Highlights
-
•
Cycling time trial decreases after caffeine supplementation intake.
-
•
Cycling performance is affected by CYP1A2 genotype.
-
•
Acute caffeine intake improves cycling time trial only in individuals with A allele.
-
•
Wingate and CMJ performance does not change after caffeine supplementation, irrespective of genotype.
Keywords: Countermovement jump test, Endurance, Ergogenic aid, Gene polymorphism, Wingate
Abstract
Background
The ergogenic effects of caffeine intake on exercise performance are well-established, even if differences exist among individuals in response to caffeine intake. The genetic variation of a specific gene, human cytochrome P450 enzyme 1A2 (CYP1A2) (rs762551), may be one reason for this difference. This systematic review and meta-analysis aimed to comprehensively evaluate the influence of CYP1A2 gene types on athletes' exercise performance after caffeine intake.
Methods
A literature search through 4 databases (Web of Science, PubMed, Scopus, and China National Knowledge Infrastructure) was conducted until March 2023. The effect size was expressed as the weighted mean difference (WMD) by calculating fixed effects meta-analysis if heterogeneity was not significant (I2 ≤ 50% and p ≥ 0.1). Subgroup analyses were performed based on AA and AC/CC genotype of CYP1A2.
Results
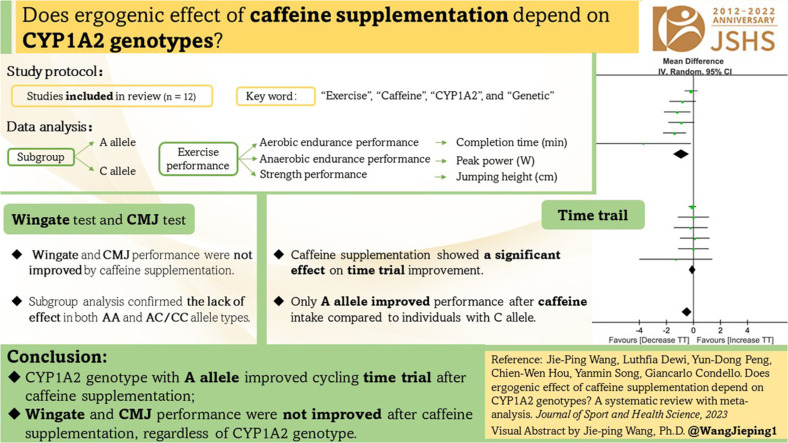
The final number of studies meeting the inclusion criteria was 12 (n = 666 participants). The overall analysis showed that the cycling time trial significantly improved after caffeine intake (WMD = –0.48, 95% confidence interval (95%CI): –0.83 to –0.13, p = 0.007). In subgroup analyses, acute caffeine intake improved cycling time trial only in individuals with the A allele (WMD = –0.90, 95%CI: –1.48 to –0.33, p = 0.002), but not the C allele (WMD = –0.08, 95%CI: –0.32 to 0.17, p = 0.53). Caffeine supplementation did not influence the Wingate (WMD = 8.07, 95%CI: –22.04 to 38.18, p = 0.60) or countermovement jump test (CMJ) performance (WMD = 1.17, 95%CI: –0.02 to 2.36, p = 0.05), and these outcomes were not influenced by CYP1A2 genotype.
Conclusion
Participants with the CYP1A2 genotype with A allele improved their cycling time trials after caffeine supplementation. However, compared to placebo, acute caffeine supplementation failed to increase the Wingate or CMJ performance, regardless of CYP1A2 genotype.
Graphical abstract
1. Introduction
Caffeine is a well-established ergogenic agent, known to enhance athletic performance.1 In particular, endurance performance can be improved when a dose ranging from 3 to 6 mg per kilogram of body weight is consumed.1,2 Although numerous studies have demonstrated an improvement in endurance performance (i.e., a reduction in time trial) and power performance (increase in jumping height) following caffeine supplementation,2, 3, 4 a substantial interindividual variability still exists in response to caffeine ingestion.5 Therefore, some individuals may experience beneficial effects from caffeine consumption, while others may experience no effects or even adverse reactions, such as palpitation and insomnia. In fact, a significant proportion of individuals fail to experience performance improvements, as nearly 30% of athletes report adverse effects (Table 1). This variability in responses could be partly attributed to genetic polymorphisms, which are among the factors contributing to individual differences.6 In particular, genetic variation of the human cytochrome P450 enzyme 1A2 (CYP1A2) gene (rs762551) has a substantial impact on both caffeine intake and caffeine metabolism.7
Table 1.
Individual responses to exercise performance after consuming caffeine with adverse effects.
| Study | Exercise mode | Caffeine dosage | Ingestion time | Sample size | Did not improve | Adverse effect |
|---|---|---|---|---|---|---|
| Acker-Hewitt et al. (2012)60 | 20-km cycling | 6 mg/kg | 60 min prior | 10 male cyclists | 4 participants | Not identified |
| Astorino et al. (2011)61 | 10-km cycling | 5 mg/kg | 60 min prior | 12 male cyclists | 4 participants | 5 participants (increased energy, anxiety, mild tremor, and nausea) |
| Astorino et al. (2012)62 | 8.2-km cycling | 6 mg/kg | 60 min prior | 10 young females | 4 participants | Not identified |
| Beaumont et al. (2017)63 | 60-min cycling | 6 mg/kg | 60 min prior | 8 healthy males | 2 participants | No adverse effects |
| Christensen et al. (2014)64 | 6-min maximal test on a rowing ergometer | 3 mg/kg | 60 min prior | 12 elite rowers (11 males and 1 female) | 3 participants | Not identified |
| Church et al. (2015)65 | 5-km running | 3 mg/kg | 60 min prior | 20 participants (10 males and 10 females) | 8 participants (4 males and 4 females) | Not identified |
| Desbrow et al. (2012)66 | Cycling time trial | 3 mg/kg | 90 min prior | 16 well-trained male cyclists | 2 participants | Not identified |
| 6 mg/kg | 4 participants | |||||
| Giráldez-Costas et al. (2022)67 | Backwards throw | 3 mg/kg | 45 min prior | 13 trained shot putters | 6 participants | 3 participants (nervousness, gastrointestinal problems, activeness, muscular pain, headache, and increased urine production) |
| Standing shot put | 2 participants | |||||
| Complete shot put | 3 participants | |||||
| Gonçalves et al. (2017)68 | Cycling time trial | 6 mg/kg | 60 min prior | 40 male cyclists | 20 participants | 16 participants (tachycardia, increased wakefulness and attention) |
| Graham-Paulson et al. (2016)69 | 3 × 20-m sprint tests | 4 mg/kg | 70 min prior | 12 male wheelchair rugby players | 5 participants | 5 participants (increased spasticity, struggling with decision-making, headaches, and nausea |
| Pitchford et al. (2014)70 | Cycling time trial | 3 mg/kg | 90 min prior | 9 well-trained male cyclists | 3 participants | Not identified |
| Potgieter et al. (2013)26 | Olympic-distance triathlon | 6 mg/kg | 60 min prior | 26 triathlon athletes (14 males and 12 females) | 6 participants | 11 participants (shakiness, heart palpitations, and gastro-intestinal tract disturbances). |
| Richardson et al. (2016)71 | Squat and bench press | 5 mg/kg | 60 min prior | 9 resistance-trained males | 3 participants | Not identified |
| Santos Rde et al. (2013)72 | 4-km cycling | 5 mg/kg | 60 min prior | 8 trained male cyclists | 2 participants | Not identified |
