Abstract
Dengue virus (DENV) nonstructural protein 5 (NS5), consisting of methyltransferase and RNA-dependent RNA polymerase (RdRp) domains, is critical for viral RNA synthesis within endoplasmic reticulum-derived replication complexes in the cytoplasm. However, a significant proportion of NS5 is localized to the nucleus of infected cells for DENV2, 3, and 4, whereas DENV1 NS5 is localized diffusely in the cytoplasm. We still have an incomplete understanding of how the DENV NS5 subcellular localization is regulated. Within NS5, two putative nuclear localization signal (NLS) sequences have been identified: NLSCentral residing in the palm of the RdRp domain as well as the recently discovered NLSC-term residing in the flexible region at the C-terminal of the RdRp domain. We have previously shown that DENV2 NS5 nuclear localization can be significantly reduced by single-point mutations to the NLSC-term. Here, we present biochemical, virological, and structural data demonstrating that the relative importance of either NLS in NS5 nuclear localization is unique to each of the four DENV serotypes. DENV1 NS5′s cytoplasmic localization appears to be due to a functionally weak interaction between its NLSCentral and importin-α (IMPα), while DENV2 NS5 is almost exclusively nuclear through its NLSC-term’s strong interaction with IMPα. Both NLSs of DENV3 NS5 appear to contribute to directing its nuclear localization. Lastly, in the case of DENV4, the regulation of its NS5 nuclear localization remains an enigma but appears to be associated with its NLSC-term.
Keywords: dengue virus serotypes, nuclear localization signal, importin, importin-α cocrystal structure
Dengue virus (DENV) is a member of the flavivirus genus that includes Zika virus (ZIKV), yellow fever virus (YFV), West Nile virus (WNV), and Japanese encephalitis virus (JEV), which collectively cause significant global healthcare burden. In infected cells, the 11-kilobase DENV single-stranded positive-sense RNA genome is translated by host ribosomes into a single linear polyprotein precursor that is cleaved by viral and host proteases into three structural (C, prM, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5), of which NS5 is the largest (∼100 kDa, 900 amino acids) and most conserved protein across the four DENV serotypes (DENV1–4) and across the flavivirus genus.1,2
DENV NS5 bears N-terminal methyltransferase (MTase) and C-terminal RNA-dependent RNA polymerase (RdRp) enzymatic activities and is essential for viral RNA capping and synthesis within endoplasmic reticulum (ER)-derived replication complexes. Additionally, DENV2 NS5 has been shown to induce the expression and secretion of cytokines IL-6 and IL-8,3,4 while NS5 of DENV1–4 and ZIKV has also been shown to promote the degradation of human STAT2, leading to inhibition of type I IFN signaling and establishing an environment conducive for viral replication.5−8
Despite this vital cytoplasmic role, flavivirus NS5 has been observed within the nucleus of infected cells, most notably in the case of DENV, ZIKV, and YFV,9−14 but less so for WNV, JEV, Usutu virus, and Duck Tembusu virus.15−18 It has been proposed that nuclear NS5 is important for modulation of the host immune response; previous studies have focused on the association between nuclear NS5 and induction of type I IFN response4,19,20 as well as interactions between DENV2 NS5 and the spliceosome.21,22 However, it remains an open question as to whether nuclear NS5 plays a critical role in DENV pathogenesis. A functional, virological approach to answering this question is to generate mutant viruses with relocalized NS5 for comparison with the wild-type DENV, in order to assess the impact of nuclear NS5 on defined pathogenesis markers. To achieve this goal, the NS5 sequences and host factors that allow them to facilitate NS5 nuclear import need to be clearly understood.
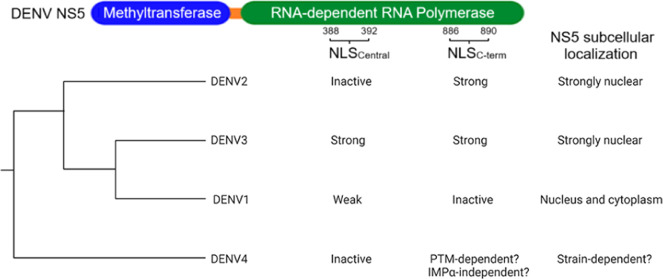
At ∼100 kDa, NS5 is too large to passively diffuse into the nucleus.23 Active nuclear import of NS5 is thought to rely on recognition of its nuclear localization signal (NLS) sequence by the host importin-α/β (IMPα/β) pathway,12 whereby efficiency of nuclear transport is determined by NLS/IMPα binding affinity.23 The best-characterized NLSs are the classical NLSs (cNLSs), which can be categorized as either monopartite or bipartite, and are characterized by clusters of basic amino acids: the monopartite cNLS has a K–(K/R)–X–(K/R) motif (positions P2–P5; X corresponds to any residue).23 Interestingly, the nuclear-to-cytoplasmic localization ratio (Fn/c) of NS5 in infected cells differs among DENV1–4, as shown by our group and others. While DENV1 NS5 is mostly cytoplasmic, DENV2 NS5 and DENV3 NS5 are primarily nuclear.12 In the case of DENV4, the H241 strain has been reported to have mostly cytoplasmic NS5;10 however, our group has reported that the EDEN4 strain has predominantly nuclear NS5.12 This differential NS5 subcellular localization is observed despite NS5 being ∼70% conserved across DENV1–4.2 Two putative classical monopartite NLS regions have been identified in flavivirus NS5,14 which we refer to as NLSCentral and NLSC-term, for clarity following recent convention.20 Initial studies to characterize the DENV2 NS5 NLS focused on the NLSCentral region (residues 380–398, previously known as the α/βNLS24−27). However, we recently discovered a novel NLS in the DENV NS5 C-terminal (residues 881–900 in DENV2, designated NLSC-term) that we found to be the primary determinant of NS5 nuclear localization, at least in the case of DENV2.12 Interestingly, we discovered that the NLSC-term P–1 proline residue (P884 in DENV2) was important for presenting the NLSC-term in the trans conformation for IMPα-binding and nuclear import; DENV1 NS5 has a threonine at this position (T883) and is mostly cytoplasmic.12 Crucially, a single P884T substitution is sufficient to significantly relocalize DENV2 NS5 from the nucleus to the cytoplasm without attenuating viral replication.12,19 It should be noted that the P–1 residue in DENV3 (P884) and DENV4 (P885) is also a proline.
In this work, we initially sought to build on our prior studies with DENV2 P884T by first investigating the role of the NLSC-term P–1 residue in determining the subcellular localization of NS5 of the remaining DENV serotypes in the context of infection. Intriguingly, we found that the NLSC-term was not the major determinant of DENV3 and 4 NS5 subcellular localization, unlike that in the case of DENV2 NS5. Using a combination of biochemical, structural, and virological approaches to clarify the relationship between NLS-IMPα interactions and NS5 subcellular localization, we found that the contribution of either NLS to NS5 nuclear localization varies for each DENV serotype. DENV2 NS5 appears to be exclusively driven by its NLSC-term, while the mostly cytoplasmic DENV1 NS5 is the result of its functionally weak NLSs. For DENV3 NS5, both NLSs appear to be able to direct nuclear localization. We also found that DENV4 NS5 nuclear localization occurs despite the poor affinity of either NLS to IMPα, suggesting that the yet-unknown DENV4-specific mechanism may involve post-translational modifications of residues at the C-terminal region and/or other parts of the protein.
Results
Variable Impact of the NLSC-term P–1 Residue on the Subcellular Localization of DENV NS5 in Infected Cells
We previously showed that DENV2 NS5 nuclear localization was driven primarily by the NLSC-term and demonstrated the importance of the NLSC-term P–1 proline in presenting this NLS in the trans conformation for IMPα-binding.12,19 Building on this work, we investigated the role of this NLSC-term P–1 residue in the other DENV serotypes. We generated viruses containing the analogous mutation in DENV3 (P884T) and DENV4 (P885T) and the reverse T883P mutation in DENV1 and infected Huh7 cells for immunofluorescence assay (IFA) to visualize their NS5 subcellular localization. DENV2 wild-type (WT) and P884T mutant infectious clone-derived viruses that had previously been generated were used as controls.19
NS5 from the infectious clone-derived DENV1 WT virus localized predominantly to the cytoplasm (Fn/c = 0.5, Figure 1), consistent with the parental clinical isolate12 (Fn/c = 0.8, see), whereas a single T883P substitution was sufficient to significantly relocalize DENV1 NS5 to the nucleus (Fn/c = 7.6, Figure 1). This confirms previous work with recombinantly expressed DENV1 T883P NS5,12 showing the importance of the NLSC-term P–1 position in directing DENV1 NS5 localization.
Figure 1.
Impact of NLSc-term P–1 mutations on the subcellular localization of DENV1–4 NS5 in infected cells. (A) Huh7 cells were infected with WT and NLSC-term P–1 mutant DENV (GenBank accession numbers: DENV1 (EU081230), DENV2(EU081177), DENV3 (EU081190), and DENV4 (GQ398256)) at MOI = 1 and fixed at 24 hpi. Cells were analyzed for the presence of NS5 (green) and dsRNA (red), with nuclei stained by DAPI (blue). Digitized images were captured using a Zeiss LSM 710 upright confocal microscope with 63× oil immersion lens. The scale bar represents 50 pm. (B) Image analysis using ImageJ software was performed on digitized images of ≥20 cells to determine Fn/c for each of the viruses from (A). Fn/c values were quantified and plotted, with each point representing a quantified cell. The dashed line indicates Fn/c = 1, whereby equivalent levels of nuclear and cytoplasmic NS5 are quantified. The Kruskal–Wallis nonparametric test was used to test for statistical significance. Results are from two to three independent experiments.
For DENV3 and DENV4, the role of the NLSC-term P–1 position was less clear-cut. We found that the DENV3 and DENV4 WT NS5 localized to the nucleus (Fn/c = 18 and 14, Figure 1), consistent with their respective parental clinical isolates (Fn/c = 13 and 4.9, see12). We observed some relocalization of DENV3 P884T NS5 to the cytoplasm, but it was not nearly as significant as that seen in DENV2, and DENV3 P884T NS5 was still predominantly nuclear (Fn/c = 3.8, Figure 1). This phenotype is also observed for DENV4 P885T NS5 (Fn/c = 4.9, Figure 1), although this finding in DENV4 is complicated by the observation that mutations to the DENV4 genome tend to quickly revert back to the wild-type sequence following routine virus expansion in C6/36 cells, suggesting that this region may have functional significance in the context of infection. We will further explore this in a later section.
