Abstract
Human milk provides essential nutrition for infants and holds many health benefits for infants and mothers. When a mother's own milk is not available for her infant, the World Health Organization recommends feeding donor human milk (DHM) from a human milk banking facility. DHM is human milk produced, collected then donated to a human milk bank (HMB). HMBs serve many vital functions, including screening donor mothers, then collecting, processing, storing, and allocating DHM to recipients. The first HMB opened in 1909, and today there are more than 700 HMBs globally. Unfortunately, HMB facilities are not present in all locales, with notable gaps in South Asia and Africa. Additionally, there are no global standards to guide HMB operational procedures. Even though most HMBs attempt to employ quality control systems to provide safe DHM, differences in community needs, resource availability, and a range of methods and policies to execute processes result in significant variations in DHM quality and HMB operations. Robust and collaborative systems that ensure safe and equitable access to DHM are needed. In this paper, we present a global snapshot of current human milk banking practices; review an interdisciplinary framework to guide and support HMB activities as an integrated part of health and newborn care systems; discuss factors that contribute to HMB sustainability; outline barriers to scaling HMBs worldwide; and highlight knowledge, policy, and research gaps. Developing global HMB guidance and rigorous, adaptable standards would strengthen efforts to improve newborn health.
Keywords: donor milk, human milk, human milk bank
Key messages
Safe donor human milk (DHM) from a human milk bank (HMB) is a WHO recommendation when the mother's own milk is not available.
Over 700 HMBs exist globally, with varying operational models and a wide range of practices due to lack of global guidelines and the current practice of adaptation to local needs and health systems.
Scaling up of HMBs to meet the global demand for DHM would be facilitated by the development of global standards to guide integration with breastfeeding promotion and quality controlled processing to ensure provision of safe DHM.
1. INTRODUCTION
Human milk provides a host of nutritional, immunological, and physiological benefits to infants, and it is widely recognised as a pillar of child survival globally (Victora et al., 2016). Breastfeeding within the first hour following birth, exclusive breastfeeding for the first 6 months of life, and continued breastfeeding for 2 years or more in conjunction with nutritionally adequate and age‐appropriate complementary foods are all standard infant feeding indicators established by the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF).
Enhanced focus on innovative methods to strengthen breastfeeding and reduce neonatal mortality have come to the forefront with increasing momentum to support and meet the sustainable development goals (SDGs). These are particularly relevant for SDG 2 to improve nutrition, SDG 3 to prevent child mortality and decrease the risk of noncommunicable diseases, and SDG 4 to support cognitive development and education (Adhanom Ghebreyesus & Lake, 2017; United Nations, 2017; United Nations Department of Economic and Social Affairs, 2015). In addition, as stated by WHO, “breastfeeding is also an enabler to ending poverty, promoting economic growth, and reducing inequalities” (Adhanom Ghebreyesus & Lake, 2017).
In accordance with many national and international feeding guidelines, low birthweight (LBW) and very low birthweight (VLBW) infants should preferentially be fed mother's own milk (MOM). However, current infant feeding indicators do not track how many vulnerable infants lack access to their own mother's milk. Anecdotal evidence from worldwide observations suggests that up to 40% of infants in neonatal wards may not have access to their mother's milk in the first hours or days of life, perhaps even longer (Israel‐Ballard, 2018). High‐risk mothers and infants are separated at birth in many settings—often admitted to different units, floors, or buildings. Even if the mother can express her milk, there may not be mechanisms to safely store, transport, and feed it to her infant.
In the absence of MOM, the 2022 WHO Recommendations for Care of the Preterm or Low Birthweight Infant recommends feeding donor human milk (DHM) obtained from a human milk banking facility, when possible (WHO, 2011, 2022). DHM is human milk produced and collected in excess of an infant's current and future needs donated by a mother to a human milk bank (HMB) for use by a recipient infant that is not the mother's infant. It is prescribed when MOM is not available in support of an exclusive human milk diet (The Lancet, 2016). In most instances, DHM is fed to infants based on clinical necessity, and its optimal use is as an alternative when MOM is not available, with the goal of serving as a temporary bridge to full breastfeeding used while the mother builds her milk supply. As the mother's supply increases through frequent breastfeeding or expression, the infant will transition to direct breastfeeding or expressed MOM. DHM will gradually decrease until it is no longer required. Under these conditions, DHM should serve as a replacement for infant formula feeding, and its use should support breastfeeding efforts, not undermine them.
DHM availability is often limited, and allocation is typically made based on the risk of morbidity and mortality, prioritising the smallest and sickest infants to avoid the potential health risks associated with formula use. Case‐by‐case decision‐making is warranted for premature, LBW, or ill infants since DHM may be appropriate even if a mother is unable or unwilling to breastfeed (Brandstetter et al., 2018). Care providers may consider long‐term DHM use based on clinical need and availability in some instances.
2. HUMAN MILK BANKING: HISTORY AND OVERVIEW
An HMB is a service established to recruit breast milk donors, collect donated human milk, and then process, screen, store, and distribute DHM to meet infants' nutritional needs (PATH, 2019a). Though informal milk sharing and wet nursing are centuries‐old traditions, formal human milk banking began in 1909 when the first HMB was opened in Vienna, Austria. The global presence of HMBs has since expanded dramatically since then. The WHO and UNICEF first recognised DHM from an HMB as an alternative to MOM in 1980 (Moro, 2018). However, when HIV was identified in human milk later that same decade (Simmer & Hartmann, 2009), compromised trust in the safety of DHM significantly stalled HMB growth and expansion for years (Moro, 2018).
The current supply of DHM is constrained by the limited number of HMBs and the geographic locations where they exist. At present, there are more than 700 HMBs in over 60 countries—though the majority are in North America, Europe, and Brazil. Unfortunately, there are few HMBs in regions with the greatest burden of sick and vulnerable infants, including sub‐Saharan Africa and South Asia, as illustrated in Figure 1. Despite the need in these regions, operational models for HMB which are appropriate for low‐resourced settings have been lacking, making it challenging for these regions to establish feasible and sustainable HMB programmes. WHO has recently called for research on implementation models in low‐ and middle‐income settings that would facilitate expansion of HMB and ensure quality, safety and appropriate use of DHM (WHO, 2022).
