Abstract
The Borna disease virus (BDV) is the prototype member of the Bornaviridae, and it replicates in the cell nucleus. The BDV p24P and p40N proteins carry nuclear localization signals (NLS) and are found in the nuclei of infected cells. The BDV p10 protein does not have an NLS, but it binds with P and/or N and is translocated to the nucleus. Hence, p10 may play a role in the replication of BDV in the cell nucleus. Here, we show that the P-binding domain is located in the N terminus of p10 and that S3 and L16 are important for the interaction.
The Borna disease virus (BDV) belongs to a new family of enveloped, negative, nonsegmented, single-stranded RNA viruses. The virus causes central nervous system disease in diverse species. It replicates and transcribes in the nuclei of infected cells (1, 2), and hence, viral proteins found in the nucleus may be important to the virus life cycle. Recently, members of our group (5) and others (8, 11) reported the expression of a 10-kDa protein, p10, which is encoded by open reading frame x1 (ORFx1) (nucleotides [nt] 1223 to 1486). p10 is found in the nucleus and the cytoplasm of BDV-infected cells, although it does not carry a nuclear localization signal (NLS) (5, 8, 11). Transient transfection studies have shown that, alone, p10 is localized in the cytoplasm but that upon cotransfection with the viral P protein, p24, or the viral N protein, p40, it is imported to the nucleus (5, 8). The p40 and the p24 proteins both carry an NLS (4, 9, 10) and are found in the nucleus of BDV-infected cells (4, 6, 9). In vitro protein-protein studies have revealed the direct interaction of p10 with p40 (5) and with p24 (8). The nuclear translocation of the p10 protein after interaction with p40 and/or p24 suggests that p10 also may play a role in BDV replication in the nucleus. Here, we report on our study to characterize the site on p10 wherein interaction with p24 occurs.
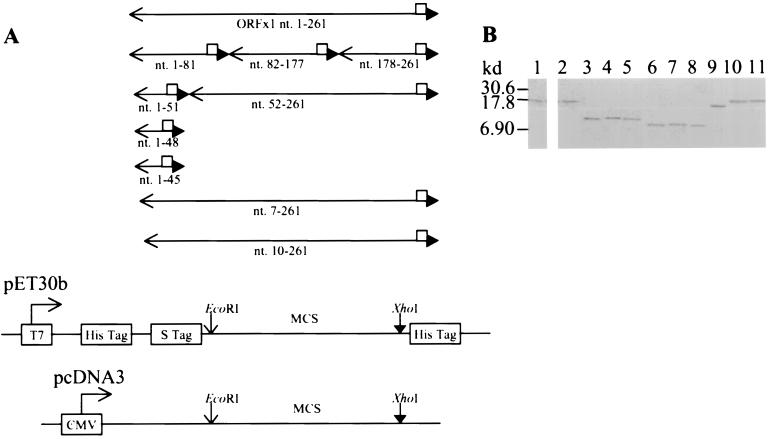
A series of prokaryotic and eukaryotic expression vectors expressing full-length and mutant p10 were constructed (Fig. 1A; Table 1) by the use of specific primers and PCR. The cDNA of ORFx1 previously cloned into the pGEX4T-3 vector to yield the pGEX-ORFx1 plasmid (5) was used as the template in the PCR. The amplified DNA fragments tagged with a sequence coding for the FLAG epitope were digested by EcoRI and XhoI and cloned into the pET-30b vector. This was in frame and downstream of the sequence encoding the His and S tags. The amplified DNA fragments were also cloned into the pcDNA3 eukaryotic expression vector under the control of the Cytomegalovirus intermediate promoter. Sequencing (7) was performed to ensure that the constructs did not have any errors.
FIG. 1.