Taken together, the Fn/c analysis of the NS5 NLSC-term P–1 mutants for DENV 1–4 by IFA suggests that the role of the NLSC-term in driving NS5 to the nucleus varies between each DENV serotype. The growth curves for the WT and the corresponding NS5 NLSC-term P–1 mutants for the serotypes are not significantly affected by changes in the subcellular location of NS5, even in the case of DENV1 where there is a significant shift from cytoplasmic to nuclear localization. This is similar to what we observed previously for DENV212 (Figure S1). This prompted us to take a step back to holistically examine the contributions of both NLSs to NS5 subcellular localization, relying on in silico prediction, biochemical binding assays, and cocrystallization as well as ectopic expression of the NS5 WT and mutants as needed.
Differential Binding Affinities of DENV NS5 NLSCentral and NLSC-term to IMPα
Two putative classical monopartite NLS regions have been identified in flavivirus NS5.14,20 Both the NLSCentral and the NLSC-term reside within the RdRp domain (Figure 2A,B). The NLSCentral region (residues 380–398 in DENV2) resides just upstream of motif G of the RdRp and has been previously demonstrated to be critical for the nuclear localization of ZIKV NS5.13,28 Meanwhile, the NLSC-term region (residues 881–900 in DENV2) resides in the C-terminus of the RdRp domain, a highly flexible region that is unresolved in almost all published structures of DENV or ZIKV NS5, with the notable exception of the DENV3 NS5 structure obtained by Choi’s group.29 We first performed in silico analysis by entering the full NS5 sequence into cNLS Mapper prediction software that scores the strength of any detected NLS sequence based on the contribution of each amino acid residue in the sequence.30 A protein bearing an NLS with a score of 8–10 is predicted to be exclusively localized to the nucleus, while one bearing an NLS with a score between 3 and 7 is predicted to be localized to both the nucleus and the cytoplasm. Finally, proteins without a detected putative NLS (assigned a 0 score) or bearing an NLS assigned a score of 1 or 2 are predicted to be localized to the cytoplasm. The NLSCentral region of DENV1–4 scored 6, 0, 8.5, and 0, respectively, while the NLSC-term region scored 2, 7.5, 6.5, and 0, respectively (Figure 2A and Tables S1–S4). The results from cNLS Mapper for the NLSC-term are consistent with our previous biochemical studies into their IMPα-binding affinity, as the high-scoring NLSC-term of DENV2 and DENV3 binds strongly to IMPα.12 Thus, the comparable high scores for the NLSCentral of DENV1 and 3 (6 and 8.5, respectively) suggest that they will be able to bind IMPα and may be functional in directing NS5 subcellular localization. This difference in scores across serotypes is despite fairly high conservation of the 2 NLS regions between the serotypes (63–79 and 50–80% pairwise identity for NLSCentral and NLSC-term, respectively) (Figure S2).
Figure 2.
Binding affinity of DENV NS5 NLScentral and NLSC-term to IMPα is serotype-specific. (A) Sequence alignment of NLScentral and NLSC-term for DENV1–4 and related flaviviruses. Residues in red have been shown to bind to the major groove of the host receptor lmportin-α in NLS peptide-lmportin α cocrystals in this (E) and previous work.1−3 The indicated cNLS scores were computed by cNLS Mapper with full-length DENV1–4 NS5 as input. The GenBank accession numbers are as follows: DENV1 (EU081230), DENV2 (EU081177), DENV3 (EU081190), DENV4 (GQ398256), DENV4 H241 (AY947539), ZIKV (KJ776791), YFV (NC 002031.1), WNV (NC_001563.2), JEV (M55506), USUV (MN813491), and TMUV (KM233707). (B) NLScentral resides in the N-terminal region of the RdRp, close to the enzymatic site, while the NLSC-term is in the flexible C-terminal region of the RdRp. (C) Direct binding between the indicated FITC-labeled DENV NLS peptides and IMPα isoforms was assessed by fluorescence polarization assay. Curves were plotted in GraphPad Prism from three independent experiments. (D) Strong correlation between the strength of NLScentral or NLSC-term as scored by cNLS Mapper and in vitro NLS/IMPα binding affinity. >ULOQ = above upper limit of quantitation. (E) Structure of IMPα bound to NLScentral of DENV1, DENV3, and ZIKV, resolved to 2.75, 2.1, and 2.2 Å, respectively, as well as an overlay of all three. See Table S5 for refinement statistics. The schematic highlights the hydrogen bonds and salt bridges (in boldface) forming between residues of IMPα and the corresponding NLScentral. ZIKV NLS/IMPα structure obtained from PDB: 5W41.
We next sought to investigate the role of both NLSCentral and NLSC-term in all four DENV serotypes, by first examining the NLS-IMPα binding affinity. NS5 nuclear import via the host IMPα/IMPβ pathway relies on recognition of its positively charged NLS by the nuclear import receptor IMPα.12,13 The human genome encodes for seven isoforms of IMPα, grouped into three subfamilies.31 We first adopted a biochemical approach to quantify the binding affinity of both the NLSCentral-IMPα and the NLSC-term-IMPα interaction for each serotype, by performing a fluorescence polarization (FP) assay using recombinantly expressed IMPαΔIBB (human isoforms 1, 3, and 7, representing each of the three IMPα subfamilies,31 each lacking the autoinhibitory N-terminal IMPβ-binding domain) and FITC-tagged DENV NLS peptides spanning the residues indicated in Figure 2A.
For the NLSC-term, our measurements of the NLSC-term-IMPα affinity showed trends consistent with previous data from solid-phase binding or pulldown assays.12 The DENV2 and 3 NLSC-term exhibited strong affinity for IMPα1 (Kd = 90 ± 10 and 140 ± 20 nM, respectively), IMPα3 (Kd = 80 ± 10 and 150 ± 30 nM, respectively), and IMPα7 (Kd = 180 ± 30 and 190 ± 40 nM, respectively), whereas the affinity of DENV1 and 4 NLSC-term for IMPα1 was not determinable within the range of our assay (Figure 2C,D). Given our observation that the NLSC-term P–1 residue impacts NS5 subcellular localization in the context of infection for DENV1 and 2 but less so for DENV3 and 4 (Figure 1), we also examined the impact of this residue on IMPα-binding affinity. As expected, we saw that T883P significantly promoted the binding of DENV1 NLSC-term to IMPα by ∼5-fold, whereas P884T substitution reduces the binding affinity of IMPα for the NLSC-term of both DENV2 and DENV3 by ∼10-fold (Figure S3).
For the NLSCentral, DENV3 exhibited strong affinity for IMPα1, IMPα3, and IMPα7 (Kd = 36 ± 4, 160 ± 50, and 100 ± 40 nM, respectively), consistent with the cNLS Mapper prediction (Figure 2C,D) and our hypothesis that both NLSCentral and NLSC-term are functional in DENV3 NS5. DENV1 NLSCentral displayed barely detectable affinity for IMPα1 (Kd > 700 nM) and weak but detectable affinity for the IMPα3 and α7 isoforms (Kd = 400 ± 100 and 360 ± 40 nM, respectively) (Figure 2C,D), consistent with the observation that DENV1 NS5 is primarily localized in the cytoplasm during infection (Figure 1). In contrast, neither DENV2 nor DENV4 NLSCentral exhibited binding affinity for IMPα (Figure 2C,D). We further validated the DENV1–4 NLSCentral/IMPα-binding data using a qualitative electrophoretic mobility shift assay (EMSA). We found that only the NLSCentral of DENV1 and DENV3 bound to the IMPαs, whereas the NLSCentral of DENV2 and DENV4 failed to bind IMPα (Figure S4A). As the P2 lysine of the NLS is deemed to contribute ∼50% of the binding affinity for IMPα,32 we examined the impact of substituting the P2 lysine to alanine (K388A) or arginine (K388R) on the FITC-DENV3 NLSCentral peptide/IMPα interaction. As measured by both FP and EMSA, we found that both K388A and K388R mutations drastically reduced the binding affinity of DENV3 NLSCentral for IMPα1, 3, and 7, beyond the measurable limit of our assay (Figure S4B–C).
With the in silico and biochemical assays suggesting that the NLSCentral of DENV1 and 3 may be functional, we sought to cocrystallize all four DENV NS5 NLSCentral with IMPα for structural studies, as we had previously done with the NLSC-term of DENV2 and 3.12 Consistent with the biochemical binding assays, we were successful in crystallizing IMPα with the NLSCentral of DENV1 and DENV3 and resolved the structures to 2.7 and 2.1 Å, respectively (see Table S5 for data collection and refinement statistics). We were able to resolve residues 387–392 of the DENV1 NLSCentral and 383–392 of the DENV3 NLSCentral binding within the armadillo (ARM) repeats, ARM2–4, major binding site in a monopartite fashion (Figure 2E). These structures are remarkably similar to the recently solved structure of IMPα bound to NLSCentral of ZIKV.12,13 We used PDBePISA33 to determine the interactions between NLSCentral and IMPα. IMPα/NLSCentral interactions were strongly conserved between DENV1 and DENV3 (Figure 2E; see Table S6 for detailed analysis of specific interactions). Overall, the NLSCentral of both DENV1 and DENV3 binds IMPα in a manner very similar to the recently solved structure of IMPα bound to NLSCentral of ZIKV.12,13 In contrast, we were unable to cocrystallize the NLSCentral of DENV2 or DENV4 with IMPα, likely due to insufficient binding affinity and consistent with the in silico and biochemical assays.
Together with the previously determined structure of DENV3 NLSC-term bound to IMPα2, this suggests that DENV3 has uniquely evolved redundancy of both NLSs to direct nuclear localization of NS5. Overall, our in silico, structural, and biochemical data confirm that IMPα is able to bind to the monopartite NLSCentral of DENV1 and DENV3 NS5 but not DENV2 or DENV4. This suggests that the NLSCentral may play a greater role in the nuclear import of DENV1 and 3 NS5 than previously thought: both NLSCentral and NLSC-term may play a role in DENV3 NS5 nuclear import, while the comparatively weaker DENV1 NLSCentral–IMPα affinity correlates with the low but detectable presence of DENV1 NS5 in the nucleus during infection (Figure 1, see also ref (12)). Meanwhile, the mechanism for DENV4 nuclear import is still unclear, given that neither NLS displayed affinity for any of the IMPα isoforms (Figure 2C,D).
