Abstract
BACKGROUND
Many patients with heart failure and preserved ejection fraction have no overt volume overload and normal resting left atrial (LA) pressure.
OBJECTIVES
This study sought to characterize patients with normal resting LA pressure (pulmonary capillary wedge pressure [PCWP] <15 mm Hg) but exercise-induced left atrial hypertension (EILAH).
METHODS
The REDUCE LAP-HF II (A Study to Evaluate the Corvia Medical, Inc. IASD System II to Reduce Elevated Left Atrial Pressure in Patients With Heart Failure) trial randomized 626 patients with ejection fraction ≥40% and exercise PCWP ≥25 mm Hg to atrial shunt or sham procedure. The primary trial outcome, a hierarchical composite of death, heart failure hospitalization, intensification of diuretics, and change in health status was compared between patients with EILAH and those with heart failure and resting left atrial hypertension (RELAH).
RESULTS
Patients with EILAH (29%) had similar symptom severity, but lower natriuretic peptide levels, higher 6-minute walk distance, less atrial fibrillation, lower left ventricular mass, smaller LA volumes, lower E/e′, and better LA strain. PCWP was lower at rest, but had a larger increase with exercise in EILAH. Neither group as a whole had a significant effect from shunt therapy vs sham. Patients with EILAH were more likely to have characteristics associated with atrial shunt responsiveness (peak exercise pulmonary vascular resistance <1.74 WU) and no pacemaker (63% vs 46%; P < 0.001). The win ratio for the primary outcome was 1.56 (P = 0.08) in patients with EILAH and 1.51 (P = 0.04) in those with RELAH when responder characteristics were present.
CONCLUSIONS
Patients with EILAH had similar symptom severity but less advanced myocardial and pulmonary vascular disease. This important subgroup may be difficult to diagnose without invasive exercise hemodynamics, but it has characteristics associated with favorable response to atrial shunt therapy. (A Study to Evaluate the Corvia Medical, Inc. IASD System II to Reduce Elevated Left Atrial Pressure in Patients With Heart Failure [REDUCE LAP-HF TRIAL II]; NCT03088033)
Keywords: exercise capacity, exercise hemodynamics, heart failure with preserved ejection fraction, pulmonary capillary wedge pressure, randomized controlled trial
Exertional dyspnea in heart failure with preserved ejection fraction (HFpEF) is due, at least in part, to elevated left atrial (LA) pressure during physical activity.1 However, not all patients with HFpEF have evidence of overt volume overload. This subgroup of patients usually has normal pulmonary capillary wedge pressure (PCWP) at rest, but marked rises in PCWP with exertion. Patients with normal left ventricular (LV) filling pressures at rest, but with exercise-induced left atrial hypertension (EILAH) can be particularly difficult to diagnose because the physical findings associated with congestion are frequently absent.2 In addition, chest radiography and resting echocardiography may be normal and natriuretic peptide levels are often below established thresholds for a HF diagnosis. Treatment of the subgroup of patients with EILAH is also frequently challenging, because the use of diuretic agents or vasodilators may lead to worsening renal function, orthostatic hypotension, or other untoward effects.
The REDUCE LAP-HF II (A Study to Evaluate the Corvia Medical, Inc. IASD System II to Reduce Elevated Left Atrial Pressure in Patients With Heart Failure; NCT03088033) trial evaluated an interatrial shunt device (Corvia Atrial Shunt, IASD System II, Corvia Medical) in patients with HF and left ventricular ejection fraction (LVEF) ≥40%.3 The device is designed to lower LA pressure, particularly during exertion.4 Atrial shunts have the theoretical advantage of producing little left-to-right shunting when LA pressures are normal and hence closer to right atrial (RA) pressure, while allowing increased left-to-right shunting when LA pressures rise excessively, such as during exercise. Thus, atrial shunts may be ideally suited to help relieve symptoms of exertional dyspnea in patients with EILAH.
The present study sought to: 1) compare the clinical, echocardiographic, and invasive hemodynamic characteristics of patients with EILAH versus those with resting left atrial hypertension (RELAH); and 2) determine whether there are differential effects of atrial shunt treatment in these groups, using data from the REDUCE LAP-HF II trial.
METHODS
REDUCE LAP-HF II STUDY DESIGN AND OBJECTIVES.
The objectives and design of the REDUCE LAP-HF II study have been previously described in detail.3 Briefly, REDUCE LAP-HF II was a multicenter, international, randomized, double-blind, sham-controlled trial of the Corvia Atrial Shunt in adults with HF and LVEF ≥40%. After comprehensive noninvasive and invasive hemodynamic screening, eligible participants were randomly assigned 1:1 to receive the Corvia Atrial Shunt (IASD System II) or a sham control procedure.5,6 The primary endpoint was a hierarchical composite of: 1) time to cardiovascular mortality or nonfatal ischemic stroke to 12 months; 2) HF hospitalizations, health care facility visits for intravenous diuresis, or urgent intensification of oral diuresis up to 24 months; and 3) change in KCCQ (Kansas City Cardiomyopathy Questionnaire) overall summary score from baseline to 12 months, analyzed by Finkelstein-Schoenfeld methodology when the last randomized patient reached 12-month follow-up.7 For patients with the same number of HF events during the available follow-up time, the “losing patient” was determined based on the time to the first HF event. Follow-up echocardiography was performed to evaluate shunt flow and cardiac chamber size/function.
The REDUCE LAP-HF II trial has been registered at Clinicaltrials.gov (NCT03088033) and followed the Declaration of Helsinki. Approval from the responsible local authorities and ethics committees were obtained prior to inclusion of patients, and all patients provided written informed consent.
SELECTION OF STUDY PATIENTS: INCLUSION AND EXCLUSION CRITERIA.
Major inclusion criteria were age ≥40 years, chronic NYHA functional class II with a history of functional class III or current functional class III symptoms of HF, LVEF ≥40%, ongoing diuretic therapy, echocardiographic evidence of diastolic dysfunction, and either: 1) HF hospitalization or intensification of oral diuretic agents in the prior 12 months; or 2) elevated B-type natriuretic peptide (BNP) levels stratified by cardiac rhythm.7 Additional inclusion criteria included site-determined invasively measured PCWP ≥25 mm Hg during supine ergometer exercise and PCWP ≥5 mm Hg above RA pressure and appropriate septal anatomy for atrial shunt device placement.3 Key exclusion criteria have been previously published and included > mild right ventricular dysfunction, > mild tricuspid regurgitation, RA pressure >14 mm Hg at rest, or pulmonary vascular resistance (PVR) >3.5 WUs at rest or during exercise.3
BASELINE ECHOCARDIOGRAPHY AND INVASIVE EXERCISE HEMODYNAMICS.
Echocardiography was performed according to a standardized protocol. Measurements were done by the central echocardiography core laboratory (University of Pennsylvania, Philadelphia, Pennsylvania, USA). Speckle-tracking was used for strain measurements of all 4 cardiac chambers in the apical 4-chamber view using TomTec Cardiac Performance Analysis software at the Northwestern University Echocardiography Core Laboratory (Chicago, Illinois, USA).
Right heart and pulmonary artery pressures were measured using a fluid-filled catheter with a properly zeroed pressure transducer. Hemodynamic measurements were performed at rest, legs up position (ie, passive leg raise) and during exercise using a supine bicycle ergometer. Graded exercise was performed at 20-W increments (3 minutes at each stage) until exhaustion. Pressure measurements were repeated at each stage and thermodilution cardiac output was recorded at baseline and peak exercise. Hemodynamics were measured at a core laboratory (Cardiovascular Clinical Studies, Boston, Massachusetts, USA), taking the average of ≥3 beats. All assessments were made blinded to treatment group.
Estimated plasma volume was calculated with previously established methods that include hematocrit, body weight, and sex-specific constants.8
CLINICAL OUTCOMES AND SAFETY ENDPOINTS.
A blinded clinical events committee adjudicated all serious adverse events and deaths, with a focus on cardiovascular death, nonfatal ischemic stroke, HF events (HF hospitalizations, urgent intravenous diuretic therapy in a health care setting, or intensification of oral diuretics), and safety outcomes.
STATISTICAL ANALYSIS.
For this analysis, we divided the REDUCE LAP-HF II trial cohort into 2 groups based on resting PCWP (all randomized patients were required to have site-reported PCWP ≥25 mm Hg during exercise). EILAH was defined as resting PCWP <15 mm Hg. RELAH was defined as resting PCWP ≥15 mm Hg. Baseline characteristics are summarized as counts and percentages for categorical variables and median (25th, 75th percentile) for continuous variables. The 2 groups were compared using chi-squared tests (or Fisher exact test) for categorical variables and Student’s t-tests for normally distributed variables or Wilcoxon rank sum test for non-normally distributed continuous variables. The Benjamini-Hochberg false discovery rate method was used to control for multiple comparisons of baseline characteristics. Values of P < 0.03 (corresponding to false discovery rate–adjusted values of P < 0.05) were considered statistically significant (Tables 1 to 4).
