Abstract
Keloids are characterized by fibroblast hyperproliferation and excessive accumulation of extracellular matrix (ECM) and are a major global health care burden among cutaneous diseases. However, the function of long noncoding RNA (lncRNA)-mediated ECM remodeling during the pathogenesis of keloids is still unclear. Herein, we identified a long noncoding transcript, namely, lymphocyte-specific protein 1 pseudogene 5 (LSP1P5), that modulates ECM component deposition in keloids. First, high-throughput transcriptome analysis showed that LSP1P5 was selectively upregulated in keloids and correlated with more severe disease in a clinical keloid cohort. Therapeutically, the attenuation of LSP1P5 significantly decreased the expression of ECM markers (COL1, COL3, and FN1) both in vitro and in vivo. Intriguingly, an antifibrotic gene, CCAAT enhancer binding protein alpha (CEBPA), is a functional downstream candidate of LSP1P5. Mechanistically, LSP1P5 represses CEBPA expression by hijacking Suppressor of Zeste 12 to the promoter of CEBPA, thereby enhancing the polycomb repressive complex 2-mediated H3K27me3 and changing the chromosomal opening status of CEBPA. Taken together, these findings indicate that targeting LSP1P5 abrogates fibrosis in keloids through epigenetic regulation of CEBPA, revealing a novel antifibrotic therapeutic strategy that bridges our current understanding of lncRNA regulation, histone modification and ECM remodeling in keloids.
Keywords: LSP1P5, keloid, extracellular matrix component deposition, CEBPA, H3K27me3, PRC2, chromosomal accessibility, RNA therapy, skin fibrosis
Graphical abstract

Gu and colleagues identified the long noncoding RNA (lncRNA) LSP1P5, which is involved in the deposition of extracellular matrix (ECM) components and the severity of keloid disease. Suppression of LSP1P5 diminishes fibrosis by modulating CEBPA expression via epigenetic mechanisms, offering an innovative therapeutic approach for keloids.
Introduction
Keloid is a human-specific stigmatized form of scarring that is often encountered in the clinic during plastic and reconstructive surgery. Burns, trauma, surgery, or even unremarkable skin lesions such as folliculitis can cause keloids, which are commonly located on the anterior chest, scapula, lower abdomen, and ear and impose major burdens on patients, including physical discomfort, psychological distress, aesthetic concerns, and financial expenses.1 Due to the substantial heterogeneity of this disease, keloids are notoriously difficult to treat effectively. The recurrence rate after surgical resection alone has been reported to reach 45%–100%.2
At present, dermal fibroblasts are believed to be responsible for this pathological process and are activated into myofibroblasts, causing excessive deposition of extracellular matrix (ECM) and resulting in fibrotic repair and aberrant scarring.3 Classically, many studies have shown that the transforming growth factor β (TGF-β)/Smad signaling pathway is the canonical pathway involved in the development of keloids and functions by affecting fibroblast proliferation, apoptosis, ECM deposition, and cytokine production.4 However, this pathway is ubiquitously involved in multiple biological processes in different organs and systems, which makes it difficult to screen for a specific therapeutic target. Although several medications or cellular therapeutics are currently available, none of them achieve complete elimination of keloids.5
With the rise of epigenetic research for decades, the study of noncoding RNAs (ncRNAs), DNA methylation, and histone modulation has led to a new era of keloid research.6,7 A series of ncRNA microarrays has been constructed for keloid and normal skin tissue, and hundreds and thousands of dysregulated ncRNAs have been identified. Duan et al. identified a group of keloid-specific RNAs including mRNAs, long noncoding RNAs (lncRNAs) and microRNAs (miRNAs), and constructed a competing endogenous RNA (ceRNA) network.8 Similarly, Alghamdi et al. identified many differentially methylated cytosine-phosphoguanine (CpG) sites and differentially expressed genes in keloids via genome-wide scanning.9 Moreover, work on histone modification in keloids has focused mostly on aberrant histone acetylation and the use of drugs such as trichostatin-A (TSA), a histone deacetylase (HDAC) inhibitor that reduces fibrosis.10,11 To our knowledge, no other histone modifications including methylation, phosphorylation, ubiquitination, and SUMOylation, have been reported in keloids. Undoubtedly, these studies lay the foundation for epigenetic research on keloids, but without in-depth mechanistic studies, these complicated networks have not revealed the etiology of keloids, let alone therapeutic targets for this disease.
In this study, we screened a human-specific lncRNA transcript, namely, lymphocyte-specific protein 1 pseudogene 5 (LSP1P5), that functions to maintain fibrosis in keloids. First, we observed that nuclear LSP1P5 is upregulated in both keloid tissue and fibroblasts. Functional analysis revealed that LSP1P5 is necessary to maintain high levels of ECM components, including collagen type I (COL1), collagen type III (COL3), and fibronectin 1 (FN1), both in vitro and in vivo. Through RNA sequencing (RNA-seq), formaldehyde-assisted isolation of regulatory elements (FAIRE), chromatin immunoprecipitation (XChIP), and cleavage under targets and tagmentation (CUT&Tag), CCAAT enhancer binding protein alpha (CEBPA) was identified as a downstream candidate of LSP1P5 and shown to exhibit antifibrotic effects on the skin. After LSP1P5 knockdown, CEBPA was significantly upregulated. Mechanistically, LSP1P5 hijacks polycomb repressive complex 2 (PRC2) to the promoter of CEBPA by interacting with Suppressor of Zeste 12 (SUZ12), catalyzing the trimethylation of lysine 27 on histone 3 (H3K27me3) of CEBPA, and thereby influencing the chromosomal opening status which results in the silencing of CEBPA. Overall, this study revealed a human-specific epigenetic regulatory pathway involved in ECM component deposition, which bridges our current understanding of lncRNA regulation, histone modification and ECM remodeling in keloids.
Results
The nuclear lncRNA LSP1P5 is elevated in keloids
To identify functional long noncoding targets in keloids, we performed a genome-wide transcriptome analysis (https://www.ncbi.nlm.nih.gov/geo/, GEO: GSE212954) to identify lncRNAs that are aberrantly expressed in keloid tissues compared with adjacent normal tissues (n = 4; Figure 1A; clinical information is displayed in Table S1). Notably, we observed a distinct transcriptomic pattern in keloids, with 865 upregulated and 2,602 downregulated protein-coding mRNAs (Figure S1). Circos and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses further revealed that the expression of ECM-related genes and signaling pathways is elevated in keloid tissues (Figures 1B and 1C), which indicates that keloids are a profibrotic disease.12 Importantly, 1,459 long noncoding RNAs were highly expressed in keloid tissues. Due to the pivotal role of fibroblasts in the pathogenesis of keloids, we investigated whether these lncRNAs are aberrantly expressed in keloid-derived fibroblasts (KDFs). Among these upregulated long noncoding transcripts, lnc-LSP1P5, a known lncRNA (NCBI Entrez Gene ID: 645166), exhibited the most elevated expression status in keloids (Figure 1D, fold change = 4.12, p < 0.01). LSP1P5 encompasses three transcripts (GenBank: NR_027354.2, NR_027355.2 and NR_027356.2) that share a homologous exon (the second exon, marked in yellow in Figure S2A). Using rapid amplification of cDNA ends (RACE) detection, we investigated the 5′ and 3′ boundaries of this lncRNA based on this shared exon. RACE analysis revealed a transcript measuring 1,118 base pairs (bp) in length, comprising four exons, which exhibited complete sequence identity with the nominated transcript, GenBank: NR_027354.2 (Figures S2B and S2C). Moreover, the Coding Potential Calculator 2.0 (CPC2) suggested that LSP1P5 is a noncoding RNA (Figure S3B). To further investigate the coding potential of LSP1P5 lncRNA, by following previous protocols,13 a series of 5′ UTR-ORF-GFPmut constructs were generated (Figure S3C). As expected, substantial expression of the GFP fusion protein was observed in known peptide-coding lncRNA HOXB-AS3 and wild-type GFP-transfected fibroblasts.14 However, we did not detect the expression of the ORFs of LSP1P5 or HOTAIR (Figure S3D). These results showed that LSP1P5 serves as a noncoding transcript in our experiments. Additionally, according to the modified Vancouver Scar Scale (mVSS) (Table S2), LSP1P5 correlated with an advanced keloid stage, further revealing the functional role of LSP1P5 in keloids (Figure 1E). Furthermore, according to RNA fluorescence in situ hybridization (RNA FISH) (Figures 1F and 1G) and a cellular fractionation assay (Figure 1H), LSP1P5 was localized mainly to the nuclei of fibroblasts in keloids. LSP1P5 was also localized primarily to the nucleus in normal skin, but its expression level was lower than that in keloids. Taken together, these results suggest that the expression of the nuclear lncRNA LSP1P5 is elevated in keloids.
Figure 1.
