Highlights
-
•
The findings are consistent in showing greater deoxygenation in the muscles during exercise, reflecting the oxygen (O2) utilization.
-
•
Muscle O2 availability becomes compromised when an individual reaches the exhaustion point by reducing blood flow and decreasing muscle O2 extraction.
-
•
Cerebral tissue experiences an increase in O2 delivery with increased exercise intensity, suggesting a greater engagement of the brain areas to sustain the exercise.
-
•
At the exhaustion point, cerebral oxygenation reaches a plateau or decline, potentially resulting in motor failure during exercise.
Keywords: Near-infrared spectroscopy, Hemodynamic, Blood flow, Brain, Muscle metabolism
Abstract
Background
Near-infrared spectroscopy (NIRS) technology has allowed for the measurement of cerebral and skeletal muscle oxygenation simultaneously during exercise. Since this technology has been growing and is now successfully used in laboratory and sports settings, this systematic review aimed to synthesize the evidence and enhance an integrative understanding of blood flow adjustments and oxygen (O2) changes (i.e., the balance between O2 delivery and O2 consumption) within the cerebral and muscle systems during exercise.
Methods
A systematic review was conducted using PubMed, Embase, Scopus, and Web of Science databases to search for relevant studies that simultaneously investigated cerebral and muscle hemodynamic changes using the near-infrared spectroscopy system during exercise. This review considered manuscripts written in English and available before February 9, 2023. Each step of screening involved evaluation by 2 independent authors, with disagreements resolved by a third author. The Joanna Briggs Institute Critical Appraisal Checklist was used to assess the methodological quality of the studies.
Results
Twenty studies were included, of which 80% had good methodological quality, and involved 290 young or middle-aged adults. Different types of exercises were used to assess cerebral and muscle hemodynamic changes, such as cycling (n = 11), treadmill (n = 1), knee extension (n = 5), isometric contraction of biceps brachii (n = 3), and duet swim routines (n = 1). The cerebral hemodynamics analysis was focused on the frontal cortex (n = 20), while in the muscle, the analysis involved vastus lateralis (n = 18), gastrocnemius (n = 3), biceps brachii (n = 5), deltoid (n = 1), and intercostal muscle (n = 1). Overall, muscle deoxygenation increases during exercise, reaching a plateau in voluntary exhaustion, while in the brain, oxyhemoglobin concentration increases with exercise intensity, reaching a plateau or declining at the exhaustion point.
Conclusion
Muscle and cerebral oxygenation respond differently to exercise, with muscle increasing O2 utilization and cerebral tissue increasing O2 delivery during exercise. However, at the exhaustion point, both muscle and cerebral oxygenation become compromised. This is characterized by a reduction in blood flow and a decrease in O2 extraction in the muscle, while in the brain, oxygenation reaches a plateau or decline, potentially resulting in motor failure during exercise.
Graphical Abstract
1. Introduction
Near-infrared spectroscopy (NIRS) is a non-invasive tool for monitoring changes in both cerebral and skeletal muscle oxygenation during exercise.1, 2, 3, 4 NIRS operates on the distinct absorption properties of chromophores within the near-infrared range of 700–1000 nm.5,6 The near-infrared light is able to penetrate several centimeters into biological tissues (reaching approximately 2–3 cm in adult heads and 4–5 cm in muscle), where hemoglobin and myoglobin (only in muscles) absorb the light photons.7,8 The NIRS signal offers information about oxygenation changes in the small blood vessels (i.e., arterioles, capillaries, venules) because large blood vessels may completely absorb light photons.5,8 The absorption rate of the light photons is different for oxygenated (absorption peak around 850 nm) and deoxygenated chromophores (absorption peak around 760 nm), which allows photodetectors to differentiate them.5,6 The difference in the tissue absorbency allows us to understand the balance between oxygen (O2) delivery and O2 consumption in the cerebral cortex and skeletal muscles.5
Different NIRS-based measurement techniques have been developed to measure tissue oxygenation. These include continuous-wave-based systems that emit light with a constant intensity, frequency-domain systems that modulate light intensity using a sinusoidal function, and time-domain systems that measure light's time of flight in addition to intensity.3,8,9 Among these, continuous-wave NIRS systems are the most commonly used in research.8,9 However, these systems determine changes in chromophore concentrations from a baseline value (relative changes) rather than absolute concentrations.5,7 Despite this limitation, NIRS technology enables real-time quantitative monitoring of crucial physiological measures. Using the modified Beer–Lambert law, it is possible to calculate changes in concentration of oxyhemoglobin ([HbO2]) and deoxyhemoglobin ([HHb]) over time, indicating the balance between O2 delivery and extraction in the local tissue, respectively.2,4, 5, 6, 7, 8,10, 11, 12 In addition, the sum of [HbO2] and [HHb] (total hemoglobin [tHb]) is considered an index of localized blood flow in tissue, and the difference between [HbO2] and [HHb] (characterized as [Hbdiff]) is used as an index of tissue O2 extraction. The ratio between [HbO2] and [tHb], which represents the tissue saturation (TSI, expressed in percentage), reflects an index of changes in tissue O2 saturation, indicating the balance between O2 delivery and tissue O2 consumption.4,7,12 Overall, the NIRS-derived parameter can be an indicator of the physiological status (i.e., blood flow and local O2 consumption) of both cerebral and muscular systems during exercise.12
NIRS systems have been validated against gold-standard techniques in humans. For example, cerebral NIRS data have been validated against functional magnetic resonance imaging, which is considered the gold standard for neuroimaging. Previous studies have demonstrated a strong correlation between functional magnetic resonance imaging blood O2 level-dependent signal and NIRS measures ([HbO2] and [HHb]) during motor13,14 and cognitive15,16 tasks. Moreover, existing evidence has reported the validity of muscle NIRS measurements (e.g., [HbO2] and [HHb]) in humans by comparing these measures with venous blood because NIRS signals are related to changes in venous saturation under varying oxygenation statuses of human muscles.17, 18, 19 Therefore, NIRS is a valid and reliable technique to measure both cerebral and muscle hemodynamics.
Real-time cerebral oxygenation analysis has important applications in the fields of clinical and sports sciences, contributing to understanding brain function during exercise. Neural activation in response to a stimulus causes increased energy demands in the activated brain area. This demand leads to rapid vasodilatation, increasing cerebral blood flow in such areas, to meet the increased metabolic needs and O2 uptake with an increased O2 supply.20,21 Since the vasodilatation precedes and overshoots the need, the increased cortical activity is associated with increased [HbO2] and decreased [HHb].20 In fact, several studies have analyzed the cerebral hemodynamics, especially of the prefrontal cortex (PFC), during physical exercises.4,6,20 The results showed alterations in brain oxygenation according to the exercise intensity, indicating an increase in cerebral oxygenation (which has been linked to increased cortical activity1,20,22,23) during moderate aerobic exercise, possibly due to the requirement to control movement.20,24,25 However, it decreases when the individual reaches voluntary exhaustion (maximal intensity exercise), reflecting impaired central involvement during neuromuscular control.26, 27, 28
In the skeletal muscle system, NIRS has been applied to assess oxidative metabolism in different muscles (e.g., arm, leg, and respiratory muscles) and exercise intensity (e.g., moderate, heavy, and severe exercise).2,4,6 The increase in [HbO2] and decrease in [HHb] are indications of O2 availability, while the reduction in [HbO2] and increase in [HHb] indicate the O2 consumption in the local muscle. The use of NIRS can aid in understanding the efficiency of the muscular system in providing and using O2 during exercise. For example, lower O2 availability has been considered indicative of compromised cardiac output and muscle blood flow.29, 30, 31 Taken together, the application of NIRS in both cerebral and muscle systems during an exercise session may contribute to the understanding of central and peripheral hemodynamic and metabolic processes.
Recent advancements in NIRS technology have allowed for the simultaneous measurement of both cerebral and skeletal muscle oxygenation. The synchronized analysis of these systems facilitates a comprehensive understanding of the blood flow during exercise sessions, enhancing our knowledge about possible mechanisms for how muscles and the brain cope with O2 demand and the wider balance between O2 delivery and utilization. Since this technology has been growing and is now successfully used in laboratory and sports settings, this systematic review aimed to synthesize the evidence and enhance an integrative understanding of blood flow adjustments and O2 changes (i.e., the balance between O2 delivery and O2 consumption) within the cerebral and muscle systems during exercise. The review focused on healthy adults and “pure” exercise (e.g., without any condition that could affect the individual oxygenation response, such as local hypoxia, altitude conditions, or any manipulation of the environment/individual)28,32, 33, 34 in order to exclude any confounding effect.
