Abstract
Background
The role of social environment, that is, the aggregate effect of social determinants of health (SDOHs), in determining dementia is unclear.
Methods
We developed a novel polysocial risk score for dementia based on 19 SDOH among 5 199 participants in the Health and Retirement Study, United States, to measure the social environmental risk. We used a survival analysis approach to assess the association between social environment and dementia risk in 2006–2020. We further studied the interaction between social environment and lifestyles, and explored racial disparities.
Results
The study participants (mean age = 73.4 years, SD = 8.3; 58.0% female; 11.6% African American) were followed up for an average of 6.2 years, and 1 089 participants developed dementia. Every 1-point increase in the polysocial risk score (ranging from 0 to 10) was associated with a 21.6% higher risk (adjusted hazard ratio [aHR] = 1.21, 95% confidence intervals [95% CI] = 1.15–1.26) of developing dementia, other things being equal. Among participants with high social environmental risk, regular exercise and moderate drinking were associated with a 43%–60% lower risk of developing dementia (p < .001). In addition, African Americans were 1.3 times (aHR = 2.28, 95% CI = 1.96–2.66) more likely to develop dementia than European Americans, other things being equal.
Conclusion
An adverse social environment is linked to higher dementia risk, but healthy lifestyles can partially offset the increased social environmental risk. The polysocial risk score can complement the existing risk tools to identify high-risk older populations, and guide the design of targeted social environmental interventions, particularly focusing on improving the companionship of the older people, to prevent dementia.
Keywords: Dementia, Lifestyle, Polysocial risk score, Racial disparities, Social environment
Background
Dementia is a pressing issue among older adults that causes significant societal and economic burdens in the United States and globally (1,2). There is currently no cure for most dementias, therefore understanding risk factors for dementia is crucial to guide prevention strategies. Researchers have been focusing on exploring the role of genetic risk and lifestyles in developing dementia. Several long-term cohort studies showed that older adults with higher genetic risk have at least a 30% higher risk of developing dementia (3–5). Polygenic risk score is often used to assess genetic risk for Alzheimer’s disease (6,7). It was developed based on genome-wide association studies (GWAS) incorporating multiple risk alleles and mutations in relevant genes among European populations, though the methods have been evolving to apply to other populations (8). Studies further showed that healthy lifestyle practices could help offset the increased genetic risk in determining dementia, such as moderate drinking, regular exercise, nonsmoking, and a balanced diet (4,5,9).
The social environment influences disease pathways and is another dimension of risk factors affecting health outcomes besides genetics and lifestyle (10). The social environment consists of multiple social determinants of health (SDOH) encompassing “conditions in the environments where people are born, live, learn, work, play, worship, seek healthcare, and age” (11). There is substantial evidence suggesting a strong association between socioeconomic deprivation and cognitive impairment and decline (12–15). Higher levels of education, income, social connectedness, and social engagement are associated with a reduced risk of developing dementia (16–19). However, there is a lack of quantitative evidence on how these SDOHs collectively influence dementia, which could be more informative in predicting dementia than a single SDOH and potentially complement existing risk assessment tools to identify high-risk populations (20,21). In addition, it remains unclear whether healthy lifestyle interventions can mitigate the dementia risk posed by adverse social environments.
The measurement of aggregate social environment risk remains a topic of debate. A social deprivation index was developed in 2012 (22), and has been used to predict cognitive function and decline in the United States and Europe (23,24). However, this index only focuses on socioeconomic factors and fails to capture several important SDOHs, such as social support or engagement that are essential for maintaining cognitive function. The concept of the polysocial risk score was introduced in 2020 as an aggregate of SDOHs, which could help predict disease onset and health outcomes, providing valuable insights for prevention, clinical practice, and policymaking (25). With the availability of large data sets, it is now possible to create a polysocial risk score that incorporates multiple SDOHs to measure social environment risk. We identified 11 studies that developed, validated, and applied polysocial risk scores for diverse health outcomes and populations by the end of 2023. Two studies targeted dementia (26,27), while others had outcomes including diabetes (28–30), heart diseases (31,32), skin diseases (33,34), disability (35), and mortality (36). The results of these studies revealed the potential of polysocial risk scores in measuring social environment risk to predict health outcomes. The 2 studies on dementia, one among the UK population and another among the U.S. population, used different methods to develop the polysocial risk score. The UK study summed up the score of 15 preidentified SDOHs and ran a mutually adjusted analysis to obtain an individual score ranging from 0 to 10 (26), whereas the U.S. study ran stepwise logistic models and selected 11 out of 24 SDOHs that were significantly associated with dementia risk to calculate the score (27). However, their methods of developing the polysocial risk score could be further optimized, largely due to the collinearity issue among multiple SDOHs. Further, neither study assessed whether healthy lifestyle could mitigate the dementia risk posed by unfavorable social environments, which is a key research question of the current study.
This study uses longitudinal cohort data from the Health and Retirement Study (HRS) in 2006−2020 in the United States to (1) develop a polysocial risk score for dementia to measure social environmental risk; (2) assess the association between social environmental risk and dementia, controlling for genetic predisposition measured by a polygenetic risk score; and (3) explore the interaction between social environment and lifestyle with dementia risk, despite genetic predisposition. In addition, we also explore the differences in risk of developing dementia by race, considering the racial disparities in dementia burden and access to healthcare in the United States (2).
Method
Study Design and Participants
We conducted a longitudinal analysis using data from the HRS, a cohort study that has been surveying a representative sample of people aged 50 years and older in the United States since 1992. In 2006, the HRS started to collect blood and saliva samples and additional psychosocial information among a randomly selected half of the sample. Detailed information about the design and cohort profile of the HRS has been published (37,38). The HRS received ethical approval from the Institute for Social Research and Survey Research Center, University of Michigan (IRB protocol: HUM00061128), and each respondent gave written consent prior to the interview (for more information, please visit: http://hrsonline.isr.umich.edu/).
Participants in our study were aged 60 years and older, without dementia at baseline, had participated in enhanced face-to-face interviews and had records of polygenetic risk scores. Previous studies often used the cutoff point of 60 years or 65 years to select participants to study dementia risk as they have a higher risk of developing dementia (4,9,39). We included participants aged 60 years and older for a larger sample size, consistent with several other studies (1,40). We used 2006 as the baseline year and followed up participants every 2 years until 2020. The final sample comprised 5 199 study participants, including 603 African Americans and 4 596 European Americans. Further information about the sample selection can be found in Supplementary Figure S1.