Figure 1.
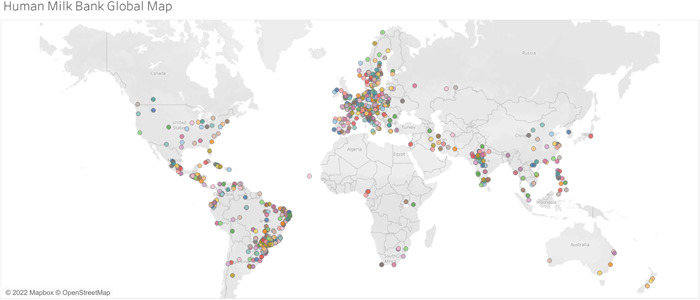
Global human milk banking map. The map data have been updated as of November 2022 and was retrieved from Tableau Public (Human Milk Bank Global Map, 2019).
3. KEY PRINCIPLES FOR HUMAN MILK BANKING ACTIVITIES
In the absence of established global guidance for HMB activities and systems, in 2012 a team of human milk banking technical experts from around the world jointly developed a set of fundamental principles to guide and support robust HMBs. The foundation of any HMB should include quality assurance, breastfeeding promotion and support, auditing and tracking, and guidance for the provision of donor milk. Atop the foundation sit the five key pillars of safety; quality; networking and information sharing; awareness, advocacy, and promotion; and sustainability, as shown in Figure 2 (PATH, 2013).
Figure 2.
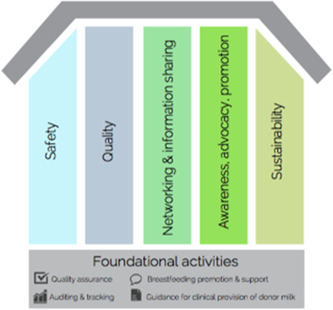
Foundation and key pillars of a human milk bank (PATH, 2013).
While the pillars supporting HMB operations are interrelated, each maintains a distinct set of aims and activities. Activities to support HMB safety include efforts to reduce pathogens, toxins, and contaminants. Regular audits, ongoing staff training, clear and transparent data management, product security, and compliance with local and national policies are also paramount. Quality measures include retention of biological and nutritional properties of donor milk as well as operational, technical, and managerial consistency and quality assurance. Networking and information sharing promote learning and foster transparency of activities and results. Awareness, advocacy, and promotion activities are grounded by lactation and breastfeeding support and counselling. In addition, these efforts respond to and advocate for the need for the HMB and donors and inform local, national, and international policy. Finally, sustainability measures are vital to establishing appropriate supply and demand for the HMB. Supporting mothers to maintain an adequate breastmilk supply, seeking engagement from local stakeholders, and maintaining financial integrity and sound business practices are also vital for HMB sustainability (PATH, 2019a).
4. HMB PROCESSES
HMBs perform numerous, complex processes, including donor recruitment and screening, milk handling and processing, and allocation of donor milk to recipients (Figure 3, PATH, 2019a). Because of a lack of universal guidelines, HMB operators face many questions in developing appropriate processes, and individual HMBs may adapt their operations based on local context, resources, and needs. For example, operational policies may permit mothers to express breastmilk in the home or the HMB. Similarly, pasteurisation processes may vary, and DHM prioritisation and allocation may differ.
Figure 3.
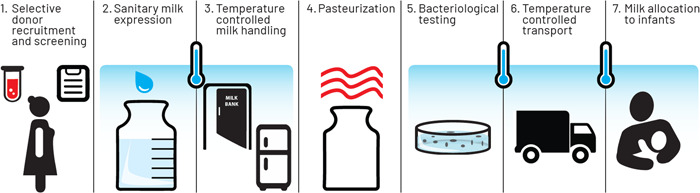
Steps for ensuring safety in human milk banking (PATH, 2019a).
As a result of disparate systems, DHM collection, processing, storage, and allocation processes are heterogeneous. Subsequently, the quality and composition of the final DHM product varies. It is important to note, however, that though HMB systems globally share many collective goals, restrictive, standardised HMB regulations are neither feasible nor appropriate for all settings because of the spectrum of diversity in culture, disease risk, government oversight, resources, and maternal and newborn care systems present around the world (Food and Agriculture Organisation [FAO], 1998; PATH, 2019a).
5. OPERATIONAL MODELS OF HMBS
HMBs operate in a variety of forms due to need, health system structure, resource availability, and local policies. Operational models for HMBs (and DHM processors) often include a combination of the following: commercial/for‐profit or nonprofit, centralised or decentralised, community‐based or facility‐based, and independent/vertical or integrated. A comparison of different structures is presented in Table 1. Although benefits and challenges exist for each operational model, much of the current literature focuses on the importance of a nonprofit, integrated approach to human milk banking that promotes best practices for maternal and newborn care and fosters equitable access to an exclusive human milk diet.
Table 1.
Categories of human milk bank operational models utilised around the world (PATH, 2019a).
| Financial structure | |
|---|---|
| Nonprofit | Commercial, for profit |
|
|
| Oversight structure | |
|---|---|
| Centralised | Decentralised |
|
|
| Facility location | |
|---|---|
| Hospital/facility‐based | Community‐based |
|
|
| Vertical and integrated systems | |
|---|---|
| Independent/vertical system | Integrated system |
|
|
Ethical considerations for for‐profit and commercial DHM programmes can be considered through multiple angles: human rights, potential exploitation of vulnerable populations, equity and fairness, and quality assurance. For example, the provision of financial incentives is counter to the WHO's Principles on the Donation and Management of Blood, Blood Components and Other Medical Products of Human Origin and the Conventions for the Protection of Human Rights and Dignity of the Human Being with Regard to the Application of Biology and Medicine, both of which maintain language that includes human milk (Council of Europe, 1999; Miracle et al., 2011; Shelley & Ginsberg, 2018; World Health Assembly, 2017). From an equity perspective, the increased cost of DHM obtained from a for‐profit company may promote commoditization of DHM and negatively influence access for those in need. And, from a quality and safety viewpoint, DHM sterilisation is employed by some commercial organisations though it has been shown to negatively impact concentrations of protein, fat, immune factors, and human milk oligosaccharides (Shelley & Ginsberg, 2018).