2. Methods
This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022295233) and written following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines updated in 202035 and the Cochrane recommendation for systematic review.36
2.1. Search strategy
Searches were conducted up to February 9, 2023, on PubMed, Embase, Scopus, and Web of Science. The search strategy included terms related to exercise, NIRS measurements, and cerebral and muscular systems. The full search strategy is presented in Table 1. Manuscripts identified through database searches were downloaded to a reference manager software where duplicates were excluded. Two of 3 authors (DOS, VSB, and PCRS) independently performed the initial screen by reviewing the titles and abstracts. Full texts were reviewed when the information in the title and abstract was unclear. The third author made the final decision in case of inconsistencies between the 2 authors.
Table 1.
Search strategy.
| Search | Query |
|---|---|
| #1 | Exercise OR physical activity OR physical exercise OR fitness OR training OR sport* OR rehabilitation OR endurance OR aerobic OR strength OR resistance OR cycling OR swim* OR walk* OR treadmill (Title/Abstract/Keywords) |
| AND | |
| #2 | Functional neuroimaging OR “near infrared spectroscopy” OR “functional near infrared spectroscopy” OR “fNIRS” OR “NIRS” OR Hemodynamic* OR oxim* OR spectroscopy OR haemoglobin OR hemoglobin OR oxyhemoglobin OR deoxyhemoglobin OR oxygenation OR tissue saturation (Title/Abstract/Keywords) |
| AND | |
| #3 | Cortex OR cortical OR brain OR cerebral OR central (Title/Abstract/Keywords) |
| AND | |
| #4 | Muscle OR muscular OR skeletal muscle OR peripheric (Title/Abstract/Keywords) |
Abbreviations: fNIRS = functional near-infrared spectroscopy; NIRS = near-infrared spectroscopy.
2.2. Eligibility criteria
Manuscripts were included if they simultaneously investigated cerebral and muscle hemodynamic changes using the NIRS system (regardless of system brands) during exercise and included only healthy adults. For this review, we considered manuscripts written in English; cross-sectional, randomized, and non-randomized controlled trials; and observational studies. We excluded pre-print and open-label studies, review articles, commentaries, conference abstracts, study protocols, and manuscripts composed of populations of adolescents or children. In addition, we excluded manuscripts that have manipulated the basal condition of individuals (e.g., by using local hypoxia protocol, interfering with blood flow or the respiratory system, simulating altitude conditions, manipulating climatic conditions, or administering any type of supplementation, medication, or tobacco).
2.3. Data extraction
Data from each study were independently extracted by 2 of the 6 authors (DOS, VSB, PCRS, FMR, ABM, and RV) and then synthesized into a table format. Any uncertainty or incomplete information arising from the extracted data was resolved through discussion with a third author. The extracted variables included: (a) study design; (b) participants’ characteristics; (c) exercise protocol; (d) comparisons performed in the study; methods used to collect and analyze the NIRS data of (e) cerebral and (f) muscular systems; (g) additional measurements assessed in the study; (h) main results; and (i) conclusions.
2.4. Methodological quality assessment
Two authors (2 of the 6 authors; DOS, VSB, PCRS, ABM, FMR, and RV) independently assessed the methodological quality (risk of bias) of the included studies using the Joanna Briggs Institute Critical Appraisal Checklist.37 Disagreements in the assessments between the reviewers were resolved by a third author (DOS or VSB). This appraisal assesses the methodological quality of a study and determines the extent to which a study has addressed the possibility of bias in its design, conduct, and analysis.37 Since all studies included were cross-sectional, we used a Joanna Briggs Institute Critical Appraisal Checklist specific to cross-sectional studies. The assessment is based on 8 critical aspects of the study: (a) the criteria for inclusion in the sample were clearly defined; (b) the study subjects and setting were described in detail; (c) the exposure was measured in a valid and reliable way; (d) objective, standard criteria were used for measurement of the condition; (e) confounding factors were identified; (f) strategies to deal with confounding factors were stated; (g) the outcomes were measured in a valid and reliable way; and (h) appropriate statistical analysis was used.37 Each item was scored as 1 (the study does fulfill the specified criteria), 0 (the study does not fulfill the stated criteria), unclear (the criteria are not clearly identified in the report), or not applicable (the criteria were not relevant to the study), thus yielding a maximum of 8 points. Studies were judged to be low (>70%), moderate (50%–70%), and high risk of bias (<50%).
3. Results
3.1. Study selection
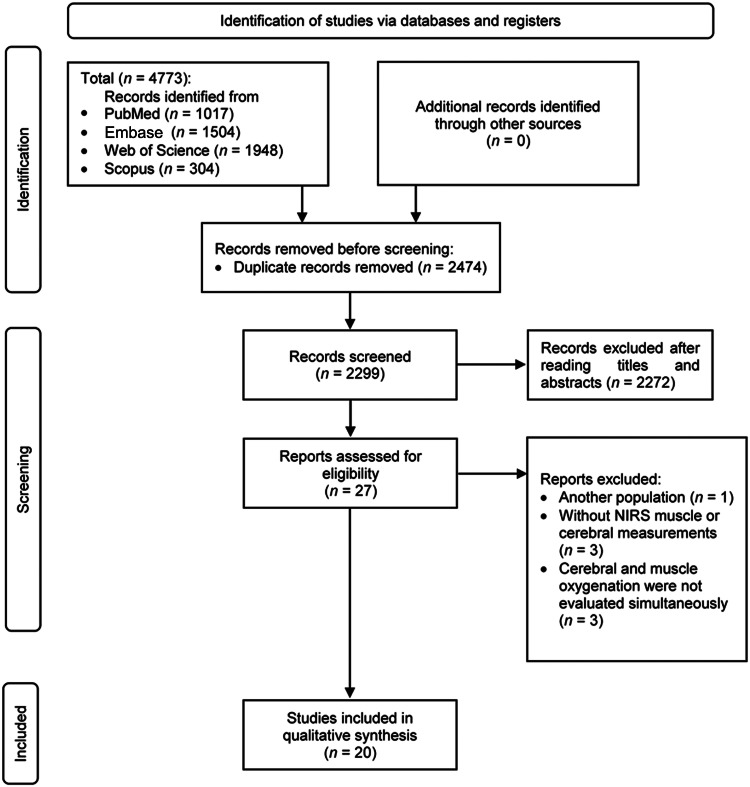
A total of 4773 records were identified through the initial search. After removing 2474 duplicates and 2272 records based on titles and abstracts, 27 studies were identified for full-text screening. Of these, 7 studies were excluded due to the following reasons: adolescent population (n = 1); without NIRS muscle or cerebral measurements (n = 3); cerebral and muscle oxygenation were not evaluated simultaneously (n = 3). Finally, 20 articles were included in the systematic reviews. The summary of the study selection is presented within the PRISMA flow diagram (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart showing the screening process. NIRS = near-infrared spectroscopy.