Varying NLS Contributions to DENV1 and DENV3 NS5 Subcellular Localization in Transfected Cells
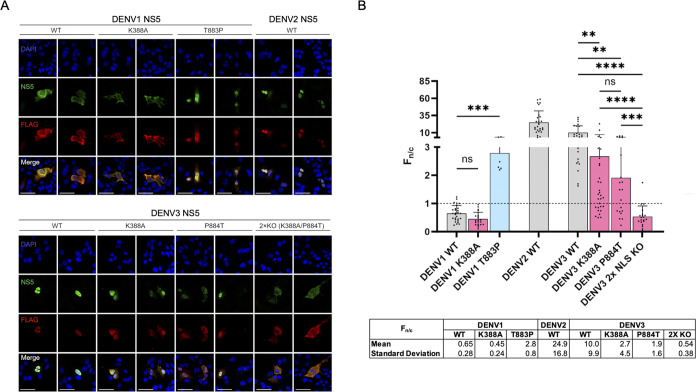
As our biochemical and virological data suggest that NLSCentral plays a role in the nuclear localization of DENV1 and 3 NS5, we sought to investigate the contributions of each NLS in NS5 nuclear import in vivo by performing IFA on Huh7 cells expressing recombinant wild-type and mutant DENV1 and DENV3 NS5 proteins, specifically DENV1 WT, K388A, T883P, DENV3 WT, K388A, P884T, and the double NLS mutant K388A/P884T (termed “2 × NLS KO”). Due to the previously reported lethality of mutations in the NLSCentral, we decided to first take this recombinant protein approach. We chose to use a plasmid with DENV NS5 engineered to be expressed downstream of ubiquitin such that cellular hydrolases cleave ubiquitin in a manner that mimics the cleavage of NS4B away from NS5 by the NS2B/3 protease during DENV infection. This has previously been shown to be important for NS5 function, particularly its ability to mediate STAT2 degradation.5,6 Reassuringly, we found the localization phenotype for the DENV1 WT, T883P, DENV2 WT, DENV3 WT, and P884T recombinant NS5 proteins to be consistent with that observed in infected cells (Figure 1), with an Fn/c of 0.65, 2.79, 24.9, 9.97, and 1.92, respectively (Figure 3). In the case of DENV1, the single K388A substitution slightly reduces NS5 nuclear accumulation compared with the wild type (Fn/c = 0.45), although the reduction in Fn/c is not statistically significant. For DENV3, although the single K388A or P884T mutations individually reduced nuclear accumulation of NS5, the Fn/c ratios of these mutants (2.68 and 1.92, respectively) indicate that they were still primarily nuclear. When we generated a mutant bearing both mutations, this 2× NLS KO mutant NS5 was predominantly cytoplasmic to an extent comparable to that of DENV1 NS5 (Fn/c = 0.54, Figure 3). This strongly demonstrates that both NLSCentral and NLSC-term contribute to directing DENV3 NS5 into the nucleus.
Figure 3.
Varying NLS contributions to DENV1 and DENV3 NS5 subcellular localization in transfected cells. (A) Huh7 cells were transfected with NS5-FLAG constructs as indicated and fixed at 24 hpt (GenBank accession numbers: DENV1 (EU081230), DENV2 (EU081177), and DENV3 (EU081190)). Cells were analyzed for NS5 (green) and FLAG (red) to verify proper folding of NS5, with nuclei stained by DAPI (blue). The scale bar represents 50 μm. (B) Image analysis using ImageJ software was performed on digitized images of ≥20 cells to determine Fn/c. Fn/c values were quantified and plotted, with each point representing a quantified cell. The dashed line indicates Fn/c = 1 whereby equivalent levels of nuclear and cytoplasmic NS5 are quantified. The Kruskal–Wallis nonparametric test was used to test for statistical significance. Results are from two to three independent experiments.
DENV4 NS5 Subcellular Localization Regulation Extends beyond NLS/Importin Binding
Our results demonstrate a causal link between the strength of the NLS/IMPα interaction and the ability of NS5 to localize to the nucleus for DENV1–3 NS5. In contrast, the localization of DENV4 NS5 is predominantly nuclear (Fn/c = 14, Figure 1) despite a lack of detectable binding affinity of either NLSCentral or NLSC-term for IMPα (Figure 2C,D). Hence, we sought to unravel the mechanism behind DENV4 NS5 subcellular localization regulation. Our initial attempt to apply the same recombinant expression approach to interrogating this was thwarted by a surprising observation that unlike the consistent phenotype for DENV1–3 NS5, DENV4 NS5 subcellular localization differs between infected and transfected cells. Recombinantly expressed DENV4 NS5 is localized to the cytoplasm of >50% of cells while being predominantly nuclear in others, as illustrated in the violin plot (Fn/c = 1.6, Figure 4). In contrast, DENV4 NS5 is strongly localized to the nucleus in the context of infection (Fn/c = 14, Figures 1A and 4). This divergence leads us to hypothesize that there are additional factors and conditions involved, such as post-translational modifications that occur only in the context of DENV4 infection.
Figure 4.
DENV4 NS5 subcellular localization differs between infected and transfected cells. (A) Huh7 cells were transfected with FLAG-tagged pEF-Ub DENV2 and DENV4 NS5 plasmids (GenBank accession numbers: DENV2 (EU081177) and DENV4 (GQ398256)) and fixed and stained as in Figure 3A. The scale bar represents 50 μm. (B) Image analysis of transfected NS5 (A) and NS5 during infection (Figure 1A) is performed as in Figure 1. Fn/c values were quantified and plotted as violin plots to show the spread of Fn/c for each virus. Each point refers to a single cell. The dashed line indicates Fn/c = 1, whereby equivalent levels of nuclear and cytoplasmic NS5 are quantified. The median is indicated by a yellow line. The Kruskal–Wallis nonparametric test was used to test for statistical significance. Results are from two to three independent experiments.
To explore this, we used the Musitedeep34,35 algorithm to predict post-translational modifications of DENV4 NS5 (EDEN4 strain) and found that residues S891, S894, and S896 were predicted as potential phosphorylation sites (Figure 5A). While S891 and S896 are highly conserved among 375 DENV4 sequences in the Virus Variation Resource database,36 the S894 residue is a serine in only ∼42.5% of the DENV4 sequences and a phenlyalanine in ∼56% of the DENV4 sequences including the H241 strain that is mainly cytoplasmic. Interestingly, phosphorylation of residues proximal to NLS regions is known to regulate nuclear import.37,38 This suggests that this S894 residue, as predicted by Musitedeep, may be a critical determinant of DENV4 NS5 nuclear localization. We set out to further investigate the role of the DENV4 NLSC-term in NS5 subcellular localization, including testing the hypothesis that phosphorylation of one or more of these residues regulates the NLSC-term/IMPα interaction and NS5 nuclear import. We generated a series of mutant viruses (listed in Figure 5B): (1) Two chimeric NLSC-term mutants: a DENV2-DENV4 Cter17 virus whereby the NS5 C-terminal 17 amino acids of the DENV2 genome were substituted with the corresponding region of DENV4 NS5 and the reverse chimera DENV4-DENV2 Cter18 virus whereby the NS5 C-terminal 18 amino acids of the DENV4 genome were substituted with the corresponding region of DENV2 NS5. (2) Single-substitution mutants targeting the NS5 NLSC-term P–1 proline and the P2 lysine, which our data for DENV1–3 show are important for NLS:IMPα binding (Figures S3–S4, see also refs (12,32)). (3) Mutations to candidate phospho-sites S891, S894, and S896 to potentially abolish phosphorylation.
Figure 5.
Role of NLSc-term in DENV4 NS5 nuclear localization. (A) WebLogo analysis of DENV4 sequences (375 sequences obtained from the virus variation resource database). Red triangles indicate residues predicted by MusiteDeep to be phosphorylated. The residue in pink is the P–1 proline that is important for NLSC-term/IMPα binding, while the blue residues are the corresponding P1–P5 residues identified as NLSc-term binding determinants in DENV2 and 3 NS5. (B) DENV infectious clones were generated with the indicated mutations to investigate the role of NLSc-term in DENV4 NS5 subcellular localization. DENV2 residues are in red. (C) Huh7 cells were infected with the DENV2 and DENV4 WT and mutant viruses as indicated (GenBank accession: DENV2 (EU081177) and DENV4 (GQ398256)). Cells are fixed with 24 hpi and stained as in Figure 1. The scale bar represents 50 μm. The panels for DENV2 P884T, DENV4 WT, and DENV4 P885T are as in Figure 1. (D) Image analysis is performed as in Figure 4B, and Fn/c values are quantified and plotted as in Figure 4B. The dashed line indicates Fn/c = 1, whereby equivalent levels of nuclear and cytoplasmic NS5 are quantified. Median intensity is indicated by a yellow line. The Kruskal–Wallis nonparametric test was used to test for statistical significance. Results are from two to three independent experiments.
All of these mutant viruses were replication-competent as assessed by qRT-PCR and IFA. While the DENV2-DENV4 Cter17 virus had plaque morphology similar to the DENV2 parental virus, none of the DENV4 WT or mutant viruses formed clearly discernible plaques (Figure S5).
Mutations to NLSC-term Impact DENV4 NS5 Nuclear Localization but Are Prone to Reversion
To characterize the importance of the NLSC-term in regulating DENV4 NS5 nuclear import, we infected Huh7 cells with our series of DENV4 WT and mutant viruses (Figure 5B) to assess their respective NS5 subcellular localization by IFA. Remarkably, although both DENV2 WT NS5 and DENV4 WT NS5 are predominantly nuclear, chimeric DENV2-DENV4 Cter17 NS5 is localized almost entirely to the cytoplasm (Figure 5C,D, Fn/c = 0.38). Given that this chimeric virus has the NLSCentral of DENV2 and the NLSC-term of DENV4, neither of which have strong affinity for IMPα (Figure 2A,C–E), this result validates our previous in vitro data and further demonstrates that the DENV4 NLSC-term is nonfunctional in a DENV2 context, suggesting that DENV4 NS5 nuclear localization requires additional domains of DENV4 NS5 or a host cell environment specific to DENV4 infection. In contrast, the reverse chimera DENV4-DENV2 Cter18 NS5 is strongly nuclear (Fn/c = 22, Figure 5C,D), as expected due to the presence of the dominant DENV2 NLSC-term. All other single-substitution mutant viruses had mixed NS5 localization phenotypes in a manner similar to ectopically expressed DENV4 NS5 (Figure 4); some cells had strongly nuclear NS5, whereas others had strongly cytoplasmic NS5, as illustrated in the violin plots (Figure 5D). While most of the mutations had vastly mixed localization phenotypes, S894A did result in the most significant relocalization of NS5 to the cytoplasm of infected cells (Fn/c = 1.8, Figure 5C,D).
