Abstract
Borna disease virus (BDV) infection of newborn rats leads to a persistent infection of the brain, which is associated with behavioral and neuroanatonomical abnormalities. These disorders occur in the absence of lymphoid cell infiltrates, and BDV-induced cell damage is restricted to defined brain areas. To investigate if damage to synaptic structures anteceded neuronal loss in BDV neonatally infected rats, we analyzed at different times postinfection the expression levels of growth-associated protein 43 and synaptophysin, two molecules involved in neuroplasticity processes. We found that BDV induced a progressive and marked decrease in the expression of these synaptic markers, which was followed by a significant loss of cortical neurons. Our findings suggest that BDV persistent infection interferes with neuroplasticity processes in specific cell populations. This, in turn, could affect the proper supply of growth factors and other molecules required for survival of selective neuronal populations within the cortex and limbic system structures.
Borna disease virus (BDV) is a nonsegmented, negative-stranded RNA virus, prototype of a new family, Bornaviridae, within the order Mononegavirales (9, 39). In a wide variety of animal species, BDV causes central nervous system (CNS) disease characterized by behavioral abnormalities and diverse pathology (20, 35). There is evidence that BDV can infect humans, and some data suggest that it might be associated with certain neuropsychiatric disorders (3, 4, 10, 11, 24, 26, 34, 37). Adult Lewis rats experimentally infected with BDV develop an immune system-mediated biphasic behavioral disease (31, 40). In contrast, neonatally infected rats develop a persistent tolerant infection (PTI) associated with distinct behavioral and neuroanatomical disturbances without encephalitis (1, 2, 6, 14, 31). Hence, the BDV PTI model offers the possibility to investigate direct effects of BDV infection on brain function in the absence of immunopathology-related brain damage.
BDV exhibits a noncytolytic multiplication in all culture cell systems assayed to date. However, BDV persistence in the rat brain is associated with discrete neuronal damage, limited to specific neuroanatomic areas. Rats with BDV PTI display cortical shrinkage, cerebellar hypoplasia and degeneration of granule cell neurons of the dentate gyrus (DG) (1, 2, 36). There is also evidence that Purkinje cell neurons of the cerebellum degenerate (15). The mechanisms involved in BDV-mediated degeneration of specific neuronal populations are unknown. The proliferating properties of the targeted neurons may play a role in this virally induced cell death (21). Brains of rats with BDV PTI are characterized by a chronic astrocytosis and microgliosis, as well as a sustained upregulation of specific proinflammatory cytokines (38). Moreover, the expression level of tissue factor, which is likely to play important roles in brain homeostasis and plasticity, is strongly increased in the brains of rats with PTI (19). Nevertheless, the cellular and molecular bases for the cognitive impairment of rats with BDV PTI remain to be determined. In this study, we examined whether persistent BDV infection affected synaptic density and neuronal plasticity, both of which have been implicated in neural functions such as learning and memory. The growth-associated protein 43 (GAP-43) and synaptophysin (SYN) are well-established reliable markers of neuroplasticity and synaptic density, respectively (18, 27, 29, 41). GAP-43 is a presynaptic membrane phosphoprotein which accumulates in neuronal growth cones. SYN is a 38-kDa calcium-binding protein present in the membranes of presynaptic vesicles. GAP-43 and SYN immunoreactivity (IR) can be used to estimate neuronal plasticity and the number of synaptic events, respectively. Here, we report a semiquantitative assessment of SYN and GAP-43 IR in BDV- and sham-infected rats. We show that BDV neonatally infected rats display a progressive decrease in synaptic density and plasticity, especially in cortex and hippocampus, which preceded a significant dropout of cortical neurons in infected rats. We discuss the implications of these findings in the context of BDV-induced cognitive impairment in rats with PTI.
MATERIALS AND METHODS
Infection of rats.
Litters of Lewis rat pups (Charles River Laboratories, Hollister, Calif., and St. Aubin les Elbeuf, France) were inoculated intracranially, within 24 h of birth, with either a 20% (wt/vol) stock of BDV-infected rat brain homogenate or virus diluent as a control (sham inoculation). We used the fourth brain passage in newborn rats of the Giessen strain He/80 (17). Procedures used for infections were as described elsewhere (19).
Preparation of tissue for histology.
On days 7, 10, 15, 21, 25, 35, 40, 45, and 60 postinoculation (p.i.), the rats were deeply anesthetized and perfused with phosphate-buffered saline followed by 4% paraformaldehyde. The brains were removed and postfixed in the same solution before being dehydrated and embedded in paraffin, using standard histological procedures (5). Sections (5 to 7 μm thick) were cut with a rotary microtome, mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburg, Pa.), and stored at 4°C before use. Adjacent series of sections for each animal were incubated with the indicated antibodies.
Immunohistochemistry.