3.2. Study characteristics
Supplementary Table 1 shows the characteristics of the 20 studies included in the systematic review. The studies were published from 2008 to 2022 and involved a total of 290 young or middle-aged adults. The sample size varied between 1 and 28 participants (median = 12). Ten studies recruited only male participants,38, 39, 40, 41, 42, 43, 44, 45, 46, 47 4 studies only female,48, 49, 50, 51 and 5 studies recruited both sexes.52, 53, 54, 55, 56 One study did not report the sex.57 Six studies mentioned that participants were physically active,39,40,42,44,47,52 7 studies did not specify the level of physical activity,41,43,45,53, 54, 55, 56 3 studies included both short-term and long-term aerobically trained participants,38,48,49 1 study included cyclists,57 1 study well-trained cyclists or triathletes,46 1 study elite synchronized swimmers,50 and 1 sub-elite futsal players.51 Different types of exercises were used to assess cerebral and muscle hemodynamic changes, such as cycling,38,39,42,44,46, 47, 48, 49,53,55,57 treadmill,45 knee extension,40,41,51,54,56 isometric contraction of the biceps brachii (BB),41,43,52 and duet swim routines.50
3.3. NIRS data acquisition and analysis
In all studies, the NIRS data were assessed during exercise. In addition, the analyses were performed considering the difference between data collected during exercise and at pre-test while resting (baseline). Thus, the delta (∆ = NIRS data during exercise – NIRS data during baseline) was considered in all analyses. In the cerebral measurements, 11 studies evaluated only the left PFC,38,40,41,44,46,48, 49, 50,52,54,57 3 studies assessed the right PFC,39,43,55 1 study evaluated the right frontal cortex,45 1 study assessed both left and right PFC,51 1 study assessed the PFC (without specifying the hemisphere) and motor cortex,47 1 study evaluated the contra-lateral frontal lobe,56 1 study assessed the frontal cortex,42 and 1 study evaluated the midline forehead.53 In the muscular measurements, 11 studies different sets were used in the optodes placement: (a) 6 studies placed them in the right vastus lateralis (VL);39,46,47,50,51,57 (b) 2 studies in the VL without specifying the body side;40,42 (c) 2 studies in the dominant VL;54,56 (d) 2 studies in both left and right VL;44 (e) 2 studies in the left VL and left gastrocnemius;38,48 (f) 2 studies in both right VL and right BB;45,55 (g) 1 study in the BB and dominant VL;41 (h) 1 study in the left BB;43 (i) 1 study in both left and right VL and left gastrocnemius;49 (j) 1 study in the right BB;52 and (k) 1 study in the left VL, left deltoid, and intercostal muscle.53
An important parameter involved in estimating the hemoglobin concentration is the differential pathlength factor (DPF), which may be incorporated into the modified Beer–Lambert law. DPF is a multiplicative correction factor that accounts for the mean optical pathlength the light travels within the tissue to the light source-detector separation distance.7,58 In the cerebral analysis, 5 studies reported the DPF: 4 studies used age-dependent factor45,48,49,55 and 1 study used 5.93.57 In the muscle analysis, 3 studies reported the DPF: 2 studies used 5.51 (for the leg) and 4.16 (for the arm),45,55 and 1 used 3.8357 as the factor.
In cerebral and muscle analysis, the sampling frequency varied between 0.5 Hz and 50 Hz. Three studies did not inform the sampling frequency.41,42,53 Regarding the filter, only 4 studies presented the methods used to filter the NIRS signals.41,47,51,53
Sixteen studies analyzed [HHb],38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49,51,52,55,57 13 studies analyzed [HbO2],39, 40, 41, 42, 43, 44, 45, 46, 47,51,52,55,57 12 studies analyzed [tHb],39, 40, 41, 42, 43, 44, 45, 46, 47,51,52,55 8 studies analyzed TSI,39, 40, 41,44,45,50,51,55 2 studies analyzed [Hbdiff],46,52 2 studies analyzed cerebral and muscle blood volume and oxygenation,54,56 and 1 study analyzed O2 extraction53 as outcome for both cerebral and muscular measurements.
3.4. Cerebral and muscle hemodynamic changes during exercise
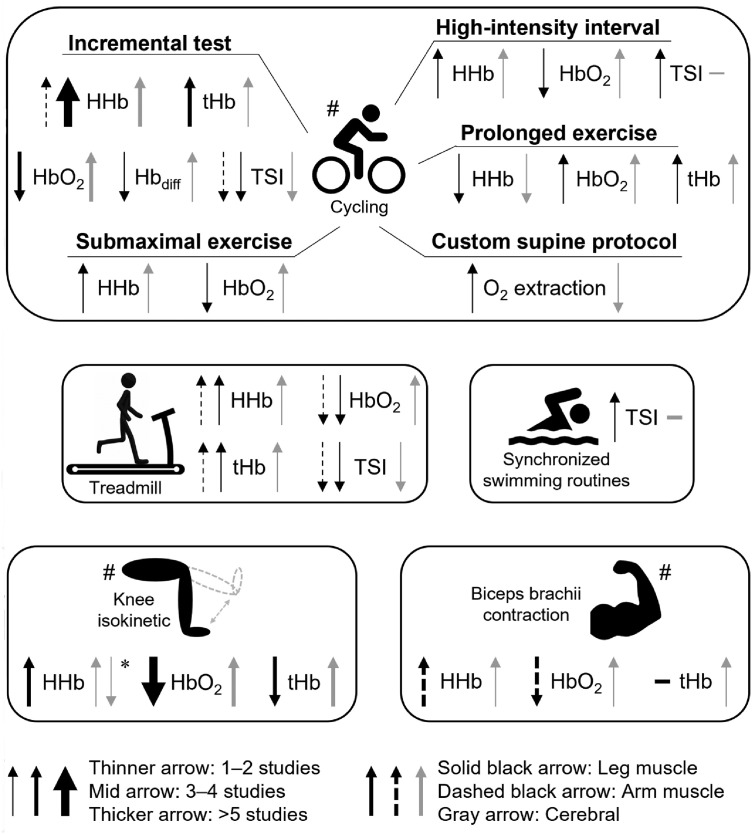
Fig. 2 summarizes the main results and Supplementary Table 2 presents a synthesis of the results and conclusions of each study. Overall, during an incremental cycling test, muscle (e.g., VL) [HHb]38,39,44,46,48,49,55,57 and [tHb]39,46,55 increased, while muscle [HbO2]39,44,46,57 and [Hbdiff]46 decreased. Studies revealed an increase in cerebral (i.e., PFC) [HHb],46,55,57 [HbO2],39,46,55,57 [tHb],39,46 and [Hbdiff].46 Similarly, in the high-intensity interval cycling exercise, VL [HbO2] decreased and PFC [HbO2] increased, whereas both VL and PFC [HHb] progressively increased until the end.44 In response to the submaximal cycling exercise, there was an increase in VL [HHb]47 and a decrease in VL [HbO2],47 as well as an increase in both PFC [HbO2]47 and [HHb].47 In contrast, prolonged cycling resulted in a decrease in VL [HHb] and an increase in VL [HbO2] and [tHb]. Cortical [HbO2] and [tHb] increased, while cortical [HHb] decreased during prolonged cycling.42 In a custom supine pedaling protocol, there was an increase in VL O2 extraction, whereas cerebral O2 extraction had a linear decrease.53
Fig. 2.
Summary of main results. Most studies used cycling exercises (n = 11), with different protocols. Other types of exercises used were treadmill exercise (n = 1), duet swim routines (n = 1), knee isokinetic exercise (n = 5), and contraction of biceps brachii (n = 3). The arrows indicate an increase or decrease in NIRS variables in the leg muscle (solid black), arm muscle (dashed black), and cerebral tissue (gray). The thickness of the arrows indicates the number of studies that found such results. Detailed results of each study can be found in Supplementary Table 1, including the effects of fatigue state on the cerebral and muscle oxygenation responses. * Contradictory results. # Fatigue state may alter the results (Supplementary Table 1). Hbdiff = difference between HbO2 and HHb; HbO2 = oxyhemoglobin; HHb = deoxyhemoglobin; NIRS = near-infrared spectroscopy; tHb = total hemoglobin; TSI = tissue saturation.
In a treadmill exercise, 1 study45 reported the muscle hemodynamic in active muscle (i.e., VL), less active muscle (i.e., BB), and cerebral tissue (i.e., frontal cortex), which showed that both leg and arm muscles decreased [HbO2] and TSI, while [HHb] and [tHb] increased. BB muscle presented higher deoxygenation at peak exercise vs. VL muscle. In the cerebral cortex, TSI decreased, while [HbO2], [HHb], and [tHb] increased. In elite-level synchronized swimmers, the VL TSI dropped during dynamic leg-kicking exercises and the PFC hemodynamic was unchanged throughout the exercise.50
During isometric knee flexion/extension, individuals presented a decrease in VL [HbO2]40,44,51,54,56 and [tHb],40,54,56 and an increase in VL [HHb].40,44,51 In the PFC, individuals presented an increase in [HbO2],40,51,54,56 [HHb],40 and [tHb].40,54,56 In contrast, 1 study found a decrease in PFC [HHb] during knee extension.51 During isometric contractions of BB, studies found a decrease in muscle [HbO2]41,43,52 and an increase in muscle [HHb].41,43,52 In addition, cerebral [HbO2]43,52 and [tHb]52 increased during isometric contractions, while [HHb] decreased.43,52
3.5. Methodological quality assessment (risk of bias)
The methodological quality ranged from 2 (25%; indicating high risk of bias) to 8 (100%; indicating low risk of bias). Nearly all the studies demonstrated good overall quality, as shown in Table 2; 16 studies were classified as having low risk,38, 39, 40,42,44, 45, 46,48, 49, 50, 51, 52,54, 55, 56, 57 2 studies as moderate risk,47,53 and 2 studies as high risk of bias.41,43
Table 2.
