Abstract
When the growth kinetics of immature hepatitis A virus provirions and mature virions were monitored, distinct eclipse phases were noted for both types of particles. Strikingly, uncoating of virions occurred around 4 h postinfection, while uncoating of provirions occurred predominantly between 8 and 10 h postinfection. It is proposed that the heterogenous mixture of infectious hepatitis A virus particles (virions and provirions) typically present in inocula is responsible for the normally asynchronous nature of hepatitis A virus uncoating kinetics.
Human hepatitis A virus (HAV), a member of the family Picornaviridae originally classified in the Enterovirus genus, is now the type member of the Hepatovirus genus (12, 31). The reclassification of HAV was based on a number of differences between HAV and other enteroviruses, including an extreme stability against elevated temperature and low pH (25, 26) and a slow and inefficient replicative cycle in cell culture (see references 27 and 31). The uncoating of HAV has been demonstrated to be a slow and asynchronous process (3, 5, 9, 30). By contrast, the attachment to and penetration of cells by HAV appear to be as efficient as for other picornaviruses (3, 8, 29, 32).
Intact, antigenic particles from HAV-infected cells or cell culture supernatants have recently been demonstrated to be of three types: (i) virions, containing capsid proteins VP1, VP2, VP3, and possibly VP4 and viral RNA; (ii) provirions, or “immature” virions, containing VP1, VP0, VP3, and viral RNA; and (iii) procapsids, which are empty HAV particles with the same capsid composition as provirions but lacking RNA (2, 7, 23). Provirions have been implicated as direct precursors of mature picornavirus virions via the cleavage of VP0 (to VP2 and VP4) in an RNA-dependent manner (6).
HAV provirions, while infectious, have a lower specific infectivity than mature virions (6, 7). We proposed that the mixture of particle types (i.e., of virions and provirions and of particles with intermediate levels of VP0 and VP2) in viral inocula was responsible for the asynchronous nature of the HAV uncoating process, on the basis that provirions might uncoat more slowly than virions. Here we report that using an HAV inoculum with a high virion content resulted in rapid as well as synchronous uncoating.
Single-cycle growth kinetics of HAV and PV.
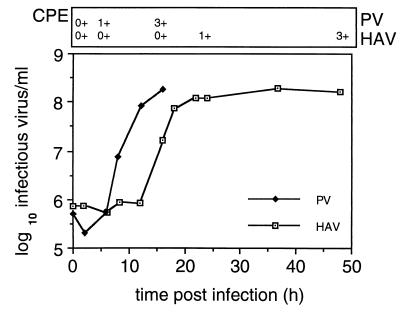
Typical single-cycle growth kinetics of HAV and poliovirus (PV) are shown in Fig. 1. An African green monkey cell line, BS-C-1, was infected with HAV strain HM175A.2 (1, 9) or PV type 1 (Mahoney), and the accumulation of infectious virus within cells was determined after various incubation times at 37°C.
FIG. 1.
Kinetics of HAV and PV replication. Monolayers of BS-C-1 cells were infected for 90 min at 4°C with HAV or PV before being shifted to 37°C. Infectious virus was assayed throughout the respective growth cycles by RIFA for HAV or plaque assay for PV. Cytopathic effect (CPE) was graded on a scale of 0+ to 4+, where 0+ represents a healthy cell monolayer and 4+ is destruction of more than 60% of cells. Much of the PV yield would be in the supernatant from 12 h postinfection.
In PV-infected cells, eclipse of the inoculated virus was evident at 2 h, reflecting rapid uncoating of the virus, followed by logarithmic accumulation of virus to 12 h. From 12 h, infectious virus accumulated slowly and much of the virus at this time was found in the supernatant. By contrast, eclipse of the HM175A.2 inoculum appeared to be incomplete, with much of the input virus being recovered up to 12 h postinfection. From 12 to 24 h, there was logarithmic virus growth, with cells finally showing distinct cytopathic effects at 48 h postinfection. Although the final yield of HAV varied from experiment to experiment, it was typically 50- to 200-fold lower than that of PV.