TABLE 1.
Demographics, Comorbidities, and Medications
| Total (N = 626) | RELAH (n = 444) | EILAH (n = 182) | P Value | |
|---|---|---|---|---|
| Age, y | 72 (66–77) | 73 (68–78) | 71 (64–76) | 0.003 |
| Female | 385 (61.5) | 266 (59.9) | 119 (65.4) | 0.20 |
| Race/ethnicity | 0.45 | |||
| Asian | 10 (2.5) | 6 (2.2) | 4 (3.3) | |
| Black or African American | 24 (6.0) | 17 (6.1) | 7 (5.7) | |
| White | 361 (90.2) | 250 (89.9) | 111 (91.0) | |
| Hypertension | 551 (88.3) | 398 (89.6) | 153 (85.0) | 0.10 |
| Obesity, BMI >30 kg/m2 | 381 (60.9) | 275 (61.9) | 106 (58.2) | 0.39 |
| Diabetes | 230 (36.7) | 178 (40.1) | 52 (28.6) | 0.007 |
| Chronic kidney disease, eGFR <60 mL/min/1.73 m2 | 347 (56.9) | 267 (61.5) | 80 (45.5) | <0.001 |
| Prior coronary percutaneous interventions | 169 (27.2) | 116 (26.4) | 53 (29.3) | 0.46 |
| Coronary artery bypass graft | 54 (8.7) | 41 (9.3) | 13 (7.2) | 0.39 |
| Prior valve surgery/intervention | 38 (14.3) | 28 (14.9) | 10 (13.0) | 0.69 |
| Atrial fibrillation | 324 (51.8) | 261 (58.8) | 63 (34.6) | <0.001 |
| Atrial flutter | 65 (10.5) | 48 (10.9) | 17 (9.3) | 0.55 |
| Atrial fibrillation or flutter on baseline ECG | 108 (17.4) | 94 (21.3) | 14 (7.7) | <0.001 |
| Anemia | 232 (37) | 173 (39.0) | 59 (32.4) | 0.12 |
| Any pacemaker | 116 (18.6) | 91 (20.7) | 25 (13.7) | 0.043 |
| Smoking history | 0.97 | |||
| Never smoked | 322 (51.5) | 227 (51.2) | 95 (52.2) | |
| Former smoker | 283 (45.3) | 202 (45.6) | 81 (44.5) | |
| Current smoker | 20 (3.2) | 14 (3.2) | 6 (3.3) | |
| COPD | 126 (20.2) | 93 (21.0) | 33 (18.1) | 0.42 |
| Pulmonary embolism | 32 (5.1) | 20 (4.5) | 12 (6.6) | 0.28 |
| Loop diuretics | 514 (82.1) | 382 (86.0) | 132 (72.5) | <0.001 |
| Loop diuretic dose, mg furosemide equivalents | 40 (20–80) | 40 (20–80) | 40 (20–60) | 0.12 |
| ACE inhibitors | 152 (24.3) | 109 (24.5) | 43 (23.6) | 0.81 |
| ARBs | 236 (37.7) | 167 (37.6) | 69 (37.9) | 0.94 |
| Beta-blockers | 438 (70.0) | 322 (72.5) | 116 (63.7) | 0.029 |
| MRAs | 325 (51.9) | 216 (48.6) | 109 (59.9) | 0.011 |
| SGLT2 inhibitors | 16 (2.6) | 14 (3.2) | 2 (1.1) | 0.14 |
| Aspirin | 241 (38.5) | 157 (35.4) | 84 (46.2) | 0.012 |
| Number of antihypertensive medications | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.046 |
Values are median (IQR) or n (%). Values of P < 0.03 are considered to be significantly different after adjustment for multiple comparisons using the false discovery rate method.
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; COPD = chronic obstructive pulmonary disease; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; EILAH = exercise-induced left atrial hypertension; MRA = mineralocorticoid receptor antagonist; RELAH = resting left atrial hypertension; SGLT2 = sodium-glucose cotransporter 2.
TABLE 4.
Invasive Exercise Hemodynamic Data
| Total (N = 626) | RELAH (n = 444) | EILAH (n = 182) | P Value | |
|---|---|---|---|---|
| Resting hemodynamics | ||||
| Aortic systolic pressure, mm Hg | 144.0 (129.0–159.0) | 146.0 (132.0–160.0) | 137.0 (125.0–153.0) | <0.001 |
| Heart rate, beats/min | 70.0 (63.0–80.0) | 70.0 (62.0–80.0) | 70.0 (63.0–79.0) | 0.82 |
| RA pressure, mm Hg | 9.0 (7.0–12.0) | 10.0 (8.0–13.0) | 6.0 (5.0–8.0) | <0.001 |
| PA systolic pressure, mm Hg | 40.0 (34.0–49.0) | 44.0 (38.0–53.0) | 32.0 (28.0–35.0) | <0.001 |
| PA diastolic pressure, mm Hg | 19.0 (15.0–23.0) | 21.0 (18.0–25.0) | 14.5 (12.0–16.0) | <0.001 |
| Mean PA pressure, mm Hg | 26.3 (21.3–32.0) | 29.0 (24.7–33.7) | 20.3 (17.7–22.7) | <0.001 |
| PCWP, mm Hg | 18.0 (14.0–23.0) | 21.0 (17.0–25.0) | 12.0 (10.0–13.0) | <0.001 |
| PCWP to RA pressure gradient, mm Hg | 8.0 (5.0–12.0) | 11.0 (7.0–14.0) | 5.0 (3.0–6.0) | <0.001 |
| Transpulmonary gradient, mm Hg | 8.0 (6.0–11.0) | 8.0 (5.7–10.7) | 9.0 (6.7–11.0) | 0.016 |
| TAPSE/PA systolic pressure ratio, mm/mm Hg | 0.5 (0.4–0.6) | 0.4 (0.3–0.6) | 0.6 (0.5–0.8) | <0.001 |
| Stroke volume, mL | 74.6 (62.6–89.3) | 75.0 (62.5–89.2) | 73.8 (64.0–89.7) | 0.91 |
| Cardiac output, L/min | 5.2 (4.4–6.2) | 5.2 (4.4–6.3) | 5.2 (4.4–6.2) | 0.99 |
| Systemic vascular resistance, WU | 17.0 (13.7–20.9) | 16.8 (13.6–20.9) | 17.2 (14.0–20.7) | 0.72 |
| Pulmonary vascular resistance, WU | 1.5 (1.1–2.1) | 1.4 (1.0–2.1) | 1.7 (1.2–2.1) | 0.062 |
| PA pulse pressure/stroke volume ratio, mm Hg/mL | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.2 (0.2–0.3) | <0.001 |
| Legs up hemodynamics | ||||
| RA pressure, mm Hg | 11.0 (8.0–15.0) | 12.0 (10.0–16.0) | 8.5 (6.5–11.0) | <0.001 |
| PCWP, mm Hg | 22.0 (18.0–27.0) | 25.0 (21.0–30.0) | 17.0 (14.0–19.5) | <0.001 |
| 20-W exercise hemodynamics | ||||
| Right atrial pressure, mm Hg | 17.0 (13.0–21.0) | 18.0 (15.0–22.0) | 14.0 (11.0–17.0) | <0.001 |
| PA systolic pressure, mm Hg | 64.0 (54.0–75.0) | 69.5 (58.0–79.0) | 55.0 (48.0–63.0) | <0.001 |
| PA diastolic pressure, mm Hg | 30.0 (27.0–37.0) | 32.0 (29.0–39.0) | 27.0 (23.0–30.0) | <0.001 |
| Mean PA pressure, mm Hg | 42.0 (36.0–48.7) | 45.3 (38.7–51.3) | 36.0 (32.0–41.0) | <0.001 |
| PA pulse pressure, mm Hg | 32.0 (25.0–41.5) | 35.0 (28.0–44.0) | 28.0 (21.0–35.0) | <0.001 |
| PCWP, mm Hg | 32.0 (27.0–38.0) | 34.0 (29.0–40.0) | 27.0 (24.0–31.0) | <0.001 |
| PCWP to RA pressure gradient, mm Hg | 15.0 (11.0–19.0) | 16.0 (12.0–21.0) | 13.0 (10.0–17.0) | <0.001 |
| Peak exercise hemodynamics | ||||
| Capacity, W | 40.0 (20.0–60.0) | 40.0 (20.0–60.0) | 40.0 (40.0–60.0) | <0.001 |
| Total duration of exercise, min | 7.0 (5.0–10.5) | 7.0 (5.0–10.0) | 9.0 (6.0–11.0) | <0.001 |
| Aortic systolic pressure, mm Hg | 160.0 (141.0–181.5) | 161.0 (142.0–182.0) | 158.0 (140.0–181.0) | 0.52 |
| Peak heart rate, beats/min | 100.0 (86.0–113.0) | 98.0 (85.0–112.0) | 104.0 (92.0–114.0) | 0.004 |
| RA pressure, mm Hg | 18.0 (14.0–22.0) | 19.0 (16.0–24.0) | 15.0 (12.0–18.0) | <0.001 |
| PA systolic pressure, mm Hg | 69.0 (60.0–80.0) | 72.0 (62.0–81.0) | 62.0 (56.0–69.0) | <0.001 |
| PA diastolic pressure, mm Hg | 34.0 (29.0–40.0) | 35.0 (30.0–40.0) | 30.0 (27.0–35.0) | <0.001 |
