With 1 million new cases in the world each year, breast cancer is the commonest malignancy in women and comprises 18% of all female cancers. In the United Kingdom, where the age standardised incidence and mortality is the highest in the world, the incidence among women aged 50 approaches two per 1000 women per year, and the disease is the single commonest cause of death among women aged 40-50, accounting for about a fifth of all deaths in this age group. There are more than 14 000 deaths each year, and the incidence is increasing particularly among women aged 50-64, probably because of breast screening in this age group.
Worldwide incidence of cancers in women (1980)
| Site of cancer | No of cases (1000s) | % of total |
| Breast | 572 | 18 |
| Cervix | 466 | 15 |
| Colon and rectum | 286 | 9 |
| Stomach | 261 | 8 |
| Endometrium | 149 | 5 |
| Lung | 147 | 5 |
| Ovary | 138 | 4 |
| Mouth and pharynx | 121 | 4 |
| Oesophagus | 108 | 4 |
| Lymphoma | 98 | 3 |
Of every 1000 women aged 50, two will recently have had breast cancer diagnosed and about 15 will have had a diagnosis made before the age of 50, giving a prevalence of breast cancer of nearly 2%.
Risk factors for breast cancer
Age
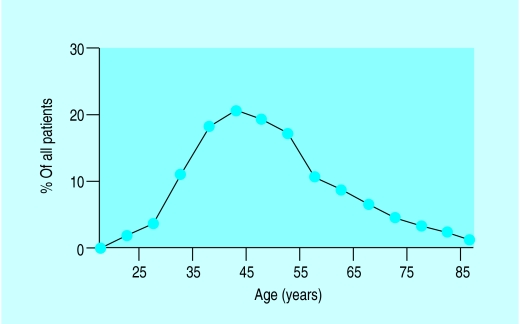
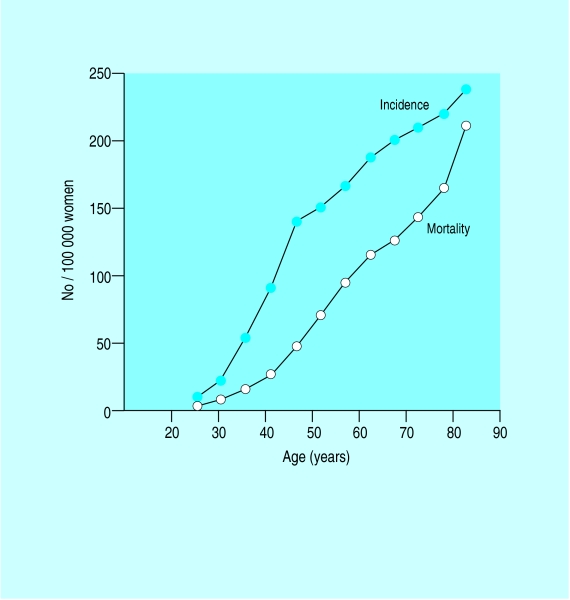
The incidence of breast cancer increases with age, doubling about every 10 years until the menopause, when the rate of increase slows dramatically. Compared with lung cancer, the incidence of breast cancer is higher at younger ages. In some countries there is a flattening of the age-incidence curve after the menopause.
