Abstract
We analyzed the protective mechanisms induced against respiratory syncytial virus subgroup A (RSV-A) infection in the lower and upper respiratory tracts (LRT and URT) of BALB/c mice after intraperitoneal immunization with a recombinant fusion protein incorporating residues 130 to 230 of RSV-A G protein (BBG2Na). Mother-to-offspring antibody (Ab) transfer and adoptive transfer of BBG2Na-primed B cells into SCID mice demonstrated that Abs are important for LRT protection but have no effect on URT infection. In contrast, RSV-A clearance in the URT was achieved in a dose-dependent fashion after adoptive transfer of BBG2Na-primed T cells, while it was abolished in BBG2Na-immunized mice upon in vivo depletion of CD4+, but not CD8+, T cells. Furthermore, the conserved RSV-A G protein cysteines and residues 193 and 194, overlapping the recently identified T helper cell epitope on the G protein (P. W. Tebbey et al., J. Exp. Med. 188:1967–1972, 1998), were found to be essential for URT but not LRT protection. Taken together, these results demonstrate for the first time that CD4+ T cells induced upon parenteral immunization with an RSV G protein fragment play a critical role in URT protection of normal mice against RSV infection.
Respiratory syncytial virus (RSV) causes frequent and repeated infections in humans worldwide that are responsible for mild to severe clinical symptoms. In adults, infection is generally confined to the upper respiratory tract (URT), while infection of the lower respiratory tract (LRT) accounts for severe pneumonia and bronchiolitis in infants and immunocompromised individuals (44). Reinfections are common despite the development of mucosal and systemic immune responses which indeed fail to confer protection, although they progressively diminish the respiratory disease. Identification of the components necessary for the induction of a complete and safe immune protective response is a prerequisite for the development of an efficient RSV vaccine.
Evidence suggests that protection of the LRT may be achieved primarily through high levels of circulating antibodies (Abs), whereas protection of the URT may be primarily mediated by secretory immunoglobulin A's (IgAs) (26, 27, 52). In addition, T cells play an important mechanistic role in respiratory tract protection since prolonged virus shedding or severe/fatal RSV infection occurs in patients with deficiencies in cellular immunity (16).
Among RSV proteins, F and G glycoproteins generate the most potent immune protective responses in animal models (10, 40). F protein is highly conserved among all RSV isolates; it induces cross-reactive Abs as well as a predominant T helper 1 (Th1)-type T-cell response and virus-specific cytotoxic CD8+ T cells (21, 31, 33, 49). In contrast, apart from a conserved central domain incorporating two disulfide bonds (9, 48), G protein is characterized by an extensive variability between and even within RSV subgroups, which might play a role in repeated infections. This protein confers protective immunity that tends to be group specific. In addition, priming of mice with purified G protein results in adverse anti-RSV Th2-type T-cell responses upon RSV subgroup A (RSV-A) challenge, responsible for extensive lung eosinophilia (1, 17, 45). This immunopathologic response has been recently associated with the presence of a Th cell epitope located between residues 184 and 198 of RSV G protein (47).
In a novel approach to RSV vaccines, we recently reported that a fusion protein, designated BBG2Na, induces a strong and long-lasting protection against RSV infection in mice without priming for RSV-enhanced pathology (11, 36, 37). Interestingly, this protein comprises residues 130 to 230 of RSV-A (Long strain) G protein (G2Na), including the conserved central domain and the immunopathology-associated Th cell epitope, fused to the albumin binding region of streptococcal protein G (BB). Surprisingly, protection is induced in both the LRT and URT and is maintained for at least 48 weeks after three intraperitoneal (i.p.) injections of 20 μg of alum-adsorbed BBG2Na (37). Such a protective efficacy has never previously been reported with other subunit vaccines administered similarly. In the lungs, viral clearance is achieved within 24 h following intranasal (i.n.) challenge. In contrast, complete elimination of nasal RSV-A requires 2 to 3 days. Passive transfer of immune sera confirmed the capacity of anti-BBG2Na serum Abs to prevent and eliminate RSV-A in the LRT (37). In contrast, URT infection was not affected, suggesting that URT and LRT protection rely on separate immune mechanisms.