| Mean PA pressure, mm Hg | 45.3 (39.7–52.0) | 47.5 (41.7–54.0) | 41.0 (36.7–46.3) | <0.001 |
| PCWP, mm Hg | 34.0 (29.0–40.0) | 36.0 (32.0–42.0) | 30.0 (27.0–35.0) | <0.001 |
| PCWP to RA pressure gradient, mm Hg | 16.0 (12.0–21.0) | 17.0 (13.0–21.0) | 15.5 (11.0–19.0) | 0.005 |
| Transpulmonary gradient, mm Hg | 10.7 (7.0–15.3) | 11.0 (7.3–15.7) | 10.0 (6.7–14.3) | 0.16 |
| Stroke volume, mL | 52.8 (43.5–65.3) | 53.5 (44.4–67.6) | 51.6 (41.1–62.4) | 0.083 |
| Cardiac output, L/min | 8.0 (6.4–10.1) | 7.9 (6.1–9.7) | 8.8 (7.0–11.1) | <0.001 |
| Systemic vascular resistance, WU | 11.5 (9.0–15.0) | 12.0 (9.1–15.4) | 10.7 (8.2–14.6) | 0.010 |
| PVR, WU | 1.3 (0.8–2.0) | 1.4 (0.9–2.1) | 1.1 (0.8–1.7) | 0.001 |
| Exercise PVR <1.74 WU, % | 46 | 60 | 0.001 | |
| Workload-corrected PCWP, mm Hg/W/kg | 76.6 (51.8–123.9) | 86.0 (58.6–145.7) | 57.8 (40.6–90.0) | <0.001 |
| PCWP/CO slope, mm Hg/L/min | 5.9 (3.6–10.5) | 6.2 (3.6–11.0) | 5.4 (3.6–9.7) | 0.31 |
| Change from rest to peak exercise | ||||
| ΔPCWP to RA gradient, mm Hg | 7 (3–12) | 6 (2–10) | 10 (7–14) | <0.001 |
| ΔPCWP, mm Hg | 16.0 (12.0–21.0) | 15.0 (10.0–20.0) | 19.0 (15.0–23.5) | <0.001 |
| ΔCO, L/min | 2.7 (1.6–4.2) | 2.4 (1.5–3.9) | 3.5 (2.2–4.8) | <0.001 |
| ΔPVR, WU | 0.21 (0.58–0.32) | 0.06 (0.46–0.433) | 0.41 (0.84–0.05) | <0.001 |
Values are median (IQR), unless otherwise indicated. Values of P < 0.03 are considered to be significantly different after adjustment for multiple comparisons using the false discovery rate method.
We used multivariable logistic regression analyses to determine which clinical characteristics and echocardiographic parameters were most closely associated with EILAH. Variables included in the logistic regression models were chosen based on clinical relevance or association with EILAH at a significance level of <0.05 on univariate analyses. We used Cox regression analyses and Kaplan-Meier curves (with log rank P value calculated) to determine the potential association between resting LA pressure and HF events in the overall study and stratified by treatment groups.
The overall primary and secondary endpoints (including safety endpoints) in the trial were stratified by EILAH vs RELAH. We used the win ratio to examine the primary hierarchical outcome,9 negative binomial regression adjusted for number of prior year HF hospitalizations or total HF events, and linear regression adjusted for baseline value for change in KCCQ overall summary score and NYHA functional class. Finally, we calculated Pearson correlation coefficients for the correlation between peak exercise PVR, cardiac rhythm device at baseline, and both RELAH and EILAH resting PCWP and ΔPCWP (peak exercise − resting PCWP) given the importance of peak exercise PVR and rhythm devices in defining responders to the atrial shunt device.10 For the analyses presented in Table 5, 2-tailed P < 0.05 was considered statistically significant.
TABLE 5.
Efficacy Outcomes Stratified by Eilah Status Alone and Combined With Responder Subgroups
| Sham/IASD, n | Win Ratio | 95% CI | P Value | HF Event, IRR | 95% CI | P Value | Change in KCCQ | 95% CI | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| EILAH | ||||||||||
| All EILAH | 88/92 | 1.06 | 0.73–1.53 | 0.78 | 0.70 | 0.28–1.70 | 0.43 | −0.1 | −5.8 to 5.7 | 0.98 |
| EILAH + peak PVR <1.74 WUs | 59/67 | 1.46 | 0.93–2.29 | 0.10 | 0.52 | 0.18–1.50 | 0.23 | +6.1 | −0.5 to 12.7 | 0.07 |
| EILAH + no pacemaker | 77/79 | 1.10 | 0.74–1.63 | 0.64 | 0.59 | 0.21–1.68 | 0.32 | −1.0 | −7.2 to 5.2 | 0.76 |
| EILAH + peak PVR <1.74 WUs and no pacemaker | 50/57 | 1.56 | 0.96–2.54 | 0.08 | 0.53 | 0.15–1.85 | 0.32 | +5.6 | −1.6 to 12.7 | 0.13 |
| RELAH | ||||||||||
| All RELAH | 224/217 | 0.98 | 0.78–1.23 | 0.85 | 1.44 | 0.92–2.25 | 0.11 | +1.3 | −2.5 to 5.1 | 0.50 |
| RELAH + peak PVR <1.74 WUs | 123/132 | 1.25 | 0.92–1.70 | 0.15 | 0.73 | 0.39–1.35 | 0.32 | +4.9 | −0.1 to 9.9 | 0.06 |
| RELAH + no pacemaker | 184/166 | 1.12 | 0.86–1.45 | 0.39 | 1.22 | 0.71–2.09 | 0.47 | +2.7 | −1.6 to 6.9 | 0.23 |
| RELAH + peak PVR <1.74 WUs and no pacemaker | 102/103 | 1.51 | 1.07–2.13 | 0.02 | 0.44 | 0.02–0.99 | 0.047 | +6.0 | 0.3–11.7 | 0.04 |
All 626 randomized patients were included in the presentation of baseline variables. Five patients were excluded after randomization and the remaining 621 patients were included in the outcomes analyses.
Analyses were conducted in Stata (version 17.0, StataCorp) and R (version 3.5.1, R Foundation for Statistical Computing).
RESULTS
ENROLLMENT OF REDUCE LAP II STUDY PARTICIPANTS.
Patients in the EILAH group were slightly younger, with similar distribution of sex, race/ethnicity, and obesity (Table 1). The EILAH group was less likely to have hypertension, diabetes, chronic kidney disease, and current or prior atrial fibrillation. Patients with EILAH had higher estimated glomerular filtration rate, lower use of loop diuretic agents and beta-adrenergic blocking agents, but higher use of mineralocorticoid receptor agonists (Table 1, Supplemental Table 1).
NYHA functional class and KCCQ score (44.5 vs 45.8) were similarly impaired in the group with EILAH compared to those with RELAH (Table 2). Although heart rate was comparable, the patients with EILAH had lower systolic blood pressure and higher 6-minute walk distance. Those with EILAH also had lower BNP and/or N-terminal pro-BNP, higher estimated glomerular filtration rate, and slightly higher bilirubin levels, but no differences in hemoglobin, electrolytes, or liver enzymes. Median BNP in patients with sinus rhythm was 56.0 pg/mL in patients with EILAH vs 110.0 pg/mL in those with RELAH and pg/mL vs 216.8 pg/mL for those in atrial fibrillation or flutter (Table 2). Median N-terminal pro-BNP in sinus rhythm was 215.0 pg/mL for patients with EILAH and 405.0 pg/mL for those with RELAH, and in those with atrial fibrillation the values were 721.0 and 1225.0 pg/mL, respectively. Estimated plasma volume was lower in patients with EILAH vs those with RELAH (mean difference: −113 mL [IQR: 6–220 mL]; P = 0.039) (Table 2).
TABLE 2.
