ABSTRACT
The eukaryotic translation initiation factor eIF4E acts as a multifunctional factor that simultaneously influences mRNA processing, export, and translation in many organisms. Its multifactorial effects are derived from its capacity to bind to the methyl-7-guanosine cap on the 5’end of mRNAs and thus can act as a cap chaperone for transcripts in the nucleus and cytoplasm. In this review, we describe the multifactorial roles of eIF4E in major mRNA-processing events including capping, splicing, cleavage and polyadenylation, nuclear export and translation. We discuss the evidence that eIF4E acts at two levels to generate widescale changes to processing, export and ultimately the protein produced. First, eIF4E alters the production of components of the mRNA processing machinery, supporting a widescale reprogramming of multiple mRNA processing events. In this way, eIF4E can modulate mRNA processing without physically interacting with target transcripts. Second, eIF4E also physically interacts with both capped mRNAs and components of the RNA processing or translation machineries. Further, specific mRNAs are sensitive to eIF4E only in particular mRNA processing events. This selectivity is governed by the presence of cis-acting elements within mRNAs known as USER codes that recruit relevant co-factors engaging the appropriate machinery. In all, we describe the molecular bases for eIF4E’s multifactorial function and relevant regulatory pathways, discuss the basis for selectivity, present a compendium of ~80 eIF4E-interacting factors which play roles in these activities and provide an overview of the relevance of its functions to its oncogenic potential. Finally, we summarize early-stage clinical studies targeting eIF4E in cancer.
KEYWORDS: Cap binding protein, capping, eIF4E, gene expression, m7G cap, mRNA export, mRNA maturation, mRNA processing, splicing, translation
Overview
The processing and translation of mRNAs are central to the functionality of the ultimate proteome, and thus are foundational underpinnings of subsequent cell physiology. Here, we review the multiple roles of the eukaryotic translation initiation factor 4E (eIF4E) in mRNA processing and translation, allowing it to adapt the proteome in response to cellular contexts. eIF4E directly binds to the methyl-7-guanosine (m7G) “cap”, a moiety found at the 5’end of most mRNAs, pre-mRNAs and some non-coding RNAs. The m7G cap is required for subsequent mRNA maturation, translation and for the biochemical activity of many non-coding RNAs. In this way, the cap provides a physical anchor by which the fate of the RNAs can be controlled. The cap is bound by cap-binding proteins which can aid in the recruitment of the appropriate maturation factors e.g. RNA processing, export and translation. The two major cap-binding proteins in mammalian cells are eIF4E and the cap-binding protein NCBP2 (also known as CBP20). Thus, these proteins are positioned to chaperone transcripts and modulate RNA fate.
This review focuses on eIF4E, the firscap-binding protein which was identified in the 1970s [1,2]. This protein is conserved in eukaryotes including fungi, plants and humans [1,3,4]. eIF4E is found in both the cytoplasm and nucleus, positioning it to act in translation and potentially RNA stability in the cytoplasm, and in mRNA processing and export in the nucleus. In the cytoplasm, eIF4E is found diffusely throughout, localized at the ER where bulk translation occurs [5,6], and in various granules including stress granules [7] and P-Bodies [8]. In the nucleus, eIF4E is also found in a variety of locals which are described below.
Here, we first describe our functional understanding of eIF4E unified by its overarching role as a cap chaperone which in turn is impacted by its subcellular localization and partner proteins. Given the importance of these partner proteins for understanding the diversity of eIF4E function and subsequent RNA fate, we assembled a compendium of nearly 80 proteins that associate, directly or indirectly with eIF4E, and play roles in modulating its activities (Table 1) [9–14]. We also discuss evidence that eIF4E influences RNA metabolism using at least a two-pronged mechanism. First, eIF4E can indirectly influence the fate of some mRNAs by modulating the levels of specific components of the mRNA processing machinery thereby re-writing the processing landscape. Second, eIF4E physically interacts with many capped mRNAs in the nucleus and cytoplasm to influence their processing and/or translation. Indeed, despite the universality of the cap, eIF4E elicits effects on subsets of mRNAs rather than all capped transcripts and we discuss here some of the relevant determinants for eIF4E sensitivity in the context of the RNA regulon model [15,16]. Using the RNA regulon parlance, these RNA elements are referred to as Untranslated Sequence Element for Regulation (USER) codes. These USER codes confer specificity usually be recruiting protein co-factors that serve as adaptors between eIF4E, the RNA containing the USER codes, the cap and the appropriate maturation machinery [17–27]. Finally, we discuss evidence that eIF4E’s functions in mRNA export, capping, and splicing as well as translation, are dysregulated in human cancers and contribute to oncogenesis in cell models. This will include a discussion of clinical studies targeting eIF4E which led to remissions and other objective clinical responses in some patients [28–33].
Table 1.
Summary of eIF4E partner proteins.
| Interactor | Localization | Techniques | Cell Line -Tissue | Year of publication | PMID | |
|---|---|---|---|---|---|---|
| 1 | ACVRL1/HHT | In vitro assay | Reconstituted Complex - Affinity Capture-Western | THP1 | 2015 | 25915158 |
| 2 | ASLX1 | Total Extract | AP-MS | HEK293T | 2022 | 36180891 |
| 3 | ASLX2 | Total Extract | AP-MS | HEK293T | 2022 | 36180891 |
| 4 | ASLX3 | Total Extract | AP-MS | HEK293T | 2022 | 36180891 |
| 5 | BCD | In vitro assay | Pull-Down (m7GTP-agarose) - Pull-Down | Drosophilia embryos | 2002 | 12368268 |
| 6 | BIRC2/cIAP1 | Total Extract | Coimmunoprecipitation - Pull Down | HEK293T | 2017 | 28852129 |
| 7 | CEFL1 | Total Extract | BioID | HEK293 | 2018 | 29395067 |
| 8 | CPEB | Total Extract | Pull-Down | oocyte extract | 1999 | 10635326 |
| 9 | CPSF1 | Nucleus | Coimmunoprecipitation | U2OS | 2019 | 31042468 |
| 10 | CPSF3 | In vitro assay | Coimmunoprecipitation | U2OS | 2019 | 31042468 |
| 11 | CRM1/XPO1 | Nucleus - In vitro assay | Coimmunoprecipitation - size exclusion chormatography | U2OS | 2009 - 2017 | 19262567 - 28325843 |
| 12 | CTNNB1/ß-catenin | Total Extract | Pull-Down (m7GTP-agarose) | A10 - HEK293T | 2018 | 30361391 |
| 13 | CYFIP1 | Total Extract | Pull-Down (m7GTP-agarose) - Coimmunoprecipitation | Total mouse brain - primary neurons | 2008 | 18805096 |
| 14 | DCC | Cytoplasm | Co-fractionation - Coimmunoprecipitation | Commissural neuron | 2010 | 33930296 |
| 15 | DDX3 | Nucleus | Coimmunoprecipitation | U2OS | 2009 | 19262567 |
| 16 | eIF3A | Total Extract | Coimmunoprecipitation | MCF7 | 2019 | 30573685 |
| 17 | eIF3B | Total Extract | Coimmunoprecipitation | 293T - MCF7 - HEK293E | 2006 - 2008 - 2019 | 16541103 - 18423201 - 30573685 |
| 18 | eIF3F | Total Extract | Coimmunoprecipitation | MCF7 | 2019 | 30573685 |
| 19 | eIF4A1 | Total Extract | Pull Down - Coimmunoprecipitation | MCF10A - CRL2324 - HTB20 - KT1 | 2006 - 2012 | 16648488 |
| 20 | eIF4A2 | Total Extract | BioID | HEK293 | 2018 | 29395067 |
| 21 | eIF4EBP1 | Total Extract | Coimmunoprecipitation - Pull Down- crystallography- NMR | Sf9 | 1994- 1995 | 7935836 - 7651417 |
| 22 | eIF4EBP2 | Total Extract | Pull Down - Crystallography | HEK293 - HEK293E | 1994 - 2010 | 7935836 - 20347422 |
| 23 | eIF4EBP3 | Total Extract | Coimmunoprecipitation - Pull-Down | HeLa | 1998 | 9593750 |
| 24 | eIF4EBP3 | Cytoplasm | Coimmunoprecipitation | T cells - HEK293 - HeLa | 2002 | 12482586 |
| 25 | eIF4ENIF1/4E-T | Total Extract | Coimmunoprecipitation - Far western | HeLa | 2000 | 10856257 |
| 26 | eIF4G | Total Extract - Cytoplasm - In vitro assay | Purification of eIF4F complex - Pull Down - Coimmunoprecipitation - NMR - Crystallography | Rabbit reticulocyte - Sf9 - U2OS | 1983 - 1995 - 2009 -2016 | 6853548 - 8521827 - 19262567 - 27773676 |
| 27 | eIF4G2 | Total Extract | Coimmunoprecipitation | BY4741 | 2023 | 37449412 |
| 28 | eIF4G3 | Total Extract | Coimmunoprecipitation - Far western | HeLa | 1998 | 9418880 |
| 29 | ELAVL1/HuR | Nucleus | Coimmunoprecipitation | U2OS | 2023 | 36843541 |
| 30 | Emx2 | Cytoplasm | Coimmunoprecipitation | COS-7 | 2004 | 15247416 |
| 31 | Engrailed 2 | Cytoplasm | Coimmunoprecipitation | COS-7 | 2004 | 15247416 |
| 32 | Fi(2)d | Nucleus | Coimmunoprecipitation | Drosophila embryo | 2011 | 21829374 |
| 33 | FMR1 | Total Extract | Pull-Down (m7GTP-agarose) | A10 - HEK293T | 2018 | 30361391 |
| 34 | G3BP1 | Total Extract | BioID | HEK293 | 2018 | 29395067 |
| 35 | GEMIN5 | In vitro assay | Pull-Down | - | 2006 | 16739988 |
| 36 | hnRNPA1 | Nucleus | Coimmunoprecipitation | U2OS | 2009 | 19262567 |
| 37 | HOX11 | In vitro assay | pulldown - NMR | - | 2003 | 12554669 |
| 38 | HOXA9 | Cytoplasm - Nucleus - In vitro assay | Pull-Down - Coimmunoprecipitation-Circular Dichroism | U937 - leukemia patients | 2005 | 15657436 |
| 39 | HSPB2 | Total Extract | Coimmunoprecipitation | LNCaP | 2010 | 20101233 |
| 40 | Importin 8 | In vitro assay | Pull-Down - Coimmunoprecipitation - NMR | 2016 | 27114554 | |
| 41 | LRPPRC | Nucleus - In vitro assay | Coimmunoprecipitation - Colocalization - Pulldownm - NMR - Size exclusion chromatography | U2OS | 2009 - 2017 | 19262567 - 28325843 |
| 42 | Maskin | Total Extract | Pull-Down | oocyte extract | 1999 | 10635326 |
| 43 | METTL3 | Total Extract - Cytoplasm | Coimmunoprecipitation | HeLa - H1299 | 2018 | 30232453 |
| 44 | MNKNK1 | Total Extract | Coimmunoprecipitation | 293T | 1999 | 9878069 |
| 45 | mTOR | Total Extract | Coimmunoprecipitation | HEK293E | 2008 | 18423201 |
| 46 | NCBP1/CBP80 | Total Extract | Coimmunoprecipitation | HeLa | 2013 | 23665581 |
| 47 | OTUD6B | Total Extract | Coimmunoprecipitation | H1299 | 2017 | 27864334 |
| 48 | Otx2 | Cytoplasm | Coimmunoprecipitation | COS-7 | 2004 | 15247416 |
| 49 | PABPC1 | Total Extract | Coimmunoprecipitation - BioID | HeLa - HEK293 | 2013 - 2018 | 29395067 |
| 50 | PATL1 | Total Extract | BioID | HEK293 | 2018 | 29395067 |
| 51 | PHAS-I | Total Extract | Far western | - | 1994 | 7935836 |
| 52 | PML | Total Extract - In vitro assay - Nucleus | Co-localization - Pull-Down - NMR - Circular Dichroism - Reconstituted Complex - CoImmunoprecipitation | NIH3T3 - human 551 fibroblasts - U937 - K562 - HEK293T | 2000 - 2001 - 2002 | 10763819 - 11500381 - 12167712 |
| 53 | PRH/HHEX | Total Extract -Cytoplasm - Nucleus | Coimmunoprecipitation - Co-localization - PullDown- Ciruclar Dichroism | leukemia patients - U937 | 2003 - 2005 | 14645512 |
| 54 | PRPF19 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 55 | PRPF31 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 56 | PRPF6 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 57 | PRPF8 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 58 | RNF113A | Total Extract | Peptide Pull-Down | SCLC | 2022 | 35819319 |
| 59 | RNMT | Nucleus - In vitro assay | Coimmunoprecipitation - Pull-Down - NMR | U2OS | 2022 | 35026230 |
| 60 | RPS6KB1 | Total Extract | Coimmunoprecipitation | HEK293E | 2008 | 18423201 |
| 61 | SF3B1 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 62 | Snf | Nucleus | Coimmunoprecipitation | Drosophila embryo | 2011 | 21829374 |
| 63 | SNRNP200 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 64 | SRSF3 | Cytoplasm | Coimmunoprecipitation | HEK293T | 2016 | 27381497 |
| 65 | STUB1/CHIP | Total Extract | Coimmunoprecipitation - Pull-Down | HEK293T | 2006 - 2017 | 16720573 - 28852129 |
| 66 | Sxl1 | Nucleus | Coimmunoprecipitation | Drosophila embryo | 2011 | 21829374 |
| 67 | TRIM22 | Total Extract | Coimmunoprecipitation | 293T -17 | 2012 | 22509910 |
| 68 | U1-70K/SnRNP70 | Nucleus | Coimmunoprecipitation | Drosophila embryo | 2011 | 21829374 |
| 69 | U2AF1/U2AF35/U2AF38 | Nucleus | Coimmunoprecipitation | U2OS - Drosophila embryo | 2023 - 2011 | 36843541 - 21829374 |
| 70 | U2AF2 | Nucleus | Coimmunoprecipitation | U2OS - NOMO | 2023 | 36843541 |
| 71 | U2AF50/Mud2 | Nucleus | Coimmunoprecipitation | Drosophila embryo | 2011 | 21829374 |
| 72 | UAP56 | Nucleus | Coimmunoprecipitation | U2OS | 2009 | 19262567 |
| 73 | UPF1 | Total Extract | Coimmunoprecipitation | HeLa | 2013 | 23665581 |
| 74 | VPg | In vitro assay | NMR - ITC - Fluorescence assay- XLMS | U2OS | 2019 | 31712417 |
| 75 | YTHDF2 | Total Extract | BioID | HEK293 | 2018 | 29395067 |
| 76 | Z protein (Lassa and LCMV) | In vitro assay | Pull-Down - NMR - Circular Dichroism | - | 2001 - 2010 | 11575918 - 20212144 |
| 77 | ZFP36 | Total Extract | BioID | HEK293 | 2018 | 29395067 |
| 78 | ZRANB1 | Total Extract | Coimmunoprecipitation | HepG2 | 2022 | 35944360 |
This list is not exhaustive and thus the absence of factors here should not be interpreted as factors not binding. In many cases, there are multiple references for interactions, in these cases the earliest relevant references were used and, in some cases, multiple references supporting the original finding are also included.
Introduction to the eIF4E cap-binding protein
Protein-coding mRNAs are in the minority in cells constituting 1–5% of total RNAs, with the remainder comprised of non-coding RNAs (ncRNAs), including ribosomal rRNAs [34]. To fish out mRNAs from this ncRNA sea, eIF4E recognizes the cap structure, which is not found on tRNAs or rRNAs [35–39]. Briefly, the cap is linked to the 5’ end of eukaryotic mRNAs via a 5’-5’ pyrophosphate linkage. The cap is important for many cellular functions, including mRNA stability, as it confers resistance to 5’exoribonuclease activity [40–42]. Also, the binding of eIF4E to the cap reduces the degradation of mRNAs by competing with mRNA decay enzymes [43–46].