Geographical variation
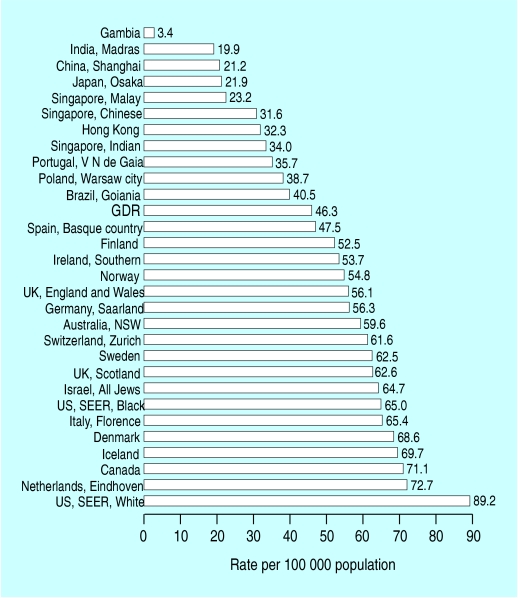
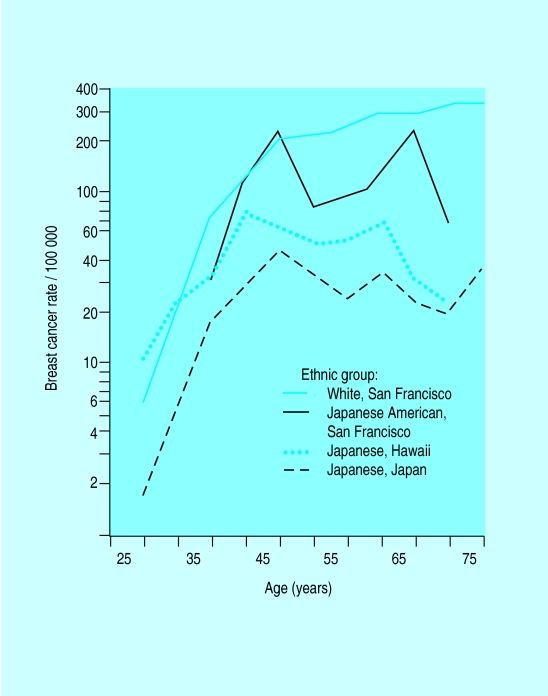
Age adjusted incidence and mortality for breast cancer varies by up to a factor of five between countries. The difference between Far Eastern and Western countries is diminishing but is still about fivefold. Studies of migrants from Japan to Hawaii show that the rates of breast cancer in migrants assume the rate in the host country within one or two generations, indicating that environmental factors are of greater importance than genetic factors.
Age at menarche and menopause
Women who start menstruating early in life or who have a late menopause have an increased risk of developing breast cancer. Women who have a natural menopause after the age of 55 are twice as likely to develop breast cancer as women who experience the menopause before the age of 45. At one extreme, women who undergo bilateral oophorectomy before the age of 35 have only 40% of the risk of breast cancer of women who have a natural menopause.
Established and probable risk factors for breast cancer
| Factor | Relative risk | High risk group |
| Age | >10 | Elderly |
| Geographical location | 5 | Developed country |
| Age at menarche | 3 | Menarche before age 11 |
| Age at menopause | 2 | Menopause after age 54 |
| Age at first full pregnancy | 3 | First child in early 40s |
| Family history | ⩾2 | Breast cancer in first degree relative when young |
| Previous benign disease | 4-5 | Atypical hyperplasia |
| Cancer in other breast | >4 | |
| Socioeconomic group | 2 | Groups I and II |
| Diet | 1.5 | High intake of saturated fat |
| Body weight: | ||
| Premenopausal | 0.7 | Body mass index >35 |
| Postmenopausal | 2 | Body mass index >35 |
| Alcohol consumption | 1.3 | Excessive intake |
| Exposure to ionising radiation | 3 | Abnormal exposure in young females after age 10 |
| Taking exogenous hormones: | ||
| Oral contraceptives | 1.24 | Current use |
| Hormone replacement therapy | 1.35 | Use for ⩾10 years |
| Diethylstilbestrol | 2 | Use during pregnancy |
Age at first pregnancy
Nulliparity and late age at first birth both increase the lifetime incidence of breast cancer. The risk of breast cancer in women who have their first child after the age of 30 is about twice that of women who have their first child before the age of 20. The highest risk group are those who have a first child after the age of 35; these women appear to be at even higher risk than nulliparous women. An early age at birth of a second child further reduces the risk of breast cancer.
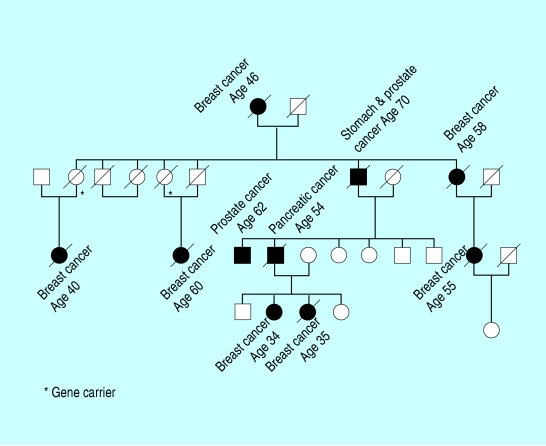
Familial breast cancer—criteria for identifying women at substantial increased risk
The following categories identify women who have three or more times the population risk of developing breast cancer
A woman who has:
One first degree relative with bilateral breast cancer or breast and ovarian cancer or
One first degree relative with breast cancer diagnosed under the age of 40 years or one first degree male relative with breast cancer diagnosed at any age or
Two first or second degree relatives with breast cancer diagnosed under the age of 60 years or ovarian cancer at any age on the same side of the family or
Three first or second relatives with breast and ovarian cancer on the same side of the family
First degree relative is mother, sister, or daughter. Second degree female relative is grandmother, granddaughter, aunt, or niece
Criteria for identifying women at very high risk in whom gene testing might be appropriate
Families with four or more relatives affected with either breast or ovarian cancer in three generations and one alive affected relative
Family history
Up to 10% of breast cancer in Western countries is due to genetic predisposition. Breast cancer susceptibility is generally inherited as an autosomal dominant with limited penetrance.
