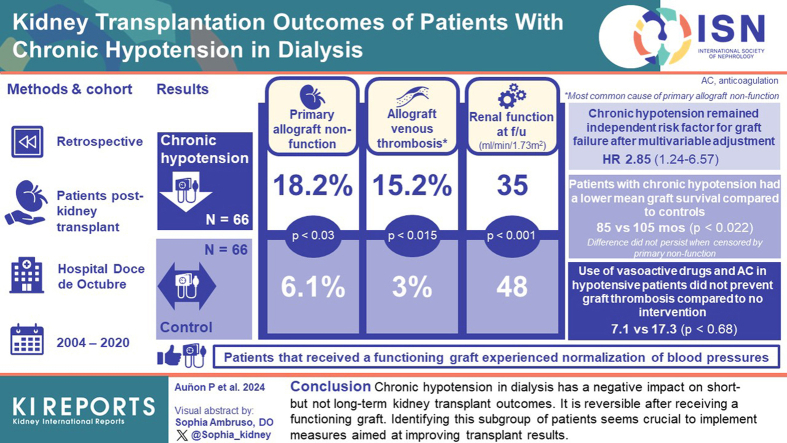
Abstract
Introduction
Persistent chronic hypotension affects 5–10% of dialysis patients. It seems to be reversible after receiving a functioning graft, but data regarding its influence on transplant outcomes are scarce. We analyze the evolution of patients with chronic hypotension in dialysis who undergo kidney transplantation at our center.
Methods
A retrospective observational study was conducted. Sixty-six patients with chronic hypotension (defined as systolic blood pressure ≤ 100 mm Hg at the time of transplantation) were identified. A control group of 66 non-hypotensive patients was assigned. The evolution of both groups was compared.
Results
Hypotensive patients had higher rates of primary non-function (18.2% vs. 6.1%; P = 0.03) mainly due to venous thrombosis of the allograft, worse renal function at the end of follow-up (eGFR of 35 mL/min/1.73 m2 vs 48 mL/min/1.73 m2, P = 0.001) but there was no statistical difference in graft survival after censoring for primary non-function. After multivariable adjustment, chronic hypotension remained an independent predictor factor for graft failure (adjusted HR of 2.85; 95% CI: 1.24–6.57; P = 0.014). Use of vasoactive drugs and anticoagulation in hypotensive patients was associated with 7.1% of venous graft thrombosis compared to 17.3% in those with no intervention (P = 0.68). Receiving a functioning graft implied blood pressure normalization in patients with chronic hypotension.
Conclusion
Chronic hypotension in dialysis has a negative impact on short-term kidney transplant outcomes but a lower impact on long-term results. It is reversible after receiving a functioning graft. Identifying this subgroup of patients seems crucial to implement measures aimed at improving transplant results.
Keywords: chronic hypotension, graft survival, kidney transplantation, outcomes, primary nonfunction, venous allograft thrombosis
Graphical abstract
See Commentary on Page 1571
Persistent chronic hypotension, defined as SBP < 100 mm Hg that is sustained between dialysis sessions,1 affects approximately 5% to 10% of patients on dialysis. It is more prevalent among patients on hemodialysis for a prolonged period of time. This hemodynamic condition is associated with high morbidity and has a negative impact on the quality of life of patients who suffer from it. Hypotensive patients frequently experience dizziness, asthenia, and weakness during the interdialytic period, symptoms that are exacerbated during dialysis sessions, hindering an adequate ultrafiltration and dialysis dose, together with a higher rate of vascular access complications such as arteriovenous fistulas thrombosis.2,3 Besides, several epidemiologic studies have linked low predialysis blood pressure with a higher mortality risk.4,5
The pathophysiology of persistent chronic hypotension is unknown. In most patients, there is no effective hypovolemia, structural heart disease, liver cirrhosis, dysautonomia, or adrenal insufficiency that justifies low blood pressure; and the underlying cause remains unidentified after a wide and systematic investigation. Chronic hypotension seems to be mediated by a decrease in peripheral vascular resistance with a preserved cardiac output, caused by functional and nonstructural changes, because high vascular compliance is evident. The uremic and proinflammatory environment would favor an overproduction of vasodilator agents (nitric oxide, adrenomedullin) followed by an increased sympathetic response and renin-angiotensin-aldosterone system activation, but without a sufficient effector response due to downregulation of adrenergic and angiotensin II receptors.2,3,6,7
In addition, baroreceptor dysfunction induced by uremia itself could contribute to the increased basal sympathetic activity in these patients, making it difficult to achieve a greater compensatory response in a situation of low blood pressure.3
There is no effective pharmacologic intervention for this complication. Midodrine, an oral α1 adrenergic agonist, has been used for this purpose based on small series and case reports, with diverse and discrete results.8, 9, 10
Most importantly, receiving a functioning graft seems to reverse persistent chronic hypotension. Several cases or series of patients with persistent hypotension have reported the normalization of blood pressure after kidney transplantation.1,10, 11, 12 Therefore, renal transplantation seems to be the only effective solution for persistent chronic hypotension in dialysis. However, this hemodynamic circumstance could make it difficult to perform a successful transplant. Data regarding the influence of this hemodynamic condition on kidney transplantation are scarce. Some studies suggest a deleterious effect of persistent chronic hypotension on kidney transplant outcomes, finding an association with a higher risk of delayed graft function (DGF) and PNF, and a lower death-censored graft survival in a subgroup of patients.13, 14, 15
In this study, we analyze the evolution of patients with persistent chronic hypotension in dialysis who underwent kidney transplantation at our center.