From this set of growth curves, it was clear that the growth of HAV was unusual, as it was slow and lacked a well-defined eclipse phase. This phenomenon has also been reported elsewhere (3, 5, 10, 30). However, the exact duration and kinetics of the HAV uncoating period vary in different studies, and this is most likely due in part to the use of different strains of HAV and their source, the temperature of growth, the type of culture medium, and the cell type and passage number used. In this regard, exact kinetics cannot be expected to be identical.
Kinetics of uncoating of light-sensitive HAV and PV.
To confirm that the eclipse phase of HAV observed in growth curves was due to asynchronous uncoating of the virus, we took advantage of the fact that most picornaviruses can be rendered light sensitive by growing them in the presence of neutral red, including PV (3, 18–20). When light-sensitive viruses are incubated with susceptible cells, the viral RNA remains light sensitive only as long as the RNA is contained inside the capsid (11, 15, 20). Conversion from photosensitivity to resistance is generally thought to be concomitant with virus uncoating and release of dye into the cytoplasm.
Light-sensitive HM175A.2 was prepared by growth in the presence of 0.003% (wt/vol) neutral red in the dark; PV was grown in the same manner but with 0.01% neutral red. Less neutral red had to be used for the growth of HAV due to the toxic effects of neutral red in culture medium over longer culture periods. PV and HAV cultures were harvested after 12 and 36 h at 37°C, respectively. Virus was then purified as for stock virus (9), except that all procedures were carried out in the dark.
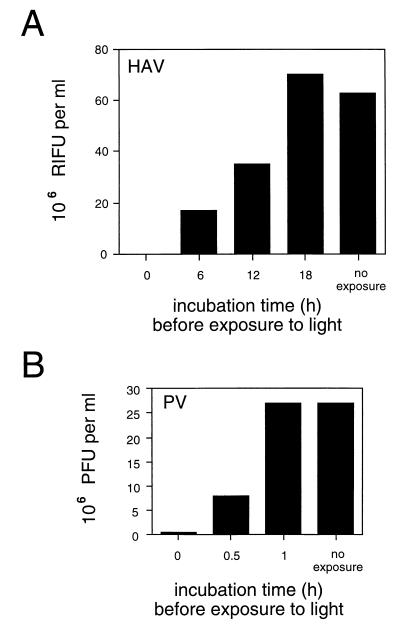
Serial 10-fold dilutions of light-sensitive HM175A.2 and PV were used to infect cultures of BS-C-1 cells by adsorption at 4°C in the dark. Cells were then shifted to 37°C and the proportion of virus which had become photoresistant was determined after exposure of cultures to light at various intervals. The number of infectious centers in each culture was then compared to that of cultures not exposed to light (Fig. 2). HAV infectivity was determined using a radioimmunofocus assay (RIFA) (3, 17), and PV infectivity was determined by plaque assay.
FIG. 2.
Kinetics of HAV and PV uncoating. Cultures of BS-C-1 cells were infected with serial dilutions of light-sensitive virus at 4°C in the dark. Cells were shifted to 37°C for various times and then exposed to light. After exposure, overlay medium was added to allow the number of particles uncoated during the incubation period to be estimated by RIFA for HAV or plaque assay for PV. RIFU, radioimmunofocus-forming units.
While uncoating of PV had occurred within 1 h (Fig. 2B), uncoating of HAV was not complete until 18 h postinfection (Fig. 2A). Approximately 25% of HAV particles had uncoated by 6 h postinfection, and 50% had uncoated by 12 h postinfection. It thus appears that not all input HAV particles are uncoated at the same time. Together (Fig. 1 and 2A), these experiments suggest that slow and asynchronous release of HAV RNA occurred.
Infectivity of HAV virions and provirions.
HAV inocula, whether derived from infected cells or supernatants, contain a mixture of mature virions and immature provirions (6). HAV RNA-containing particles with a higher proportion of capsid protein VP2 than VP0 have a greater specific activity than those containing a higher proportion of VP0 than VP2 (7). Furthermore, when HAV provirions are converted to virions by autocatalytic cleavage of VP0 to VP2 at 37°C, infectivity is likewise increased (6). These experiments indicated that HAV provirions are precursors of mature virions. Moreover, when HAV provirions (around 130S) are converted to virions in vitro, they then resediment in linear sucrose density gradients with the same sedimentation coefficient as mature virions produced in vivo (160S [data not shown]) (2, 23). Therefore, VP2-containing particles synthesized from VP0-containing particles in vitro have all the characteristics of HAV virions.