This means that it can be transmitted through either sex and that some family members may transmit the abnormal gene without developing cancer themselves. It is not yet known how many breast cancer genes there may be. Two breast cancer genes, BRCA1 and BRCA2, which are located on the long arms of chromosomes 17 and 13 respectively, have been identified and account for a substantial proportion of very high risk families—ie those with four or more breast cancers among close relatives. Both genes are very large and mutations can occur at almost any position, so that molecular screening to detect mutation for the first time in an affected individual or family is technically demanding. Certain mutations occur at high frequency in defined populations. For instance, some 2% of Ashkenazi Jewish women carry either BRCA1 185 del AG (deletion of two base pairs in position 185), BRCA1 5382 ins C (insertion of an extra base pair at position 5382) or BRCA 6174 del T (deletion of a single base pair at position 6174), while BRCA2 999 del 5 (deletion of five base pairs at position 999) accounts for about half of all familial breast cancer in Iceland. Inherited mutations in two other genes, p53 and PTEN, are associated with familial syndromes (Li-Fraumeni and Cowden's respectively) that include a high risk of breast cancer but both are rare. These are almost certainly other (as yet unidentified) genes that increase the risk of disease by only a moderate degree—perhaps three or four-fold above the general population level. These are unlikely to generate florid multi-case families but they are probably rather common and therefore account for a substantial part of the overall genetic contribution to breast cancer.
Many families affected by breast cancer show an excess of ovarian, colon, prostatic, and other cancers attributable to the same inherited mutation. Patients with bilateral breast cancer, those who develop a combination of breast cancer and another epithelial cancer, and women who get the disease at an early age are most likely to be carrying a genetic mutation that has predisposed them to developing breast cancer. Most breast cancers that are due to a genetic mutation occur before the age of 65, and a woman with a strong family history of breast cancer of early onset who is still unaffected at 65 has probably not inherited the genetic mutation.
A woman's risk of breast cancer is two or more times greater if she has a first degree relative (mother, sister, or daughter) who developed the disease before the age of 50, and the younger the relative when she developed breast cancer the greater the risk. For example, a woman whose sister developed breast cancer aged 30-39 has a cumulative risk of 10% of developing the disease herself by age 65, but that risk is only 5% (close to the population risk) if the sister was aged 50-54 at diagnosis. The risk increases by between four and six times if two first degree relatives develop the disease. For example, a woman with two affected relatives, one who was aged under 50 at diagnosis, has a 25% chance of developing breast cancer by the age of 65.
Previous benign breast disease
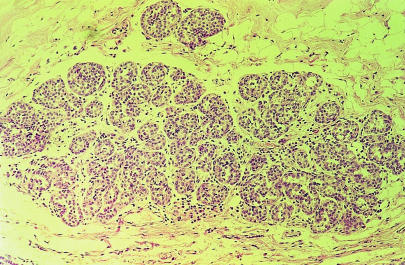
Women with severe atypical epithelial hyperplasia have a four to five times higher risk of developing breast cancer than women who do not have any proliferative changes in their breast. Women with this change and a family history of breast cancer (first degree relative) have a ninefold increase in risk. Women with palpable cysts, complex fibroadenomas, duct papillomas, sclerosis adenosis, and moderate or florid epithelial hyperplasia have a slightly higher risk of breast cancer (1.5-3 times) than women without these changes, but this increase is not clinically important.