Procedures were similar to those previously described (11, 12). Briefly, sections were baked at 65°C for 1 h, deparaffinized in xylene, and hydrated. They were treated for 30 min in methanol containing 0.3% H2O2 to quench endogenous peroxidase activity and washed extensively in Tris-buffered saline (TBS). After a blocking step for 1 h in TBS containing 3% horse serum, the sections were incubated overnight at 4°C with monoclonal anti-GAP-43 (diluted 1:100; Sigma-Aldrich, St. Louis, Mo.) or anti-SYN (diluted 1:5; Boehringer Mannheim, Meylan, France) diluted in TBS. Subsequently, the sections were incubated in biotinylated horse anti-mouse immunoglobulin G (diluted 1:75; Vector Laboratories, Burlingame, Calif.), followed by an avidin-biotin-peroxidase complex (diluted 1:100; Vector Laboratories), for 45 min each. Between each incubation, the sections were extensively washed in TBS. Peroxidase activity was revealed by immersing the slides in 0.5 mg of diaminobenzidine (Sigma-Aldrich) per ml in 100 mM Tris-HCl (pH 7.4) containing 0.03% H2O2, and the sections were dehydrated and mounted in Eukitt (Kindler GmbH & Co., Freiburg, Germany). Stainings with the BDV nucleoprotein (11) and glial fibrillary acidic protein (GFAP; Dakopatts, Carpinteria, Calif.) rabbit antibodies were done similarly, with the following modifications: (i) the sections were permeabilized by treatment with 0.5% Triton before staining, (ii) we used a biotinylated goat anti-rabbit secondary antibody (diluted 1:100; Vector Laboratories), and (iii) sections were counterstained with Harris hematoxylin before mounting. Parvalbumin expression was assessed by incubation with a monoclonal antiparvalbumin antibody (diluted 1:2,000; Sigma-Aldrich), followed by incubation with a fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (diluted 1:500; Diagnostics Pasteur, Marnes-la-Coquette, France). After extensive washes in TBS, the sections were mounted in Vectashield (Vector Laboratories).
Semiquantitative assessment of SYN and GAP-43 IR by microdensitometry.
SYN and GAP-43 immunolabeled sections were analyzed with a Leica 570 C Quantimet as described elsewhere (28). Briefly, the average optical density (OD) of the reaction product was measured in six different fields, including the frontal cortex, molecular layer of the hippocampus, granule cell layers of the DG and cerebellum, thalamus (lateral posterior thalamic nucleus), and basal ganglia (caudate putamen), for at least two sections for each animal. The area of interest was delineated on the video screen with a mouse-type cursor. All OD measurements were done under the same optical and lighting conditions. The OD of a blank field in each slide was subtracted to arrive at a corrected OD value, expressed as the percentage of the median of a given experimental point. This procedure has been previously used by us and validated in experimental models of denervation and reinnervation (27), as well as in human neurodegenerative disorders (29).
Morphometric analysis.
Neuronal cell counts were performed in 5-μm-thick sections of five each control and infected rats (45 days p.i.) that were stained with cresyl violet, dehydrated, and mounted in Eukitt. Quantitative analysis of the sections (cortical thickness and number of cells) was done using the Leica 570 C Quantimet as described elsewhere (42). Morphometric determinations of the number of stained cells along the cortical ribbon for two sections for each animal were determined using the 40× objective. This approach has been previously shown to yield results comparable to those obtained by stereological procedures, but in addition provides information as to cell size (16).
Statistical analysis.
For statistical analysis, we used the nonparametric Mann-Whitney U test (from the Statview package), with a minimum accepted level of significance of 0.05 (P < 0.05).
RESULTS
Clinical assessment of rats with BDV PTI.
A total of 120 rats from 20 different litters were used in this study. All BDV- and sham-infected rats appeared clinically healthy over the observation period, except for the previously reported growth retardation in the infected group (2). By the end of experiment, the weight of BDV PTI rats was approximately 60% of the weight of the control littermates.
Altered GAP-43 and SYN expression in rats with BDV PTI.