Methods
Patients
We conducted a retrospective observational study, in which we evaluated 2308 consecutive kidney transplants performed at the Hospital Doce de Octubre between 2004 and 2020. We identified 66 patients with “chronic hypotension” or “persistent hypotension” in their medical history who had SBP ≤ 100 mm Hg, not on antihypertensive treatment, at the time of transplantation (on a single reading, the day of hospital admission before transplant).
A control group of 66 nonhypotensive patients was assigned. For each hypotensive case we used as control (1:1), whenever possible, the other kidney recipient from the same donor to minimize the impact of donor source on transplantation outcomes (n = 38). For the rest of cases (n = 28), in which the contralateral kidney was discarded or sent to another transplant center without it being possible to obtain information on its evolution, we selected as control the nearest transplantation in time, with the same type of donor (donation after brain death or donation after circulatory death or living donation] and similar age (± 5 years old).
This study was conducted according to the Declaration of Helsinki. Institutional review board statement was waived because this study involved retrospective review of patient charts, the information was recorded by the investigator in a way that the identity of the human subjects could not be ascertained directly or through identifiers linked to the subjects, the investigator did not contact the subjects, and the investigator would not reidentify subjects.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Use of Vasoactive Drugs and Anticoagulation
Fourteen patients with chronic hypotension were treated with vasoactive drugs and prophylactic anticoagulation after kidney transplantation. The aim was to improve graft perfusion, and thus minimize the risk of acute tubular necrosis and graft thrombosis, eventually avoiding the PNF of the graft.
Use of vasoactive drugs and anticoagulation to prevent thrombosis-associated PNF was implemented on an individual basis by the treating physician and not based on a standard protocol.
Noradrenaline was initiated in the immediate posttransplant period to achieve mean arterial pressure ≥ 70 mm Hg and SBP ≥ 100 to 110 mm Hg. Low-molecular weight heparin (enoxaparin 20–40 mg subcutaneous per day) was administered from day 2 or day 3 after transplantation depending on the individual risk of bleeding. Both were maintained until graft function was observed.
Aims and Outcomes
Primary End Point
The primary end point was to compare the evolution of both groups in terms of PNF; graft thrombosis; delay in graft function; serum creatinine at months 1, 3, 6, 12 and at the end of follow-up; and renal graft survival.
Secondary End Points
The effectiveness of vasoactive drugs and anticoagulation in preventing venous graft thrombosis (and therefore PNF) was evaluated in a subgroup of hypotensive patients (n = 14). Blood pressure evolution after successful kidney transplantation among patients with chronic hypotension was also analyzed. In addition, we assessed pretransplant and posttransplant factors predicting renal graft survival.
Follow-Up and Data Collection
The baseline (at the time of presentation) and follow-up data were collected retrospectively from medical records following a uniform protocol and included recipient, donor, and transplantation variables. All unexplained renal dysfunction episodes were evaluated by renal biopsy and classified based on Banff criteria valid at that time (2003–2019)16, 17, 18, 19, 20, 21, 22, 23 and were treated according to histologic findings.
Definitions
PNF was defined as permanent lack of graft function from the time of transplantation that led to the continuation of chronic dialysis or retransplantation.
DGF was defined as the need for dialysis during the first week after renal transplantation. Graft survival was defined as the absence of end-stage renal disease. End-stage renal disease was defined as an estimated glomerular filtration rate < 15 ml/min per 1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration equation) or a need for chronic dialysis.
Immunosuppressive Treatment
Patients received immunosuppressive regimen following our center protocol. All patients were treated with a triple drug regimen of glucocorticoids, tacrolimus, and mycophenolic acid or azathioprine as maintenance therapy. Induction was made with antithymocyte globulin in hyperimmunized recipients or those with a previous transplant, and anti-CD25 monoclonal antibody was used in living donors and those with extended criteria to reduce anticalcineurin exposition. Patients with a donor after circulatory death also received antithymocyte globulin, delaying the introduction of anticalcineurin until functional graft recovery.
Statistical Analysis
Continuous variables were expressed as mean ± SD. Categorical variables were shown as absolute and relative frequencies (percentage). Two-sample comparisons were performed with the use of the χ2 test or the nonparametric Fisher exact test for categorical variables, as appropriate. The t-test or Wilcoxon rank-sum test were used for continuous variables, as appropriate. Kaplan-Meier survival analysis was used to assess the time to reach end-stage kidney disease, and differences between groups (hypotensive patients vs. control) were compared by using the log-rank test. Cox proportional hazards models were used to analyze risk factors associated with kidney graft failure, using a backward stepwise process. The selection of covariates in multivariable models was based on univariate associations and biologic plausibility. All P-values were 2-tailed and a value < 0.05 was considered as criteria for statistical significance. Analyses and plots were performed using STATA version 12.1 (StataCorp, College Station, TX).