Radiation
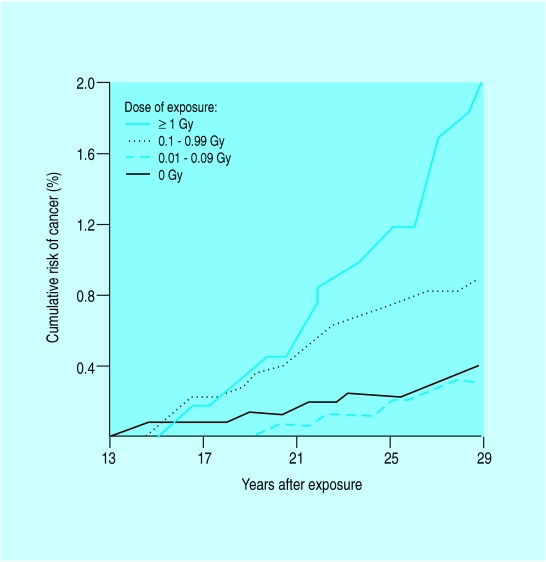
A doubling of risk of breast cancer was observed among teenage girls exposed to radiation during the Second World War. Ionising radiation also increases risk later in life, particularly when exposure is during rapid breast formation. Mammographic screening is associated with a net decrease in mortality from breast cancer among women aged over 50.
Lifestyle
Diet
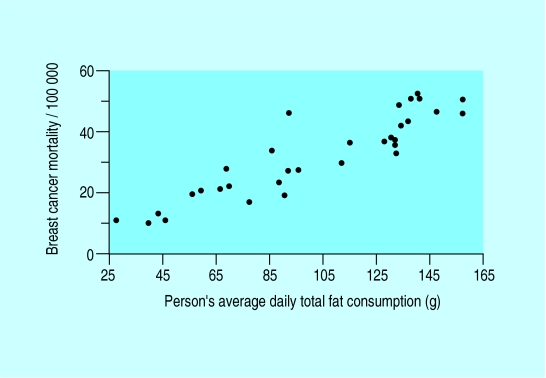
Although there is a close correlation between the incidence of breast cancer and dietary fat intake in populations, the true relation between fat intake and breast cancer does not appear to be particularly strong or consistent.
Weight
Obesity is associated with a twofold increase in the risk of breast cancer in postmenopausal women whereas among premenopausal women it is associated with a reduced incidence.
Alcohol intake
Some studies have shown a link between alcohol consumption and incidence of breast cancer, but the relation is inconsistent and the association may be with other dietary factors rather than alcohol.
Smoking
Smoking is of no importance in the aetiology of breast cancer.
Oral contraceptive
While women are taking oral contraceptives and for 10 years after stopping these agents, there is a small increase in the relative risk of developing breast cancer. There is no significantly increased risk of having breast cancer diagnosed 10 or more years following cessation of the oral contraceptive agent. Cancers diagnosed in women taking the oral contraceptive are less likely to be advanced clinically than those diagnosed in women who have never used these agents, relative risk 0.88 (0.81-0.95). Duration of use, age at first use, dose and type of hormone within the contraceptives appear to have no significant effect on breast cancer risk. Women who begin use before the age of 20 appear to have a higher relative risk than women who begin oral contraceptive use at an older age. This higher relative risk applies at an age when the incidence of breast cancer is however very low.
Relative risk of breast cancer in relation to use of oral contraceptives
| Relative risk | 95% CI | |
| >10 years after stopping | 1 | |
| Current user | 1.24 | 0.96-1.05 |
| 1-5 years since stopping | 1.16 | 1.08-1.23 |
| 5-9 years since stopping | 1.07 | 1.02-1.13 |
Hormone replacement therapy
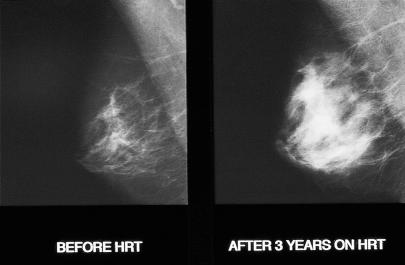
Among current users of HRT and those who have ceased use 1-4 years previously the relative risk of having breast cancer diagnosed increases by a factor of 1.023 (1.011-1.036) for each year of use. This increase is consistent with the effect of a delay in the menopause, because the relative risk of breast cancer increases in never users by a factor of 1.028 (1.021-1.034) for each year older at the menopause. The risk of breast cancer appears higher with combined oestrogen and progestogen combinations. HRT increases breast density and reduces the sensitivity and specificity of breast screening. Cancers diagnosed in women taking HRT tend to be less advanced clinically than those diagnosed in women who have not used HRT. Current evidence suggests that HRT does not increase breast cancer mortality.
Prevention of breast cancer
Screening as currently practised can reduce mortality but not incidence, and then only in a particular age group. Advances in treatment have produced significant but modest survival benefits. A better appreciation of factors important in the aetiology of breast cancer would raise the possibility of disease prevention.