Development of the Polysocial Risk Score for Dementia
We adopted the “Healthy People 2030” framework proposed by the U.S. Department of Health and Human Services and considered the data availability in the HRS to identify key SDOHs to construct the polysocial risk score. The Healthy People initiative started in 1980 and aims to guide health promotion and disease prevention in the United States by identifying public health priorities (41). Healthy People 2030 is the fifth iteration of the initiative, and SDOH is one of its priorities (42). We included 19 SDOHs in 5 domains outlined in Healthy People 2030 to develop the polysocial risk score for dementia (Supplementary Table S1).
(1) Economic stability: annual personal income, annual total household income, total household wealth, poverty status, and employment status;
(2) Education access and quality: highest education obtained;
(3) Healthcare access and quality: health insurance coverage and long-term care insurance coverage;
(4) Neighborhood and built environment: home type, rural/urban residence, and neighborhood safety;
(5) Social and community context: region of living, marital status, religious activity involvement, living arrangement, social support, social cohesion, lifetime stressful events, and discrimination.
To construct the polysocial risk score, we adopted the principal component analysis (PCA) approach and selected 6 principal components to capture the data variance of the 19 SDOHs. The PCA approach transforms the original variables into uncorrelated principal components, simplifying data complexity to address collinearity while capturing the variance in the data (43). Therefore, it has been widely used in relevant studies to distill key information among a large set of variables (43–45). We also conducted a decomposition analysis to calculate the contribution of each SDOH to the data variance based on their eigenvalues (see Supplementary Material for details on how we conducted the PCA and decomposition analysis). Lastly, we rescaled the polysocial risk score to range from 0 to 10 for easier application and interpretation. We also categorized the score into tertiles for categorical comparison and interaction analysis. We further tested the internal validity of the polysocial risk score in predicting dementia using receiver operating characteristic (ROC) analyses (see Supplementary Material for detailed methods and results).
Dementia
The primary outcome of the study was incident dementia. We used the Langa-Weir classification, one of the most widely used algorithmic tools to identify dementia based on cognitive assessment results (46,47). It calculates a composite score ranging from 0 to 27, which sums up the results of the immediate and delayed word recall test (0–20), the serial 7s test (0–5), and the backward counting test (0–2). Participants are classified into 3 categories: with dementia (0–6), cognitively impaired but without dementia (7–11), and cognitively healthy (12–27,48,49). In the current study, we followed the standard approach and used a binary variable to identify dementia (scored 0–6).
Genetic Predisposition
We measured participants’ genetic predisposition for dementia using polygenic risk scores for AD, calculated by the International Genomics of Alzheimer’s Project (IGAP) in 2019 based on a GWAS meta-analysis. The IGAP included 25 Alzheimer’s disease risk loci to construct the polygenic risk score based on 46 data sets comprising 21 982 cases and 41 944 normal controls (50). We used the polygenic risk score that included only variants with a significant association (p value < .01) with the outcome in the GWAS, as well as 2 imputed SNPs comprising APOE-ε4 status (rs7412 and rs429358). The polygenic risk scores were standardized separately within the European American and African American subsamples to follow a standard normal distribution (50).
Participants were categorized into low, intermediate, and high genetic risk groups based on the quintiles of their polygenic risk scores within the European American and African American subsamples. Specifically, we classified those in the lowest quintile as low risk, those in the 2nd–4th quintiles as intermediate risk, and those in the highest quintile as high risk, following previous genetic risk studies (4,51).
Lifestyle and Other Covariates
Smoking, drinking, and physical activity were included as key variables to measure participants’ lifestyle risk. Smoking status was categorized as currently smoking or not. Considering the U-shape relationship between drinking and dementia risk, drinking status was categorized into 2 groups: no or heavy drinking (0 drinks per week or >14 drinks per week) and moderate drinking (1–14 drinks per week). The cutoff point was selected based on previous research findings and reflected the 2020–2025 Dietary Guidelines for Americans (40,52,53). Regular physical activity was defined as moderate activity on at least 5 days per week or vigorous activity once a week, following previous studies and adjusted based on the American Heart Association guideline (4,54).
We further included covariates of demographic variables and known risk factors for developing dementia, which were sex, age, race, depression, hypertension, diabetes, and hearing impairment, largely based on the report by the Lancet Commission on dementia prevention, intervention, and care published in 2020 (40). Depressive symptoms were measured by the Center for Epidemiological Studies Depression (CES-D) mean score. Hypertension and diabetes were self-reported outcomes of doctors’ diagnoses. Hearing impairment was measured by whether the participant used any hearing aid.
Statistical Analysis
We described baseline characteristics of the entire study sample, by polygenic risk score and polysocial risk score categories. We tested for differences using ANOVA tests for continuous variables and chi-square tests for categorical variables. We used multiple imputations by chained equations with 20 sets of imputations for variables with missing values based on key socioeconomic variables, including sex, race, and education (Supplementary Table S1).
We adopted a survival analysis approach to examine the relationship between polysocial risk score and the risk of incident dementia. Participants, who were all at risk when entering the cohort in 2006, were followed up until the date of onset of dementia, death, loss to follow-up, or the interview until 2020, whichever occurred first. We used the competing-risk framework that treated death as a competing risk to calculate the cumulative incidence of developing dementia during follow-up (55). We ran 4 separate sets of Cox proportional hazard regression models to assess the association between polysocial risk score and dementia incidence. The first model included polysocial risk score as the independent variable and only adjusted for age. The second model added the polygenetic risk score and the third added lifestyle variables. The fourth model was a full model that was adjusted for a complete set of covariates. We also tested for interactions between polysocial risk score and lifestyle risk with dementia. Racial disparities were explored by assessing the association between race and the outcome, as well as the interactions between polysocial risk score and race with the outcome.
We verified the proportional hazards assumption using the Schoenfeld residuals technique and the assumption held (p = .50, global test results for departures from proportionality in the full model). We reported the hazard ratio (HR) for the Cox regression model results and the interaction results, with 95% confidence intervals and statistical significance level. All analyses were conducted using Stata SE, version 16 (StataCorp LLC, College Station, TX).
