Abstract
An important limitation of DNA immunization in nonhuman primates is the difficulty in generating high levels of antigen-specific antibody responses; strategies to enhance the level of immune responses to DNA immunization may be important in the further development of this vaccine strategy for humans. We approached this issue by testing the ability of molecular adjuvants to enhance the levels of immune responses generated by multicomponent DNA vaccines in rhesus macaques. Rhesus macaques were coimmunized intramuscularly with expression plasmids bearing genes encoding Th1 (interleukin 2 [IL-2] and gamma interferon)- or Th2 (IL-4)-type cytokines and DNA vaccine constructs encoding human immunodeficiency virus Env and Rev and simian immunodeficiency virus Gag and Pol proteins. We observed that the cytokine gene adjuvants (especially IL-2 and IL-4) significantly enhanced antigen-specific humoral immune responses in the rhesus macaque model. These results support the assumption that antigen-specific responses can be engineered to a higher and presumably more desirable level in rhesus macaques by genetic adjuvants.
Numerous vaccines that stimulate the production of protective antibodies have proven successful for combating diseases such as hepatitis A and B, measles, and poliomyelitis. As a novel and important vaccination technique, nucleic acid or DNA immunization delivers DNA constructs encoding specific immunogens directly into the host (10–12, 14, 15). These expression cassettes transfect host cells, which become the in vivo protein source for the production of antigen. This antigen then is the focus of the resulting humoral and cellular immune responses. Nucleic acid immunization is being explored as an immunization strategy against a variety of infectious diseases (10–12, 14, 15).
To support the ultimate use of this vaccine technology in humans, it may be important to translate the results originally observed in small-animal systems to similar levels in primate model systems (4). The nonhuman primates represent an important and relevant model for vaccine evaluation (2, 7). These animals are the closest species to humans, and there are numerous challenge models for various infectious agents.
On the other hand, it has been reported that primates may have a limited ability to produce DNA vaccine-encoded proteins through direct genetic inoculation into muscle (3). An exact mechanism for producing such proteins is unclear, and a major challenge of investigating DNA immunization in nonhuman primates is the difficulty in eliciting potent immune responses. For instance, DNA immunizations alone in primates were not sufficient to generate high levels of antigen-specific antibody responses (6). Intramuscular immunization of a human immunodeficiency virus type 1 (HIV-1) gp120 DNA vaccine construct using a large dose (2 mg of DNA given eight times at 4-week intervals) in rhesus macaques elicited only a low level of antigen-specific binding and no detectable neutralizing antibodies (6). These observations of reduced humoral immunogenicity of DNA vaccines in nonhuman primates suggest the need for higher doses in humans. Thus, strategies to enhance the level of immune responses to DNA immunization may be important in the further development of this vaccine strategy for humans.
Several groups, including ours, have been investigating the use of molecular adjuvants as a method of enhancing and modulating immune responses induced by DNA immunogens. Codelivery of these molecular adjuvants consisting of an expression plasmid bearing genes coding for immunologically relevant molecules, including costimulatory molecules, cytokines, and chemokines, with DNA vaccine constructs led to modulation of the magnitude and direction (humoral or cellular) of the immune responses induced in mice (1a, 2a, 5, 9, 16). It has been reported recently that the modulation of immune responses through this approach may modulate disease progression in several mouse challenge models (9, 16). These results support the idea that disease can be modulated by the use of cytokine adjuvants, at least in mice; however, the effects of this strategy in nonhuman primates have not been extensively reported.
In this study, we examined the use of cytokine cDNAs to enhance the level of humoral immune responses generated by DNA vaccines in rhesus macaques. We coimmunized rhesus macaques with expression plasmids bearing genes encoding either Th1 (interleukin 2 [IL-2] and gamma interferon [IFN-γ])- or Th2 (IL-4)-type cytokines and DNA vaccine constructs encoding HIV-1 MN Env and Rev (pCEnv) and simian immunodeficiency virus (SIV) mac239 Gag and Pol (pCSGag/pol) proteins. We observed that antigen-specific humoral immune responses could be modulated positively in the macaque models using this approach.
Five groups of two rhesus macaques each were immunized with specific DNA vaccine constructs. The first group was immunized with constructs coding for HIV-1 MN Env and Rev (pCEnv) and Rev-independent SIV Gag and Pol (pCSGag/pol) antigens along with a control vector, pCDNA3. The second group was immunized with pCEnv plus pCSGag/pol plus IL-2 constructs. The third and fourth groups were immunized with pCEnv plus pCSGag/pol plus IL-4 and pCEnv plus pCSGag/pol plus IFN-γ, respectively. The last control group was immunized with a control vector, pCDNA3. These macaques were immunized with 200 μg of each DNA at weeks 0, 6, and 12 and boosted with 500 μg of each DNA at week 28. These constructs were formulated and mixed prior to injection into the quadriceps muscle (1).