To identify these mechanisms, we investigated the relative contributions of Abs and lymphocyte populations to the anti-RSV protection of mouse LRT and URT. We also used a panel of site-specific and deletion mutants to map the residues implicated in BBG2Na-mediated protection. Our data demonstrate that different epitopes and separate immune mechanisms account for LRT and URT protection in mice after immunization with this recombinant RSV G protein fragment. In addition, we demonstrate for the first time that CD4+ T cells play an essential role in RSV protection of the URT.
MATERIALS AND METHODS
Gene assembly, vector constructions, and expression and purification of BBG2Na and derived deletion and substitution mutants.
Gene assembly, vector constructions, expression, and first-step protein purification of BBG2Na and BBG2ΔCa (BBGnat and BBGcys, respectively, in reference 37) were undertaken as previously described (37). Gsera was derived from G2Na by alternative PCR site-directed mutagenesis (30), such that the conserved Cys residues at positions 173, 176, 182, and 186 were each mutated to Ser. Six deletion mutants were generated by PCR from a G2Na template using a single 5′ oligonucleotide (5′-CGA GAA TTC CAT GCA GAC CCA GCC GAG-3′), incorporating a unique EcoRI site (underlined), and a series of nested 3′ oligonucleotides (5′-ATCAAGCTTATTTGTTCGGGATAC-3′, 5′-ATCAAGCTTACGGTTTTTTGTTCGGGATACG-3′, 5′-ATCAAGCTTATTTGCCCGGTTTTTTGTTC-3′, 5′-ATCAAGCTTAGGTTTTTTGCCCGG-3′, 5′-ATCAAGCTTAGGTCGTGGTTTTTTGCCCG-3′, and 5′-ATCAAGCTTACGGGATGGTTTTGC-3′), each incorporating a unique HindIII site (underlined). Resultant gene fragments encode residues 140 to 200 (G200a), 140 to 198 (G198a), 140 to 196 (G196a), 140 to 194 (G194a), 140 to 192 (G129a), and 140 to 190 (G190a), respectively, of the RSV-A G protein (Fig. 1).
FIG. 1.
BBG2Na and derived deletion and substitution mutants. Mutant proteins were derived from G2Na by replacement of the codons for the conserved RSV G protein Cys (∗) residues 176 and 182 (for G2DCa) and 173, 176, 182 and 186 (for G2Sera) by codons for serine (S). Deletion proteins BBG200a, BBG198a, BBG196a, BBG194a, BBG192a, and BBG190a were obtained from a gene derived from G2Na, encoding a unique N-terminal amino acid 140 and progressively truncated by two amino acids from the C terminus to positions 200, 198, 196, 194, 192, and 190, respectively. These constructs were expressed with BB, an albumin binding region of streptococcal protein G, as the fusion partner.
All PCR amplicons were digested with EcoRI and HindIII, purified, and subcloned into pvaBB308 expression vector (pABP308 in reference 28). Gene fragment sequences were confirmed by Taq DNA polymerase cycle sequencing on an automated DNA sequencer (model 373A; Perkin-Elmer Applied Biosystems, Foster City, Calif.). Resultant vectors pvaBBG2Sera, pvaBBG200a, pvaBBG198a, pvaBBG196a, pvaBBG194a, pvaBBG192a, and pvaBBG190a encoded the fusion proteins BBG2Sera, BBG200a, BBG198a, BBG196a, BBG194a, BBG192a, and BBG190a, respectively. Following transformation of Escherichia coli RV 308 (24), each recombinant protein was produced as intracellular inclusion bodies, recovered, and renatured as previously described (37, 43). BBG2Na, BBG2DCa, and BBG2sera were purified to homogeneity by a two-step process including affinity chromatography on human serum albumin-Sepharose and reverse-phase high-performance liquid chromatography (HPLC) as previously described (37). After an albumin-Sepharose affinity chromatography step, BBG200a, BBG198a, BBG196a, BBG194a, BBG192a, and BBG190a were further purified by anion-exchange chromatography and reverse-phase HPLC. Lyophilized proteins were analyzed for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 15% homogeneous gels under reducing conditions. Protein content was determined by the Pierce bicinchoninic acid assay.