The three-dimensional structures of both cap-free and cap-bound eIF4E have been determined using NMR, and X-ray crystallography, with structures highly conserved across organisms [47–50]. Human eIF4E is composed of a six-stranded ß-sheet and three α-helices (Figure 2) [47,49,50]. The α-helices form the dorsal surface of eIF4E, which interacts with multiple-binding proteins involved in the recruitment of eIF4E for translation, as well as other functions (Table 1, Figure 2). The ß-sheet forms a concave surface distal to the dorsal surface, forming a cap-binding site which more recently was identified as also a site for specific protein partners[27,51,52,53]. The methyl-7-guanosine moiety of the cap intercalates between two aromatic residues, usually tryptophans (Figure 2). The phosphate groups of the cap associate with an adjacent positive surface and these phosphates increase the affinity of eIF4E for the cap by ~ 4-fold [55–57]. The dissociation constant for eIF4E and the phosphorylated cap analogues such as m7GTP or m7GpppG range from ~1–500 nM depending on the buffer conditions used, with ~500 nM binding observed in phosphate buffers and pH7.2 while tighter binding is observed at pH 8 in HEPES buffer [50,58–61]. A comparison of apo-eIF4E and m7G cap-bound human eIF4E revealed only minor alterations in the overall conformation, with changes mainly localized to the cap-binding site [50]. Interestingly, there are widescale changes to overall electrostatic charge of eIF4E in the apo and cap-bound forms whereby the cap-free eIF4E has a highly basic surface in the phosphate-binding portion of the cap-binding site while cap-binding causes a closing of the cap-binding site and reduction in the basic character due to interactions with the phosphate moiety [50]. Indeed, some proteins that bind to the cap-binding region of eIF4E exploit this charge, specifically binding cap-free eIF4E [51,53]. Binding of eIF4G to the dorsal surface leads to ~2-fold increased cap-affinity of eIF4E (Figure 2) [52,53,63,64]. This occurs through allosteric changes directed from the dorsal surface to the cap-binding site, as observed by NMR [50,65]. eIF4E can also bind other types of RNA cap structures e.g. 2,2,7-trimethyl guanosine (TMG), as shown by the crystal structures of human and nematode eIF4E with TMG [59]. This modification is found in UsnRNAs, HIV-1 RNAs, and some mRNAs particularly in nematodes [59,66–70] although the affinity of mouse eIF4E for TMG is only about 3 µM [59,71]. Given the abundance of UsnRNAs, even with this weak affinity, eIF4E may bind TMG caps in cells, this remains to be directly tested.
Figure 2.
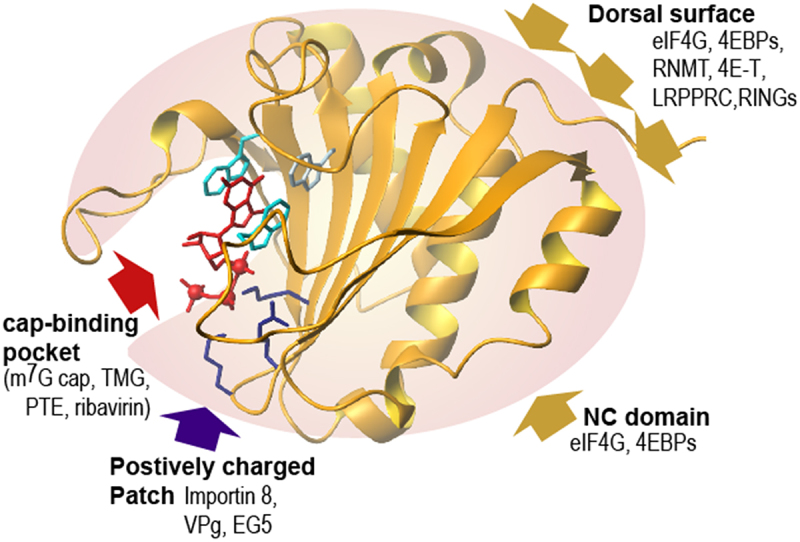
The human eIF4E structure binding the m7G cap (in red) and known interactions: eIF4E structure and relative position of regions utilized by known partner proteins. The red arrow indicates the cap-binding site with the associated tryptophans in light blue, the purple arrow indicates the positively charge patch, where Importin 8, VPg and EG5 bind with the 2 lysines and the arginine shown. The dorsal surface is also shown and as is the surface used by the NC (non- canonical domain) of eIF4G and 4EBPs with gold yellow arrows (Adapted from [52], PDB3AM7).
eIF4E in mRNA translation
It has been known for many years that eIF4E recruits mRNAs to the ribosome through its cap-binding activity although not all mRNAs are sensitive to modulation of eIF4E [14,17,20,26,52,72]. eIF4E is a component of eIF4F, a protein complex including eIF4G and eIF4A/B (see Table 1) [73,74]. eIF4E simultaneously binds the capped mRNAs with its cap-binding site, and eIF4G, using its dorsal surface (Figure 2) [56,75–77]. The helicase eIF4A is recruited to this complex via eIF4G, inducing unwinding of the 5’UTR and recruitment of eIF3 bound to the small subunit of the ribosome resulting in the formation of the 43S pre-initiation complex; for further details regarding translation initiation consult the following reviews [61,74,78].
Notably, eIF4E influences translation efficiency, defined as the number of polysomes per transcript, for ~30–50% of transcripts [79,80]. Translational sensitivity to eIF4E appears to be characteristic of a given mRNA and its compliment of USER codes. For example, mRNAs with complex 5’UTRs are more sensitive to eIF4E than other mRNAs. A broader discussion about translation USER codes is provided in section 7 (Roots of RNA selectivity and in eIF4E-dependent processes). Importantly, there is substantial evidence for alternative forms of cap-dependent translation that do not rely on eIF4E, where other cap-binding factors such as eIF3d recruit mRNAs to other scaffold proteins, such as DAP5/eIF4G2 [52,81,82]. Aside from eIF4E (also known as eIF4E–1), two other eIF4E family members, eIF4E–2 (also known as 4EHP) and eIF4E–3, are cap-binding proteins that have been implicated as positive or negative regulators of translation for specific mRNAs under different stress conditions [82–85,87–90].
eIF4E goes nuclear
The relevance of nuclear eIF4E was initially overlooked. This is in part due to the prevailing idea that cap-binding proteins were functionally compartmentalized based on work in the 1970s through to the 1990s. In this model, eIF4E was considered the cytoplasmic cap-binding protein and NCBP2 with its accessory protein NCBP1 forming the heterodimer CBC, the nuclear cap-binding protein. Indeed, CBC is mainly limited at steady-state to the nucleus and plays well-established roles in transcriptional elongation and RNA maturation, U snRNA export, splicing and formation of mRNA export complexes with the bulk export NXF1/NXT1 receptor [91–95]. However, studies revealed that CBC was also involved in the pioneer round of translation in the cytoplasm [96,97]. Thus, the nuclear border no longer appears to provide an absolute demarcation of function for the two major cap-binding proteins.
eIF4E was first reported to have a nuclear localization in the early 1990s. Indeed, eIF4E is found in the nucleus in a variety of organisms including human, yeast, and Drosophila [98–103]. Interestingly in some cell lines and human tissue specimens, eIF4E at steady state is predominantly nuclear with reports of up to 70% of eIF4E being found in the nucleus, as observed using confocal microscopy (Figure 1) [98,99,101,102,104–107]. In the nucleus, eIF4E is found in different populations i.e. diffusely localized as well as concentrated in different nuclear structures while excluded from nucleoli. These nuclear structures may in some instances co-localize with nuclear speckles [99], some structures colocalize with mRNA export targets of eIF4E and a subset of eIF4E colocalize with PML nuclear bodies but in an RNA-free form [51,98-101,106-109]. Importantly, eIF4E physically associates with capped mRNAs in the nuclear as well as cytoplasmic compartments indicative of functionality in RNA biology in both locations [18,19,25,109,111,112]. Notably, to date nearly 30 proteins have been discovered to physically interact with eIF4E in the nucleus, these have provided important clues into its nuclear activities (Table 1). It is important to note that eIF4E overexpression or its genetic reduction only rarely alters steady-state levels of transcripts suggesting that eIF4E is not modulating steady-state transcription or stability [54,100,113,114]. Below, we review the major nuclear activities of eIF4E in the general order of mRNA processing rather than in the order of their discovery.
Figure 1.
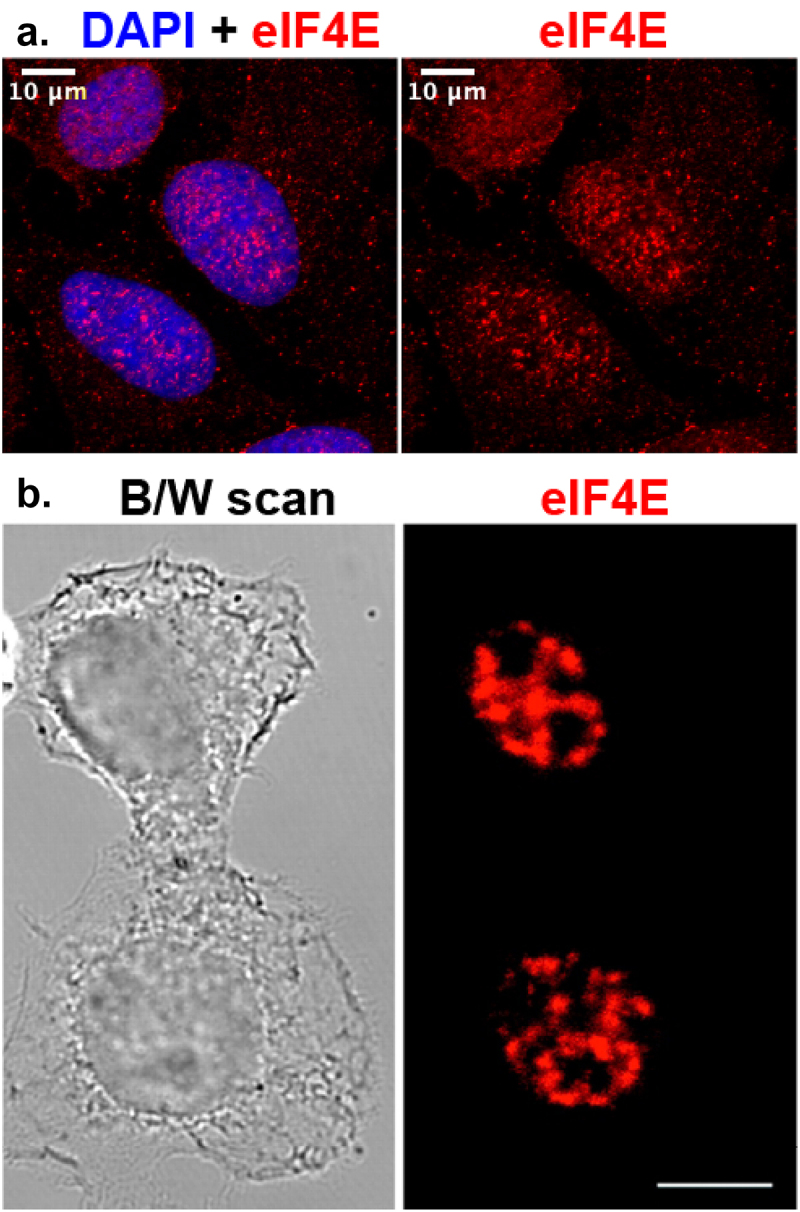
A, Single section of confocal imaging of U2OS cells stained for eIF4E (sc-271480 anti-eIF4E antibody, red) and DAPI (blue, nuclear dye) showing nuclear and cytoplasmic localization. Left panel shows the overlap between DAPI and eIF4E staining. The right panel shows eIF4E signal alone. B, Confocal imaging of eIF4E in the nucleus of HeLa cells with the antibody 10C6 from [101]. Reprint with permission from Dostie J, Lejbkowicz F, Sonenberg N. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles [99]. White bar = 10μm.
eIF4E & m7G capping
Initially, capping on the 5’ end of mRNAs was widely considered to be constitutive. However, it has become evident that steady-state capping is a highly regulated process that ultimately affects the translation capacity of coding mRNAs and the biochemical activities of non-coding RNAs [114–119]. The extent of capping for a given mRNA can be altered depending on the developmental or differentiation stage in plants and animals and could be influenced by the expression of oncogenes such as Myc and eIF4E [114,116,117,120].
Capping is a three-step process that involves the RNA guanylyltransferase and 5’ phosphatase (RNGTT), methyltransferase (RNA guanine-7- methyltransferase), and the accessory RAM proteins [121]. RNGTT and RNMT are required for capping and ultimately cell survival [122–128]. RNGTT removes the 5’ phosphate of the 5’ triphosphate on the pre-mRNA or non-coding RNA using its 5’ phosphatase activity, which produces a 5’-diphosphate-RNA and serves as a substrate for the addition of guanosine via a distinct 5’-5’ pyrophosphate link [129]. The resulting cap guanylate is then methylated by RNMT, which uses S-adenosyl methionine (SAM) as the methyl donor [130–132]. RAM binds to RNMT and increases its methylation activity [132,133]. Although the localization of RNMT and RNGTT is mainly nuclear, these factors have been observed in the cytoplasm and linked to the re-capping of mRNAs after de-capping [118,133–135].
Several methods to measure capping efficiency of RNAs have been developed and include use of m7G cap antibody IPs and various enzymatic methods [35]. Consistent with previous studies [116,117,136,137], new methods revealed that steady-state capping of specific mRNAs in human cells was lower than anticipated (~30–50% for most RNAs examined) suggesting that capping efficiency and thus mRNA activity is regulated [114]. Consistent with its capacity to increase the levels of the capping enzymes, eIF4E overexpression increased the capping efficiency of specific subsets of mRNAs and long non-coding mRNAs to some cases over 90%; while others were either unchanged or reduced [114]. eIF4E overexpression increased capping of transcripts encoding factors implicated in cellular proliferation and transformation, such as Myc, CycD1, Mdm2, CTNNB, and ABALON. Consistent with its “landscape effect”, eIF4E overexpression increased, while its knockdown decreased, the production of the capping machinery (Table 2). RNGTT, RNMT, and RAM transcripts are nuclear export targets, while RNGTT and RNMT transcripts are also translational targets of eIF4E [114]. eIF4E was also found to directly interact with the RNMT enzyme suggesting another means by which eIF4E could impact capping efficiency [62]. It was observed that not all mRNAs found in nuclear eIF4E-RIPs had increased capping upon eIF4E overexpression, and not all capping targets interact with nuclear eIF4E. This suggests that the increased capping efficiency for mRNAs not bound to eIF4E is a result of eIF4E-induction of RNMT, RNGTT, and RAM proteins. A USER code was identified for capping, cap sensitivity element (CapSE) which conferred increased capping efficiency on a LacZ-CapSE reporter in eIF4E-overexpressing cells. In this case, it is possible that via increased production of capping enzymes and also through its interaction with RNMT (possibly in coordination with other factors), eIF4E enhances recruitment of capping enzymes to mRNAs containing the CapSE element, explaining largely overlapping but distinct lists of mRNAs for which capping is modulated upon RNMT or eIF4E overexpression [35,114,136,138]. Indeed, RNMT overexpression increased the capping efficiency of specific mRNAs and induced the oncogenic transformation of mammary epithelial cells [136]. Myc, an eIF4E target [72,139,140], increased capping for specific groups of mRNAs by increasing phosphorylation of RNA polymerase II, which promotes the recruitment of capping machinery to specific genes [130,137,141], and upregulates the expression of S-adenosyl homocysteine hydrolase (SAHH), which is necessary for neutralization of the inhibitory SAH by-product of methylation reactions [142]. Finally, direct interactions of eIF4E with the methyltransferase domain of RNMT were observed using NMR and biochemical methods [62] (Table 1, Figures 2, 3). RNMT binds to the dorsal surface of eIF4E which leads to a direct competition between RNMT and other dorsal surface associated factors such as LRPPRC (related to eIF4E-dependent RNA export, see below), 4EBP1, and eIF4G. This supports a model whereby RNMT-eIF4E complexes are biochemically distinct from eIF4E’s translation and RNA export complexes (Figure 2). Moreover, eIF4E and RAM compete for overlapping binding sites on RNMT. Consistently, eIF4E did not immunoprecipitate with RAM in cells. Further studies are needed to establish the effect of eIF4E on RNMT-capping activity. In all, eIF4E is positioned to have multiple impacts on capping, a greater understanding of which will benefit from further mechanistic dissection.