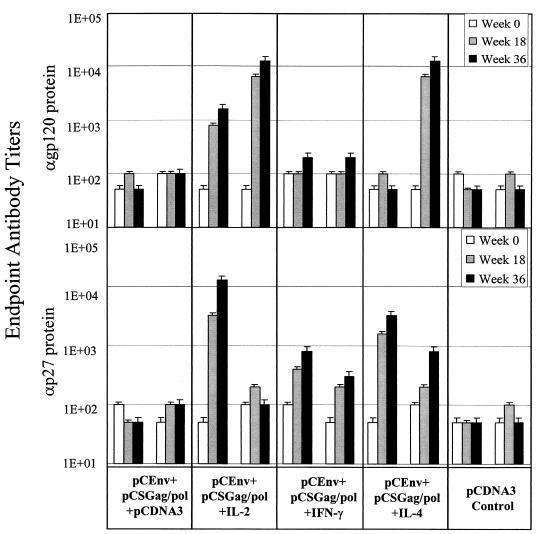
Both pre- and postimmunization serum samples from the immunized macaques were collected, and binding reactivities to recombinant HIV-1 envelop and SIV Gag proteins were determined by enzyme-linked immunosorbent assay. As shown in Fig. 1, macaques immunized with pCEnv plus pCSGag/pol plus pCDNA3 had minimal levels of anti-envelope or anti-Gag antibody responses following immunization. On the other hand, a significant enhancement of the levels of anti-envelope or anti-Gag antibodies was observed in the animals immunized with pCEnv plus pCSGag/pol plus IL-2. In fact, the magnitude of antibody response enhancement with IL-2 codelivery in macaques was even greater than that observed in mice: a 4-fold increase in endpoint titer was seen in mice compared to an over 100-fold increase in titer in macaques against envelope and Gag proteins (Fig. 1) (5). Similarly, IL-4 coimmunization also positively modulated the antigen-specific antibody responses. The group immunized with pCEnv plus pCSGag/pol plus IL-4 developed a significant level of anti-envelope-specific antibodies and a high antibody response against Gag. Macaques immunized with pCEnv plus pCSGag/pol plus IFN-γ had a more moderate response against both envelop and Gag proteins. These results demonstrate that antigen-specific antibody responses can be driven to a higher and presumably more desirable level through the use of cytokine genetic adjuvants in rhesus macaques.
FIG. 1.
Modulation of antibody responses in rhesus macaques. Five groups of two rhesus macaques were immunized with 200 μg of each DNA vaccine construct at weeks 0, 6, and 12 and boosted with 500 μg of each DNA at week 28. Serum samples were collected from the immunized macaques at weeks 0, 18, and 36. Binding reactivities to recombinant HIV-1 gp120 envelope and SIV p27 Gag proteins (ImmunoDiagnostics, Inc., Bedford, Mass.) were determined by enzyme-linked immunosorbent assay as previously described (1). Specific binding (absorbance at 450 nm) was calculated by subtracting A450 values from serum samples bound to bovine serum albumin (control) from A450 values from serum samples bound to gp120, that is, the A450s of experimental wells minus the A450s of control wells. The endpoint antibody titers for immunized rhesus macaques were determined as previously described (1).
We also examined the ability of the antibodies from immunized macaques to neutralize homologous HIV-1 MN or heterologous HIV-1 IIIB (Table 1). Although IL-2 or IL-4 coimmunization resulted in enhanced levels of serum antibody responses, these animals showed only a minimal level at best of neutralizing antibodies against the homologous HIV-1 MN isolate. On the other hand, similar to what occurs with protein vaccines, none of the serum antibodies were able to neutralize HIV IIIB, a divergent virus. Although these neutralizing titers were still low overall, they illustrate the potential of this approach. Higher dosages and frequencies of injections are expected to further enhance the observed levels of neutralizing antibodies. Combinations of envelopes might broaden the observed responses.
TABLE 1.