Sensitivity Analysis
We conducted 3 sensitivity analyses. First, we simply used the median values, instead of multiple imputations by chained equations, to impute the missing data in the SDOH to develop the polysocial risk score and run the Cox regression models. Second, we used the cumulative incidence function model that treated death as a competing risk to reestimate the regression results. Third, we categorized drinking into 3 groups: no drinking, moderate drinking, and heavy drinking to reassess the role of moderate drinking.
Results
At baseline, of the 5 199 participants, 3 015 (58%) were female and 603 (11.6%) were African American. Their mean age was 73.4 (SD = 8.3). They were followed up for an average of 6.2 years (median: 6.0 [interquartile range, IQR: 3.8−8.5]). A total of 1 089 (20.9%) participants developed dementia during the 14 years follow-up. Participants in the low social environment risk group were more likely to be younger, female, European American, with a healthier lifestyle and better mental and physical health (Table 1). There were no significant differences at baseline between participants with different genetic risks (Table 1).
Table 1.
Baseline Characteristics of the Study Participants: Total and Different Genetic and Social Environment Risk Groups
| Total | Polygenetic Risk Score | p Value for Difference | Polysocial Risk Score | p Value for Difference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline covariates | Low risk (n = 1 041) | Interm. risk (n = 3 119) | High risk (n = 1 039) | Low risk: 0–4.1 (n = 1 733) |
Interm. risk: 4.1–5.1 (n = 1 733) |
High risk: 5.1–10 (n = 1 733) |
|||
| Age | 73.4 (8.3) | 74.3 (8.5) | 73.2 (8.3) | 73.2 (8.1) | .001 | 72.7 (7.7) | 73.6 (8.4) | 73.9 (8.6) | <.001 |
| Race | |||||||||
| African Ancestry | 4 596 (88.4%) | 121 (11.6%) | 362 (11.6%) | 120 (11.5%) | .998 | 144 (8.3%) | 200 (11.5%) | 259 (15.0%) | <.001 |
| European Ancestry | 603 (11.6%) | 920 (88.4%) | 2 757 (88.4%) | 919 (88.5%) | 1 589 (91.7%) | 1 533 (88.5%) | 1 474 (85.1%) | ||
| Female | 3 017 (58.0%) | 631 (60.6%) | 1 778 (57.0%) | 608 (58.5%) | .116 | 929 (53.6%) | 995 (57.4%) | 1 093 (63.1%) | <.001 |
| No drinking | 3 470 (66.8%) | 723 (69.4%) | 2 064 (66.2%) | 683 (65.8%) | .346 | 1 127 (65.0%) | 1 146 (66.1%) | 1 197 (69.1%) | .007 |
| Moderate drinking | 1 567 (30.2%) | 287 (27.6%) | 960 (30.1%) | 320 (30.1%) | 555 (32.0%) | 542 (31.3%) | 470 (27.1%) | ||
| Physically active | 1 882 (36.2%) | 373 (35.8%) | 1 130 (36.2%) | 379 (36.5%) | .953 | 731 (42.2%) | 614 (35.4%) | 537 (31.0%) | <.001 |
| No current smoking | 4 651 (89.4%) | 937 (90.0%) | 2 789 (89.4%) | 925 (89.0%) | .762 | 1 629 (94.0%) | 1 560 (90.0%) | 1 462 (84.4%) | <.001 |
| Depression* | 1.3 (1.8) | 1.2 (1.7) | 1.3 (1.8) | 1.4 (1.8) | .156 | 0.9 (1.4) | 1.2 (1.8) | 1.8 (2.0) | <.001 |
| Hypertension | 3 031 (58.3%) | 612 (58.8%) | 1 826 (58.5%) | 593 (57.1%) | .663 | 948 (54.7%) | 1 013 (58.5%) | 1 070 (61.7%) | <.001 |
| Diabetes | 948 (18.2%) | 185 (17.8%) | 576 (18.5%) | 187 (18.0%) | .860 | 258 (14.9%) | 321 (18.5%) | 369 (21.3%) | <.001 |
| Hearing impairment | 774 (14.9%) | 174 (16.7%) | 446 (14.3%) | 154 (14.8%) | .165 | 264 (15.2%) | 255 (14.7%) | 255 (14.7%) | .884 |
Notes:
*Measured by the Center for Epidemiological Studies Depression (CES-D) mean score (0–8) covering 8 items: feeling depressed, everything an effort, restless sleep, was happy, feeling alone, feeling sad, could not get going and enjoyed life. A higher score indicates more negative feelings of the respondent in the past week.
The polysocial risk score (ranging from 0 to 10) followed a normal distribution with a mean of 4.6 and a standard deviation of 1.3 at baseline. Six principal components were obtained from the PCA results, which accounted for 43.1% of the total variance of the 19 SDOHs. The decomposition analysis results showed that the top 5 SDOHs that contributed most to variance were marital status (4.0%), living arrangement (4.0%), household income (3.9%), personal income (3.5%), and education (2.9%; Figure 1). The ROC area was 0.78 (95% CI: 0.77, 0.79), showing good prediction ability of the polysocial risk score for dementia (Supplementary Figure S3 for the ROC curve).
Figure 1.
Contribution of the 19 SDOHs to the dementia polysocial risk score.