| Skinner et al. (2013)73 | 40-km cycling | 6 mg/kg | 60 min prior | 14 trained male cyclists and triathletes | 5 participants | 4 participants (headaches, increased alertness, nausea, light-headedness, and muscle cramping) |
| When peak serum caffeine concentrations will coincide with onset of exercise. | 9 participants | 4 participants (headaches, increased alertness, nausea, light-headedness, and muscle cramping) | ||||
| Smirmaul et al. (2017)74 | Incremental exercise test on a cycle ergometer | 4 mg/kg | About 70 min prior | 7 male adults | 1 participant | Not identified |
| Stadheim et al. (2013)75 | 8-km cross-country double poling performance tests | 6 mg/kg | 75 min prior | 10 trained male cross-country skiers | 2 participants | Not identified |
| Dos Santos et al. (2023)39 | 8-km cross-country double poling time trial | 4.5 mg/kg | 73 min prior | 13 sub-elite male cross-country skiers | 3 participants | Not identified |
| Wang et al. (2022)76 | Sprint triathlon | 110 mg | 60 min prior | 12 male triathlon athletes | 5 participants | Not identified |
| 220 mg | 9 participants |
The CYP1A2 isozyme of cytochrome P450 is only present in the liver, where it represents around 15% of the total cytochromes. It is responsible for more than 90% of caffeine metabolism, and it decomposes caffeine into 3 metabolites: paraxanthine (81.5%), theobromine (10.0%), and theophylline (5.4%).8 The substitution of A to C at position 163 (rs762551) of the CYP1A2 genotype decreases enzyme inducibility after caffeine intake and determines the metabolic rate of caffeine.9 This rate is reflected in the ratio of caffeine to metabolites in plasma or urine.9 Individuals carrying the C allele (163A/C and 163C/C) are categorized as “slow metabolizers”, which demonstrate a slow clearance of caffeine because they metabolize caffeine at a slower rate compared to individuals homozygous for the A allele (163A/A), who are classified as “fast metabolizers”.10 It is worth noting that fast metabolizers constitute approximately 46% of the overall population.11 Therefore, the evidence shows that the A allele of the CYP1A2 genotype may be more responsive than the C allele is to caffeine ingestion.
The potential influence of polymorphism of CYP1A2 genotype on exercise performance in response to caffeine intake has been reviewed in previous work12 that covered all sources of caffeine intake, including caffeine gum and mouth rinse. A preferential benefit of A/A genotype in response to caffeine ingestion was found for trained individuals in 5 studies.13, 14, 15, 16, 17 However, one study showed that individuals with A/A genotype had a great occurrence of insomnia caused by caffeine intake compared with C allele carriers.16 One study on CYP1A2 genotypes also found that individuals with A/A genotype performed better than those with C allele.18 Contrary to this result, a study found that people with A/C genotype performed better in the 3-km time trial than those with A/A genotype.19 In addition, other studies found that there was no difference in the performance of different genotypes after caffeine intake.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 The inconclusive nature of these results could be attributed to factors such as small sample sizes, variations in caffeine delivery methods, differences in training status, and diverse exercise performance outcomes.
To comprehensively assess the impact of CYP1A2 genotypes on exercise performance following an acute caffeine supplementation, a systematic review with meta-analysis is essential. Since the variety of pre-exercise caffeine intervention (i.e., ingesting, mouth rinse) may cause different responses, we only focused on the intervention of caffeine ingestion. Moreover, subgroup analyses are required to account for genetic variations, ensuring a detailed evaluation of the role of CYP1A2 genotypes in athletes' performance enhancement through caffeine intake.
2. Methods
2.1. Study protocol and registration
The present work has been registered to the International Prospective Register for Systematic Reviews (PROSPERO; registration number: CRD42022376187).
The literature search was performed for relevant studies (including a range of publications through to March 2023) across 4 databases: Web of Science, PubMed, Scopus, and China National Knowledge Infrastructure, according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline.31 The keywords included “exercise”, “caffeine”, “CYP1A2”, and “genetic”.
2.2. Inclusion and exclusion criteria
Studies were selected based on the Population, Intervention, Comparison, Outcomes and Study model (Table 2).32 Intervention studies conducting caffeine ingestion were included. The primary outcome of the present meta-analysis included CYP1A2 genotypes, while the secondary outcome was exercise performances. Studies were excluded if: (a) CYP1A2 genotypes were not classified; (b) there was a mixed intervention (i.e., if caffeine was not the only supplement); (c) exercise performances were not measured; (d) placebo data were not available; and (e) there was a lack of detailed results and full-text data.
Table 2.
Eligibility criteria of studies based on the PICOS model.
| Parameter | Inclusion criteria |
|---|---|
| Population | Healthy individuals |
| Intervention | Acute caffeine ingestion |
| Comparator | Placebo |
| Outcome | Exercise performance |
| Study design | Randomized and non-randomized control trials |
Abbreviation: PICOS = Population, Intervention, Comparator, Outcome, Study design.
2.3. Data extraction
The initial review of records from all databases and the eligibility of studies were conducted by the primary investigator, then those results were confirmed by at least 2 separate investigators. The records were imported into Endnote (Version X9.3.3; Clarivate Analytic, Philadelphia, PA, USA) and were automated and manually screened. Once the screening of the included studies was finalized, the data were categorized by the characteristics of participants (sample size, age, and sex) and the exercise mode. The outcome data were expressed as the weighted mean difference (WMD). If the full-text article data were presented only in a figure format, WebPlotDigitizer (Ankit Rohatgi, 2019, V.4.2; WebPlotDigitizer, Pacifica, CA, USA) was used to extract the data from the figures.