We assessed the stability of each of our NLSC-term mutations in the viruses used in Figure 5C,D that were generated following two rounds of virus expansion as per routine protocol. Unusually, we detected evidence of reversion to the respective WT residues by Sanger sequencing in each of the DENV4 mutant viruses we generated during viral expansion via two passages in C6/36 cells, ranging from ∼25% reversion for S894A to almost complete reversion for P2 lysine mutants K888A and K888R (summarized in Table 1). Such reversions are atypical based on our experience in generating recombinant viruses using reverse genetics, although it should be noted that our analysis is qualitative based on Sanger sequencing. In contrast, no mutations were detected in the genomes of the DENV2 WT, DENV4 WT, or the DENV2-DENV4 Cter17 chimeric virus. This suggests that the NLSC-term region of the DENV4 genome is under strong selection pressure, especially residues such as K888 that is adjacent to R888 in DENV2 (R889 in DENV4) previously found to be essential for de novo initiation through a direct interaction with 3′SL.39 This evidence of reversion of these mutants suggests that there is a mixed population of viruses infecting the Huh7 cells for IFA studies (Figure 5C,D), which might account for the variation in the NS5 localization phenotype from cell to cell. While this clouded our ability to interrogate the effect of each mutation, these data nonetheless demonstrate that mutations to the NLSC-term have an impact on DENV4 NS5 nuclear localization. A deeper investigation of this is beyond the scope of this current study.
Table 1. Summary of Reversions of DENV4 Mutant Viruses (GenBank Entry GQ398256) Following Two Passages in C6/36 Cells, as Assessed by Sanger Sequencinga.
Representative results from two independent expansions of wild-type and mutant viruses.
Additionally, we biochemically investigated whether phosphorylation of Y890, S891, S894, or S896 may promote binding of FITC-tagged DENV4 NLSC-term peptides to IMPα in a gel-shift EMSA. We observed that both WT and phospho-NLSC-term peptides migrated toward the anode at the same rate whether in the absence (Figure S6A, lanes 4–8) or presence of IMPα (Figure S6A, lanes 9–23), indicating that none of our phospho-NLSC-term peptides were able to bind to IMPα. Similarly, none of these phospho-NLSC-term peptides displayed binding affinity for IMPα as measured by FP, within the optimized range of our assay (data not shown). We considered that DENV4 NS5 may utilize an IMPα-independent pathway for nuclear import and assessed if DENV4 NLSC-term may instead bind to IMPβ or 14–3–3. Using EMSA, we similarly observed that none of our WT or phosphorylated peptides were able to bind to IMPβ (Figure S6B) or 14–3–3 isoforms ϵ, ζ, or σ (data not shown). It is therefore still unclear how the DENV4 NLSC-term drives NS5 nuclear import, and further in-depth studies beyond the scope of this study are necessary to fully understand the mechanisms behind NS5 nuclear localization.
Discussion
In this study, we took biochemical, structural, and virological approaches to investigate the serotype-specific regulation of the subcellular localization of DENV NS5. Having previously shown the importance of the NS5 NLSC-term P–1 residue in directing DENV2 NS5 subcellular localization,12 we first undertook a virological approach to investigate the importance of this residue in the other DENV serotypes (Figure 1). We then investigated the interaction between IMPα and the NLS sequences of NS5 from all four serotypes of DENV and its role in driving subcellular localization. We demonstrated that our biochemical binding data obtained using FITC-tagged NLS peptides covering NLSCentral and NLSC-term (Figure 2C–E) agree well with in silico predictions (Figure 2A) and our experimental findings on subcellular localization using infectious clones with specific mutations or ectopic expression of NS5, whereby the contribution of NLSCentral and NLSC-term to NS5 nuclear localization is unique to each DENV serotype.
Serotype-Specific Mechanisms of NS5 Nuclear Import
DENV1
Although the core NLSC-term P1–P5 binding motif is intact, a P–1 proline is required for optimal NLSC-term/IMPα binding and nuclear import (Figures 1–2 and S3). Hence, the native NLSC-term with a P–1 threonine is unable to bind IMPα to drive nuclear import. The NLSCentral is able to bind weakly to and cocrystallize with IMPα (Figure 2C–E) but functionally does not appear to drive significant amounts of DENV1 NS5 nuclear import. Taken together, there is little nuclear import of DENV1 NS5 and this accounts for its primarily cytoplasmic localization (Figures 1–3).
DENV2
The lack of NLSCentral/IMPα binding affinity (Figure 2C,D) validates our previous data that DENV2 NS5 nuclear localization is driven dominantly by strong NLSC-term.
For both DENV1 and DENV2, a single mutation of the NLSC-term P–1 residue is able to dramatically relocalize NS5. We were successful in generating a mutant DENV1 virus in which a single NS5 T883P substitution is sufficient to significantly relocalize NS5 from the cytoplasm to the nucleus of both infected and transfected cells (Figures 1 and S7). This mutant T883P virus can be used as a tool to understand the impact of NS5 relocalization on DENV1 pathogenesis, in a manner similar to our previous studies with DENV2 P884T.12,19 Our studies show that this mutant did not replicate significantly differently from the WT (Figure S1). This finding is also similar to our previous demonstration for DENV2 and its NS5 P884T mutant, suggesting that the subcellular localization of NS5 is not crucial for virus replication itself, at least in a tissue culture system, but nuclear NS5 has been implicated in the modulation of the host immune response19,21 and hence might play a role in pathogenesis or host response to virus infection. Hence, future work will focus on studying pathogenesis upon Aedes aegypti or mouse infection, with particular focus on the stability of the T883P mutation.
DENV3
DENV3 has evolved redundant NLS in its NS5, with both NLSCentral and NLSC-term playing roles in driving its nuclear localization. Here, we provide multiple lines of evidence to support this conclusion. The strong affinity of IMPα for both NLSCentral and NLSC-term (Figure 2C–E) in in vitro biochemical assays is supported by cellular data showing that the single P884T mutation only partially relocalizes NS5 to the cytoplasm of infected cells (Figure 1) and further supported by data of cells transfected with DENV3 NS5 single and 2× NLS KO mutants (Figure 3). Notably, in parallel to our study, an independent group found that substituting both the NLSCentral P3 and P5 arginines with alanine (R389A/R391A) significantly relocalized lentivirus-expressed DENV3 NS5 to the cytoplasm,40 supporting the role of this region in driving DENV3 NS5 to the nucleus.
DENV4
Unlike DENV2–3, DENV4 NS5 nuclear localization occurs despite the poor affinity of IMPα for either NLSCentral or NLSC-term (Figure 2C–E), suggesting a unique mechanism for achieving nuclear localization. The observation that NS5 is strongly nuclear in DENV4-infected cells (Figure 1) and yet can be either nuclear and/or cytoplasmic in cells expressing recombinant DENV4 NS5 (Figure 4) suggests that changes in the host cell induced by DENV infection could be involved in promoting nuclear localization such as the recruitment of the host factor or post-translational modification of DENV4 NS5. Mutations to residues in the DENV4 NLSC-term partially relocalize NS5 from the nucleus to the cytoplasm, strongly suggesting that the NLSC-term region plays a role in driving the nuclear import of DENV4 NS5. However, phosphorylation of individual residues Y890, S891, S894, and S896D did not promote binding of an FITC-tagged DENV4 NLSC-term peptide to either IMPα or IMPβ (Figure S6). Previous attempts with phosphomimics Y890E, S891E, and S896E also failed to promote DENV4 NLSC-term:IMPα binding in a GST-pulldown assay.41 The mechanism by which the DENV4 NLSC-term drives NS5 nuclear import is thus still unclear, and further studies beyond the scope of the current study are necessary to address this gap in our understanding.
Possible Mechanisms Driving DENV4 NS5 Nuclear Accumulation
Given that DENV4 NS5 nuclear accumulation occurs despite the poor affinity of IMPα for either of the two NLSs (Figure 2A,C–E), it is still unclear whether DENV4 NS5 nuclear accumulation relies on the IMPα/β pathway or some other mechanism. Interestingly, there is precedent for a flavivirus NS5 using a mechanism independent of the IMPα/β pathway to achieve nuclear import. YFV NS5 is reported to localize strongly to the nucleus,9 and yet neither the NLSCentral nor the NLSC-term is predicted to be functional by cNLS Mapper (Figure 2A).
Our mutagenesis studies in DENV4 NS5 that focus on the NLSC-term region suggest that this region, particularly the P8 S894 residue, plays a role in driving DENV4 NS5 nuclear localization (Figure 5). It has previously been hypothesized that DENV4 NS5 nuclear localization may be phosphorylation-regulated;12 however, our FP and EMSA data indicate that phosphorylation of single amino acid residues in the DENV4 NLSC-term does not promote the binding affinity for IMPα (Figure S6). We cannot rule out the possibility that phosphorylation of multiple residues in the DENV4 NLSC-term is required to promote the affinity for IMPα. Indeed, early studies found that a hyperphosphorylated form of DENV2 NS5 was localized to the nucleus.42 We have preliminary data that DENV4 NS5 is also phosphorylated during infection, although the precise residues that are phosphorylated are still not known. Further studies will need to be conducted to verify whether the NLSC-term region or other domains of DENV4 NS5 are phosphorylated. Meanwhile, several isoforms of 14–3–3 have been known to bind to and regulate the subcellular localization of phosphorylated substrates. Our preliminary explorations demonstrated that neither the WT nor phosphorylated NLS peptides of DENV4 bound to 14–3–3 isoforms ϵ, ζ, or σ (data not shown). Thus, although NLSC-term clearly contributes to DENV4 nuclear localization, it is still unclear whether this phenotype is regulated by phosphorylation of the NLSC-term region.