The nucleus-localized lncRNA LSP1P5 is highly expressed in keloids
(A) Schematic diagram of the RNA sequencing workflow for paired keloid and normal skin tissues (n = 4). (B) Circos analysis of differentially expressed genes. (C) KEGG analysis showed activated signaling pathways in keloid tissues. (D) lncRNA expression in KDFs (n = 5) and NDFs (n = 5). The value of one NDF sample was set as 1. The relative values were normalized to the GAPDH expression level and are presented as the mean ± SD. ns, not significant, ∗p < 0.05, ∗∗p < 0.01. (E) A correlation analysis between LSP1P5 and VSS. GAPDH was used to normalize the qRT-PCR data and the 2-ΔΔCt method was used to calculate the relative expression levels. The LSP1P5 expression level of K9 was defined as 1, and the expression levels were normalized to the fold changes. (F and G) RNA FISH of LSP1P5 in fibroblasts (F, Scale bar, 25 μm) and keloid tissue (G, Scale bar, 100 μm). (H) qRT-PCR analysis of MALAT1, GAPDH, and LSP1P5 in the nucleus or cytoplasm of fibroblasts. KDF, keloid-derived fibroblast; NDF, normal skin-derived fibroblast; VSS, Vancouver Scar Scale.
Silencing LSP1P5 attenuates ECM component deposition in vitro and in vivo
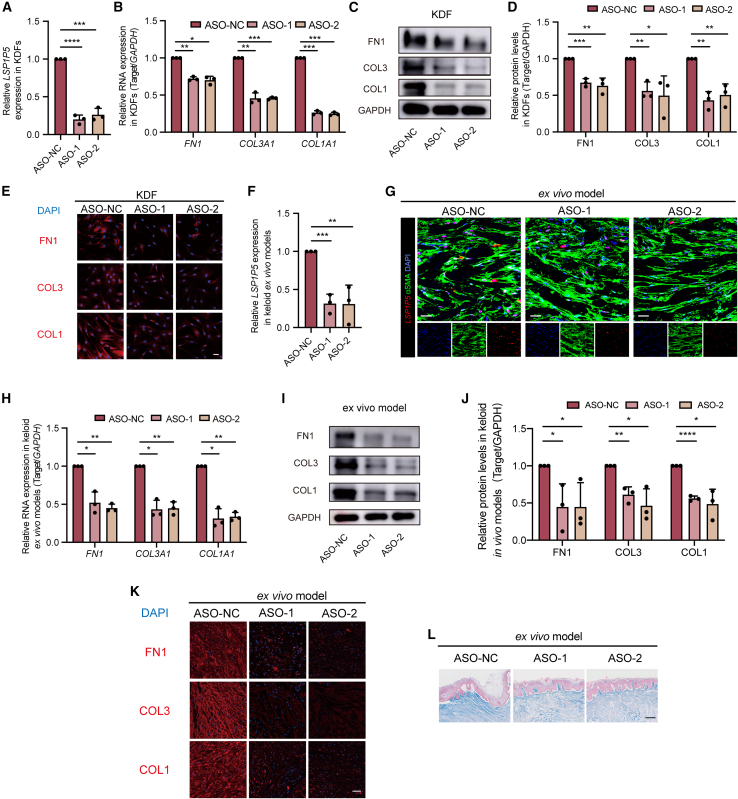
To explore the functional role of LSP1P5 in fibroblasts, we first silenced LSP1P5 in KDFs by transfecting two individual antisense oligonucleotides (ASOs). LSP1P5 expression was significantly decreased (approximately 0.2-fold) upon transfection of the two ASOs (Figure 2A). Moreover, decreased expression of FN1, COL1, and COL3 was observed in the LSP1P5-silenced KDFs according to qRT‒PCR (Figure 2B), western blot (WB) (Figures 2C and 2D), and immunofluorescence (Figure 2E) assays. Additionally, the knockdown of LSP1P5 resulted in negligible changes in cell proliferation and migration of KDFs (Figure S4), and the RNA and protein expression of ECM components in normal skin-derived fibroblasts (NDFs) (Figures S5A–S5D). These results suggest that LSP1P5 serves as a profibrotic factor in KDFs. Similarly, anti-LSP1P5 ASOs also exhibited efficient inhibitory efficacy in a keloid ex vivo model (Figures 2F and 2G), with significant reductions in FN1, COL1, and COL3 following LSP1P5 inhibition (Figures 2H–2K). In addition, Masson staining of keloid tissue from the ex vivo model demonstrated a significant decrease in collagen signal intensity following the delivery of anti-LSP1P5 ASOs (Figure 2L). In contrast, the expression of alpha-smooth muscle actin (αSMA), the MMP family and TIMP1 was unchanged upon deprivation of LSP1P5, suggesting that LSP1P5 functions as an on-target agent for ECM component deposition rather than for intracellular cytoskeleton remodeling and ECM degradation (Figure S6).
Figure 2.
Knockdown of LSP1P5 attenuates ECM component deposition in KDFs and ex vivo keloid models
(A) LSP1P5 expression after ASO knockdown in KDFs. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (B) The RNA expression of ECM-related genes, including COL1A1, COL3A1, and FN1, in KDFs transfected with different ASOs. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (C and D) Western blotting analysis of COL1, COL3, and FN1 protein levels in KDFs. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (E) IF of the COL1, COL3, and FN1 proteins in KDFs. Scale bar, 50 μm. (F) RNA expression of LSP1P5 in ex vivo keloid models. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗∗p < 0.01, ∗∗∗p < 0.001. (G) RNA FISH of LSP1P5 in ex vivo keloid models. Scale bar, 100 μm. (H) RNA expression of ECM-related genes, including COL1A1, COL3A1, and FN1, in different ASO-treated ex vivo keloid models. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01. (I and J) Western blotting assays of COL1, COL3, and FN1 protein levels in ex vivo keloid models. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. (K) IF of COL1, COL3, and FN1 proteins in ex vivo keloid models. Scale bar, 100 μm. (L) Masson’s trichrome staining in ex vivo keloid models. Scale bar, 200 μm.
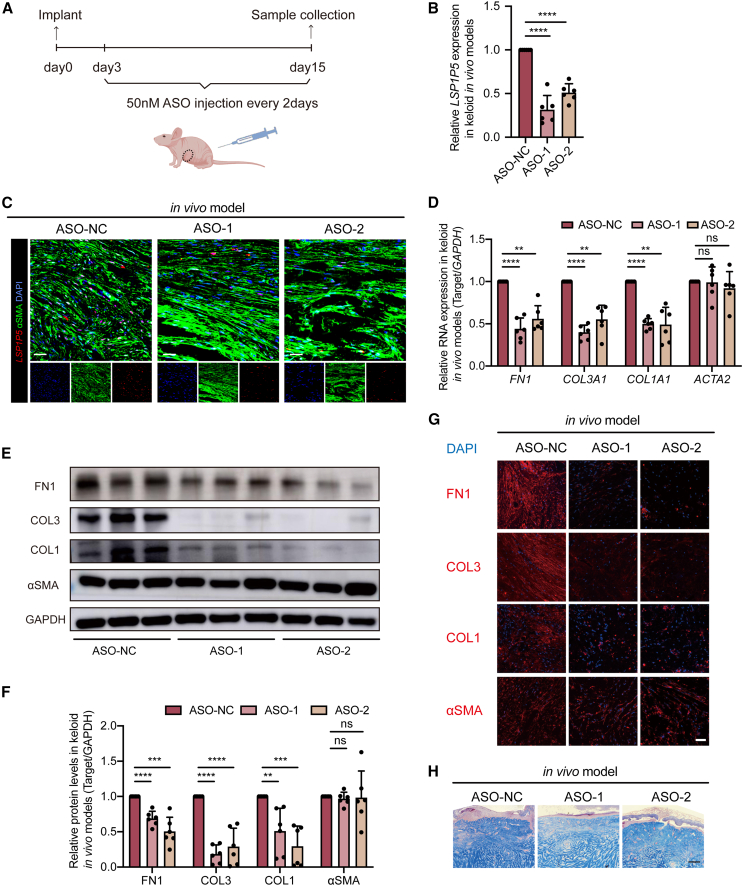
We then established an in vivo orthotopic keloid model by embedding patient-derived keloid samples into immune-deficient mice (Figure 3A). After regular injection of ASOs (50 nM in situ every 2 days) for 15 days, we observed attenuated expression of LSP1P5 (Figures 3B and 3C). Consistently, both qRT‒PCR (Figure 3D), immunoprecipitation (Figures 3E and 3F), and IF (Figure 3G) showed that FN1, COL1, and COL3 were significantly silenced in the LSP1P5-deficient groups, while αSMA expression did not change. Notably, Masson staining of keloid xenografts also revealed decreased collagen deposition after silencing LSP1P5 (Figure 3H). Taken together, these in vivo results agree with our observations in both cells and ex vivo models, demonstrating the profibrotic function of LSP1P5.
Figure 3.
Knockdown of LSP1P5 attenuates ECM component deposition in in vivo keloid models
(A) Study design of keloid in vivo models (n = 12 biologically independent nude mice). (B) RNA expression of LSP1P5 in in vivo keloid models. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗∗∗∗p < 0.0001. (C) RNA FISH of LSP1P5 in in vivo keloid models. Scale bar, 100 μm. (D) RNA expression of ECM-related genes, including COL1A1, COL3A1, FN1, and ACTA2 in different ASO-treated keloid models in vivo. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ns, not significant, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. (E and F) COL1, COL3, FN1, and αSMA protein levels in in vivo keloid models. The value of the control ASO-treated group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ns, not significant, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (G) IF of the COL1, COL3, FN1, and αSMA proteins in in vivo keloid models. Scale bar, 100 μm. (H) Masson’s trichrome staining in in vivo keloid models. Scale bar, 200 μm.