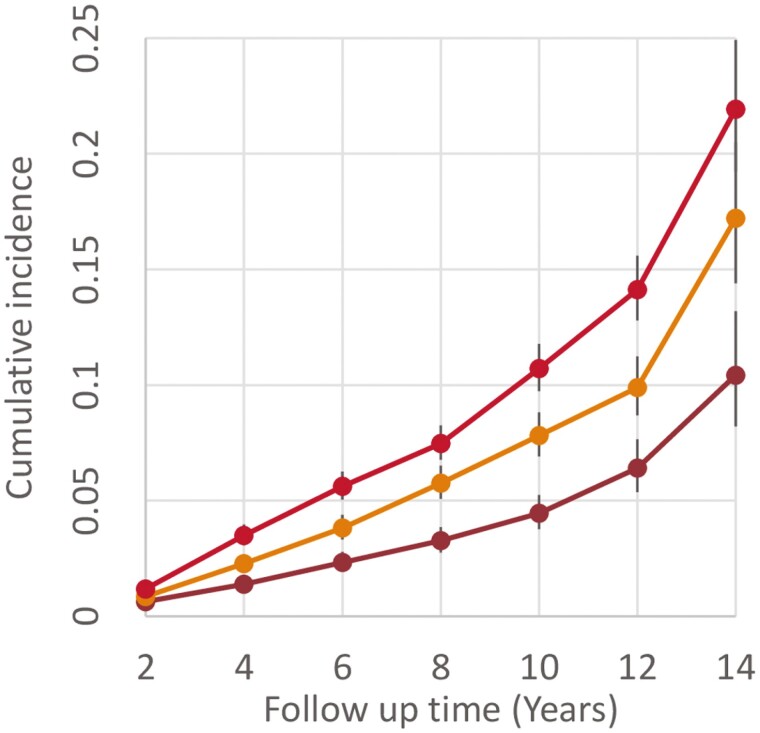
The risk of incident dementia increased monotonically with the polysocial risk score (Figure 2). The incident rate of dementia per 1 000 person-years for those in the low, intermediate, and high social environment risk groups was 12.77 (95% CI: 11.12, 14.65), 21.66 (95% CI: 19.49, 24.07), and 32.24 (95% CI: 29.64, 35.07), respectively. After adjusting for genetic risk, lifestyle and all the covariates, every 1-point increase in the polysocial risk score was associated with a 21.6% (aHR = 1.21, 95% CI: 1.15–1.26) higher risk of developing dementia (Table 2). When treating the polysocial risk score as a categorical variable, the full model showed that compared with the low-risk group, those in the intermediate and high social environment risk had a 48.5% (aHR = 1.49, 95% CI: 1.25, 1.76) and 86.1% (aHR = 1.86, 95% CI: 1.59, 2.18) higher risk of developing dementia, other things being equal (Supplementary Table S2 for results of all the 4 models).
Figure 2.
Cumulative incidence of dementia during follow-up by social environmental risk groups.
Table 2.
Association Between Social Environment Risk and Incident Dementia: Cox Model Results
| Events/Total | Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Social environment risk | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| — | 1.32*** | 1.26 | 1.38 | 1.32*** | 1.26 | 1.38 | 1.26*** | 1.20 | 1.31 | 1.21*** | 1.15 | 1.26 | |
| Genetic risk | |||||||||||||
| Low risk | 203/1 041 | — | — | — | Ref. | Ref. | Ref. | ||||||
| Interm. risk | 665/3 119 | — | — | — | 1.19** | 1.02 | 1.38 | 1.21** | 1.04 | 1.40 | 1.22** | 1.05 | 1.42 |
| High risk | 221/1 039 | — | — | — | 1.26** | 1.05 | 1.51 | 1.30** | 1.08 | 1.56 | 1.34** | 1.12 | 1.61 |
| Lifestyle | |||||||||||||
| Physically active | |||||||||||||
| No | 894/3 317 | — | — | — | — | — | — | Ref. | Ref. | ||||
| Yes | 195/1 882 | — | — | — | — | — | — | 0.55*** | 0.48 | 0.65 | 0.57*** | 0.49 | 0.66 |
| Weekly alcohol consumption | |||||||||||||
| 0 or >14 units | 929/3 581 | — | — | — | — | — | — | Ref. | Ref. | ||||
| 1–14 units | 160/1 348 | — | — | — | — | — | — | 0.38*** | 0.32 | 0.44 | 0.40*** | 0.34 | 0.47 |
| Smoking | |||||||||||||
| Yes | 64/548 | — | — | — | — | — | — | Ref. | Ref. | ||||
| No | 1 025/4 651 | — | — | — | — | — | — | 1.03 | 0.81 | 1.32 | 1.05 | 0.83 | 1.33 |
| Race | |||||||||||||
| European Ancestry | 863/4 596 | — | — | — | — | — | — | — | — | — | Ref. | ||
| African Ancestry | 226/603 | — | — | — | — | — | — | — | — | — | 2.27*** | 1.95 | 2.65 |
| Age | 1.09*** | 1.08 | 1.10 | 1.09*** | 1.08 | 1.10 | 1.09*** | 1.08 | 1.09 | 1.09*** | 1.08 | 1.10 | |
| Depression | |||||||||||||
| CES-D score | 1.03** | 1.00 | 1.07 | ||||||||||
| Hypertension | |||||||||||||
| No | Ref. | ||||||||||||
| Yes | 0.98 | 0.86 | 1.12 | ||||||||||
| Diabetes | |||||||||||||
| No | Ref. | ||||||||||||
| Yes | 0.99 | 0.87 | 1.14 | ||||||||||
| Hearing aid | |||||||||||||
| No | Ref. | ||||||||||||
| Yes | 1.20** | 1.06 | 1.36 | ||||||||||
Notes: HR = hazard ratio. Model 1 = polysocial risk score and age; Model 2 = polysocial risk score, polygenetic risk score and age; Model 3 = polysocial risk score, polygenetic risk score, lifestyle and age; Model 4 = polysocial risk score, polygenetic risk score, lifestyle, age, and other covariates including sex, race, depression, hypertension, diabetes, and hearing capacity.
**Statistically significant at 5%.
***Statistically significant at 1%.
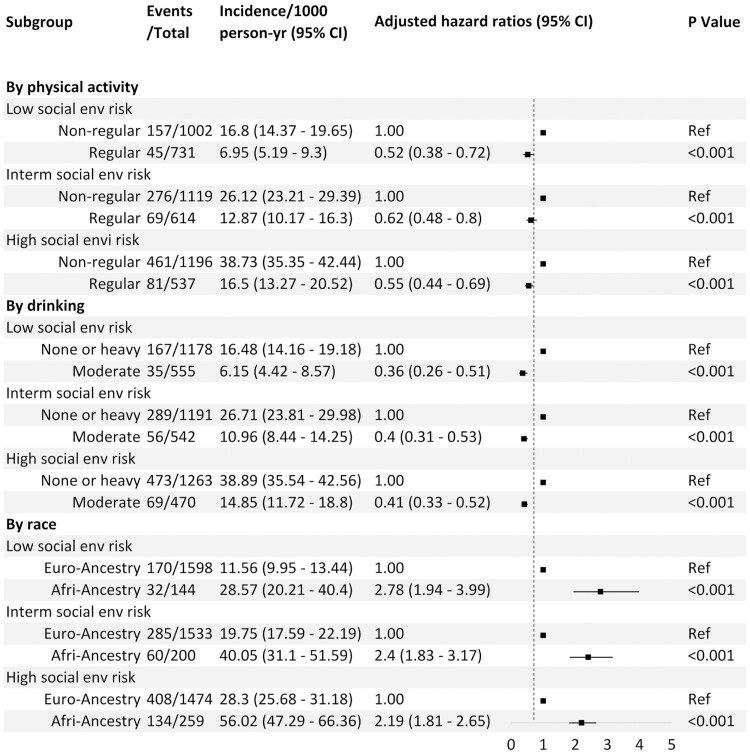
We found a 42.9% (aHR = 0.57, 95% CI: 0.49, 0.66) risk reduction in developing incident dementia among those with regular physical activity, and a 59.8% (aHR = 0.40, 95% CI: 0.34, 0.47) risk reduction among those with moderate drinking compared with the no drinking group (Table 2). We found no statistically significant association between smoking and dementia (Table 2). We did not find any significant interaction between social environment and regular exercise (p for interaction > .4), moderate drinking (p for interaction > .7), or smoking (p for interaction > .5) with regard to dementia risk. Regular exercise and moderate drinking were significantly strong protectors across all social environment risk groups, even in the high-risk group (Figure 3).