Results
Study Population
A total of 122 patients were included in the study, 66 hypotensive patients and 66 controls. Baseline characteristics of graft recipients are presented in Table 1. The mean recipient age was 55 years and there was male predominance (63.6%). Glomerulonephritis, hypertension or nephroangiosclerosis, chronic interstitial nephropathy, and diabetes were the most common causes of end-stage renal disease.
Table 1.
Graft recipients baseline characteristics in both groups
| Baseline | Hypotensive patients (n = 66) | Controls (n = 66) | P-value |
|---|---|---|---|
| Age, yr (mean ± SD) | 54.4 ± 13.7 | 56 ± 13.1 | 0.50 |
| Gender (males), n (%) | 39 (59.1%) | 45 (68.2) | 0.28 |
| Cause of ESRD, n (%) | 0.63 | ||
| Chronic interstitial nephropathy | 13 (19.7) | 6 (9.1) | |
| Glomerulonephritis | 11 (16.7) | 10 (15.3) | |
| Hypertension | 9 (13.6) | 11 (16.7) | |
| Diabetic nephropathy | 5 (7.6) | 11 (16.7) | |
| Polycystic kidney disease | 7 (10.6) | 9 (13.6) | |
| Vesicoureteral reflux | 3 (4.5) | 3 (5.8) | |
| Renal aplasia/ hypoplasia | 2 (3.8) | 4 (6.1) | |
| Lupus | 1 (1.5) | 0 (0) | |
| Others | 7 (10.6) | 2 (3.8) | |
| Unknown | 7 (10.6) | 8 (12.1) | |
| Presence of diabetes | 11 (16.7) | 15 (22.7) | 0.39 |
| Dialysis modality (hemodialysis / peritoneal dialysis/ both), n (%) | 55 (83.3) / 3 (4.5) / 7 (10.7) | 43 (65.2) / 17 (25.8) / 4 (6.1) | 0.06 |
| Months on dialysis | 65.7 | 57.9 | 0.014 |
| History of AVF thrombosis, n (%) | 23 (35.4) | 9 (13.6) | 0.004 |
| Antiphospholipid syndrome, n (%) | 1 (1.5) | 0 (0) | |
| History of previous transplants, n (%) | 27 (40.9) | 15 (22.7) | 0.025 |
| Hyperimmunized patients, n (%) | 13 (19.7) | 9 (13.6) | 0.35 |
| Blood type, n (%) | 0.59 | ||
| A | 30 (45.4) | 23 (34.8) | |
| B | 5 (7.6) | 8 (12.1) | |
| AB | 4 (6.1) | 5 (7.6) | |
| O | 27 (40.9) | 29 (43.9) | |
| Pretransplant BP, mm Hg (mean ± SD) | |||
| Systolic | 90.4 ± 9.9 | 139.2 ± 21.1 | <0.001 |
| Diastolic | 56.9 ± 11.7 | 80.2 ± 14.6 | <0.001 |
| Pretransplant hematocrit, % (mean ± SD) | 39.9 ± 5.5 | 38.6 ± 5.3 | 0.152 |
AVF, arteriovenous fistula; BP, blood pressure; ESRD, end-stage renal disease.
When comparing both groups (hypotensive patients and controls), there were no differences in the recipient age or gender, the cause of end-stage renal disease, the presence of diabetes, or the dialysis modality. However, patients with chronic hypotension had been on dialysis for a longer period of time compared to nonhypotensive patients (65.7 vs. 57.9 months; P = 0.014); they had, more often, a history of arteriovenous fistula thrombosis (35.4% vs. 13.6%; P = 0.004); and there were more patients in the hypotensive group who had received previous transplant(s) (40.9% vs. 22.7%; P = 0.025), but with this not implying a significant greater prevalence of hyperimmunization.
Baseline characteristics of donors and transplantation are shown in Table 2. No differences between both groups were observed. The mean donor age was 54 years and mean serum creatinine was 0.88 mg/dl. Regarding the source of kidneys, the vast majority (80.3%) came from donation after brain death followed by donation after circulatory death (15.2%) and LD (4.5%). Cold ischemia time was 18.6 hours.
Table 2.
Donor and transplantation baseline characteristics in both groups
| Baseline | Hypotensive patients (n = 66) | Controls (n = 66) | P-value |
|---|---|---|---|
| Donor age, yr (mean ± SD) | 54.5 ± 16.2 | 53.8 ± 16.2 | 0.80 |
| Donor gender males, n (%) | 33 (50) | 39 (59.1) | 0.41 |
| Donor serum creatinine, mg/dl (mean ± SD) | 0.90 ± 0.51 | 0.85 ± 0.39 | 0.52 |
| Cold ischemia time, h (mean ± SD) | 18.5 ± 6.2 | 18.7 ± 6.8 | 0.85 |
| Type of donor, n (%) | 1 | ||
| DBD | 53 (80.3) | 53 (80.3) | |
| EC-DBD | 27 (40.9) | 28 (42.4) | 1 |
| DCD | 10 (15.2) | 10 (15.2) | |
| Living donor | 3 (4.5) | 3 (4.5) | |
| Number of HLA mismatches (mean ± SD) | 4.1 ± 1.2 | 4 ± 1.4 | 0.554 |
DBD, donation after brain death; DCD, donation after circulatory death; EC-DBD, donation after brain death with expanded criteria; HLA, human leukocyte antigens.