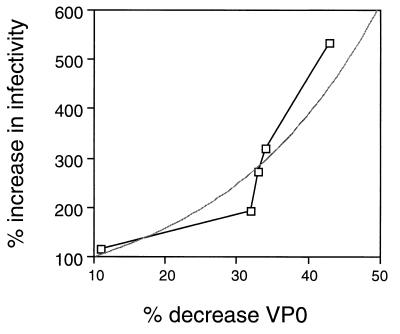
To directly study the relationship between the infectivity of HAV particles and VP0 content, five separate preparations of HAV provirions containing between 50 and 89% VP0 were incubated at 37°C for autocatalytic cleavage to occur. Cleavage of VP0 to VP2 was determined by Western immunoblotting (6, 7), and the VP0 proportion of the VP0-plus-VP2 sum was expressed as a percentage. The resulting decrease in VP0 content and the corresponding increase (n-fold) in infectivity were then calculated (Table 1). The increase in infectivity of the cleaved aliquots was converted to a percent increase in infectivity and plotted against the change in VP0 (and thus VP2) content of the HAV particle. The calculation used should thus negate the variability in specific infectivity that resulted from the use of multiple preparations, which was necessary because half of each preparation was required to give a reliably quantitatable signal in immunoblotting for the cleaved and uncleaved samples. Interestingly, VP0 cleavage led to an exponential increase in specific infectivity, with infectivity increasing approximately twofold for each 25% of VP0 cleaved (Fig. 3). The exponential increase in the specific infectivity of particles in relation to VP2 content suggests that multiple sites of cleavage are required to form infectious particles.
TABLE 1.
Infectivity of HAV provirions before and after VP0 cleavage
| VP0 content (%)a
|
Incubation at 37°C (days) | Infective titer (RIFU/ml)b
|
Increase in infectivity (fold)c | ||
|---|---|---|---|---|---|
| −70°C | 37°C | −70°C | 37°C | ||
| 52 | 20 | 4 | 4.2 × 108 | 8.1 × 108 | 1.9 |
| 50 | 16 | 4 | 2.1 × 108 | 6.7 × 108 | 3.2 |
| 52 | 19 | 4 | 5.5 × 106 | 1.5 × 107 | 2.7 |
| 89 | 78 | 1 | 2.8 × 108 | 3.2 × 108 | 1.1 |
| 65 | 22 | 5 | 7.5 × 108 | 4.0 × 109 | 5.3 |
Aliquots were examined for HAV VP0 and VP2 by Western immunoblotting and data were converted to percent VP0.
Determined by RIFA (with errors of less than ±2 × 10y). RIFU, radioimmunofocus-forming units.
After self-cleavage at 37°C.
FIG. 3.
Relationship between VP0 self-cleavage and specific infectivity. In five separate experiments, provirions were purified from infected cells and duplicate aliquots were placed at −70 or 37°C for various times. The percent conversion of VP0 to VP2 was determined, and the corresponding increase in infectivity was determined (Table 1). The percent decrease in VP0 content was then calculated and plotted against the increase in infectivity. It should be noted that the percent decrease in VP0 content of these preparations correlated absolutely with the increase in VP2 content in all cases. The lighter line indicates an exponential curve fit.
These data provide further evidence that the provirion is a precursor of the mature virion in picornavirus morphogenesis. Cleavage of VP0, as the final step in morphogenesis, is likely to be necessary for uncoating of the virus particle in endosomes (13, 14, 22, 24). In comparison to mature PV virions, which retain one to three copies of VP0, PV particles in which VP0 is not myristylated fail to cleave VP0 and are noninfectious (21), and human rhinovirus particles with a high VP0 content have been shown to be deficient in delivery of RNA to the cell interior after attachment (16). Recent studies of PV empty capsids have further defined a role for VP0 cleavage in allowing the extrusion of VP4 during structural rearrangements subsequent to binding (4). If HAV uses a similar uncoating mechanism, uncoating may not occur until VP0 cleavage within particles is complete. It will be difficult to determine whether there is a threshold level of VP2 which must be reached before viral uncoating can occur. Particles that fail to cleave sufficient numbers of VP0 within endosomes may subsequently be degraded in lysosomes or perhaps expelled at the cell surface.