Physical Exam, Symptoms, Health Status, Exercise Capacity, and Laboratory Data
| Total (N = 626) | RELAH (n = 444) | EILAH (n = 182) | P Value | |
|---|---|---|---|---|
| Height, cm | 167.3 (160.0–174.2) | 167.6 (160.0–175.0) | 165.3 (160.0–174.0) | 0.42 |
| Weight, kg | 89.8 (76.9–105.0) | 91.0 (77.0–105.1) | 87.6 (76.0–102.1) | 0.056 |
| Body mass index, kg/m2 | 32.0 (27.7–37.0) | 32.2 (28.0–37.4) | 31.6 (27.1–35.7) | 0.074 |
| Body surface area | 2.0 (1.9–2.2) | 2.0 (1.9–2.2) | 2.0 (1.8–2.2) | 0.067 |
| Heart rate, beats/min | 70 (63–80) | 70 (62–80) | 70 (63–79) | 0.82 |
| Systolic blood pressure, mm Hg | 143 (128–158) | 145 (131–160) | 137 (125–152) | 0.001 |
| Diastolic blood pressure, mm Hg | 74 (67–83) | 74 (67–83) | 75 (68–81) | 0.57 |
| NYHA functional class | 0.22 | |||
| II | 132 (21.4) | 88 (20.1) | 44 (24.6) | |
| III | 484 (78.6) | 349 (79.9) | 135 (75.4) | |
| MAGGIC risk score | 23 (18–26) | 23 (19–27) | 21 (17–25) | <0.001 |
| KCCQ clinical summary score at baseline | 50.0 (35.4–67.1) | 49.5 (34.9–66.9) | 52.1 (36.5–67.2) | 0.57 |
| KCCQ overall summary score at baseline | 45.8 (29.2–62.5) | 45.8 (28.5–62.2) | 44.5 (30.2–63.5) | 0.82 |
| 6MWD at baseline, m | 301.3 (236.0–380.0) | 295.5 (225.0–367.8) | 326.2 (263.0–415.0) | <0.001 |
| H2FPEF score | 6 (5–8) | 6 (5–8) | 5 (4–6) | <0.001 |
| At least 1 hospitalization for HF in the past 12 months | 171 (27.3) | 113 (25.5) | 58 (31.9) | 0.10 |
| BNP, atrial fibrillation/flutter, pg/mL | 213.5 (119.0–380.3) | 216.8 (126.7–425.0) | 160.3 (81.8–305.6) | 0.36 |
| BNP, sinus rhythm, pg/mL | 91.9 (40.8–170.0) | 110.0 (60.0–220.0) | 56.0 (29.3–104.6) | <0.001 |
| NT-proBNP, atrial fibrillation/flutter, pg/mL | 1,147.5 (643.0–1,792.0) | 1,225.0 (647.0–1,900.0) | 721.0 (453.5–965.5) | 0.026 |
| NT-proBNP, sinus rhythm, pg/mL | 329.0 (158.3–653.0) | 405.0 (200.0–788.0) | 215.0 (104.6–427.0) | <0.001 |
| Hemoglobin, g/dL | 12.7 (11.4–13.8) | 12.7 (11.3–13.8) | 12.7 (11.6–13.8) | 0.70 |
| eGFR, mL/min/1.73 m2 | 56.5 (42.0–68.0) | 55.0 (40.0–66.0) | 60.0 (46.5–69.5) | 0.005 |
| Estimated plasma volume, mL | 3,234 (2,834–3,649) | 3,152 (2,808–3,458) | 0.039 |
Values are median (IQR) or n (%). Values of P < 0.03 are considered to be significantly different after adjustment for multiple comparisons using the false discovery rate method.
6MWD = 6-minute walk distance; BNP = B-type natriuretic peptide; eGFR = estimated glomerular filtration rate; HF = heart failure; KCCQ = Kansas City Cardiomyopathy Questionnaire; MAGGIC = Meta-Analysis Global Group in Chronic Heart Failure; NT-proBNP = N-terminal pro– B-type natriuretic peptide; other abbreviations as in Table 1.
Baseline cardiac structure and function assessed by echocardiography are shown in Table 3 (additional data in Supplemental Table 2). The EILAH group had similar LV volumes, lower LV mass (77.4 vs 82.7 g/m2), similar LVEF, and higher absolute value of LV global longitudinal strain. In terms of diastolic parameters, patients with EILAH had lower E velocity and higher A velocity, with lower E/A and E/e′ (10.8 vs 13.2). Maximal and minimal LA volumes were lower, whereas LA peak longitudinal strain was significantly higher (24.5% vs 18.1%) in patients with EILAH. RA volume was lower, but tricuspid annular plane systolic excursion and right ventricular free wall longitudinal strain were not different. Estimated RA pressure was the same in both groups, but estimated pulmonary artery systolic pressure was lower in those with EILAH (28.0 vs 33.0 mm Hg).
TABLE 3.
Echocardiographic Data
| Total (N = 626) | RELAH (n = 444) | EILAH (n = 182) | P Value | |
|---|---|---|---|---|
| Septal wall thickness, cm | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 0.39 |
| Posterior wall thickness, cm | 0.9 (0.8–1.0) | 0.9 (0.9–1.0) | 0.9 (0.8–1.0) | 0.017 |
| LV end-diastolic dimension, cm | 4.8 (4.4–5.2) | 4.9 (4.5–5.3) | 4.7 (4.3–5.0) | 0.002 |
| LV end-systolic dimension, cm | 3.5 (3.1–4.0) | 3.5 (3.2–4.0) | 3.4 (3.0–3.8) | 0.015 |
| LV mass indexed to BSA, g/m2 | 80.8 (65.3–98.4) | 82.7 (67.2–101.6) | 77.4 (63.6–93.0) | 0.005 |
| LV end-diastolic volume, mL | 217.8 (171.1–287.4) | 223.4 (170.3–298.2) | 211.2 (173.4–264.2) | 0.096 |
| LV end-systolic volume, mL | 101.6 (75.9–137.8) | 102.1 (77.0–145.8) | 97.5 (75.6–125.0) | 0.089 |
| Ejection fraction, % | 60.0 (55.0–65.0) | 60.0 (55.0–65.0) | 60.0 (55.0–65.0) | 0.14 |
| Mitral E velocity, cm/s | 86.0 (69.0–108.0) | 91.0 (72.0–114.0) | 76.0 (62.0–93.0) | <0.001 |
| Mitral A velocity, cm/s | 75.0 (55.0–94.0) | 72.5 (53.0–93.0) | 80.0 (60.0–95.0) | 0.020 |
| E/A ratio | 1.1 (0.8–1.6) | 1.2 (0.8–1.8) | 0.9 (0.7–1.3) | <0.001 |
| Septal e′ velocity, cm/s | 6.0 (5.0–7.0) | 6.0 (5.0–7.0) | 6.0 (5.0–7.0) | 0.28 |
| Lateral e′ velocity, cm/s | 8.0 (6.0–10.0) | 8.0 (6.0–10.0) | 8.0 (6.0–10.0) | 0.48 |
| Average E/e′ ratio | 12.5 (9.6–17.1) | 13.2 (10.0–17.8) | 10.8 (8.8–14.0) | <0.001 |
| LA maximal volume indexed to BSA, mL/m2 | 31.6 (24.9–40.6) | 33.0 (26.0–41.3) | 28.4 (23.1–36.5) | <0.001 |
| LA minimal volume indexed to BSA, mL/m2 | 20.3 (14.5–28.1) | 21.8 (15.7–30.4) | 17.0 (13.1–22.7) | <0.001 |
| Ratio of LA to RA volume | 1.3 (1.0–1.6) | 1.2 (1.0–1.5) | 1.3 (1.1–1.6) | 0.068 |
| LA emptying fraction, % | 35.3 (25.4–43.8) | 32.8 (22.5–42.9) | 39.6 (31.4–46.1) | <0.001 |
| RV end-diastolic dimension, cm | 3.7 (3.4–4.1) | 3.8 (3.4–4.2) | 3.7 (3.3–4.0) | 0.094 |
| RV s′ velocity, cm/s | 11.0 (10.0–14.0) | 11.0 (10.0–14.0) | 11.0 (10.0–14.0) | 0.77 |
| TAPSE, cm | 2.0 (1.8–2.3) | 2.0 (1.7–2.3) | 2.0 (1.8–2.3) | 0.17 |
| RA volume indexed to BSA, mL/m2 | 25.2 (19.0–33.1) | 26.8 (20.4–35.6) | 22.4 (17.3–28.6) | <0.001 |
| Peak TR velocity, cm/s | 261.0 (236.0–292.0) | 265.5 (240.0–300.0) | 250.0 (227.0–275.0) | <0.001 |
| Estimated PA systolic pressure, mm Hg | 31.0 (26.0–39.0) | 33.0 (27.0–40.0) | 28.0 (24.0–33.0) | <0.001 |
| Estimated RA pressure, mm Hg | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 0.15 |
| Stroke volume, L | 66.1 (55.4–81.6) | 65.9 (53.8–79.8) | 68.4 (56.0–83.1) | 0.23 |
| LV global longitudinal strain, % | −17.7 (−15.4 to −20.2) | −17.3 (−15.0 to −19.9) | −18.9 (−16.8 to −20.8) | <0.001 |
| RV free wall strain, % | −22.4 (−17.9 to −26.1) | −22.0 (−17.6 to −25.6) | −23.1 (−18.5 to −26.8) | 0.067 |
| LA reservoir strain, % | 20.3 (14.2–26.9) | 18.1 (13.0–25.3) | 24.5 (17.0–28.9) | <0.001 |
| RA reservoir strain, % | 24.0 (17.8–31.3) | 22.2 (17.1–29.1) | 27.1 (22.2–33.1) | <0.001 |
Values are median (IQR). Values of P < 0.03 are considered to be significantly different after adjustment for multiple comparisons using the false discovery rate method.