To exclude off-target effects of the ASOs, we performed gain-of-function experiments in KDFs and keloid ex vivo and in vivo models. The results showed that the decrease in ECM component deposition caused by knockdown of LSP1P5 could be rescued upon restoration of LSP1P5 both in vitro and in vivo (Figure S7). In addition, overexpression of LSP1P5 in NDFs did not affect ECM component deposition (Figures S5I and S5J).
The antifibrotic factor CEBPA is inhibited by endogenous LSP1P5
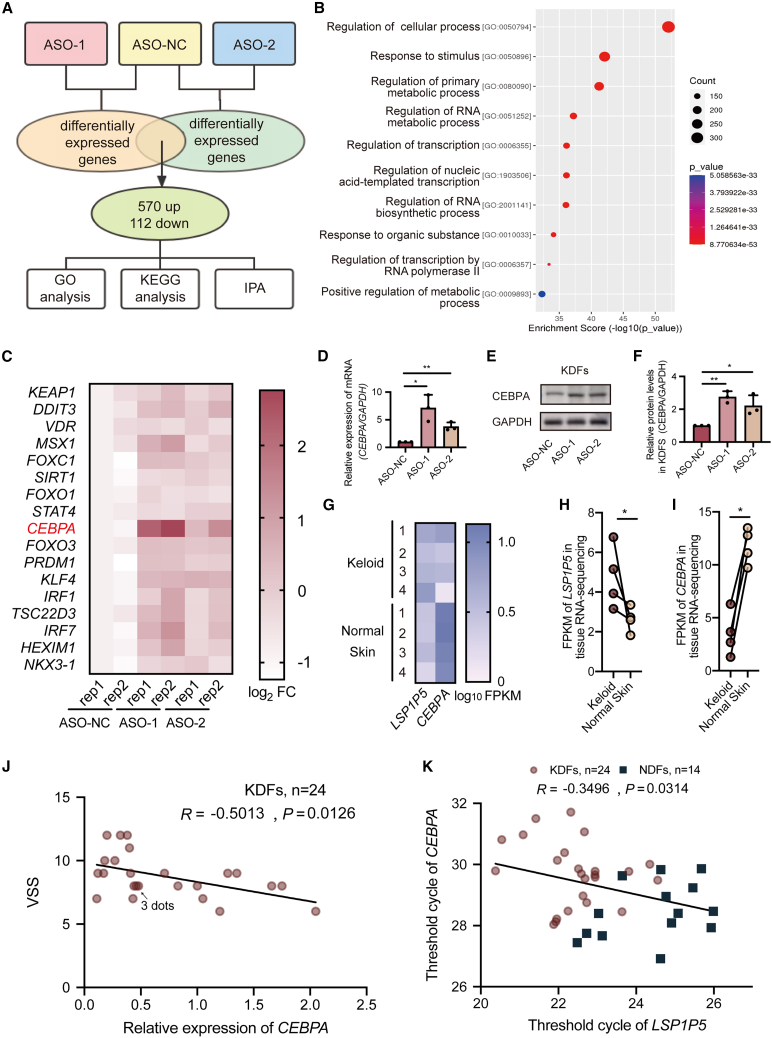
To identify the functional downstream candidates of LSP1P5, we performed a genome-wide transcriptome analysis after inhibiting LSP1P5 in KDFs (Sequence Read Archive, SRA: PRJNA972830). Notably, silencing LSP1P5 resulted in a marked change in the transcriptional profile, with 570 upregulated genes and 112 downregulated genes (Figure 4A). These dysregulated genes were enriched in metabolic reprogramming and transcription-related signaling pathways (Figure 4B). To further explore the central target of LSP1P5, we performed upstream regulatory analysis via Ingenuity Pathway Analysis (IPA) software, which identified 17 transcription factors as important regulators of the dysregulated transcriptional pattern (Figure 4C). Among these 17 transcription factors, CEBPA was the most upregulated gene upon treatment with both ASOs (Figures 4C–4F). Most importantly, CEBPA has been noted to be an important antifibrotic factor in pulmonary and hepatic fibrosis.15,16,17 The increased expression of CEBPA is consistent with our observation that LSP1P5 promotes fibrosis under keloid conditions. Consistent with these findings, we also noted a decrease in the expression of CEBPA in keloid samples (Figures 4G–4I), which was negatively correlated with the clinical severity of keloids, as shown by the mVSS (R = −0.5013, p < 0.05) (Figure 4J). Moreover, a significant negative correlation between LSP1P5 and CEBPA was observed (R = −0.3496, p < 0.05) in a clinical cohort that included 24 keloid and 14 normal control samples (Figure 4K). Taken together, these data indicate that CEBPA might serve as an important downstream target of LSP1P5, which is silenced by LSP1P5.
Figure 4.
The transcription factor CEBPA serves as a target of LSP1P5
(A) Study design involving RNA sequencing (SRA: PRJNA972830) and subsequent bioinformatics analysis. (B) GO analysis was performed and revealed the functions of the upregulated DEGs. (C) Heatmap of the expression of key transcription factors according to the RNA-seq data (SRA: PRJNA972830). FPKM, fragments per kilobase of exon model per million mapped fragments. The ASO-NC-rep1 group was used as the control group, and the results are presented as the log2 of the fold change (FC), which was calculated as log2(FPKMtest+1)-log2(FPKMcontrol+1). (D–F) The mRNA and protein levels of CEBPA upon LSP1P5 knockdown. The value of the control group (ASO-NC) was set as 1. The data are presented as the mean ± SD. ∗p < 0.05; ∗∗p < 0.01. (G–I) Heatmap (G) and bar graphs (H and I) of the expression of LSP1P5 and CEBPA in paired keloid and normal skin tissues according to RNA-seq data (GSE212954). The results are presented as log10 of the FPKM. ∗p < 0.05. (J) Correlation analysis between VSS and the relative expression of CEBPA in KDFs (n = 24, R = −0.5013, p < 0.05). The relative values were normalized to the GAPDH expression level, and the CEBPA expression level of K9 (the sample with LSP1P5 expression close to the average level in the keloid group) was defined as 1. (K) Correlation analysis between VSS and the expression of CEBPA and LSP1P5 in fibroblasts (KDFs, n = 24; NDFs, n = 14, R = −0.3496, p < 0.05).
CEBPA negatively regulates ECM component deposition in keloid fibroblasts
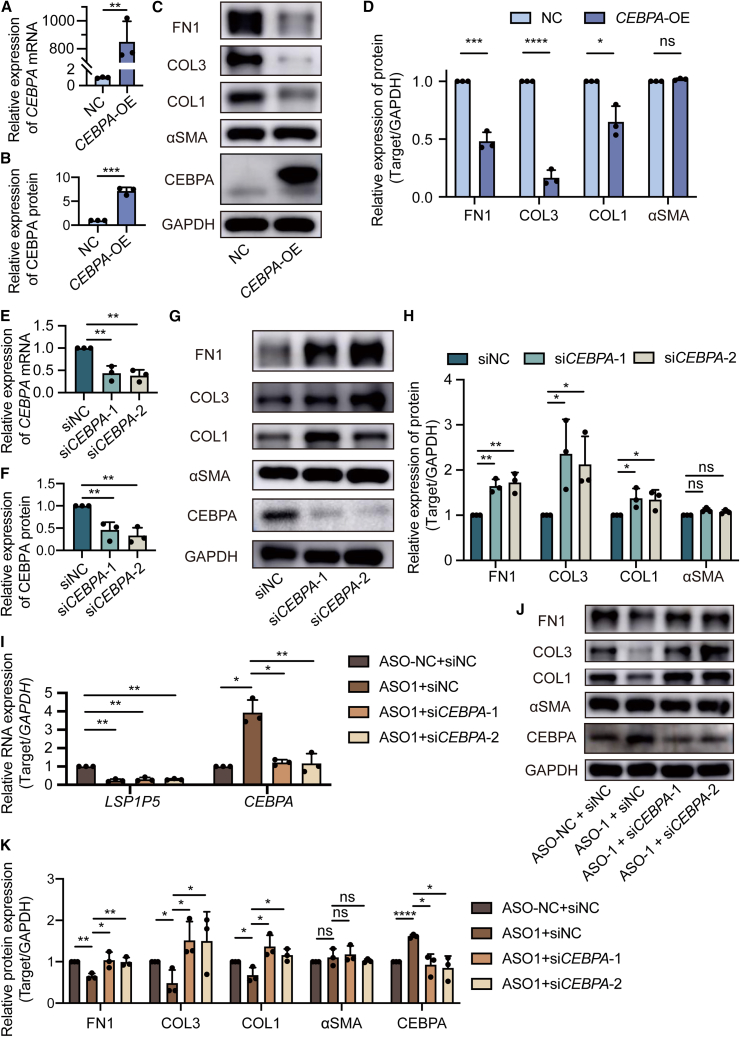
Although CEBPA has been recognized as an important antifibrotic factor, its role in keloids remains unclear. We then explored the function of CEBPA in keloid pathogenesis via gain- and loss-of-function assays. Notably, the exogenous overexpression of CEBPA resulted in a reduction in FN1, COL1, and COL3 in KDFs (Figures 5A–5D). We then attempted to silence CEBPA by transfecting two individual short interfering RNAs (siRNAs). Importantly, CEBPA silencing significantly upregulated FN1, COL1, and COL3 expression (Figures 5E–5H). These data confirmed that CEBPA is an antifibrotic factor in keloids.