Patients With Chronic Hypotension
The cause of hypotension remained unknown in most cases. Only 2 patients had structural heart disease that could contribute to chronic hypotension: one patient had rheumatic heart disease with mild-to-moderate tricuspid regurgitation, and another one suffered from idiopathic dilated cardiomyopathy with a left ventricular ejection fraction of 50% without associated valvular disease. Six patients had a history of adrenal insufficiency, and 5 of them were under hormone replacement therapy (fludrocortisone ± hydrocortisone), with low blood pressure persisting despite this treatment. No patient was receiving midodrine at the time of transplantation.
Short-Term Outcomes
DGF was significantly more frequent in patients with chronic hypotension than those in the control group (85.9% vs. 53.2%; P < 0.001) (Table 3). Hypotensive patients presented a higher rate of PNF, which was seen in 12 of 66 hypotensive patients (18.2%) compared to 4 of 66 patients (6.1%) in the control group (P = 0.03). This PNF was mainly due to venous thrombosis of the allograft, present in 10 hypotensive patients (15.2%) and in 2 patients in the control group (3%), (P = 0.015). In the remaining cases, PNF was explained by biopsy-proven acute tubular necrosis (observed in 2 hypotensive patient) or acute antibody-mediated rejection (which occurred in 2 control patients) (Table 3).
Table 3.
Outcomes
| Outcomes | Hypotensive patients (n = 66) | Controls (n = 66) | P-value |
|---|---|---|---|
| Delayed graft function, n (%) | 45 (85.9)a | 33 (53.2)b | <0.001 |
| Primary nonfunction, n (%) | 12 (18.2) | 4 (6.1) | 0.03 |
| Venous thrombosis of the renal allograft | 10 (15.2) | 2 (3) | 0.015 |
| Acute AMR | 0 (0) | 2 (3) | 0.45 |
| ATN | 2 (3) | 0 (0) | 0.45 |
| Serum creatinine, mg/dl (mean ± SD) | |||
| 1 mo | 2.7 ± 2.0 | 1.7 ± 0.7 | <0.001 |
| 3 mo | 1.8 ± 0.8 | 1.6 ± 0.5 | 0.04 |
| 12 mo | 1.6 ± 0.6 | 1.5 ± 0.5 | 0.20 |
| Last follow-up | 2.7 ± 2 | 1.7 ± 0.9 | 0.001 |
| eGFR, ml/min per 1.73 m2, (mean ± SD) | |||
| 1 mo | 33 ± 19 | 45 ± 20 | 0.006 |
| 3 mo | 42 ± 18 | 48 ± 19 | 0.11 |
| 12 mo | 46 ± 17 | 50 ± 17 | 0.16 |
| Last follow-up | 35 ± 21 | 48 ± 20 | 0.001 |
| Acute Rejection | 12 (18.2) | 14 (21.2) | 0.5 |
| AMR | 1 (1.5) | 3 (4.5) | |
| ACR | 11 (16.7) | 11 (16.7) | |
| Borderline | 4 (6) | 5 (7.6) | |
| Renal survival, yr (mean ± SD) | 0.008 | ||
| 1 yr | 78.6 | 90.8 | |
| 3 yr | 73.6 | 90.8 | |
| 5 yr | 61.6 | 87.1 | |
| 8 yr | 61.6 | 82.6 | |
| Renal survivalc, yr (mean ± SD) | 0.061 | ||
| 1 yr | 94.4 | 96.7 | |
| 3 yr | 88.3 | 96.7 | |
| 5 yr | 78.1 | 92.9 | |
| 8 yr | 74 | 88 | |
| Graft failure at the end of follow-up, % | 32.3 | 13.6 | 0.013 |
| Deaths, n (%) | 4 (6.1) | 7 (10.6) | 0.33 |
| Follow-up, mo (mean ± SD) | 42 ± 36 | 49 ± 35 | 0.19 |
ACR, acute cellular rejection; AMR, antibody-mediated rejection; ATN, acute tubular necrosis; eGFR, estimated glomerular filtration rate.
Primary nonfunction in 12 patients.
Primary nonfunction in 4 patients.
Renal survival censored by primary nonfunction.