BSA = body surface area; LA = left atrial; LV = left ventricular; PA = pulmonary artery; RA = right atrial; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; TR = tricuspid regurgitation; other abbreviations as in Table 1.
Using logistic regression analysis, we found that lack of atrial fibrillation, lack of loop diuretic therapy, lower systolic blood pressure, higher 6-minute walk distance, and lower natriuretic peptide levels were all associated with EILAH (Supplemental Table 3). Lower baseline LV end-diastolic dimension was independently associated with EILAH, whereas LV mass and E/e′ were not associated.
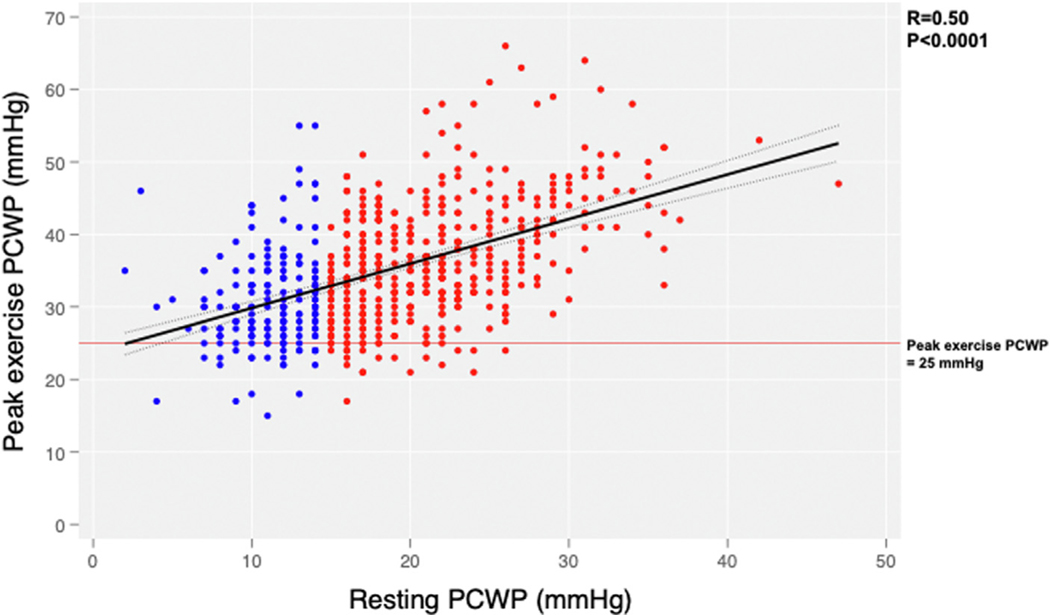
Invasive hemodynamic measurements at rest revealed lower RA pressure; lower pulmonary artery systolic, diastolic, and mean pressure; markedly lower PCWP (by definition); slightly higher mixed venous oxygen saturation; but no difference in cardiac output or systemic or pulmonary vascular resistance (1.4 vs 1.7 WUs; P = 0.06) in EILAH vs RELAH groups (Table 4, additional data in Supplemental Table 4). RA pressure, pulmonary arterial pressure, and PCWP increased in both groups with legs up and further with supine bicycle exercise, but these pressures remained lower in the group with EILAH. Average watts achieved was the same in both groups (40 W). At peak exercise, cardiac output was higher, PCWP was lower and PCWP/cardiac output slope was lower in the EILAH group. There was a significant positive association between resting PCWP and peak PCWP for the overall population (r = 0.50; P < 0.001) (Figure 1).
FIGURE 1.
Relationship Between Resting and Peak Exercise PCWP
Patients with resting pulmonary capillary wedge pressure (PCWP) <15 mm Hg (exercise-induced left atrial hypertension) are shown in blue and those with resting PCWP ≥15 mm Hg (resting left atrial hypertension) in red. The mean PCWP at peak exercise was lower in the exercise-induced left atrial hypertension group.
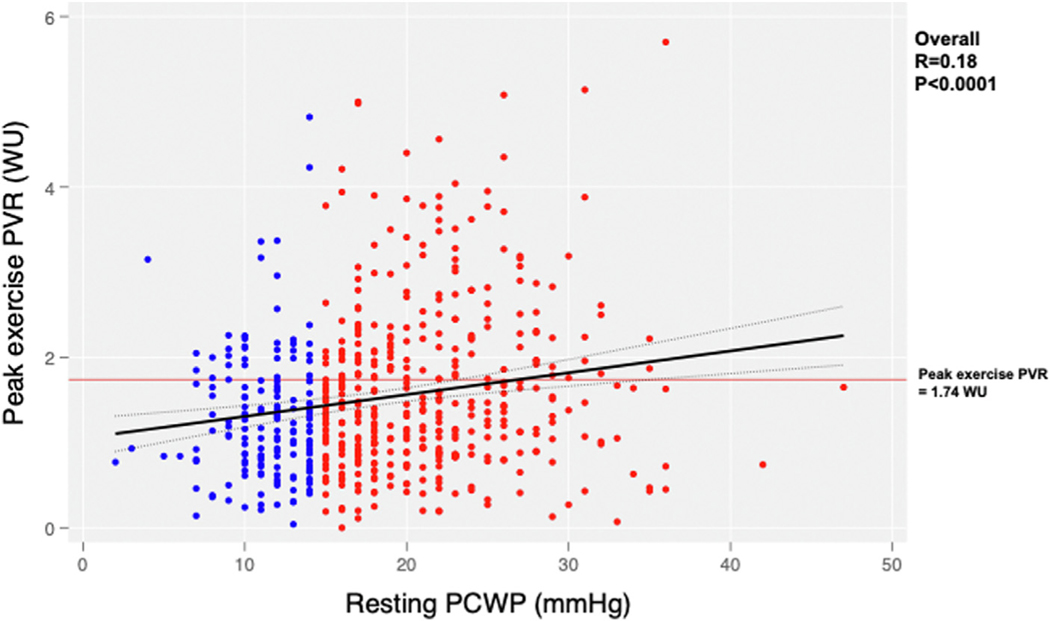
The primary outcome for shunt therapy was not statistically significant in either the EILAH (win ratio: 1.08; P = 0.69) or RELAH groups as a whole (win ratio: 0.98; P = 0.85) (Table 5). Because exercise PVR <1.74 WUs and absence of cardiac rhythm device have previously been identified as potential markers of response to atrial shunt therapy,10 we investigated these parameters. There was a modest but significant association between resting PCWP and peak exercise PVR in patients who have EILAH, with lower peak exercise PVR in patients with EILAH vs in those with RELAH (1.1 vs 1.4 WU; P < 0.001) (Table 3, Figure 2). Patients with EILAH were more likely than those with RELAH to have peak exercise PVR ≤1.74 WUs (60% vs 46%; P = 0.001) (Table 4, Figure 2) and to not have a pacemaker (13.7% vs 20.7%; P = 0.043).
FIGURE 2.
Relationship Between Resting PCWP and Peak Exercise PVR
Patients with resting PCWP <15 mm Hg (exercise-induced left atrial hypertension) are shown in blue and those with resting PCWP ≥15 mm Hg (resting left atrial hypertension) in red. The mean pulmonary vascular resistance (PVR) at peak exercise was lower in the exercise-induced left atrial hypertension group (1.1 vs 1.4 WU). Patients with peak exercise PVR <1.74 WU have previously been identified as a group with beneficial response to atrial shunt therapy. Abbreviation as in Figure 1.