Mice.
Specific-pathogen-free female BALB/c, CB17 scid/scid (SCID), and nu/nu BALB/c inbred mice, aged 8 to 9 weeks, were purchased from IFFA CREDO (l'Arbresle, France) and kept under specific-pathogen-free conditions. They were given sterilized mouse maintenance diet AO4 (Usine d'Alimentation Rationnelle, Villemoissin-sur-Orge, France) and water ad libitum. For maternal antibody experiments, breeding cages were checked daily for new births, and the day of birth was recorded as the day the litter was found. Pups were kept with mothers until weaning at the age of 4 weeks.
ELISA.
BB-, BBG2Na-, and RSV-A-specific IgG titers were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (37). ELISA titers were expressed as the reciprocal of the last dilution with an optical density of >0.15 and at least twofold that of the control well to which no sample was added.
Immunizations.
BALB/c mice were confirmed seronegative for RSV-A before inclusion in the experiments. They were given i.p. two doses of either 20 μg of BBG2Na at a 3-week interval before mating (for experiments of mother-to-offspring Ab transfer), 50 μg of BBG2Na at a 2-week interval (for cell transfer experiments), or 20 μg at a 2-week interval of BBG2Na or BBG2Na-derived mutant proteins (for in vivo T-cell depletion and protection experiments). Control mice received phosphate-buffered saline (PBS) or 105 50% tissue culture infectious doses (TCID50) of RSV-A injected similarly. All immunizations were performed in 200-μl volumes in PBS with 20% (vol/vol) Al(OH3) (alum; Superfos BioSector, Vedbaek, Denmark).
Preparation of lymphocyte populations and adoptive cell transfer.
Ten days after the last immunization, BALB/c mice were sacrificed. Their spleens and mesenteric lymph nodes were removed, processed into single-cell suspensions in RPMI 1640 (Gibco, Cergy Pontoise, France) with 10% fetal calf serum, and incubated on a nylon wool column for 90 min at 37°C. The nonadherent cells were carefully eluted and washed in PBS. T lymphocytes were obtained after incubation of these cells for 45 min at room temperature with microbeads (Dynal, Compiègne, France) previously coated with anti-CD19 and/or anti-CD8 Abs (Pharmingen Inc., San Diego, Calif.). Attached B and/or CD8+ T cells were eliminated with a magnet. B-cell preparations were obtained by gently teasing the nylon wool in cold RPMI 1640. Recovered cells were incubated on a plastic dish overnight at 37°C and 45 min at room temperature with microbeads previously coated with anti-CD4 and anti-CD8 Abs to remove the remaining macrophages and T cells, respectively. Preparations of CD3+ T, CD4+ T, and B cells (>95% viability) were more than 98% pure as controlled by flow cytometry. They were transferred into H-2-identical SCID mice within 3 h after RSV-A infection by i.p. injection of 5 × 106, 10 × 106, or 15 × 106 CD3+ T cells, 20 × 106 B cells alone or together with 2 × 106 CD4+ T cells, or 2 × 106 CD4+ T cells alone.
Virus preparation, challenge procedures, and virus titration.