Table 2.
Summary of RNA processing factors that are regulated by eIF4E.
| Sensitive Factor | eIF4E elevation | eIF4E reduction | Cell/tissue | Mode of eIF4E mediated regulation | Process | Doi |
|---|---|---|---|---|---|---|
| RPL13 | elevation | reduction | NIH3T3, MEFs | - | Translation | 10.1371/journal.pone.0000242 |
| RPL23 | elevation | reduction | NIH3T3, MEFs | Translation | Translation | 10.1371/journal.pone.0000242 |
| RPL26 | elevation | reduction | NIH3T3, MEFs | - | Translation | 10.1371/journal.pone.0000242 |
| RPL29 | elevation | reduction | NIH3T3, MEFs | - | Translation | 10.1371/journal.pone.0000242 |
| RPL32 | elevation | reduction | NIH3T3, MEFs | - | Translation | 10.1371/journal.pone.0000242 |
| RPL34 | elevation | reduction | NIH3T3, MEFs | Translation | Translation | 10.1371/journal.pone.0000242 |
| RPL35 | elevation | reduction | NIH3T3, MEFs | - | Translation | 10.1371/journal.pone.0000242 |
| RPL39 | elevation | reduction | NIH3T3, MEFs | - | Translation | 10.1371/journal.pone.0000242 |
| RanBP2 | reduction | elevation | U2OS | indirect protein stability | Nuclear transport | 10.1016/j.celrep.2012.07.007 |
| RanBP1 | elevation | reduction | U2OS | export | Nuclear transport | 10.1016/j.celrep.2012.07.007 |
| Gle1 | elevation | reduction | U2OS | export | Nuclear transport | 10.1016/j.celrep.2012.07.007 |
| DDX19 | elevation | reduction | U2OS | export | Nuclear transport | 10.1016/j.celrep.2012.07.007 |
| CPSF1 | elevation | - | U2OS | export | CPA | 10.1016/j.celrep.2019.04.008 |
| CPSF2 | elevation | - | U2OS | export | CPA | 10.1016/j.celrep.2019.04.008 |
| CPSF3 | elevation | - | U2OS | export | CPA | 10.1016/j.celrep.2019.04.008 |
| CPSF4 | elevation | - | U2OS | - | CPA | 10.1016/j.celrep.2019.04.008 |
| FIPL1 | elevation | - | U2OS | export | CPA | 10.1016/j.celrep.2019.04.008 |
| SYMPL | elevation | - | U2OS | export | CPA | 10.1016/j.celrep.2019.04.008 |
| WDR33 | elevation | - | U2OS | export | CPA | 10.1016/j.celrep.2019.04.008 |
| RNMT | elevation | reduction | U2OS, AMLp | - | Capping | 10.1073/pnas.2002360117 |
| RNGTT | elevation | reduction | U2OS, AMLp | - | Capping | 10.1073/pnas.2002360117 |
| RAM | elevation | reduction | U2OS | - | Capping | 10.1073/pnas.2002360117 |
| U2AF1 | elevation | - | U2OS | export | Splicing | 10.15252/embj.2021110496 |
| U2AF2 | elevation | reduction | U2OS, AMLp, NOMO-1 | export, translation | Splicing | 10.15252/embj.2021110496 |
| SF3B1 | elevation | reduction | U2OS, AMLp, NOMO-1 | export, translation | Splicing | 10.15252/embj.2021110496 |
| PRPF6 | elevation | reduction | U2OS, AMLp, NOMO-1 | export | Splicing | 10.15252/embj.2021110496 |
| PRPF8 | elevation | reduction | U2OS, AMLp, NOMO-1 | export, translation | Splicing | 10.15252/embj.2021110496 |
| SNRNP200 | elevation | reduction | U2OS, AMLp, NOMO-1 | export | Splicing | 10.15252/embj.2021110496 |
| PRPF19 | elevation | reduction | U2OS, AMLp, NOMO-1 | - | Splicing | 10.15252/embj.2021110496 |
| PRPF31 | elevation | - | U2OS | export | Splicing | 10.15252/embj.2021110496 |
This list is not exhaustive and thus the absence of factors here should not be interpreted as factors not binding. Dashes indicate unknown mechanism. Other mechanisms than those listed could also influence the factors’ production. AMLp refers to primary AML patient specimen; MEFS to mouse embryonic fibroblasts.
Figure 3.
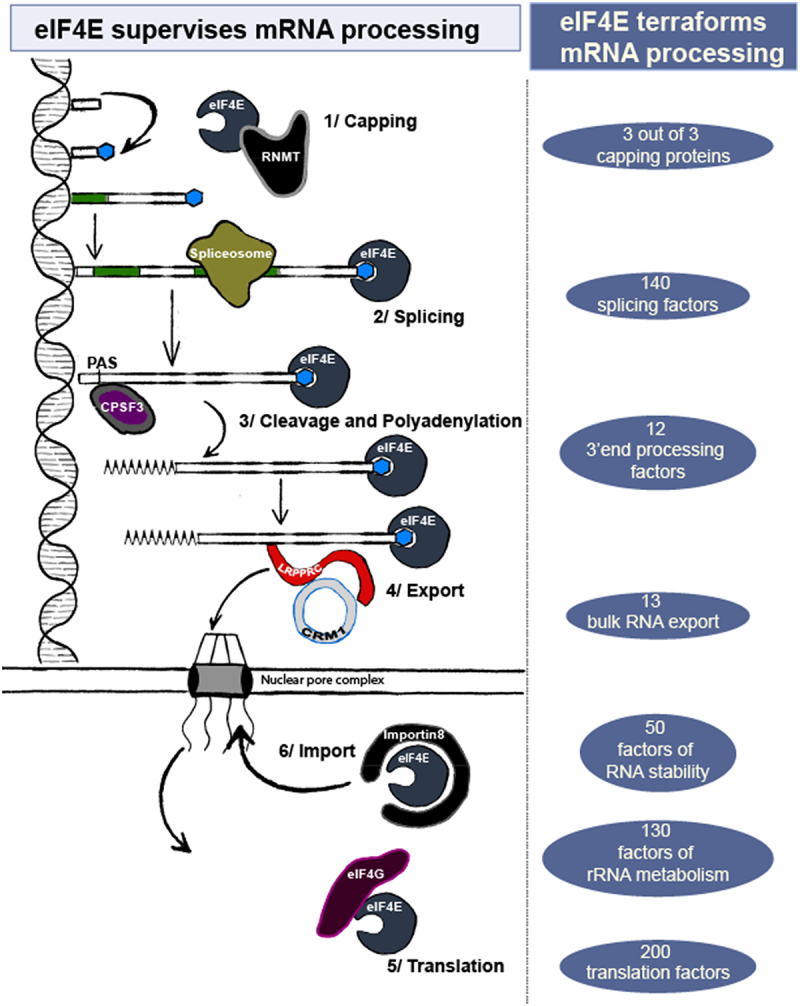
eIF4E can directly influence RNA fate or indirectly through its capacity to terraform the RNA processing landscape. On the left, interactions between eIF4E target mRNAs and the noted machineries are shown. On the right, numbers of mRNAs in a given process were identified in nuclear, endogenous eIF4E RIP-Seq [54] GSE63265_LY1_4EIP_allreps_counts.Txt.gz and/or RNA-seq splicing data segregated on high and normal-eIF4E AML specimens [100] (https://leucegene.ca/). These strongly suggest that eIF4E can influence a broad array of factors responsible for RNA processing thereby terraforming the RNA processing landscape. Functional categories were assigned using Metascape (metascape.Org).
eIF4E and splicing
The removal of introns and ligation of exons, a process known as splicing, is a central step in mRNA maturation [143]. Splicing is modulatory and can produce alternative mRNAs by altering splicing in the following ways: mutually exclusive exons (MXE), intron retention (IR), exon skipping (ES), alternative 5’ splice sites, or alternative 3’ splice sites, which can affect the sequence and structure of the resultant proteins, and ultimately their function [144]. This process is executed by the spliceosome composed of more than 150 proteins known as splicing factors (SF) and five uridine-rich small nuclear RNAs (UsnRNAs; U1, U2, U4, U5, and U6) [145]. Splicing defects occur frequently in genetic diseases and cancers, such as AML where 30% of genes are aberrantly spliced [146–151]. They can be due to somatic mutations of splice factors, in particular SF3B1, SRSF2 and U2AF1 in myelodysplastic syndromes [149–152] but aberrant splicing is more widespread in AML than these mutations suggesting that other mechanisms also impact splicing [148].
The potential implication of nuclear eIF4E in splicing has been suggested by experiments that showed potential co-localization with the splicing factor SC-35/SRSF2 [99]. Evidence of direct eIF4E involvement in splicing came for the first time from studies of female-specific alternative splicing of Drosophila msl-2 and Sxl pre-mRNAs in a genetic screen [153]. In this study, an interaction between eIF4E and the components of the U2 snRNP were observed implicating eIF4E in the regulation of alternative splicing (Table 1). The first large-scale study investigating the effect of eIF4E in splicing demonstrated it impacted thousands of splicing events in multiple mammalian cellular contexts including in AML [100]. eIF4E overexpression alone was sufficient to alter nearly a thousand splice events in U2Os cells without inducing any known SF mutations. Moreover, in clinical AML samples segregated on high- and normal-eIF4E AML, ~4000 splicing events were affected based on eIF4E expression in the absence of SF mutations [100]. eIF4E elevation in some instances increased and in others impaired splicing events. RNAs sensitive to eIF4E tended to have very long introns, but more work is needed to identify a splicing USER code. eIF4E physically associated with the spliceosome in an RNA- and cap-dependent manner in normal-eIF4E U2OS cells and in high-eIF4E AML contexts (Table 1), indicating that eIF4E overexpression is not required for these interactions and that these still occur in high-eIF4E disease contexts. Using RNA immunoprecipitation, it was observed that in the nucleus eIF4E interacted with both pre- and mature mRNAs for specific transcripts, suggesting that it could play a direct role in chaperoning mRNAs to the spliceosome. As in the case of capping enzymes in the preceding section, eIF4E can terraform the splicing protein landscape [100] (Figure 3). Specifically, eIF4E increased mRNA export and, in some cases, also translation of components of each of the major splicing complexes including SF3B1, SNRNP200, PRPF6, PRPF8, PRPF31, U2AF1, and U2AF2 (Table 2; Figure 3). Further, eIF4E did not alter UsnRNAs levels, suggesting that eIF4E affects the composition of spliceosomes, rather than their number [100]. In all, eIF4E influences specific splicing events for thousands of RNAs and modulated the expression of many components of the spliceosome.
eIF4E and 3’ end processing
The maturation of mRNAs includes, in most cases, the addition of a poly-A tail to the 3’end using a process referred to as cleavage and polyadenylation (CPA). This is a two-step process that includes the recognition of the polyadenylation site (PAS) by the CPSF3 enzyme and co-factors, followed by the cleavage of a CA dinucleotide near the PAS and subsequent addition of a polyA tail by polyA polymerase [154,155]. Multiple potential PAS sites exist in 70% of eukaryotic mRNAs, and the selection of a PAS can affect the stability, localization, transport, translation of an mRNA, and the final form of the resulting protein [156]. This selection of a given PAS is known as alternative polyadenylation and can even create truncated proteins with alternative C-terminal domains or other functional changes [157]. eIF4E physically associates with at least two components of the polyadenylation machinery, including the cleavage enzyme CPSF3 in mammalian cells (1) [158,159]. eIF4E overexpression increased PAS cleavage of a reporter construct using a 4ESE element. Furthermore, eIF4E overexpression affects the cleavage of CCND1 and MCL1, two well-known nuclear export targets of eIF4E [158,159]. Similar to other mRNA maturation steps, eIF4E also affects the protein levels of the CPA machinery (CPSF1, CPSF2, CPSF3, CPSF4, FIP1L1, SYMPL, and WDR33) by increasing the export of their corresponding mRNAs, suggesting that eIF4E could also modulate CPA through modifying the corresponding RNA processing factor landscape in the nucleus (Table 2; Figure 3). Further studies are needed to better understand the role of eIF4E in APA.
eIF4E-dependent nuclear mRNA export
The export of mRNAs from the nucleus to the cytoplasm through the nuclear pore is a key mechanism to control cytoplasmic availability of transcripts to the ribosome and is also an important RNA surveillance mechanism. While it was originally considered a constitutive housekeeping function, in the last 20 years it has become clear that this process is highly regulated [160–164]. First, we describe the bulk mRNA export pathway and then describe the role of eIF4E in selective mRNA export. Generally, mRNAs form cargo-receptor-mRNP complexes within the nucleus, which engage proteins of the nuclear basket of the nuclear pore complex (NPC), permitting transit through nuclear pores and release into the cytoplasm [165–167]. To be efficiently exported from the nucleus, generally mRNAs must be correctly processed with the addition of the m7G cap, properly spliced, and undergo 3’ end formation (typically addition of a poly-A tail) [168–171].
The majority of mRNAs associate with the NPC by employing the bulk export pathway mediated by the heterodimeric complex NXF1:NXT1 [95,172,173]. The nuclear RNA export factor 1 (NXF1 or TAP, tip-associated protein) contains an NTF2-like domain which associates with the NTF2-related export protein (NXT1 or p15) which binds to the NPC and the cargo mRNA [174–176]. NXF1:NXT1 binds the transcription export (TREX) complex, which consists of UAP56, CIP29, and Aly/Ref proteins, and a multimeric THO complex [95]. The TREX complex is assembled at the 5’end of mRNAs and is dependent on the presence of the cap and exon junction complexes (EJC) which mark splice sites [177]. During transcription, NCBP2 recognizes the cap co-transcriptionally while the accessory protein NCBP1 recruits Aly/Ref to the nascent mRNA [178,179]. UAP56 mediates the assembly of THO, CIP29, and Aly/Ref on mRNA in ATP-dependent manner [178]. Aly/Ref seems to interact with both the 5’ and 3’ ends of mRNA through the THO complex, and by binding to NXF1:NXT1 bridges the binding of cargo mRNAs with export receptors [180–183]. The recruitment of Aly/Ref occurs in a cap-dependent manner via interaction with CBC [91,184], via the EJC [185] and also potentially through direct interaction with 5-methylcytosine (m5C) to promote the export of specific mRNAs [186]. In addition to TREX, alternative complexes, such as TREX2 and AREX, have been implicated in the export of specific subsets of mRNAs with specific USER codes using the NXF1:NXT1 pathway. Additional adaptor proteins are involved in the NXF1:NXT1 pathway such as serine- and arginine-rich (SR) proteins that bind and export intronless histone 2A and other spliced mRNAs [187,188]. Thus, there is diversity even in the bulk mRNA export pathway, where specific subsets of mRNAs can be differentially exported by using at least two classes of adaptors, Aly/REF and SR, to engage NXF1/Nxt1. These events are typically considered to employ NCBP2 engagement of the cap [189].