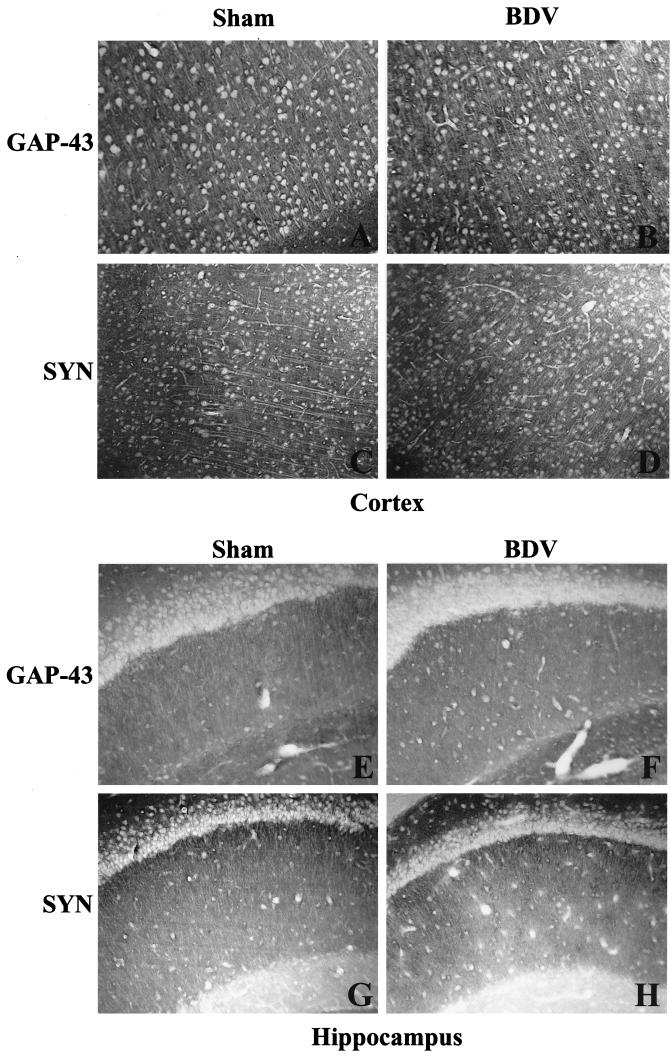
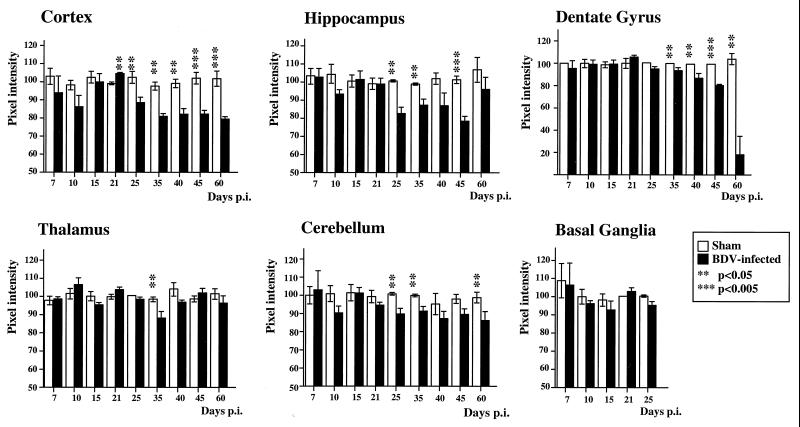
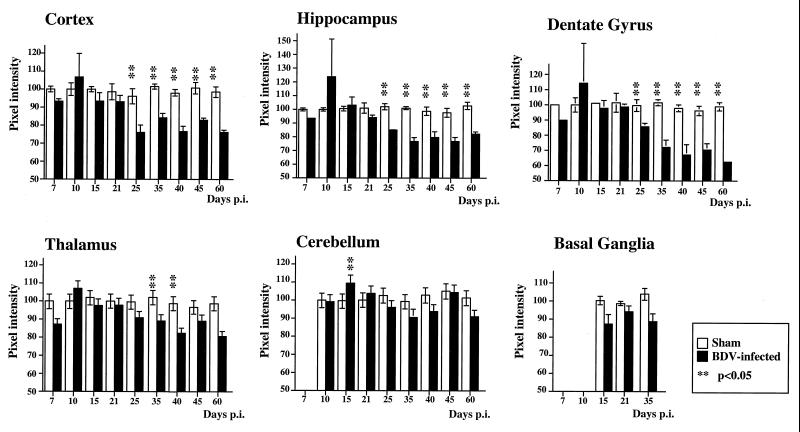
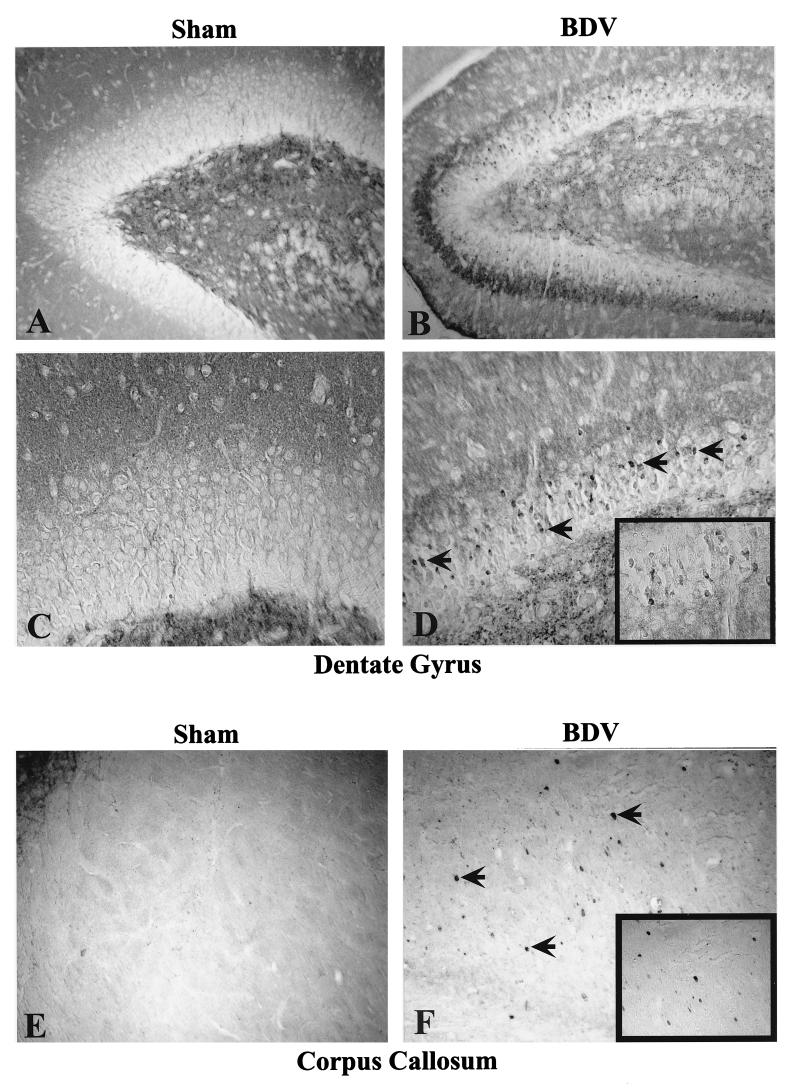
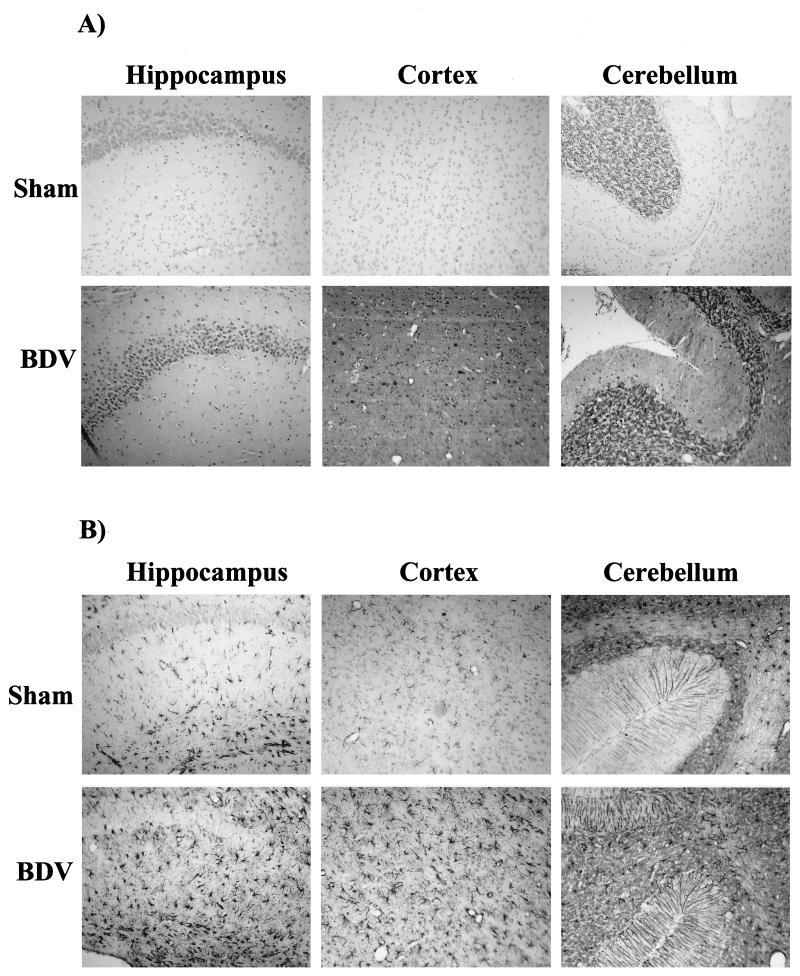
To determine if persistent BDV infection leads to synaptic damage, sections from sham- and BDV-infected rats were immunolabeled with antibodies against the synaptic marker, SYN, and the marker of neuroplasticity and regeneration, GAP-43. Consistent with previous studies (27), SYN and GAP-43 immunolabeled the neuropil with a characteristic granular pattern, following a laminar distribution (Fig. 1). Semiquantitative analysis revealed that IR (expressed as mean ± standard error of the mean [SEM]) for GAP-43 (Fig. 2) and SYN (Fig. 3) were both selectively decreased in specific CNS areas of infected animals. Decrease of these two markers in the DG paralleled the kinetics of DG granule cell degeneration previously found in rats with BDV PTI (2). In addition, GAP-43 expression was decreased in the cortex and hippocampus and was unchanged in the thalamus, cerebellum, and basal ganglia (Fig. 2), whereas SYN IR was decreased in the cortex, hippocampus, and thalamus of infected animals but unchanged in the cerebellum and basal ganglia (Fig. 3). Moreover, a closer observation of SYN immunostaining revealed an abnormal pattern in the distribution of its IR in BDV-infected animals (Fig. 4). SYN IR, which usually accumulates in synaptic terminals, was clustered in the inner molecular layer of the DG and also present in the cell bodies of DG granule neurons by 25 days p.i., prior to their degeneration (Fig. 4B and D). By day 45 p.i., SYN was also found accumulating in dystrophic axon figures in the corpus callosum, which are often linked with axonal transport dysfunction (Fig. 4F). Such axonal spheroids were also observed more occasionally within the neocortex (not shown).