6. INTEGRATION OF HUMAN MILK BANKING INTO HEALTH SYSTEMS
In a presentation made at the 21st International Congress of Nutrition, UNICEF representatives provided a statement suggesting that “human milk banks should not be isolated initiatives; [they] need to be established and managed as part of a comprehensive programme for the protection, promotion, and support of breastfeeding” (France & Clark, 2017).
When HMBs are integrated into health systems, they offer a systems strengthening mechanism for newborn nutrition and foster equitable access to an exclusive human milk diet. Fortified newborn nutrition systems influence how DHM is used, help prevent DHM overuse, and promote exclusive human milk feeding by supporting kangaroo mother care and other best practices that build on the revised Baby‐Friendly Hospital Initiative (BFHI) approach (DeMarchis et al., 2017; WHO, n.d.), including protecting, promoting, and supporting breastfeeding (WHO, United Nations Children's Fund, 2018). Integrated HMBs also align with the WHO/UNICEF Ten Steps to Successful Breastfeeding (2018), which advocate for early breastfeeding initiation immediately after birth, exclusive breastfeeding, and discharge planning to ensure ongoing lactation support and care (Figure 4).
Figure 4.
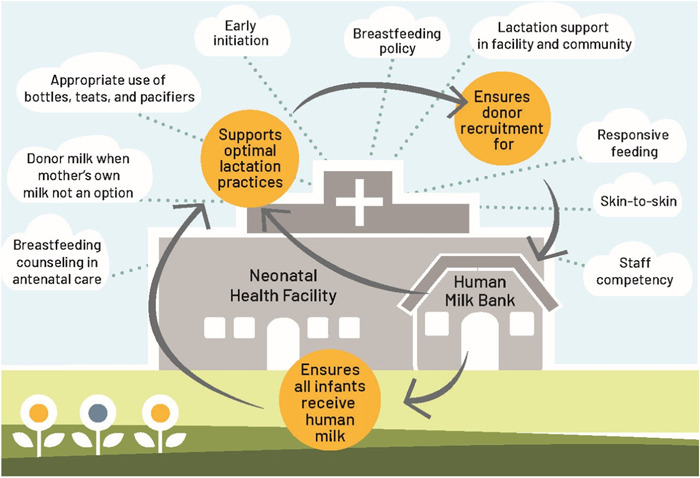
Components of an integrated human milk bank programme as part of the Baby‐Friendly Hospital Initiative (BFHI) (World Health Organization, United Nations Children's Fund UNICEF, 2018).
Integrated HMBs serve as a centralised community hub and resource centre to establish lactation, support mothers to continue breastfeeding successfully, and serve to accept, process, and distribute safe, high‐quality DHM to infants in need. Data suggest that individual and community awareness of the importance of breastfeeding increases with the presence of an HMB in a hospital, neonatal ward, or in the local community. In this research, as a result of increased awareness, breastfeeding rates upon discharge improved (Adhisivam et al., 2017; Arslanoglu et al., 2013).
Integration of HMBs into existing maternal and infant health strategies can also strengthen infrastructure, human resources, and technical and financial sustainability by creating a reinforced system for improving health. However, for true integration into existing neonatal and maternal health systems, HMBs must be carefully planned. A phased implementation, including stakeholder and governmental engagement, community needs assessment, capacity‐building, and impact demonstration, is required.
Establishing a foundation using a phased approach helps to ensure community ownership and engagement, sustainability, and standardised evaluation measures across the human milk banking system—an approach that has been successfully demonstrated in government‐led HMB programmes in LMIC settings, including India, Vietnam, and Kenya. By design, truly integrated HMBs function as community hubs and resource centres to support families by helping to establish lactation, support mothers to continue breastfeeding successfully, and serve to accept, process, and distribute safe, high‐quality DHM (DeMarchis et al., 2017).
HMBs may face upfront challenges in fostering partnerships and developing communication networks to support collaboration. However, partnerships are vital to realising the goals of supporting maternal and newborn health and increasing equitable access to DHM and MOM. Additionally, support from healthcare facilities and the community is needed to ensure that the system is appropriate for the local context considering infrastructure, human resources, and the technical and financial means required for implementation and sustainability.
7. STRATEGIES SUPPORTING SUSTAINABILITY OF HMB SYSTEMS
HMB sustainability should be included in strategy development, stakeholder engagement, and resource planning. HMB systems sustainability depends upon DHM supply and demand; monitoring and research initiatives; financial sustainability; communications, policy, and advocacy; collaboration; and defined roles and responsibilities of core HMB staff (PATH, 2019a). As shown in Figure 5, each domain is inextricably linked to the others, and a deficiency in any one area can potentially undermine the efforts of the HMB programme (PATH, 2019a).
Figure 5.
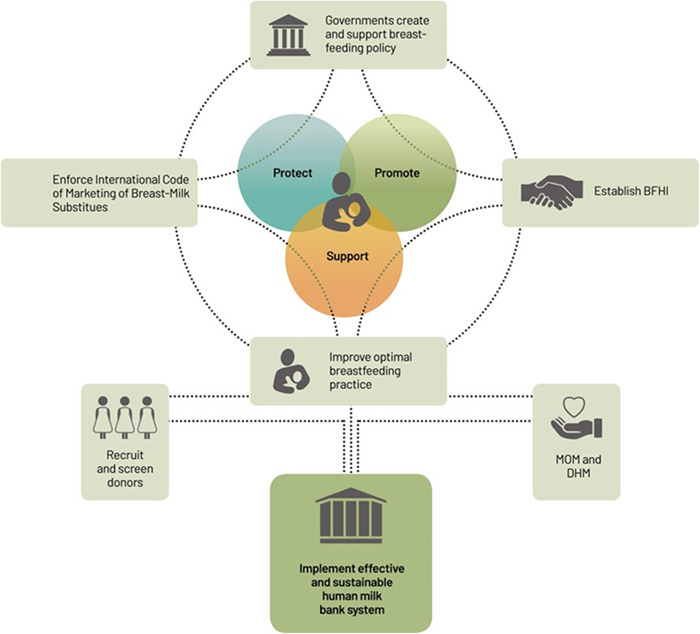
Effective human milk bank implementation requires investment in policies by the government to protect, promote, and support breastfeeding (PATH, 2019a).