RSV-A (Long strain; ATCC VR-26; American Type Culture Collection, Manassas, Va.) was propagated in HEp-2 cells (ECACC 86030501; European Collection of Animal Cell Cultures, Porton Down, Salisbury, United Kingdom) as previously described (48). The virus stock was prepared from the supernatant of a 48- to 72-h culture and stored at −196°C until use. Mice were anesthetized with a 4/1 (vol/vol) mixture of ketamine (Imalgene 500; Rhône Mérieux, Lyon, France) and xylazine (Rompun 2%; Bayer, Puteaux, France) (2.5 ml/kg of body weight before i.n. instillation of 105 TCID50 RSV-A). They were sacrificed 5 to 11 days later following anesthesia and total intracardiac puncture. Removal and processing of lungs, nasal tract lavage fluids (NTL) and virus titrations were undertaken as previously described (37). The limit of detection of virus in lung tissues and for NTL were log10 1.45/g and 0.45/ml, respectively.
In vivo depletion of T-cell subsets.
Rat GK1-5 (anti-CD4) and H35 17.2 (anti-CD8) hybridoma cells were kindly provided by S. Izui. These cells (107) were injected i.p. to pristane-primed nu/nu BALB/c mice. Ascitic fluids were pooled and titrated by flow cytometry by staining of normal spleen cells, using a fluorescein isothiocyanate-conjugated goat anti-rat Ab as a secondary reagent. Mice were depleted of CD4+ and/or CD8+ T cells by i.p. injections of 300-μl PBS aliquots containing GK1-5 and H35 17.2 ascitic fluids diluted 1/2 and 1/6, respectively. These treatments were performed on days 2 and 1 before challenge and again on days 1 and 3 postchallenge. Mice were sacrificed on day 5. Depletion was confirmed by flow cytometric analysis of cells from blood, spleen, and nasal tract-associated lymphoid tissues (NALT), using double staining with combinations of anti-CD3 (Caltag, San Francisco, Calif.) and anti-CD4 (Caltag) or anti-CD8 (Caltag) Abs.
Statistical analyses.
Statistical analyses were done using the t test and Kolmogorov-Smirnov (for low sample numbers) tests of the Statigraphic software program (Manugistics, Rockville, Md.).
RESULTS
Protection of LRT but not URT after mother-to-offspring Ab transfer.
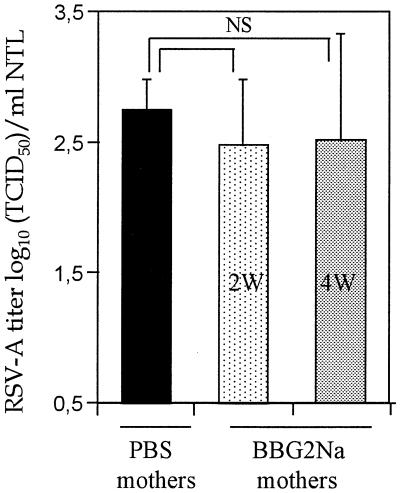
In contrast to active immunization, passive transfer of BBG2Na-immune sera to naive adult mice remains without effect on URT infection, although it prevents lung RSV-A infection (37). The detection of transferred Abs in the serum, bronchoalveolar lavage fluid, and nasal tract of the recipient mice 24 to 48 h after i.p. or i.n. passive transfer demonstrates that immunoglobulins rapidly transit from one compartment to another (Plotnicky-Gilquin, personal observation). Therefore, we first asked whether the lack of URT protection following passive Ab transfer could be explained by the generation of lower serum BBG2Na IgG Ab titers following passive transfer than those observed following BBG2Na immunization. This hypothesis was assessed with a neonatal murine model in which transfer of maternal Ab from mother to pups is so efficient as to result in IgG Ab titers in pups similar to those in their immunized mothers (5). As previously demonstrated, serum BBG2Na Abs in the offspring of BALB/c mothers immunized twice with BBG2Na prior to mating reached means ± standard deviation (SD) of 4.25 ± 0.08 and 5.23 ± 0.11 log10 in 2- and 4-week-old litters, respectively (5). These Ab titers are thus similar to those observed in BBG2Na-immunized adult BALB/c mice, in which URT protection is observed. In addition, nasal Ab titers to BBG2Na in the offsprings were 2.3 ± 0.6 and 1.9 ± 0.57 log10, respectively (versus <0.3 log10 in naive mice), which were also similar to the titers observed in BBG2Na-immunized mice (2.16 ± 0.51 log10). When challenged with RSV-A, lungs of all of these 2- and 4-week-old pups from BBG2Na-immuned mothers were protected, with virus absent or at the limit of detection of the assay in the LRT (1.74 ± 0.38 log10 TCID50/g of lung) (5). In contrast, they all demonstrated URT infection (Fig. 2); there were indeed no significant differences in nasal tract RSV-A titers (mean, 2.48 ± 0.54 log10 TCID50/ml of NTL for 2- and 4-week-old mice) compared to control litters born from PBS-control mothers (2.80 ± 0.28 log10 TCID50/ml of NTL). Thus, even high titers of anti-RSV-A Abs transferred from mother to pups did not prevent URT infection, although it efficiently protected the LRT from RSV-A challenge (5).