Figure 5.
LSP1P5 modulates keloid ECM component deposition through its downstream target, CEBPA
(A) CEBPA mRNA levels in KDFs. The value of the control group (NC) was set as 1. The data are presented as the mean ± SD. ∗∗p < 0.01. (B–D) The protein levels of FN1, COL3, COL1, αSMA, and CEBPA in KDFs. The value of the control group (NC) was set as 1. The data are presented as the mean ± SD. ns, not significant, ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (E) CEBPA mRNA levels in KDFs. The value of the control group (siNC) was set as 1. The data are presented as the mean ± SD. ∗∗p < 0.01. (F–H) The protein levels of FN1, COL3, COL1, αSMA, and CEBPA in KDFs. The value of the control group (siNC) was set as 1. The data are presented as the mean ± SD. ns, not significant, ∗p < 0.05; ∗∗p < 0.01. (I) The mRNA levels of LSP1P5 and CEBPA. The value of the control group (ASO-NC + siNC) was set as 1. The data are presented as the mean ± SD. ∗p < 0.05; ∗∗p < 0.01. (J and K) The protein levels of FN1, COL3, COL1, αSMA, and CEBPA. The value of the control group (ASO-NC + siNC) was set as 1. The data are presented as the mean ± SD. ns, not significant, ∗p < 0.05; ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
To confirm that CEBPA serves as an important functional candidate of LSP1P5, we restored the expression of CEBPA by these two individual siRNAs (Figure 5I). As a result, the abrogation of CEBPA disrupted the anti-ECM component deposition efficacy of LSP1P5 silencing, with rescued expression of FN1, COL1, and COL3 (Figures 5J and 5K). Consistent with previous observations, the αSMA level remained unaltered in both the CEBPA gain- and loss-of-function assays. Taken together, these data confirmed that CEBPA serves as an important functional candidate for LSP1P5.
LSP1P5 hijacks PRC2 to epigenetically silence CEBPA
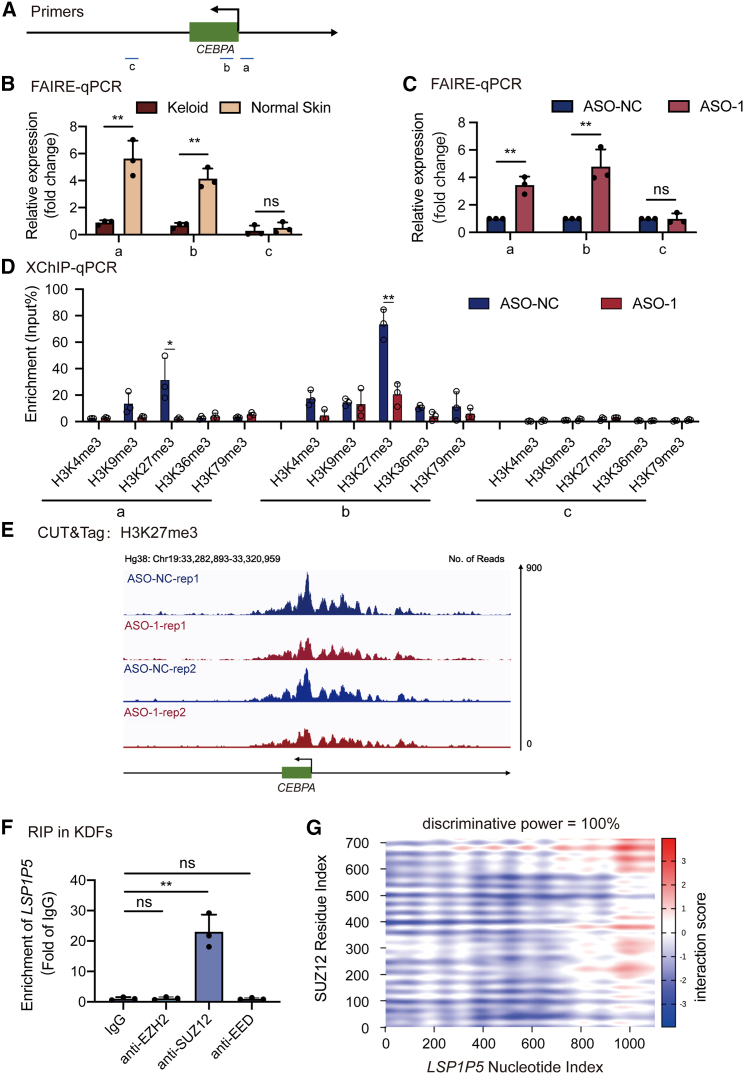
Nuclear lncRNAs regulate gene expression by regulating the chromosomal accessibility of downstream targets. To further elucidate the molecular basis underlying the transcriptional regulation of CEBPA by LSP1P5, we first explored the chromosomal status of CEBPA. Notably, CEBPA was more closely related in keloid samples according to the FAIRE assay, which was consistent with the repressed expression of CEBPA in keloids (Figure 6B). Moreover, silencing LSP1P5 significantly promoted the chromosomal opening of CEBPA in keloid fibroblasts (Figure 6C). Taken together, these data indicated that LSP1P5 regulates CEBPA through chromosomal remodeling.
Figure 6.
LSP1P5 recruits PRC2 to promote H3K27me3 in the CEBPA gene
(A) Schematic graph of primer locations. (B and C) Quantification of FAIRE at the CEBPA gene in fibroblasts derived from keloid and normal skin. The data are presented as the mean ± SD. ns, not significant, ∗∗p < 0.01. (D) X-ChIP-qPCR of multiple histone methylation. The data are presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01. (E) The reads at the CEBPA gene of CUT&Tag using the H3K27me3 antibody. (F) RIP of the PRC2 core in KDFs. The data are presented as the mean ± SD. ns, not significant, ∗∗p < 0.01. (G) An RNA-protein interaction prediction assay conducted with catRAPID showed that LSP1P5 interacted with SUZ12.
Since diverse histone modifications are responsible for switching the chromosomal state,18 we subsequently explored the histone modification status (including H3K4me3, H3K9me3, H3K27me3, H3K36me3, and H3K79me3) following the decrease in LSP1P5. Notably, H3K27me3 was decreased in the promoter region of CEBPA, while other histone modification patterns were unchanged (Figure 6D). Most importantly, the CUT&Tag assay further demonstrated decreased H3K27me3 signal intensity in the CEBPA promoter in LSP1P5-deficient KDFs (Figure 6E). Furthermore, since H3K27me3 is decorated by polycomb repressive complex 2 (PRC2), we were interested in testing the physical interaction between LSP1P5 and the following PRC cores: Enhancer of Zeste 2 (EZH2), SUZ12, and embryonic ectoderm development (EED). Notably, both the RNA immunoprecipitation (RIP) (Figure 6F) assay and the RNA-protein interaction prediction assay (Table S3; Figure 6G) revealed that LSP1P5 strongly interacts with SUZ12, a PRC2 scaffold organizer, in KDFs. Based on catRAPID, a public algorithm used to accurately estimate the binding propensity of proteins and RNA, SUZ12 was found to rank second among the LSP1P5-binding proteins (Table S3). A further RNA-protein interaction prediction assay revealed an almost definite bridge between LSP1P5 and SUZ12 (Figure 6G). To verify this, we synthesized biotin-labeled sense/antisense LSP1P5 RNAs and performed a pulldown assay followed by mass spectrometry. Notably, we captured a robust signal of SUZ12 in LSP1P5 (peptide number = Q15022, rank 14th, unique peptide = 3). However, the SUZ12 signal was not detected in the LSP1P5-antisense group. The IP-MS results have been uploaded to the Mendeley Data:https://doi.org/10.17632/kknj4nj96z.1.
To further confirm the ability of SUZ12 to bind to LSP1P5 to promote H3K27me3 modifications and repress CEBPA expression, we silenced SUZ12 in both wild-type and LSP1P5-deficient KDFs. As a result, the abrogation of SUZ12 led to markedly increased CEBPA expression (Figures 7A–7C, lanes 1 and 3) but only resulted in a minimal increase in LSP1P5-silenced fibroblasts (Figures 7A–7C, lanes 2 and 4). Additionally, we performed an XChIP‒qPCR assay to assess the levels of H3K27me3 modifications in each experimental group. The reduction in H3K27me3 modification at the CEBPA promoter region caused by knockdown of LSP1P5 was abolished upon knockdown of SUZ12, indicating the indispensability of SUZ12 in this process (Figure 7D). To further validate that CEBPA is regulated by LSP1P5, CEBPA promoter sequences (−1 to −2,000 bp) were inserted into the pGL3 vector, which was subjected to luciferase reporter assays in either LSP1P5-overexpressing or LSP1P5-silenced KDFs. Similarly, the exogenous overexpression of LSP1P5 led to decreased luciferase intensity, while a remarkable upregulation of the CEBPA was observed upon the inhibition of LSP1P5 (Figure S8).