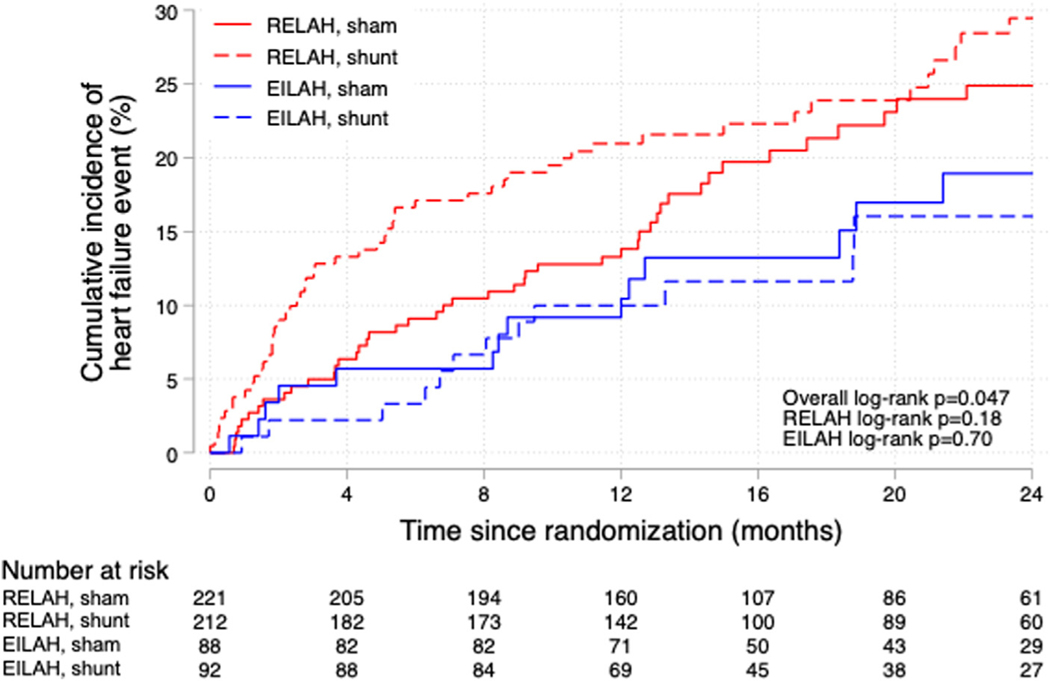
Looking at individual components of the primary endpoint, the HF event rate was lower in the EILAH group than in the RELAH group (overall log rank P = 0.047) (Figure 3). However, there was no statistical difference in the HF event rates with atrial shunt device vs sham procedure in the 2 groups. The smaller size of the EILAH subgroup reduces the power for such comparisons. Both groups had comparable increases in RA and right ventricular volumes after shunt therapy (Supplemental Table 5). Patients in the EILAH group had more improvement in NYHA functional class in response to shunt therapy than those with RELAH (Supplemental Figure 1). Safety events were not different in the 2 groups (Supplemental Table 6).
FIGURE 3.
Kaplan-Meier Curves for HF Events Broken Down by EILAH and RELAH and Sham or Atrial Shunt Therapy
Kaplan-Meier curves for heart failure (HF) events broken down by the classification of resting PCWP <15 mm Hg (exercise-induced left atrial hypertension [EILAH], shown in blue) and resting PCWP ≥15 mm Hg (resting left atrial hypertension [RELAH], shown in red) and randomization to sham or atrial shunt therapy. Patients with EILAH had fewer HF events overall, regardless of therapy. The overall log rank score indicates differences between the 4 groups. However, for the entire EILAH and RELAH groups HF events were not different in the sham or treatment groups. The trend toward lower events in the patients with EILAH who were randomized to treatment is likely related to lower peak pulmonary vascular resistance and fewer subjects with pacemakers in this group. Abbreviations as in Figure 1
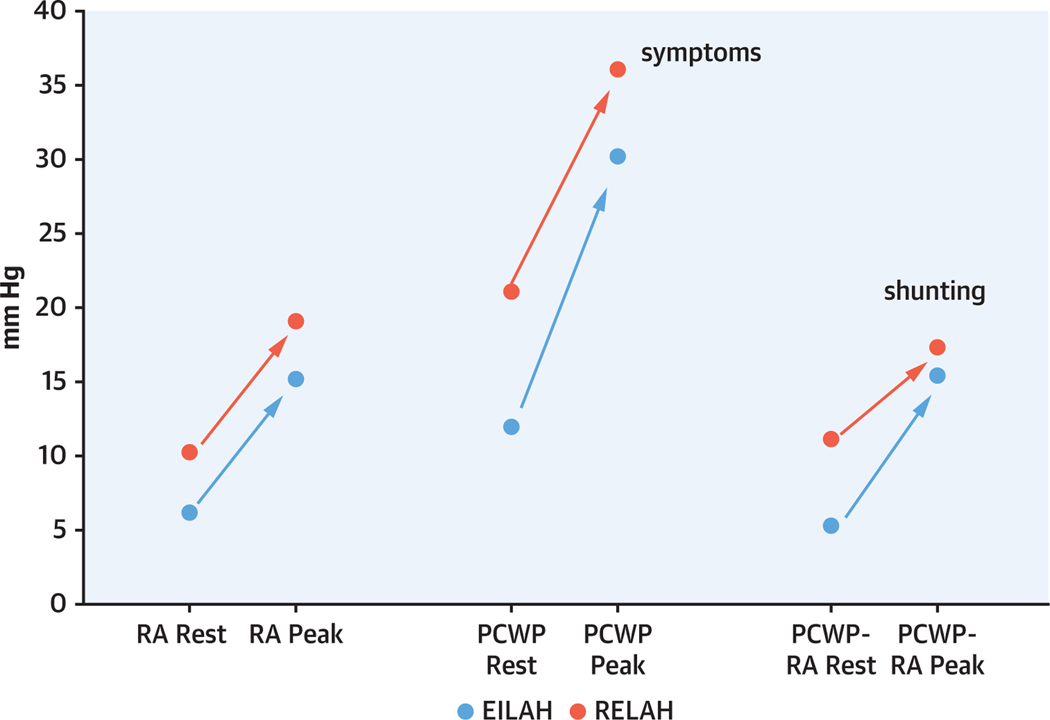
The pressure gradient from LA to RA is the major driver of flow through the atrial shunt. Patients with EILAH had lower RA pressure at rest and the magnitude of increase was comparable to those with RELAH at peak exercise. Although it was lower at rest, PCWP increased more in EILAH than in RELAH at peak exercise (ΔPCWP 19.0 mm Hg vs 15.0 mm Hg, respectively). These changes resulted in a lower PCWP to RA pressure gradient at rest in EILAH, but nearly equal gradients at peak exercise in the 2 groups (Table 4, Figure 4).
FIGURE 4.
Changes in Hemodynamics From Baseline to Peak Exercise in the EILAH and RELAH Groups
Changes in hemodynamics from baseline to peak exercise in the patients with resting PCWP <15 mm Hg (EILAH, shown in blue) and resting PCWP ≥15 mm Hg (RELAH, shown in red). Dots represent median values for each group. Patients with EILAH had lower right atrial (RA) pressure, lower PCWP, and lower PCWP-RA pressure gradient at rest. RA pressure increased comparably at peak exercise in both groups, whereas PCWP increased more in the EILAH groups. This resulted in nearly equivalent PCWP-RA pressure gradient at peak exertion in both groups. The peak PCWP is likely a major driver of symptoms, whereas the PCWP-RA pressure gradient is the main driver of left-to-right shunting. Abbreviations as in Figures 1 and 3.
DISCUSSION
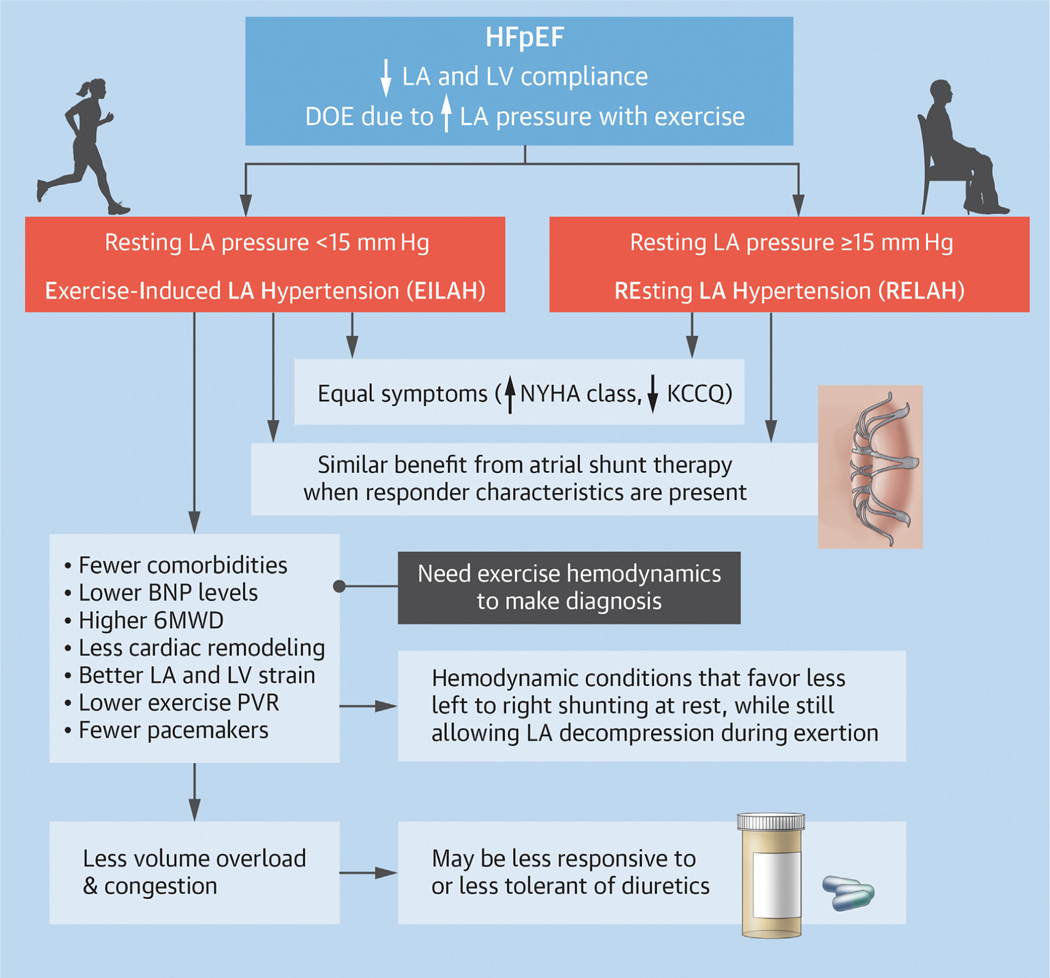
In this large, randomized trial of a novel device therapy in hemodynamically defined HFpEF, almost one-third of subjects had a resting PCWP <15 mm Hg but elevation of PCWP to ≥25 mm Hg with supine exercise. Symptom severity was similar in the groups with normal or elevated resting PCWP (both groups predominantly NYHA functional class III with similar KCCQ clinical summary score). However, those with EILAH had ~50% lower natriuretic peptide levels; fewer comorbidities; and many clinical, echocardiographic, and hemodynamic markers of less advanced or better compensated HF. This constellation of features can make patients with EILAH difficult to identify on routine clinical evaluation and may limit the use of traditional therapies such as diuretic agents and vasodilators. However, these characteristics also highlight the finding that the EILAH group is enriched with the responder phenotype for atrial shunt therapy (Central Illustration).