2.4. Data analysis
A subgroup analysis was conducted to distinguish the outcomes based on different CYP1A2 genotype for individual exercise performance. CYP1A2 genotypes were categorized into AA and AC/CC. Exercise performance included aerobic endurance performance, anaerobic endurance performance, and strength performance. Aerobic endurance performance was determined as the completion time (expressed in min) during cycling time trial. Anaerobic endurance performance was measured by peak power (expressed in W) of Wingate test, while strength performance was defined by jumping height (expressed in cm) of a countermovement jump test (CMJ).
2.5. Quality assessment
The quality of the included studies was assessed using 5 domains according to the revised Cochrane Risk of Bias tool for randomized trials: (a) randomization process, (b) deviations from intended interventions, (c) missing outcome data, (d) measurement of the outcome, and (e) selection of the reported result. The overall risk-of-bias was defined as “low risk” if all domains were at low risk of bias, “some concerns” if containing at least 1 domain at some concerns status but not at high risk of bias for any domain, and “high risk” if at least 1 study was judged in some concerns for multiple domains.33
2.6. Statistical analysis
Meta-analysis was performed using Review Manager (RevMan Version 5.4.1; Cochrane, London, UK). Due to the involvement of continuous data (mean ± SD), WMD with 95% confidence intervals (95%CIs) were used to represent the final analysis. Means and SD from placebo and caffeine conditions were collected. Subgroup analyses were performed for outcomes in relation to the type of CYP1A2 genotype. The forest plots were produced to display WMD, SD, and the overall effect of Z score. If publications reported the standard error only, the SD was calculated using the following formula, where n represented the number of participants:
To assess the heterogeneity, tau-squared (τ2), χ2 Cochran's Q (x2) test, and I2 statistic were performed. A value of τ2 > 1 indicated variability between studies. Q test measured the variation around a weighted mean, in which p < 0.10 was considered as significant heterogeneity.34 I2 statistic was used to assess the effect consistency across the studies, with I2 interpreted as follows: (a) I2: 0%–29% showing no important heterogeneity, (b) I2: 30%–49% showing moderate heterogeneity, (c) I2: 50%–74% showing substantial heterogeneity, (d) I2: 75%–100% showing considerable heterogeneity.35,36 Meta-analysis was performed using a fixed-effects model when heterogeneity was not significant (I2 ≤ 50% and p ≥ 0.1) and a random-effects model was used when heterogeneity was significant (I2 > 50% or p < 0.1). p < 0.05 was considered statistically significant.
3. Results
3.1. Literature search
3.1.1. Selection process
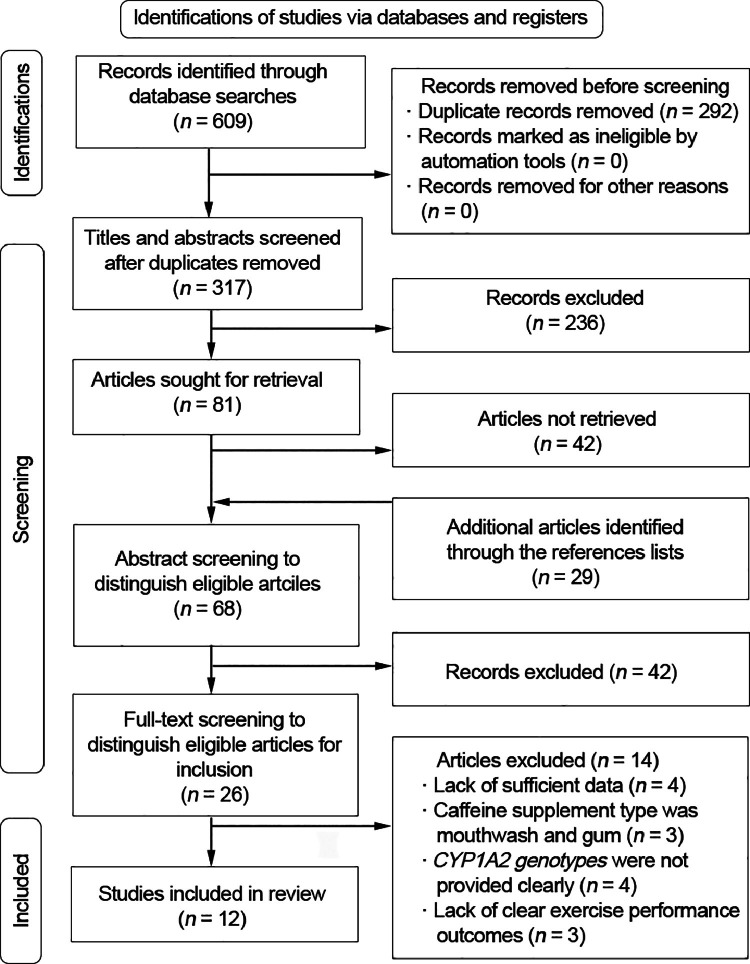
The number of identified articles from the 4-database search and selection process are shown in Fig. 1. A total of 609 intervention studies were retrieved from the database search, and 292 duplicated and ineligible articles were excluded. After the screening phase, including title and abstract screening, 26 articles were left. The authors excluded 14 articles from this meta-analysis due to: (a) lack of sufficient data,14,20,21,25 (b) caffeine supplement type was mouthwash and gum,19,37,38 (c) CYP1A2 genotypes were not clearly provided,26,28,39,40 and (d) lack of clear exercise performance outcomes.24,41,42 The screening resulted in 12 eligible articles that were used for the current quantitative analysis.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram defining the electronic search and selection process.
3.1.2. Quality assessment in individual studies
Among the included studies, no study scored in the high-risk bias, 3 studies scored in the moderate-risk bias,15,29,43 and 9 studies scored in the low-risk bias.13,16, 17, 18,22,23,27,30,44 Results of the quality assessment are shown in Supplementary Fig. 1.
3.1.3. Characteristics of studies
The characteristics of the studies included in the meta-analysis are summarized in Table 3.13,15, 16, 17, 18,22,23,27,29,30,43,44 For all studies the sample was composed of young adults (aged 15 ± 2 to 29 ± 7 years) comprising active (n = 525) and sedentary (n = 141) individuals. The dosages of caffeine supplementation varied from 2 mg/kg to 6 mg/kg and were ingested acutely before conducting the performance test. The exercise regimen in the current meta-analysis consisted of cycling time trial (n = 4),13,17,22,44 Wingate test (n = 4),18,23,27,30 and CMJ test (n = 5).15,16,23,29,43
Table 3.