Figure 3.
Risk of incident dementia according to social environmental risk, lifestyle, and race.
We found racial disparities in developing dementia. African Americans had a 1.3-fold higher risk (aHR = 2.27, 95% CI: 1.95, 2.65) of developing dementia compared to European Americans, all things being equal (Table 2 and Figure 3). We did not find any interactions between social environment and race regarding dementia risk (p > .3). This association was consistent across all social environment risk groups (Figure 3).
We observed a similar pattern of associations in the 3 sensitivity analyses (Supplementary Table S3). Using a simpler method of missing data imputation did not alter the direction or magnitude of the coefficients. The results obtained from the cumulative incidence function-based proportional hazard model were also highly consistent with the main findings. Categorizing drinking into 3 groups and using no drinking as reference group generated consistent results: we still found that moderate drinking was associated with a reduced risk of dementia (HR = 0.40, 95% CI: 0.34, 0.47), and found no interaction between alcohol and dementia risk (p for interaction > .2; Supplementary Table S3).
Discussion
This study advances our understanding of the quantitative relationship between social environmental risk and dementia, and underscores that healthy lifestyles could help offset the increased social environmental risk in determining dementia. We used robust methods to aggregate the effect of 19 SDOHs to develop the polysocial risk score ranging from 0 to 10 for measuring the social environment risk. In addition, we included a comprehensive set of risk factors for dementia, including genetic risk, to assess the association between social environment and dementia, and explored the interaction between lifestyles and social environment. In this 14-year longitudinal study, we found that a 1-point increase in the polysocial risk score (ranging from 0 to 10) was associated with a 21.6% higher risk of developing dementia among older people, controlling for genetic risk and lifestyle factors. Regular exercise and moderate drinking were found to protect participants from developing dementia across all social environment risk groups. Furthermore, the findings provided supporting evidence for the racial disparities in the risk of developing dementia between European Americans and African Americans.
The dementia cases are estimated to triple and reach 153 million by 2050, unless effective prevention strategies are taken to address the risk factors, especially among high-risk populations (1). The polysocial risk score for dementia developed in this study has the potential to help identify high-risk populations with social environmental data, complement the existing risk assessment tools and enrich the design of interventions targeting important drivers of social environment risk. Different from traditional methods that used unweighted sum or stepwise regressions (28,31,56), we developed the polysocial risk score using a PCA approach, which can indirectly address potential collinearity issues among the SDOH (43). The strong association between the polysocial risk score and dementia found in this study suggests its potential to be used in combination with other existing risk assessment tools in clinical settings to identify high-risk groups, or to guide the design of relevant interventions.
The decomposition analysis results demonstrate that among the 19 SDOHs, the top 5 SDOHs that contributed most to the social environmental risk are marital status, living arrangement, household income, personal income, and education. The findings are consistent with previous studies to underscore the significant role of social contact in protecting older people from dementia (19,39,40). Although it is difficult to design interventions targeting income or education, future intervention studies or government programs may consider designing prevention strategies to improve the companionship of older people, especially among those who are widowed or isolated, to prevent dementia.
Our findings confirm that dementia disproportionately affects individuals with an unfavorable social environment, consistent with prior research (15,17–19,39,57–59). Regular exercise and moderate drinking emerged as strong protectors against dementia in our study, which is consistent with previous research findings (53,60). Our study further advances this understanding by demonstrating that: (1) healthy lifestyles have similar protective effect against dementia among all social environmental risk groups (indicated by the lack of interactions), and (2) the impact of an unfavorable social environment is not deterministic: regular exercise and moderate drinking are associated with a 46%–53% lower risk of developing dementia among the high social environmental risk group, other things being equal. It provides strong evidence that having a good lifestyle can also offset the increased social environmental risk, similar to its role in mitigating the increased genetic risk for dementia (4,5). There is a debate on whether moderate drinking is associated with decreased dementia risk. The results of our study support this argument and are consistent with many original studies with robust study designs and with systematic reviews (40,53,61,62). A plausible hypothesis, therefore, is that moderate drinking could protect the older participants from dementia risk via promoting social interactions. Alcohol consumption usually implies more social interaction activities, and there has been very strong evidence showing the protective role of social connectedness on dementia risk (19,39). Therefore, we should be cautious when interpreting the results and future studies can further study the relationship and the role of drinking when more data become available. Further, despite the protective role of healthy lifestyles, ensuring good adherence to a healthy lifestyle among older adults is challenging due to age-specific barriers, such as poor physical functioning and living alone. Exploring reliable predictors of healthy lifestyle to overcome these barriers and intervening at midlife could be an effective approach to take for researchers and policymakers (63,64).
Our study provides further evidence of persistent health disparities between African Americans and European Americans. Being an African American was associated with a 1.3-fold increased risk of dementia, all things being equal. These disparities were consistent across different levels of social environment risk, providing supporting evidence for the existence of structural racism. It is defined as “the totality of ways in which societies foster racial discrimination, through mutually reinforcing inequitable systems of housing, education, employment, earnings, benefits, credit, media, healthcare, and criminal justice” (1,2). In this study, the observed disparities in dementia risk between African Americans and European Americans reflect the accumulated risks that African Americans face from birth due to racism. For example, those from ethnic minorities often encounter structural racism that reduces their chances to obtain quality education and high-income jobs, consequently limiting their access to neighborhoods with better health access, public transport, food security, green space, and other resources. This structural racism might be the fundamental driver behind the racial disparities in developing dementia found in the United States in both our study and previous studies (59,65–68).