First, comprehensive support for and promotion of exclusive breastfeeding is the cornerstone of improving infant health and cultivating sustainable DHM supply and demand. Lactation support services, including access to lactation professionals, peer support groups, referrals to necessary maternal health services, and expression equipment, are also essential to the sustainability of the donor pool. Additionally, outreach and awareness campaigns are crucial for developing a broad and diverse donor pool, increasing public knowledge and trust in the HMB, clarifying misconceptions about DHM, and leveraging financial support resources to ensure that safe donor milk is always available.
Second, rigorous monitoring and data collection systems are needed to document impact, ongoing evaluation, and programme enhancements that result in the sustainable and contextually appropriate expansion of HMB programmes. Innovative research, alongside standardised quality control measures, fosters sustainability by informing action to improve the safety and efficiency of human milk banking programmes. It also produces data to increase community and investor confidence in human milk banking systems' health and cost benefits.
Third, sustainable, consistent, and ample funding is required for HMB start‐up and ongoing operational costs. National health funds, government resources, charitable organisations, private funders, and other sponsors often support HMB operations. When part of a hospital or health facility, the facility may directly provide staffing, equipment, and office space, as well as sponsor milk handling and treatment efforts (Minh et al., 2017).
Fourth, HMB leaders should use communication, policy, and advocacy efforts to support HMB sustainability via targeted messaging that raises awareness and understanding of optimal nutrition for infant feeding and the need for human milk banking systems (PATH, 2019b). Maintaining familiarity with guidelines, regulations, and policies that guide milk donation and human milk banking practices and fostering regular communication and partnerships with regulatory bodies creates a policy promotion and advocacy network. Policy and advocacy efforts may also support the integration of HMB strategies into existing maternal and newborn health policies.
Fifth, collaborative networks of local, national, and regional HMB programmes and professionals establish the potential for linked quality assurance systems and a sense of community. Although several national and regional associations exist, there is no singular global platform for knowledge sharing and policy development among HMBs. Establishing collaborative channels and sharing experiences and data through HMB networks helps teams learn to apply innovative equipment, embrace best practices, and remain updated on current research, policies, and technologies. As a diverse network of experts, the international HMB community could be better harnessed to improve and promote knowledge sharing and quality standards among HMBs worldwide to help ensure the safety and sustainability of the human milk banking system (PATH, 2013).
Finally, HMB leadership, staff training, and development are vital to the functionality of an HMB system. Investing in facility leaders who maintain a strong HMB knowledge base and ties to the local community is critical for securing perpetual financial, logistical, and community support. Strong leadership may also facilitate the recruitment of essential HMB staff, advocates, and volunteers to aid in the recruitment of donors, support mothers, and promote a sustainable system that promotes breastfeeding and lactation support. Strong facility leaders should also build and maintain communication networks within the national and international HMB community to share learning and best practices to ensure the safety and quality of DHM.
8. POLICY FRAMEWORKS SUPPORTING THE USE OF DHM
The WHO and other global health leaders have recognised the benefits of using DHM over infant formula for sick and vulnerable newborns. And they have recommended the scale‐up of HMBs to encourage the provision of safe DHM as a strategy for improving neonatal health and survival (WHO, 2008). Additionally, recommendations for the provision of DHM for sick and vulnerable newborns are included in several other globally adopted frameworks and documents that aim to improve newborn health and survival, including, but not limited to, the following: Global Strategy for Infant and Young Child Feeding (WHO, United Nations Children's Fund, 1990), Breastfeeding and the Use of Human Milk (Eidelman et al., 2005), Integrated Management of Pregnancy and Childbirth: Pregnancy, Childbirth, Postpartum and Newborn Care: A guide for essential practice (WHO, United Nations Population Fund, World Bank, & UNICEF, 2015), Implementation Guidance: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services—the Revised Baby‐Friendly Hospital Initiative (WHO, 2020, 2022; WHO, United Nations Children's Fund, 2018).
DHM inclusion in health‐focused frameworks and documents is not enough to protect and safeguard human milk; global and regional policies and legally binding frameworks are needed. The absence of legal frameworks protecting DHM, donors, and recipients presents a significant limitation for HMBs. Oversight and regulatory requirements for HMBs may be established at the local, regional, national, or international levels. However, in the absence of universal guidance, there are multiple complementary programmes that could apply to or be extended by additional policy guidance. Examples include The International Code of Marketing of Breastmilk Substitutes (WHO, 1981), the WHO Guidelines for Donation and Management of Blood and Medical Products of Human Origin (World Health Assembly, 2017), and The Baby Friendly Hospital Initiative (BFHI) (WHO, United Nations Children's Fund, 2018).
9. CLASSIFICATION AND REGULATION OF DHM AS A FOOD, NUTRITION THERAPY, MEDICAL PRODUCT OF HUMAN ORIGIN, OR UNDEFINED
An essential aspect of DHM regulation and HMB oversight includes delineating how human milk is classified. Classification is vital and carries implications for accessibility; cost; payment and insurance coverage; regulatory oversight; and protections for donors, recipients, and DHM products. Countries, regions, and locales have elected to classify human milk in four ways: as a food, as a nutrition therapy or medical/functional food, as a medical product of human origin, or in an undefined category. Each category has advantages and disadvantages, and classification should align with local regulatory frameworks (PATH, 2019a).
In Brazil, for example, DHM is classified as a food because it is viewed as a product that contains macro and micronutrients needed to support infant growth and development. HMBs are tasked with ensuring safety and preventing contamination, and regulatory oversight is provided to ensure compliance with national‐level food safety standards. Potential benefits of classifying human milk as food may include featuring the nutritional importance of human milk, cost‐effectiveness, utilisation of existing principles and safeguards (including HACCP), and standardisation of quality and safety management. Challenges may include ineligibility for insurance reimbursement, public perception that regulatory measures are inadequate, possible private or informal DHM sales, and insufficient consideration of the risks and complexities human milk presents beyond those associated with manufactured food products.