Methodological quality assessment based on Joanna Briggs Institute (JBI) Critical Appraisal Checklist.
| Criteria and score |
Total score/% | Risk of bias | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | |||
| Bhambhani et al. (2014)52 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7/87.5 | Low |
| Buzza et al. (2016)49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Buzza et al. (2019)48 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Buzza et al. (2020)38 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Caen et al. (2019)39 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Cherouveim et al. (2022)40 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Ellwien et al. (2017)53 | U | 0 | 1 | 1 | U | U | 1 | 1 | 4/50.0 | Moderate |
| Formenti et al. (2018)51 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Ganesan et al. (2013)41 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | U | 2/25.0 | High |
| Jones and Cooper (2018)50 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7/87.5 | Low |
| Kounalakis and Geladas (2012)42 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7/87.5 | Low |
| Matsuura et al. (2011)54 | U | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7/87.5 | Low |
| Oka and Asgher (2021)43 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3/37.5 | High |
| Peltonen et al. (2013)55 | U | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7/87.5 | Low |
| Pereira et al. (2009)56 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7/87.5 | Low |
| Perentis et al. (2021)44 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Racinais et al. (2014)57 | U | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6/75.0 | Low |
| Rissanen et al. (2012)45 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Rupp and Perry (2008)46 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8/100 | Low |
| Rupp et al. (2013)47 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5/62.5 | Moderate |
Notes: Criteria #1: Were the criteria for inclusion in the sample clearly defined? #2: Were the study subjects and the setting described in detail? #3: Was the exposure measured in a valid and reliable way? #4: Were objective, standard criteria used for measurement of the condition? #5: Were confounding factors identified? #6: Were strategies to deal with confounding factors stated? #7: Were the outcomes measured in a valid and reliable way? #8: Was appropriate statistical analysis used? (1) indicates the article does fulfill the specified criteria, (0) indicates the article does not fulfill the stated criteria, or (U) indicates that it is unclear whether the article fulfills the criteria.
4. Discussion
This systematic review aimed to synthesize the evidence and enhance an integrative understanding of blood flow adjustments and O2 changes (i.e., the balance between O2 delivery and O2 consumption) within the cerebral and muscle systems during exercise. We examined 20 studies, of which most (80%) had good methodological quality. In summary, the studies focused on the PFC area for cortical analysis; however, there were discrepancies in the muscles assessed (e.g., 11 different sets were used in the muscle optodes placement). Most of the studies analyzed the change of [HHb], [HbO2], and [tHb]. The studies involved small sample sizes and varied in terms of individuals' physical activity level, exercise protocol, NIRS data processing, and NIRS outcomes, which makes the results difficult to generalize. Despite this, the studies showed relevant information about changes in cerebral and muscle oxygenation during different types of exercise and intensity efforts.
4.1. Hemodynamic changes during cycling
Eleven studies examined cycling exercise. However, the protocols varied between studies (e.g., prolonged cycling, constant-power test until exhaustion, incremental cycling test until voluntary exhaustion, or high-intensity interval exercise). Overall, the studies reported that muscle [HHb] increased and [HbO2] decreased during cycling exercises, changes that suggest an increased muscle tissue O2 extraction. However, exercising until voluntary exhaustion may limit the O2 extraction, reducing blood flow or decreasing muscle tissue oxygenation. Although interesting results have been found relative to muscle oxygenation, there is a gap in the evidence regarding different muscles. The focus of most of these studies was the VL muscle (the primary muscle involved in cycling exercise), but the analyses of different muscles (e.g., less involved muscles during cycling exercise) may help to advance knowledge of the redistribution of blood flow during exercise.59 In cerebral (e.g., PFC) measurements, the response to cycling exercise is different between studies. While some studies found both cerebral [HbO2] and [HHb] increased during submaximal and exhaustive exercise,39,46,57 other studies reported contradictory findings, such as no significant changes in [HbO2] with an increase in [HHb] and [tHb] throughout the exercise,44 or an increase in cerebral [HbO2] and decrease in the [HHb] during exercise.42 The discrepancies observed among studies findings (i.e., the lack of consistency) might be attributed to variations in cycling protocols. Moreover, population characteristics such as physical fitness levels, age, and sex could act as additional factors contributing to contradictory results. These variations in exercise protocols and participant demographics across studies could potentially account for the observed discrepancies.
Although all studies focused on analyzing the frontal cortex, Rupp and colleagues47 verified both PFC and motor cortex hemodynamics during 3 successive 80-min cycling bouts. Interestingly, they found different oxygenation responses, in particular, during the first 80 min, where motor cortex [HbO2] decreased, and PFC [HbO2] increased in response to exercise. These results demonstrate the specificity of O2 delivery and activation patterns in motor-related cortical areas during submaximal exercise. However, more studies are necessary to confirm the motor cortex oxygenation response during exercise.
Cerebral and muscle oxygenation responses are related to exercise intensity. Healthy young men cycling at 40 revolutions per minute (r/min) or 80 r/min for 90 min presented lower values of muscle [tHb] and [HbO2] at 80 r/min than 40 r/min after 30 min of exercise, while both [HHb] and TSI remained the same between cadences. In addition, cerebral TSI was lower at 80 r/min than at 40 r/min during the exercise and both [tHb] and [HbO2] were lower at 80 r/min than 40 r/min at the end of exercise.42 During submaximal cycling exercise, healthy young adults had an increase in leg O2 extraction from rest to 130% of resting heart rate (HR) and then stabilized. In the brain, the O2 extraction has a linear decrease with exercise level (130%, 150%, and 170% of resting HR).53 These findings suggest that higher cycling intensity is related to compromised muscle and cerebral blood volume and cerebral oxygenation.42 A possible explanation may be attributed to the reduction in cardiac output in the higher intensity. A compromised cardiac output may affect regional muscle oxygenation and cause local vasoconstriction or inhibit vasodilatation,42 limiting O2 delivery to skeletal muscles, which may lead to an anaerobiosis process and, as a consequence, inhibit muscle contraction and induce muscle fatigue.60,61 Similarly, in the brain, impaired cardiac output may affect cerebral blood volume and cerebral blood flow,42 inducing central fatigue.62 As a result, a reduction in cardiac output may precede task failure.60
Cerebral and muscle hemodynamics are altered in voluntary exhaustion. Cyclists performing an incremental cycling test until exhaustion presented a non-linear increase in both cerebral [HHb] and [HbO2] concomitantly to the first ventilatory threshold. Cerebral [HHb] further increased, while [HbO2] reached a plateau after the second ventilatory threshold. VL [HbO2] displayed a non-linear decrease, while VL [HHb] increased between the first and second ventilatory thresholds, following an attenuation after the second ventilatory threshold.57 Similarly, during progressive maximal cycling, well-trained cyclists who exercised to exhaustion showed a continuous increase in the VL and PFC [tHb] until the second ventilatory threshold and then stabilized. Also, the individuals increased VL [HHb] during exercise, whereas both VL [HbO2] and [Hbdiff] decreased continuously from the beginning of the test until exhaustion. Cerebral [HHb] increased during exercise until the end. In contrast, cerebral [HbO2] and [Hbdiff] increased during exercise to second ventilatory thresholds, then dropped until the end-point of the test (exhaustion).46 Taken together, these results suggest that the metabolic and ventilatory events may affect both cerebral and muscle oxygenation levels during an incremental test.46,57 Muscle fatigue has been related to a reduction in microcirculation and disturbances in oxidative metabolism, which may impair muscle function.63 Furthermore, a decrease in the PFC oxygenation before exhaustion (motor performance failure) implies the role of the PFC in the reduction of motor output at the cessation of exercise.46
Different muscles respond differently to exercise. For example, during incremental cycling exercise, deoxygenation in the BB was more severe than in the leg muscle.55 A possible explanation for such difference may be the exercise pressor reflex function.64,65 Basically, the exercise pressor reflex is a function related to the autonomic nervous system's response to increased metabolic demand during physical activity. This function is a chain of events that is not directly related to an increase in O2 delivery, but it implies the O2 supply to active muscles while decreasing the O2 for non-active muscles.64,65 Thus, exercise pressor reflex functions may contribute to circulatory adjustment, resulting in a redistribution of blood flow between more and less active muscles. Also, previous studies reported a higher [HHb] in the VL compared to gastrocnemius muscles during ramp incremental cycling or square-wave constant load (SWCL) cycling.38,48,49 The variations in muscle fiber characteristics38,48,49 and the recruitment pattern of such muscles during different exercise intensities (e.g., intercostal muscle and arm muscle deoxygenation increased the contribution during maximal cycling exercise) may influence the oxygenation response during exercise.