Our study has several strengths. First, we adopted a novel tool, the polysocial risk score approach, to quantify the combined impact of multiple SDOHs to measure the social environment and used the PCA approach to address the collinearity issue. Second, we included several dementia risk factors, including genetic, lifestyle, and social environment risk, and explored the interactions between social environment and healthy lifestyles with dementia risk. Third, our study sample included participants from both African and European Americans and explored racial disparities. Fourth, our study had a long follow-up period and a relatively large sample size, enhancing the robustness and generalizability of our findings.
We also acknowledge the following limitations. First, the measurement of dementia relied on the Langa-Weir cognitive assessment due to a lack of access to medical records. It is a probable dementia classification in nature, and ideally further studies can use a combination of medical records and case finding based on adjudicated diagnosis within the study to measure dementia, if resources allow. Second, the SDOHs included in constructing the polysocial risk score were comprehensive, but not exhaustive due to data limitations. Future studies could include more SDOHs, such as food access, green space, frequency and quality of contacts, presence of a confidante, and loneliness, among others. Third, we relied on self-reported data in the HRS to measure health status. Fourth, constrained by the data availability in the HRS, the measurement of physical activity only captured frequency and did not include diet when measuring lifestyle risk. In addition, we used hearing aid usage as a proxy to measure hearing impairment, which excluded undiagnosed or uncorrected hearing impairment. Future studies can refine the measurement when data become available. Fifth, the polygenetic score of the African Americans released by HRS was derived based on the GWAS results of non-Hispanic Whites, which might not have the same predictive capacity though these were the best data we could access. Further, the polygenetic risk scores were only available for European and African Americans in the HRS, and we were unable to include individuals from other ethnic backgrounds, such as Hispanics and Asian Americans, in our analyses. Lastly, given that we analyzed the predictive validity of the polysocial risk score only in the HRS sample, future studies can test its predictive validity using other data sets.
Conclusion
An unfavorable social environment is linked to an increased risk of dementia, but healthy lifestyles can protect older people from developing dementia across all social environmental risk groups. The polysocial risk score can complement the existing risk assessment tools to identify high-risk individuals and assist in the design of targeted social environment interventions to prevent dementia.
Supplementary Material
Acknowledgments
The authors are deeply grateful to the Health and Retirement Study (HRS) team for their data collection. This analysis uses data or information from the Harmonized HRS data set and Codebook, Version D as of August 2023 developed by the Gateway to Global Aging Data. DOI: https://doi.org/10.25549/4smz-hp46. For more information, please refer to https://g2aging.org/.
Contributor Information
Shu Chen, School of Risk and Actuarial Studies, UNSW Business School, UNSW Sydney, Sydney, New South Wales, Australia; Australian Research Council Centre of Excellence in Population Ageing Research (CEPAR), Kensington, New South Wales, Australia.
Shanquan Chen, International Centre for Evidence in Disability, London School of Hygiene and Tropical Medicine, London, UK.
Katja Hanewald, School of Risk and Actuarial Studies, UNSW Business School, UNSW Sydney, Sydney, New South Wales, Australia; Australian Research Council Centre of Excellence in Population Ageing Research (CEPAR), Kensington, New South Wales, Australia.
Yafei Si, School of Risk and Actuarial Studies, UNSW Business School, UNSW Sydney, Sydney, New South Wales, Australia; Australian Research Council Centre of Excellence in Population Ageing Research (CEPAR), Kensington, New South Wales, Australia.
Hazel Bateman, School of Risk and Actuarial Studies, UNSW Business School, UNSW Sydney, Sydney, New South Wales, Australia; Australian Research Council Centre of Excellence in Population Ageing Research (CEPAR), Kensington, New South Wales, Australia.
Bingqin Li, Social Policy Research Centre, UNSW Sydney, Sydney, New South Wales, Australia.
Xiaolin Xu, Department of Big Data in Health Science, School of Public Health, Zhejiang University, Hangzhou, Zhejiang, China; Centre of Clinical Big Data and Analytics, The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Suraj Samtani, Centre for Healthy Brain Ageing (CHeBA), School of Clinical Medicine, Discipline of Psychiatry and Mental Health, Faculty of Medicine and Health, UNSW Sydney, Sydney, New South Wales, Australia.
Chenkai Wu, Global Health Research Center, Duke Kunshan University, Kunshan, Jiangsu, China.
Henry Brodaty, Centre for Healthy Brain Ageing (CHeBA), School of Clinical Medicine, Discipline of Psychiatry and Mental Health, Faculty of Medicine and Health, UNSW Sydney, Sydney, New South Wales, Australia.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
This work was supported by UNSW Sydney and the ARC Centre of Excellence in Population Ageing Research (CEPAR), Australia (grant number: CE17010005). Dr. S.C.’s work was supported by the PENDA, funded by the UK Foreign, Commonwealth and Development Office. The Health and Retirement Study (HRS) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. The development of the harmonized HRS was funded by the National Institute on Aging (R01 AG030153, RC2 AG036619, and 1R03AG043052). Dr. S.S. declares funding from the Dementia Australia Resesarch Foundation, although not for the current manuscript.
Conflict of Interest
H.B. is or has been an advisory board member or consultant to Biogen, Eisai, Eli Lilly, Medicines Australia, Roche, and Skin2Neuron. H.B. is a Medical/Clinical Advisory Board member for Montefiore Homes and Cranbrook Care.
Data Availability
The HRS data are all publicly available to researchers. Researchers can access and download the HRS data from its website (https://hrs.isr.umich.edu/data-products).
Author Contributions
S.C. designed the study under the supervision of K.H., B.L., and H.B. S.C. extracted the data, conducted statistical analyses in Stata (StataCorp LLC, College Station, TX), formulated tables, and figures, and drafted the manuscript. All authors contributed to commenting, editing, and approval of the final manuscript. All accept responsibility for submitting for publication.