Characteristics of studies included in the meta-analysis.
| Study | Information of participants |
Dosage (mg/kg) |
Exercise mode | Outcome | Conclusion | ||
|---|---|---|---|---|---|---|---|
| Sample size and sex (male/female) |
Genetic ratio A/C |
Age (year) (mean ± SD) |
|||||
| Giersch et al. (2018)22 | 20 participants (20/0) |
8/12 | 25 ± 8 | 6 | 3-km cycling | Caffeine ingestion made a 2.2% improvement | Serum caffeine concentration C > A after 1 h; caffeine improved performance, but no difference between allele types |
| Grgic et al. (2020)23 | 22 trained males (20/0) | 13/9 | 28 ± 5 | 3 | CMJ test; Wingate test |
Caffeine ingestion enhanced power output in the Wingate test and vertical jump height | Caffeine improved performance, but no difference between allele types |
| Guest et al. (2018)13 | 101 athletes (101/0) |
49/52 | 24 ± 5 | 2; 4 | 10-km cycling | Performance increased 4.8% for 2 mg/kg caffeine and 6.8% for 4 mg/kg caffeine in participants with AA; performance decreased 13.7% for 4 mg/kg caffeine in participants with CC | Only those with the A allele improved |
| Guest et al. (2022)44 | 100 athletes (100/0) |
49/51 | 25 ± 4 | 2; 4 | 10-km cycling | 2 mg/kg caffeine decreased completion time by 1.7 min for participants with AA | Only those with the A allele improved |
| Minaei et al. (2022)18 | 16 trained males (16/0) |
6/10 | 22 ± 7 | 6 | Wingate test | Participants with AA improved 5.8% following caffeine ingestion | Only those with the A allele improved |
| Muñoz et al. (2020)15 | 31 handball players (16 /15) |
14/17 | 24 ± 3 | 3 | CMJ test; sprint test; handgrip strength test |
Jump height increased 3.4% for participants with AA and 4.3% for those with the C-allele | A > C when pitching 7 m after caffeine intake |
| Puente et al. (2018)16 | 19 basketball athletes (10/9) | 10/9 | 28 ± 5 | 3 | CMJ test | Caffeine increased jump height 2.9% in participants with AA; no significant effect for those with the C-allele | Only those with the A allele improved |
| Salinero et al. (2017)27 | 21 participants (14/7) |
5/16 | 29 ± 7 | 3 | Wingate test | Caffeine ingestion increased peak power by 1.7% | Caffeine improved performance, but no difference between allele types |
| Sicova et al. (2021)30 | 100 athletes (100/0) |
46/53 | 25 ± 4 | 2; 4 | Wingate test | No difference | Caffeine had no effect, and genes did not modify the effects of caffeine |
| Spineli et al. (2020)29 | 100 adolescents (no mention) | 49/51 | 16 ± 2 | 6 | handgrip strength test; CMJ test; |
No difference | Caffeine improved performance, but no difference on between allele types |
| Womack et al. (2012)17 | 35 cyclists (35/0) |
16/19 | 25 ± 7 | 6 | 40-km cycling | 4.9% decrease in completion time for participants with AA; completion time increased 1.8% in those with the C allele | The A allele showed a greater ergogenic effect |
| Wong et al. (2021)43 | 102 athletes (102/0) |
50/52 | 25 ± 4 | 2; 4 | CMJ test; handgrip strength test | Participants with CC experienced a 12.8% decrease in handgrip strength with 4 mg/kg of caffeine | Handgrip strength in those with the CC genotype declined in response to 4 mg/kg of caffeine |
Abbreviation: CMJ = countermovement jump test.
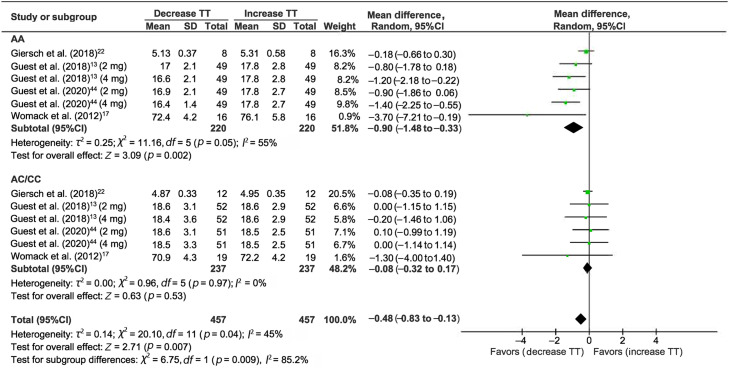
3.2. The effect of caffeine on the time trial
A total of 4 studies measured CYP1A2 genotype differences for cycling time trial after caffeine supplementation (Fig. 2).13,17,22,44 Regardless of the allele genotype, caffeine supplementation showed a small but significant effect on time trial improvement (WMD = –0.48, 95%CI: –0.83 to –0.13, p = 0.007). However, the subgroup analysis demonstrated a large effect size among individuals with the A allele who ingested caffeine (WMD = –0.90, 95%CI: –1.48 to –0.33, p = 0.002) compared to individuals with the C allele, who did not confirm the effect on time trial when caffeine was ingested (WMD = –0.08, 95%CI: –0.32 to 0.17, p = 0.53). In the present meta-analysis, the average decrease in completion time among individuals with the A allele following caffeine ingestion across the included studies was 5.8%, whereas it was only 0.6% for individuals with the C allele.
Fig. 2.
Forest plot of standardized mean difference for caffeine intake on TT outcome. 95%CI = 95% confidence interval; df = degree of freedom; I2 = inconsistency between studies; TT = time trial; WMD = standardized mean difference.
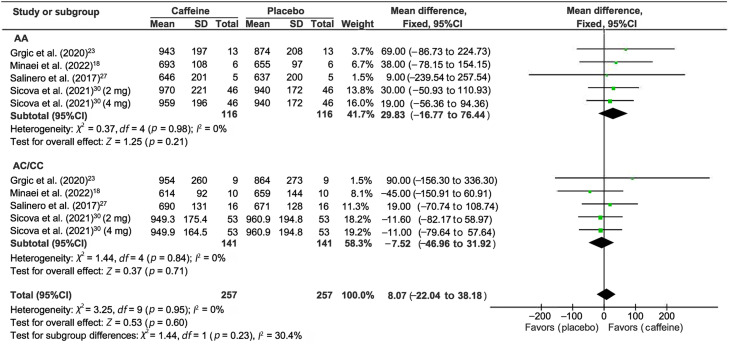
3.3. The effect of caffeine on the Wingate test
Four studies measured CYP1A2 genotype differences for the Wingate test after caffeine supplementation (Fig. 3).18,23,27,30 When gene type was not considered, caffeine supplementation did not have a beneficial effect on the Wingate performance (WMD = 8.07, 95%CI: –22.04 to 38.18, p = 0.60). Subgroup analysis confirmed the lack of effect in both AA (WMD = 29.83, 95%CI: –16.77 to 76.44, p = 0.21) and AC/CC (WMD = –7.52, 95%CI: –46.96 to 31.92, p = 0.71) allele types.
