requiring hospitalizations, ESKD, and death. In those with kidney involvement (n = 71), there were no differences in the mean (SD) eGFR increase at weeks 26 and 52, proteinuria at weeks 26 and 52, and resolution of hematuria.
Comparison of Patients by Prednisone Discontinuation Status at Most Recent Follow-Up
At the time of induction therapy, 97% (n = 89) of patients were started on prednisone and 3% (n = 3) were not started on prednisone. In those who were started on prednisone (n = 89), 28% (n = 25) continued prednisone and 72% (n = 64) stopped prednisone at the most recent follow-up of mean (SD) 231 (177) days. The mean (SD) time to discontinuation of prednisone was 97 (81) days from induction therapy and 62 (71) days from avacopan initiation. There were no differences between the ANCA serotype, clinical phenotype, relapsing disease, or organ involved between the 2 groups. There were no differences in mean (SD) eGFR increase at weeks 26 and 52, clinical remission at weeks 26 and 52, disease relapse, infections requiring hospitalizations, ESKD, and death.
Prednisone Outcomes Among Oral Steroid Users (≥30 days) Stratified by Avacopan Initiation Time
89% (n = 82) were on steroids 30 days after induction. By Kaplan-Meier analysis, those on avacopan before day 30 were more likely to discontinue steroids (compared to on or after day 30). The probability of being off steroids at week 26 was 52% (vs. 33%), and at week 52 was 20% (vs. 19%) in both groups, respectively (Figure 3). Of the 10 patients not on steroids, 3 never started oral steroids with induction, and 7 discontinued steroids before 30 days (with 6 already on avacopan).
Figure 3.
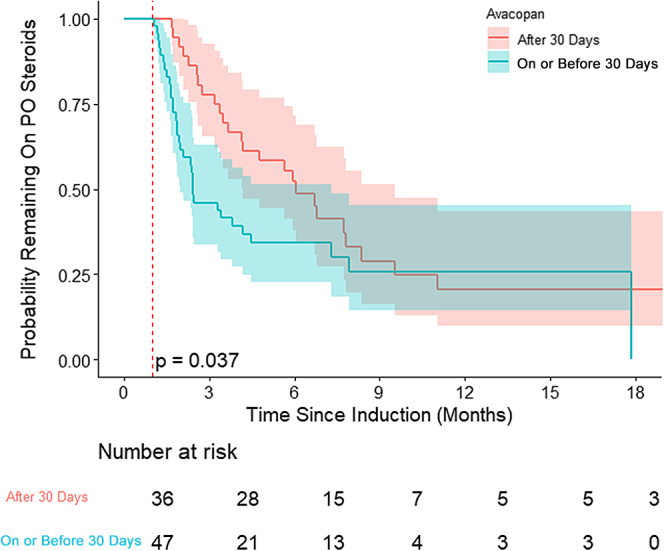
Kaplan-Meier curve of PO steroid discontinuation among those on PO steroids stratified by timing of avacopan initiation. PO, by mouth.
Safety
Avacopan was stopped in 33% (n = 30) of patients, with 13% (n = 12) after completion of 52 weeks of treatment, and 20% (n = 18) stopping prior to 52 weeks due to adverse events. Common reasons for discontinuation included transaminitis (n = 4, 2 patients less than 3× normal and 2 patients higher than 3× normal (in 200–500 range) and gastrointestinal-related side effects (n = 3). Other reported events that led to discontinuation of therapy included worsening kidney function, worsening proteinuria, need to increase prednisone for ongoing sinus disease, COVID-19, fingertip necrosis, chest pain, shortness of breath, neuropathy, new malignancy diagnosis, pruritus, and financial cost burden.
Discussion
The results of this large postmarketing retrospective multicenter study of avacopan use in AAV demonstrates several key findings. First, we observed high remission rates in real-world use. Second, we observed its use in populations that were excluded in the ADVOCATE trial, including advanced kidney disease, kidney replacement therapy, and patients who received PLEX. Third, we observed a wide variation of steroid use with avacopan, in contrast to the ADVOCATE trial. Fourth, we noted substantial delays in avacopan initiation. Fifth, we observed an overall acceptable safety profile.