FIG. 2.
Absence of nasal tract protection following mother-to-offspring Ab transfer. Female adult mice were immunized twice with 20 μg of BBG2Na or PBS in 20% alum at a 3-week interval prior to mating. Offspring were kept with mothers until 2 (2W) or 4 (4W) weeks of age, bled, and challenged with RSV-A (5). Results represent means ± SD of nasal RSV-A titers measured in NTL of these mice (seven pups from BBG2Na-immunized mothers and nine from PBS-immunized mothers) at day 5 postchallenge. NS, not significant.
RSV-A clearance in LRT but not URT after transfer of BBG2Na-primed B cells.
Another possibility for Ab-mediated URT protection in BBG2Na-immunized mice is the production of Abs by resident B cells, which would result in the presence of local protective Abs. In BBG2Na-immunized mice, Abs detected in the nasal tract were always of the IgG isotype (37). Thus, to induce local BBG2Na-primed B cells within the nasal tract, cells were adoptively transferred from BBG2Na-immunized mice into RSV-A-infected SCID mice. Experiments performed under these conditions demonstrated that adoptively transferred cells migrate and are detectable after a few days in the nasal tract of SCID mice (data not shown). BALB/c were immunized twice with BBG2Na and sacrificed for preparation of the B-cell suspensions as described in Materials and Methods. B cells were injected into SCID mice infected 2 or 3 h previously with RSV-A, as indicated in Fig. 3.
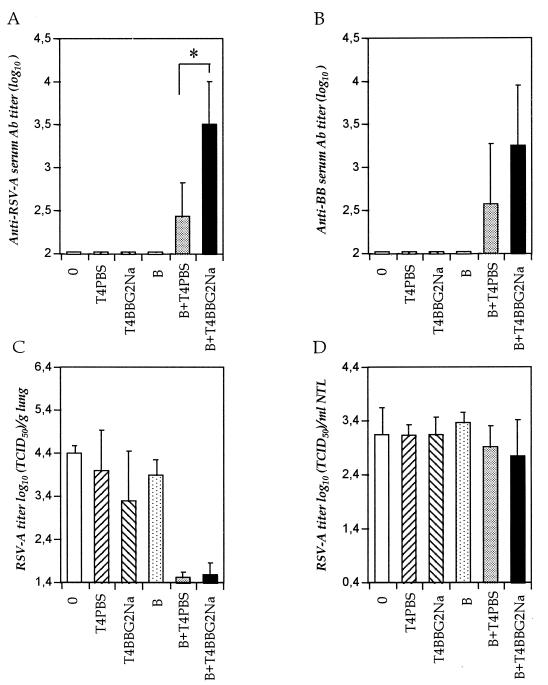
FIG. 3.
Transfer of BBG2Na-primed B cells. Purified B-cell suspensions were prepared from spleens and mesenteric lymph nodes of BBG2Na-immunized mice. They were injected i.p. into RSV-A-infected SCID mice, either alone (15 × 106 cells) or together with 2 × 106 purified CD4+ T4PBS or T4BBG2Na. Control SCID mice were not grafted (0) or received CD4+ T cells only. (A and B) Means ± SD of anti-RSV-A and anti-BB serum Ab titers, respectively; (C and D) means ± SD of RSV-A titers in lungs and NTL, respectively. The data were determined from two experiments 10 days after the cell transfers for groups including a total of seven to eight animals. ∗, P < 0.05 calculated by t test using the null hypothesis.