Construction of eukaryotic and prokaryotic vectors expressing full-length and mutant p10. (A) PCR performed with specific primer pairs (available upon request) amplified the DNA fragments encoding the full-length p10 (nt. 1-261) consisting of 87 residues, the N-terminal 27 residues (nt. 1-81), the 32-residue peptide 28-59 (nt. 82-177), the C-terminal 28-residue peptide 60-87 (nt. 178-261), the N-terminal 17 residues (nt. 1-51), the N-terminal 16 residues (nt. 1-48), the N-terminal 15 residues (nt. 1-45), and the N-terminal truncated p10Δ2 (nt. 7-261), p10Δ3 (nt. 10-261), and p10Δ17 (nt. 52-261). The cDNA of ORFx1 previously cloned into the pGEX4T-3 vector (Pharmacia) to yield the pGEX-ORFx1 plasmid (5) was used as the template in the PCR. One primer contained an EcoRI site plus 3 bases at the 5′ end, while the second primer had the DNA sequence encoding the FLAG epitope, followed by an XhoI site, plus 3 bases at the 5′ end. The amplified DNA fragments were digested by EcoRI and XhoI and cloned into the pET-30b vector (Novagen), in frame and downstream of the sequence encoding for the His and S tags, to yield the pET-ORFx1-FLAG, pET1-81-FLAG, pET82-177-FLAG, pET178-261-FLAG, pET1-51-FLAG, pET1-48-FLAG, pET1-45-FLAG, pET7-261-FLAG, pET10-261-FLAG, and pET52-261-FLAG plasmids, respectively. Cloning of the ORFx1 DNA fragments containing nt 1 to 51 and nt 52 to 261 into the pcDNA3 eukaryotic expression vector yielded the pc1-51-FLAG plasmid (encoding a 2.9-kDa protein) and the pc52-261-FLAG plasmid (encoding a 8.9-kDa protein), respectively. Sequencing to show that all the constructs did not have errors was performed by the method of Sanger et al. (7). □, FLAG sequence; ←, EcoRI site; ▸, XhoI site. (B) Expression of the prokaryotic constructs yielded the fusion protein p10-FLAG (lanes 1 and 2) and the FLAG-tagged p10 deletion mutation proteins (lanes 3 to 11). These were identified with the use of the anti-FLAG monoclonal antibody (Sigma) in Western blot analysis. The p10-FLAG protein was also identified with the use of anti-p10 serum in Western blot analysis. Alkaline phosphatase-conjugated protein A/G (A+G) (Pierce) and 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP-NBT) (Sigma) reactive to the alkaline phosphatase-conjugated protein A/G were used for the detection of the proteins. The 17-kDa His-S-p10-FLAG was identified by the anti-p10 serum (lane 1) and by the anti-FLAG monoclonal antibody (lane 2). The anti-FLAG monoclonal antibody also identified the His-S-tagged 10.1-kDa peptide 1-27-FLAG encoded by pET1-81-FLAG (lane 3), the 10.7-kDa peptide 28-59-FLAG encoded by pET82-177-FLAG (lane 4), the 10.2-kDa peptide 60-87-FLAG encoded by pET178-261-FLAG (lane 5), the 8.9-kDa peptide 1-17-FLAG encoded by pET1-51-FLAG (lane 6), the 8.7-kDa peptide 1-15-FLAG encoded by pET1-45-FLAG (lane 7), the 8.8-kDa peptide 1-16-FLAG encoded by pET1-48-FLAG (lane 8), the 15-kDa p10Δ17-FLAG encoded by pET52-261-FLAG (lane 9), the 16.9-kDa p10Δ2-FLAG encoded by pET7-261-FLAG (lane 10), and the 16.8-kDa p10Δ3-FLAG encoded by pET10-261-FLAG (lane 11).
TABLE 1.