FIG. 1.
Distribution of GAP-43 and SYN IR in sham- and neonatally BDV-infected rats, 40 days p.i. Cortex (A to D) and hippocampus (E to H) from control and infected rats exhibit the characteristic granular immunolabeling of the neuropil but not the cell bodies. Magnification, ×100.
FIG. 2.
Quantitative analysis of GAP-43 IR in the cortex, hippocampus, DG, thalamus, cerebellum, and basal ganglia from control and BDV-infected rats. Values of pixel intensities (percentage of median) are mean ± SEM, each bar representing the result of at least eight independent measurements. Double (P < 0.05) and triple (P < 0.005) asterisks indicate the level of statistical significance (determined by the Mann-Whitney U test). Loss of GAP-43 IR in the DG of neonatally infected rats followed the kinetics of DG granule cell degeneration observed in these animals. Compared to age-matched controls, GAP-43 levels are significantly reduced in the cortex and hippocampus starting at about day 25 p.i. No significant differences are observed elsewhere.
FIG. 3.
Quantitative analysis of SYN IR in the cortex, hippocampus, DG, thalamus, cerebellum, and basal ganglia from control and BDV-infected rats. Values of pixel intensities (percentage of median) are mean ± SEM, each bar representing the result of at least eight independent measurements. Double asterisks (P < 0.05) indicate the level of statistical significance (determined by the Mann-Whitney U test). Loss of SYN IR in the DG of neonatally infected rats precedes and follows the kinetics of DG granule cell degeneration observed in these animals. Compared to age-matched controls, SYN levels are significantly reduced in the cortex, hippocampus, and thalamus.
FIG. 4.
Abnormal SYN immunostaining in BDV-infected rat brain. Note the clustering of SYN IR within the inner molecular layer of the DG at 25 days p.i. (compare panels A and B). SYN staining is also found in the cell bodies of dentate granule cells (arrowed cells in panel D, shown at a larger magnification in the boxed area). By day 45 p.i., the corpus callosum, which is usually negative for SYN staining in control rats (panel E), exhibits a patched IR in infected animals (arrows in panel F and boxed area). The immunostaining accumulates in axonal blobs, indicating an abnormal axoplasmic flow and a poor transport of SYN to the synaptic terminals. Magnification, ×150.
Patterns of neuronal loss in BDV-infected rats.
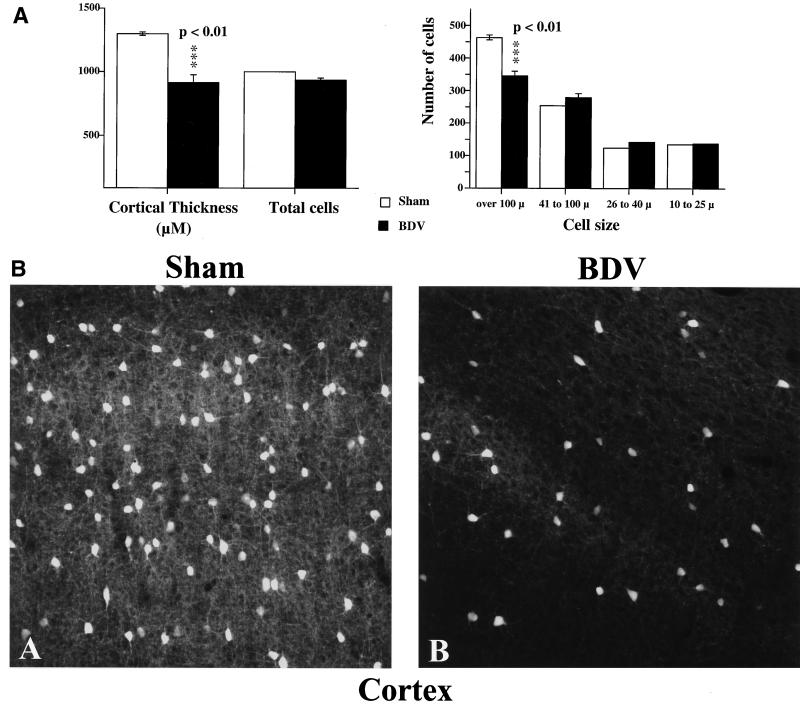
To examine the impact of synaptic density and plasticity alterations on neuronal viability, sections from sham- and BDV-infected rats were stained with cresyl violet and analyzed with a Quantimet 570C (Fig. 5A). We found that by day 45 p.i., BDV-infected animals exhibited a marked cortical shrinkage (about 30%), accompanied by a selective loss of cells with a diameter of greater than 100 μm (P < 0.01) (Fig. 5A). Based on their size, it is probable that dying cells were cortical neurons. Moreover, staining with an antibody, specific to parvalbumin, a calcium-binding protein present in virtually all gamma-aminobutyric acid (GABA)-ergic neurons in the cortex (7), showed that this neuronal population was severely depleted in BDV-infected rats by day 45 p.i. (Fig. 5B).