In France, DHM is classified as a medical product of human origin along with blood products, stem cells, and other tissues. Regulation requires a framework that defines the system, standards, and legal specifications and ensures compliance and enforcement. It also aims to address public and private activities. Potential benefits of DHM under the medical product of human origin classification may include (1) improved insurance reimbursement support and public safety perception, (2) cross‐utilisation of existing tissue regulating guidelines and principles, (3) high‐level quality management and accountability supported by government entities, and (4) enhanced public protection that prohibits informal sale. On the other hand, challenges may include increased costs associated with regulation that may be unnecessary given the unique properties of human milk that are not common to other types of human tissue, limited or restricted DHM access because of cost or procurement restrictions, and nationally insufficient regulatory frameworks for surveillance activities.
In the United Kingdom and India, DHM is in an undefined class that allows a mother to donate her expressed milk for a voluntary donation without remuneration. Regulatory guidelines include testing and processing procedures and self‐reported maternal health information. In addition, an HMB regulatory body functions as a liaison between independent HMBs and governmental agencies, and HMB inspections are employed to ensure quality control and adherence to guidelines. Potential benefits include employment of well‐established guidelines that have been validated across several countries, acknowledgement of human milk in a distinct classification (not a food, tissue, or nutrition therapy), and utilisation of existing health department inspection procedures to aid in cost containment. However, national endorsement by an existing regulatory body or the creation of a new regulatory entity may be challenging, and insurance reimbursement for DHM may be difficult.
Classification of human milk as a medical product of human origin may serve as a viable compromise in developing regulatory frameworks around human milk banking and DHM use. As a nutrition therapy or medical or functional food, DHM could be viewed as food intended for the dietary management of a particular condition or disease and, as nutrition therapy, would generally be administered under the supervision of a physician. Governmental regulation would be included in food and drug safety, consisting of classification criteria, safety protocols, and guidelines for sale and use. Potential benefits might include representation of the therapeutic benefits of DHM and its immunoglobulins, insurance coverage for DHM as a therapeutic product, recipient savings if DHM were included in bundled hospital costs, and enhanced oversight in comparison to food products. Challenges may include regulatory barriers to access and costs associated with regulatory oversight, especially if the classification is not otherwise used in governmental systems.
10. EXISTING REGULATORY FRAMEWORKS AND OVERSIGHT ENTITIES FOR HMBS
Government oversight and regulatory requirements for HMBs differ among countries, and oversight entities maintain significant influence over the selection and adoption of operational procedures. HMBs are tasked with engaging local and national agencies to determine how to best utilise available resources to ensure the safest and highest‐quality donor milk. For example, in the United States, the Food and Drug Administration provides suggestions for HMB operations but not oversight.
Current posted FDA website information (accessed on 25 January 2022, content current as of 22 March 2018) states that the FDA does not provide oversight for human milk banking activities in the United States. The organisation recommends that families consult a healthcare provider and consider possible safety risks, including chemical contaminants, medications, and infectious diseases, before initiating feedings with DHM (United Nations Department of Economic and Social Affairs, 2018).
If an individual elects to use DHM for infant feeding, the FDA suggests using milk that has been sourced from an HMB and not milk obtained directly from another individual or via the internet. Though the agency recommends consulting the individual state departments of health and HMBANA for more information, the United States Food and Drug Administration website (2018) clearly states that “FDA has not been involved in establishing these voluntary guidelines or state standards” (paras. 6).
Operational variation may occur in individual countries or regions based on what is dictated by the local context. There are many national and regional‐level HMB oversight organisations and associations globally, including those of Austria, France, Spain, the United Kingdom, India, Brazil, South Africa, and the Philippines. The Human Milk Banking Association of North America (HMBANA; the association of the United States and Canada) and the European Milk Banking Association (EMBA; the association inclusive of members representing many European countries) are the two largest, most‐recognised HMB organisations. HMBANA and EMBA do not provide authority or oversight (regulatory or voluntary) outside their respective locales. However, their publications and work are often seen as models for other HMB programmes. Though these organisations serve as respected global leaders, their structure, operations, and activities differ significantly across membership structures, governance, membership criteria, guideline endorsement, and other factors.
11. BARRIERS TO EFFECTIVE SCALE‐UP
Unfortunately, DHM access is thwarted on many fronts. First, there is a lack of supportive national and international policy development and alignment. While neonatal care and nutrition policy efforts exist, they outline different priorities, especially concerning human milk feeding options for preterm and LBW infants. For example, the recent Survive and Thrive Key Findings outline neonatal care guidelines and discourage separating infants and mothers since keeping them together promotes breastfeeding and skin‐to‐skin contact. However, the guidelines do not explicitly address infant feeding in cases where breastfeeding may not be possible because of prematurity, LBW, separation, or maternal or infant illness (WHO, 2019).
Second, there are many existing gaps in knowledge of infant feeding practices. Data on breastfeeding indicators are typically collected via Demographic Health Surveys or Multiple Indicator Surveys that use recall data that may not accurately reflect infant feeding in the hospital setting. Furthermore, there is no data on how many infants globally lack MOM, how often human milk is shared informally, and what the need for DHM may be. Recent newborn and nutrition publications have lacked focus on newborn breastfeeding behaviours, and the authors have stated this resulted from a lack of data (Every Newborn Series, 2014; Maternal Child Nutrition Series, 2013; The Lancet, 2016).
Third, there is limited sponsorship of innovative systems and technologies to optimise HMB operations and processes, ensure DHM quality and safety, and promote low‐cost global access to DHM. Opportunities for innovation are many and include donor recruitment strategies, human milk collection and tracking, DHM treatment methods to eliminate pathogens, rapid point‐of‐care diagnostics for microbial assays, packaging for collection, storage, and sampling of DHM, long‐term DHM storage, and long‐distance transportation systems.