References
- 1. Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. [DOI] [PubMed] [Google Scholar]
- 3. Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–1458. 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. 10.1001/jama.2019.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25(9):1364–1369. 10.1038/s41591-019-0547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sugrue LP, Desikan RS.. What are polygenic scores and why are they important? JAMA. 2019;321(18):1820–1821. 10.1001/jama.2019.3893 [DOI] [PubMed] [Google Scholar]
- 7. Leonenko G, Baker E, Stevenson-Hoare J, et al. Identifying individuals with high risk of Alzheimer’s disease using polygenic risk scores. Nat Commun. 2021;12(1):4506. 10.1038/s41467-021-24082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark K, Leung YY, Lee WP, Voight B, Wang LS.. Polygenic risk scores in Alzheimer’s disease genetics: methodology, applications, inclusion, and diversity. J Alzheimers Dis 2022;89(1):1–12. 10.3233/jad-220025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faul JD, Ware EB, Kabeto MU, Langa KM, Llewellyn DJ, Galama T.. Lifestyle and genetic risk: revisiting the association with incident dementia. Alzheimers Dement. 2020;16(S10):e044220. [Google Scholar]
- 10. Yen IH, Syme SL.. The social environment and health: a discussion of the epidemiologic literature. Annu Rev Public Health. 1999;20(1): 287–308. 10.1146/annurev.publhealth.20.1.287 [DOI] [PubMed] [Google Scholar]
- 11. Gómez CA, Kleinman DV, Pronk N, et al. Addressing health equity and social determinants of health through healthy people 2030. J Public Health Manag Pract. 2021;27(Suppl 6):S249–S257. 10.1097/PHH.0000000000001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiao C, Botticello A, Fuh JL.. Life-course socio-economic disadvantage and late-life cognitive functioning in Taiwan: results from a national cohort study. Int Health. 2014;6(4):322–330. 10.1093/inthealth/ihu046 [DOI] [PubMed] [Google Scholar]
- 13. Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM.. Education and cognitive functioning across the life span. Psychol Sci Public Interest. 2020;21(1):6–41. 10.1177/1529100620920576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyu J, Burr JA.. Socioeconomic status across the life course and cognitive function among older adults: an examination of the latency, pathways, and accumulation hypotheses. J Aging Health. 2016;28(1):40–67. 10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- 15. Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM.. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol. 2017;186(7):805–814. 10.1093/aje/kwx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. 10.1001/jama.287.6.742 [DOI] [PubMed] [Google Scholar]
- 17. Elovainio M, Lahti J, Pirinen M, et al. Association of social isolation, loneliness and genetic risk with incidence of dementia: UK Biobank Cohort Study. BMJ Open. 2022;12(2):e053936. 10.1136/bmjopen-2021-053936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matthews FE, Stephan BCM, Robinson L, et al. ; Cognitive Function and Ageing Studies (CFAS) Collaboration. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7(1):11398. 10.1038/ncomms11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samtani S, Mahalingam G, Lam BCP, et al. ; SHARED consortium for the Cohort Studies of Memory in an International Consortium (COSMIC). Associations between social connections and cognition: a global collaborative individual participant data meta-analysis. Lancet Healthy Longev. 2022;3(11):e740–e753. 10.1016/S2666-7568(22)00199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anstey KJ, Zheng L, Peters R, et al. Dementia risk scores and their role in the implementation of risk reduction guidelines. Front Neurol. 2021;12:765454. 10.3389/fneur.2021.765454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anstey KJ, Kootar S, Huque MH, Eramudugolla R, Peters R.. Development of the CogDrisk tool to assess risk factors for dementia. Alzheimers Dement. 2022;14(1):e12336. 10.1002/dad2.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butler DC, Petterson S, Phillips RL, Bazemore AW.. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2 Pt 1):539–559. 10.1111/j.1475-6773.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hofbauer LM, Rodriguez FS.. Association of social deprivation with cognitive status and decline in older adults. Int J Geriatr Psychiatry. 2021;36(7):1085–1094. 10.1002/gps.5555 [DOI] [PubMed] [Google Scholar]
- 24. Hofbauer LM, Rodriguez FS.. Validation of a social deprivation index and association with cognitive function and decline in older adults. Int Psychogeriatr. 2021;33(12):1309–1320. 10.1017/S1041610221000995 [DOI] [PubMed] [Google Scholar]
- 25. Figueroa JF, Frakt AB, Jha AK.. Addressing social determinants of health: time for a Polysocial Risk Score. JAMA. 2020;323(16):1553–1554. 10.1001/jama.2020.2436 [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Zeng Z, Zhuang Z, et al. Polysocial and polygenic risk scores and all-cause dementia, Alzheimer’s disease, and vascular dementia. J Gerontol A Biol Sci Med Sci. 2023;79:glad262. [DOI] [PubMed] [Google Scholar]
- 27. Wu C, Ping Y, Chen X, Odden M, Prina M.. Deciphering racial and ethnic disparities in dementia and cognitive function: a polysocial score approach. Innov Aging. 2022;6(Suppl 1):1–1. 10.1093/geroni/igac059.001 [DOI] [Google Scholar]
- 28. Zhao Y, Li Y, Zhuang Z, et al. Associations of polysocial risk score, lifestyle and genetic factors with incident type 2 diabetes: a prospective cohort study. Diabetologia. 2022;65:2056–2065. 10.1007/s00125-022-05761-y [DOI] [PubMed] [Google Scholar]
- 29. Huang Y, Guo J, Donahoo WT, et al. A Fair individualized polysocial risk score for identifying increased social risk in type 2 diabetes. Res Sq. 2023:rs.3–rs.3684698. 10.21203/rs.3.rs-3684698/v1 [DOI] [Google Scholar]
- 30. Lo D, Zia H, Tsiang K, Zia T, Visaria A.. Investigating Polysocial Risk Score In Association With Type 2 Diabetes: A US Perspective [Internet]. NY, USA; 2023. [cited February 18, 2024]. https://papers.ssrn.com/abstract=4575159 [Google Scholar]
- 31. Javed Z, Valero-Elizondo J, Dudum R, et al. Development and validation of a polysocial risk score for atherosclerotic cardiovascular disease. Am J Prevent Cardiol. 2021;8:100251. 10.1016/j.ajpc.2021.100251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang J, Sheng C, Wu YY, Yan LL, Wu C.. Association of joint genetic and social environmental risks with incident myocardial infarction: results from the health and retirement study. J Am Heart Assoc. 2023;12(6):e028200. 10.1161/JAHA.122.028200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen P, Yang Z, Fan Z, et al. Associations of polysocial risk score with incident rosacea: a prospective cohort study of government employees in China. Front Public Health. 2023;11 [cited February 18, 2024]. https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2023.1096687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tian R, He Q, Yang Y, Nong X, Wang S.. Associations of polysocial risk score, lifestyle and genetic factors with incident psoriasis: a larger-scale prospective cohort study. Public Health. 2023;225:320–326. 10.1016/j.puhe.2023.10.034 [DOI] [PubMed] [Google Scholar]