Summary of the full-length and mutant ORFx1 constructs described in Fig. 1 legend
| Construct | ORFx1 nt | Residues of p10a |
|---|---|---|
| pET-ORFx1-FLAG | 1–261 | 1–87 |
| pET181-FLAG | 1–81 | 1–27 |
| pET82-177-FLAG | 82–177 | 28–59 |
| pET178-261-FLAG | 178–261 | 60–87 |
| pET1-51-FLAG | 1–51 | 1–17 |
| pET1-48-FLAG | 1–48 | 1–16 |
| pET1-45-FLAG | 1–45 | 1–15 |
| pET7-261-FLAG | 7–261 | 3–87 (p10Δ2) |
| pET10-261-FLAG | 10–261 | 4–87 (p10Δ3) |
| pET52-261-FLAG | 52–261 | 18–87 (p10Δ17) |
| pc1-51-FLAG | 1–51 | 1–17 |
| pc52-261-FLAG | 52–261 | 18–87 (p10Δ17) |
Designations for mutated proteins are in parentheses.
Inclusion of the FLAG epitope at the 3′ end ensured detection of the expressed recombinant proteins. A commercially available anti-FLAG monoclonal antibody was used throughout this study to detect the full-length and the p10 mutants. As shown in Fig. 1B, the p10 fusion protein (Fig. 1B, lanes 1 and 2) expressed by the pET-ORFx1-FLAG plasmid was detected by our p10-specific antiserum (Fig. 1B, lane 1) and by the FLAG-specific monoclonal antibody (Fig. 1B, lane 2) in Western blot analysis. All of the recombinant p10 deletion mutants were also detected by the anti-FLAG monoclonal antibody (Fig. 1B, lanes 3 to 11).
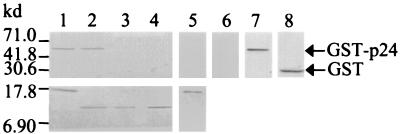
By the use of an in vitro protein-binding assay, Schwemmle and colleagues (8) reported the interaction of p10 with p24. We have also developed a comparable assay system, which members of our group used to detect the specific interaction between p10 and p40 (5). The interaction domains on p24 for binding with p10 have been reported previously (8). To study the domain on p10 that interacts with p24, we first confirmed that p10 directly interacted with p24 equally well in our in vitro assay system. The glutathione-S-transferase (GST) and GST-p24 fusion proteins were expressed, according to the manufacturer's protocol (Pharmacia) in Escherichia coli transformed with the pGEX4T-3 and pGEX-p24 (3) plasmids, respectively. The proteins were then purified by use of glutathione-Sepharose 4B (Pharmacia) affinity chromatography. Likewise, the p10 fusion protein was expressed and purified, according to the manufacturer's protocol (Novagen), from the pET-ORFx1-FLAG-transformed E. coli. The purified p10 fusion protein was cross-linked by the anti-FLAG monoclonal antibody to protein-G beads. The bound protein was then allowed to interact with either the purified GST or the GST-p24 fusion protein overnight at 4°C. Analyses of the proteins bound to the beads by Western blotting showed that the GST-p24 protein had bound to the p10-FLAG protein cross-linked to the solid phase (Fig. 2, lane 1), while the control GST fusion partner had not (Fig. 2, lane 5). The GST-p24 protein did not cross-react with the anti-FLAG monoclonal antibody (Fig. 2, lane 6). To determine whether the p24-binding domain is located in the middle section or at the N or C terminus of p10, peptide 1-27, peptide 28-59 and peptide 60-87 fused to FLAG were expressed from the pET1-81-FLAG, pET82-177-FLAG, and pET178-261-FLAG plasmids, respectively, and cross-linked to protein-G beads. In vitro interaction with the purified GST-p24 showed that the GST-p24 fusion protein bound to peptide 1-27 (Fig. 2, lane 2) and not to peptide 28-59 (Fig. 2, lane 3) or to peptide 60-87 (Fig. 2, lane 4). Thus, the p24-binding domain is located in the N-terminal 27 amino acid residues of p10. As the GST-p24 did not bind to peptide 28-59 or peptide 60-87, these results also provided an important internal control demonstrating the specificity of the interaction experiments.
FIG. 2.