Fourth, while milk banking associations and other organisations have published standards and guidance (PATH, 2019a; Tyebally Fang et al., 2021), global HMB operating standards have been lacking. As a result, DHM quality control and HMB operations are inconsistently developed and applied at the local, national, and institutional levels resulting in a range of practices and outcomes globally. Minimum standards for quality, safety, and appropriate use are needed, and a set of basic requirements should be established and universally applied to all HMBs. Due to this need and critical, the WHO is currently employing a formal systematic process to develop global guidance for HMB.
Finally, there are persistent challenges in integrating HMBs into existing nutrition and neonatal and maternal care programmes across health systems. Many HMBs have been established as independent facilities dedicated to collection, processing, and storing human milk, rather than as linked, integrated components of national or facility‐based breastfeeding promotion programmes. Best practices suggest increased impact with a comprehensive, integrated approach to human milk banking activities, including community education and engagement to raise awareness of HMB activities and normalise human milk feeding for all infants.
12. THE IMPACT OF THE COVID‐19 PANDEMIC ON HUMAN MILK BANKING PRACTICES AND OPERATIONS
At the onset of the COVID‐19 pandemic, fear around viral transmission via breastfeeding resulted in mother‐infant separation and provision of alternative feeds. Although the WHO recommendations remained in support of breastfeeding, it was many months before many other agencies or national policies followed (Olonan‐Jusi et al., 2021; Spatz et al., 2021). This complex situation included fears of SARS‐CoV‐2 virus in DHM. Research eventually documented both that transmission via breastfeeding was unfounded (Krogstad et al., 2021; Perez et al., 2022) and that Holder pasteurisation of DHM inactivated potential SARS‐CoV‐2, thus rendering DHM safe (Unger et al., 2020).
Regardless, the impact of the pandemic on human milk banking globally was profound (Shenker et al., 2021; Shenker & Virtual Collaborative Network of Human Milk Banks, 2020; Olonan‐Jusi et al., 2021). In many regions, fear of mother‐to‐child transmission, increased requests for DHM. Simultaneously, HMBs struggled with varying milk donations due to lockdowns and with operational stability due to staffing in the midst of pandemic environments.
Facing a pandemic in the absence of global guidelines or immediate response mechanisms for HMBs presented a massive challenge in the initial months due to limited information, no global communication platform, and the need to rapidly update safety protocols related to DHM. In response to this critical gap, a Virtual Communications Network of milk bank leaders was formed on 17 March 2020 (Shenker et al., 2021). In the initial operating months, the network gained more than 80 members from 34 countries and to date has grown to 120 members from 45 countries. This platform enabled the group to actively and in real‐time discuss COVID‐19 challenges and how to mitigate issues related to donor milk safety and HMB operations. This communications network, which had never previously existed to connect HMBs at a global level, allowed for sharing of best practices, safety data, research, and policies to guide an aligned approach in addressing challenges in staffing, donor recruitment, safe handling of human milk, and increased demand for DHM.
This network was created in response to emergency pandemic needs and has since become the Global Alliance of Human Milk Banks and Associations (GAMBA) with the vision of continuing a worldwide collaborative voice to advance HMB safety and quality (Human Milk Foundation, 2022).
13. KNOWLEDGE GAPS AND FUTURE DIRECTIONS
Human milk banking processes are still in their infancy, and there are active efforts to address data, research, and knowledge gaps that continue to present challenges for HMB leadership organisations, human milk banking operations, and policymakers. Though indirect, these challenges also impact DHM donors and recipients.
Opportunities exist wherein regulation, research, collaboration, and knowledge development could produce tangible programmatic improvements. Leaders and policymakers can provide regulatory and financial support and can engage HMB and DHM stakeholders with expertise in maternal and infant care, microbiology, lactation, pharmacology, nutrition, nursing, finance, research, and law to ensure the safety of human milk donors and recipients and promote the appropriate allocation of DHM. Researchers can study infant feeding practices globally to create improved knowledge to inform further programme development. Furthermore, the study of the short and long‐term impacts of DHM use, exploration of the need for and effects of human milk fortification, understanding the long‐term cost‐effectiveness of DHM feeding, and development of innovations to screen for toxins, bacteria, and other components would be beneficial. Individual HMBs and HMB networks can embrace opportunities for collaboration to identify and share best practices and assist in research generation and data collection, support trials of technology‐enabled solutions, and aid policymakers in developing realistic policy solutions that support HMB operations. And breastfeeding and human milk advocates can continue to educate the professionals and the public about the potential health benefits and cost‐effectiveness of an exclusive human milk diet—especially for vulnerable infants.
14. CONCLUSION
Exclusive human milk diets are vital for infant health and survival, especially for small, sick, and vulnerable newborns. Efforts to support exclusive breastfeeding are critical. However, when mother's own breastmilk is not available, DHM from a HMB can provide a temporary alternative.
Collective and collaborative efforts are needed to expand HMB services, increase DHM availability, and improve HMB practices globally. With global engagement, framework, policy alignment, and regulatory support, programmatic improvements to improve maternal support systems and neonatal outcomes may be both possible and realistic.
AUTHOR CONTRIBUTIONS
Kiersten Israel‐Ballard and Emily LaRose contributed equally to the writing and editing of this paper. Kimberly Mansen provided guidance, direction, and in‐depth review. They are all equally responsible for content.
CONFLICT OF INTEREST STATEMENT
Kiersten Israel‐Ballard serves on the Board of Directors for the Human Milk Banking Association of North America. Kimberly Mansen serves on the Board of Directors for the Northwest Mothers Milk Bank in Oregon. The remaining author declares no conflict of interest.
ACKNOWLEDGEMENTS
Funding for this report is from the Family Larsson‐Rosenquist Foundation.