The SCID mice remained seronegative for RSV-A except when BBG2Na-primed B cells were transferred together with low numbers of CD4+ T cells (Fig. 3A). In these animals, RSV-A serum Ab titers reached 2.43 ± 0.39 and 3.5 ± 0.5 log10 when CD4+ T cells from PBS- and BBG2Na-immunized mice (T4PBS and T4BBG2Na) were transferred, respectively, versus <1.95 log10 in the other groups. Thus, B cells alone failed to produce Abs, while T-to-B cell cooperation was required to restore Ab secretion. Anti-BB Abs were also detected (Fig. 3B), indicating that the Abs were produced by memory B cells. These cells cooperated with either naive or BBG2Na-primed T cells, although the latter cells appeared more efficient. In addition, anti-BBG2Na IgGs were detected in the NTL of mice grafted with BBG2Na-primed B and T cells and reached mean ELISA titers of 1.14 ± 0.26 log10, versus <0.3 log10 in ungrafted animals or after transfer of B or T cells alone. This level of Abs exceeded the titers (≤0.6 log10) measured in naive mice after several independent passive transfer experiments undertaken with sufficient IgGs to result in serum Ab titers of approximately 3 log10, thereby suggesting the production of immunoglobulins locally within the nasal tract.
In accordance with the passive Ab transfer experiments, the LRT of anti-RSV-A-seropositive SCID mice were protected from challenge (Fig. 3C). Lung RSV-A clearance was thus uniquely dependent on the production of Abs under the conditions described. In contrast, despite the presence of the nasal Abs in the seropositive mice, RSV-A titers in the URT were similar in all groups (Fig. 3D). Thus, under the conditions described, nasal anti-BBG2Na IgGs had no effect on RSV-A infection in the URT.
T cells clear both LRT and URT RSV-A infection.
Since high serum Ab titers and nasal IgGs failed to protect the URT of naive mice, we assessed the antiviral efficacy of adoptively transferred TBBG2Na into RSV-A-infected SCID mice. TBBG2Na, TPBS, and T cells from RSV-A-immunized mice (TRSV-A) were injected into SCID mice at 5 × 106 (5 M), 10 × 106 (10 M), or 15 × 106 (15 M) cells per mouse, within 3 h after i.n. infection with RSV-A. Controls consisted of SCID mice that were infected but not grafted. Figure 4 shows RSV-A titers measured in the LRT and URT of SCID mice 5, 7, and 9 days after the adoptive T-cell transfer.
FIG. 4.
Adoptive transfer of T cells. Purified T-cell suspensions were prepared from spleens and mesenteric lymph nodes of RSV-A (TRSV-A)-, BBG2Na (TBBG2Na)-, or PBS (TPBS)-immunized mice. They were transferred into RSV-A-infected SCID mice at 5 × 106 (5 M), 10 × 106 (10 M), or 15 × 106 (15 M) cells per mouse. Means RSV-A titers of lung (A) and NTL (B) were determined in groups of four to seven animals 5, 7, and 9 days after the cell transfer and compared with mean RSV-A titers of four to six ungrafted animals per day of sacrifice. SD values are not represented for clarity. ∗, P < 0.05 calculated by the Kolmogorov-Smirnov test.
As indicated in Fig. 4A, 15 and 10 M TBBG2Na efficiently eliminated lung virus by 9 days postinfection, while 5 M cells were ineffective. These results were comparable to those for TRSV-A, which eliminated virus in LRT by day 9 at all cell concentrations tested. In contrast, TPBS had no impact on LRT infection, indicating that elimination of RSV-A in the other groups was not due to the development of a primary anti-RSV-A response. All SCID mice were confirmed seronegative for RSV-A before and after the T-cell transfer (data not shown). Therefore, TBBG2Na are able to clear LRT RSV-A infection in the absence of detectable Ab.