Leg muscles (but not arm muscle or cerebral) deoxygenation differs between sexes. Peltonen and colleagues55 compared men vs. women during incremental cycling protocol and revealed that VL [HHb] increased in both sexes but significantly more in men than women at peak exercise. Additionally, cerebral and arm muscle oxygenation showed similarities in both sexes. The discrepancy in leg muscle deoxygenation may be attributed to a sex-specific response in matching O2 delivery and utilization with systemic circulation, vascular resistance, and blood pressure.55,66 Previous studies have indicated that men tend to have higher cardiac output and blood volume compared to women.67 These factors appear to contribute to reduced peripheral resistance in active leg muscles, thus creating conditions conducive to efficient deoxygenation.55 Furthermore, variations in factors such as vascular responsiveness, regulation of blood flow to muscles, and blood perfusion may exist between men and women,67 leading to differences in muscle deoxygenation. Additionally, hormonal differences (i.e., testosterone and estrogen) between men and women may impact muscle oxygenation.68,69 Sex hormones influence various vasoactive substances and pathways involved in vascular smooth muscle contraction, potentially affecting deoxygenation levels during exercise.70 In contrast, similar oxygenation levels observed in inactive muscles and the cerebral tissue suggest that both men and women tend to exhibit similar regulation of regional blood flow.55 Therefore, the disparity in leg muscle deoxygenation demonstrates that O2 delivery and utilization are sex-specific, whereas this distinction is not observed in the arm or cerebral regions.
Beyond sex, the current training load and age affect the central and peripheral hemodynamics. Three studies38,48,49 used the same cycling exercise protocol (ramp incremental cycling and SWCL cycling) and compared the current training load in different age groups and sexes (women: 18–30 years; women and men: 40–60 years). Buzza et al.48 found that, in the ramp incremental test, long-term trained young women presented higher [HHb] in the VL and gastrocnemius compared to those who were short-term trained, but not in the SWCL test. In contrast, Buzza et al.49 reported that short-term trained and long-term trained older women (40–60 years) presented no difference in [HHb] in the gastrocnemius during ramp incremental and SWCL test exercises. They found that only VL [HHb] increased during exercise. These findings indicate that O2 extraction is reduced in older women compared to young women. Both studies did not find a difference in PFC [HHb] between groups during the ramp incremental and SWCL tests, which suggest that age and training status do not seem to affect O2 extraction in the PFC in adult women during exercise.48 The third study analyzed both short- and long-term trained men and identified that [HHb] was greater in gastrocnemius but not the VL in long- compared to short-term trained during the SWCL test. There was no difference during the ramp incremental test. In addition, in 90% of gas exchange threshold during SWCL test, long-term trained presented greater [HHb] in the PFC compared to short-term trained, suggesting that long-term vs. short-term trained older men utilize more O2 in the PFC. Taken together, these results provided evidence that O2 extraction is age, sex, and training load dependent.
4.2. Hemodynamic changes during treadmill exercise and synchronized swimming routines
There is a discrepancy between the number of studies that have simultaneously analyzed cerebral and muscle hemodynamic changes during cycling (n = 11), walking/running (n = 1), or swimming (n = 1). A possible explanation may be the NIRS technology used in the studies. The use of a stationary cycle ergometer instead of a treadmill or swimming may facilitate data collection; however, there are wireless NIRS systems available that allow individuals greater freedom of movement. Further studies are required to explore the hemodynamic changes in different exercises to better understand the balance of cerebral and muscle O2 supply and demand according to exercise contexts.
Despite the small number of studies using treadmill and swimming exercises, the results are in line with the findings in cycling studies. Rissanen and colleagues45 analyzed the oxygenation responses in active (i.e., VL) and less active muscles (i.e., BB) and cerebral tissue (i.e., frontal cortex) of young men during incremental treadmill exercise. The study showed that [HbO2] and TSI decreased from baseline walking to peak exercise while [HHb] and [tHb] increased in both leg and arm muscles. Cerebral measurements showed that TSI decreased while [HbO2], [HHb], and [tHb] increased. Deoxygenation at peak exercise was greater in the arm muscle compared to the leg muscle and cerebral tissue, which could also be explained by the exercise pressor reflex function,64,65 as mentioned above. The authors concluded that O2 delivery to BB (less active muscle) may be limited by an accelerated increase in ventilation at high running intensities. In addition, a greater capacity for blood O2 carrying is associated with a greater level of VL deoxygenation at peak treadmill exercise.
Jones and Cooper50 examined changes in peripheral muscle and brain oxygenation in elite-level synchronized swimmers. They observed a rapid decrease in VL TSI (%) during dynamic leg-kicking exercise, indicating an initial constriction accompanied by heightened O2 consumption. Interestingly, cerebral oxygenation, analyzed in the PFC, remained constant throughout the exercise, leading the authors to propose a protective mechanism of the cerebral system. Similar results were found during maximal dry breath-holds, where cerebral oxygenation was maintained compared to reduced muscle oxygenation.71 Taken together, these findings are in line with cerebral autoregulation mechanisms. This mechanism contributes to cerebral blood flow regulation, which is key to maintaining a constant nutrient and O2 supply, thus enabling adequate neural function.72 Therefore, the human diving response is O2-conserving in supplying O2 to the brain, while the muscle O2 stores are depleted.71
4.3. Hemodynamic changes during knee isokinetic exercise
Knee extension induces muscle deoxygenation. Previous studies reported an increase in VL [HHb] and a reduction in VL [HbO2] during unilateral knee extension.40,44,51 In addition, a slow speed of movement during exercises promotes higher VL [HHb] than exercises performed with a normal speed.51 Taken together, these results demonstrated the O2 utilization in local tissue during exercise and slow movement may elicit larger muscle deoxygenation, maximizing the levels of metabolic stress.51
The PFC oxygenation response during knee extension is contradictory. Cherouveim et al.40 revealed a rapid increase in both PFC [HbO2] and [tHb], while PFC [HHb] slowly increased during knee flexion. Formenti et al.51 found a decrease in PFC [HHb] and an increase in PFC [HbO2] during the exercise. In contrast, Perentis and colleagues44 did not find significant changes in PFC [HHb] and [HbO2]. Such differences may be related to the different protocols used in these studies, such as knee isokinetic exercise performed at 60°/s and an intensity corresponding to 60% of the maximal voluntary contraction,40 unilateral knee extensions at ∼50% of 1 repetition maximum in 3 different paces (1 s, 3 s, and 5 s for both concentric and eccentric phases),51 and high-intensity interval isokinetic exercise (7 sets of 5 repetitions with 15-s intervals between sets).44 Furthermore, voluntary exhaustion was achieved by participants in only 1 out of 3 studies,51 of which might have contributed to conflicting results in cerebral oxygenation.