Identification of the p24P-binding domain to the N-terminal 27 amino acids of p10 by in vitro protein-protein interaction. GST-p24 (2.5 μg; 500 μM) were allowed to interact overnight at 4°C with 0.5 μg (500 μM) of p10-FLAG or FLAG-tagged p10 deletion mutation proteins, which had been cross-linked by the anti-FLAG monoclonal antibody to protein-G beads (Sigma). As a control, GST (1.5 μg; 500 μM) was allowed to interact overnight at 4°C with 0.5 μg of p10-FLAG, bound to solid phase. The interactions were carried out under stringent conditions (1× phosphate-buffered saline, 0.02% sodium dodecyl sulfate [SDS], and 0.1% Triton) to ensure specific binding. The bound proteins were washed five times (0.1 M Tris [pH 8.0], 0.5 M NaCl, 0.02% SDS, and 0.1% Triton) and resolved by denaturing SDS–15% polyacrylamide gel electrophoresis. The proteins were then electrotransferred onto a nitrocellulose membrane, and Western blotting was performed with anti-p24 antiserum (top blot, lanes 1 through 4 and 7), anti-GST antibody (top blot, lanes 5 and 8) or with the anti-FLAG monoclonal antibody (top blot, lane 6; bottom blot, lanes 1 through 5). GST-p24 interacted with p10-FLAG (lane 1) and peptide 1-27-FLAG (lane 2) but not with peptide 28-59-FLAG (lane 3) or peptide 60-87-FLAG (lane 4). The GST fusion partner in GST-p24 did not react with p10-FLAG (lane 5). The GST protein alone was stained by the anti-GST antibody (Pharmacia) (lane 8). The GST-p24 protein alone did not cross-react with the anti-FLAG monoclonal antibody (lane 6) but was stained by the p24-specific antiserum (lane 7). Protein A/G (A+G) cross-linked to alkaline phosphatase reactive to BCIP-nitroblue tetrazolium was used to detect the antibody-antigen interactions.
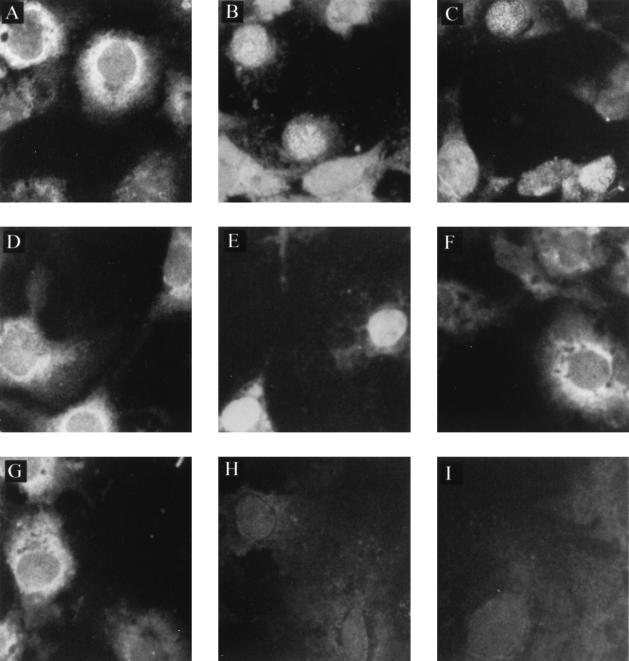
Computer analyses of the N-terminal 27 residues of p10 by use of PC/Gene (Intelligenetics, Inc.) showed that residues 1 to 17 (1MSSDLRLTLLELVRRLN17) form a leucine-rich α-helical domain. We hypothesized that the interaction domain is located in this region. To verify this hypothesis, we constructed the eukaryotic and prokaryotic expression plasmids that expressed either the N-terminal 17 residues of p10 tagged by FLAG (pc1-51-FLAG and pET1-51-FLAG [Fig. 1 and Table 1]) or the FLAG-tagged deletion mutant missing the first 17 amino acids of p10 (pc52-261-FLAG and pET52-261-FLAG [Fig. 1 and Table 1]). We have previously reported that p10 is localized in the cytoplasm of Cos-7 cells transfected with the pcORFx1-FLAG plasmid alone (5), while p24 is localized in the nucleus of Cos-7 cells transfected with the pcDL-p24 plasmid alone (9). Since p24 carries an NLS and p10 does not, subcellular localization of p10 and the p10 mutants tagged by FLAG after cotransfection with the pcDL-p24 plasmid in Cos-7 cells would reveal whether interaction with p24 had occurred. If interaction had occurred, p10 would be found in the nucleus (8); if not, p10 would remain in the cytoplasm. As shown in Fig. 3, p10 was localized predominantly in the cytoplasm of Cos-7 cells transfected with the pcORFx1-FLAG plasmid alone (Fig. 3A), and p24 was localized in the nucleus of Cos-7 cells transfected with the pcDL-p24 plasmid alone (Fig. 3B). In contrast, p10 became localized in the nucleus and the cytoplasm when Cos-7 