After traveling through the central channel of the NPC, mRNP-export complexes arrive at the cytoplasmic fibrils on the cytoplasmic face of the NPC, where they undergo cargo release and export-factor recycling. The major component of cytoplasmic fibrils, RanBP2 (also known as Nup358), plays a central role in cargo release and recycling for both bulk and specific export pathways [190,191]. RanBP2 is associated with the NPC via Nup88 and Nup214. Through its multiple domains also recruits NXF1, RanGAP, Ran, and CRM1/XPO1 [192,193]. For most mRNAs, binding of the ATP-dependent DEAD box helicase (DDX19) and its co-factor Gle1 mediates the release of cargo to the cytoplasm. This process is inositol-hexakisphosphate (InsP6)-dependent, suggesting a possible involvement of intracellular signaling in the regulation of mRNA export. Here, the Gle1–InsP6 complex stimulates the binding of DDX19 to cargo mRNA, which then triggers ATP hydrolysis and cargo release [194–197]. It is important to note that not all mRNAs are exported with equal efficiency in all contexts using this pathway, and also that some factors such as RanBP2, Nup214, and Nup88 are not exclusively used for this export pathway, suggesting general plasticity and modularity of the system.
The other major NPC receptor is CRM1/XPO1 (Chromosomal Maintenance 1/Exportin 1). This pathway is used by a specific subset of mRNAs and other types of RNAs (e.g. UsnRNAs). CRM1 is a member of the karyopherin family and directly interacts with nucleoporins (Nups) at the nuclear basket of the NPC to permit export [86,190]. CRM1 does not bind mRNA directly but interacts with its cargoes by recognizing a leucine-rich nuclear export signal (NES) found in many shuttling and adaptor proteins that, in turn, bind the RNA or other RNA-binding proteins [198]. Similar to bulk mRNA export, subsets of mRNAs with specific USER codes are exported using the CRM1 pathway in combination with different adaptor proteins. One of these is the HuR/ELAV1 protein, which binds CRM1 and AU-rich elements (AREs) USER codes within target mRNAs [199]. While some ARE-containing mRNAs were found accumulated in the nucleus upon treatment with the CRM1 inhibitor leptomycin B, other ARE containing mRNAs are exported independently of HuR/ELAV1 suggesting there are likely additional USER codes and export modalities [200]. Additionally, CRM1 can interact with NXF3 (a member of the NXF1/TAP family), which does not directly bind Nups and thus uses CRM1 to export mRNAs in a tissue-specific manner [201]. CRM1 binds mRNP-cargoes in the nucleus in the presence of the GTP-bound form of Ran [190]. Once in the cytoplasm, CRM1-mRNP complexes associate with the Ran GTPase-activating protein (RanGAP) and either RanBP1 or RanBP2, enabling GTP hydrolysis mediated by the Ran protein, and dissociation of CRM1–cargo complexes release the mRNAs and permit recycling of export factors [190]. Similar to the bulk mRNA export pathway, Nup88, Nup214, and RanBP2 also play critical roles in the recycling and release steps for CRM1-dependent export as in case of bulk mRNA [190,202–204].
eIF4E overexpression was reported to modify the mRNA export of CCDN1 and ODC transcripts in mammalian cells and interestingly that CCDN1 was only an mRNA export and not a translation target of eIF4E [72]. At that time, it was thought to be an indirect effect of eIF4E, likely via enhancing translation of components of the export machinery. However, subsequent studies demonstrated that eIF4E bound to capped mRNAs in the nucleus and to CRM1 indicative of a direct effect of eIF4E on mRNA export [18,19,25,205,206]. eIF4E-dependent export targets contain a ~50 nucleotide USER code known as an eIF4E sensitivity element (4ESE), which is comprised of a paired stem loop element located in their 3’UTR [18,19,25,27]. Indeed, LacZ-4ESE, but not LacZ, were sensitive to eIF4E-dependent export. Other USER codes for eIF4E-dependent mRNA export likely exist. Inhibition of CRM1 with leptomycin B decreased the export of 4ESE containing mRNAs, but not of bulk mRNAs, and led to nuclear accumulation of eIF4E [19,206]. Moreover, genetic reduction of NXF1 did not impair eIF4E-dependent mRNA export but reduced bulk mRNA export [19]. Similar to the bulk pathway, eIF4E export mRNPs contained UAP56, hnRNPA1, and DDX3; however, REF/Aly did not interact with eIF4E, suggesting compositional differences in these complexes (Table 1). For example, the leucine-rich pentatricopeptide repeat C-terminus protein (LRPPRC) acts as an assembly platform for eIF4E-dependent export by directly binding to CRM1, 4E-SE mRNAs and eIF4E as seen by NMR, size-exclusion chromatography and biochemical assays (Figure 2) [25,27]. Importantly, eIF4E overexpression leads to enhanced mRNA export for a subset of mRNAs. Endogenous 4ESE mRNAs such as CCND1 are targets of both bulk and eIF4E/CRM1 pathways; this is likely because endogenous mRNAs contain multiple, competing USER codes [25]. Conversely, the genetic or pharmacological reduction of eIF4E impairs the export of these specific mRNAs [54,58,100,114]. As for capping, splicing and APA, eIF4E overexpression increased the levels of some NPC-associated proteins, including RanBP1, Gle1, and DDX19, and interestingly decreased the major cytoplasmic fibril protein RanBP2 [113] again influencing the RNA maturation landscape (Table 2; Figure 3). Overexpression of the zinc finger domain of RanBP2 inhibited eIF4E-dependent mRNA export, whereas siRNA knockdown of RanBP2 enhanced this export path. It seems that eIF4E downregulates the expression of RanBP2 to reduce sequestration of cargoes on these long fibrils, and at the same time, increased RanBP1 production to expedite the release and recycling of its export cargoes, since this configuration is likely to be less sterically hindered compared to sequestration on the large RanBP2 fibrils [113,207]. Interestingly, RanBP2 hypomorph mice have more spontaneous tumors than their littermate controls [208] consistent with the oncogenic phenotype associated with eIF4E and its capacity to lower RanBP2 levels. Gle1, DDX19, and RanBP1 factors are also required for NXF1 mediated export, and by increasing their production, eIF4E can potentially modulate bulk mRNA export, but this has not yet been directly examined. At the same time, eIF4E-dependent export shares components with bulk and other CRM1 mediated export pathways, indicating another level of crosstalk between different export pathways.
Roots of RNA selectivity in eIF4E-dependent processes
As is evident from the above discussion, the cap is not enough to imbue transcripts with eIF4E-sensitivity. Indeed, from the perspective of translation, it is well known that not all capped mRNAs have their translation equally efficiency influenced by eIF4E [17,26,79]. eIF4E binds many but not all capped transcripts in the nucleus. For example, eIF4E RIP Seq studies indicated that eIF4E associated with >3000 transcripts in the nucleus of human lymphoma cells [54]. The selectivity in terms of physical association with mRNAs implies that eIF4E in the nucleus is somehow restricted to mRNAs with specific USER codes in addition to the cap. Indeed, capping of transcripts is required for eIF4E association for transcripts monitored to date as eIF4E mutants deficient in cap-binding and cap-analog-based competition studies demonstrate a necessity for the cap [18,19]. Below we describe information related to this selectivity.
Thus, the question arises if the cap is required but not sufficient for eIF4E specificity, what cooperating USER codes are required? The 4ESE USER code for eIF4E-dependent mRNA export is a structural element that is sufficient to induce the export of reporter mRNAs, and its deletion represses export of endogenous mRNAs [18,27]. For the case of LacZ mRNA export studies, eIF4E immunoprecipated with LacZ-4ESE mRNAs but not LacZ indicating that addition of the 50 nucleotide 4ESE USER code suffices to imbue eIF4E-binding properties in the nucleus. Many eIF4E-dependent mRNA export targets contain 4ESE elements such as CCND1 and PIM1 [18,19,54]. This specific USER code directly binds LRPPRC in the CRM1/XPO1 export complex [27]. The 4ESE is also implicated in eIF4E-dependent CPA cleavage of LacZ-4ESE relative to LacZ presumably by binding other proteins than found in the export complex, and suggesting that it is not an export-dedicated signal but can also mediate eIF4E specificity for other mechanisms [158]. In terms of capping, a cap sensitivity element (CapSE) has been identified [114] which physically interacts with RNMT, suggesting an eIF4E-RNMT-CapSE complex could form. Future studies will better elucidate this mechanism. The specific targets of eIF4E in alternative splicing tend to have long introns but no USER code has been discovered yet, although AU-rich elements (ARE) have been implicated given the physical association between the ARE binding protein HuR/ELAVL1 protein and eIF4E [100].
In the cytoplasm, eIF4E also elicits specific rather than global effects on the translation of transcripts. Specifically, eIF4E does not increase the translation efficiency of all transcripts, but rather impacts about 30–50% of global translation [17,79,209]. The cap is still required, but the accompanying USER codes needed for impacting translation differ significantly from those used for mRNA processing and export described above. The translation efficiency of mRNAs possessing highly structured or cytosine-enriched regulator of translation (CERT) elements within their 5’UTRs are more sensitive to eIF4E levels, and in this way, these structured elements serve as USER codes for their translation [20,23,26]. Using crosslinking immunoprecipitation (CLIP) a recent study suggested that eIF4E is associated with mRNAs containing the 5’Terminal Oligo Pyrimidine (5’TOP) motifs and that this interaction is specifically reduced upon rapamycin treatment, resulting in decreased translation of these TOP-mRNAs [210].
While the cap appears to be a defining feature of what is likely at least a bipartite USER code, there is strong evidence that eIF4E also has the capacity to bind moieties other than the cap in its cap-binding sites (Figure 2). For example, human and nematode eIF4E also bind TMG which is usually associated with UsnRNAs in mammalian cells but is associated with mRNA in nematodes. The TMG-eIF4E crystal structure is highly homologous to those of cap-eIF4E structures [59]. When in the 5’UTR, the 4ESE element from Histone H4 RNA bound to the cap-binding surface of eIF4E to positively regulate translation [211]. In NMR studies, the 4ESE was also found to bind the positive surface of the cap-binding site of eIF4E but with much weaker affinity than the cap [27]. Through this same surface, eIF4E physically associated with the PTE (Panicum mosaic virus translation element), an RNA element of the Pea enation virus required for viral protein translation [212]. Structural studies revealed that the plant potyvirus viral protein genome-linked (VpG) directly binds the cap-binding surface of eIF4E and this protein can act as a cap to recruit the RNA to the translation machinery [53]. NMR studies showed that the human kinesin EG5/KIF11 (kinesin family member 11) also binds directly to the cap-binding site, suggesting that eIF4E could be transported around the cell in an RNA-free form, but further work needs to be done to investigate this possibility [53]. Finally, NMR and biophysical studies demonstrated that the nuclear importer Importin 8 also binds to the cap-binding site of eIF4E competing for cap-binding (see below) [51].
While USER codes for eIF4E are one of the most notable sources of selectivity, it appears likely that mRNAs are competing in the nucleus for NCBP2 and eIF4E, and perhaps other factors. Cap affinity for NCBP2 is of about 10 nM when bound to its NCBP1 partner [213] and 1–500 nM for eIF4E, depending on in vitro conditions [50,58–60,64,87,214]. Competition between these cap-binding proteins and target mRNAs was observed in mammalian cells where it is likely that USER codes and their associated co-factors play an important role in the selection of which cap-binding protein to bind. For example, in nucleoplasmic fraction, LacZ RNAs were immunoprecipitated by NCBP1 while LacZ-4ESE RNAs were immunoprecipitated by eIF4E [25]. Moreover, eIF4E overexpression led to higher levels of CCDN1 RNA enriched in nuclear eIF4E immunoprecipitations compared to vector [25]. The transition from the pioneer-round of translation to steady-state translation must involve similar exchange between these cap-binding factors. Localized concentrations of cap-binding proteins seem key. For example, CBC or eIF4E can act in the pioneer round of translation [96,97,215–217]. CBC promotes translation in the vicinity of the nuclear membrane after mRNA cytoplasmic export in which case CBC would be part of the nuclear export complex for many of these transcripts [215,216]. In all, we posit that USER codes and their concomitant recruitment of co-factors route RNAs between eIF4E and NCBP2 driven fates.
Nuclear trafficking of eIF4E
At steady state, eIF4E is found in both the nuclear and cytoplasmic compartments in many cell types and organisms as described above. Indeed, eIF4E can be depleted from or accumulate in the nucleus depending on conditions, suggesting active mechanisms of transport and/or retention are in play. However, eIF4E lacks a classic or proline-tyrosine nuclear localization sequence [51]. Early studies had suggested that the nuclear import of eIF4E was mediated by the eIF4E-transporter, 4ET, but it now appears that 4ET plays a role in trafficking eIF4E within the cytoplasm to P-bodies [8,206]. The best-defined mechanism to date for nuclear eIF4E import involves Importin 8 [51] . Biochemical and NMR studies showed that cap-free eIF4E directly interacts with Importin 8 utilizing the positively charged surface region of the cap-binding site. Indeed, Importin 8 and cap binding to eIF4E are mutually exclusive. For example, cap analogs dissociated eIF4E-Importin 8 complexes, as observed by NMR and biophysical methods. This provides a molecular mechanism explaining the effects of increased cap addition in in vitro nuclear import assays reduced nuclear import of eIF4E [27,51]. Furthermore, this observation has functional implications, where eIF4E mRNA export complexes just arriving in the cytoplasm should not be recycled into the nucleus prior to mRNA cargo release because they will not form complexes with Importin 8 when mRNA-cargo bound. Additionally, cytoplasmic eIF4E engaged in translation would not be an import cargo. Knockdown of Importin 8 (but not Importin 9) led to cytoplasmic accumulation of eIF4E and nuclear accumulation of mRNA export targets, whereas overexpression of Importin 8 enhanced eIF4E-dependent mRNA export. Consequently, depletion of Importin 8 decreased eIF4E-mediated oncogenic transformation in human U2Os cells. Although this study showed that Importin 8 is a direct mediator of eIF4E nuclear import, it did not exclude the possibility that other karyopherins/importins are involved in the regulation of eIF4E localization in different contexts. Importin 8 is also regulated by eIF4E providing another example of the landscape effect, whereby eIF4E increases the mRNA export of Importin 8 transcripts, thereby increasing the cytoplasmic availability and ultimate Importin protein levels. Reciprocally, Importin 8 overexpression increases nuclear eIF4E localization at steady-state and increases levels of eIF4E targets (MCL-1 and CCND1), while conversely, knockdown of Importin 8 decreases their protein levels.