Ability of serum samples collected from immunized macaques at week 36 to neutralize HIV-1a
| Immunization group | Monkey | Neutralizing antibody titer
|
|
|---|---|---|---|
| Anti- HIV-1 MN | Anti- HIV-1 IIIB | ||
| pCEnv + pCSGag/pol + pCDNA3 | 1 | ||
| 2 | |||
| pCEnv + pCSGag/pol + IL-2 | 3 | ||
| 4 | 1:4 | ||
| pCEnv + pCSGag/pol + IFN-γ | 5 | ||
| 6 | |||
| pCEnv + pCSGag/pol + IL-4 | 7 | ||
| 8 | 1:64 | ||
| Control | 9 | ||
| 10 | |||
The ability of sera to neutralize viral infection in vitro was assessed according to described methods (8, 13). All serum samples were heat inactivated at 56°C for 40 min prior to use. T-cell-line-adapted HIV-1 isolates IIIB and MN were obtained from the National Institutes of Health AIDS Research and Reagent Reference Program repository. Dilutions of experimental sera were aliquoted in quadruplicate wells of a 96-well microtiter plate (25 μl per well). Culture media without antibody and preimmune macaque sera served as controls for baseline virus growth. An equal volume of virus stock (25 μl), containing 50 50% tissue culture infective doses of HIV-1 MN or IIIB, was added to each well. After 30 min at 37°C, 3 × 104 MT-2 target cells (100 μl) were added and incubated overnight at 37°C. Cells were then washed extensively to remove p24 antigen and plasma anti-p24 antibody and transferred to a 96-well microtiter plate with culture media. Inhibition of target cell infection was assessed by quantitative p24 measurement of cell supernatants during the early virus growth phase (days 4 to 6 for the viruses in this study) (Coulter [Hialeah, Fla.] enzyme immunoassay). The serum dilution causing a 90% reduction in p24 antigen was calculated by linear regression analysis.
These results indicate that the cytokine gene adjuvants can positively enhance antibody responses in rhesus macaques by intramuscular injections of DNA vaccines. This simple strategy has important implications for vaccines and immunotherapy approaches using the DNA platform. The use of molecular adjuvants (especially IL-2 or IL-4) to enhance antibody responses might be important in disease models such as hepatitis B, where the generation of antibodies is sufficient to provide protective immunity.
Acknowledgments
This work was supported in part by grants from the NIH to D.B.W. and from NHLBI/NIH to K.E.U.
We thank R. Ciccarelli from WLV for thoughtful discussion and providing material for this study.
REFERENCES
- 1.Boyer J D, Ugen K E, Wang B, Agadjanyan M G, Gilbert L, Bagarazzi M, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 1a.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleuken-2. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulskotte E G, Geretti A-M, Osterhaus A D. Towards an HIV-1 vaccine: lessons and studies in macaque models. Vaccine. 1998;97:904–915. doi: 10.1016/s0264-410x(97)00292-2. [DOI] [PubMed] [Google Scholar]
- 2a.Iwasaki A, Stiernholm B J, Chan A K, Berstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 3.Jiao S, Williams P, Berg R K, Hodgeman B A, Liu L, Repetto G, Wolff J A. Direct gene transfer into non-human primate myofibers in vivo. Hum Gene Ther. 1992;3:21–33. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 4.Kim J J, Weiner D B. DNA/genetic vaccination for HIV. Springer Semin Immunopathol. 1997;19:174–195. doi: 10.1007/BF00870267. [DOI] [PubMed] [Google Scholar]
- 5.Kim J J, Trivedi N N, Nottingham L, Morrison L, Tsai A, Hu Y, Mahalingam S, Dang K, Ahn L, Doyle N K, Wilson D M, Chattergoon M A, Chalian A A, Boyer J D, Agadjanyan M G, Weiner D B. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M-E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx P, Compans R, Getties A, Staas J, Gilley R, Mulligan M, Yamshchikov G, Chen D, Eldridge J. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 8.Montefiori D C, Robinson W E, Mithell W M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1988;85:9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sin J-I, Kim J J, Boyer J D, Ciccarelli R B, Higgins T J, Weiner D B. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501–509. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D, DeVit M, Johnston S. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 11.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 12.Ulmer J B, Donnelly J, Parker S E, Rhodes G H, Felgner P L, Dwarki V L, Gromkowski S H, Deck R, DeVitt C M, Friedman A, Hawe L A, Leander K R, Marinez D, Perry H, Shiver J W, Montgomery D, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 13.VanCott T C, Mascola J R, Kaminski R W, Kalyanaraman V, Hallberg P L, Burnett P R, Ulrich J T, Rechtman D J, Birx D L. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 16.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]