Fig. 3.
Forest plot of standardized mean difference for caffeine intake on Wingate test outcome relative to placebo. 95%CI = 95% confidence interval; df = degree of freedom; I2 = inconsistency between studies; WMD = standardized mean difference.
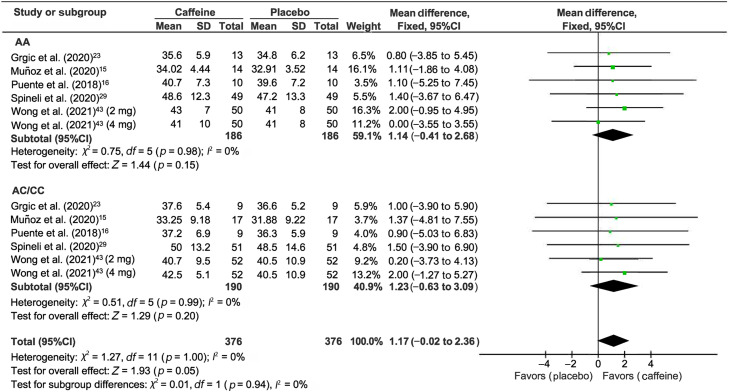
3.4. The effect of caffeine on the CMJ test
Five studies measured CYP1A2 genotype differences for the CMJ test after caffeine supplementation (Fig. 4).15,16,23,29,43 CMJ performance was not improved by caffeine supplementation (WMD = 1.17, 95%CI: –0.02 to 2.36, p = 0.05). Subgroup analysis confirmed the lack of effect in both AA (WMD = 1.14, 95%CI: –0.41 to 2.68, p = 0.15) and AC/CC (WMD = 1.23, 95%CI: –0.63 to 3.09, p = 0.20) allele types.
Fig. 4.
Forest plot of standardized mean differences for caffeine intake on CMJ outcome relative to placebo. 95%CI = 95% confidence interval; CMJ = countermovement jump test; df = degree of freedom; I2 = inconsistency between studies; WMD = standardized mean difference.
4. Discussion
To our knowledge, the present review provides a comprehensive quantitative analysis to confirm the polymorphism effect of CYP1A2 genotype on exercise performance in response to caffeine intake. In the current meta-analysis, we found that acute caffeine supplementation significantly decreased the completion time during a cycling time trial in individuals with the A allele but not in individuals with the C allele. We observed no significant effects of acute caffeine supplementation on peak power in the Wingate test or on the CMJ test, and these outcomes were not influenced by CYP1A2 genotype. These findings suggest that the influence of CYP1A2 genotype on caffeine metabolism is complex and may interact with specific aspects of athletic performance.
The current meta-analysis elucidated the impact of CYP1A2 genotype polymorphism on aerobic endurance. Notably, caffeine intake demonstrated performance improvements exclusively for participants with the AA genotype. In a 10 km cycling time trial, distinct genetic profiles revealed discernible disparities. Despite C allele carriers’ demonstrated higher serum caffeine concentrations compared to that of A allele carriers 1 h after intake, AA homozygotes exhibited a performance enhancement of approximately twice the amount as the C allele carriers.22 The greater magnitude of the effect was particularly pronounced among A allele carriers, suggesting a potential association with exercise duration.13,17,44 Although the precise mechanism through which caffeine enhances exercise performance remains to be fully elucidated, a leading hypothesis suggests that caffeine exerts a central effect by antagonizing adenosine receptors.45 This action inhibits the adverse effects of adenosine on nerve transmission, arousal, and pain perception. Additionally, caffeine stimulates skeletal muscle contractility through the excitation-contraction coupling mechanism.46,47 Biologically, caffeine acts as an adenosine receptor antagonist, alleviating endogenous adenosinergic inhibition, thereby triggering muscle contraction via calcium release.48,49 The genetic variability in CYP1A2 genotype may determine the 10-km cycling time trial outcome, as A allele carriers exhibit faster metabolism, resulting in a more rapid biological effect. Conversely, C allele carriers may necessitate a longer ingestion period to induce performance-enhancing effects. Moreover, theophylline and 1,7-dimethylxanthine (paraxanthine), which are metabolites of caffeine and adenosine antagonists, exhibit higher binding affinities for adenosine receptors and may play a bigger role in improving exercise performance than caffeine itself.50 This could explain the greater benefits for AA homozygotes compared to C allele carriers. Across the studies included in this meta-analysis, caffeine ingestion typically occurred about 60 min prior to exercise. Future research should consider both the duration of endurance exercise and the timing of caffeine intake before exercise to optimize performance outcomes.
For the current meta-analysis, peak power during the Wingate test was not improved by caffeine supplementation regardless of gene type. Caffeine doses may influence the efficacy of caffeine supplementation for improving anaerobic performance in individuals with the A allele. Among them, the study conducted by Sicova et al.30 demonstrated that performance did not improve in both supplemented groups with caffeine dosages of 2 mg/kg or 4 mg/kg. However, when participants ingested 6 mg/kg of caffeine, A allele carriers improved their anaerobic capacity.18 In addition, an improvement in exercise performance was obtained with the ingestion of 3 mg/kg of caffeine in 2 studies, without any influence of gene type.23,27 Up to now, the influence of genetic variability for the effect of caffeine ingestion on anaerobic capacity have remained inconclusive. A meta-analysis measuring the effect of caffeine ingestion on Wingate performance regardless of gene variability has been conducted previously, and it showed a small effect on enhancing power output.51 The discrepancy between our results and those of the previous meta-analysis can be explained by the fact that only studies considering gene variability were included in our meta-analysis.
When jumping performance was evaluated, the current meta-analysis demonstrates that caffeine intake did not improve jumping height, regardless of genotype. Among the included studies, caffeine intake increased jumping height of A allele participants only in the Puente study,16 while CYP1A2 genotype did not influence jumping height in most other studies.15,23,29,43 Although jumping ability after caffeine intake is not affected by gene type, improved sport-related performances in handball and basketball were observed in individuals with the A allele compared to those with the C allele after caffeine was supplemented.15,16
Our meta-analysis confirmed that the ergogenic effect of caffeine is associated with CYP1A2 genotype variability, providing evidence of the benefits of caffeine supplementation (with proper dosage and timing) before exercise training. The CYP1A2 activity influenced by the A allele has been previously reported.9 In addition, other factors can affect the activity of CYP1A2 genotypes,10,52 such as diet (cruciferous vegetables: broccoli and cabbage,53 and high curcumin foods54), smoking status,8 drinking habits,55 exogenous estrogens,53,56 oral contraceptives, and other drugs (fluvoxamine, omeprazole).57 In a previous meta-analysis, smoking habits was associated with the CYP1A2 activity in individuals with homozygous AA and heterozygous A/C.58 Therefore, it is crucial to note that dietary control and habits may influence study outcomes. However, for the studies included in the present meta-analysis, dietary records and smoking status were not reported. The choice of caffeine delivery method for enhancing exercise performance assumes an important value, given the varying absorption rates between ingestion and the chewing method.59 Importantly, ingestion involves an interaction with the gastrointestinal tract, potentially leading to an extended absorption period. Increasing peak power output in participants with the A allele after an acute caffeine supplementation with dosage 6 mg/kg had showed similar improvement with C allele.18 Therefore, more studies are required to confirm the results.