Effect of VP0 processing on HAV replication in single-cycle growth curves.
To examine the biological differences between HAV virions and provirions, single-cycle growth kinetics of HAV were examined following infection of cells with inocula with a known VP0/VP2 content. Replication of the same pools of HAV was studied with or without prior autocatalytic cleavage of VP0 at 37°C, resulting in inocula with different levels of provirions and virions.
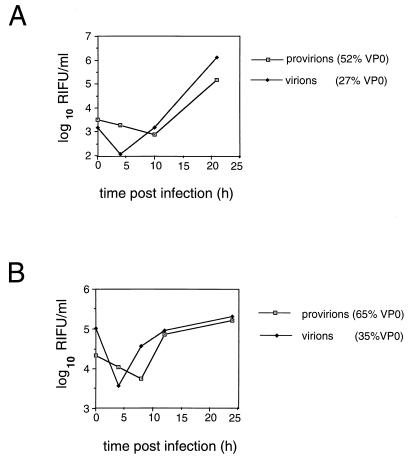
Examination of the provirion growth curve in Fig. 4A shows the lack of a distinct eclipse phase, where an HAV pool containing approximately equal proportions of VP0 and VP2 was used as the inoculum. By contrast, a much clearer eclipse phase was seen in cells infected with an inoculum containing predominantly virions (formed by self-cleavage of the original pool), indicating that the uncoating of HAV virions occurred in a more synchronized manner (Fig. 4A; virions), as is typical of other picornaviruses.
FIG. 4.
Single-cycle growth curves of HAV virions and provirions. In two separate experiments, pools of provirions were used to infect BS-C-1 cells with or without prior self-cleavage of VP0 at 37°C. (A) VP0 content of particles was approximately 52% before cleavage and 27% after self-cleavage at 37°C for 3 days. (B) VP0 content of particles was 65% before cleavage and 22% after self-cleavage at 37°C for 4 days. Virions in panel A were diluted 1:5 prior to infection, providing an approximately equal input titer. RIFU, radioimmunofocus-forming units.
When single-cycle growth kinetics of HAV provirions (65% VP0 content in the viral pool) were compared to those of HAV virions derived from the same pool after 4 days of self-cleavage at 37°C (now containing only 22% VP0), a distinct eclipse phase was detected in growth cycles of both HAV provirions and HAV virions (Fig. 4B), although slightly less clearly defined for the provirion pool.
The observed differences in growth kinetics are unlikely to reflect the different multiplicities of infection obtained with provirion and virion pools, on the basis that the virion pool is intrinsically more infectious. In the experiment whose results are shown in Fig. 4A, the virion pool had been diluted fivefold relative to the provirion pool to obtain a similar input multiplicity of infection, and a clear difference between growth curves was seen. In the experiment whose results are shown in Fig. 4B, the relative multiplicities of infection were not corrected and yet the residual infectivity in the virion pool was seen to vary 16-fold relative to that in the provirion pool over the first 4 h postinfection.
These results indicate that the uncoating of HAV provirions is delayed relative to that of virions, leading to either (i) asynchronous uncoating with a lack of a clearly defined eclipse when the inoculum contains both species of particles (Fig. 4A) or (ii) synchronous but delayed uncoating when the inoculum contains a very high proportion of provirions (Fig. 4B). The protracted time within the cell, during which provirions are presumably converted to virions prior to uncoating, is likely to result in the lower specific infectivity of these particles.