Another partner protein known to modulate eIF4E localization is the proline-rich homeodomain protein (PRH), also known as haematopoietically expressed homeodomain (Hex). PRH binds directly to the dorsal surface of eIF4E [107]. PRH overexpression leads to cytoplasmic accumulation of eIF4E, inhibition of its mRNA export activity, and suppression of eIF4E-dependent proliferation and transformation. PRH contains a consensus eIF4E-binding site and associates with eIF4E in a manner similar to eIF4E binding proteins (4EBP1) or eIF4G potentially inhibiting the translation activity of eIF4E [107]. PRH expression is tissue-specific where it is found in normal myeloid cells and is decreased and/or mislocalized expression in M4 and M5 AML patient specimens, which have correspondingly elevated eIF4E levels, nuclear localization, and mRNA export activity [103]. Overexpression of the dominant negative inhibitor of NF-κB (IκB-SR) in primary AML patient specimens restored the expression and reduced nuclear localization of PRH and eIF4E to a phenotype similar to that observed in bone marrow cells from healthy volunteers [107]. Additionally, overexpression of IkB-SR in CML primary specimens decreased the levels of HoxA9 protein and its translocation from the nucleus and decreased accumulation of eIF4E into nuclear bodies [103,108]. HoxA9 protein is a positive regulator of eIF4E-dependent mRNA export and translation of specific target mRNAs and competes with PRH to bind to eIF4E [108]. Immunoprecipitation studies have shown that overexpression of HoxA9 leads to a decreased interaction of PRH with eIF4E [108]. In addition to HoxA9 and PRH, other homeobox proteins, such as Hox11, Bicoid, Emx-2, and Engrailed 2, directly bind to eIF4E positioning it as a widescale, direct regulator of developmental processes [103,107,108,218–221]. Furthermore, overexpression of PML, a negative regulator of eIF4E mediated export leads to increased recruitment of eIF4E to PML bodies without changing eIF4E protein levels [18,98,109]. PML, PRH, HOXA9, HOX11 and Bicoid all bind to the dorsal surface of eIF4E, while the binding surface has not been experimentally determined for the others listed. This interplay of regulatory factors indicates the multifactorial and context-dependent regulation of eIF4E localization.
eIF4E: direct control
eIF4E has a wide array of protein partners that are positioned to influence its biochemical and biological activities in both the nucleus and cytoplasm (Table 1). Many of the proteins listed in Table 1 physically associate with eIF4E but do not directly interact with it. In this section, we focus on different structural and biochemical modalities used by direct-binding partners of eIF4E and how this could influence activity. Common structural strategies have evolved to modify eIF4E function and/or recruit eIF4E to the processing/export/translation machinery. Proteins that bind to the dorsal surface can impair or promote eIF4E activity. For example, both eIF4G and LRPPRC are required for eIF4E’s translation and nuclear mRNA export functions, respectively (Figure 2). Competition with other factors impairs these functions e.g. the translation factor eIF4G and the translation inhibitor 4EBP1 compete for eIF4E using the same eIF4E consensus motif (YXXXXLΦ where Φ is hydrophobic residue and X is any residue) as well as additional interactions with the non-canonical domains to bind to its dorsal surface (Figure 2) [76,222,223]. Several homeodomains also directly bind the dorsal surface eIF4E including Bicoid, HoxA9, PRH using this consensus motif (Table 1) [107,108,220]. By contrast, while LRPPRC binds to the dorsal surface of eIF4E, as shown by NMR, it appears to use a different structural motif to associate with this surface based on its structures although high-resolution structure of the complex is needed to validate this [27]. Structural and biochemical studies have also demonstrated that PML and a related arenavirus protein Z employ their RING domains which interact with an overlapping but distinct portion of the dorsal surface of eIF4E compared to eIF4G and other proteins that use the consensus-binding motif [65,224,225]. These are particularly interesting because unlike eIF4G and 4EBP1, the addition of PML and Z substantially reduced cap-binding of eIF4E (by nearly 100-fold) through allosteric effects, as observed by NMR [65] while, by contrast, binding to eIF4G and 4EBP1 are associated with moderate increases in cap-binding affinity for eIF4E [71,226]. Consistently, eIF4E associated with PML nuclear bodies in cells but the PML-associated eIF4E fraction did not contain endogenous or model RNA targets presumably due to reduced cap-affinity elicited by PML [18,19,224,225]. Another RING protein, HHARI RING binds to the eIF4E family member eIF4E2 [227]. These observations highlight that there are multiple structural means exemplified by eIF4G (consensus motif users), LPRPRC and RING domains to associate with the dorsal surface of eIF4E which can elicit different effects on eIF4E’s functional state.
While it was originally considered that eIF4E protein-binding was restricted to its dorsal surface and mRNA restricted to its cap-binding surface, recent studies indicate that this is not the case (Figure 2). As described above, some protein factors bind to the cap-binding surface and thereby directly impair eIF4E’s cap-binding activity. The three reported to date are Importin 8, VPg, and EG5/KIF11 based on NMR and biochemical studies [27,51,53]. VPg appears to enable the viral protein to act as a cap and engage eIF4E for translation since VPg is covalently attached to the viral RNA during infection. EG5/KIF11 and VPg binding are highly homologous using structurally similar domains to bind the cap-binding site. EG5 is a mammalian kinesin suggesting that this protein may traffic eIF4E in the cell in an RNA-free state [53]. The mode of Importin 8 binding provides an RNA surveillance mechanism whereby only RNA-free eIF4E and LRPPRC reenter the nucleus, reducing futile export cycles by ensuring cargo release in the cytoplasm prior to reentry for subsequent cycles of export. Another surface appears relevant to the control of eIF4E, that used by the 4GI–1 inhibitor. While original studies suggested this molecule bound the dorsal surface, crystal structures revealed that it rather binds near to but not overlapping with the cap-binding site [228]. More studies into factors that bind this site will certainly bring new insights into the regulation of eIF4E.
Phosphorylation on serine 209 is an important control point of eIF4E. eIF4E is phosphorylated by MAP kinase-interacting serine/threonine-protein kinase MNK1 and MNK2 kinases which also phosphorylate other proteins [52,229,230]. Its phosphorylation contributes to its oncogenic potential in cells and animal models [111,112,231–233]. Mouse models with mutations in this phosphorylation site demonstrated that phosphorylation is not required for viability and these mice appear to develop normally [231]. However, there appears to be impacts on stress response [234]. Biochemically speaking, impaired phosphorylation of eIF4E reduces mRNA export by about 50% [111,112], the impact on translation is controversial with reports indicating that phosphorylation is or is not required for translation [235,236]. The impacts of phosphorylation on the other eIF4E activities remain to be tested.
eIF4E terraforms the landscape
As described above, eIF4E appears to terraform the cellular mRNA processing landscape. Indeed, eIF4E modulated the production of 3/3 of capping enzymes and at least 8 splicing factors, 7 CPA, 4 export factors as confirmed by western blot (Table 2, Figure 3). Given this, we examined published endogenous, nuclear eIF4E RIP data and AML patient splicing data segregated on eIF4E level to obtain a global assessment of the RNA processing factors potentially under the control of eIF4E [54,100] (eIF4E-RIP seq transcripts: GSE63265_LY1_4EIP_allreps_counts.txt.gz). Functional information was obtained using Metascape. We found that ~140 transcripts encoded factors associated with splicing, 3 code for the 3 capping proteins, 12 factors involved in CPA, 13 in mRNA export, 50 in RNA stability, 130 in rRNA metabolism and 200 associated with translation. It is yet to be determined what level(s) these transcripts are regulated by eIF4E. In addition, eIF4E influences its own localization by altering Importin 8 production but does not influence CRM1/XPO1 at least under conditions examined to date. In all, this supports a model whereby eIF4E drives simultaneous, widescale reprogramming of multiple mRNA processing events by terra-forming the RNA processing and translation factor landscape. Thus, eIF4E can modulate mRNA processing without physically interacting with some target transcripts allowing it to have much broader impacts that predicted by RNA immunoprecipitation studies alone. This is in addition to direct impacts of eIF4E found by the ~3000 RNAs it physically associates with in the nuclear fraction as well as most of those found in the cytoplasm.
eIF4E in cancer
eIF4E was the first translation factor reported to have an oncogenic capacity and its overexpression led to increased tumor growth and metastasis in mouse models [237,238]. In patient specimens, eIF4E levels and/or phosphorylation status are elevated in many cancers and this is generally correlated with poor prognosis [100,239–244]. The contribution of eIF4E dysregulation depends on cancer type. For example, in head and neck cancers eIF4E is elevated in >98% of patients while in breast cancers, only some subtypes have dysregulated eIF4E [32,243,244]. eIF4E elevation and nuclear accumulation are associated with poor outcomes in a subset of AML patients [29,100,103]. In primary patient specimens, eIF4E elevation has been shown to impact splicing, capping, export and translation of specific mRNAs that typically encode oncoproteins as described in the above sections, with its CPA activity not yet tested in patient specimens [28,100,103,114]. In cell lines, eIF4E rescues cells from a variety of apoptotic stresses, reduces contact inhibition, increases growth in soft agar and increased cell motility [60,237,245–249]. Various studies using separation-of-function mutants demonstrated that the nuclear functions of eIF4E contributed to its oncogenic potential and the importance of the nuclear role was later verified in clinical trials [28–30]. For example, the S53A mutant, active in translation but inactive in mRNA export, cannot transform or rescue cells from apoptosis, whereas the W73A mutant, active in export and apoptotic rescue but not in translation, retains the oncogenic capacity similar to wildtype eIF4E [51,98,103,112,113,245,250. Overexpression of eIF4E or of the export competent W73A mutant rescued cells from serum starvation-induced apoptosis, whereas the export deficient but translationally active S53A mutant did not [245,246]. eIF4E remodeling of the NPC can also be linked to its oncogenic potential via reduction of RanBP2 as discussed in the mRNA export section. Indeed, RanBP2 overexpression counteracts the oncogenic potential of eIF4E [113]. Studies using CCND1-4ESE constructs also indicated that eIF4E driven mRNA export was related to increased oncogenic capacity of cells [18]. Direct links have been forged with nuclear import of eIF4E with its oncogenic capacity. Indeed, addition of a traditional nuclear localization signal to eIF4E increased its nuclear localization and its oncogenic activity [51].
Given these findings, it is not surprising that targeting eIF4E in patients has become an area of intense interest. The first clinical studies to target eIF4E involved the use of the old antiviral drug ribavirin which binds the cap-binding site of eIF4E thereby competing with capped RNAs in vitro as observed by NMR, mass spectrometry and biochemical methods and eIF4E immunoprecipitated with 3H ribavirin in live cells [29,51,54,58,110,251]. It is important to note that the ribavirin binding to eIF4E occurs under physiological conditions whereas in conditions that cause both eIF4E and ribavirin to aggregate binding such as pH 8.0 in HEPES buffer, binding is impaired [58,110,252,253]. Ribavirin inhibits eIF4E mediated splicing, mRNA export and translation, with CPA and capping not yet assessed; these findings parallel impacts of the genetic knockdown of eIF4E [54,100,114,205]. Ribavirin reduces growth of eIF4E-dependent tumours in mouse models [54,249,254–259,260].
The clinical efficacy of targeting eIF4E with ribavirin has been assessed by multiple groups in early stage clinical trials in AML, in oral carcinomas and in castration-resistant prostate cancer [28–33]. Two key questions are studied in most of these trials: 1. does ribavirin target eIF4E in patients and 2. does targeting eIF4E lead to clinical benefit? In the first clinical studies, relapsed and refractory AML patients were monitored as a function of ribavirin monotherapy. Of the 15 evaluable patients 3 achieved remissions, 3 blast response which indicate at least 50% reduction in AML bone marrow blasts, 6 stable disease and 3 progressive disease [29]. Molecular studies revealed that eIF4E was indeed targeted by orally delivered ribavirin and this was tightly correlated to clinical response. Reduced mRNA export was observed for eIF4E targets in responding patients, for example. However, the most striking molecular observation was the impact ribavirin had on the subcellular localization of eIF4E [29]. Prior to treatment, patients who were pre-screened for elevated eIF4E prior to trial entry, had nuclear accumulations of eIF4E and highly elevated RNA export, the only nuclear function of eIF4E known at that time. During clinical response to ribavirin, patients had a massive re-localization of eIF4E to the cytoplasm and at relapse, patients had a reemergence of nuclear eIF4E enrichment; a molecular feature observed in subsequent trials as well [28–30]. Indeed, ribavirin competed with Importin 8 for the cap-binding site of eIF4E consistent with impaired nuclear entry of eIF4E in AML upon ribavirin treatment [51]. At relapse, patients lost the capacity to uptake ribavirin into their AML cells and/or ribavirin was chemically modified by cells, so it no longer bound to eIF4E [251]. This is correlated with increased nuclear eIF4E levels and ribavirin resistance. Ribavirin combination with low-dose cytarabine (Ara-C) led to similar results in patient as monotherapy but with perhaps longer responses [28] and combination with Vismodegib showed promise in targeting ribavirin resistance [30]. Ribavirin treatment sometimes also reduced levels of eIF4E in AML blasts and oral cancers, which had not been observed in cell lines and has been attributed to selection of low-eIF4E cancer cells during ribavirin treatment [29,31,32]. HPV-related cancers are interesting as the virus induces eIF4E [261]). In these stage IV oral cancers, ribavirin, afatinib and low-dose chemotherapy regimens yielded responses that were not dissimilar to those with standard induction chemotherapy but with less toxicity. These findings may allow detoxification of the chemotherapy in future through inclusion of ribavirin. Here, 6/10 patients had unconfirmed partial remissions and 75% disease-free two-year survival [32]. In a ribavirin monotherapy trial studying pharmacodynamics in oral cancers, phospho-eIF4E levels were reduced in post 14-day ribavirin treatment relative to pre-treatment biopsies for 4/6 patients [31]. On the therapeutic arm, best response was stable disease lasting 222 days for one patient and 173 for another with the remaining patients achieving shorter (~2 months) stable disease [31]. Taken together, this suggests that targeting eIF4E alone may not be sufficient to elicit responses in these cancers. Other groups employed alternative strategies to target eIF4E in patients. For example, antisense oligonucleotides directed against eIF4E were used to lower eIF4E protein levels. While these studies were promising in mouse models of prostate cancer [80], there were no objective clinical improvements in the solid tumor patients tested with the best responses achieved were 2/22 stable of short duration patients and little impact on eIF4E levels in these patients likely underpinning the lack of clinical efficacy [262]. New means to deliver antisense oligonucleotides will likely improve this methodology. Targeting eIF4E activity by inhibiting its phosphorylation using MNK inhibitors in patients is ongoing (ClinicalTrials.gov NCT02040558 (accessed on 9 February 2024)), the results of which are not yet known. These studies also strongly support the notion that eIF4E targeting alone will not be sufficient to induce long-term remissions in patients. Thus, identification of efficacious combinations is the challenge for the development of next-generation therapeutics targeting eIF4E.
The end of the beginning?
In this review, we described the multifactorial nature of the cap-chaperone eIF4E which underpins its capacity to impact the processing, export and translation of thousands of mRNAs simultaneously. We discussed several systemic effects of eIF4E that are the foundation of these impacts. For example, eIF4E terraforms the RNA processing landscape through its effects on the splicing, export and translation of many transcripts that encode these machineries (Tables 1 and 2, Figure 3). Additionally, eIF4E physically interacts with these machineries (Table 1). Consistent with its broad range of activities, eIF4E has been reported to interact with ~80 proteins, with evidence that ~15 of these directly bind to eIF4E e.g. Importin 8, RNMT and eIF4G (Table 1). While the traditional view has been that protein co-factors bind the dorsal surface of eIF4E and only RNA binds the cap-binding site, more recent studies demonstrate that several factors directly bind to the cap-binding site elucidating a major new mechanism for the control of eIF4E activity through direct competition for capped mRNA binding (e.g. Importin 8, VPg, EG5/KIF11) (Figure 2). Studies into eIF4E provide an important conceptual model as to how multifactorial activities can be selectively coordinated via USER codes, as predicted by the RNA regulon theory [15,16]. For some mRNAs, eIF4E binds both the pre-mRNA and mature mRNA further supporting a direct, as well as indirect, role in mRNA processing as illustrated by its role in the modulation of splicing, CPA, capping, nucleo-cytoplasmic trafficking, and translation machineries (Table 2). eIF4E’s impact on the proteomic output of the cell can be regulated by modulation of the status of the mRNAs, eIF4E levels, eIF4E localization, and the abundance and availability of eIF4E partners and of other cap-binding proteins such as NCBP2. Dysregulation of these eIF4E activities is observed in many cancers and targeting eIF4E has led to clinical benefit in AML and head and neck cancer. Clearly, there are many open questions relevant to establishing these activities in molecular detail some of which are provided below.
Open questions:
How does competition between other cap-binding proteins such as NCBP2 impact eIF4E activity and what are the effects on RNA processing?
What are the corresponding biological consequences of this competition?
What are the complement of USER codes for activities?
What are the protein partners of these USER codes?
Is it possible to develop inhibitors for specific eIF4E activities (rather than the global effects of ribavirin acting as a cap competitor or ASOs reducing eIF4E protein levels)?
How does nuclear eIF4E move within the nuclear compartment and associate with the right mRNA-processing machinery at the right time?
What are the best pathways to target in combination with eIF4E to treat high-eIF4E cancers?
Acknowledgments
We thank Michael Osborne for reading and helpful comments and for assistance generating Figure 2.