Long-Term Outcomes
Renal Function
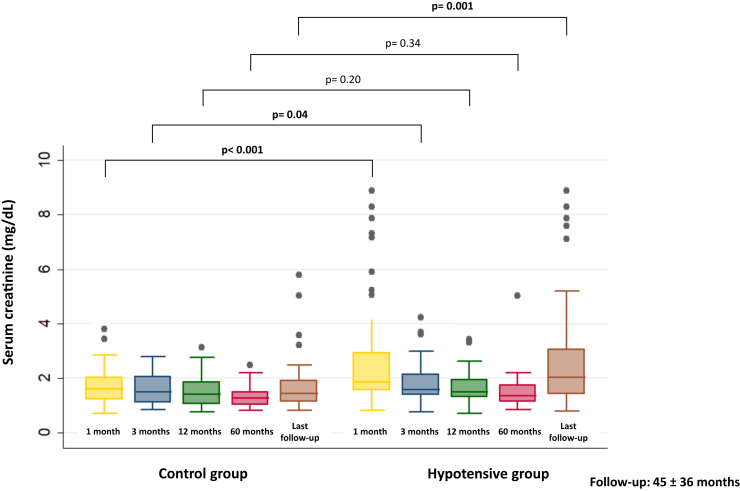
Patients with chronic hypotension had significantly worse serum creatinine and estimated glomerular filtration rate at 1 month after transplantation compared to controls (2.7 mg/dl vs. 1.7 mg/dl; P < 0.001 and 33 ml/min vs. 45 ml/min, P = 0.006, respectively). This difference was no longer statistically significant from month 3 after transplantation but became significant again at the end of follow-up, with a mean estimated glomerular filtration rate of 35 ml/min in the hypotensive group compared to 48 ml/min in the control group (P = 0.001). (Table 3, Figure 1)
Figure 1.
Evolution of serum creatinine in both groups.
Renal Survival
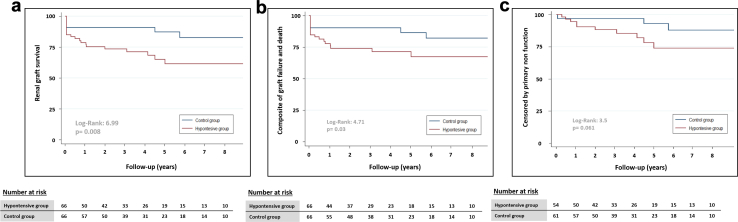
Patients with chronic hypotension had a mean graft survival of 81 months, which was significantly lower than the 105 months observed in control patients (P = 0.022). One, 3, 5 and 8-year graft survival was significantly worse in hypotensive patients (78.6%, 73.6%, 61.6%, and 61.6%, respectively vs. 90.8%, 90.8%, 87.1%, and 82.6% in control group; P = 0.008) (Table 3, Figure 2). There were differences when graft survival was censored for patient death (77.5%, 73.8%, 71.1%, and 67.1%, respectively in hypertensive patients vs. 90.2%, 90.2%, 86.6%, and 82%, respectively in control group). When graft survival was censored by PNF, this difference was no longer significant (Table 3, Figure 2). The mean follow-up period was similar in both groups (42 ± 36 months in hypotensive patients vs. 49 ± 35 months in controls; P = 0.19)
Figure 2.
Renal graft survival in both groups; (a) renal graft survival, (b) composite of graft failure and death, and (c) censored by primary nonfunction.
Acute Rejection
There were 26 biopsy-proven episodes of acute rejection (19.5%) (Table 3). Acute rejection episodes are described in Supplementary Table S1. Incidence of acute rejection was similar in both groups, with 12 events (18.2%) in 10 patients in the hypotensive group compared to 14 episodes in controls (21.2%) (P = 0.5). There was 1 episode of acute antibody-mediated rejection among the hypotensive patients and 3 episodes in the control group, despite the more common history of previous transplants in the hypotensive group. Acute rejection resulted in graft loss in 2 patients, both from the control group.
Impact of Chronic Hypotension on Graft Survival
The incidence of graft failure at the end of follow-up in patients with chronic hypotension was higher than in controls (32.3% vs. 13.6%; P = 0.013). Chronic hypotension was the only factor associated with kidney graft failure on univariable analysis. Patients with chronic hypotension who received a kidney transplant had a significantly higher risk of graft failure with a hazard ratio of 2.77 (95% confidence interval: 1.22–6.26; P = 0.01). After multivariable adjustment for recipient as well as donor and transplantation characteristics, chronic hypotension remained an independent predictor factor for kidney graft failure (adjusted hazard ratio of 2.85; 95% confidence interval:1.24–6.57; P = 0.014). (Table 4)
Table 4.
Univariate and multivariate analysis for predictor of kidney graft failure
| Variable | Univariable analysis: HR | P-value | Multivariable analysis: adjusted HR | P-value |
|---|---|---|---|---|
| Age of recipient, per 10 yr | 1.10 (0.83–1.45) | 0.51 | ||
| Male gender | 1.07 (0.51–2.27) | 0.86 | ||
| Age of donor, per 5 yr | 3.24 (0.97–10.90) | 0.057 | 1.15 (0.99–1.32) | 0.055 |
| Dialysis modality | 0.34 | |||
| Peritoneal dialysis | 1.00 (Reference) | |||
| Hemodialysis | 2.56 (0.60–10.8) | |||
| Both | 2.09 (0.29–14.85) | |||
| Time on dialysis, per yr | 1.01 (0.94–1.09) | 0.73 | 1.07 (0.97–1.18) | |
| History of thrombosis | 1.42 (0.63–3.25) | 0.41 | ||
| History of previous transplants | 0.55 (0.22–1.35) | 0.17 | 0.40 (0.12–1.33) | 0.13 |
| Chronic hypotension | 2.77 (1.22–6.26) | 0.01 | 2.85 (1.24–6.57) | 0.014 |
| Delayed graft function | 1.22 (0.38–4.01) | 0.73 | ||
| Vasoactive drugs + anticoagulation use | 1.21 (0.36–4.04) | 0.75 | ||
| HLA mismatches | 1.15 (0.86–1.53) | 0.33 | ||
| Type of donor | 0.60 | 0.10 | ||
| LD | 1.00 (Reference) | 1.00 (Reference) | ||
| DBD | 0.72 (0.17–3.13) | 0.38 (0.08–1.73) | ||
| DCD | 1.00 (0.21–4.85) | 0.63 (0.13–3.17) | ||
| Early acute rejection | 1.68 (0.68–4.13) | 0.28 | ||
| Late acute rejection | 0.50 (0.07–3.71) | 0.45 | ||
| Infection | 1.89 (0.41–8.62) | 0.41 |
DBD, donation after brain death; DCD, donation after circulatory death; HLA, human leukocyte antigens; HR, hazard ratio; LD, living donor.