Interestingly, DENV4 NS5 subcellular localization can be uniquely strain-specific among the various DENV serotypes. While we have described it to be predominantly nuclear for EDEN4,12 an American genotype strain isolated from a Singapore 2005 outbreak,43 others have reported the DENV4 prototype strain H241,10 an Asian genotype strain isolated from Philippines in 1956, to be strongly cytoplasmic. The NS5 of these two strains differs by 16 amino acids (Table S7), including at the NLSC-term P8 residue, which is a serine-894 in the strongly nuclear EDEN4 NS5 but a phenylalanine-894 in the strongly cytoplasmic H241 NS5 (Table S7). We have found that H241 NS5 is strongly cytoplasmic in the context of infection (data not shown), yet the single S894F mutation in EDEN4 NS5 does not produce a strongly cytoplasmic NS5 (Figure 5).
Finally, given that DENV4 NS5 is predominantly nuclear in the context of infection but cytoplasmic in the context of transfection, it is possible that activation of an antiviral state in the cell during infection triggers the localization of NS5 to the nucleus. In YFV, it has been observed that, upon stimulation with IFNβ, NS5 is polyubiquitinated and subsequently sequesters STAT2 in the cytoplasm, preventing it from translocating to the nucleus and thus inhibiting type I IFN signaling.44
Taken together, the regulation of DENV NS5 subcellular localization is serotype-specific, with the two NLS sequences (NLSCentral and NLSC-term) playing varying roles in DENV1–3. In contrast, the mechanism driving DENV4 NS5 subcellular localization remains an enigma, although our studies here have provided some clues, including the dependence of DENV infection conditions for NS5 nuclear localization and the fact that DENV4 NS5 is phosphorylated in infected cells. These will form the basis of further and deeper studies to uncover the mechanisms regulating the DENV4 and NS5 subcellular localization.
Methods
Cell Lines
Huh-7 (JCRB) cells were maintained in high-glucose Dulbecco’s modified Eagle medium (DMEM, Gibco) containing l-glutamine supplemented with 10% [v/v] fetal bovine serum (FBS) and 1% [v/v] penicillin and streptomycin (P/S). BHK-21 cells (ATCC) were maintained in RPMI1640 medium (Gibco) supplemented with 10% FBS and 1% P/S. All mammalian cells were grown at 37 °C in 5% CO2. C6/36 (ATCC) cells were maintained in RPMI1640 medium containing 25 mM HEPES (Gibco), supplemented with 10% FBS and 1% P/S at 28 °C, in the absence of CO2.
Plaque Assay
2 × 105 BHK-21 cells were seeded in a 24-well plate and incubated overnight. Viral inocula were serially diluted with RPMI 1640 serum-free medium and inoculated on the cell monolayer for 1 h. Virus inocula were then removed, and the cells were overlaid with 0.8% carboxylmethyl cellulose (CMC) and further incubated for 4–5 days. Infected cells were fixed with 3.7% formaldehyde and stained with 1% crystal violet for plaque visualization.
Reverse Genetics Approach to Obtain DENV1–4 Viruses (Genbank Accession: DENV1 (EU081230), DENV2 (EU081177), DENV3 (EU081190), and DENV4 (GQ398256))
The full-length DENV2 WT (EU081177) and NS5 P884T, R890A, and R891A infectious cDNA clones used in this study were generated previously.19 A novel DENV1 infectious cDNA infectious clone was synthesized and cloned (Genscript) based on a DENV1 (EU081230) clinical isolate, with the introduction of 16 mutations to silence cryptic prokaryotic promoters without changing the amino acid sequence (Figure S7), a strategy that has been successfully utilized to establish reverse genetics systems for DENV2, JEV and ZIKV MR766, and H/PF/2013 strains.45−47 NS5 T883P mutation was introduced into the subclone pWSK29 DENV1 fragment 3 using a QuikChange II XL site-directed mutagenesis kit (Stratagene) (refer to Table S8 for the list of primers used) following manufacturer’s instructions. Successful mutagenesis was confirmed by Sanger sequencing. Each of the genome-length DENV1 mutant cDNA clones was constructed by excising fragment 3 containing the respective mutations with SacI and SacII and inserted into the subclone pWSK29 DENV1 fragment 2 + 3 that was similarly cut with the enzymes above. The full-length cDNA clones were linearized with SacII and purified using the phenol-chloroform method. RNA was then in vitro transcribed from the linearized plasmid using a T7 mMESSAGE mMACHINE kit (Ambion). The in vitro-transcribed RNA was transfected into BHK-21 or C6/36 cells using previously described conditions.48 Supernatants from the transfected C6/36 cells were collected on day 7 after transfection (P1 virus) and passaged once in C6/36 cells to obtain the P2 virus stock. Given the inclusion of the 16 CEP-silencing mutations, next-generation sequencing (described below) of the virus stock was performed to confirm the stability of the DENV1 full-length infectious clone genome (Figure S7).
DENV3 and 4 viruses were generated using a Gibson assembly approach as previously described.49,50 Briefly, six genome fragments that together encode for the complete DENV3 (EU081190) or DENV4 (GQ398256) viral genomes were maintained in pGEM-T Easy Vector (Promega). Plasmids containing the wild-type or mutant sequence introduced by a QuikChange II XL site-directed mutagenesis kit (Stratagene) were sequence-verified using Sanger sequencing. All six viral genome fragments together with a pUC19 vector were amplified using primer pairs as in Table S8 with NEB Q5 Hot-Start High-Fidelity Master Mix (New England Biolabs). Amplified fragments were gel-purified, and equimolar (0.1 pmol) amounts of each genome fragment and vector were assembled using NEBuilder HiFi DNA Assembly Master Mix (New England Biolabs) at 50 °C for 60 min to generate infectious clones. 5 μL of each assembled infectious clone was transfected into 2 × 105 HEK293T cells in a 24-well tissue culture plate using 3 μL of Lipofectamine 2000 (Thermo Fisher Scientific) as per the manufacturer’s protocols. Media containing the infectious clone-derived virus were collected 72 h post-transfection and passaged in C6/36 cells in T25 tissue culture flasks to propagate the virus.
All viruses used in this study were grown in C6/36 cells, titered in BHK-21 cells, and stored at −80 °C. Viral RNA from supernatants was extracted for sequence verification of the full-length genome by Sanger sequencing using a previously described protocol,51 analyzed using Geneious Prime 2023.1.1 (Biomatters), and used for all subsequent infection studies.
Plasmids for Transfection of Huh7 Cells
DENV NS5 was cloned into a pEF vector52 to have a C-terminal 3× FLAG tag. NS5 was engineered to be expressed downstream of ubiquitin, such that cellular hydrolases cleave ubiquitin in a manner that mimics the cleavage of NS4B away from NS5 by the NS2B/3 protease during DENV infection.5,6 Mutations were introduced into the pEF-Ub-DENV NS5-FLAG plasmid using a QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Inc.).
Viral Infection and Plasmid Transfection
For infection, 2 × 105 Huh7 cells were seeded in 12-well plates overnight. Cells were then incubated with 500 μL of DENV inoculum in serum-free media at indicated MOI for 1 h, before inocula were replaced with growth media prior to incubation for the indicated durations. For transfection, 2 × 105 Huh7 cells were seeded in 12-well plates overnight. Cells were transfected with indicated plasmids using FuGENE 6 with a transfection reagent/DNA ratio of 3:1, as per the manufacturer’s protocol (Promega).
RNA Extraction and Quantitative RT-PCR
At indicated time points, supernatants were collected for DENV RNA extraction using a QIAamp Viral RNA Mini kit (Qiagen). Extracted DENV RNA was used for full-length genome Sanger sequencing as described above or for quantitative RT-PCR using a iTaq Universal SYBR Green One-Step kit (Biorad) according to the manufacturer’s instructions. Total RNA from cell lysates was isolated using TRizol (Invitrogen) and subjected to cDNA synthesis using the Improm II reverse transcription system (Promega) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using iQ SYBR Green supermix (Bio-Rad) according to the manufacturer’s instructions for detection of intracellular DENV RNA. Primer sequences are available upon request. The values of the intracellular viral genome numbers were normalized to actin levels and expressed relative to mock-infected cells. The primers for DENV1–4 strand-specific reverse transcription and quantitative RT-PCR have been described previously.51
Virus Next-Generation Sequencing
Deep sequencing of the viruses generated in this study was performed as previously described49 to examine the genomic stability. Briefly, viral genomic RNA was isolated from the DENV1, 2, 3, and 4 supernatant using a QIAamp Viral RNA Mini Kit without carrier RNA. Library prep was completed using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina. The quality of the libraries was assessed by a DNA1000 Bioanalyzer chip (Agilent Technologies) prior to paired-end sequencing on the Illumina MiSeq (2 × 250bp) sequencing system in the Duke-NUS Genome Biology Facility. Reads were trimmed and assessed for quality using Cutadapt 2.8 and FastQC, respectively. The typical MiSeq coverage for each nucleotide position ranges from 6000 to 8000 reads/nucleotide, and samples with an overall read depth of less than 3000 were excluded from variant call analysis. Reads were aligned to reference sequences using an in-house pipeline using the bowtie package.53
Immunofluorescence and Confocal Laser Scanning Microscopy
Infected or transfected cells grown in 12-well plates containing #1.0 glass coverslips were fixed with ice-cold methanol for 20 min at −20 °C and washed twice with ice-cold 1× phosphate-buffered saline (PBS). Nonspecific binding was blocked by 1 h of incubation with 1% bovine serum albumin in PBS before 1 h of incubation of cells with the following primary antibodies as indicated: anti-NS5 (5R3),54 anti-FLAG M2 (Sigma #F1804), anti-V5 (Cell Signaling D3H8Q), or anti-dsRNA J2 (Scicons). After being washed in ice-cold 1× PBS, cells were incubated with secondary antibodies coupled to AlexaFluor 488 or Alexa-Fluor 594 (Invitrogen). Cell nuclei were visualized using DAPI (Sigma, 1:10,000 in H2O). Coverslips were mounted using the ProLong Gold antifade reagent (Invitrogen) on glass slides, and confocal laser scanning microscopy was performed using a Zeiss LSM 710 upright confocal microscope (Carl Zeiss, Germany) with a 63× oil immersion lens. Image processing was performed with ImageJ software55 to determine the nuclear-to-cytoplasmic fluorescence ratio (Fn/c) of each cell using the formula Fn/c = (Fn – Fb)/(Fc – Fb), where Fn is the nuclear fluorescence, Fc is the cytoplasmic fluorescence, and Fb is the background fluorescence.12 The mean Fn/c ± SD was calculated for ≥20 cells.