Funding Statement
This work was supported by NIH R01 CA80728, NIH R01 CA98571, Canadian Institutes of Health Research PJT159785, and the Canada Research Chair in Molecular Biology of the Cell Nucleus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Author contributions
J.-C.M., B.C.-K., K.L.B.B. have contributed to conception, writing, and reviewing of the article. All authors have read and agreed to the published version of the manuscript.
References
- [1].Sonenberg N, Morgan MA, Merrick WC, et al. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5’-terminal cap in mRNA. Proc Natl Acad Sci. U.S.A. 1978;75(10):4843–28. doi: 10.1073/pnas.75.10.4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Filipowicz W, Furuichi Y, Sierra JM, et al. A protein binding the methylated 5’-terminal sequence, m7GpppN, of eukaryotic messenger RNA. Proc Natl Acad Sci. U.S.A. 1976;73(5):1559–1563. doi: 10.1073/pnas.73.5.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Altmann M, Muller PP, Pelletier J, et al. A mammalian translation initiation factor can substitute for its yeast homologue in vivo. J Biol Chem. 1989;264(21):12145–12147. [PubMed] [Google Scholar]
- [4].Joshi B, Lee K, Maeder DL, et al. Phylogenetic analysis of eIF4E-family members. BMC Evol Biol. 2005;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reid DW, Chen Q, Tay AS, et al. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell. 2014;158(6):1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reid DW, Nicchitta CV.. Primary role for endoplasmic reticulum-bound ribosomes in cellular translation identified by ribosome profiling. J Biol Chem. 2012;287(8):5518–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frydryskova K, Masek T, Borcin K, et al. Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules. BMC Mol Biol. 2016;17(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ferraiuolo MA, Basak S, Dostie J, et al. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Bio. 2005;170(6):913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bartish M, Abraham MJ, Goncalves C, et al. The role of eIF4F-driven mRNA translation in regulating the tumour microenvironment. Nat Rev Cancer. 2023;23(6):408–425. [DOI] [PubMed] [Google Scholar]
- [10].Borden KLB. The eukaryotic translation initiation factor eIF4E unexpectedly acts in splicing thereby coupling mRNA processing with translation: eIF4E induces widescale splicing reprogramming providing system-wide connectivity between splicing, nuclear mRNA export and translation. BioEssays. 2024;46(1):e2300145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen X, An Y, Tan M, et al. Biological functions and research progress of eIF4E. Front Oncol. 2023;13:1076855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hernandez G. The versatile relationships between eIF4E and eIF4E-interacting proteins. Trends Genet. 2022;38(8):801–804. [DOI] [PubMed] [Google Scholar]
- [13].Hernandez G, Vazquez-Pianzola P. eIF4E as a molecular wildcard in metazoans RNA metabolism. Biol Rev Camb Philos Soc. 2023;98(6):2284–2306. [DOI] [PubMed] [Google Scholar]
- [14].Mars JC, Ghram M, Culjkovic-Kraljacic B, et al. The Cap-Binding Complex CBC and the Eukaryotic Translation Factor eIF4E: Co-Conspirators in Cap-Dependent RNA Maturation and Translation. Cancers (Basel). 2021;13(24):6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8(7):533–543. [DOI] [PubMed] [Google Scholar]
- [16].Keene JD, Lager PJ. Post-transcriptional operons and regulons co-ordinating gene expression. Chromosome Res. 2005;13(3):327–337. [DOI] [PubMed] [Google Scholar]
- [17].Clemens MJ, Bommer UA. Translational control: the cancer connection. Int J Biochem Cell Biol. 1999;31(1):1–23. [DOI] [PubMed] [Google Scholar]
- [18].Culjkovic B, Topisirovic I, Skrabanek L, et al. eIF4E promotes nuclear export of cyclin D1 mRnas via an element in the 3‘UTR. J Cell Bio. 2005;169(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Culjkovic B, Topisirovic I, Skrabanek L, et al. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Bio. 2006;175(3):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23(18):3189–3199. [DOI] [PubMed] [Google Scholar]
- [21].Dua K, Williams TM, Beretta L. Translational control of the proteome: relevance to cancer. Proteomics. 2001;1(10):1191–1199. [DOI] [PubMed] [Google Scholar]
- [22].Jia Y, Polunovsky V, Bitterman PB, et al. Cap-dependent translation initiation factor eIF4E: an emerging anticancer drug target. Med Res Rev. 2012;32(4):786–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koromilas AE, Lazaris-Karatzas A, Sonenberg N. mRnas containing extensive secondary structure in their 5’ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. Embo J. 1992;11(11):4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miras M, Miller WA, Truniger V, et al. Non-canonical Translation in Plant RNA Viruses. Front Plant Sci. 2017;8:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Topisirovic I, Siddiqui N, Lapointe VL, et al. Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export-competent RNP. Embo J. 2009;28(8):1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Truitt ML, Conn CS, Shi Z, et al. Differential Requirements for eIF4E Dose in Normal Development and Cancer. Cell. 2015;162(1):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Volpon L, Culjkovic-Kraljacic B, Sohn HS, et al. A biochemical framework for eIF4E-dependent mRNA export and nuclear recycling of the export machinery. RNA. 2017;23(6):927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Assouline S, Culjkovic-Kraljacic B, Bergeron J, et al. A phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica. 2015;100(1):e7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Assouline S, Culjkovic B, Cocolakis E, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114(2):257–260. [DOI] [PubMed] [Google Scholar]
- [30].Assouline S, Gasiorek J, Bergeron J, et al. Molecular targeting of the UDP-glucuronosyltransferase enzymes in high-eukaryotic translation initiation factor 4E refractory/relapsed acute myeloid leukemia patients: a randomized phase II trial of vismodegib, ribavirin with or without decitabine. Haematologica. 2023;108(11):2946–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burman B, Drutman SB, Fury MG, et al. Pharmacodynamic and therapeutic pilot studies of single-agent ribavirin in patients with human papillomavirus-related malignancies. Oral Oncol. 2022;128:105806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dunn LA, Fury MG, Sherman EJ, et al. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck. 2018;40(2):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kosaka T, Maeda T, Shinojima T, et al. A clinical study to evaluate the efficacy and safety of docetaxel with ribavirin in patients with progressive castration resistant prostate cancer who have previously received docetaxel alone. J Clin Oncol. 2017;35(15_suppl):e14010–e14010. [Google Scholar]
- [34].Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Borden K, Culjkovic-Kraljacic B, Cowling VH. To cap it all off, again: dynamic capping and recapping of coding and non-coding RNAs to control transcript fate and biological activity. Cell Cycle. 2021;20(14):1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Furuichi Y, Shatkin AJ. Viral and cellular mRNA capping: past and prospects. Adv Virus Res. 2000;55:135–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Galloway A, Cowling VH. mRNA cap regulation in mammalian cell function and fate. Biochim Biophys Acta, Gene Regul Mech. 2019;1862(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kominami R, Muramatsu M. Heterogeneity of 5’ -termini of nucleolar 45S, 32S and 28S RNA in mouse hepatoma. Nucleic Acids Res. 1977;4(1):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Qiu L, Jing Q, Li Y, et al. RNA modification: mechanisms and therapeutic targets. Mol Biomed. 2023;4(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Furuichi Y, LaFiandra A, Shatkin AJ. 5’-Terminal structure and mRNA stability. Nature. 1977;266(5599):235–239. [DOI] [PubMed] [Google Scholar]
- [41].Murthy KG, Park P, Manley JL. A nuclear micrococcal-sensitive, ATP-dependent exoribonuclease degrades uncapped but not capped RNA substrates. Nucleic Acids Res. 1991;19(10):2685–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shimotohno K, Kodama Y, Hashimoto J, et al. Importance of 5’-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977;74(7):2734–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gao M, Fritz DT, Ford LP, et al. Interaction between a poly(A)-specific ribonuclease and the 5’ cap influences mRNA deadenylation rates in vitro. Mol Cell. 2000;5(3):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grudzien E, Kalek M, Jemielity J, et al. Differential inhibition of mRNA degradation pathways by novel cap analogs. J Biol Chem. 2006;281(4):1857–1867. [DOI] [PubMed] [Google Scholar]
- [45].Schwartz DC, Parker R. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRnas in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19(8):5247–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schwartz DC, Parker R. mRNA decapping in yeast requires dissociation of the cap binding protein, eukaryotic translation initiation factor 4E. Mol Cell Biol. 2000;20(21):7933–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Marcotrigiano J, Gingras AC, Sonenberg N, et al. Cocrystal structure of the messenger RNA 5’ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell. 1997;89(6):951–961. [DOI] [PubMed] [Google Scholar]
- [48].Monzingo AF, Dhaliwal S, Dutt-Chaudhuri A, et al. The structure of eukaryotic translation initiation factor-4E from wheat reveals a novel disulfide bond. Plant Physiol. 2007;143(4):1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tomoo K, Shen X, Okabe K, et al. Crystal structures of 7-methylguanosine 5’-triphosphate (m(7)GTP)- and P(1)-7-methylguanosine-P(3)-adenosine-5’,5’-triphosphate (m(7)GpppA)-bound human full-length eukaryotic initiation factor 4E: biological importance of the C-terminal flexible region. Biochem J. 2002;362(Pt 3):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Volpon L, Osborne MJ, Borden KL. NMR assignment of human eukaryotic translation initiation factor 4E (eIF4E) in its cap-free form. J Biomol NMR. 2006;36(Suppl 1):65. [DOI] [PubMed] [Google Scholar]
- [51].Volpon L, Culjkovic-Kraljacic B, Osborne MJ, et al. Importin 8 mediates m7G cap-sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. Proc Natl Acad Sci USA. 2016;113(19):5263–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Borden KLB, Volpon L. The diversity, plasticity, and adaptability of cap-dependent translation initiation and the associated machinery. RNA Biol. 2020;17(9):1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Coutinho de Oliveira L, Volpon L, Rahardjo AK, et al. Structural studies of the eIf4E-VPg complex reveal a direct competition for capped RNA: Implications for translation. Proc Natl Acad Sci USA. 2019;116(48):24056–24065 do i:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Culjkovic-Kraljacic B, Fernando TM, Marullo R, et al. Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B-cell lymphomas. Blood. 2016;127(7):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Osborne MJ, Borden KL. The eukaryotic translation initiation factor eIF4E in the nucleus: taking the road less traveled. Immunol Rev. 2015;263(1):210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Volpon L, Osborne MJ, Borden KLB. Biochemical and Structural Insights into the Eukaryotic Translation Initiation Factor eIF4E. Curr Protein Pept Sci. 2019;20(6):525–535. [DOI] [PubMed] [Google Scholar]
- [57].Zuberek J, Jemielity J, Jablonowska A, et al. Influence of electric charge variation at residues 209 and 159 on the interaction of eIF4E with the mRNA 5' terminus. Biochemistry. 2004;43(18):5370–5379. [DOI] [PubMed] [Google Scholar]
- [58].Kentsis A, Topisirovic I, Culjkovic B, et al. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci USA. 2004;101(52):18105–18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu W, Jankowska-Anyszka M, Piecyk K, et al. Structural basis for nematode eIF4E binding an m(2,2,7)G-Cap and its implications for translation initiation. Nucleic Acids Res. 2011;39(20):8820–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tamarkin-Ben-Harush A, Vasseur JJ, Debart F, et al. Cap-proximal nucleotides via differential eIF4E binding and alternative promoter usage mediate translational response to energy stress. Elife. 2017;6:21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].von der Haar T, Gross JD, Wagner G, et al. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol. 2004;11(6):503–511. [DOI] [PubMed] [Google Scholar]
- [62].Osborne MJ, Volpon L, Memarpoor-Yazdi M, et al. Identification and Characterization of the Interaction Between the Methyl-7-Guanosine Cap Maturation Enzyme RNMT and the Cap-Binding Protein eIF4E. J Mol Biol. 2022;434(5):167451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Saha B, Bhardwaj U, Goss DJ. Thermodynamically Favorable Interactions between eIF4E Binding Domain of eIF4GI with Structured 5'-Untranslated Regions Drive Cap-Independent Translation of Selected mRnas. Biochemistry. 2023;62(11):1767–1775. [DOI] [PubMed] [Google Scholar]
- [64].von Der Haar T, Ball PD, McCarthy JE. Stabilization of eukaryotic initiation factor 4E binding to the mRNA 5’-Cap by domains of eIF4G. J Biol Chem. 2000;275(39):30551–30555. [DOI] [PubMed] [Google Scholar]
- [65].Volpon L, Osborne MJ, Capul AA, et al. Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E. Proc Natl Acad Sci USA. 2010;107(12):5441–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mattaj IW. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986;46(6):905–911. [DOI] [PubMed] [Google Scholar]
- [67].Saponara AG, Enger MD. “Occurrence of N2,N2,7-trimethylguanosine in minor RNA species of a mammalian cell line”. Nature. 1969;223(5213):1365–1366. [DOI] [PubMed] [Google Scholar]
- [68].Wallace A, Filbin ME, Veo B, et al. The nematode eukaryotic translation initiation factor 4E/G complex works with a trans-spliced leader stem-loop to enable efficient translation of trimethylguanosine-capped RNAs. Mol Cell Biol. 2010;30(8):1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wurth L, Gribling-Burrer AS, Verheggen C, et al. Hypermethylated-capped selenoprotein mRnas in mammals. Nucleic Acids Res. 2014;42(13):8663–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yedavalli VS, Jeang KT. Trimethylguanosine capping selectively promotes expression of Rev-dependent HIV-1 RNAs. Proc Natl Acad Sci USA. 2010;107(33):14787–14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Niedzwiecka A, Marcotrigiano J, Stepinski J, et al. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5’ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol. 2002;319(3):615–635. [DOI] [PubMed] [Google Scholar]
- [72].Rousseau D, Kaspar R, Rosenwald I, et al. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci U S A. 1996;93(3):1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Andreou AZ, Klostermeier D. The DEAD-box helicase eIF4A: paradigm or the odd one out? RNA Biol. 2013;10(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Brito Querido J, Diaz-Lopez I, Ramakrishnan V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat Rev Mol Cell Biol. 2023;25:168–186. [DOI] [PubMed] [Google Scholar]
- [75].Gross JD, Moerke NJ, von der Haar T, et al. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell. 2003;115(6):739–750. [DOI] [PubMed] [Google Scholar]
- [76].Gruner S, Peter D, Weber R, et al. The Structures of eIF4E-eIF4G Complexes Reveal an Extended Interface to Regulate Translation Initiation. Mol Cell. 2016;64(3):467–479. [DOI] [PubMed] [Google Scholar]
- [77].Marcotrigiano J, Gingras AC, Sonenberg N, et al. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell. 1999;3(6):707–716. [DOI] [PubMed] [Google Scholar]
- [78].Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11(2):113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Graff JR, Boghaert ER, De Benedetti A, et al. Reduction of translation initiation factor 4E decreases the malignancy of ras-transformed cloned rat embryo fibroblasts. Int J Cancer. 1995;60(2):255–263. [DOI] [PubMed] [Google Scholar]
- [80].Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117(9):2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].de la Parra C, Ernlund A, Alard A, et al. A widespread alternate form of cap-dependent mRNA translation initiation. Nat Commun. 2018;9(1):3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jeong SJ, Park S, Nguyen LT, et al. A threonyl-tRNA synthetase-mediated translation initiation machinery. Nat Commun. 2019;10(1):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Joshi B, Cameron A, Jagus R. Characterization of mammalian eIF4E-family members. Eur J Biochem. 2004;271(11):2189–2203. [DOI] [PubMed] [Google Scholar]