CENTRAL ILLUSTRATION.
Dyspnea on Exertion Is the Most Common Symptom in HFpEF and Is Due in Part to Elevation of LA Pressure During Exertion
Some patients have normal left atrial (LA) pressure at rest, but have exercise-induced left atrial hypertension (EILAH). Compared to those with resting left atrial hypertension (RELAH), the group with EILAH had fewer comorbidities, lower natriuretic peptide levels, less cardiac remodeling, and better 6-minute walk distance (6MWD). They were more likely to have characteristics associated with favorable response to atrial shunt therapy (lower exercise pulmonary vascular resistance [PVR] and fewer implanted pacemakers). Hemodynamics at rest and with exertion suggest that patients with EILAH may have less left-to-right shunting at rest, but equivalent shunting (LA decompression) during exercise. BNP = B-type natriuretic peptide; DOE = dyspnea on exertion; HFpEF = heart failure with preserved ejection fraction; KCCQ = Kansas City Cardiomyopathy Questionnaire; LV = left ventricle.
Medical therapy of HFpEF has been challenging. Most pharmacologic interventions that target neurohumoral activation either failed to show significant clinical benefit, or the magnitude of benefits have been relatively small.11–15 Recently, sodium-glucose cotransporter 2 inhibitors have been associated with reduced HF hospitalizations16 and modestly improved quality of life metrics in patients with HFpEF.17,18 Nonetheless, additional approaches are needed to further reduce hospitalizations and mortality and to improve symptoms in patients with HFpEF. The relatively large subgroup of patients with HFpEF who do not have overt volume overload or vascular congestion would theoretically be less likely to benefit from neurohormonal antagonism and particularly from diuretic therapy. Patients with isolated EILAH in previous pharmacologic HFpEF trials may have contributed to the apparently low efficacy of the therapies that were evaluated.
Patients with EILAH have less cardiac remodeling, better LV and LA strain, lower right- and left-sided filling pressures at rest and peak exercise, higher exercise cardiac output, and lower exercise PVR; these findings are consistent with another recent study, where roughly one-third of patients with HFpEF were also found to have normal resting PCWP.19 Importantly, patients with EILAH also have a higher prevalence of characteristics previously shown to identify a group with good response to atrial shunt therapy (lower RA and LA volumes, fewer pacemakers, and lower exercise PVR). It is possible that patients with EILAH had more effective diuresis. However, the finding that loop diuretic use was lower in patients with EILAH (72.5% vs 86.0%; P < 0.001) (Table 2) does not support that idea.
The reasons for the equivalently high symptom burden despite other more favorable clinical and resting hemodynamic characteristics are of interest. We hypothesize that HF symptoms may be more apparent or bothersome if they are relatively new, or if they represent a change from a higher level of baseline function. It is possible that patients with EILAH may feel very debilitated compared to their usual level of function, but still have better functional capacity than patients with more advanced HF do. Also, it is very interesting that the patients with EILAH had a larger absolute rise in PCWP from rest to peak exercise compared to those with RELAH (19.0 vs 15.0 mm Hg; P < 0.001) (Table 4). This large change with exertion might be related to their relatively high symptomatology, even though resting hemodynamics are less impaired. In other words, exercise PCWP may be more strongly associated with symptoms of HF than resting PCWP is. Prior studies support the notion that exercise PCWP is related to exercise capacity.20 It is important to recognize patients with EILAH, because they may be viewed by clinicians as exaggerating their symptoms, or they may be erroneously labeled as having noncardiac causes of their symptoms. This may be particularly true if they are intolerant of diuretic therapy.
Our data show that neither group, as a whole, had a significant improvement in the primary endpoint with atrial shunt therapy. However, patients with EILAH were more likely than those with RELAH to have peak exercise PVR ≤1.74 WUs and no pacemaker, which are characteristics previously shown to identify a group with good response to atrial shunt therapy.10 When these characteristics are present, both groups have similarly favorable win ratios for the primary endpoint (Table 5). The EILAH group is enriched with many other characteristics that may be associated with beneficial response to shunt therapy such as less atrial fibrillation and better LV, LA, and RA strain.
Patients with EILAH have a lower PCWP to RA pressure gradient at rest. However, the gradient increases to nearly the same level in patients with EILAH and those with RELAH during exercise. This hemodynamic milieu should favor less left-to-right shunting under resting conditions but allow for similar unloading of the LA during exertion. If this interpretation is correct, patients with EILAH might be at lower risk for long-term adverse effects on the right heart and pulmonary circulation caused by chronic left-to-right shunting, but still derive symptomatic benefit from the shunt during exercise. The changes in PCWP to RA gradient during exercise further highlight the need for some sort of provocative testing to better understand the pathophysiology of the HF symptoms and the response to treatment in specific groups of patients.
STUDY LIMITATIONS.
The relatively smaller size of the subgroup with EILAH (only one-half as many patients as those with RELAH) in the current study reduces the power to detect improvements in outcomes. This likely explains why the win ratio was similar in patients with EILAH and in those with RELAH who had exercise PVR <1.74 WUs and no pacemaker, but this was not statistically significant in the patients with EILAH. It is important to recognize that this classification scheme (EILAH vs RELAH) is not static. Patients may shift between groups over time, depending on medical therapies and other factors. However, this is also true for other clinical phenotypic characteristics that are not fixed such as degree of blood pressure control, diabetes control, presence of atrial fibrillation (or rate control), changes in overweight/obesity, ejection fraction changes, and so on. Despite the potentially fluid nature of the hemodynamics, characterization of HFpEF using the EILAH/RELAH paradigm is potentially useful and would be worth studying in other populations. Indeed, it is possible that more aggressive medical therapy prior to shunt implantation could be of benefit in those with RELAH and might reduce the potential for adverse effects from chronic left-to-right shunting. The use of 15 mm Hg as the definition of elevated resting PCWP is not universally accepted, although it is often used clinically and in research studies.21 The use of a different cut point might have yielded somewhat different results. We did not measure plasma volume in this study. Estimated plasma volume was lower in the EILAH group, which is compatible with our hypothesis that part of the difference between groups is the shift of volume into the central circuit (ie, relatively higher stressed volume). However, estimates of plasma volume only have moderate correlations with directly measured plasma volume.8 Further study will be required to confirm the hypothesis that differences in total and stressed blood volume contribute to the HFpEF phenotype. Lastly, the design of the trial required that patients had additional evidence of HF such as a HF hospitalization, need for intravenous diuretics, or elevated natriuretic peptide levels. These inclusion criteria likely eliminated a significant number of patients with EILAH, so our results may not be reflective of this group as a whole.
STUDY IMPLICATIONS.
Because there is evidence of less advanced myocardial disease and fewer coexisting conditions in the patients with EILAH, they could have more potential for reversibility of the disease processes and hence, larger potential gains in exercise capacity and quality of life. On the other hand, the fact that they are healthier overall could make it more difficult to demonstrate reductions in HF hospitalizations or mortality. Larger trials with longer duration of follow-up will likely be needed to detect changes in these outcomes. Nonetheless, improvements in quality of life or ambulatory activity are increasingly valued as measures of treatment success and these might be particularly suitable for testing in larger, adequately powered trials of patients with EILAH and RELAH. Combining atrial shunts with other newer approaches such as greater splanchnic nerve ablation, which targets stressed blood volume,22 might be a good approach in future studies.