Israel‐Ballard, K. , LaRose, E. , & Mansen, K. (2024). The global status of human milk banking. Maternal & Child Nutrition, 20(S4), e13592. 10.1111/mcn.13592
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Adhanom Ghebreyesus, T. , & Lake, A. (2017). Breastfeeding is not a one‐woman job. World Health Organization Newsroom. https://www.who.int/news-room/commentaries/detail/breastfeeding-is-not-a-one-woman-job
- Adhisivam, B. , Vishnu Bhat, B. , Banupriya, N. , Poorna, R. , Plakkal, N. , & Palanivel, C. (2017). Impact of human milk banking on neonatal mortality, necrotizing enterocolitis, and exclusive breastfeeding—Experience from a tertiary care teaching hospital, south India. Journal of Maternal‐Fetal and Neonatal Medicine, 2017, 1–4. [DOI] [PubMed] [Google Scholar]
- Arslanoglu, S. , Moro, G. E. , Bellù, R. , Turoli, D. , De Nisi, G. , Tonetto, P. , & Bertino, E. (2013). Presence of human milk bank is associated with elevated rate of exclusive breastfeeding in VLBW infants. JPME, 41(2), 129–131. 10.1515/jpm-2012-0196 [DOI] [PubMed] [Google Scholar]
- Berkley, S. , Dybul, M. , Godal, T. , & Lake, A. (2014). Integration and innovation to advance newborn survival. The Lancet, 384(9938), e22–e23. 10.1016/s0140-6736(14)60691-7 [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Bahl, R. , Lawn, J. E. , Salam, R. A. , Paul, V. K. , Sankar, M. J. , Blencowe, H. , Rizvi, A. , Chou, V. B. , & Walker, N. , Lancet Newborn Interventions Review Group, & Lancet Every Newborn Study Group . (2014). Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? The Lancet, 384(9940), 347–370. 10.1016/S0140-6736(14)60792-3 [DOI] [PubMed] [Google Scholar]
- Brandstetter, S. , Mansen, K. , DeMarchis, A. , Nguyen Quyhn, N. , Engmann, C. , & Israel‐Ballard, K. (2018). A decision tree for donor human milk: An example tool to protect, promote, and support breastfeeding. Frontiers in Pediatrics, 6, 324. 10.3389/fped.2018.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of Europe . (1999). Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: Convention on human rights and biomedicine. https://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/164
- DeMarchis, A. , Israel‐Ballard, K. , Mansen, K. A. , & Engmann, C. (2017). Establishing an integrated human milk banking approach to strengthen newborn care. Journal of Perinatology, 37(5), 469–474. 10.1038/jp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman, A. I. , Richard, J. , & Schanler, R. J. (2005). Breastfeeding and the use of human milk. Pediatrics, 115(2), 496–506. 10.1542/peds.2004-2491 [DOI] [PubMed] [Google Scholar]
- Every Newborn Series . (2014). The lancet . https://www.thelancet.com/series/everynewborn
- Food and Agriculture Organization (FAO) . (1998). Food quality and safety system—A training manual on food hygiene and the hazard analysis and critical control point (HACCP) system. [Google Scholar]
- France, B. , & Clark, D. (2017). Donor human milk and the international code of marketing of breastmilk substitutes: Ethical challenges and implications of sharing, selling, and donating human milk, IUNS 21st International Congress of Nutrition. Buenos Aires. www.iuns-icn2017.com [Google Scholar]
- Human Milk Bank Global Map . (2019). Human milk bank global map tableau . https://public.tableau.com/app/profile/human.milk.bank.global.map/viz/HumanMilkBankGlobalMap_0/HumanMilkBankGlobalMap
- Human Milk Foundation . (2022). The global alliance of milk banks and associations (GAMBA) . https://humanmilkfoundation.org/about-hmf/gamba/
- Israel‐Ballard, K. (2018). Strengthening systems to ensure all infants receive human milk: Integrating human milk banking into newborn care and nutrition programming. Breastfeeding Medicine, 13(8), 524–526. 10.1089/bfm.2018.0133 [DOI] [PubMed] [Google Scholar]
- Krogstad, P. , Contreras, D. , Ng, H. , Tobin, N. , Chambers, C. D. , Bertrand, K. , Bode, L. , & Aldrovandi, G . (2021). No Evidence of infectious SARS‐CoV‐2 in human milk: Analysis of a cohort of 110 lactating Women. medRxiv [Preprint]. 10.1101/2021.04.05.21254897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maternal Child Nutrition Series . (2013). The lancet . https://www.thelancet.com/series/maternal-and-child-nutrition
- Minh, H. V. , Mai, V. Q. , & Anh, T. T. , Hanoi University of Public Health . (2017). The cost of establishing the first human milk bank in Viet Nam (Conference presentation). Experience and Learning from Launching the First Human Milk Bank in Vietnam Workshop.