Corroborating the influence of voluntary exhaustion in both muscle and cerebral oxygenation, 2 studies54,56 reported an increase in cerebral oxygenation and cerebral blood volume along with a decline in muscle oxygenation and muscle blood volume during the static and dynamic knee extensions until voluntary exhaustion. The results were independent of sex and intensity.54 These findings suggest that muscle fatigue was not due to a lack of cerebral oxygenation throughout the contractions but due to intramuscular factors, such as reduced blood volume and O2 availability in the muscle tissue during resistance exercise.54,56
4.4. Hemodynamic changes during arm contraction
BB contraction increases local deoxygenation. Similar to knee isokinetic exercise, the BB muscle, during isometric or isokinetic contraction, presented an increase in [HHb] and a decrease in [HbO2],41,43,52 such that [HHb] increases progressively with increasing intensity (20%, 40%, and 60% of maximal voluntary contraction)52 and increases even more with muscle fatigue.43 These findings imply a greater O2 utilization by the muscle fiber during BB contraction, mainly in high-intensity exercises.41,43,52
In the brain, [HbO2] and [tHb] increased steadily with the increasing intensity during isometric contractions, while [HHb] decreased from 20% to 40% and remained reduced at the same level from 40% to 60% maximal voluntary contraction.52 Also, PFC [HbO2] increased and [HHb] decreased at the beginning of exercise, but [HHb] increased when exercise intensity reached the exhaustion point.43 The increased [HbO2] indicates enhanced cerebral oxygenation (that may be interpreted as increased cortical activation) which, combined with motor recruitment (evidenced by increased root mean square electromyography in the muscle), demonstrates the role of the PFC in the motor unit recruitment to maintain the target force.52 The increased PFC [HHb] may be a physiological indicator for analysis of the limit of exercise and fatigue caused by exhaustive exercise.43 In addition, muscle fatigue during BB contraction is mediated by peripheral O2 availability instead of impaired cerebral oxygenation,52 corroborating previous studies that found similar results during knee isokinetic exercise.54,56
4.5. Studies limitations and future directions
The major limitations of this study are in regard to NIRS processing. Not all studies reported detailed information about sampling frequency, signal correction by DPF, or the applied filter methods. Studies that reported these aspects of NIRS processing showed great variability. For example, 5 out of 20 studies reported that the DPF was used in cerebral analysis, and in 2 of them it represented different values (age-dependent factor or 5.93). In the muscle analysis, 3 studies reported the DPF, which again varied between studies. The utilization of a fixed DPF value among subjects, typically ranging between 3 and 6,2,58,73 is common in NIRS analyses. However, accurate assessment of hemodynamic changes depends on the use of precise DPF values in NIRS signal calculations.74 DPF values should ideally vary between subjects due to differences in the anatomical structure and tissue composition of the brain (e.g., skull thickness and cerebrospinal fluid)74 and muscle (e.g., subcutaneous adipose tissue thickness and muscle condition).75 The use of constant DPF does not account for possible variation between subjects; as a result, the hemodynamics estimation may not be accurately calculated, causing serious crosstalk errors.74,75 Hence, employing a constant DPF for all subjects is not recommended.7 Several studies have proposed equations or algorithms to precisely calculate the DPF.73, 74, 75, 76 However, to the best of our knowledge, there is no consensus on better DPF estimates. Further investigation is necessary to determine the ideal DPF algorithm or equation for both muscular and cerebral NIRS. Additionally, we recommend clearly documenting all processing steps to enhance study comparison and reproducibility.
It is notable that all studies have evaluated the frontal cortex. The focus on the frontal cortex instead of other cortex areas (e.g., motor cortex) during exercise is not exclusive, but it is prevalent for several reasons: (a) the frontal cortex is more accessible for analysis compared to other parts of the head or cortex, which may be covered by hair, potentially obstructing or absorbing NIRS light;77 (b) it is known that the PFC has projection to pre-motor areas and plays a crucial role in movement planning, goal-directed behavior, and decision-making;78 additionally, the PFC is associated with various features induced by exercise, such as affective processing,79 cognitive functions,80 and mood status;81 (c) previous study suggests that the patterns of hemodynamic responses to exercise are similar in the PFC and motor areas;82 and (d) as previously mentioned in the discussion, there is consistent evidence showing that inadequate PFC oxygenation is related to central fatigue and, consequently, impairs motor performance.6,20 These factors demonstrate the important contribution of PFC analysis during exercise. Nonetheless, future studies should explore other cortical areas, like motor areas, to gain a deeper understanding of the involvement of different brain regions during both maximal and submaximal exercise and their roles in performance.
Regarding muscle oxygenation, since previous studies showed different hemodynamic responses between muscles, further studies are necessary to provide information about the dynamic between O2 delivery and utilization in different sites of the body. In addition, cardiac output should be measured simultaneously with hemodynamic changes in different sites of the body for a better understanding of blood flow redistribution during exercise. Finally, although hemodynamic changes during cycling and knee extension exercises have been extensively researched, investigations into other exercises (e.g., treadmill, swimming) are scarce.
While we excluded studies that manipulated the basal condition of individuals (e.g., blood flow restriction and exercise in hypoxia) to eliminate any confounding effects, NIRS data analysis may offer crucial insights into O2 supply responses in various exercise contexts. Existing evidence demonstrates the significance of the NIRS system for comprehending cerebral and muscle hemodynamic responses during exercise, especially with additional manipulations affecting oxygenation status.6 For instance, previous studies have compared changes in muscle and cerebral tissue oxygenation between normoxia and hypoxia during exercise.28,83, 84, 85, 86, 87, 88 Therefore, a systematic understanding of both central and peripheral oxygenation changes in situations where O2 availability is limited could pave the way for further comprehensive reviews.
5. Conclusion
The present systematic review provides an overview of simultaneous O2 delivery and utilization in both muscle and cerebral tissue during exercise. Overall, muscle and cerebral oxygenation respond differently to exercise. While muscle shows an increase in O2 utilization (indicated by increased [HHb]) during exercise, cerebral tissue experiences an increase in O2 delivery (indicated by increased [HbO2]) with increased exercise intensity. These adjustments suggest an increase in O2 consumption to maintain muscle activity and a greater engagement of the PFC to sustain the exercise. However, when an individual reaches the exhaustion point, both muscle and cerebral oxygenation become compromised. This is characterized by a reduction in blood flow and a decrease in O2 extraction in the muscle; and, in the brain, the oxygenation reaches a plateau or decline, potentially resulting in motor failure during exercise. Additionally, our systematic review revealed that different muscles respond differently to exercise in terms of oxygenation (e.g., less active muscles exhibit greater deoxygenation than more active muscles); muscle but not cerebral deoxygenation differs between sexes; and O2 extraction is age- and training level-dependent.
Despite the relevant evidence compiled in this systematic review, there are some limitations that should be considered with caution when interpreting the data, such as variations in exercise protocols, analysis of only a few brain areas (i.e., predominantly frontal areas) and muscles (i.e., predominantly in one active muscle), small sample sizes, and insufficient information about data processing. Therefore, future studies are necessary to confirm the results identified in our review. Finally, based on this review, we believe that advances in knowledge in the area of exercise physiology and physical training will continue in an integrative way, further improving the interpretation of different types of exercise and intensities of effort, and their impacts on hemodynamic adjustments in the brain and muscles.
Acknowledgments
Acknowledgments
This work was supported by the São Paulo Research Foundation (FAPESP) (Grant Numbers 20/11946-6, 18/05821-6, 17/10201-4, 09/08535-5, 19/20894-2, and 19/10666-2), the Brazilian National Council for Scientific and Technological Development (CNPq) (Grant Numbers 164937/2020-0, 309832/2021-7, 308117/2018-2, 307718/2018-2, and 409521/2021-3), the Pró-Reitoria de Pesquisa (PROPe) of São Paulo State University (UNESP), and the IDOR/Pioneer Science Initiative (www.pioneerscience.org).
Authors’ contributions
DOS conceived the idea for the review; conducted search, study selection, data extraction, and quality assessment; and wrote the first draft of the manuscript; CAG and FBMG conceived the idea for the review and commented on previous versions of the manuscript; VSB, PCRS, FMR, ABM, and RV conducted search, study selection, data extraction, and quality assessment; and commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2024.03.003.