The limited number of included studies may lead to inaccurate interpretations of the anaerobic and power outcomes. Therefore, more studies are required to obtain consistency in the results. Our meta-analysis offers a comprehensive estimation of the effect for individual exercise categories, as well as an aggregated effect for subgroup analysis, to derive conclusions regarding caffeine's ergogenic effects with respect to distinct genetic alleles.
The results of previous studies collected up to the present time could guide us toward a new approach: Profiling endurance athletes with an aim to enhance the impact of caffeine consumption on their endurance performance, particularly before a competition. A higher caffeine dose could compensate for the slower metabolism associated with the C allele, potentially improving endurance performance in athletes who possess this genetic variant.
5. Conclusion
Regardless of the CYP1A2 variability, an acute caffeine supplementation improved cycling time trial but not Wingate or CMJ performance. When considering CYP1A2 genotype, only A allele carriers improved their performance in the cycling time trial; the Wingate and CMJ performances had no associations with gene type. Therefore, individual differences should be considered when consuming caffeine to enhance exercise performance.
Authors’ contributions
JW conceived the study, took part on the screening process and data extraction, and drafted the manuscript; LD took part on the screening process and data process and participated in writing the first draft on the manuscript; YP helped to draft the manuscript and performed the statistical analysis; CWH participated in its design; YS also participated in its design and assisted in drafting the manuscript; GC formulated the review criteria and helped to revise the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2023.12.005.
Supplementary materials
References
- 1.Southward K, Rutherfurd-Markwick KJ, Ali A. The effect of acute caffeine ingestion on endurance performance: A systematic review and meta-analysis. Sports Med. 2018;48:1913–1928. doi: 10.1007/s40279-018-0939-8. [DOI] [PubMed] [Google Scholar]
- 2.Guest NS, VanDusseldorp TA, Nelson MT, et al. International society of sports nutrition position stand: Caffeine and exercise performance. J Int Soc Sports Nutr. 2021;18:1. doi: 10.1186/s12970-020-00383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grgic J. Effects of caffeine on resistance exercise: A review of recent research. Sports Med. 2021;51:2281–2298. doi: 10.1007/s40279-021-01521-x. [DOI] [PubMed] [Google Scholar]
- 4.Shearer J, Graham TE. Performance effects and metabolic consequences of caffeine and caffeinated energy drink consumption on glucose disposal. Nutr Rev. 2014;72(Suppl. 1):S121–136. doi: 10.1111/nure.12124. [DOI] [PubMed] [Google Scholar]
- 5.Pickering C, Kiely J. Are the current guidelines on caffeine use in sport optimal for everyone? Inter-individual variation in caffeine ergogenicity, and a move towards personalised sports nutrition. Sports Med. 2018;48:7–16. doi: 10.1007/s40279-017-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southward K, Rutherfurd-Markwick K, Badenhorst C, Ali A. The role of genetics in moderating the inter-individual differences in the ergogenicity of caffeine. Nutrients. 2018;10:1352. doi: 10.3390/nu10101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl) 2010;211:245–257. doi: 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;(200):33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- 9.Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nehlig A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev. 2018;70:384–411. doi: 10.1124/pr.117.014407. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 12.Grgic J, Pickering C, Del Coso J, Schoenfeld BJ, Mikulic P. CYP1A2 genotype and acute ergogenic effects of caffeine intake on exercise performance: A systematic review. Eur J Nutr. 2021;60:1181–1195. doi: 10.1007/s00394-020-02427-6. [DOI] [PubMed] [Google Scholar]
- 13.Guest N, Corey P, Vescovi J. El-Sohemy A. Caffeine, CYP1A2 genotype, and endurance performance in athletes. Med Sci Sports Exerc. 2018;50:1570–1578. doi: 10.1249/MSS.0000000000001596. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi R. The effect of CYP1A2 genotype on the ergogenic properties of caffeine during resistance exercise: A randomized, double-blind, placebo-controlled, crossover study. Ir J Med Sci. 2019;188:337–345. doi: 10.1007/s11845-018-1780-7. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz A, López-Samanes Á, Aguilar-Navarro M, et al. Effects of CYP1A2 and ADORA2A genotypes on the ergogenic response to caffeine in professional handball players. Genes (Basel) 2020;11:933. doi: 10.3390/genes11080933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puente C, Abián-Vicén J, Del Coso J, Lara B, Salinero JJ. TheCYP1A2-163C>A polymorphism does not alter the effects of caffeine on basketball performance. PloS One. 2018;13:e0195943. doi: 10.1371/journal.pone.0195943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Womack CJ, Saunders MJ, Bechtel MK, et al. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J Int Soc Sports Nutr. 2012;9:7. doi: 10.1186/1550-2783-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minaei S, Jourkesh M, Kreider RB, et al. CYP1A2 genotype polymorphism influences the effect of caffeine on anaerobic performance in trained males. Int J Sport Nutr Exerc Metab. 2022;32:16–21. doi: 10.1123/ijsnem.2021-0090. [DOI] [PubMed] [Google Scholar]
- 19.Pataky MW, Womack CJ, Saunders MJ, et al. Caffeine and 3-km cycling performance: Effects of mouth rinsing, genotype, and time of day. Scand J Med Sci Sports. 2016;26:613–619. doi: 10.1111/sms.12501. [DOI] [PubMed] [Google Scholar]
- 20.Carswell AT, Howland K, Martinez-Gonzalez B, Baron P, Davison G. The effect of caffeine on cognitive performance is influenced by CYP1A2 but not ADORA2A genotype, yet neither genotype affects exercise performance in healthy adults. Eur J Appl Physiol. 2020;120:1495–1508. doi: 10.1007/s00421-020-04384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davenport AD, Jameson TSO, Kilroe SP, et al. A randomised, placebo-controlled, crossover study investigating the optimal timing of a caffeine-containing supplemeGiersch GEnt for exercise performance. Sports Med Open. 2020;6:17. doi: 10.1186/s40798-020-00246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giersch GE, Boyett JC, Hargens TA, et al. The effect of the CYP1A2− 163 C>A polymorphism on caffeine metabolism and subsequent cycling performance. J Caffeine Adenosine Res. 2018;8:65–70. [Google Scholar]