cells were cotransfected with the pcORFx1-FLAG and the pcDL-p24 plasmids (Fig. 3C), suggesting that p10 expressed in the cytoplasm had been translocated into the nucleus in the presence of p24. This confirmed the earlier results of Schwemmle et al. (8). We stained cells singly transfected with the p24 P-expressing plasmid pcDL-p24 with anti-FLAG monoclonal antibody for use as controls. As with the Western blot study shown in Fig. 2, lane 6, we did not observe any cross-reactivity and staining of p24 by the anti-FLAG antibody (Fig. 3I). Transfection of Cos-7 cells with the pc1-51-FLAG plasmid expressing the first 17 amino acid residues of p10 (1MSSDLRLTLLELVRRLN17-FLAG) yielded fluorescence predominantly in the cytoplasm after staining with the anti-FLAG monoclonal antibody (Fig. 3D). However, nuclear staining was detected after the Cos-7 cells were cotransfected with the pc1-51-FLAG and the pcDL-p24 plasmids (Fig. 3E), suggesting nuclear translocation of the FLAG-tagged peptide in the presence of p24. Transfection of Cos-7 cells with the pc52-261-FLAG plasmid expressing the mutant missing the first 17 residues of p10 yielded predominantly cytoplasmic fluorescence after staining with the anti-FLAG monoclonal antibody (Fig. 3F). Nuclear translocation of this p10 mutant was not observed after cotransfection of Cos-7 cells with the pc52-261-FLAG and pcDL-p24 plasmids. Only a predominantly cytoplasmic staining was observed (Fig. 3G). In all cases, mock-transfected cells were not stained by the anti-p24 antiserum or by the anti-FLAG monoclonal antibody. A representation of these results is given in Fig. 3H. Taken together, these in vivo observations strongly suggest that the N-terminal 17 residues of p10 (1MSSDLRLTLLELVRRLN17) interact with p24.
FIG. 3.
Subcellular localization of p10 and p10 deletion mutants in Cos-7 cells cotransfected with p24P. Cos-7 cells (American Tissue Culture Collection, Rockville, Md.) were cultured at a concentration of 2.5 × 105 cells per 35-mm-diameter tissue culture, in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin (Life Technologies, Gaithersburg, Md.) per ml. After overnight culture at 37°C to achieve a 50 to 60% confluency, the cells were transfected with plasmid DNA by the use of Lipofectamine (Life Technologies) according to the manufacturer's protocol. At 48 h posttransfection, aliquots of cells were spotted onto slides and left at 37°C for 16 h, such that the same transfected culture could be assayed for more than one protein by immunofluorescence. Cells were then fixed and permeabilized on the slides with 3.7% formaldehyde for 20 min followed by 0.5% Triton for 10 min. The Cos-7 cells were transfected with the pcORFx1-FLAG (10 μg) alone (A), the pcDL-p24 plasmid (20 μg) alone (B and I), or with both constructs (C). The Cos-7 cells were also transfected with pc1-51-FLAG alone (D) or pc52-261-FLAG alone (F) or cotransfected with pc1-51-FLAG plus pcDL-p24 (E) and pc52-261-FLAG plus pcDL-p24 (G). Mock-transfected cells (H) served as control. The cells were stained with the anti-FLAG monoclonal antibody (A and C to I) or the p24-specific antiserum (B). A fluorescein isothiocyanate-conjugated protein G (Sigma) was used to detect the staining under an epifluorescence microscope. Magnification, ×400.