We observed higher rates of clinical remission in our postmarketing study, 90% at week 26 and 84% at week 52 compared to the ADVOCATE trial’s findings of 72% and 66% at 26 and 52 weeks, respectively.3 The large discrepancy is likely due to methodologic differences. First, definitions of clinical remission varied. In the ADVOCATE trial, remission was defined as a BVAS of 0 and being off GC for 4 weeks before week 26, and sustained remission, which was defined as remission at week 26 and at week 52, as well as being off GC for 4 weeks before week 52. In contrast, our study defined remission as the absence of vasculitic activity as clinically determined by the individual investigator. Steroid cessation was not a defining criterion for remission. Given that BVAS is primarily a research tool, it is not always used in real-world practice. Second, our study was enriched with patients receiving combination of rituximab and low-dose cyclophosphamide as their base induction regimen (n = 43; 47%). Observational studies have shown higher rates of clinical remission with combination therapy due to the premise that simultaneously addressing different steps in the pathophysiology of AAV (rituximab targeting CD20 positive B cells and cyclophosphamide targeting CD20 negative plasmablasts and plasma cells, as well as innate immune responses) results in more rapid achievement of disease control and rapid-tapering of high-dose glucocorticoids.9, 10, 11 Randomized controlled trials are needed to better evaluate the efficacy and safety of combination therapy with avacopan. Third, the mean cumulative oral prednisone dose at week 52 in our cohort was 2212 mg, compared to 1676 mg in the ADVOCATE avacopan arm. The additional anti-inflammatory properties of GCs may have acted synergistically with avacopan, leading to higher overall efficacy. Fourth, maintenance therapy was started in the majority of patients in our cohort compared to ADVOCATE where rituximab-induced patients did not receive maintenance therapy and this practice may account for the higher remission rate in our cohort at week 52.
Another observation in our study was the successful use of avacopan in populations that were excluded from the ADVOCATE trial. Avacopan and its main metabolite (“M1” – a mono-hydroxylated form of avacopan that represents ∼12% of drug plasma levels and has similar efficacy to avacopan) are hepatically metabolized by CYP3A4. Its route of elimination is primarily fecal. After oral administration of avacopan, ∼77% (7% as unchanged avacopan) is recovered in feces whereas 10% (< 0.1% unchanged) is recovered in urine.12 Therefore, depressed kidney function should not significantly alter pharmacodynamics or pharmacokinetics. In our study, we did not observe any increase in infections requiring hospitalization among patients with eGFR < 15 ml/min per 1.73 m2 compared to those with eGFR ≥ 15 ml/min per 1.73 m2. On the contrary, we observed a numerically higher but not statistically significant increase in eGFR from baseline to week 26 and week 52 among patients with eGFR < 15 ml/min per 1.73 m2, compared to those with eGFR ≥15 ml/min per 1.73 m2.
Second, patients receiving kidney replacement therapy (n = 10, 11%) were studied in our cohort. Avacopan has a molecular weight of 581.6 g/mol, it is uncharged, poorly water soluble, and nearly all protein-bound. As such, dialysis is not expected to alter plasma concentrations significantly. Five out of 9 patients (56%) on kidney replacement therapy at the outset recovered their kidney function. A previous case report of 3 patients with AAV who were on dialysis recovered their kidney function and were on avacopan.13 We present promising data on the use of avacopan in this important subset of patients.
Third, a key unstudied population are those receiving PLEX. As mentioned, avacopan and its main (M1) metabolite are more than 99.9% bound to plasma proteins. Although this gives access for PLEX to remove drug from the circulation, this is countered by the drug’s large apparent volume of distribution (345 l), long elimination half-life of 4 days, daily dosing, and dosing after PLEX on treatment days.12 Both PLEX and avacopan are advantageous in their immediate ability to attenuate disease activity while acting with distinct mechanisms. When to use PLEX remains controversial, and the addition of avacopan as a treatment option may further add variations in treatment strategies. However, randomized studies are necessary for validation.
In addition, 2 key differences we observed in the real-world use of avacopan have been the wide variation in GC exposure and the delays in avacopan initiation. In ADVOCATE, there was less GC use in the avacopan group compared to the prednisone group (mean cumulative dose 1676 mg vs. 3847 mg, respectively), representing a 57% reduction in cumulative dose exposure. The GC use in the avacopan group was allowed at the discretion of the investigator and was not to exceed 20 mg at study entry and must be tapered off by week 4. In our cohort, 64% of patients received pulse methylprednisolone with a mean (SD) cumulative dose of 1.3 (1.3) g, followed by a mean (SD) cumulative oral prednisone dose of 2.2 (1.6) g by week 52. In contrast, the low dose prednisone arm of PEXIVAS used approximately 2.3 g of cumulative prednisone equivalence in a 70 kg individual.14 We speculate the increased GC in real-world practice is related to the delay in initiating avacopan. In our cohort, it took a median of 3 weeks to start avacopan after initiating induction therapy. This long duration undermines the time-sensitive utility of the drug, and practitioners were likely to rely on GC during this time. In fact, among patients with 1 year of follow up from induction, patients who started avacopan ≥30 days after induction therapy had a 15-point increase in eGFR from baseline to week 26 and a 15-point increase in eGFR from baseline to week 52, whereas those who started avacopan <30 days after induction therapy experienced a 27 and 25 point increase in eGFR from baseline to week 26 and 52, respectively, though differences were not statistically significant. Time to nadir proteinuria was significantly shorter in those who began avacopan sooner. Moreover, those starting avacopan ≥30 days after induction therapy received substantially more prednisone and were less likely to stop prednisone compared to those who initiated avacopan <30 days after induction therapy. The former group also had more infections requiring hospitalization, likely related to the higher GC use. Lastly, we found that it took a median of 40 days to discontinue GCs after avacopan initiation. Based on the authors anecdotal experience, the reasons for delay were nonavailability of avacopan on hospital inpatient formularies, logistical barriers in the drug company’s quick start program, and obstacles in the insurance company’s drug approval process, particularly at the outset after US Food and Drug Administration approval. As the use of avacopan increases, logistical barriers are expected to improve.