Figure 7.
The ability of SUZ12 to bind to LSP1P5 to promote H3K27me3 modifications
(A) The RNA expression of LSP1P5, SUZ12, and CEBPA in KDFs transfected with different ASOs or siRNAs. The values for the control ASO- and siRNA-treated groups (ASO-NC + siNC) were set as 1. The data are presented as the mean ± SD. ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (B and C) WB analysis of SUZ12 and CEBPA in KDFs transfected with different ASOs or siRNAs. The values for the control ASO- and siRNA-treated groups (ASO-NC + siNC) were set as 1. The data are presented as the mean ± SD. ns, not significant, ∗p < 0.05, ∗∗p < 0.01. (D) Enrichment of the CEBPA promoter region detected by X-ChIP-qPCR with H3K27me3 antibody. The data are presented as the mean ± SD. ns, not significant, ∗∗p < 0.01.
Taken together, these results suggest that LSP1P5 modulates CEBPA expression by interacting with SUZ12 and promotes H3K27me3 modifications in the CEBPA promoter.
Discussion
Since Nature and Science noted the vital importance of exploring noncoding sequences in the genome, research on ncRNAs has become popular in the past decade.19,20,21 Numerous studies have shown the functional roles of lncRNAs in various diseases, including tissue fibrosis. lncRNAs play key regulatory roles in the fibrosis of various organs, including the liver, heart, lung, kidney, and skin, affecting all phases of fibrosis, namely, inflammation, proliferation, and remodeling.22,23 For instance, the lncRNA HOTAIR promotes skin fibrosis by enhancing EZH2-mediated H3K27me3, thereby activating the profibrotic activity of dermal fibroblasts. In addition, skin fibrosis in patients with systemic sclerosis usually originates from the hands and feet, where HOTAIR is highly expressed.24 Keloids are a typical type of pathological scar and fibrotic skin disorder; however, to date, the epigenetic research in this field has been conducted at the macroscopic genome-wide scanning and ceRNA network mapping stages but has not elucidated the underlying mechanisms involved. In this study, we report a long noncoding nuclear transcript, LSP1P5, that acts as a necessary fibrosis maintenance factor to modulate ECM component deposition. By hijacking PRC2 to promote H3K27me3 modification, LSP1P5 hinders the opening of chromatin at the antifibrotic gene CEBPA, which further impedes transcription and ultimately causes CEBPA silencing in keloids (Figure 8).
Figure 8.
Schematic of LSP1P5-guided CEBPA silencing in keloids
LSP1P5 interacts with SUZ12 and recruits PRC2 to promote H3K27me3 modifications. This phenomenon hinders the opening of chromatin at the CEBPA gene, which further impedes transcription. As CEBPA is an antifibrotic factor, silencing CEBPA causes abnormal ECM deposition and promotes keloid formation.
Activation of the TGF-β signaling pathway plays a profibrotic role in fibrosis, including keloids.25,26 In multiple fibrotic diseases, TGF-β1 binds to its receptor complex and activates the Smad family complex, promoting the macrophage-to-myofibroblast transition (MMT) process.27 Importantly, Yingying Zhang et al. identified a novel Smad3-dependent lncRNA, LRNA9884, that was upregulated in the diabetic kidneys of db/db mice.28 The promoter region of LRNA9884 contains a Smad3-binding site, and deletion of Smad3 blocks the upregulation of LRNA9884 in the diabetic kidney. In addition, lnc-TSI binds to the MH2 domain of Smad3, blocking its interaction with TGF-β receptor I, which specifically inhibits TGF-β-induced Smad3 phosphorylation and downstream profibrotic gene expression.29,30 Moreover, the lnc-TSI acted as an upstream signaling molecule in regulating the TGF-β signaling pathway. Our results indicated that LSP1P5 was upregulated in TGF-β-stimulated fibroblasts, suggesting its possible involvement as a downstream effector of TGF-β (Figure S9). Since TGF-β/Smad signaling serves as an important trigger for diverse fibrotic diseases, the underlying mechanism of the involvement of LSP1P5/CEBPA signaling requires further investigation.
CEBPA is a classical transcription factor that recognizes and binds to the CCAAT sequence in the promoter of target genes and exerts transcriptional regulatory effects, thereby affecting the expression and activity of target genes.31 Numerous studies have confirmed that CEBPA is a critical protective factor in the fibrotic processes of the lung, liver, and kidney. In patients with chronic obstructive pulmonary disease (COPD), the activity of CEBPA in airway epithelial cells is decreased, and conditional knockdown of CEBPA in mouse lung epithelial cells leads to bronchiolitis including fibrosis.15 In pulmonary fibroblasts derived from idiopathic pulmonary fibrosis (IPF) patients, CEBPA expression was significantly downregulated, and the overexpression of CEBPA in fibroblasts inhibited profibrotic gene expression and reduced the deposition of ECM.16 For hepatic fibrosis, MTL-CEBPA, a small activating RNA (saRNA) oligonucleotide therapy (CEBPA-51) formulated with liposomal nanoparticles that upregulate hepatic CEBPA expression, was confirmed to reverse matrix deposition in cirrhosis and fibrosis in preclinical studies.17 In addition to these findings, our study revealed the antifibrotic role of CEBPA in keloids. As the downstream candidate gene with the most significant upregulation upon LSP1P5 knockdown, CEBPA was assessed by gain- and loss-of-function assays, which revealed its antifibrotic ability to inhibit the protein expression of ECM components and reverse the process of fibrosis. In contrast to previous studies in which CEBPA served as an important repressor of pathological fibrosis, along with decreased αSMA signals, this reduction was not observed in our research. Since the fibrotic process plays a context-dependent role in diverse pathological conditions, this could be attributed to the discrepancy between different disease models and organs or cell types.
Additionally, there is currently a lack of rigorous experimental studies, such as RNA-seq, to screen and confirm the downstream mechanisms by which it exerts its effects. Therefore, only the downstream mechanisms and pathways can be inferred based on the current research. A previous IPF study illustrated that CEBPA overexpression antagonizes TGF-β signaling.16 CEBPA, which is a critical receptor that mediates TGF-β signaling, was also reported to negatively regulate TGFBR2 promoter activity and expression.32 Therefore, we speculated that CEBPA might mediate the transcriptional regulation of key genes involved in TGF-β signaling and further influence fibrosis in keloids.
Notably, the chromatin assembly of CEBPA was closer in KDFs than in WT cells, and the silencing of LSP1P5 promoted chromosomal opening. These results prompted us to focus on histone modifications closely related to chromatin assembly, and in the present study, H3K27me3 was found to be involved. PRC2 is a protein complex that contains histone methyltransferase activity that mediates H3K27me3 modification to silence gene transcription.33 Additionally, this process is usually triggered by lncRNAs. For example, XIST initiates X chromosome inactivation by coating the inactive X chromosome, enriching PRC2 and H3K27me3.34,35 The lncRNA MALAT1 displaces PRC2 from binding to the LTR promoter, which releases the epigenetic silencing of HIV-1 replication.36 The function of PRC2 relies mainly on its core subunits, which include EZH2, SUZ12, and EED. Among them, SUZ12 acts as a scaffold bridging a catalytic lobe with a targeting and regulatory lobe and functions in nucleic acid binding.37 Here, we suggest that LSP1P5 interacts with PRC2 in a SUZ12-dependent manner. This concept was supported by both the public database (catRAPID) and RIP results in our study. EZH2 is the catalytic center that directly mediates the trimethylation of lysine residues on histones. CEBPA was reported to be a methylation-sensitive gene, and EZH2 can bind to the promoter region of the CEBPA gene, catalyzing H3K27me3 and silencing the CEBPA gene.38,39 This finding is consistent with the results of the XChIP and CUT&Tag assays in our study. To our knowledge, we are the first to study the interaction between lncRNAs and H3K27me3 in keloids and to perform H3K27me3 CUT&Tag in KDFs, which will aid in the development of a genome-wide understanding of H3K27me3 in keloids.
Recently, RNA therapies targeting nucleic acids, including single-stranded ASOs, have attracted increased interest, and ASOs were tested in the present study.40 In a mouse model of myocardial infarction, the use of ASOs targeting the lncRNA Wisper in cardiac fibroblasts significantly inhibited cardiac fibrosis and improved cardiac function, highlighting the potential of the lncRNA Wisper as a therapeutic target for cardiac fibrosis.41 Eteplirsen, an ASO drug used to treat Duchenne muscular dystrophy (DMD), works by restoring the translational reading frame of DMD through specific skipping of exon 51 in defective gene variants.42 In addition, several ASO drugs are in phase III clinical trials.43 In our study, we designed two ASO sequences to effectively knock down LSP1P5, which also showed good potential for clinical use in keloid treatment.