The N-terminal 17 residues of p10 contain a leucine-rich α-helical domain that is compatible with such a motif serving as a nuclear export signal (NES). It is tempting to postulate that this NES may be responsible for the predominantly cytoplasmic localization of p10. However, the p10Δ17 protein lacking the putative NES was also predominantly localized in the cytoplasm. This suggests that the NES may not play an important role in localizing the p10 protein to the cytoplasm in our experiments. To study whether the NES may direct the p10 localized in the nucleus to migrate to the cytoplasm will require detailed quantitative analysis of the p10 and p10Δ17 expression levels in the cytoplasm and the nucleus. These experiments are beyond the scope of this study, in which we have identified the location of the P-binding domain on p10.
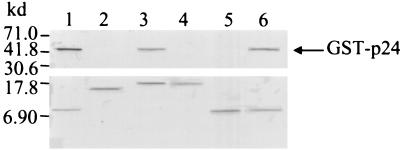
In vitro protein-protein interaction studies confirmed that the N-terminal 17 residues of p10 interacted with p24. The first 17 residues of p10 fused to the FLAG epitope (1MSSDLRLTLLELVRRLN17-FLAG) and the mutant p10Δ17-FLAG expressed from pET1-51-FLAG and pET52-261-FLAG, respectively, were allowed to interact with the purified GST-p24 protein. After the protein interaction, the resultant products were analyzed by Western blotting. Figure 4 shows that the GST-p24 reacted with the 1MSSDLRLTLLELVRRLN17-FLAG cross-linked to the beads (Fig. 4, lane 1) but not with p10Δ17-FLAG cross-linked to beads (Fig. 4, lane 2).
FIG. 4.
Residues S3 and L16 are important for the binding of p10 to p24 P. Interaction of GST-p24 (2.5 μg; 500 μM) with FLAG-tagged p10 deletion mutation proteins (0.5 μg; 500 μM) bound to solid phase was performed as described in the legend to Fig. 2. The bound proteins were resolved by denaturing SDS–15% polyacrylamide gel electrophoresis and electrotransferred onto a nitrocellulose membrane, and Western blotting was performed with anti-p24 antiserum (top blot) or with the anti-FLAG monoclonal antibody (bottom blot). GST-p24 interacted with the peptide 1-17-FLAG (lane 1), p10Δ2-FLAG (lane 3), and the peptide 1-16-FLAG (lane 6), but not with p10Δ17-FLAG (lane 2), p10Δ3-FLAG (lane 4), or the peptide 1-15-FLAG (lane 5).
Once the p24 interaction domain had been identified as residues 1 to 17 of p10, we created additional p10 deletion mutants to further characterize this interaction domain by in vitro protein-protein interaction experiments. We produced the proteins p10Δ2-FLAG and p10Δ3-FLAG from the pET7-261-FLAG and the pET10-261-FLAG plasmids, respectively. The N-terminal 15 (1MSSDLRLTLLELVRR15) and 16 (1MSSDLRLTLLELVRRL16) residues of p10 tagged by FLAG were expressed by the pET1-45-FLAG and the pET1-48-FLAG plasmids, respectively. We then allowed these recombinant mutants to bind to the purified GST-p24 protein. After the protein interaction, the resultant products were analyzed by Western blotting. Figure 4 shows that GST-p24 reacted with p10Δ2-FLAG (Fig. 4, lane 3) and 1MSSDLRLTLLELVRRL16-FLAG (Fig. 4, lane 6), but not with the p10Δ3-FLAG (Fig. 4, lane 4) and 1MSSDLRLTLLELVRR15-FLAG (Fig. 4, lane 5) mutants. As expected, we also found that P10Δ1 interacts with p24 (data not shown). These results suggested that S3 and L16 are important for the interaction of p10 and p24.