FIG. 5.
Cell loss in the cortex of neonatally BDV-infected animals, 45 days p.i. (A) Morphometric analysis. Results are expressed as mean ± SEM. Averages are based on results from five each sham-inoculated and rats with BDV PTI (two sections per animal). The level of statistical significance (determined by Mann-Whitney U test) is indicated by triple asterisk (P < 0.01). BDV-infected rats display significant cortical shrinkage (about 30%) and loss of cells with a diameter of >100 μm compared to noninfected age-matched controls. (B) Immunohistochemical staining for parvalbumin in the cortex of sham- and BDV-infected rats, 45 days p.i. Parvalbumin, a calcium-binding protein, labels GABA-ergic neurons in the cortex. Similar cortical areas are shown for each animal. Note the significant decrease in numbers of stained cells and processes in the infected animal. Magnification, ×250.
Viral load and degree of astrocytosis in rats with BDV PTI.
To study the load and distribution of BDV antigen and the degree of reactive astrogliosis in the brains of BDV PTI and control rats, sections from BDV-infected and sham-inoculated control rats were immunolabeled with antibodies against the BDV nucleoprotein and GFAP, a marker for astrocytes (Fig. 6). Consistent with previous findings by us and others (1, 15, 38), soon after inoculation (7 to 15 days p.i.), expression of BDV antigen was mainly localized to limited regions of the brain, i.e., the cortex, CA3 and CA4 regions of the hippocampus, and Purkinje cells of the cerebellum (not shown). By 3 weeks p.i., BDV antigen was strongly expressed in virtually all brain areas (Fig. 6A), with no apparent decrease of expression level over time. Viral antigen was detected within the cell bodies. As previously described, we also observed a more diffuse staining of the tissue, likely due to high levels of viral nucleoprotein in the neuropil (38). Of interest, only low levels of BDV antigen expression were detected in neurons of the DG prior to their degeneration (not shown). No staining was seen in sham-inoculated animals. Immunostaining with the GFAP antibody confirmed the previously described (19) upregulation of GFAP expression in brains of rats with BDV PTI by 3 weeks p.i. (Fig. 6B). Astrogliosis was most prominent in the hippocampus, cortex, and cerebellum of BDV-infected rats.
FIG. 6.
Detection of BDV antigen and GFAP expression in control and infected rat brains, 35 days p.i. (A) Expression of BDV nucleoprotein. Sections were immunostained with an antibody specific for BDV nucleoprotein. There is a strong nuclear staining in abundant pyramidal cells of the hippocampus and neocortex, as well as in Purkinje and granule cells of the cerebellum. Diffuse staining of the neuropil is also observed. There was no staining in sham-inoculated animals. Magnification, ×80. (B) Analysis of GFAP expression in similar fields. Neonatally infected rats display a significant increase in the number of GFAP-positive astroglial cells in the hippocampus (molecular layer and dentate gyrus), cortex, and cerebellum. Consistent with this activation, astrocytes are significantly hypertrophied in infected brains. Magnification, ×80.
DISCUSSION
Viral infections of the CNS can cause behavioral disturbances that are associated with specific neuronal injury (25, 30, 43). This neuronal damage can occur as a result of direct cytolysis due to virus multiplication or as a consequence of the host immune responses against the infectious agent (22, 44). However, viruses can also persist in the CNS in the absence of the hallmarks of cell destruction and inflammation, but causing disturbances in specialized functions of cells (13, 32, 33). This, in turn, can disrupt CNS homeostasis and alter normal brain function. Thus, we have previously reported that persistent lymphocytic choriomeningitis virus infection of the mouse CNS causes altered synaptic plasticity and cognitive function without destruction of brain cells (12).