Despite the significant findings of this study, it is important to acknowledge and address certain limitations that may have impacted the interpretability and generalizability of our results. First, in vitro cultured cells and models do not fully recapitulate the state of keloids in vivo. Further attempts to maintain keloid features in this field should be addressed by the use of keloid organoids in the subsequent studies. Second, the mVSS values in this study were scored by the same surgeon (Dr. Tao Zan), which might introduce subjective bias and diagnostic bias. Third, it is essential to acknowledge that the RNA-seq and CUT&Tag experiments were performed with only two replicates, which may not provide sufficient statistical power but for illustrative purposes.
In conclusion, this study revealed a human-specific epigenetic pathway underlying keloid ECM component deposition in which LSP1P5 is upregulated in keloids and hijacks PRC2 to silence CEBPA through the catalysis of H3K27me3, subsequently promoting ECM component deposition. This study extends the understanding of keloids from the novel perspective of crosstalk between lncRNA regulation and histone modification. Hopefully, ASO drugs targeting LSP1P5 combined with novel material delivery technologies will show potential for effectively treating keloids and other fibrotic skin disorders.
Materials and methods
Cells, clinical samples, and ethics statement
Keloid and normal skin samples were obtained from patients who underwent surgery at the Department of Plastic and Reconstructive Surgery at Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. All patients were well informed about the use of the specimens and signed written informed consent forms in accordance with the guidelines approved by the Shanghai Ninth People’s Hospital Ethics Committee (patient information is summarized in Table S1). The ethics permit number for the use of clinical samples collected during surgery was 2018-129-T107.
Cell culture and treatment
Isolation and culture of human keloid and normal skin fibroblasts were conducted as previously described.44 Specifically, specimens obtained during surgery were cut into 5 mm × 5 mm pieces and soaked in 0.3% dispase II (0.3 g/mL; Gibco, 17105041) at 4°C for 12 h. Then, the epidermis was removed, and the dermis was minced and incubated in collagenase NB4 (3 mg/mL; Nordmark, S1745401) at 37°C for 4 h to isolate dermal fibroblasts. Fibroblasts were cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin-streptomycin (Gibco, USA) and then incubated at 37°C in a humidified atmosphere with 5% CO2. After approximately 4–5 days, the isolated dermal fibroblasts began to adhere, and after another 48 h of culture, the density reached approximately 90%. At this time point, fibroblasts were passaged at a 1:3 ratio. Afterward, the fibroblasts were cultured under the conditions mentioned above, and passages were performed every 72 h for a maximum of six passages.
RNA isolation and quantitative reverse-transcription PCR (qRT‒PCR)
Total RNA was extracted using TRIzol reagent (Solarbio, China). Complementary DNA was synthesized from 2000 ng of RNA with PrimeScript RT Master Mix (TaKaRa, Japan). PCR was performed on an ABI QuantStudio 6 Flex system using SYBR Premix (TaKaRa, Japan) according to the manufacturer’s instructions. The sequences of the primers used are summarized in Table S4.
RNA FISH
RNA FISH was carried out using a Ribo Fluorescent In Situ Hybridization Kit (RiboBio, China) following the manufacturer’s instructions. The LSP1P5, U6, and 18S probes were designed and synthesized by RiboBio. Briefly, fibroblasts were seeded on glass coverslips 24 h before FISH was performed. After hybridization, fluorescence detection was performed with a confocal laser scanning microscope (Nikon). The probe for LSP1P5 FISH is provided in Table S5.
Cytoplasmic and nuclear RNA isolation
The nuclei and cytoplasm of the fibroblasts were separated by using a Nuclear Extraction Kit (Active Motif, USA) following the manufacturer’s instructions. RNA was extracted from these fractions using TRIzol (Solarbio, China). qRT-PCR was performed to analyze LSP1P5, MALAT1, and GAPDH expression.
RNA interference
LSP1P5 knockdown in fibroblasts and ex vivo/in vivo models was achieved by transfection with ASOs. Knockdown of CEBPA in fibroblasts was achieved by transfection with siRNAs. The sequences of the ASOs and siRNAs used are listed in Table S6. The primers were synthesized by Zorin Biotechnology (China). Transfection of ASOs and siRNAs was performed using Lipofectamine 3000 transfection reagent (Thermo Fisher, USA) according to the manufacturer’s instructions. Forty-eight to 72 h after transfection, RNA and protein were extracted from the transfected cells for further experiments.
RNA-seq and data analysis
Total RNA was extracted from keloid/normal skin tissue or fibroblasts using TRIzol reagent (Solarbio, China). Poly-T oligo-attached magnetic beads were used to enrich eukaryotic mRNA. After fragmentation, the mRNA was converted into individual cDNA libraries. After cluster generation, the library preparations were sequenced on an Illumina NovaSeqTM 6000 platform. Gene expression levels were quantified by fragments per kilobase of exon model per million mapped reads (FPKM). The DESeq2 algorithm was used to identify DEGs, and a false discovery rate (FDR) < 0.05 and | log2(fold change) | ≥1 were used as the thresholds. The RNA-seq data were deposited in the GEO database (GEO: GSE212954) and SRA database (SRA: PRJNA972830). Sequencing was conducted by Kangcheng Biotech, Inc. (Shanghai, China).
Gene Ontology (GO) analysis of the designated genes was performed using DAVID (http://david.abcc.ncifcrf.gov/). Fisher’s exact test was used to identify the significant GO categories, and the FDR was used to correct the p values. GO terms with p < 0.05 were considered significantly different. Enrichment maps were created using Cytoscape 3.7.0. The downstream candidate genes were screened using IPA via the Shanghai Jiao Tong University School of Medicine library.
WB and IF analyses
WB analysis and IF staining were performed according to the protocol described in our previous study.45 The antibodies used for WB and IF are listed in Table S7. The original WB images are available in Figure S10.
Histological analysis
Tissues were first fixed with 4% paraformaldehyde overnight and then embedded in paraffin. Sections (5 μm) were processed and stained with a Masson’s Trichrome Stain Kit following a standardized protocol (Solarbio, China).
Keloid ex vivo model
Ex vivo explant culture was conducted according to Professor Wei Liu’s published work with some adjustments.46 Briefly, human keloid samples were obtained from surgical excision, cut into 3 mm × 2 mm × 2 mm fragments and incubated in DMEM containing 10% FBS. The medium was subsequently replaced with DMEM containing 10% FBS and 50 nM ASO the next day. After 72 h of treatment, the tissue fragments were fixed overnight in 4% paraformaldehyde for further histological analysis or immediately frozen in liquid nitrogen for mRNA or protein extraction.
Keloid in vivo model
All the procedures for establishing the model were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Committee of Animal Care and Use for Research and Education of Shanghai Jiao Tong University School of Medicine. The ethics permit number for the animal keloid model study was SH9H-2021-A215-1. Briefly, keloid tissue was cut into 4 mm × 4 mm × 4 mm pieces and embedded subcutaneously in the axilla of immune-deficient mice. Then 50 nM ASO was injected every 2 days beginning on day 3. On day 15, the treated tissue fragments were fixed overnight in 4% paraformaldehyde for further histological analysis or immediately frozen in liquid nitrogen for mRNA or protein extraction.
Overexpression lentiviral packaging and transfection
LSP1P5 and CEBPA overexpression and negative control plasmids with marked GFP were synthesized by Zorin Biotechnology (China). Primers for constructing the overexpression vectors are shown in Table S8. Then, the lentiviral packaging kit (Zorin, China) was used to generate lentiviruses in 293T cells according to the manufacturer’s instructions. Fibroblasts were seeded in six-well dishes at a density of approximately 50%, and 24 h after the seeding, the cells were transfected with the overexpression lentivirus or negative control lentivirus supplemented with 10 μg/mL polybrene for 12 h. Forty-eight to 72 h after the transfection, the transfection efficiency was evaluated briefly by observing the green fluorescence of the cells via a fluorescence microscope. Moreover, RNA and protein were extracted from the transfected cells for further experiments.
FAIRE
Cell fixation and shearing were conducted using a ChIP-IT Express Shearing Kit (Active Motif, No. 53032, USA) according to the manufacturer’s instructions. Briefly, 37% formaldehyde was added to a final concentration of 1%, followed by the addition of 2.5 M glycine to quench the formaldehyde. The fixed cell samples were then scraped and transferred to a centrifuge tube and centrifuged at 2500 rpm for 10 min at 4°C. Subsequently, the fixed cells were resuspended in cold lysis buffer supplemented with protease inhibitor cocktail (PIC) and PMSF and incubated for 30 min on ice. After centrifugation at 5,000 rpm for 10 min at 4°C, the sediment was resuspended in shearing buffer containing PIC and PMSF and sonicated (25% power, 15 pulses of 20 s each, with a 30-s rest on ice between each pulse) to obtain an average DNA fragment size of approximately 200∼300 bp. After centrifugation at 15,000 rpm for 10 min at 4°C, the supernatant containing the DNA fragments was collected.