Our major concern with studying the P-binding domain was that the mutational deletions might inadvertently remove the p10 epitope(s) that can be marked by the anti-p10 antiserum. We therefore introduced the FLAG epitope tag in the deletion mutants and used an anti-FLAG monoclonal antibody for detection, thus overcoming the problem. All the mutants could be detected by the FLAG-specific monoclonal antibody in Western blot analysis, as shown in Fig. 1B. The in vitro protein-protein interactions demonstrated that the P-binding domain is located in the first third of the N terminus of p10, while the in vivo cotransfection studies suggested that it most likely involves the first 17 amino acids. Confirmation came from the in vitro studies. The p10Δ17 mutant did not interact with p24, whereas peptide 1MSSDLRLTLLELVRRLN17 did. However, neither p10Δ3 nor 1MSSDLRLTLLELVRR15 bound to p24, suggesting S3 and L16 are likely important to the interaction.
Computer predictions suggest that the first 17 residues, 1MSSDLRLTLLELVRRLN17, of p10 have an α-helical conformation, but the importance of the α-helix to the p10-p24 interaction is not clear. Residues 3 to 16 (3SDLRLTLLELVRRL16) also maintain an α-helical conformation. While we have demonstrated that S3 and L16 are important for p10 binding with p24, site-directed mutagenesis studies, in the long term, will locate and confirm all of the important amino acid residues in the P-binding domain. It will then be important to perform quantitative studies to determine the binding kinetics and the affinity of the p10 and p24 proteins for each other, as well as to study the biologic function of BDV p10.
Acknowledgments
We thank Jessica Costa for technical support.
This work was supported in part by grants from the National Institute of Mental Health, U.S. Public Health Service (MH57740), and Salem-Teikyo University.
REFERENCES
- 1.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Shoya Y, Koda T, Takashima I, Lai P K, Ikuta K, Kakinuma M, Kishi M. Nuclear targeting activity associated with the amino terminal region of Borna disease virus nucleoprotein. Virology. 1998;243:188–197. doi: 10.1006/viro.1998.9049. [DOI] [PubMed] [Google Scholar]
- 5.Malik T H, Kobayashi T, Ghosh M, Kishi M, Lai P K. Nuclear localization of the protein from the open reading frame x1 of the Borna disease virus was through interactions with the viral nucleoprotein. Virology. 1999;258:65–72. doi: 10.1006/viro.1999.9715. [DOI] [PubMed] [Google Scholar]
- 6.Pyper J M, Gartner A E. Molecular basis for the differential subcellular localization of the 38- and 39-kilodalton structural proteins of Borna disease virus. J Virol. 1997;71:5133–5139. doi: 10.1128/jvi.71.7.5133-5139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwemmle M, Salvatore M, Shi L, Richt J, Lee C H, Lipkin W I. Interactions of Borna disease virus P, N and X proteins and their functional implications. J Biol Chem. 1998;273:9007–9012. doi: 10.1074/jbc.273.15.9007. [DOI] [PubMed] [Google Scholar]
- 9.Shoya Y, Kobayashi T, Koda T, Ikuta K, Kakinuma M, Kishi M. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J Virol. 1998;72:9755–9762. doi: 10.1128/jvi.72.12.9755-9762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thierer J, Riehle H, Grebenstein O, Binz T, Herzog S, Thiedemann N, Stitz L, Rott R, Lottspeich F, Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992;73:413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- 11.Wehner T, Ruppert A, Herden C, Frese K, Becht H, Richt J A. Detection of a novel Borna disease virus encoded 10 kDa protein in infected cells and tissues. J Gen Virol. 1997;78:2459–2466. doi: 10.1099/0022-1317-78-10-2459. [DOI] [PubMed] [Google Scholar]