Neonatal BDV infection of the rat provides an important system to study the mechanisms whereby viruses can interfere with cognitive, neurodevelopmental, and behavioral functions of the CNS without encephalitis (1). In this study, we have shown that rats with BDV PTI display significant alterations in the expression levels of GAP-43 and SYN, two molecules that play key roles in synaptic density and plasticity (18, 27, 29, 41). These virally mediated disturbances in synaptic protein expression were restricted mainly to the limbic system and did not appear to correlate directly with either viral load or astrogliosis. Thus, GAP-43 and SYN levels of expression were not altered in the cerebellum of infected rats, despite a prominent astrocytosis and high viral load in this brain region. The progressive loss of neurons in DG and cortex likely contributed to the decreased expression of GAP-43 and SYN. However, a downregulation in the expression of these synaptic markers preceded the observed neuronal degeneration.
Closer examination of SYN staining showed that by 3 weeks p.i., neurons appeared to be affected in axonal transport, as revealed by the accumulation of this protein in the cell bodies of granule cells in the DG, or in axonal spheroids in the corpus callosum. This is consistent with the cholinergic abnormalities found by Gies et al. in brains of BDV acutely infected rats, prior to the onset of encephalitis (17). These cholinergic abnormalities had also been linked to abnormal axoplasmic flow, suggesting that BDV infection of neurons may interfere with such processes.
Morphometric analysis showed a loss of about 30% of neuronal bodies in the cortical area of rats with PTI, indicating that contrary to the commonly accepted idea, BDV persistence causes a progressive neuronal degeneration in areas outside the DG and cerebellum. The selective decrease in cells with a diameter of >100 μm and with positive parvalbumin staining suggest that both pyramidal and GABA-ergic cortical neurons exhibit selective vulnerability to BDV infection. It is worth noting that a 15% reduction in cortical neurons, lower than the reduction described here, has been associated with cognitive impairment in rats (8). There is compelling evidence, both in cultured cells and in vivo, that BDV is noncytolytic (2, 23). Based on the findings reported here, it is plausible that BDV-induced synaptic and axoplasmic flow damage leads to impairment in the uptake and trafficking of growth factors required for proper neuronal function. This, in turn, may result in degeneration of specific neuronal populations. Localized synaptic alterations were apparent at a time when virus antigen was present in virtually all brain areas. Therefore, different neuronal populations may differ in vulnerability to a number of factors, including not only viral load and reactive astrocytosis but likely also local changes in the production of cytokines and other molecules involved in maintaining proper brain function. This, in turn, may promote regional alteration in neural cell communication, leading to injury.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (program PRFMMIP) and by the Institut Pasteur and the Centre National de la Recherche Scientifique (D.G.D. and S.S.); by grant NS12428 (J.C.T.); by grants AG5131, AG10689, MH45294, MH59745, and DA 12065 (E.M.); and by an Overseas Researcher Scholarship from the Ministry of Education, Science, Sports and Culture of Japan (M.W.).
REFERENCES
- 1.Bautista J R, Rubin S A, Moran T H, Schwartz G J, Carbone K M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Dev Brain Res. 1995;90:45–53. doi: 10.1016/0165-3806(96)83485-7. [DOI] [PubMed] [Google Scholar]
- 2.Bautista J R, Schwartz G J, de la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–36. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 3.Bode L. Human infections with Borna disease virus and potential pathogenic implications. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 103–130. [DOI] [PubMed] [Google Scholar]
- 4.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 5.Brahic M, Ozden S. Simultaneous detection of cellular RNA and proteins. In: Wilkinson R G, editor. In situ hybridization—a practical approach. Oxford, England: IRL Press; 1992. pp. 85–104. [Google Scholar]
- 6.Carbone K M, Park S W, Rubin S A, Waltrip II R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celio M R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 8.Chen K S, Masliah E, Mallory M, Gage F. Synaptic loss in cognitively impaired aged rats is ameliorated by chronic human NGF infusion. Neuroscience. 1995;68:19–27. doi: 10.1016/0306-4522(95)00099-5. [DOI] [PubMed] [Google Scholar]
- 9.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Torre J C, Bode L, Dürrwald R, Cubitt B, Ludwig H. Sequence characterization of human Borna disease virus. Virus Res. 1996;44:33–44. doi: 10.1016/0168-1702(96)01338-x. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grässer F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 12.de la Torre J C, Mallory M, Brot M, Gold L, Koob G, Oldstone M B, Masliah E. Viral persistence in neurons alters synaptic plasticity and cognitive functions without destruction of brain cells. Virology. 1996;220:508–515. doi: 10.1006/viro.1996.0340. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre J C, Oldstone M B A. The anatomy of viral persistence: mechanisms of persistence and associated disease. Adv Virus Res. 1996;46:311–343. doi: 10.1016/s0065-3527(08)60075-5. [DOI] [PubMed] [Google Scholar]