- 23.Grgic J, Pickering C, Bishop DJ, Schoenfeld BJ, Mikulic P, Pedisic Z. CYP1A2 genotype and acute effects of caffeine on resistance exercise, jumping, and sprinting performance. J Int Soc Sports Nutr. 2020;17:21. doi: 10.1186/s12970-020-00349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein CS, Clawson A, Martin M, et al. The effect of caffeine on performance in collegiate tennis players. J Caffeine Res. 2012;2:111–116. [Google Scholar]
- 25.McGrath MC. Massey University; Manawatu: 2015. The significance of CYP1A2 genotype on caffeine metabolism and exercise performance. [Master's thesis] [Google Scholar]
- 26.Potgieter S. Stellenbosch University; Stellenbosch: 2013. The effect of caffeine supplementation on Olympic-distance triathletes and triathlon performance in the Western Cape, South Africa. [Dissertation] [Google Scholar]
- 27.Salinero JJ, Lara B, Ruiz-Vicente D, et al. CYP1A2 genotype variations do not modify the benefits and drawbacks of caffeine during exercise: A pilot study. Nutrients. 2017;9:269. doi: 10.3390/nu9030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southward K. Massey University; Albany: 2016. Effect of caffeine ingestion on aspects of endurance performance and cognition in CYP1A2 heterozygous A/C male recreational athletes: A thesis presented in partial fulfilment for the requirements of a Master of Science in Sport and Exercise Science at Massey University. [Master's thesis] [Google Scholar]
- 29.Spineli H, Pinto MP, Dos Santos BP, et al. Caffeine improves various aspects of athletic performance in adolescents independent of their 163 C > A CYP1A2 genotypes. Scand J Med Sci Sports. 2020;30:1869–1877. doi: 10.1111/sms.13749. [DOI] [PubMed] [Google Scholar]
- 30.Sicova M, Guest NS, Tyrrell PN. El-Sohemy A. Caffeine, genetic variation and anaerobic performance in male athletes: A randomized controlled trial. Eur J Appl Physiol. 2021;121 doi: 10.1007/s00421-021-04799-x. 3499–13. [DOI] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown P, Brunnhuber K, Chalkidou K, et al. How to formulate research recommendations. BMJ. 2006;333:804–806. doi: 10.1136/bmj.38987.492014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Hoaglin DC. Misunderstandings about Q and “Cochran's Q test” in meta-analysis. Stat Med. 2016;35:485–495. doi: 10.1002/sim.6632. [DOI] [PubMed] [Google Scholar]
- 35.Higgins J, Thomas J, Chandler J, et al. Cochrane; Chichester: 2022. Cochrane handbook for systematic reviews of interventions. Version 6.3 (updated February 2022) [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Algrain HA, Thomas RM, Carrillo AE, et al. The effects of a polymorphism in the cytochrome P450 CYP1A2 gene on performance enhancement with caffeine in recreational cyclists. J Caffeine Res. 2016;6:34–39. [Google Scholar]
- 38.Figueiredo N, Queiroz M, Felício FP, et al. Acute caffeine mouth rinsing does not improve 10-km running performance in CYP1A2 C-allele carriers. Clin Nutr ESPEN. 2021;42:93–97. doi: 10.1016/j.clnesp.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Dos Santos MPP, Spineli H, Dos Santos BP, et al. The effect of caffeine on exercise performance is not influenced by ADORA2A genotypes, alone or pooled with CYP1A2 genotypes, in adolescent athletes. Eur J Nutr. 2023;62:1041–1050. doi: 10.1007/s00394-022-03045-0. [DOI] [PubMed] [Google Scholar]
- 40.Glaister M, Chopra K, Pereira De Sena AL, Sternbach C, Morina L, Mavrommatis Y. Caffeine, exercise physiology, and time-trial performance: No effect of ADORA2A or CYP1A2 genotypes. Appl Physiol Nutr Metab. 2021;46:541–551. doi: 10.1139/apnm-2020-0551. [DOI] [PubMed] [Google Scholar]
- 41.Yoshihara T, Zaitsu M, Shiraishi F, et al. Influence of genetic polymorphisms and habitual caffeine intake on the changes in blood pressure, pulse rate, and calculation speed after caffeine intake: A prospective, double blind, randomized trial in healthy volunteers. J Pharmacol Sci. 2019;139:209–214. doi: 10.1016/j.jphs.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Thomas RM, Algrain HA, Ryan EJ, et al. Influence of a CYP1A2 polymorphism on post-exercise heart rate variability in response to caffeine intake: A double-blind, placebo-controlled trial. Ir J Med Sci. 2017;186:285–291. doi: 10.1007/s11845-016-1478-7. [DOI] [PubMed] [Google Scholar]
- 43.Wong O, Marshall K, Sicova M, Guest NS, García-Bailo B. El-Sohemy A. CYP1A2 genotype modifies the effects of caffeine compared with placebo on muscle strength in competitive male athletes. Int J Sport Nutr Exerc Metab. 2021;31:420–426. doi: 10.1123/ijsnem.2020-0395. [DOI] [PubMed] [Google Scholar]
- 44.Guest NS, Corey P, Tyrrell PN, El-Sohemy A. Effect of caffeine on endurance performance in athletes may depend on HTR2A and CYP1A2 genotypes. J Strength Cond Res. 2022;36:2486–2492. doi: 10.1519/JSC.0000000000003665. [DOI] [PubMed] [Google Scholar]
- 45.Davis JK, Green JM. Caffeine and anaerobic performance: Ergogenic value and mechanisms of action. Sports Med. 2009;39:813–832. doi: 10.2165/11317770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Weber A, Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968;52:750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- 48.Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl. 1):S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 49.Szent-Györgyi AG. Calcium regulation of muscle contraction. Biophys J. 1975;15:707–723. doi: 10.1016/S0006-3495(75)85849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daly JW, Butts-Lamb P, Padgett W. Subclasses of adenosine receptors in the central nervous system: Interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grgic J. Caffeine ingestion enhances Wingate performance: A meta-analysis. Eur J Sport Sci. 2018;18:219–225. doi: 10.1080/17461391.2017.1394371. [DOI] [PubMed] [Google Scholar]