The intriguing question is why HAV produces high numbers of provirions, which are slow to uncoat, in contrast to other picornaviruses. We propose this is related to the target of infection in vivo, the liver. Hepatocytes are unusual because they lack a direct excretory mechanism to mediate viral exocytosis from the same membrane surface (28). Therefore, in order for the host to excrete viral particles, HAV must be transported by transcytosis back to the apical surface, where the particles can enter the bile canaliculi and begin the pathway for excretion from the host. If virus particles were capable of fusing with transcytotic vesicles during cross-cellular transport, they would not reach the apical membrane and would not be available for excretion and subsequent dissemination.
Acknowledgments
N.E.B. was supported by a Commonwealth Postgraduate Research Award, and D.A.A. was supported by the BHP Community Trust. This work was supported in part by the Macfarlane Burnet Centre for Medical Research and the World Health Organization Programme for Vaccine Development.
We thank J. Mills for critical reading of the manuscript, S. Locarnini and S. Borovec for helpful discussions, and P. Edwards and D. Hugo for excellent technical assistance.
REFERENCES
- 1.Anderson D A. Cytopathology, plaque assay, and heat inactivation of hepatitis A virus strain HM175. J Med Virol. 1987;22:45–56. doi: 10.1002/jmv.1890220106. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D A, Ross B C. Morphogenesis of hepatitis A virus: isolation and characterization of subviral particles. J Virol. 1990;64:5284–5289. doi: 10.1128/jvi.64.11.5284-5289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson D A, Locarnini S A, Ross B C, Coulepis A G, Anderson B N, Gust I D. Single-cycle growth kinetics of hepatitis A virus in BS-C-1 cells. In: Brinton M A, Rueckert R R, editors. Positive strand RNA viruses. New York, N.Y: Alan R. Liss; 1987. pp. 497–507. [Google Scholar]
- 4.Basavappa R, Gómez-Yafal A, Hogle J M. The poliovirus empty capsid specifically recognizes the poliovirus receptor and undergoes some, but not all, of the transitions associated with cell entry. J Virol. 1998;72:7551–7556. doi: 10.1128/jvi.72.9.7551-7556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop N E. Examination of potential inhibitors of hepatitis A virus uncoating. Intervirology. 1998;41:261–271. doi: 10.1159/000024948. [DOI] [PubMed] [Google Scholar]
- 6.Bishop N E, Anderson D A. RNA-dependent cleavage of VP0 capsid protein in provirions of hepatitis A virus. Virology. 1993;197:616–623. doi: 10.1006/viro.1993.1636. [DOI] [PubMed] [Google Scholar]
- 7.Bishop N E, Anderson D A. Hepatitis A virus subviral particles: purification, accumulation, and relative infectivity of virions, provirions, and procapsids. Arch Virol. 1997;142:2147–2160. doi: 10.1007/s007050050232. [DOI] [PubMed] [Google Scholar]
- 8.Bishop N E, Anderson D A. Early interactions of hepatitis A virus with cultured cells: viral elution and the effect of pH and calcium ions. Arch Virol. 1997;142:2161–2178. doi: 10.1007/s007050050233. [DOI] [PubMed] [Google Scholar]
- 9.Bishop N E, Hugo D L, Borovec S V, Anderson D A. Rapid and efficient purification of hepatitis A virus from cell culture. J Virol Methods. 1994;47:203–216. doi: 10.1016/0166-0934(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 10.Cho M W, Ehrenfeld E. Rapid completion of the replication cycle of hepatitis A virus subsequent to reversal of guanidine inhibition. Virology. 1991;180:770–780. doi: 10.1016/0042-6822(91)90090-x. [DOI] [PubMed] [Google Scholar]
- 11.Eggers H H, Waidner E. Effect of 2-(α-hydroxybenzyl)-benzimidazole and guanidine on the uncoating of echovirus 12. Nature. 1970;227:952–953. doi: 10.1038/227952a0. [DOI] [PubMed] [Google Scholar]
- 12.Francki R I B, Fauquet C M, Knudson D L, Brown F, editors. Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses. Arch Virol. 1991;1991(Suppl. 2):1–450. [Google Scholar]
- 13.Hellen C U T, Wimmer E. Maturation of poliovirus capsid proteins. Virology. 1992;187:391–397. doi: 10.1016/0042-6822(92)90440-z. [DOI] [PubMed] [Google Scholar]