- 35. Tang J, Chen Y, Liu H, Wu C.. Examining racial and ethnic differences in disability among older adults: a polysocial score approach. Maturitas. 2023;172:1–8. 10.1016/j.maturitas.2023.03.010 [DOI] [PubMed] [Google Scholar]
- 36. Zhao Y, Li Y, Zhuang Z, Song Z, Wang W, Huang N, et al. Associations of a Polysocial Risk Score With Total and Cause-Specific Mortality: A Prospective Cohort Study [Internet]. NY, USA; 2022. [cited February 18, 2024]. https://papers.ssrn.com/abstract=4037267 [Google Scholar]
- 37. Crimmins E, Faul J, Kim J, Guyer H, Langa K, Ofstedal MB, et al. Documentation of Biomarkers in the 2006 and 2008 Health and Retirement Study. MI, USA: Institute for Social Research, University of Michigan; 2013. [Google Scholar]
- 38. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR.. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poey JL, Burr JA, Roberts JS.. Social connectedness, perceived isolation, and dementia: does the social environment moderate the relationship between genetic risk and cognitive well-being? Gerontologist. 2017;57(6):1031–1040. 10.1093/geront/gnw154 [DOI] [PubMed] [Google Scholar]
- 40. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. US CDC National Center for Health Statistics. Healthy People—Healthy People Homepage [Internet]. 2023. [cited March 23, 2023]. https://www.cdc.gov/nchs/healthy_people/index.htm [Google Scholar]
- 42. US Department of Health and Human Services. Social Determinants of Health—Healthy People 2030 | health.gov [Internet]. 2020. [cited February 13, 2023]. https://health.gov/healthypeople/priority-areas/social-determinants-health [Google Scholar]
- 43. Wold S, Esbensen K, Geladi P.. Principal component analysis. Chemometr Intell Lab Syst. 1987;2(1):37–52. [Google Scholar]
- 44. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D.. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 45. Liu Z, Chen X, Gill TM, Ma C, Crimmins EM, Levine ME.. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: evidence from the Health and Retirement Study. PLoS Med. 2019;16(6):e1002827. 10.1371/journal.pmed.1002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crimmins EM, Kim JK, Langa KM, Weir DR.. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66B(suppl_1):i162–i171. 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gianattasio KZ, Wu Q, Glymour MM, Power MC.. Comparison of methods for algorithmic classification of dementia status in the Health and Retirement Study. Epidemiology. 2019;30(2):291–302. 10.1097/EDE.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crimmins EM, Saito Y, Kim JK.. Change in cognitively healthy and cognitively impaired life expectancy in the United States: 2000–2010. SSM Popul Health. 2016;2:793–797. 10.1016/j.ssmph.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ware E, Gard A, Schmitz L, Faul J.. HRS Polygenic Scores – Release 4. MI, USA: Survey Research Center, Institute for Social Research, University of Michigan; 2020. [Google Scholar]
- 51. Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349–2358. 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025 and Online Materials | Dietary Guidelines for Americans [Internet]. 2020. [cited February 14, 2023]. https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials [Google Scholar]
- 53. Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. Br Med J. 2018;362:k2927. 10.1136/bmj.k2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121(4):586–613. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 55. Fine JP, Gray RJ.. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. 10.2307/2670170 [DOI] [Google Scholar]
- 56. Ping Y, Oddén MC, Stawski RS, Abdel Magid HS, Wu C.. Creation and validation of a polysocial score for mortality among community-dwelling older adults in the USA: the Health and Retirement Study. Age Ageing. 2021;50(6):2214–2221. 10.1093/ageing/afab174 [DOI] [PubMed] [Google Scholar]
- 57. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R.. A Systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. 2019;70(s1):S165–S186. 10.3233/JAD-190181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Röhr S. Social determinants of brain health need to be addressed in risk reduction of cognitive decline and dementia. Int Psychogeriatr. 2021;33(12):1249–1251. 10.1017/S104161022100260X [DOI] [PubMed] [Google Scholar]
- 59. Adkins-Jackson PB, George KM, Besser LM, et al. The structural and social determinants of Alzheimer’s disease related dementias. Alzheimers Dement. 2023;19(7):3171–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Piumatti G, Moore SC, Berridge DM, Sarkar C, Gallacher J.. The relationship between alcohol use and long-term cognitive decline in middle and late life: a longitudinal analysis using UK Biobank. J Public Health. 2018;40(2):304–311. 10.1093/pubmed/fdx186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jeon KH, Han K, Jeong SM, et al. Changes in alcohol consumption and risk of dementia in a nationwide cohort in South Korea. JAMA Network Open. 2023;6(2):e2254771. 10.1001/jamanetworkopen.2022.54771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ilomaki J, Jokanovic N, Tan ECK, Lonnroos E.. Alcohol consumption, dementia and cognitive decline: an overview of systematic reviews. Curr Clin Pharmacol. 2015;10(3):204–212. 10.2174/157488471003150820145539 [DOI] [PubMed] [Google Scholar]
- 63. Schutzer KA, Graves BS.. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–1061. 10.1016/j.ypmed.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 64. Chen M, Yerramalla MS, van Hees VT, et al. Individual barriers to an active lifestyle at older ages among Whitehall II study participants after 20 years of follow-up. JAMA Network Open. 2022;5(4):e226379. 10.1001/jamanetworkopen.2022.6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT.. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 66. Kornblith E, Bahorik A, Boscardin WJ, Xia F, Barnes DE, Yaffe K.. Association of race and ethnicity with incidence of dementia among older adults. JAMA. 2022;327(15):1488–1495. 10.1001/jama.2022.3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen C, Zissimopoulos JM.. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement. 2018;4:510–520. 10.1016/j.trci.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275–284. 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HRS data are all publicly available to researchers. Researchers can access and download the HRS data from its website (https://hrs.isr.umich.edu/data-products).