Lastly, we observed an acceptable safety profile with avacopan use. The most frequent cause for drug discontinuation was abnormalities in serum aminotransferases (4.3%), which is consistent with findings from the ADVOCATE trial (5.4%).3
The study has several strengths and weaknesses. The greatest strengths are the large size of the cohort receiving avacopan, the inclusion of patients across a broad spectrum of AAV severity, and the treatment strategies encountered in clinical practice. The main weaknesses are inherent to data collection in retrospective studies, as well as its uncontrolled nature, thereby making the outcome analysis susceptible to confounding by indication, and variable duration of follow up. In addition, the efficacy of avacopan in the more granulomatous manifestations of AAV (e.g., subglottic stenosis, pseudotumor, among others) remains unknown, as this subset of patients were not well-represented in our cohort and ADVOCATE.
In summary, the relatively new approval of avacopan warranted postmarketing analysis. We found high rates of remission, no concerning safety signals, and successful use in previously unstudied patient populations, including those with low GFR and patients receiving dialysis. Moreover, we observed a wide variety of steroid use, along with substantial delays in avacopan initiation.
Disclosure
RZ has served as a consultant for and received speaker fees from Amgen. FBC has served as a consultant for Amgen, Valenza Bio, Travere Therapeutics, and Aurinia Pharmaceuticals. FBC has received speaking fees from Amgen, Aurinia Pharmaceuticals, and Calliditas Therapeutics. SESC has served as a consultant for Sanofi; and received grants from GSK, Astra-Zeneca, and Bristol-Myers Squibb. DB has received grants from Astra-Zeneca and honoraria from AbbVie, Alexion, Amgen, and Novartis. CD has received honoraria from ChemoCentryx and Amgen. AG has received grants from Amgen, Aurinia, Travere, Vera, Chinook, and Acerylin; and consulting fees from Aurinia, Chinook, Kaneka, Travere, and GSK. IA has received consulting fees from Aurinia, George Clinicals, Sanofi, and Travere. AB has received fees from Amgen. KDJ has received consulting fees from GSK, Secretrome, Calliditas, George Clinicals, and PMV Pharmaceuticals. VKD has received consulting fees from Amgen, Novartis, Merck, Forma therapeutics, and Bayer. JLN has received consulting fees from ChemoCentryx and Amgen. DG has received consulting fees from Amgen, ChemoCentryx, Calliditas, GSK, and Otsuka. All the other authors declared no competing interests.
References
- 1.Zonozi R., Niles J.L., Cortazar F.B. Renal involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Rheum Dis Clin North Am. Rheum Dis Clin North Am. 2018;44:525–543. doi: 10.1016/j.rdc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Kronbichler A., Bajema I.M., Bruchfeld A., Mastroianni Kirsztajn G., Stone J.H. Diagnosis and management of ANCA-associated vasculitis. Lancet. 2024;403:683–698. doi: 10.1016/S0140-6736(23)01736-1. [DOI] [PubMed] [Google Scholar]
- 3.Jayne D.R., Merkel P.A., Schall T.J., Bekker P., ADVOCATE study group Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384:599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar F.B., Niles J.L., Jayne D.R.W., et al. Renal recovery for patients with ANCA-associated vasculitis and low eGFR in the ADVOCATE trial of avacopan. Kidney Int Rep. 2023;8:860–870. doi: 10.1016/j.ekir.2023.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennette J.C. Overview of the 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603–606. doi: 10.1007/s10157-013-0869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhtyar C., Lee R., Brown D., et al. Modification and validation of the birmingham vasculitis activity score (version 3) Ann Rheum Dis. 2009;68:1827–1832. doi: 10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 7.de Groot K., Harper L., Jayne D.R., et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 8.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine-and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merino-Vico A., van Hamburg J.P., Tas S.W. B Lineage cells in ANCA-associated vasculitis. Int J Mol Sci. 2021;23:387. doi: 10.3390/ijms23010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortazar F.B., Muhsin S.A., Pendergraft W.F., 3rd, et al. Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney Int Rep. 2018;3:394–402. doi: 10.1016/j.ekir.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati K., Edwards H., Prendecki M., et al. Combination treatment with rituximab, low-dose cyclophosphamide and plasma exchange for severe antineutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2021;100:1316–1324. doi: 10.1016/j.kint.2021.08.025. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Biotechnology Information PubChem compound summary for CID 49841217, avacopan. https://pubchem.ncbi.nlm.nih.gov/compound/Avacopan
- 13.Cortazar F.B., Cerda J., Dhanani R., Roglieri J., Santoriello D. Avacopan in patients with rapidly progressive glomerulonephritis requiring dialysis. Kidney Int Rep. 2023;8:1687–1691. doi: 10.1016/j.ekir.2023.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh M., Merkel P.A., Peh C.A., et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382:622–631. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]