- Miracle, D. J. , Szucs, K. A. , Torke, A. M. , & Helft, P. R. (2011). Contemporary ethical issues in human milk‐banking in the United States. Pediatrics, 128(6), 1186–1191. 10.1542/peds.2010-2040 [DOI] [PubMed] [Google Scholar]
- Moro, G. E. (2018). History of milk banking: From origin to present time. Breastfeeding Medicine, 13(S1), S‐16–S‐17. 10.1089/bfm.2018.29077.gem [DOI] [PubMed] [Google Scholar]
- Olonan‐Jusi, E. , Zambrano, P. G. , Duong, V. H. , Anh, N. T. T. , Aye, N. S. S. , Chua, M. C. , Kurniasari, H. , Moe, Z. W. , Ngerncham, S. , Phuong, N. T. T. , & Datu‐Sanguyo, J. (2021). Human milk banks in the response to COVID‐19: A statement of the regional human milk bank network for Southeast Asia and beyond. International Breastfeeding Journal, 16(1), 29. 10.1186/s13006-021-00376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATH . (2013). Bill & melinda gates foundation grand challenges initiative. [Google Scholar]
- PATH . (2019a). Strengthening human milk banking: A resource toolkit for establishing & integrating human milk bank programs—A global implementation framework. Version 2.0 (p. 2019). https://www.path.org/programs/maternal-newborn-child-health-and-nutrition/strengthening-human-milk-banking-resource-toolkit-0/ [Google Scholar]
- PATH . (2019b). Strengthening human milk banking: A resource toolkit for establishing, operating, and integrating human milk bank programs: Communications toolkit. [Google Scholar]
- Perez, S. E. , Luna Centeno, L. D. , Cheng, W. A. , Marentes Ruiz, C. J. , Lee, Y. , Congrave‐Wilson, Z. , Powell, R. L. , Stellwagen, L. , & Pannaraj, P. S. (2022). Human milk SARS‐CoV‐2 antibodies up to 6 months after vaccination. Pediatrics, 149(2), e2021054260. 10.1542/peds.2021-054260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley, T. , & Ginsberg, H. G. (2018). Bioethics in practice: The ethics surrounding the use of donor milk. The Ochsner Journal, 18(1), 17–19. [PMC free article] [PubMed] [Google Scholar]
- Shenker, N. , Staff, M. , Vickers, A. , Aprigio, J. , Tiwari, S. , Nangia, S. , Sachdeva, R. C. , Clifford, V. , Coutsoudis, A. , Reimers, P. , Israel‐Ballard, K. , Mansen, K. , Mileusnic‐Milenovic, R. , Wesolowska, A. , Goudoever, J. , Hosseini, M. , Klotz, D. , Grøvslien, A. H. , & Weaver, G. , Virtual Collaborative Network of Milk Banks and Associations . (2021). Maintaining human milk bank services throughout the COVID‐19 pandemic: A global response. Maternal & Child Nutrition, 17(3), e13131. 10.1111/mcn.13131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker, N. , Virtual Collaborative Network of Human Milk and Associations . (2020). Maintaining safety and service provision in human milk banking: a call to action in response to the COVID‐19 pandemic. The Lancet, Child & Adolescent Health, 4(7), 484–485. 10.1016/S2352-4642(20)30134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer, K. , & Hartmann, B. (2009). The knowns and unknowns of human milk banking. Early Human Development, 85(11), 701–704. 10.1016/j.earlhumdev.2009.08.054 [DOI] [PubMed] [Google Scholar]
- Spatz, D. L. , Davanzo, R. , Müller, J. A. , Powell, R. , Rigourd, V. , Yates, A. , Geddes, D. T. , van Goudoever, J. B. , & Bode, L. (2020). Promoting and protecting human milk and breastfeeding in a COVID‐19 world. Frontiers in Pediatrics, 8, 633700. 10.3389/fped.2020.633700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet . (2016). Breastfeeding: Achieving the new normal. The Lancet, 387(10017), 404. 10.1016/s0140-6736(16)00210-5 [DOI] [PubMed] [Google Scholar]
- Tyebally Fang, M. , Chatzixiros, E. , Grummer‐Strawn, L. , Engmann, C. , Israel‐Ballard, K. , Mansen, K. , O'Connor, D. , Unger, S. , Herson, M. , Weaver, G. , & Biller‐Andorno, N. (2021). Developing global guidance on human milk banking. Bulletin of the World Health Organization, 99(12), 892–900. 10.2471/blt.21.286943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger, S. , Christie‐Holmes, N. , Guvenc, F. , Budylowski, P. , Mubareka, S. , Gray‐Owen, S. D. , & O'Connor, D. L. (2020). Holder pasteurization of donated human milk is effective in inactivating SARS‐CoV‐2. Canadian Medical Association Journal, 192(31), E871–E874. 10.1503/cmaj.201309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . (2017). Breastfeeding is ‘smartest investment’ families, communities and countries can make. https://www.un.org/sustainabledevelopment/blog/2017/08/breastfeeding-is-smartest-investment-families-communities-and-countries-can-make-un/
- United Nations Department of Economic and Social Affairs . (2015). Sustainable development goals knowledge platform . https://sustainabledevelopment.un.org/
- United Nations Department of Economic and Social Affairs . (2018). The sustainable development goals report (p. 2018). United Nations. https://unstats.un.org/sdgs/files/report/2018/TheSustainableDevelopmentGoalsReport2018-EN.pdf [Google Scholar]
- United States Food and Drug Administration website . (2018). Use of donor human milk. http://www.fda.gov/science-research/pediatrics/use-donor-human-milk
- Victora, C. G. , Bahl, R. , Barros, A. J. D. , França, G. V. A. , Horton, S. , Krasevec, J. , Murch, S. , Sankar, M. J. , Walker, N. , & Rollins, N. C. (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. 10.1016/s0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- World Health Assembly . (2017). Principles on the donation and management of blood, blood components and other medical products of human origin: Report by the Secretariat. World Health Organization. http://www.who.int/iris/handle/10665/274793.
- World Health Organization . (n.d.). Nutrition and food safety . http://www.who.int/nutrition/bfhi/ten-steps/en/
- World Health Organization . (1981). International code for marketing of breast‐milk substitutes. [Google Scholar]
- World Health Organization . (2008). World health assembly: Resolutions and decisions annexes. https://www.who.int/nutrition/topics/WHA61.20_iycn_en.pdf [Google Scholar]
- World Health Organization . (2011). Guidelines on optimal feeding of low birth weight infants in low‐ and middle‐income countries. [PubMed] [Google Scholar]
- World Health Organization . (2019). Survive and thrive: Transforming care for every small and sick mewborn (p. 2019). WHO/FWC/MCA/18.11. https://apps.who.int/iris/bitstream/handle/10665/276655/WHO-FWC-MCA-18.11-eng.pdf?ua=1 [Google Scholar]
- World Health Organization . (2020). Standards for improving the quality of care for small and sick newborns in health facilities. https://www.who.int/publications/i/item/9789240010765 [Google Scholar]
- World Health Organization . (2022). WHO recommendations for care of the preterm or low‐birth‐weight Infant . https://www.who.int/publications/i/item/9789240058262 [PubMed]
- World Health Organization, United Nations Children's Fund (UNICEF) . (1990). Declaration innocenti on the protection, promotion, and support of breastfeeding. Breastfeeding in the 1990s: A Global Initiative. [Google Scholar]
- World Health Organization, United Nations Children's Fund (UNICEF) . (2018). Implementation guidance: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services—The revised Baby‐friendly Hospital Initiative. WHO. http://www.who.int/nutrition/bfhi/ten-steps/en/ [Google Scholar]
- World Health Organization, United Nations Population Fund, World Bank, & UNICEF . (2015). Pregnancy, childbirth, postpartum and newborn care: A guide for essential practice (3rd ed.). WHO. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