Protein Expression and Purification for Binding and Structural Studies
The following constructs encoding IMPαs lacking the IMPβ-binding domain (IMPαΔIBB) were codon-optimized, synthesized by Genscript, and cloned into pET30a using the BamHI site: human IMPα1 (UniProtKB P52292; residues 70–529), human IMPα3 (UniProtKB O00629; residues 64–521), human IMPα7 (UniProtKB O60684; encoding residues 73–536), and mouse IMPα2 (UniProtKB P52293; encoding residues 70–529). DENV3 NS5 NLSCentral, encoding residues 371–407, was synthesized by DNA 2.0 and cloned into pGEX4T-1 using the BamHI and EcoRI sites. Plasmids were transformed into BL21 (DE3) pLysS cells and expressed via the Studier autoinduction method as described previously.56,57 Postinduction, bacterial cells were pelleted via centrifugation at 6000 rpm for 20 min and subsequently resuspended using 20 mL per 2 L of bacterial culture with HIS buffer A (50 mM phosphate buffer, 300 mM NaCl, 20 mM imidazole, pH 8) and lysed via two freeze–thaw cycles in addition to the application of 1 mL of 20 mg/mL lysozyme (Sigma-Aldrich, St. Louis, MI) and 10 μL of 50 mg/mL DNase (Sigma-Aldrich) at room temperature for 1 h. The soluble extract was harvested via centrifugation at 12,000 rpm for 30 min and filtered through a 0.45 μm low-protein affinity filter. The soluble extract was loaded and injected over a 5 mL HisTrap HP column (GE Healthcare, Chicago, IL) and washed with 20 column volumes of His buffer A on an AKTApurifier FPLC (GE Healthcare). An increasing concentration gradient of imidazole (20–500 mM) (ChemSupply, Gillman, SA, Australia) eluted the protein off the column, and resulting fractions were pooled. Size-exclusion chromatography was employed to further purify proteins using a HiLoad 26/60 Superdex 200 column (GE Healthcare), pre-equilibrated in GST buffer A (50 mM Tris and 125 mM NaCl). The corresponding fractions of elution volume and protein size were collected and concentrated using an Amicon MWCO 10 kDa filter (Merck Millipore, Burlington, MA), aliquoted, and stored at −80 °C. Samples were assessed for purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) at 165 V for 30 min on a 4–12% Bis-Tris plus gel (Thermo Fisher Scientific, Waltham, MA).
The NS5 coding sequence was constructed in the pProEX-HTb vector with an N-terminal His6 tag followed by a TEV cleavage site. Expression and purification of the recombinant full-length NS5 of DENV2 have been described previously.1Escherichia coli cells were resuspended in His Buffer A (50 mM Tris–HCl pH 7.5, 500 mM NaCl, 10% glycerol, 10 mM β-mercaptoethanol, and 10 mM imidazole) and lysed with 0.1 volume of the 10× FastBreak Cell Lysis Reagent (Promega), followed by DNase treatment and clarified by centrifugation. The supernatant was filtered using a 0.45 μm syringe filter unit, loaded on a HisTrapFF column, and eluted with a linear gradient of imidazole from 10 to 500 mM. Fractions containing His-tagged protein were bagged into a SnakeSkin (Thermo Scientific, 30,000 MWCO) membrane tubing together with TEV for His-tag cleavage and concomitantly dialyzed against His buffer A at 4 °C for 18 h or overnight. The cleavage mixture was reloaded on the HisTrapFF column to separate any possible uncleaved NS5 protein. The flow-through sample from the second run of His-affinity chromatography was concentrated and loaded on a HiPrep 26/60 Sephacryl S-200 HR column (GE Healthcare Lifesciences), which was equilibrated in S200 Buffer (20 mM HEPES at pH 7.5, 300 mM NaCl, 10% glycerol, and 2 mM DTT) for further purification by size exclusion chromatography.
The DENV3 NLSCentral/IMPα2 complex was isolated by affinity and size exclusion chromatography. Following expression using the Studier autoinduction method as described for the importins above, the cells were resuspended in GST Buffer A (50 mM Tris pH 7.4, 125 mM NaCl), lysed using two freeze–thaw cycles in addition to lysozyme and DNase treatment, and clarified by centrifugation. The supernatant was filtered using a 0.45 μm syringe filter unit, loaded on a GST Sepharose column, and washed with GST A to remove unwanted cellular proteins. Once washed, purified tagless IMPα2 was added to the column and then washed until the UV reading was stable and the DENV3 NLSCentral/IMPα2 complex was eluted with GST Buffer B (50 mM Tris pH 7.4, 125 mM NaCl, 10 mM reduced l-glutathione). The GST tag was removed by overnight thrombin cleavage at 4 °C and loaded onto a HiLoad 26/60 superdex 200 column (GE Healthcare Lifesciences) pre-equilibrated in GST A. Fractions forming the correct corresponding size were combined, concentrated, and stored at −80 °C. The presence of the complex was confirmed by SDS-PAGE.
NLS Peptides
Peptides for the DENV1–4 NS5 NLSCentral and NLSC-term were derived from the serotypes, as described above, and synthesized by Genscript. Peptides were tagged with an N-terminal FITC label attached to an AHX linker (see Table S9 for sequence information). The lyophilized peptides were resuspended in 50 mM Tris (pH 8.0) and 125 mM sodium chloride buffer to 10 mg/mL.
Electromobility Shift Assays
10 μM FITC-labeled DENV NS5 NLS peptide was mixed with 10 μM of each IMPα isoform and incubated in a total volume of 17 μL for 15 min at room temperature. Samples were supplemented with 3 μL of 50% glycerol and run on a 1% agarose TB gel for 1.5 h at 70 V in TB running buffer. The gel was imaged using a fluorescein filter with a Bio-Rad ChemiDoc MP imaging system to record the migration of the FITC-labeled peptides. The gel was then stained with Coomassie stain and destained in 10% ethanol and 10% glacial acetic acid overnight before imaging. Images of the FITC and Coomassie stains were processed and overlaid using Image Lab Software.
Fluorescence Polarization Assays
FITC-tagged DENV1–4 NLS peptides (10 nM) were incubated with twofold serially diluted IMPα concentrations (starting concentration of 15 μM) across 23 wells to a total volume of 200 μL. Fluorescence polarization measurements were recorded using a CLARIOstar Plus plate reader (BMG Labtech) in 96-well black Fluotrac microplates (Greiner Bio-One; Kremsmünster, Austria). Assays were repeated in triplicate and contained a negative control (no binding partner) and blank (Tris buffered saline, pH 8). Triplicate data were fitted to a one-site specific binding curve using GraphPad Prism 9.5.1.
Crystallization of DENV1 and the DENV3 NLSCentral/IMPα Complex
The protein complex was crystallized using the hanging drop vapor diffusion method with a final crystallization condition of 0.75 M Na citrate and 0.1 M HEPES pH 6.5 mixed in 1:1 ratio with either the DENV3 NLSCentral/IMPα2 complex or FITC-peptide/protein complex solution and crystallized at 22 °C. Rod-shaped crystals (100 μM × 10 μM × 10 μM) grew after 2 days of incubation. Crystals were collected and cryoprotected in the reservoir solution containing 20% glycerol prior to being flash-frozen to −196 °C in liquid nitrogen.
Data Collection and Structure Determination
X-ray diffraction data were obtained from the Australian Synchrotron on the MX2 beamline.58 Data were indexed and merged through iMOSFLM,59 prior to scaling and subsequent merging via Aimless.60,61 Phasing was undertaken using molecular replacement in Phaser,62 and the structure 5W41 from the Protein Data Bank (PDB) was used as a search model. Final model rebuilding and relative refining were performed in Coot63−65 and Phenix,66 respectively.
Acknowledgments
We thank Eng Eong Ooi, Justin Roby, and members of the Vasudevan laboratory for critical discussions. This research was supported by the Singapore Ministry of Health’s National Medical Research Council under its RIE2020/RIE2025 OF-IRG funding scheme (MOH-000086: MOH-OFIRG18-may-0006; MOH-OFIRG23-jul-0033) and OF-YIRG funding scheme (OFYIRG23jan-0032) and the IAF-ICP (I2301E0019) administered by Agency for Science, Technology and Research. D.D.R.A. acknowledges the support from the Australian Government New Colombo Plan Scholarship. The FP7 WeNMR (project# 261572), H2020 West-Life (project# 675858), the EOSC-hub (project# 777536), and the EGI-ACE (project# 101017567) European e-Infrastructure projects are acknowledged for the use of their web portals, which make use of the EGI infrastructure with the dedicated support of CESNET-MCC, INFN-PADOVA-STACK, INFN-LNL-2, NCG-INGRID-PT, TW-NCHC, CESGA, IFCA-LCG2, UA-BITP, SURFsara, and NIKHEF and the additional support of the national GRID Initiatives of Belgium, France, Italy, Germany, Netherlands, Poland, Portugal, Spain, U.K., Taiwan, and the US Open Science Grid.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.4c00054.