Supplementary materials
References
- 1.Herold F, Wiegel P, Scholkmann F, Müller N. Applications of functional near-infrared spectroscopy (fNIRS) neuroimaging in exercise–cognition science: A systematic, methodology-focused review. J Clin Med. 2018;7:466. doi: 10.3390/jcm7120466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrey S, Ferrari M. Muscle oximetry in sports science: A systematic review. Sport Med. 2018;48:597–616. doi: 10.1007/s40279-017-0820-1. [DOI] [PubMed] [Google Scholar]
- 3.Wolf M, Ferrari M, Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt. 2007;12 doi: 10.1117/1.2804899. [DOI] [PubMed] [Google Scholar]
- 4.Boone J, Vandekerckhove K, Coomans I, Prieur F, Bourgois JG. An integrated view on the oxygenation responses to incremental exercise at the brain, the locomotor and respiratory muscles. Eur J Appl Physiol. 2016;116:2085–2102. doi: 10.1007/s00421-016-3468-x. [DOI] [PubMed] [Google Scholar]
- 5.Pereira MIR, Gomes PSC, Bhambhani YN. A brief review of the use of near infrared spectroscopy with particular interest in resistance exercise. Sports Med. 2007;37:615–624. doi: 10.2165/00007256-200737070-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bhambhani Y. Application of near infrared spectroscopy in evaluating cerebral and muscle haemodynamics during exercise and sport. J Near Infrared Spectrosc. 2012;20:117–139. [Google Scholar]
- 7.Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol (1985) 2019;126:1360–1376. doi: 10.1152/japplphysiol.00166.2018. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari M, Quaresima V. Near infrared brain and muscle oximetry: From the discovery to current applications. J Near Infrared Spectrosc. 2012;20:1–14. [Google Scholar]
- 9.Fantini S, Sassaroli A. Frequency-domain techniques for cerebral and functional near-infrared spectroscopy. Front Neurosci. 2020;14:300. doi: 10.3389/fnins.2020.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos Trans R Soc A Math Phys Eng Sci. 2011;369:4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage. 2012;63:921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Scano A, Zanoletti M, Pirovano I, et al. NIRS-EMG for clinical applications: A systematic review. Appl Sci. 2019;9:2952. doi: 10.3390/app9152952. [DOI] [Google Scholar]
- 13.Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006;29:368–382. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehagnoul-Schipper DJ, van der Kallen BF, Colier WN, et al. Simultaneous measurements of cerebral oxygenation changes during brain activation by near-infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects. Hum Brain Mapp. 2002;16:14–23. doi: 10.1002/hbm.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijeakumar S, Huppert TJ, Magnotta VA, Buss AT, Spencer JP. Validating an image-based fNIRS approach with fMRI and a working memory task. Neuroimage. 2017;147:204–218. doi: 10.1016/j.neuroimage.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Esaki K, Hamaoka T, Rådegran G, et al. Association between regional quadriceps oxygenation and blood oxygen saturation during normoxic one-legged dynamic knee extension. Eur J Appl Physiol. 2005;95:361–370. doi: 10.1007/s00421-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 18.Hamaoka T, McCully KK. Review of early development of near-infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism. J Physiol Sci. 2019;69:799–811. doi: 10.1007/s12576-019-00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol (1985) 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 20.Perrey S. Non-invasive NIR spectroscopy of human brain function during exercise. Methods. 2008;45:289–299. doi: 10.1016/j.ymeth.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 22.Herold F, Wiegel P, Scholkmann F, Thiers A, Hamacher D, Schega L. Functional near-infrared spectroscopy in movement science: A systematic review on cortical activity in postural and walking tasks. Neurophotonics. 2017;4:41403. doi: 10.1117/1.NPh.4.4.041403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrey S. Evaluating brain functioning with NIRS in sports: Cerebral oxygenation and cortical activation are two sides of the same coin. Front Neuroergon. 2022;3 doi: 10.3389/fnrgo.2022.1022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Miyai I, Ono T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: An optical imaging study. Neuroimage. 2004;23:1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol (1985) 1999;87:1604–1608. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- 26.Bhambhani Y, Malik R, Mookerjee S. Cerebral oxygenation declines at exercise intensities above the respiratory compensation threshold. Respir Physiol Neurobiol. 2007;156:196–202. doi: 10.1016/j.resp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya K, Tanaka J, Kuboyama N, Murai S, Ogaki T. Cerebral cortex activity during supramaximal exhaustive exercise. J Sports Med Phys Fitness. 2004;44:215–219. [PubMed] [Google Scholar]
- 28.Subudhi AW, Dimmen AC, Roach RC. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol (1985) 2007;103:177–183. doi: 10.1152/japplphysiol.01460.2006. [DOI] [PubMed] [Google Scholar]
- 29.Matsui S, Tamura N, Hirakawa T, Kobayashi S, Takekoshi N, Murakami E. Assessment of working skeletal muscle oxygenation in patients with chronic heart failure. Am Heart J. 1995;129:690–695. doi: 10.1016/0002-8703(95)90317-8. [DOI] [PubMed] [Google Scholar]
- 30.Hanada A, Okita K, Yonezawa K, et al. Dissociation between muscle metabolism and oxygen kinetics during recovery from exercise in patients with chronic heart failure. Heart. 2000;83:161–166. doi: 10.1136/heart.83.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belardinelli R, Barstow TJ, Nguyen P, Wasserman K. Skeletal muscle oxygenation and oxygen uptake kinetics following constant work rate exercise in chronic congestive heart failure. Am J Cardiol. 1997;80:1319–1324. doi: 10.1016/s0002-9149(97)00672-3. [DOI] [PubMed] [Google Scholar]
- 32.Zandonai T, Tam E, Bruseghini P, et al. The effects of oral smokeless tobacco administration on endurance performance. J Sport Health Sci. 2018;7:465–472. doi: 10.1016/j.jshs.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marillier M, Rupp T, Bouzat P, et al. Cerebral haemodynamics and oxygenation during whole-body exercise over 5 days at high altitude. Exp Physiol. 2021;106:65–75. doi: 10.1113/EP088354. [DOI] [PubMed] [Google Scholar]
- 34.Puthon L, Bouzat P, Robach P, Favre-Juvin A, Doutreleau S, Verges S. Effect of ageing on hypoxic exercise cardiorespiratory, muscle and cerebral oxygenation responses in healthy humans. Exp Physiol. 2017;102:436–447. doi: 10.1113/EP085949. [DOI] [PubMed] [Google Scholar]
- 35.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4. Available at: www.training.cochrane.org/handbook. [accessed 01.09.2023].
- 37.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Available at:https://jbi-global-wiki.refined.site/space/MANUAL/4687372/Chapter+7%3A+Systematic+reviews+of+etiology+and+risk. [accessed 01.09.2023].
- 38.Buzza G, Lovell GP, Askew CD, Solomon C. A comparison of V̇O 2, and muscle and prefrontal cortex tissue oxygen extraction between short and long-term aerobically trained men aged 40–60 years. Int J Exerc Sci. 2020;13:964–978. doi: 10.70252/AVNW2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caen K, Vermeire K, Pogliaghi S, et al. Aerobic interval training impacts muscle and brain oxygenation responses to incremental exercise. Front Physiol. 2019;10:1195. doi: 10.3389/fphys.2019.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherouveim ED, Margaritelis NV, Koulouvaris P, et al. Skeletal muscle and cerebral oxygenation levels during and after submaximal concentric and eccentric isokinetic exercise. J Sports Sci. 2022;40:195–202. doi: 10.1080/02640414.2021.1983248. [DOI] [PubMed] [Google Scholar]
- 41.Ganesan G, Cotter J, Reuland W, et al. Use of diffuse optical spectroscopy to monitor muscle and brain oxygenation dynamics during isometric and isokinetic exercise. Optical Tomography and Spectroscopy of Tissue X. 2013;8578 doi: 10.1117/12.2007459. [DOI] [Google Scholar]
- 42.Kounalakis SN, Geladas ND. Cardiovascular drift and cerebral and muscle tissue oxygenation during prolonged cycling at different pedalling cadences. Appl Physiol Nutr Metab. 2012;37:407–417. doi: 10.1139/h2012-011. [DOI] [PubMed] [Google Scholar]
- 43.Oka N, Asgher U. Changes in prefrontal cortex and skeletal muscle metabolism associated with muscle fatigue: An fnirs study. Available at:https://www.scopus.com/inward/record.uri?eid=2-s2.0-85088266350&doi=10.1007%2F978-3-030-51041-1_32&partnerID=40&md5=ed29cc88e343f41a9a430e86f2ba9b72 10.1007/978-3-030-51041-1_32. [accessed 09.02.2023]. [DOI]
- 44.Perentis PA, Cherouveim ED, Malliou VJ, et al. The effects of high-intensity interval exercise on skeletal muscle and cerebral oxygenation during cycling and isokinetic concentric and eccentric exercise. J Funct Morphol Kinesiol. 2021;6:62. doi: 10.3390/jfmk6030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rissanen A-PE, Tikkanen HO, Koponen AS, et al. Alveolar gas exchange and tissue oxygenation during incremental treadmill exercise, and their associations with blood O2 carrying capacity. Front Physiol. 2012;3:265. doi: 10.3389/fphys.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rupp T, Perrey S. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur J Appl Physiol. 2008;102:153–163. doi: 10.1007/s00421-007-0568-7. [DOI] [PubMed] [Google Scholar]