- 14.Dittrich W, Bode L, Ludwig H, Kao M, Scheider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;20:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 15.Eisenman L M, Brothers R, Tran M H, Kean R B, Dickson G M, Dietzschold B, Hooper D C. Neonatal Borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J Neurovirol. 1999;5:181–189. doi: 10.3109/13550289909022000. [DOI] [PubMed] [Google Scholar]
- 16.Everall I P, DeTeresa R M, Terry R D, Masliah E. Comparison of two quantitative methods for the evaluation of neuronal number in the frontal cortex of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1202–1206. doi: 10.1097/00005072-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gies U, Bilzer T, Stitz L, Staiger J F. Disturbance of the cortical cholinergic innervation in Borna disease prior to encephalitis. Brain Pathol. 1998;8:39–48. doi: 10.1111/j.1750-3639.1998.tb00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gispen W H, Nielander H B, De Graan P N, Oestreicher A B, Schrama L H, Schotman P. Role of the growth-associated protein B-50/GAP-43 in neuronal plasticity. Mol Neurobiol. 1991;5:61–85. doi: 10.1007/BF02935540. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Dunia D, Eddleston M, Mackman N, Carbone K, de la Torre J C. Expression of tissue factor is increased in astrocytes within the central nervous system during persistent infection with Borna disease virus. J Virol. 1996;70:5812–5820. doi: 10.1128/jvi.70.9.5812-5820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosztonyi G, Ludwig H. Borna disease-Neuropathology and pathogenesis. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 39–73. [PubMed] [Google Scholar]
- 21.Gould E, McEwen B S. Neuronal birth and death. Curr Opin Neurobiol. 1993;3:676–682. doi: 10.1016/0959-4388(93)90138-o. [DOI] [PubMed] [Google Scholar]
- 22.Hardwick J M, Griffin D E. Viral effects on cellular functions. In: Nathanson N, Ahmed R, et al., editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 55–84. [Google Scholar]
- 23.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 24.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 25.Kristensson K, Norrby E. Persistence of RNA viruses in the central nervous system. Annu Rev Microbiol. 1986;40:159–184. doi: 10.1146/annurev.mi.40.100186.001111. [DOI] [PubMed] [Google Scholar]
- 26.Lipkin W I, Schneemann A, Solbrig M V. Borna disease virus: implications for human neuropsychiatric illness. Trends Microbiol. 1995;3:64–69. doi: 10.1016/s0966-842x(00)88877-0. [DOI] [PubMed] [Google Scholar]
- 27.Masliah E, Fagan A M, Terry R D, DeTeresa R, Mallory M, Gage F H. Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with GAP-43 in the dentate gyrus of the adult rat. Exp Neurol. 1991;113:131–142. doi: 10.1016/0014-4886(91)90169-d. [DOI] [PubMed] [Google Scholar]
- 28.Masliah E, Terry R D, Alford M, DeTeresa R. Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. J Histochem Cytochem. 1990;38:837–844. doi: 10.1177/38.6.2110586. [DOI] [PubMed] [Google Scholar]
- 29.Masliah E, Terry R D, DeTeresa R M, Hansen L A. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103:234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- 30.Mohammed A K, Norrby E, Kristensson K. Viruses and behavioural changes: a review of clinical and experimental findings. Rev Neurosci. 1993;4:267–286. doi: 10.1515/revneuro.1993.4.3.267. [DOI] [PubMed] [Google Scholar]
- 31.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent Borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 32.Oldstone M B. Molecular anatomy of viral persistence. J Virol. 1991;65:6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldstone M B. Viral persistence. Cell. 1989;56:517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- 34.Planz O, Rentzsch C, Batra A, Winkler T, Büttner M, Rziha H J, Stitz L. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6256. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rott R, Becht H. Natural and experimental Borna disease in animals. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 17–30. [DOI] [PubMed] [Google Scholar]
- 36.Rubin S A, Sylves P, Vogel M, Pletnikov M, Moran T H, Schwartz G J, Carbone K M. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Res Bull. 1999;48:23–30. doi: 10.1016/s0361-9230(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 37.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I the Borna Virus Study Group. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 38.Sauder C, de la Torre J C. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 39.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 40.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 75–92. [DOI] [PubMed] [Google Scholar]
- 41.Strittmatter S M, Vartanian T, Fishman M C. GAP-43 as a plasticity protein in neuronal form and repair. J Neurobiol. 1992;23:507–520. doi: 10.1002/neu.480230506. [DOI] [PubMed] [Google Scholar]
- 42.Toggas S M, Masliah E, Rockenstein E M, Rall G F, Abraham C R, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 43.Yolken R H, Torrey E F. Viruses, schizophrenia, and bipolar disorders. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinkernagel R M. Virus-induced immunopathology. In: Nathanson N, Ahmed R, et al., editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 163–180. [Google Scholar]