- 52.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: Influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–637. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- 53.Le Marchand L, Sivaraman L, Franke AA, et al. Predictors of N-acetyltransferase activity: Should caffeine phenotyping and NAT2 genotyping be used interchangeably in epidemiological studies? Cancer Epidemiol Biomarkers Prev. 1996;5:449–455. [PubMed] [Google Scholar]
- 54.Chen Y, Liu WH, Chen BL, et al. Plant polyphenol curcumin significantly affects CYP1A2 and CYP2A6 activity in healthy, male Chinese volunteers. Ann Pharmacother. 2010;44:1038–1045. doi: 10.1345/aph.1M533. [DOI] [PubMed] [Google Scholar]
- 55.Heinz AJ, de Wit H, Lilje TC, Kassel JD. The combined effects of alcohol, caffeine, and expectancies on subjective experience, impulsivity, and risk-taking. Exp Clin Psychopharmacol. 2013;21:222–234. doi: 10.1037/a0032337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eugster HP, Probst M, Würgler FE, Sengstag C. Caffeine, estradiol, and progesterone interact with human CYP1A1 and CYP1A2. Evidence from cDNA-directed expression in Saccharomyces cerevisiae. Drug Metab Dispos. 1993;21:43–49. [PubMed] [Google Scholar]
- 57.Culm-Merdek KE, von Moltke LL, Harmatz JS, Greenblatt DJ. Fluvoxamine impairs single-dose caffeine clearance without altering caffeine pharmacodynamics. Br J Clin Pharmacol. 2005;60:486–493. doi: 10.1111/j.1365-2125.2005.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koonrungsesomboon N, Khatsri R, Wongchompoo P, Teekachunhatean S. The impact of genetic polymorphisms on CYP1A2 activity in humans: A systematic review and meta-analysis. Pharmacogenomics J. 2018;18:760–768. doi: 10.1038/s41397-017-0011-3. [DOI] [PubMed] [Google Scholar]
- 59.Kamimori GH, Karyekar CS, Otterstetter R, et al. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm. 2002;234:159–167. doi: 10.1016/s0378-5173(01)00958-9. [DOI] [PubMed] [Google Scholar]
- 60.Acker-Hewitt TL, Shafer BM, Saunders MJ, Goh Q, Luden ND. Independent and combined effects of carbohydrate and caffeine ingestion on aerobic cycling performance in the fed state. Appl Physiol Nutr Metab. 2012;37:276–283. doi: 10.1139/h11-160. [DOI] [PubMed] [Google Scholar]
- 61.Astorino TA, Cottrell T, Lozano AT, Aburto-Pratt K, Duhon J. Ergogenic effects of caffeine on simulated time-trial performance are independent of fitness level. J Caffeine Res. 2011;1:179–185. [Google Scholar]
- 62.Astorino TA, Roupoli LR, Valdivieso BR. Caffeine does not alter RPE or pain perception during intense exercise in active women. Appetite. 2012;59:585–590. doi: 10.1016/j.appet.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Beaumont RE, James LJ. Effect of a moderate caffeine dose on endurance cycle performance and thermoregulation during prolonged exercise in the heat. J Sci Med Sport. 2017;20:1024–1028. doi: 10.1016/j.jsams.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Christensen PM, Petersen MH, Friis SN, Bangsbo J. Caffeine, but not bicarbonate, improves 6 min maximal performance in elite rowers. Appl Physiol Nutr Metab. 2014;39:1058–1063. doi: 10.1139/apnm-2013-0577. [DOI] [PubMed] [Google Scholar]
- 65.Church DD, Hoffman JR, LaMonica MB, et al. The effect of an acute ingestion of Turkish coffee on reaction time and time trial performance. J Int Soc Sports Nutr. 2015;12:37. doi: 10.1186/s12970-015-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desbrow B, Biddulph C, Devlin B, Grant GD, Anoopkumar-Dukie S, Leveritt MD. The effects of different doses of caffeine on endurance cycling time trial performance. J Sports Sci. 2012;30:115–120. doi: 10.1080/02640414.2011.632431. [DOI] [PubMed] [Google Scholar]
- 67.Giráldez-Costas V, Aguilar-Navarro M, González-García J, Del Coso J, Salinero JJ. Acute caffeine supplementation enhances several aspects of shot put performance in trained athletes. J Int Soc Sports Nutr. 2022;19:366–380. doi: 10.1080/15502783.2022.2096415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonçalves LS, Painelli VS, Yamaguchi G, et al. Dispelling the myth that habitual caffeine consumption influences the performance response to acute caffeine supplementation. J Appl Physiol (1985) 2017;123:213–220. doi: 10.1152/japplphysiol.00260.2017. [DOI] [PubMed] [Google Scholar]
- 69.Graham-Paulson TS, Perret C, Watson P, Goosey-Tolfrey VL. Improvement of sprint performance in wheelchair sportsmen with caffeine supplementation. Int J Sports Physiol Perform. 2016;11:214–220. doi: 10.1123/ijspp.2015-0073. [DOI] [PubMed] [Google Scholar]
- 70.Pitchford NW, Fell JW, Leveritt MD, Desbrow B, Shing CM. Effect of caffeine on cycling time-trial performance in the heat. J Sci Med Sport. 2014;17:445–449. doi: 10.1016/j.jsams.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Richardson DL, Clarke ND. Effect of coffee and caffeine ingestion on resistance exercise performance. J Strength Cond Res. 2016;30:2892–2900. doi: 10.1519/JSC.0000000000001382. [DOI] [PubMed] [Google Scholar]
- 72.Santos Rde A, Kiss MA, Silva-Cavalcante MD, et al. Caffeine alters anaerobic distribution and pacing during a 4000-m cycling time trial. PLoS One. 2013;8:e75399. doi: 10.1371/journal.pone.0075399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skinner TL, Jenkins DG, Taaffe DR, Leveritt MD, Coombes JS. Coinciding exercise with peak serum caffeine does not improve cycling performance. J Sci Med Sport. 2013;16:54–59. doi: 10.1016/j.jsams.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Smirmaul BP, de Moraes AC, Angius L, Marcora SM. Effects of caffeine on neuromuscular fatigue and performance during high-intensity cycling exercise in moderate hypoxia. Eur J Appl Physiol. 2017;117:27–38. doi: 10.1007/s00421-016-3496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stadheim HK, Kvamme B, Olsen R, Drevon CA, Ivy JL, Jensen J. Caffeine increases performance in cross-country double-poling time trial exercise. Med Sci Sports Exerc. 2013;45:2175–2183. doi: 10.1249/MSS.0b013e3182967948. [DOI] [PubMed] [Google Scholar]
- 76.Wang JP, Wei CC, Peng YD, et al. Dose caffeinated energy drink is a consideration issue for endurance performance. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.999811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.