- 47.Rupp T, Jubeau M, Millet GY, et al. Muscle, prefrontal, and motor cortex oxygenation profiles during prolonged fatiguing exercise. Adv Exp Med Biol. 2013;789:149–155. doi: 10.1007/978-1-4614-7411-1_21. [DOI] [PubMed] [Google Scholar]
- 48.Buzza G, Lovell GP, Askew CD, Solomon C. The effect of short- and long-term aerobic training years on systemic O2 utilization, and muscle and prefrontal cortex tissue oxygen extraction in young women. J Strength Cond Res. 2019;33:2128–2137. doi: 10.1519/JSC.0000000000002512. [DOI] [PubMed] [Google Scholar]
- 49.Buzza G, Lovell GP, Askew CD, Kerhervé H, Solomon C. The effect of short and long term endurance training on systemic, and muscle and prefrontal cortex tissue oxygen utilisation in 40–60 year old women. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones B, Cooper CE. Near infrared spectroscopy (NIRS) observation of vastus lateralis (muscle) and prefrontal cortex (brain) tissue oxygenation during synchronised swimming routines in elite athletes. Adv Exp Med Biol. 2018;1072:111–117. doi: 10.1007/978-3-319-91287-5_18. [DOI] [PubMed] [Google Scholar]
- 51.Formenti D, Perpetuini D, Iodice P, et al. Effects of knee extension with different speeds of movement on muscle and cerebral oxygenation. PeerJ. 2018;6:e5704. doi: 10.7717/peerj.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhambhani Y, Fan J-L, Place N, Rodriguez-Falces J, Kayser B. Electromyographic, cerebral, and muscle hemodynamic responses during intermittent, isometric contractions of the biceps brachii at three submaximal intensities. Front Physiol. 2014;5:190. doi: 10.3389/fphys.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellwein L, Samyn MM, Danduran M, Schindler-Ivens S, Liebham S, LaDisa JF. Toward translating near-infrared spectroscopy oxygen saturation data for the non-invasive prediction of spatial and temporal hemodynamics during exercise. Biomech Model Mechanobiol. 2017;16:75–96. doi: 10.1007/s10237-016-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuura C, Gomes PSC, Haykowsky M, Bhambhani Y. Cerebral and muscle oxygenation changes during static and dynamic knee extensions to voluntary fatigue in healthy men and women: A near infrared spectroscopy study. Clin Physiol Funct Imaging. 2011;31:114–123. doi: 10.1111/j.1475-097X.2010.00986.x. [DOI] [PubMed] [Google Scholar]
- 55.Peltonen JE, Hägglund H, Koskela-Koivisto T, et al. Alveolar gas exchange, oxygen delivery and tissue deoxygenation in men and women during incremental exercise. Respir Physiol Neurobiol. 2013;188:102–112. doi: 10.1016/j.resp.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Pereira MIR, Gomes PSC, Bhambhani YN. Acute effects of sustained isometric knee extension on cerebral and muscle oxygenation responses. Clin Physiol Funct Imaging. 2009;29:300–308. doi: 10.1111/j.1475-097X.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 57.Racinais S, Buchheit M, Girard O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front Physiol. 2014;5:142. doi: 10.3389/fphys.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholkmann F, Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J Biomed Opt. 2013;18 doi: 10.1117/1.JBO.18.10.105004. [DOI] [PubMed] [Google Scholar]
- 59.Manchado-Gobatto FB, Marostegan AB, Rasteiro FM, et al. New insights into mechanical, metabolic and muscle oxygenation signals during and after high-intensity tethered running. Sci Rep. 2020;10:6336. doi: 10.1038/s41598-020-63297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calbet JAL, Gonzalez-Alonso J, Helge JW, et al. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol (1985) 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- 61.Cherouveim ED, Miliotis PG, Koskolou MD, Dipla K, Vrabas IS, Geladas ND. The effect of skeletal muscle oxygenation on hemodynamics, cerebral oxygenation and activation, and exercise performance during incremental exercise to exhaustion in male cyclists. Biology (Basel) 2023;12:981. doi: 10.3390/biology12070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (1985) 2009;107:1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 63.Perrey S, Thedon T, Bringard A. Application of near-infrared spectroscopy in preventing work-related musculoskeletal disorders: Brief review. Int J Ind Ergon. 2010;40:180–184. [Google Scholar]
- 64.Grotle A-K, Macefield VG, Farquhar WB, O'Leary DS, Stone AJ. Recent advances in exercise pressor reflex function in health and disease. Auton Neurosci. 2020;228 doi: 10.1016/j.autneu.2020.102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell JH, Smith SA. Unravelling the mysteries of the exercise pressor reflex at the cellular level. J Physiol. 2008;586:3025–3026. doi: 10.1113/jphysiol.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glace BW, Kremenic IJ, McHugh MP. Sex differences in central and peripheral mechanisms of fatigue in cyclists. Eur J Appl Physiol. 2013;113:1091–1098. doi: 10.1007/s00421-012-2516-4. [DOI] [PubMed] [Google Scholar]
- 67.Bassareo PP, Crisafulli A. Gender differences in hemodynamic regulation and cardiovascular adaptations to dynamic exercise. Curr Cardiol Rev. 2020;16:65–72. doi: 10.2174/1573403X15666190321141856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Landen S, Hiam D, Voisin S, Jacques M, Lamon S, Eynon N. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J Physiol. 2023;601:419–434. doi: 10.1113/JP279499. [DOI] [PubMed] [Google Scholar]
- 69.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda) 2015;30:30–39. doi: 10.1152/physiol.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 71.Palada I, Obad A, Bakovic D, Valic Z, Ivancev V, Dujic Z. Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath-holds. Respir Physiol Neurobiol. 2007;157:374–381. doi: 10.1016/j.resp.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Mulser L, Moreau D. Effect of acute cardiovascular exercise on cerebral blood flow: A systematic review. Brain Res. 2023;1809 doi: 10.1016/j.brainres.2023.148355. [DOI] [PubMed] [Google Scholar]
- 73.Kamran MA, Mannann MMN, Jeong MY. Differential path-length factor's effect on the characterization of brain's hemodynamic response function: A functional near-infrared study. Front Neuroinform. 2018;12:37. doi: 10.3389/fninf.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Talukdar T, Moore JH, Diamond SG. Continuous correction of differential path length factor in near-infrared spectroscopy. J Biomed Opt. 2013;18 doi: 10.1117/1.JBO.18.5.056001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pirovano I, Porcelli S, Re R, et al. Effect of adipose tissue thickness and tissue optical properties on the differential pathlength factor estimation for NIRS studies on human skeletal muscle. Biomed Opt Express. 2021;12:571–587. doi: 10.1364/BOE.412447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duncan A, Meek JH, Clemence M, et al. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res. 1996;39:889–894. doi: 10.1203/00006450-199605000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Friesen CL, Lawrence M, Ingram TGJ, et al. Portable wireless and fibreless fNIRS headband compares favorably to a stationary headcap-based system. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 79.Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 80.Fernandes RM, Correa MG, dos Santos MAR, et al. The effects of moderate physical exercise on adult cognition: A systematic review. Front Physiol. 2018;9:667. doi: 10.3389/fphys.2018.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monroe DC, Patel NR, McCully KK, Dishman RK. The effects of exercise on mood and prefrontal brain responses to emotional scenes in smokers. Physiol Behav. 2020;213 doi: 10.1016/j.physbeh.2019.112721. [DOI] [PubMed] [Google Scholar]
- 82.Subudhi AW, Miramon BR, Granger ME, Roach RC. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol (1985) 2009;106:1153–1158. doi: 10.1152/japplphysiol.91475.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rupp T, Perrey S. Effect of severe hypoxia on prefrontal cortex and muscle oxygenation responses at rest and during exhaustive exercise. Adv Exp Med Biol. 2009;645:329–334. doi: 10.1007/978-0-387-85998-9_49. [DOI] [PubMed] [Google Scholar]
- 84.Woorons X, Dupuy O, Mucci P, Millet GP, Pichon A. Cerebral and muscle oxygenation during repeated shuttle run sprints with hypoventilation. Int J Sports Med. 2019;40:376–384. doi: 10.1055/a-0836-9011. [DOI] [PubMed] [Google Scholar]
- 85.Peltonen JE, Kowalchuk JM, Paterson DH, et al. Cerebral and muscle tissue oxygenation in acute hypoxic ventilatory response test. Respir Physiol Neurobiol. 2007;155:71–81. doi: 10.1016/j.resp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Raberin A, Meric H, Mucci P, Lopez Ayerbe J, Durand F. Muscle and cerebral oxygenation during exercise in athletes with exercise-induced hypoxemia: A comparison between sea level and acute moderate hypoxia. Eur J Sport Sci. 2020;20:803–812. doi: 10.1080/17461391.2019.1669717. [DOI] [PubMed] [Google Scholar]
- 87.Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: Effect of oxygenation on maximal performance. Am J Physiol Circ Physiol. 2008;294:H164–H171. doi: 10.1152/ajpheart.01104.2007. [DOI] [PubMed] [Google Scholar]
- 88.Smith KJ, Billaut F. Influence of cerebral and muscle oxygenation on repeated-sprint ability. Eur J Appl Physiol. 2010;109:989–999. doi: 10.1007/s00421-010-1444-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.