- [84].Landon AL, Muniandy PA, Shetty AC, et al. MNKs act as a regulatory switch for eIF4E1 and eIF4E3 driven mRNA translation in DLBCL. Nat Commun. 2014;5:5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Morita M, Ler LW, Fabian MR, et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol Cell Biol. 2012;32(17):3585–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nguyen KT, Holloway MP, Altura RA. The CRM1 nuclear export protein in normal development and disease. Int J Biochem Mol Biol. 2012;3(2):137–151. [PMC free article] [PubMed] [Google Scholar]
- [87].Osborne MJ, Volpon L, Kornblatt JA, et al. eIF4E3 acts as a tumor suppressor by utilizing an atypical mode of methyl-7-guanosine cap recognition. Proc Natl Acad Sci U S A. 2013;110(10):3877–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rosettani P, Knapp S, Vismara MG, et al. Structures of the human eIF4E homologous protein, h4EHP, in its m7GTP-bound and unliganded forms. J Mol Biol. 2007;368(3):691–705. [DOI] [PubMed] [Google Scholar]
- [89].Uniacke J, Perera JK, Lachance G, et al. Cancer cells exploit eIF4E2-directed synthesis of hypoxia response proteins to drive tumor progression. Cancer Res. 2014;74(5):1379–1389. [DOI] [PubMed] [Google Scholar]
- [90].Weiss B, Allen GE, Kloehn J, et al. eIF4E3 forms an active eIF4F complex during stresses (eIF4FS) targeting mTOR and re-programs the translatome. Nucleic Acids Res. 2021;49(9):5159–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Clarke BP, Angelos AE, Mei M, et al. Cryo-EM structure of the CBC-ALYREF complex. eLife. 2023;12:RP91432. [Google Scholar]
- [92].Izaurralde E, Lewis J, McGuigan C, et al. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell. 1994;78(4):657–668. [DOI] [PubMed] [Google Scholar]
- [93].Izaurralde E, Stepinski J, Darzynkiewicz E, et al. A cap binding protein that may mediate nuclear export of RNA polymerase II-transcribed RNAs. J Cell Bio. 1992;118(6):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lewis JD, Izaurralde E, Jarmolowski A, et al. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5’ splice site. Genes Dev. 1996;10(13):1683–1698. [DOI] [PubMed] [Google Scholar]
- [95].Xie Y, Ren Y. Mechanisms of nuclear mRNA export: A structural perspective. Traffic. 2019;20(11):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gao Q, Das B, Sherman F, et al. Cap-binding protein 1-mediated and eukaryotic translation initiation factor 4E-mediated pioneer rounds of translation in yeast. Proc Natl Acad Sci U S A. 2005;102(12):4258–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Maquat LE, Hwang J, Sato H, et al. CBP80-promoted mRNP rearrangements during the pioneer round of translation, nonsense-mediated mRNA decay, and thereafter. Cold Spring Harb Symp Quant Biol. 2010;75:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cohen N, Sharma M, Kentsis A, et al. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. Embo J. 2001;20(16):4547–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dostie J, Lejbkowicz F, Sonenberg N. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J Cell Bio. 2000;148(2):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ghram M, Morris G, Culjkovic-Kraljacic B, et al. The eukaryotic translation initiation factor eIF4E reprograms alternative splicing. Embo J. 2023;42(7):e110496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lejbkowicz F, Goyer C, Darveau A, et al. A fraction of the mRNA 5’ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc Natl Acad Sci U S A. 1992;89(20):9612–9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Topisirovic I, Capili AD, Borden KL. Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Mol Cell Biol. 2002;22(17):6183–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Topisirovic I, Guzman ML, McConnell MJ, et al. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol. 2003;23(24):8992–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Iborra FJ, Jackson DA, Cook PR. Coupled transcription and translation within nuclei of mammalian cells. Science. 2001;293(5532):1139–1142. [DOI] [PubMed] [Google Scholar]
- [105].Kachaev ZM, Ivashchenko SD, Kozlov EN, et al. Localization and Functional Roles of Components of the Translation Apparatus in the Eukaryotic Cell Nucleus. Cells. 2021;10(11):3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lang V, Zanchin NI, Lunsdorf H, et al. Initiation factor eIF-4E of Saccharomyces cerevisiae. Distribution within the cell, binding to mRNA, and consequences of its overproduction. J Biol Chem. 1994;269(8):6117–6123. [PubMed] [Google Scholar]
- [107].Topisirovic I, Culjkovic B, Cohen N, et al. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. Embo J. 2003;22(3):689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Topisirovic I, Kentsis A, Perez JM, et al. Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels. Mol Cell Biol. 2005;25(3):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lai HK, Borden KL. The promyelocytic leukemia (PML) protein suppresses cyclin D1 protein production by altering the nuclear cytoplasmic distribution of cyclin D1 mRNA. Oncogene. 2000;19(13):1623–1634. [DOI] [PubMed] [Google Scholar]
- [110].Kentsis A, Volpon L, Topisirovic I, et al. Further evidence that ribavirin interacts with eIF4E. RNA. 2005;11(12):1762–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Phillips A, Blaydes JP. MNK1 and EIF4E are downstream effectors of MEKs in the regulation of the nuclear export of HDM2 mRNA. Oncogene. 2008;27(11):1645–1649. [DOI] [PubMed] [Google Scholar]
- [112].Topisirovic I, Ruiz-Gutierrez M, Borden KL. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004;64(23):8639–8642. [DOI] [PubMed] [Google Scholar]
- [113].Culjkovic-Kraljacic B, Baguet A, Volpon L, et al. The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Rep. 2012;2(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Culjkovic-Kraljacic B, Skrabanek L, Revuelta MV, et al. The eukaryotic translation initiation factor eIF4E elevates steady-state m(7)G capping of coding and noncoding transcripts. Proc Natl Acad Sci USA. 2020;117(43):26773–26783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jiao X, Xiang S, Oh C, et al. Identification of a quality-control mechanism for mRNA 5’-end capping. Nature. 2010;467(7315):608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jiao Y, Riechmann JL, Meyerowitz EM. Transcriptome-wide analysis of uncapped mRnas in Arabidopsis reveals regulation of mRNA degradation. Plant Cell. 2008;20(10):2571–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lim SK, Maquat LE. Human beta-globin mRnas that harbor a nonsense codon are degraded in murine erythroid tissues to intermediates lacking regions of exon I or exons I and II that have a cap-like structure at the 5’ termini. Embo J. 1992;11(9):3271–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Otsuka Y, Kedersha NL, Schoenberg DR. Identification of a cytoplasmic complex that adds a cap onto 5’-monophosphate RNA. Mol Cell Biol. 2009;29(8):2155–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Schoenberg DR, Maquat LE. Re-capping the message. Trends Biochem Sci. 2009;34(9):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Grasso L, Suska O, Davidson L, et al. mRNA Cap Methylation in Pluripotency and Differentiation. Cell Rep. 2016;16(5):1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mao X, Schwer B, Shuman S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol Cell Biol. 1995;15(8):4167–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chu C, Shatkin AJ. Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes. Mol Cell Biol. 2008;28(19):5829–5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Pillutla RC, Yue Z, Maldonado E, et al. Recombinant human mRNA cap methyltransferase binds capping enzyme/RNA polymerase IIo complexes. J Biol Chem. 1998;273(34):21443–21446. [DOI] [PubMed] [Google Scholar]
- [124].Shafer B, Chu C, Shatkin AJ. Human mRNA cap methyltransferase: alternative nuclear localization signal motifs ensure nuclear localization required for viability. Mol Cell Biol. 2005;25(7):2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Srinivasan P, Piano F, Shatkin AJ. mRNA capping enzyme requirement for Caenorhabditis elegans viability. J Biol Chem. 2003;278(16):14168–14173. [DOI] [PubMed] [Google Scholar]
- [126].Tsukamoto T, Shibagaki Y, Niikura Y, et al. Cloning and characterization of three human cDnas encoding mRNA (guanine-7-)-methyltransferase, an mRNA cap methylase. Biochem Biophys Res Commun. 1998;251(1):27–34. [DOI] [PubMed] [Google Scholar]
- [127].Yamada-Okabe T, Doi R, Shimmi O, et al. Isolation and characterization of a human cDNA for mRNA 5’-capping enzyme. Nucleic Acids Res. 1998;26(7):1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Yue Z, Maldonado E, Pillutla R, et al. Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc Natl Acad Sci USA. 1997;94(24):12898–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chu C, Das K, Tyminski JR, et al. Structure of the guanylyltransferase domain of human mRNA capping enzyme. Proc Natl Acad Sci USA. 2011;108(25):10104–10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cowling VH. Regulation of mRNA cap methylation. Biochem J. 2009;425(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Fabrega C, Hausmann S, Shen V, et al. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell. 2004;13(1):77–89. [DOI] [PubMed] [Google Scholar]
- [132].Gonatopoulos-Pournatzis T, Dunn S, Bounds R, et al. RAM/Fam103a1 is required for mRNA cap methylation. Mol Cell. 2011;44(4):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Trotman JB, Giltmier AJ, Mukherjee C, et al. RNA guanine-7 methyltransferase catalyzes the methylation of cytoplasmically recapped RNAs. Nucleic Acids Res. 2017;45(18):10726–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mukherjee C, Patil DP, Kennedy BA, et al. Identification of cytoplasmic capping targets reveals a role for cap homeostasis in translation and mRNA stability. Cell Rep. 2012;2(3):674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Varshney D, Petit AP, Bueren-Calabuig JA, et al. Molecular basis of RNA guanine-7 methyltransferase (RNMT) activation by RAM. Nucleic Acids Res. 2016;44(21):10423–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cowling VH. Enhanced mRNA cap methylation increases cyclin D1 expression and promotes cell transformation. Oncogene. 2010;29(6):930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cowling VH, Cole MD. The Myc transactivation domain promotes global phosphorylation of the RNA polymerase II carboxy-terminal domain independently of direct DNA binding. Mol Cell Biol. 2007;27(6):2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Posternak V, Ung MH, Cheng C, et al. MYC Mediates mRNA Cap Methylation of Canonical Wnt/beta-Catenin Signaling Transcripts by Recruiting CDK7 and RNA Methyltransferase. Mol Cancer Res. 2017;15(2):213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].De Benedetti A, Harris AL. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31(1):59–72. [DOI] [PubMed] [Google Scholar]
- [140].Schmidt EV. The role of c-myc in regulation of translation initiation. Oncogene. 2004;23(18):3217–3221. [DOI] [PubMed] [Google Scholar]
- [141].Lombardi O, Varshney D, Phillips NM, et al. c-Myc deregulation induces mRNA capping enzyme dependency. Oncotarget. 2016;7(50):82273–82288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Fernandez-Sanchez ME, Gonatopoulos-Pournatzis T, Preston G, et al. S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation. Mol Cell Biol. 2009;29(23):6182–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wilkinson ME, Charenton C, Nagai K. RNA Splicing by the Spliceosome. Annu Rev Biochem. 2020;89:359–388. [DOI] [PubMed] [Google Scholar]
- [144].Wang Y, Liu J, Huang BO, et al. Mechanism of alternative splicing and its regulation. Biomed Rep. 2015;3(2):152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136(4):701–718. [DOI] [PubMed] [Google Scholar]
- [146].Adamia S, Bar-Natan M, Haibe-Kains B, et al. NOTCH2 and FLT3 gene mis-splicings are common events in patients with acute myeloid leukemia (AML): new potential targets in AML. Blood. 2014;123(18):2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Dvinge H, Bradley RK. Widespread intron retention diversifies most cancer transcriptomes. Genome Med. 2015;7(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rivera OD, Mallory MJ, Quesnel-Vallieres M, et al. Alternative splicing redefines landscape of commonly mutated genes in acute myeloid leukemia. Proc Natl Acad Sci USA. 2021;118(15):e2014967118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Saez B, Walter MJ, Graubert TA. Splicing factor gene mutations in hematologic malignancies. Blood. 2017;129(10):1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Taylor J, Lee SC. Mutations in spliceosome genes and therapeutic opportunities in myeloid malignancies. Genes Chromosomes Cancer. 2019;58(12):889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Urbanski LM, Leclair N, Anczukow O. Alternative-splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip Rev RNA. 2018;9(4):e1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Visconte V, M ON, H JR. Mutations in Splicing Factor Genes in Myeloid Malignancies: Significance and Impact on Clinical Features. Cancers (Basel). 2019;11(12):1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Graham PL, Yanowitz JL, Penn JK, et al. The translation initiation factor eIF4E regulates the sex-specific expression of the master switch gene Sxl in Drosophila melanogaster. PLOS Genet. 2011;7(7):e1002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Proudfoot NJ. Ending the message: poly(A) signals then and now. Genes Dev. 2011;25(17):1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Proudfoot NJ, Furger A, Dye MJ. Integrating mRNA processing with transcription. Cell. 2002;108(4):501–512. [DOI] [PubMed] [Google Scholar]
- [156].Tian B, Manley JL. Alternative polyadenylation of mRNA precursors. Nat Rev Mol Cell Biol. 2017;18(1):18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Mohanan NK, Shaji F, Koshre GR, et al. Alternative polyadenylation: An enigma of transcript length variation in health and disease. Wiley Interdiscip Rev RNA. 2022;13(1):e1692. [DOI] [PubMed] [Google Scholar]
- [158].Davis MR, Delaleau M, Borden KLB. Nuclear eIF4E Stimulates 3’-End Cleavage of Target RNAs. Cell Rep. 2019;27(5):1397–1408 e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Delaleau M. Monitoring eIf4E-Dependent Nuclear 3’ End mRNA Cleavage. Methods Mol Biol. 2021;2209:347–361. [DOI] [PubMed] [Google Scholar]
- [160].Bensidoun P, Zenklusen D, Oeffinger M. Choosing the right exit: How functional plasticity of the nuclear pore drives selective and efficient mRNA export. Wiley Interdiscip Rev RNA. 2021;12(6):e1660. [DOI] [PubMed] [Google Scholar]
- [161].Bonnet A, Palancade B. Regulation of mRNA trafficking by nuclear pore complexes. Genes (Basel). 2014;5(3):767–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Culjkovic-Kraljacic B, Borden KL. Aiding and abetting cancer: mRNA export and the nuclear pore. Trends Cell Biol. 2013;23(7):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Khan M, Hou S, Chen M, et al. Mechanisms of RNA export and nuclear retention. Wiley Interdiscip Rev RNA. 2023;14(3):e1755. [DOI] [PubMed] [Google Scholar]
- [164].Natalizio BJ, Wente SR. Postage for the messenger: designating routes for nuclear mRNA export. Trends Cell Biol. 2013;23(8):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Burns LT, Wente SR. From hypothesis to mechanism: uncovering nuclear pore complex links to gene expression. Mol Cell Biol. 2014;34(12):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol. 2010;11(7):490–501. [DOI] [PubMed] [Google Scholar]
- [167].Walde S, Kehlenbach RH. The Part and the Whole: functions of nucleoporins in nucleocytoplasmic transport. Trends Cell Biol. 2010;20(8):461–469. [DOI] [PubMed] [Google Scholar]
- [168].Hartenian E, Glaunsinger BA. Feedback to the central dogma: cytoplasmic mRNA decay and transcription are interdependent processes. Crit Rev Biochem Mol Biol. 2019;54(4):385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416(6880):499–506. [DOI] [PubMed] [Google Scholar]
- [170].McKee AE, Silver PA. Systems perspectives on mRNA processing. Cell Res. 2007;17(7):581–590. [DOI] [PubMed] [Google Scholar]
- [171].Stutz F, Izaurralde E. The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 2003;13(6):319–327. [DOI] [PubMed] [Google Scholar]
- [172].De Magistris P. The Great Escape: mRNA Export through the Nuclear Pore Complex. Int J Mol Sci. 2021;22(21):11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Palazzo AF, Qiu Y, Kang YM. mRNA nuclear export: how mRNA identity features distinguish functional RNAs from junk transcripts. RNA Biol. 2024;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Fribourg S, Braun IC, Izaurralde E, et al. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol Cell. 2001;8(3):645–656. [DOI] [PubMed] [Google Scholar]