- 14.Hogle J M, Syed R, Fricks C E, Icenogle J P, Flore O, Filman D J. Role of conformational transitions in poliovirus assembly and cell entry. In: Brinton M A, Heinz F X, editors. New aspects of positive-strand RNA viruses. Washington, D.C.: American Society for Microbiology; 1990. pp. 199–210. [Google Scholar]
- 15.Kato N, Eggers H J. Inhibition of uncoating of fowl plaque virus by 1-adamantamine. Virology. 1969;37:632–641. doi: 10.1016/0042-6822(69)90281-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee W-M, Monroe S S, Rueckert R R. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 1993;67:2110–2122. doi: 10.1128/jvi.67.4.2110-2122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemon S M, Binn L N, Marchwicki R H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983;17:834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madshus I H, Olsnes S, Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984;98:1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madshus I H, Olsnes S, Sandvig K. Different pH requirements for entry of the two picornaviruses, human rhinovirus 2 and murine encephalomyocarditis virus. Virology. 1984;139:346–357. doi: 10.1016/0042-6822(84)90380-5. [DOI] [PubMed] [Google Scholar]
- 20.Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967;31:702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- 21.Marc D, Masson G, Girard M, van der Werf S. Lack of myristoylation of poliovirus capsid polypeptide VP0 prevents the formation of virions or results in the assembly of noninfectious virus particles. J Virol. 1990;64:4099–4107. doi: 10.1128/jvi.64.9.4099-4107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossmann M G, Arnold E, Erickson J W, Frankenberger E A, Griffith J P, Hecht H J, Johnson J E, Kamer G, Lou M, Mosser A G, Rueckert R R, Sherry B, Vriend G. Structure of a human common cold virus and functional relationship to other picorniaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 23.Ruchti F, Siegl G, Weitz M. Identification and characterization of incomplete hepatitis A virus particles. J Gen Virol. 1991;72:2159–2166. doi: 10.1099/0022-1317-72-9-2159. [DOI] [PubMed] [Google Scholar]
- 24.Rueckert R R. Picornaviridae and their replication. In: Fields B N, et al., editors. Virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 507–548. [Google Scholar]
- 25.Scholz E, Heinricy U, Flehmig B. Acid stability of hepatitis A virus. J Gen Virol. 1989;70:2481–2485. doi: 10.1099/0022-1317-70-9-2481. [DOI] [PubMed] [Google Scholar]
- 26.Siegl G, Weitz M, Kronauer G. Stability of hepatitis A virus. Intervirology. 1984;22:218–226. doi: 10.1159/000149554. [DOI] [PubMed] [Google Scholar]
- 27.Siegl G, Nüesch J P F, Weitz M. Replication and protein processing of hepatitis A virus. In: Hollinger F B, Lemon S M, Margolis H, editors. Viral hepatitis and liver disease. Baltimore, Md: Williams and Wilkins; 1991. pp. 25–30. [Google Scholar]
- 28.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton J T, Frederick J, Meyer B. Hepatitis A virus attachment to cultured cell lines. J Infect Dis. 1991;164:1098–1103. doi: 10.1093/infdis/164.6.1098. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler C M, Fields H A, Schable C A, Meinke W J, Maynard J E. Adsorption, purification, and growth characteristics of hepatitis A virus strain HAS-15 propagated in fetal rhesus monkey kidney cells. J Clin Microbiol. 1986;23:434–440. doi: 10.1128/jcm.23.3.434-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wimmer E, Murdin A D. Hepatitis A virus and the molecular biology of the picornaviruses: a case for a new genus of the family Picornaviridae. In: Hollinger F B, Lemon S M, Margolis H, editors. Viral hepatitis and liver disease. Baltimore, Md: Williams and Wilkins; 1991. pp. 31–44. [Google Scholar]
- 32.Zajac A J, Amphlett E M, Rowlands D J, Sangar D V. Parameters influencing the attachment of hepatitis A virus to a variety of continuous cell lines. J Gen Virol. 1991;72:1667–1675. doi: 10.1099/0022-1317-72-7-1667. [DOI] [PubMed] [Google Scholar]