The supernatant was transferred to a fresh 1.5-mL tube and incubated with 1 μL of DNase-free RNase A for 30 min at 37°C. Subsequently, 300 μL of phenol/chloroform/isoamyl alcohol (Sangon, China) was added, and the sample was vortexed for 10 s and subsequently centrifuged at 12,000 × g for 5 min. The aqueous (top) layer was subsequently transferred, after which the extraction process was repeated three times, after which all the aqueous solutions were pooled. Subsequently, 1/10 volume of 3 M sodium acetate, 2 volumes of 95% ethanol, and 1 μL of 20 mg/mL glycogen were added, and the solution was incubated at −80°C for at least 30 min. The DNA was then purified using a DNA Clean-up Kit (Axygen, USA). The primers used for FAIRE-qPCR are listed in Table S4.
XChIP
XChIP assays were also conducted using a ChIP-IT Express Kit (Active Motif, No. 53008, USA). The cell fixation and shearing methods used were the same as those used for FAIRE. Subsequently, anti-H3K4me3, anti-H3K9me3, anti-H3K27me3, anti-H3K36me3, and anti-H3K79me3 antibodies were applied for immunoprecipitation, and rabbit IgG was used as a negative control. The specific information for the antibodies used is listed in Table S7. After end-to-end rotation for 4 h at 4°C, the magnetic beads were collected and washed with ChIP Buffer. The chromatin was eluted, and the crosslinking was reversed. After treatment with proteinase K, the DNA could be used immediately for PCR or stored at −80°C. The primers used for XChIP‒qPCR are listed in Table S4.
CUT&Tag
CUT&Tag assays were performed by Shanghai Jiayin Biotechnology Company according to the protocol described in Professor Steven Henikoff’s article.47 Briefly, LSP1P5 was knocked down in KDFs, and negative controls were used for CUT&Tag. A quality distribution plot and base content distribution were generated by FastQC. Clean reads were obtained from the raw reads by removing the adaptor sequences using Trimmomatic software. The clean reads were subsequently aligned to reference genome sequences using the BWA program. The fragment sizes were calculated for read pairs given a BAM file from paired-end sequencing. Several regions are sampled depending on the size of the genome and number of processors to estimate the summary statistics on the fragment lengths. Properly paired reads were used for computation. The bam file was generated by the unique mapped reads as an input file using macs2 software for callpeaks with a cutoff q value <0.05. The read distributions (from bigWig) across genes are presented as an average plot (average of the read signals across all the genes). The deepTools tool was used for this analysis. The raw data were deposited in the SRA database (SRA: PRJNA972833).
RIP
RIP was conducted using an RNA immunoprecipitation kit (Geneseed, P0101, China) according to the manufacturer’s instructions. Specifically, 1.0 × 107 fibroblasts were treated with 1 mL of RIP lysis buffer. The resulting supernatants were divided into 2 fractions: 100 μL was kept as the input, and 900 μL was incubated with specific antibody- or rabbit immunoglobulin (Ig)G-conjugated protein A/G magnetic beads in IP buffer supplemented with RNase inhibitors at 4°C for 2 h. The immunoprecipitated RNA was digested, purified, and further analyzed by qPCR. The antibodies and primers used for the RIP-qPCR experiments are listed in Tables S4 and S7, respectively. Specifically, RNA extraction after RIP could not be conducted using the column in this RIP kit because the target RNA of interest was a lncRNA. Instead, TRIzol reagent was used for subsequent experiments.
Statistical analyses
GraphPad Prism 9 was used for the statistical analyses. The values are presented as the mean ± SD. For comparison of relative expression, the control group was normally set as 1 or 100% compared with the other treatment groups. The comparative threshold cycle was applied to the qRT-PCR assay data according to the ΔΔ threshold cycle method. Differences between two groups were analyzed using the unpaired two-sided Student’s t test. The correlation between two sets of data was assessed using simple linear regression analysis. A p value less than 0.05 indicated statistical significance, and these differences are indicated with asterisks, as described in the figure legends; namely, ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Data and code availability
RNA-seq and CUT&Tag data have been deposited in the NCBI GEO database (GEO: GSE212954) and in the SRA database (SRA: PRJNA972830 and SRA: PRJNA972833).
All the other data supporting the key findings of this study are available within the article and supplemental information files or from the corresponding author upon reasonable request.
Acknowledgments
This study was supported by National Natural Science Foundation of China (82072177, 82272264, 82302805), Shanghai Clinical Research Center of Plastic and Reconstructive Surgery supported by Science and Technology Commission of Shanghai Municipality (22MC1940300), “Two Hundred Talent” Program, “Hengjie” Program of Shanghai Health Youth Talent Reward Foundation, China Postdoctoral Science Foundation (2022M722132). We thank Kangcheng Biotech, Inc. (Shanghai, China), for assistance with RNA-seq, and Jiayin Biotechnology Ltd. (Shanghai, China), for assistance with CUT&Tag.
Author contributions
S.G. and X.H. designed and performed the experiments and drafted and revised the manuscript. S.L. and Y.L. designed and performed some of the experiments. S.G., S.L., and Y.K. were responsible for sample and patient information collection and data analysis. S.G., L.T., R.X., and E.Y. discussed and participated in the data interpretation. Y.Z., M.Y., and T.Z. revised and approved the manuscript. All the authors reviewed and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2024.03.031.
Contributor Information
Yixuan Zhao, Email: yixuan1.3@163.com.
Min Yao, Email: my058@vip.sina.com.
Tao Zan, Email: zantao@sjtu.edu.cn.
Supplemental information
References
- 1.Ogawa R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast. Reconstr. Surg. 2022;149:79E–94E. doi: 10.1097/PRS.0000000000008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauglitz G.G. Springer International Publishing; 2020. Textbook on Scar Management: State of the Art Management and Emerging Technologies. [DOI] [PubMed] [Google Scholar]
- 3.Andrews J.P., Marttala J., Macarak E., Rosenbloom J., Uitto J. Keloids: The paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix Biol. 2016;51:37–46. doi: 10.1016/j.matbio.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limandjaja G.C., Niessen F.B., Scheper R.J., Gibbs S. The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T., Wang X.F., Wang Z.C., Lou D., Fang Q.Q., Hu Y.Y., Zhao W.Y., Zhang L.Y., Wu L.H., Tan W.Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson A.W., Deng Z., Allahham A., Prêle C.M., Wood F.M., Fear M.W. The epigenetics of keloids. Exp. Dermatol. 2021;30:1099–1114. doi: 10.1111/exd.14414. [DOI] [PubMed] [Google Scholar]
- 7.Lv W., Ren Y., Hou K., Hu W., Yi Y., Xiong M., Wu M., Wu Y., Zhang Q. Epigenetic modification mechanisms involved in keloid: current status and prospect. Clin. Epigenet. 2020;12:183. doi: 10.1186/s13148-020-00981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan X., Wu Y., Zhang Z., Lu Z. Identification and analysis of dysregulated lncRNA and associated ceRNA in the pathogenesis of keloid. Ann. Transl. Med. 2020;8:222. doi: 10.21037/atm.2020.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alghamdi M.A., Wallace H.J., Melton P.E., Moses E.K., Stevenson A., Al-Eitan L.N., Rea S., Duke J.M., Danielsen P.L., Prêle C.M., et al. Identification of differentially methylated CpG sites in fibroblasts from keloid scars. Biomedicines. 2020;8 doi: 10.3390/BIOMEDICINES8070181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor E.J.F., Badshah I.I., Addae L.Y., Kundasamy P., Thanabalasingam S., Abioye D., Soldin M., Shaw T.J. Histone deacetylase 2 is upregulated in normal and keloid scars. J. Invest. Dermatol. 2012;132:1293–1296. doi: 10.1038/jid.2011.432. [DOI] [PubMed] [Google Scholar]