As shown in Fig. 4B, primed T cells were also capable of eliminating virus infection of the URT. TRSV-A were the most efficient, with a significant reduction of nasal virus titers by days 5 and 7 at the highest cell numbers (P < 0.05) and complete virus elimination by day 9 at all cell concentrations tested. The kinetics of virus clearance was somewhat slower after adoptive transfer of TBBG2Na. Nonetheless, significant reduction in URT virus titers were evident by day 9 as a function of the TBBG2Na concentration transferred (P < 0.05). No protective effect was induced with TPBS. Therefore, as for the lung results, elimination of URT infection depended on the priming of donor mice and was not due to a primary anti-RSV-A response. Thus, BBG2Na immunization conferred an antiviral activity to the T cells. In contrast to serum Abs, these cells efficiently eliminated RSV-A infection in the URT after transfer into SCID mice.
BBG2Na-induced URT protection is mediated by CD4+ T-cells.
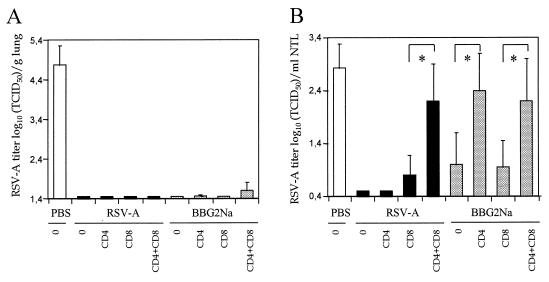
To confirm the role of T cells in BBG2Na- and RSV-A-immunized normal animals, in vivo T-cell depletions were undertaken. Mice were immunized with PBS, RSV-A, or BBG2Na and treated by i.p. injections of anti-CD4 and/or CD8 monoclonal Abs. Both cell subsets were reduced by 98 to 100% in blood, spleen, and NALT of BALB/c mice, as determined by flow cytometry (not shown).
The consequences of T-cell depletion on LRT and URT protection are shown in Fig. 5. All RSV-A and BBG2Na-immunized groups had comparable levels of serum RSV-A Abs (4.36 ± 0.34 and 4.44 ± 0.39 log10, respectively) and were protected from LRT infection, with no or minimal detectable virus, irrespective of the treatment used (Fig. 5A). Therefore, in the presence of RSV-A or BBG2Na Abs, T cells were not required for LRT protection. In contrast, nasal RSV-A titers in both groups were significantly modified after depletion of T cells (Fig. 5B). URT protection was reduced after depletion of CD8+ (but not CD4+) T cells in RSV-A-immunized mice and completely abolished after depletion of CD4+ and CD8+ T cells, indicating that although CD8+ T cells appeared more important than CD4+ lymphocytes, both T-cell subsets cooperate to achieve URT protection in these mice. In contrast, in BBG2Na-immunized mice, depletion of CD4+ T cells significantly diminished URT protective efficacy, while depletion of CD8+ T cells alone had no effect. Furthermore, no additional effect was observed after elimination of both T-cell subsets. These data demonstrate that CD4+, but not CD8+, T cells are required for the URT protection of BBG2Na-immunized mice. Indeed, to our knowledge they provide the first evidence of CD4-dependent URT protection against viral infection in normal mice following parenteral administration of a recombinant protein.
FIG. 5.
In vivo depletion of T-cell subsets. Mice were immunized twice with 105 TCID50 of RSV-A, 20 μg of BBG2Na or 20 μg of PBS in 20% alum at a 2-week interval. After bleeding they were either untreated (0) or depleted of CD4+ or/and CD8+ T cells by i.p. injections of anti-CD4 or/and anti-CD8 Abs, respectively, twice before RSV-A challenge and twice thereafter. Mean ± SD RSV-A titers of lung (A) and NTL (B) were determined for groups of seven animals at day 5 postchallenge. ∗, P < 0.05 calculated by t test using the null hypothesis.