Use of Vasoactive Drugs and Anticoagulation in Hypotensive Patients
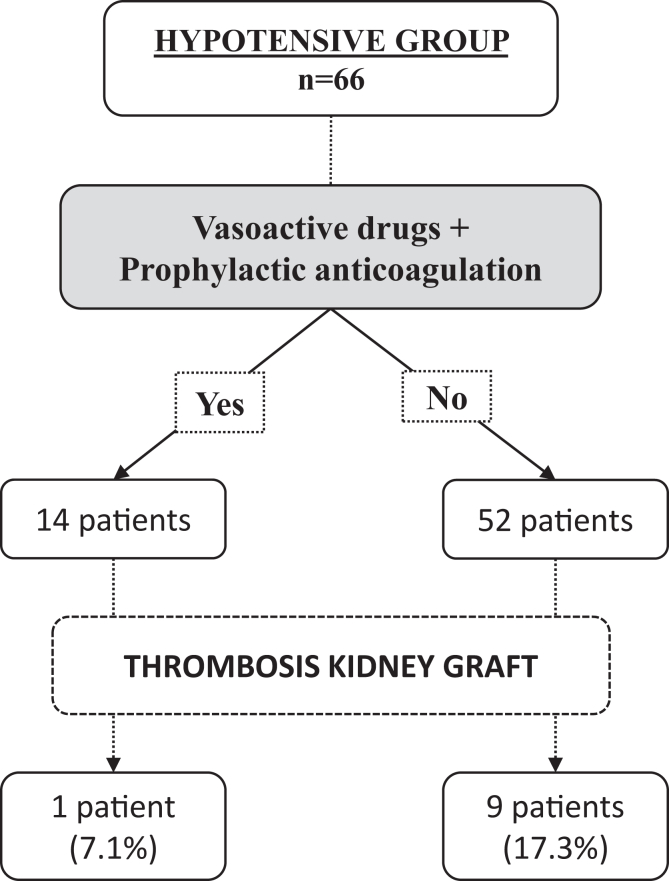
Noradrenaline and prophylactic enoxaparin were administered to 14 patients with chronic hypotension, from the immediate posttransplant period until graft function was observed. With this intervention, there was only 1 patient with venous graft thrombosis out of 14 (7.1%), compared to 9 venous graft thrombosis among the 52 hypotensive patients with no intervention (17.3%), although this difference did not reach statistical significance (P = 0.67) (Figure 3).
Figure 3.
Use of vasoactive drugs and anticoagulation in the immediate posttransplant period.
Evolution of Blood Pressure in Hypotensive Patients After Receiving a Functioning Graft
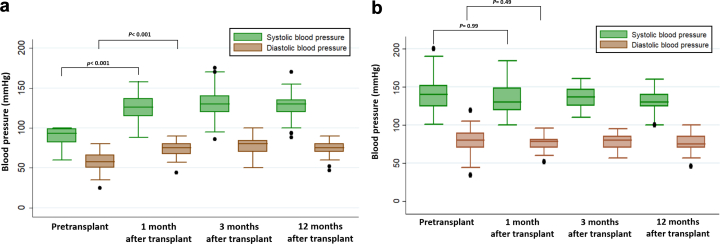
Patients with chronic hypotension who received a functioning graft experienced a normalization in their blood pressure. This phenomenon was observed from the first week after kidney transplantation and persisted during follow-up (Figure 4a). Moreover, 28 patients who were hypotensive in dialysis (representing more than a half of those with a functioning graft in the group with chronic hypotension) had become hypertensive during the first year after transplantation and were receiving a median number of 1.5 antihypertensive drugs (range 1–5) at that point. On the contrary, there were no significant blood pressure changes in the control group before and after transplantation (Figure 4b), or changes in the number of antihypertensive drugs (median number = 1; range: 0–5).
Figure 4.
Evolution of blood pressure after kidney transplantation; (a) in the hypotensive group, and (b) in the control group.
Discussion
Persistent chronic hypotension is not an uncommon complication in patients on chronic dialysis, which is associated with high morbidity and mortality, and has no effective pharmacologic treatment. Its influence on transplant outcomes has been barely explored even though it seems to have a detrimental effect on kidney transplantation results.