Mutation of the NLSC-term P–1 residue in the NLS of DENV NS5 having no significant impact on DENV replication, pairwise identity of NLSCentral and NLSC-term between DENV1–4 NS5, mutation of the NLSC-term P-1 residue abrogating binding to IMPα, NLSCentral/IMPα biochemical EMSA and FP binding assays, plaque morphology of wild-type and mutant DENV generated and used in this study, phosphorylation not promoting binding of DENV4 NLSC-term to IMPα or IMPβ, construction of a novel DENV1 infectious clone-derived virus, DENV1–4 NS5 cNLS Mapper result as scored by cNLS Mapper, data collection and refinement statistics for DENV1 NLSCentral·mIMPαΔIBB and DENV3 NLSCentral·mIMPαΔIBB, complete list of interactions of the DENV1 and 3 NS5 NLSCentral with IMPα2, single amino acid polymorphisms between EDEN4 and H241 strains of DENV4 NS5, list of primers used in this study, and list of FITC-labeled peptides for NLS-IMPα binding studies (PDF)
Author Contributions
# C.X.C. and M.J.A.T. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhao Y.; Soh T. S.; Zheng J.; Chan K. W. K.; Phoo W. W.; Lee C. C.; Tay M. Y. F.; Swaminathan K.; Cornvik T. C.; Lim S. P.; Shi P.-Y.; Lescar J.; Vasudevan S. G.; Luo D. A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication. PLoS Pathog. 2015, 11 (3), e1004682 10.1371/journal.ppat.1004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sahili A.; Lescar J. Dengue Virus Non-Structural Protein 5. Viruses 2017, 9 (4), 91 10.3390/v9040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. F.; Kaufusi P. H.; Volper E. M.; Nerurkar V. R. Maturation of dengue virus nonstructural protein 4B in monocytes enhances production of dengue hemorrhagic fever-associated chemokines and cytokines. Virology 2011, 418 (1), 27–39. 10.1016/j.virol.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin C. L.; Fitzgerald K. A.; Rothman A. L. Dengue Virus Nonstructural Protein NS5 Induces Interleukin-8 Transcription and Secretion. J. Virol. 2005, 79 (17), 11053–11061. 10.1128/JVI.79.17.11053-11061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour J.; Laurent-Rolle M.; Shi P.-Y.; García-Sastre A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. J. Virol. 2009, 83 (11), 5408–5418. 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J.; Laurent-Rolle M.; Maestre A. M.; Rajsbaum R.; Pisanelli G.; Simon V.; Mulder L. C. F.; Fernandez-Sesma A.; García-Sastre A. Dengue Virus Co-opts UBR4 to Degrade STAT2 and Antagonize Type I Interferon Signaling. PLoS Pathog. 2013, 9 (3), e1003265 10.1371/journal.ppat.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A.; Ponia S. S.; Tripathi S.; Balasubramaniam V.; Miorin L.; Sourisseau M.; Schwarz M. C.; Sánchez-Seco M. P.; Evans M. J.; Best S. M.; García-Sastre A. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19 (6), 882–890. 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Thurmond S.; Zhou K.; Sánchez-Aparicio M. T.; Fang J.; Lu J.; Gao L.; Ren W.; Cui Y.; Veit E. C.; Hong H.; Evans M. J.; O’Leary S. E.; García-Sastre A.; Zhou Z. H.; Hai R.; Song J. Structural basis for STAT2 suppression by flavivirus NS5. Nat. Struct. Mol. Biol. 2020, 27 (10), 875–885. 10.1038/s41594-020-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A.; Gaidamovich S.; Turchinskaya A.; Gould E. A. Monoclonal antibodies identify the NS5 yellow fever virus non-structural protein in the nuclei of infected cells. J. Gen. Virol. 1992, 73 (5), 1125–1130. 10.1099/0022-1317-73-5-1125. [DOI] [PubMed] [Google Scholar]
- Hannemann H.; Sung P.-Y.; Chiu H.-C.; Yousuf A.; Bird J.; Lim S. P.; Davidson A. D. Serotype-specific Differences in Dengue Virus Non-structural Protein 5 Nuclear Localization. J. Biol. Chem. 2013, 288 (31), 22621–22635. 10.1074/jbc.M113.481382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M. Y. F.; Fraser J. E.; Chan W. K. K.; Moreland N. J.; Rathore A. P.; Wang C.; Vasudevan S. G.; Jans D. A. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013, 99 (3), 301–306. 10.1016/j.antiviral.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Tay M. Y. F.; Smith K.; Ng I. H. W.; Chan K. W. K.; Zhao Y.; Ooi E. E.; Lescar J.; Luo D.; Jans D. A.; Forwood J. K.; Vasudevan S. G. The C-terminal 18 Amino Acid Region of Dengue Virus NS5 Regulates its Subcellular Localization and Contains a Conserved Arginine Residue Essential for Infectious Virus Production. PLoS Pathog. 2016, 12 (9), e1005886 10.1371/journal.ppat.1005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng I. H. W.; Chan K. W.; Tan M. J. A.; Gwee C. P.; Smith K. M.; Jeffress S. J.; Saw W. G.; Swarbrick C. M. D.; Watanabe S.; Jans D. A.; Gruber G.; Forwood J. K.; Vasudevan S. G. Zika Virus NS5 Forms Supramolecular Nuclear Bodies That Sequester Importin-alpha and Modulate the Host Immune and Pro-Inflammatory Response in Neuronal Cells. ACS Infect. Dis. 2019, 5 (6), 932–948. 10.1021/acsinfecdis.8b00373. [DOI] [PubMed] [Google Scholar]
- Tan M. J. A.; Chan K. W. K.; Ng I. H. W.; Kong S. Y. Z.; Gwee C. P.; Watanabe S.; Vasudevan S. G. The Potential Role of the ZIKV NS5 Nuclear Spherical-Shell Structures in Cell Type-Specific Host Immune Modulation during ZIKV Infection. Cells 2019, 8 (12), 1519 10.3390/cells8121519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J. M.; Kenney M. T.; Westaway E. G. West Nile virus strain Kunjin NS5 polymerase is a phosphoprotein localized at the cytoplasmic site of viral RNA synthesis. J. Gen. Virol. 2007, 88 (4), 1163–1168. 10.1099/vir.0.82552-0. [DOI] [PubMed] [Google Scholar]
- Uchil P. D.; Kumar A. V. A.; Satchidanandam V. Nuclear Localization of Flavivirus RNA Synthesis in Infected Cells. J. Virol. 2006, 80 (11), 5451–5464. 10.1128/JVI.01982-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albentosa-González L.; Clemente-Casares P.; Sabariegos R.; Mas A. Polymerase Activity, Protein-Protein Interaction, and Cellular Localization of the Usutu Virus NS5 Protein. Antimicrob. Agents Chemother. 2019, 64 (1), e01573-19 10.1128/AAC.01573-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M.; Chen S.; Zhang W.; Duan Y.; Jiang B.; Pan X.; Wang M.; Jia R.; Zhu D.; Liu M.; Zhao X.; Yang Q.; Wu Y.; Zhang S.; Huang J.; Ou X.; Mao S.; Tian B.; Gao Q.; Cheng A. Nuclear localization of duck Tembusu virus NS5 protein attenuates viral replication in vitro and NS5-NS2B3 interaction. Vet. Microbiol. 2021, 262, 109239 10.1016/j.vetmic.2021.109239. [DOI] [PubMed] [Google Scholar]
- Cheng C. X.; Tan M. J. A.; Chan K. W. K.; Watanabe S.; Wang S.; Choy M. M.; Manuel M.; Victorio C. B. L.; Ong J.; Reolo M.; Chacko A.-M.; Vasudevan S. G. In Vitro and In Vivo Stability of P884T, a Mutation that Relocalizes Dengue Virus 2 Non-structural Protein 5. ACS Infect. Dis. 2021, 7 (12), 3277–3291. 10.1021/acsinfecdis.1c00441. [DOI] [PubMed] [Google Scholar]
- Petit M. J.; Kenaston M. W.; Pham O. H.; Nagainis A. A.; Fishburn A. T.; Shah P. S. Nuclear dengue virus NS5 antagonizes expression of PAF1-dependent immune response genes. PLoS Pathog. 2021, 17 (11), e1010100 10.1371/journal.ppat.1010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio F. A.; Risso G.; Iglesias N. G.; Shah P.; Pozzi B.; Gebhard L. G.; Mammi P.; Mancini E.; Yanovsky M. J.; Andino R.; Krogan N.; Srebrow A.; Gamarnik A. V. The Dengue Virus NS5 Protein Intrudes in the Cellular Spliceosome and Modulates Splicing. PLoS Pathog. 2016, 12 (8), e1005841 10.1371/journal.ppat.1005841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi B.; Bragado L.; Mammi P.; Torti M. F.; Gaioli N.; Gebhard L. G.; García Solá M. E.; Vaz-Drago R.; Iglesias N. G.; García C. C.; Gamarnik A. V.; Srebrow A. Dengue virus targets RBM10 deregulating host cell splicing and innate immune response. Nucleic Acids Res. 2020, 48 (12), 6824–6838. 10.1093/nar/gkaa340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M.; Chang C.-W.; Róna G.; Smith K. M.; Stewart A. G.; Takeda A. A. S.; Fontes M. R. M.; Stewart M.; Vértessy B. G.; Forwood J. K.; Kobe B. Structural Biology and Regulation of Protein Import into the Nucleus. J. Mol. Biol. 2016, 428, 2060–2090. 10.1016/j.jmb.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Forwood J. K.; Brooks A.; Briggs L. J.; Xiao C.-Y.; Jans D. A.; Vasudevan S. G. The 37-Amino-Acid Interdomain of Dengue Virus NS5 Protein Contains a Functional NLS and Inhibitory CK2 Site. Biochem. Biophys. Res. Commun. 1999, 257 (3), 731–737. 10.1006/bbrc.1999.0370. [DOI] [PubMed] [Google Scholar]
- Brooks A. J.; Johansson M.; John A. V.; Xu Y.; Jans D. A.; Vasudevan S. G. The Interdomain Region of Dengue NS5 Protein That Binds to the Viral Helicase NS3 Contains Independently Functional Importin β1 and Importin α/β-Recognized Nuclear Localization Signals. J. Biol. Chem. 2002, 277 (39), 36399–36407. 10.1074/jbc.M204977200. [DOI] [PubMed] [Google Scholar]