Relationship of HRT to breast cancer development
| Time on HRT | Breast cancers over the 20 years from age 50-70 | Extra breast cancers in HRT users | Individual risk of women over 20 years |
| Never | 45 per 1000 | — | 1 in 22 |
| 5 years use | 47 per 1000 | 2 per 1000 | 1 in 21 |
| 10 years use | 51 per 1000 | 6 per 1000 | 1 in 19 |
| 15 years use | 57 per 1000 | 12 per 1000 | 1 in 17-18 |
Hormonal control
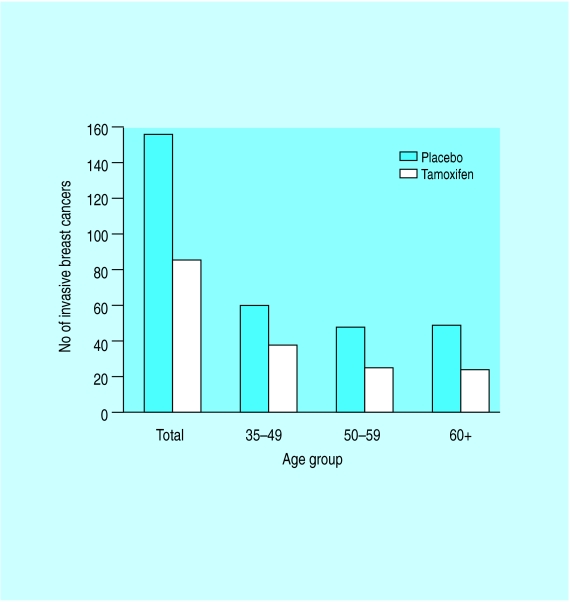
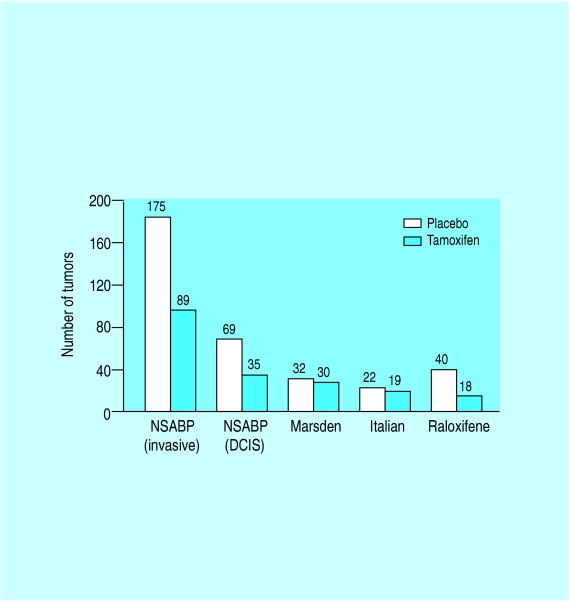
One promising avenue for primary prevention is influencing the hormonal milieu of women at risk. During trials of tamoxifen as an adjuvant treatment for breast cancer, the number of contralateral breast cancers was less than expected, suggesting that this drug might have a role in preventing breast cancer. Studies comparing tamoxifen with placebo in women at high risk of breast cancer have been reported and show conflicting results. The NSABP study randomised 3338 women with a risk equal to that of a 60 year old woman and showed a 47% reduction in the risk of invasive breast cancer and a 50% reduction in the rate of non-invasive breast cancer in women taking tamoxifen. Benefits of tamoxifen were observed in all age groups. The effect found for tamoxifen also reduced the overall incidence of osteoporotic fractures of the hip, spine and radius by 19%. It increased the relative risk of endometrial cancer by 2.5 but this risk was limited to women aged 50 or older. More women over 50 in the tamoxifen group developed deep venous thrombosis, pulmonary emboli and stroke. An Italian study and a UK study have failed to confirm the benefits of tamoxifen but overall evidence suggests there is a benefit of tamoxifen in preventing breast cancer. The ongoing UK trial should demonstrate whether this translates into a reduction in deaths from breast cancer. Raloxifene, a tamoxifen-like compound, has been evaluated in a population of 10 355 postmenopausal women being treated for osteoporosis and has demonstrated a 54% decrease in the number of breast cancers in the raloxifene group. Both the tamoxifen and raloxifene studies show a selective reduction in the incidence of oestrogen receptor positive breast cancers.