In a retrospective study designed to identify risk factors for DGF, which occurred in 8.8% of the patients, pretransplantation systolic pressure < 120 mm Hg was linked to a 3-fold increased risk of DGF.13 In our study, chronic hypotension defined as pretransplantation SBP < 100 mm Hg was associated with 85.9% of DGF. Unlike the precedent study (with an optimal cohort of young recipients with related living donors), our study is more in line with usual practice. Fifty-seven percent of our recipients' grafts came from donation after brain death with expanded criteria or donation after circulatory death, which are donation sources more prone to present basally DGF. The mean cold ischemia time was almost 19 hours. These 2 factors could explain the higher rates of DGF in our cohort, even in the control group, which reached 53%. Chronic hypotension has also been associated with PNF. Webber et al. found that mean arterial pressure during the 3 months before transplantation was an independent risk factor for PNF. The risk was the highest in patients with mean arterial pressure ≤80 mm Hg, with an odds ratio for PNF of 4.32 compared to those with mean arterial pressure >100 mm Hg.14 Consistent with this observation, our study confirms a higher rate of PNF in patients with chronic hypotension. PNF was observed in 12 of 66 hypotensive patients (18.2%), which is 3 times more frequent than PNF in the control group. However, contrary to Webber’s study (with 67% of PNF explained by persistent acute tubular necrosis), increased incidence of PNF in the present study was mainly due to venous graft thrombosis (10 of 12) and not due to acute tubular necrosis (2 of 12). Hypercoagulability was discarded in these patients; thus, graft thrombosis seems to have been directly mediated by reduced renal flow secondary to low blood pressure.
More recently, Dolla et al. performed a retrospective study identifying chronic hypotension as an independent risk factor for DGF, which was associated with a lower death-censored graft survival in those hypotensive patients who received a kidney transplant from a donor aged >50 years.15
In our study, chronic hypotension was associated with lower death-censored graft survival and worse renal function at the end of follow-up, and this hemodynamic condition remained an independent predictor factor for kidney graft failure after multivariate analysis.
However, it is very important to emphasize that the worse renal survival observed in our patients with chronic hypotension was determined mainly by the higher rate of PNF due to graft venous thrombosis in the immediate posttransplant period. When graft survival was censored by PNF, differences between hypotensive and control groups were no longer significant. Ultimately, once the immediate period after transplantation was surpassed and primary function was reached, transplant results were practically comparable to nonhypotensive patients.
Receiving a successful kidney transplant seems to be the only effective and definitive treatment for chronic hypotension in patients on dialysis. In this regard, only small series or case reports have described chronic hypotension reversibility after kidney transplantation. Muscroft et al. described a series of 8 patients with dialysis-associated hypotension, 4 of whom had severe persistent hypotension who underwent renal transplantation. Blood pressure normalized rapidly after graft function was established, suggesting the role of soluble mediators produced or excreted by the transplanted kidney. They point out the unlikely implication of tacrolimus, which was started 4 days after transplantation without a preoperative increase in blood pressure.1 Our study is the first to widely evaluate blood pressure evolution in a larger cohort of hypotensive patients and confirm the resolution of hypotension after receiving a functioning graft. Therefore, although it is essential to consider this hemodynamic circumstance in pretransplant evaluation, this should not be an absolute contraindication because the transplant itself supposes its solution.
Finally, this subgroup of patients could benefit from therapeutic measures to improve their short-term transplant evolution, and therefore equate their outcomes with those of nonhypotensive patients. Our data suggest a possible beneficial effect from vasoactive drugs and prophylactic anticoagulation initiated in the immediate posttransplant period, although the small cohort size probably did not permit achieving statistical significance. Prospective studies are needed to evaluate the efficacy of this type of intervention.
The present study has several strengths. To the best of our knowledge, this is the largest study (n = 66) investigating short-term and long-term effects of chronic hypotension on transplant outcomes and demonstrating its reversibility after achieving graft function. Furthermore, case-control matching was designed to minimize the impact of other factors related to organ source on transplant outcomes. In addition, it is the first one to propose an intervention aimed at improving results.
However, our study also has all the limitations inherent to retrospective analysis. Moreover, choosing SPB ≤ 100 mm Hg as hypotension threshold has limitations in handling a continuous variable, as a recent study demonstrates its low sensitivity in predicting DGF.24 The number of hypotensive patients undergoing preventive measures (vasoactive drugs and anticoagulation) was relatively small, which limits the statistical power to detect associations. In addition, the decision for these interventions was not fully standardized in advance, thereby introducing a possible bias. The small cohort size could also have influenced the absence of difference in graft survival after censoring for PNF, due to statistical power lack.
In conclusion, patients with chronic hypotension in dialysis have worse transplant outcomes than patients without this condition, determined by higher rates of PNF due to venous thrombosis of the allograft. Nevertheless, if the graft does not suffer thrombosis and primary function is achieved, long-term renal survival seems comparable to that of nonhypotensive patients. Chronic hypotension reverses in those patients with a functioning graft. Prospective studies are needed to evaluate possible interventions, such as the use of vasoactive drugs and anticoagulation, aimed to ameliorate transplant results in this subgroup of patients.