- Pryor M. J.; Rawlinson S. M.; Butcher R. E.; Barton C. L.; Waterhouse T. A.; Vasudevan S. G.; Bardin P. G.; Wright P. J.; Jans D. A.; Davidson A. D. Nuclear Localization of Dengue Virus Nonstructural Protein 5 Through Its Importin α/β–Recognized Nuclear Localization Sequences is Integral to Viral Infection. Traffic 2007, 8 (7), 795–807. 10.1111/j.1600-0854.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Bühler S.; Selisko B.; Davidson A.; Mulder K.; Canard B.; Miller S.; Bartenschlager R. Nuclear Localization of Dengue Virus Nonstructural Protein 5 Does Not Strictly Correlate with Efficient Viral RNA Replication and Inhibition of Type I Interferon Signaling. J. Virol. 2013, 87 (8), 4545–4557. 10.1128/JVI.03083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Tao M.; Han W.; Fan Z.; Imran M.; Cao S.; Ye J. Nuclear localization of Zika virus NS5 contributes to suppression of type I interferon production and response. J. Gen. Virol. 2021, 102 (3), 001376 10.1099/jgv.0.001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klema V. J.; Ye M.; Hindupur A.; Teramoto T.; Gottipati K.; Padmanabhan R.; Choi K. H. Dengue Virus Nonstructural Protein 5 (NS5) Assembles into a Dimer with a Unique Methyltransferase and Polymerase Interface. PLoS Pathog. 2016, 12 (2), e1005451 10.1371/journal.ppat.1005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S.; Hasebe M.; Tomita M.; Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. U.S.A. 2009, 106 (25), 10171–10176. 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumroy R. A.; Cingolani G. Diversification of importin-α isoforms in cellular trafficking and disease states. Biochem. J. 2015, 466 (1), 13–28. 10.1042/bj20141186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. M.; Di Antonio V.; Bellucci L.; Thomas D. R.; Caporuscio F.; Ciccarese F.; Ghassabian H.; Wagstaff K. M.; Forwood J. K.; Jans D. A.; Palù G.; Alvisi G. Contribution of the residue at position 4 within classical nuclear localization signals to modulating interaction with importins and nuclear targeting. Biochim. Biophys. Acta, Mol. Cell Res. 2018, 1865 (8), 1114–1129. 10.1016/j.bbamcr.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Krissinel E.; Henrick K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372 (3), 774–797. 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Wang D.; Liu D.; Yuchi J.; He F.; Jiang Y.; Cai S.; Li J.; Xu D. MusiteDeep: a deep-learning based webserver for protein post-translational modification site prediction and visualization. Nucleic Acids Res. 2020, 48 (W1), W140–W146. 10.1093/nar/gkaa275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Zeng S.; Xu C.; Qiu W.; Liang Y.; Joshi T.; Xu D. MusiteDeep: a deep-learning framework for general and kinase-specific phosphorylation site prediction. Bioinformatics 2017, 33 (24), 3909–3916. 10.1093/bioinformatics/btx496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher E. L.; Zhdanov S. A.; Bao Y.; Blinkova O.; Nawrocki E. P.; Ostapchuck Y.; Schäffer A. A.; Brister J. R. Virus Variation Resource – improved response to emergent viral outbreaks. Nucleic Acids Res. 2017, 45 (D1), D482–D490. 10.1093/nar/gkw1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes M. R.; Teh T.; Jans D.; Brinkworth R. I.; Kobe B. Structural basis for the specificity of bipartite nuclear localization sequence binding by importin-α. J. Biol. Chem. 2003, 278 (30), 27981–27987. 10.1074/jbc.M303275200. [DOI] [PubMed] [Google Scholar]
- Róna G.; Marfori M.; Borsos M.; Scheer I.; Takács E.; Toth J.; Babos F.; Magyar A.; Erdei A.; Bozoky Z.; et al. Phosphorylation adjacent to the nuclear localization signal of human dUTPase abolishes nuclear import: structural and mechanistic insights. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2013, 69 (12), 2495–2505. 10.1107/S0907444913023354. [DOI] [PubMed] [Google Scholar]
- Wang S.; Chan K. W. K.; Tan M. J. A.; Flory C.; Luo D.; Lescar J.; Forwood J. K.; Vasudevan S. G. A conserved arginine in NS5 binds genomic 3′ stem–loop RNA for primer-independent initiation of flavivirus RNA replication. RNA 2022, 28 (2), 177–193. 10.1261/rna.078949.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J. N.; Schutt W.; Mladinich M.; Sohn S.-Y.; Hearing P.; Mackow E. R. NS5 Sumoylation Directs Nuclear Responses that Permit Zika Virus to Persistently Infect Human Brain Microvascular Endothelial Cells. J. Virol. 2020, 94, e01086-20 10.1128/JVI.01086-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.Structural and Functional Characterisation of Nuclear Import Complexes in Human Health and Disease. Ph.D. Thesis; Charles Sturt University: Wagga Wagga, Australia, 2019. [Google Scholar]
- Kapoor M.; Zhang L.; Ramachandra M.; Kusukawa J.; Ebner K. E.; Padmanabhan R. Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J. Biol. Chem. 1995, 270 (32), 19100–19106. 10.1074/jbc.270.32.19100. [DOI] [PubMed] [Google Scholar]
- Low J. G.; Ooi E. E.; Tolfvenstam T.; Leo Y. S.; Hibberd M. L.; Ng L. C.; Lai Y. L.; Yap G. S.; Li C. S.; Vasudevan S. G.; Ong A. Early Dengue infection and outcome study (EDEN)-study design and preliminary findings. Ann. Acad. Med., Singapore 2006, 35 (11), 783–789. 10.47102/annals-acadmedsg.V35N11p783. [DOI] [PubMed] [Google Scholar]
- Laurent-Rolle M.; Morrison J.; Rajsbaum R.; Macleod J. M. L.; Pisanelli G.; Pham A.; Ayllon J.; Miorin L.; Martínez-Romero C.; tenOever B. R.; García-Sastre A. The Interferon Signaling Antagonist Function of Yellow Fever Virus NS5 Protein Is Activated by Type I Interferon. Cell Host Microbe 2014, 16 (3), 314–327. 10.1016/j.chom.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münster M.; Płaszczyca A.; Cortese M.; Neufeldt C. J.; Goellner S.; Long G.; Bartenschlager R. A Reverse Genetics System for Zika Virus Based on a Simple Molecular Cloning Strategy. Viruses 2018, 10 (7), 368 10.3390/v10070368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. W. K.; Bifani A. M.; Watanabe S.; Choy M. M.; Ooi E. E.; Vasudevan S. G. Tissue-specific expansion of Zika virus isogenic variants drive disease pathogenesis. eBioMedicine 2023, 91, 104570 10.1016/j.ebiom.2023.104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S.-Y.; Wu R.-H.; Yang C.-C.; Jao T.-M.; Tsai M.-H.; Wang J.-C.; Lin H.-M.; Chao Y.-S.; Yueh A. Successful Propagation of Flavivirus Infectious cDNAs by a Novel Method To Reduce the Cryptic Bacterial Promoter Activity of Virus Genomes. J. Virol. 2011, 85 (6), 2927–2941. 10.1128/JVI.01986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M. Y. F.; Saw W. G.; Zhao Y.; Chan K. W. K.; Singh D.; Chong Y.; Forwood J. K.; Ooi E. E.; Grüber G.; Lescar J.; Luo D.; Vasudevan S. G. The C-terminal 50 Amino Acid Residues of Dengue NS3 Protein Are Important for NS3-NS5 Interaction and Viral Replication. J. Biol. Chem. 2015, 290 (4), 2379–2394. 10.1074/jbc.M114.607341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siridechadilok B.; Gomutsukhavadee M.; Sawaengpol T.; Sangiambut S.; Puttikhunt C.; Chin-inmanu K.; Suriyaphol P.; Malasit P.; Screaton G.; Mongkolsapaya J. A Simplified Positive-Sense-RNA Virus Construction Approach That Enhances Analysis Throughput. J. Virol. 2013, 87 (23), 12667–12674. 10.1128/JVI.02261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. C.; Kwek S. S.; Sun B.; Yousefi M.; Ong E. Z.; Tan H. C.; Puschnik A. S.; Chan K. R.; Ooi Y. S.; Ooi E. E. A fast-growing dengue virus mutant reveals a dual role of STING in response to infection. Open Biol. 2022, 12 (12), 220227 10.1098/rsob.220227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenbury J. G.; Aw P. P. K.; Ong S. H.; Schreiber M. J.; Chow A.; Gubler D. J.; Vasudevan S. G.; Ooi E. E.; Hibberd M. L. A method for full genome sequencing of all four serotypes of the dengue virus. J. Virol. Methods 2010, 169 (1), 202–206. 10.1016/j.jviromet.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Mizushima S.; Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990, 18 (17), 5322. 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B.; Trapnell C.; Pop M.; Salzberg S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10 (3), R25 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Moreland N. J.; Tay M. Y. F.; Lee C. C.; Swaminathan K.; Vasudevan S. G. Identification and molecular characterization of human antibody fragments specific for dengue NS5 protein. Virus Res. 2014, 179, 225–230. 10.1016/j.virusres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Schneider C. A.; Rasband W. S.; Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Protein production by auto-induction in high-density shaking cultures. Protein Expression Purif. 2005, 41 (1), 207–234. 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Hoad M.; Cross E. M.; Donnelly C. M.; Sarker S.; Roby J. A.; Forwood J. K. Structural Characterization of Porcine Adeno-Associated Virus Capsid Protein with Nuclear Trafficking Protein Importin Alpha Reveals a Bipartite Nuclear Localization Signal. Viruses 2023, 15 (2), 315 10.3390/v15020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips T. M.; McPhillips S. E.; Chiu H.-J.; Cohen A. E.; Deacon A. M.; Ellis P. J.; Garman E.; Gonzalez A.; Sauter N. K.; Phizackerley R. P.; Soltis S. M.; Kuhn P. Blu-Ice and the Distributed Control System: software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron Radiat. 2002, 9 (6), 401–406. 10.1107/S0909049502015170. [DOI] [PubMed] [Google Scholar]
- Battye T. G. G.; Kontogiannis L.; Johnson O.; Powell H. R.; Leslie A. G. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67 (4), 271–281. 10.1107/S0907444910048675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. Scaling and assessment of data quality. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2006, 62 (1), 72–82. 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- Evans P. R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67 (4), 282–292. 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. J.; Grosse-Kunstleve R. W.; Adams P. D.; Winn M. D.; Storoni L. C.; Read R. J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40 (4), 658–674. 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004, 60 (12), 2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Emsley P.; Lohkamp B.; Scott W. G.; Cowtan K. Features and development of Coot. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66 (4), 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M. D.; Ballard C. C.; Cowtan K. D.; Dodson E. J.; Emsley P.; Evans P. R.; Keegan R. M.; Krissinel E. B.; Leslie A. G.; McCoy A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67 (4), 235–242. 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. D.; Afonine P. V.; Bunkóczi G.; Chen V. B.; Davis I. W.; Echols N.; Headd J. J.; Hung L.-W.; Kapral G. J.; Grosse-Kunstleve R. W.; et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66 (2), 213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.