- [175].Viphakone N, Cumberbatch MG, Livingstone MJ, et al. Luzp4 defines a new mRNA export pathway in cancer cells. Nucleic Acids Res. 2015;43(4):2353–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wiegand HL, Coburn GA, Zeng Y, et al. Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol Cell Biol. 2002;22(1):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Dufu K, Livingstone MJ, Seebacher J, et al. ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex. Genes Dev. 2010;24(18):2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Cheng H, Dufu K, Lee CS, et al. Human mRNA export machinery recruited to the 5’ end of mRNA. Cell. 2006;127(7):1389–1400. [DOI] [PubMed] [Google Scholar]
- [179].Visa N, Izaurralde E, Ferreira J, et al. A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Bio. 1996;133(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Katahira J. mRNA export and the TREX complex. Biochim Biophys Acta. 2012;1819(6):507–513. [DOI] [PubMed] [Google Scholar]
- [181].Rodriguez-Navarro S, Hurt E. Linking gene regulation to mRNA production and export. Curr Opin Cell Biol. 2011;23(3):302–309. [DOI] [PubMed] [Google Scholar]
- [182].Shi M, Zhang H, Wu X, et al. ALYREF mainly binds to the 5‘and the 3’ regions of the mRNA in vivo. Nucleic Acids Res. 2017;45(16):9640–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Viphakone N, Hautbergue GM, Walsh M, et al. TREX exposes the RNA-binding domain of Nxf1 to enable mRNA export. Nat Commun. 2012;3:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Cordiner RA, Dou Y, Thomsen R, et al. Temporal-iCLIP captures co-transcriptional RNA-protein interactions. Nat Commun. 2023;14(1):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Gromadzka AM, Steckelberg AL, Singh KK, et al. A short conserved motif in ALYREF directs cap- and EJC-dependent assembly of export complexes on spliced mRnas. Nucleic Acids Res. 2016;44(5):2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yang X, Yang Y, Sun BF, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27(5):606–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Huang Y, Steitz JA. SRprises along a messenger’s journey. Mol Cell. 2005;17(5):613–615. [DOI] [PubMed] [Google Scholar]
- [188].Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417(1):15–27. [DOI] [PubMed] [Google Scholar]
- [189].Rambout X, Maquat LE. The nuclear cap-binding complex as choreographer of gene transcription and pre-mRNA processing. Genes Dev. 2020;34(17–18):1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17(4):193–201. [DOI] [PubMed] [Google Scholar]
- [191].Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2(10):a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Bernad R, van der Velde H, Fornerod M, et al. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol. 2004;24(6):2373–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Singh BB, Patel HH, Roepman R, et al. The zinc finger cluster domain of RanBP2 is a specific docking site for the nuclear export factor, exportin-1. J Biol Chem. 1999;274(52):37370–37378. [DOI] [PubMed] [Google Scholar]
- [194].Carmody SR, Wente SR. mRNA nuclear export at a glance. J Cell Sci. 2009;122(Pt 12):1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Folkmann AW, Noble KN, Cole CN, et al. Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export. Nucleus. 2011;2(6):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Montpetit B, Thomsen ND, Helmke KJ, et al. A conserved mechanism of DEAD-box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature. 2011;472(7342):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].von Moeller H, Basquin C, Conti E. The mRNA export protein DBP5 binds RNA and the cytoplasmic nucleoporin NUP214 in a mutually exclusive manner. Nat Struct Mol Biol. 2009;16(3):247–254. [DOI] [PubMed] [Google Scholar]
- [198].Dong X, Biswas A, Suel KE, et al. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458(7242):1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Brennan CM, Gallouzi IE, Steitz JA. Protein ligands to HuR modulate its interaction with target mRnas in vivo. J Cell Bio. 2000;151(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Kimura T, Hashimoto I, Nagase T, et al. CRM1-dependent, but not ARE-mediated, nuclear export of IFN-alpha1 mRNA. J Cell Sci. 2004;117(Pt 11):2259–2270. [DOI] [PubMed] [Google Scholar]
- [201].Yang J, Bogerd HP, Wang PJ, et al. Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol Cell. 2001;8(2):397–406. [DOI] [PubMed] [Google Scholar]
- [202].Borden KLB. The Nuclear Pore Complex and mRNA Export in Cancer. Cancers (Basel). 2020;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Okamura M, Inose H, Masuda S. RNA Export through the NPC in Eukaryotes. Genes (Basel). 2015;6(1):124–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Walther TC, Pickersgill HS, Cordes VC, et al. The cytoplasmic filaments of the nuclear pore complex are dispensable for selective nuclear protein import. J Cell Bio. 2002;158(1):63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Bollmann F, Fechir K, Nowag S, et al. Human inducible nitric oxide synthase (iNOS) expression depends on chromosome region maintenance 1 (CRM1)- and eukaryotic translation initiation factor 4E (elF4E)-mediated nucleocytoplasmic mRNA transport. Nitric Oxide. 2013;30:49–59. [DOI] [PubMed] [Google Scholar]
- [206].Dostie J, Ferraiuolo M, Pause A, et al. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5’ cap-binding protein, eIF4E. Embo J. 2000;19(12):3142–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Hamada M, Haeger A, Jeganathan KB, et al. Ran-dependent docking of importin-beta to RanBP2/Nup358 filaments is essential for protein import and cell viability. J Cell Bio. 2011;194(4):597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Dawlaty MM, Malureanu L, Jeganathan KB, et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Mamane Y, Petroulakis E, Martineau Y, et al. Epigenetic activation of a subset of mRnas by eIF4E explains its effects on cell proliferation. PLOS ONE. 2007;2(2):e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Jensen KB, Dredge BK, Toubia J, et al. capCLIP: a new tool to probe translational control in human cells through capture and identification of the eIF4E-mRNA interactome. Nucleic Acids Res. 2021;49(18):e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Martin F, Barends S, Jaeger S, et al. Cap-assisted internal initiation of translation of histone H4. Mol Cell. 2011;41(2):197–209. [DOI] [PubMed] [Google Scholar]
- [212].Wang Z, Parisien M, Scheets K, et al. The cap-binding translation initiation factor, eIF4E, binds a pseudoknot in a viral cap-independent translation element. Structure. 2011;19(6):868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Wilson KF, Fortes P, Singh US, et al. The nuclear cap-binding complex is a novel target of growth factor receptor-coupled signal transduction. J Biol Chem. 1999;274(7):4166–4173. [DOI] [PubMed] [Google Scholar]
- [214].Scheper GC, van Kollenburg B, Hu J, et al. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277(5):3303–3309. [DOI] [PubMed] [Google Scholar]
- [215].Kim KM, Cho H, Choi K, et al. A new MIF4G domain-containing protein, CTIF, directs nuclear cap-binding protein CBP80/20-dependent translation. Genes Dev. 2009;23(17):2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Park Y, Park J, Hwang HJ, et al. Translation mediated by the nuclear cap-binding complex is confined to the perinuclear region via a CTIF-DDX19B interaction. Nucleic Acids Res. 2021;49(14):8261–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Rufener SC, Muhlemann O. eIF4E-bound mRnps are substrates for nonsense-mediated mRNA decay in mammalian cells. Nat Struct Mol Biol. 2013;20(6):710–717. [DOI] [PubMed] [Google Scholar]
- [218].Brunet I, Weinl C, Piper M, et al. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438(7064):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Nedelec S, Foucher I, Brunet I, et al. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proc Natl Acad Sci USA. 2004;101(29):10815–10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Niessing D, Blanke S, Jackle H. Bicoid associates with the 5’-cap-bound complex of caudal mRNA and represses translation. Genes Dev. 2002;16(19):2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Topisirovic I, Borden KL. Homeodomain proteins and eukaryotic translation initiation factor 4E (eIF4E): an unexpected relationship. Histol Histopathol. 2005;20(4):1275–1284. [DOI] [PubMed] [Google Scholar]
- [222].Matsuo H, Li H, McGuire AM, et al. Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat Struct Biol. 1997;4(9):717–724. [DOI] [PubMed] [Google Scholar]
- [223].Romagnoli A, D’Agostino M, Ardiccioni C, et al. Control of the eIF4E activity: structural insights and pharmacological implications. Cell Mol Life Sci. 2021;78(21–22):6869–6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Kentsis A, Dwyer EC, Perez JM, et al. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J Mol Biol. 2001;312(4):609–623. [DOI] [PubMed] [Google Scholar]
- [225].Kentsis A, Gordon RE, Borden KL. Self-assembly properties of a model RING domain. Proc Natl Acad Sci USA. 2002;99(2):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Siddiqui N, Tempel W, Nedyalkova L, et al. Structural insights into the allosteric effects of 4EBP1 on the eukaryotic translation initiation factor eIF4E. J Mol Biol. 2012;415(5):781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Tan NG, Ardley HC, Scott GB, et al. Human homologue of ariadne promotes the ubiquitylation of translation initiation factor 4E homologous protein, 4EHP. FEBS Lett. 2003;554(3):501–504. [DOI] [PubMed] [Google Scholar]
- [228].Papadopoulos E, Jenni S, Kabha E, et al. Structure of the eukaryotic translation initiation factor eIF4E in complex with 4EGI-1 reveals an allosteric mechanism for dissociating eIF4G. Proc Natl Acad Sci USA. 2014;111(31):E3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Joshi S, Platanias LC. Mnk kinase pathway: Cellular functions and biological outcomes. World J Biol Chem. 2014;5(3):321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Joshi S, Platanias LC. Mnk kinases in cytokine signaling and regulation of cytokine responses. Biomol Concepts. 2015;6(1):85. [DOI] [PubMed] [Google Scholar]
- [231].Furic L, Rong L, Larsson O, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA. 2010;107(32):14134–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Guo Q, Bartish M, Goncalves C, et al. The MNK1/2-eIF4E Axis Supports Immune Suppression and Metastasis in Postpartum Breast Cancer. Cancer Res. 2021;81(14):3876–3889. [DOI] [PubMed] [Google Scholar]
- [233].Proud CG. Mnks, eIF4E phosphorylation and cancer. Biochim Biophys Acta. 2015;1849(7):766–773. [DOI] [PubMed] [Google Scholar]
- [234].Martinez A, Sese M, Losa JH, et al. Phosphorylation of eIF4E Confers Resistance to Cellular Stress and DNA-Damaging Agents through an Interaction with 4E-T: A Rationale for Novel Therapeutic Approaches. PLOS ONE. 2015;10(4):e0123352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].McKendrick L, Morley SJ, Pain VM, et al. Phosphorylation of eukaryotic initiation factor 4E (eIF4E) at Ser209 is not required for protein synthesis in vitro and in vivo. Eur J Biochem. 2001;268(20):5375–5385. [DOI] [PubMed] [Google Scholar]
- [236].Morley SJ, Naegele S. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J Biol Chem. 2002;277(36):32855–32859. [DOI] [PubMed] [Google Scholar]
- [237].Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5’ cap. Nature. 1990;345(6275):544–547. [DOI] [PubMed] [Google Scholar]
- [238].Zhan Y, Dahabieh MS, Rajakumar A, et al. The role of eIF4E in response and acquired resistance to vemurafenib in melanoma. J Invest Dermatol. 2015;135(5):1368–1376. [DOI] [PubMed] [Google Scholar]
- [239].Crew JP, Fuggle S, Bicknell R, et al. Eukaryotic initiation factor-4E in superficial and muscle invasive bladder cancer and its correlation with vascular endothelial growth factor expression and tumour progression. Br J Cancer. 2000;82(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Culjkovic B, Borden KL. Understanding and Targeting the Eukaryotic Translation Initiation Factor eIF4E in Head and Neck Cancer. J Oncol. 2009;2009:981679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Lee JW, Choi JJ, Lee KM, et al. eIF-4E expression is associated with histopathologic grades in cervical neoplasia. Hum Pathol. 2005;36(11):1197–1203. [DOI] [PubMed] [Google Scholar]
- [242].Li BD, Gruner JS, Abreo F, et al. Prospective study of eukaryotic initiation factor 4E protein elevation and breast cancer outcome. Ann Surg. 2002;235(5):732–738; discussion 738-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Nathan CO, Franklin S, Abreo FW, et al. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol. 1999;17(9):2909–2914. [DOI] [PubMed] [Google Scholar]
- [244].Pettersson F, Yau C, Dobocan MC, et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin Cancer Res. 2011;17(9):2874–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Culjkovic B, Tan K, Orolicki S, et al. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Bio. 2008;181(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Polunovsky VA, Rosenwald IB, Tan AT, et al. Translational control of programmed cell death: eukaryotic translation initiation factor 4E blocks apoptosis in growth-factor-restricted fibroblasts with physiologically expressed or deregulated Myc. Mol Cell Biol. 1996;16(11):6573–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Smith MR, Jaramillo M, Liu YL, et al. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990;2(7):648–654. [PubMed] [Google Scholar]
- [248].Tan A, Bitterman P, Sonenberg N, et al. Inhibition of Myc-dependent apoptosis by eukaryotic translation initiation factor 4E requires cyclin D1. Oncogene. 2000;19(11):1437–1447. [DOI] [PubMed] [Google Scholar]
- [249].Zahreddine HA, Culjkovic-Kraljacic B, Emond A, et al. The eukaryotic translation initiation factor eIF4E harnesses hyaluronan production to drive its malignant activity. Elife. 2017;6:e29830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Joshi-Barve S, Rychlik W, Rhoads RE. Alteration of the major phosphorylation site of eukaryotic protein synthesis initiation factor 4E prevents its association with the 48 S initiation complex. J Biol Chem. 1990;265(5):2979–83. [PubMed] [Google Scholar]
- [251].Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511(7507):90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Westman B, Beeren L, Grudzien E, et al. The antiviral drug ribavirin does not mimic the 7-methylguanosine moiety of the mRNA cap structure in vitro. RNA. 2005;11(10):1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Yan Y, Svitkin Y, Lee JM, et al. Ribavirin is not a functional mimic of the 7-methyl guanosine mRNA cap. RNA. 2005;11(8):1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Chen J, Xu X, Chen J. Clinically relevant concentration of anti-viral drug ribavirin selectively targets pediatric osteosarcoma and increases chemosensitivity. Biochem Biophys Res Commun. 2018;506(3):604–610. [DOI] [PubMed] [Google Scholar]
- [255].Huq S, Casaos J, Serra R, et al. Repurposing the FDA-Approved Antiviral Drug Ribavirin as Targeted Therapy for Nasopharyngeal Carcinoma. Mol Cancer Ther. 2020;19(9):1797–1808. [DOI] [PubMed] [Google Scholar]
- [256].Huq S, Kannapadi NV, Casaos J, et al. Preclinical efficacy of ribavirin in SHH and group 3 medulloblastoma. J Neurosurg Pediatr. 2021;27(4):482–488. [DOI] [PubMed] [Google Scholar]
- [257].Pettersson F, Del Rincon SV, Emond A, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res. 2015;75(6):1102–1112. [DOI] [PubMed] [Google Scholar]
- [258].Volpin F, Casaos J, Sesen J, et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene. 2017;36(21):3037–3047. [DOI] [PubMed] [Google Scholar]
- [259].Xu M, Tao Z, Wang S, et al. Suppression of oncogenic protein translation via targeting eukaryotic translation initiation factor 4E overcomes chemo-resistance in nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2019;512(4):902–907. [DOI] [PubMed] [Google Scholar]
- [260].Zha HL, Chen W, Shi W, et al. Inhibition of Eukaryotic Initiating Factor eIF4E Overcomes Abemaciclib Resistance in Gastric Cancer. Curr Med Sci. 2023;43(5):927–934. [DOI] [PubMed] [Google Scholar]
- [261].Wang S, Pang T, Gao M, et al. HPV E6 induces eIF4E transcription to promote the proliferation and migration of cervical cancer. FEBS Lett. 2013;587(6):690–697. [DOI] [PubMed] [Google Scholar]
- [262].Hong DS, Kurzrock R, Oh Y, et al. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin Cancer Res. 2011;17(20):6582–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