Acknowledgments
Disclosure
All the authors declared no competing interests.
Author Contributions
AA and PA designed the study; PA, AG, and JG collected the data; PA, TC, and AA analyzed the data; PA and TC prepared the tables and figures; PA, TC, AG, JG, and AA drafted and revised the paper; and all authors revised the paper and approved the final version of the manuscript. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Footnotes
Table S1. Episodes of biopsy-proven acute rejection in both groups.
STROBE Checklist
Supplementary Material
Table S1. Episodes of biopsy-proven acute rejection in both groups. STROBE Checklist.
References
- 1.Muscroft L., Zehnder D., Fletcher S., et al. Rapid resolution of severe sustained low blood pressure in haemodialysis patients after successful renal transplantation. Nephrol Dial Transplant. 2012;27:4223–4227. doi: 10.1093/ndt/gfs338. [DOI] [PubMed] [Google Scholar]
- 2.Cases A., Coll E. Chronic hypotension in the dialysis patient. J Nephrol. 2002;15:331–335. [PubMed] [Google Scholar]
- 3.Palmer B.F. Why are some dialysis patients chronically hypotensive in the absence of heart disease and volume depletion? Semin Dial. 2011;24:404–405. doi: 10.1111/j.1525-139X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 4.Port F.K., Hulbert-Shearon T.E., Wolfe R.A., et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 5.Hannedouche T., Roth H., Krummel T., et al. French observatory. Multiphasic effects of blood pressure on survival in hemodialysis patients. Kidney Int. 2016;90:674–684. doi: 10.1016/j.kint.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Coll E., Larrousse M., de la Sierra A., Collado S., Jiménez W., Cases A. Chronic hypotension in hemodialysis patients: role of functional vascular changes and vasodilator agents. Clin Nephrol. 2008;69:114–120. doi: 10.5414/cnp69114. [DOI] [PubMed] [Google Scholar]
- 7.Cases A., Esforzado N., Lario S., et al. Increased plasma adrenomedullin levels in hemodialysis patients with sustained hypotension. Kidney Int. 2000;57:664–670. doi: 10.1046/j.1523-1755.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y.F., Wang J.Y., Denq J.C., Lin S.H. Midodrine improves chronic hypotension in hemodialysis patients. Am J Med Sci. 2003;325:256–261. doi: 10.1097/00000441-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Fang J.T., Huang C.C. Midodrine hydrochloride in patients on hemodialysis with chronic hypotension. Ren Fail. 1996;18:253–260. doi: 10.3109/08860229609052795. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L., Jia H., Wang R., et al. Kidney transplantation from small pediatric donors may be feasible to those who developed chronic refractory dialysis hypotension: a single-center experience. Ann Transl Med. 2020;8:683. doi: 10.21037/atm-20-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun C.Y., Wu M.S. Renal transplantation reversed intractable hypotension in a diabetic patient. Diabetes Care. 2007;30 doi: 10.2337/dc07-0074. [DOI] [PubMed] [Google Scholar]
- 12.Kim T.W., Bailard N., Coveler L.A. The anesthetic management of a child with chronic hypotension for renal transplantation. J Clin Anesth. 2006;18:297–299. doi: 10.1016/j.jclinane.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Ozdemir F.N., Ibis A., Altunoglu A., Usluogullari A., Arat Z., Haberal M. Pretransplantation systolic blood pressure and the risk of delayed graft function in young living-related renal allograft recipients. Transplant Proc. 2007;39:842–845. doi: 10.1016/j.transproceed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Webber A., Hambleton J., Chami A., et al. Mean arterial blood pressure while awaiting kidney transplantation is associated with the risk of primary nonfunction. Transplantation. 2012;93:54–60. doi: 10.1097/TP.0b013e3182398035. [DOI] [PubMed] [Google Scholar]
- 15.Dolla C., Mella A., Vigilante G., et al. Recipient pre-existing chronic hypotension is associated with delayed graft function and inferior graft survival in kidney transplantation from elderly donors. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racusen L.C., Halloran P.F. Banff SK 2003 meeting report. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant. 2004;4:1562–1566. doi: 10.1111/j.1600-6143.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 17.Solez K., Colvin R.B., Racusen L.C., et al. Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 18.Solez K., Colvin R.B., Racusen L.C., et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 19.Mengel M., Sis B., Haas M., et al. Banff 2011 Meeting report. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M., Sis B., Racusen L.C., et al. Banff 2013 meeting report. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 21.Loupy A., Haas M., Solez K., et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am J Transplant. 2017;17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas M., Loupy A., Lefaucheur C., et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. doi: 10.1111/ajt.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loupy A., Haas M., Roufosse C., et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. doi: 10.1111/ajt.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wazir S., Abbas M., Ratanasrimetha P., Zhang C., Hariharan S., Puttarajappa C.M. Preoperative blood pressure and risk of delayed graft function in deceased donor kidney transplantation. Clin Transpl. 2022;36 doi: 10.1111/ctr.14776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Episodes of biopsy-proven acute rejection in both groups. STROBE Checklist.