CONCLUSIONS
In a large, rigorously phenotyped cohort of patients with hemodynamically verified HFpEF or HF with mildly reduced ejection fraction, almost one-third had normal LV filling pressures at rest. Exercise evaluation is necessary to unmask the underlying cardiac limitations in these patients. Although they have significant impairment of quality of life, these patients appear to have a less advanced stage of myocardial and pulmonary vascular dysfunction. Importantly, they have a number of characteristics that suggest they may derive benefit from atrial shunt therapy. These findings merit further evaluation in prospective trials.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
HFpEF is associated with elevation of LA pressure during exertion. However, approximately one-third of patients with HFpEF have normal LA pressure at rest. Despite having similar symptom severity to those with elevated LA pressure at rest, these patients have evidence of an earlier or less advanced stage of HF based on lesser cardiac remodeling and dysfunction, better 6-minute walk distance, and lower natriuretic peptide levels. The patients with normal resting LA pressure have several characteristics such as lower RA volume, lower exercise PVR, and less frequent presence of implanted rhythm devices, all of which make them potentially attractive candidates for atrial shunt therapies.
TRANSLATIONAL OUTLOOK:
Therapies for HFpEF are still limited, particularly in patients without overt volume overload and normal ventricular filling pressures at rest. There is great interest in the role of atrial shunt therapies as a means to selectively lower left heart filling pressures during exercise in patients with HF. Although the approach appears to be safe and possibly effective in certain subgroups of patients, it will be extremely important to determine which subsets of patients are the optimal candidates for these therapies.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This study was sponsored by Corvia Medical Inc. Dr Litwin has received research funding from the department of Veterans Affairs, Corvia, AstraZeneca, V-Wave, Axon Therapeutics, and Eli Lilly all paid to the institution; has received consulting fees from CVRx, Axon Therapeutics, Occlutech, Eli Lilly, and Rivus Pharmaceuticals; and has received travel grants, speaker fees, and advisory board honoraria from NovoNordisk and Roche. Dr Komtebedde is employed by Corvia. Dr Burkhoff has consulted for Corvia. Dr Hasenfuß has consulted for AstraZeneca, Boehringer Ingelheim, Corvia, Impulse Dynamics, Novartis, Servier, Vifor; has received honoraria for lectures from AstraZeneca, Bayer, Impulse Dynamics, Novartis, Pfizer, Servier, and Vifor; and is a co-principal investigator to Impulse Dynamics. Dr Borlaug has received research grants from Corvia, AstraZeneca, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and has received consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr Solomon has received research grants from Alnylam, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Cytokinetics, Eidos, GlaxoSmithKline, Ionis, Lilly, MyoKardia, the National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI; and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, DiNAQOR, Tremeau, CellProthera, Moderna, American Regent, Sarepta, Lexicon, AnaCardio, and Akros. Dr Mohan has received research support from Corvia and V-Wave paid to the institution. Dr Kahwash has served as a consultant for Medtronic, Impulse Dynamics, and Cardionomic. Dr Sverdlov has received research grants from the National Heart Foundation of Australia (Future Leader Fellowships 101918 and 106025), Department of Health and Aged Care (Australia): Medical Research Future Fund (MRF2017053), New South Wales Health (Australia), Novartis Australia, Biotronik, RACE Oncology, Bristol Myers Squibb, Roche Diagnostics, and Vifor Pharma; and has received personal fees from Novartis, Bayer, Bristol Myers Squibb, AstraZeneca, Corvia, and Boehringer Ingelheim. Dr Fail has received research support paid to the institution from Corvia and Alleviant. Dr Chung has served as a consultant to Intershunt. Dr Kaye has received research support from Corvia. Dr Hummel has received research grant funding from National Institutes of Health, Veterans Affairs, American Heart Association, Novartis, Pfizer, AstraZeneca, Corvia, and Axon Therapies. Dr Zirlik has received personal consulting fees and honoraria for lectures from Abbott, Abiomed, AstraZeneca, Amarin, Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimensions, Cardiorentis, Corvia, Daichi Sankyo, Edwards Lifesciences, Eli Lilly, Janssen, Merck, Neucomed, Novo Nordisk, Novartis, Rigel, and Stealth Peptides. Dr Hayward has received research support from Corvia, Medtronic, Abbott, Roche, and Procyrion. Dr Lewis has received research funding from the National Institutes of Health (R01-HL 151841, R01-HL131029, R01-HL159514), American Heart Association (15GPSGC-24800006), Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, and SoniVie; has received honoraria for advisory boards outside of the current study from Pfizer, Merck, Boehringer Ingelheim, NXT, American Regent, Cyclerion, Cytokinetics, and Amgen; and has received royalties from UpToDate for scientific content authorship related to exercise physiology. Dr Gupta has received research support from the National Institutes of Health, Imara, Corvia, and Astellas Pharma. Dr Cikes has received institutional research grants from Abbott, Novartis, and Pfizer; has received travel grants, speaker fees, and advisory board honoraria from Abbott, Abiomed, Amicus, AstraZeneca, Bayer, Boehringer Ingelheim, GE Healthcare, Krka Pharma, LivaNova, Medtronic, Novartis, Orion Corporation, Pfizer, Sanofi, Swixx BioPharma, and Teva Pharmaceutical Industries, all outside of the present study; and has received research support from Corvia. Dr Gustafsson has received honoraria outside the present study as a consultant for Abbott, Pfizer, Ionis Pharmaceuticals, Bayer, AstraZeneca, and Alnylam; has received speaker fees from Novartis and Orion Pharma; and has received research support from Corvia. Dr Silvestry has received research support from Corvia. Dr Rowin has received research support from Corvia; and has served as a consultant for Cardiovascular Clinical Sciences. Dr Cutlip has received research support from Corvia paid to the institution. Dr Kitzman has received honoraria outside the present study as a consultant for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Rivus, Keyto, and Novartis; has received grant funding outside the present study from Novartis, Bayer, Novo Nordisk, and AstraZeneca; owns stock in Gilead Sciences; and has received research support from Corvia. Dr Shah has received research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received personal fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, Intellia Therapeutics, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BNP
B-type natriuretic peptide
- EILAH
exercise-induced left atrial hypertension
- HFpEF
heart failure with preserved ejection fraction
- LA
left atrial
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- PCWP
pulmonary capillary wedge pressure
- PVR
pulmonary vascular resistance
- RA
right atrial
- RELAH
resting left atrial hypertension
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the AuthorCenter.
REFERENCES
- 1.Pandey A, Khera R, Park B, et al. Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction: a study-level pooled analysis. J Am Coll Cardiol HF. 2018;6(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry N, Mauri L, Feldman T, et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the pivotal randomized trial to REDUCE Elevated Left Atrial Pressure in Patients with Heart Failure II (REDUCE LAP-HF II). Am Heart J. 2020;226:222–231. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JM, Borlaug BA, Komtebedde J, et al. Impact of interatrial shunts on invasive hemodynamics and exercise tolerance in patients with heart failure. J Am Heart Assoc. 2020;9(17):e016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman T, Mauri L, Kahwash R, et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364–375. [DOI] [PubMed] [Google Scholar]
- 6.Kaye DM, Hasenfuss G, Neuzil P, et al. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(12):e003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SJ, Borlaug BA, Chung ES, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399(10330):1130–1140. [DOI] [PubMed] [Google Scholar]
- 8.Ling HZ, Flint J, Damgaard M, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17(1):35–43. [DOI] [PubMed] [Google Scholar]
- 9.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33(2):176–182. [DOI] [PubMed] [Google Scholar]
- 10.Borlaug BA, Blair J, Bergmann MW, et al. Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation. 2022;145(21):1592–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17(9):559–573. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet. 2003;362(9386):777–781. [DOI] [PubMed] [Google Scholar]
- 14.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. [DOI] [PubMed] [Google Scholar]
- 16.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. [DOI] [PubMed] [Google Scholar]
- 17.Butler J, Filippatos G, Siddiqi TJ, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-Preserved trial. Circulation. 2022;145(3):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omote K, Verbrugge FH, Sorimachi H, et al. Central hemodynamic abnormalities and outcome in patients with unexplained dyspnea. Eur J Heart Fail. 2023;25(2):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolsk E, Kaye D, Borlaug BA, et al. Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2018;20(4):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered hemodynamics and endorgan damage in heart failure: impact on the lung and kidney. Circulation. 2020;142(10):998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fudim M, Fail PS, Litwin SE, et al. Endovascular ablation of the right greater splanchnic nerve in heart failure with preserved ejection fraction: early results of the REBALANCE-HF trial roll-in cohort. Eur J Heart Fail. 2022;24(8):1410–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.