- 11.Diao J.S., Xia W.S., Yi C.G., Wang Y.M., Li B., Xia W., Liu B., Guo S.Z., Sun X.D. Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch. Dermatol. Res. 2011;303:573–580. doi: 10.1007/s00403-011-1140-1. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A.J., Nikbakht N., Uitto J. Keloid Disorder: Genetic Basis, Gene Expression Profiles, and Immunological Modulation of the Fibrotic Processes in the Skin. Cold Spring Harb. Perspect. Biol. 2023;15 doi: 10.1101/cshperspect.a041245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai P., Jia R., Jia R., Pan H., Wang S., Ni H., Wang H., Zhou C., Shi Y., Ge S., et al. Dynamic chromosomal tuning of a novel GAU1 lncing driver at chr12p13.32 accelerates tumorigenesis. Nucleic Acids Res. 2018;46:6041–6056. doi: 10.1093/nar/gky366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J.Z., Chen M., Chen D., Gao X.C., Zhu S., Huang H., Hu M., Zhu H., Yan G.R. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol. Cell. 2017;68:171–184.e6. doi: 10.1016/j.molcel.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Didon L., Roos A.B., Elmberger G.P., Gonzalez F.J., Nord M. Lung-specific inactivation of CCAAT/enhancer binding protein α causes a pathological pattern characteristic of COPD. Eur. Respir. J. 2010;35:186–197. doi: 10.1183/09031936.00185008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W., Meridew J.A., Aravamudhan A., Ligresti G., Tschumperlin D.J., Tan Q. Targeted regulation of fibroblast state by CRISPR-mediated CEBPA expression. Respir. Res. 2019;20:281. doi: 10.1186/s12931-019-1253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reebye V., Huang K.W., Lin V., Jarvis S., Cutilas P., Dorman S., Ciriello S., Andrikakou P., Voutila J., Saetrom P., et al. Gene activation of CEBPA using saRNA: Preclinical studies of the first in human saRNA drug candidate for liver cancer. Oncogene. 2018;37:3216–3228. doi: 10.1038/s41388-018-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence M., Daujat S., Schneider R. Lateral Thinking: How Histone Modifications Regulate Gene Expression. Trends Genet. 2016;32:42–56. doi: 10.1016/j.tig.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Encode Consortium. Carolina N., Hill C. ENCODE Project Writes Eulogy For Junk DNA. Science. 2012;337:1159–1161. doi: 10.1126/science.337.6099.1159. [DOI] [PubMed] [Google Scholar]
- 20.Maher B. The human encyclopaedia. Nature. 2012;489:46–48. doi: 10.1038/489046a. [DOI] [PubMed] [Google Scholar]
- 21.Abascal F., Acosta R., Addleman N.J., Adrian J., Afzal V., Aken B., Akiyama J.A., Jammal O.A., Amrhein H., Anderson S.M., et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583:699–710. doi: 10.1038/s41586-020-2493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z., Jiang S., Shang J., Jiang Y., Dai Y., Xu B., Yu Y., Liang Z., Yang Y. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res. Rev. 2019;52:17–31. doi: 10.1016/j.arr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Liang X., Ma L., Long X., Wang X. LncRNA expression profiles and validation in keloid and normal skin tissue. Int. J. Oncol. 2015;47:1829–1838. doi: 10.3892/ijo.2015.3177. [DOI] [PubMed] [Google Scholar]
- 24.Wasson C.W., Abignano G., Hermes H., Malaab M., Ross R.L., Jimenez S.A., Chang H.Y., Feghali-Bostwick C.A., Del Galdo F. Long non-coding RNA HOTAIR drives EZH2-dependent myofibroblast activation in systemic sclerosis through miRNA 34a-dependent activation of NOTCH. Ann. Rheum. Dis. 2020;79:507–517. doi: 10.1136/annrheumdis-2019-216542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jun J.I., Lau L.F. Resolution of organ fibrosis. J. Clin. Invest. 2018;128:97–107. doi: 10.1172/JCI93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Cao J., Li M., Yu Y., Yang Y., Xiao X., Wu Z., Wang L., Tu Y., Chen H. Collagen triple helix repeat containing-1 inhibits transforming growth factor-β1-induced collagen type I expression in keloid. Br. J. Dermatol. 2011;164:1030–1036. doi: 10.1111/j.1365-2133.2011.10215.x. [DOI] [PubMed] [Google Scholar]
- 27.Wei J., Xu Z., Yan X. The role of the macrophage-to-myofibroblast transition in renal fibrosis. Front. Immunol. 2022;13:934377. doi: 10.3389/fimmu.2022.934377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y.Y., Tang P.M.K., Tang P.C.T., Xiao J., Huang X.R., Yu C., Ma R.C.W., Lan H.Y. LRNA9884, a novel smad3-dependent long noncoding RNA, promotes diabetic kidney injury in db/db mice via enhancing MCP-1-dependent renal inflammation. Diabetes. 2019;68:1485–1498. doi: 10.2337/db18-1075. [DOI] [PubMed] [Google Scholar]
- 29.Tang P.C.T., Chan A.S.W., Zhang C.B., García Córdoba C.A., Zhang Y.Y., To K.F., Leung K.T., Lan H.Y., Tang P.M.K. TGF-β1 Signaling: Immune Dynamics of Chronic Kidney Diseases. Front. Med. 2021;8 doi: 10.3389/fmed.2021.628519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P., Luo M.L., Song E., Zhou Z., Ma T., Wang J., Jia N., Wang G., Nie S., Liu Y., et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway. Sci. Transl. Med. 2018;10:1–14. doi: 10.1126/scitranslmed.aat2039. [DOI] [PubMed] [Google Scholar]
- 31.Lekstrom-Himes J., Xanthopoulos K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 32.Takayama K., Kawabata K., Nagamoto Y., Inamura M., Ohashi K., Okuno H., Yamaguchi T., Tashiro K., Sakurai F., Hayakawa T., et al. CCAAT/enhancer binding protein-mediated regulation of TGFβ receptor 2 expression determines the hepatoblast fate decision. Development. 2014;141:91–100. doi: 10.1242/dev.103168. [DOI] [PubMed] [Google Scholar]
- 33.Laugesen A., Højfeldt J.W., Helin K. Molecular Mechanisms Directing PRC2 Recruitment and H3K27 Methylation. Mol. Cell. 2019;74:8–18. doi: 10.1016/j.molcel.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richart L., Picod-Chedotel M.L., Wassef M., Macario M., Aflaki S., Salvador M.A., Héry T., Dauphin A., Wicinski J., Chevrier V., et al. XIST loss impairs mammary stem cell differentiation and increases tumorigenicity through Mediator hyperactivation. Cell. 2022;185:2164–2183.e25. doi: 10.1016/j.cell.2022.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Aguilar R., Spencer K.B., Kesner B., Rizvi N.F., Badmalia M.D., Mrozowich T., Mortison J.D., Rivera C., Smith G.F., Burchard J., et al. Targeting Xist with compounds that disrupt RNA structure and X inactivation. Nature. 2022;604:160–166. doi: 10.1038/s41586-022-04537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu D., Sun W.W., Li L., Ma L., Sun L., Jin X., Li T., Hou W., Wang J.H. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res. 2019;47:3013–3027. doi: 10.1093/nar/gkz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackledge N.P., Klose R.J. The molecular principles of gene regulation by Polycomb repressive complexes. Nat. Rev. Mol. Cell Biol. 2021;22:815–833. doi: 10.1038/s41580-021-00398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Ruscio A., Ebralidze A.K., Benoukraf T., Amabile G., Goff L.A., Terragni J., Figueroa M.E., De Figueiredo Pontes L.L., Alberich-Jorda M., Zhang P., et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503:371–376. doi: 10.1038/nature12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng J.X., Anastasi J., Watanabe K., Kleinbrink E.L., Grimley E., Knibbs R., Shen Q.J., Vardiman J.W. Genome-wide profiling reveals epigenetic inactivation of the PU.1 pathway by histone H3 lysine 27 trimethylation in cytogenetically normal myelodysplastic syndrome. Leukemia. 2013;27:1291–1300. doi: 10.1038/leu.2013.45. [DOI] [PubMed] [Google Scholar]
- 40.Deweerdt S. RNA therapies explained. Nature. 2019;574:S2–S3. [Google Scholar]
- 41.Micheletti R., Plaisance I., Abraham B.J., Sarre A., Ting C.C., Alexanian M., Maric D., Maison D., Nemir M., Young R.A., et al. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim K.R.Q., Maruyama R., Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Dev. Ther. 2017;11:533–545. doi: 10.2147/DDDT.S97635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F., Zuroske T., Watts J.K. RNA therapeutics on the rise. Nat. Rev. Drug Discov. 2020;19:441–442. doi: 10.1038/d41573-020-00078-0. [DOI] [PubMed] [Google Scholar]
- 44.Gu S., Huang X., Xu X., Liu Y., Khoong Y., Zhang Z., Li H., Gao Y., Zan T. Inhibition of CUB and sushi multiple domains 1 (CSMD1) expression by miRNA-190a-3p enhances hypertrophic scar-derived fibroblast migration in vitro. BMC Genom. 2021;22:613. doi: 10.1186/s12864-021-07920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X., Gu S., Liu C., Zhang L., Zhang Z., Zhao Y., Khoong Y., Li H., Gao Y., Liu Y., et al. CD39+ Fibroblasts Enhance Myofibroblast Activation by Promoting IL-11 Secretion in Hypertrophic Scars. J. Invest. Dermatol. 2022;142:1065–1076.e19. doi: 10.1016/j.jid.2021.07.181. [DOI] [PubMed] [Google Scholar]
- 46.Zhou B.Y., Liu W., Wang W.B., Wu X.L., Zhang W.J., Zhou G.D., Gao Z. Nintedanib inhibits keloid fibroblast functions by blocking the phosphorylation of multiple kinases and enhancing receptor internalization. Acta Pharmacol. Sin. 2020;41:1234–1245. doi: 10.1038/s41401-020-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaya-Okur H.S., Wu S.J., Codomo C.A., Pledger E.S., Bryson T.D., Henikoff J.G., Ahmad K., Henikoff S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-09982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq and CUT&Tag data have been deposited in the NCBI GEO database (GEO: GSE212954) and in the SRA database (SRA: PRJNA972830 and SRA: PRJNA972833).
All the other data supporting the key findings of this study are available within the article and supplemental information files or from the corresponding author upon reasonable request.