Dietary intervention
If specific dietary factors are found to be associated with an increased risk of breast cancer dietary intervention will be possible. However, reduction of dietary intake of such a factor in whole communities may well be difficult to achieve without major social and cultural changes. Weight gain by more than 10-20 kg from the weight at age 18 does seem to be associated with an increased risk.
Other preventive agents
Retinoids affect the growth and differentiation of epithelial cells, and experiments suggest that they may have a role in preventing breast cancer. A clinical trial of fenretinoid has been reported. In a study of 2972 women with breast cancer randomly allocated to fenretinoid or no treatment, no significant difference was seen in contralateral breast cancer between the two groups. There was a significant interaction with treatment and menopausal status with a beneficial effect being seen in premenopausal patients (adjusted hazard ratio 0.66, 95% CI 0.14-1.07) and an opposite trend on postmenopausal women. Selenium is another possible cancer preventing agent.
Key references
| • Bilimoria M, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. Cancer J Clin 1995;45:263-78. | • Futreal PA, Liu Q, Shattuck-Eidens D et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science 1994;266:120-2. |
| • Black DM. The genetics of breast cancer. Eur J Cancer 1994;30a:1957-61. | • Hill ADK, Doyle JM, McDermott EW, et al. Hereditary breast cancer. Br J Surg 1997;84:1334-9. |
| • Brinton LA, Devesa SS. Etiology and pathogenesis of breast cancer: incidence, demographics and environmental factors. In: Harris JR, Lippman ME, Morrow M, Hellman S, eds. Diseases of the breast. Philadelphia: Lippincott-Raven, 1996:159-68. | • Isaacs CJD, Peshkin BN, Lerman C. Evaluation and management of women with a strong family history of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Disease of the Breast. Philadelphia: Lippincott Williams & Wilkins, 2000:237-54. |
| • Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996;347:1713-27. | • Jordan CV, Costa AF. Chemoprevention. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Disease of the Breast. Philadelphia: Lippincott Williams & Wilkins, 2000:265-80. |
| • Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 without breast cancer. Lancet 1997;350:1047-59. | • Jordan VC, Glusman JE, Eckert S, et al. Raloxifene reduces incident primary breast cancers: integrated data from multicenter double blind placebo controlled, randomised trials in postmenopausal women. Breast Cancer Res Treat 1998;50:227 (abst no 2). |
| • Decker D. Prophylactic mastectomy for familial breast cancer. JAMA 1993;269:2608-9. | • Powles TJ, Eeles R, Ashley S, et al. Interim analysis of the incident breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 1998;362:98. |
| • DeMichele A, Weber BL. Inherited genetic factors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Disease of the Breast. Philadelphia: Lippincott Williams & Wilkins, 2000:221-36. | • Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Lancet 1998;362:93. |
| • Fisher B, Constantino JP, Wickerman DL et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371. | • Willett WC, Rockhill B, Hankinson SE, et al. Epidemiology and nongenetic causes of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Disease of the Breast. Philadelphia: Lippincott Williams & Wilkins, 2000:175-220. |
Figure.
Percentage of all deaths in women attributable to breast cancer
Figure.
Standardised mortality for breast cancer in different countries
Figure.
Age specific incidence and mortality for breast cancer in United Kingdom
Figure.
Annual incidence of breast cancer in Japanese women in Japan, Hawaii, and San Francisco and in white women from San Francisco
Figure.
Family tree of family with genetically inherited breast cancer
Figure.
Severe atypical lobular hyperplasia
Figure.
Relation between breast cancer mortality in various countries and fat consumption
Figure.
Cumulative risk of breast cancer in women who were aged 10-19 years when exposed to radiation from atomic bombs during the Second World War
Figure.
The effect of tamoxifen on the incidence of breast cancer in the NSABP Prevention Study in relation to age
Figure.
Mammograms of patient before and after three years of hormone replacement therapy showing increase in density caused by treatment
Figure.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) data illustrate the effects of tamoxifen on both invasive and non-invasive breast cancer. Data from the Royal Marsden and the Italian studies include only the number of invasive cancers. The raloxifene data include both invasive and non-invasive breast cancers
Footnotes
K McPherson is professor of public health epidemiology, London School of Hygiene and Tropical Medicine, London, C M Steel is professor of medical and biological sciences, University of St Andrews, St Andrews, and J M Dixon is consultant surgeon and senior lecturer in surgery, Edinburgh Breast Unit, Western General Hospital, Edinburgh.
The ABC of breast diseases is edited by J Michael Dixon.