Identification of G2Na structural elements implicated in LRT and URT protection.
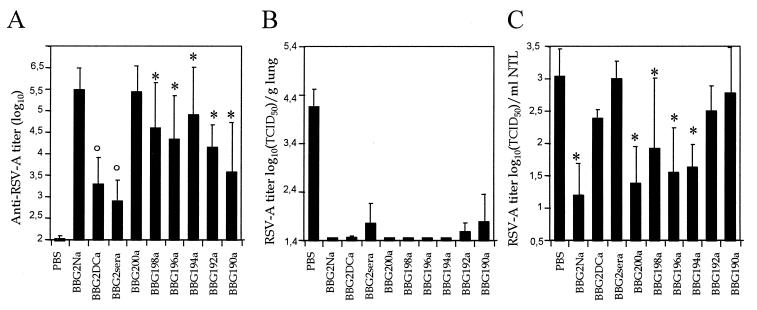
To identify the domain(s) implicated in LRT and URT protection, BALB/c mice were immunized twice i.p. with a panel of BBG2Na-derived mutants that contained site-specific mutations or were deleted by 10 amino acids at the N terminus and to various degrees at the C terminus (Fig. 1). The immunogenicity of these mutants clearly depended on the construct used. BBG2Na and BBG200a were the most immunogenic and induced similar RSV-A serum Ab titers (Fig. 6A). In contrast, deletion of residues 200 to 190 resulted in a reduction in immunogenicity relative to RSV-A. This effect was further enhanced with the mutations of Cys173 and Cys186 (BBG2DCa) or all cysteines (BBG2sera) to serines.
FIG. 6.
Immunogenicity and protective efficacy of BBG2Na-derived mutants. Mice were immunized twice with 20 μg of BBG2Na and BBG2Na-derived mutants or PBS in 20% alum at a 2-week interval and bled to determine anti-RSV-A serum Ab titers (A). Mean ± SD RSV-A titers of lung (B) and NTL (C) were determined for groups of 5 to 10 animals 5 days after challenge with RSV-A. ∗, P < 0.05 calculated by t test using the null hypothesis; °, P < 0.05 calculated using the Kolmogorov-Smirnov test.
Irrespective of their differential immunogenicity, all constructs induced efficient protection of mouse LRT against RSV-A challenge (Fig. 6B). In contrast, URT protection was a function of the construct used for immunization (Fig. 6C). Neither BBG2ΔCa nor BBG2sera induced URT-protective responses, indicating that the conserved cysteine residues were important for URT protection. In addition, while BBG2Na and the deletion constructs to BBG194a induced significant reduction in URT RSV-A titers relative to PBS-immunized mice, BBG192a and BBG190a did not. Thus, removal of residues 193 and 194 also abrogated URT-protective efficacy. Altogether, these data indicate that in contrast to LRT protection, URT protection requires the conservation of residues 173, 186, 193, and 194.
DISCUSSION
In this study, we report that i.p. immunization with a recombinant RSV G protein fragment induces anti-RSV protection of the mouse LRT through circulating Abs and a CD4+ T-cell-dependent protective response in the URT. In addition, a region critical for URT but not LRT protection was identified and located between amino acids 173 and 194.
The importance of Abs in LRT protection was previously demonstrated by passive transfer experiments in adult naive mice (37) and in pups of immune mothers (5). It is confirmed here by adoptive transfer experiments of BBG2Na-primed B cells secreting Abs into SCID mice and the inability of in vivo T-cell depletion to affect LRT protection in BBG2Na-immunized mice. The high anti-RSV-A protective efficacy of BBG2Na-immune sera after transfer into naive mice is consistent with several reports showing lung-protective efficacy of passively transferred Abs to RSV F and G proteins into mice, cotton rats, and owl monkeys (4, 18, 46). Interestingly, deletion and mutation of various amino acids on G2Na modulated the B-cell immunogenicity of the molecule but had no impact on LRT protection. This is consistent with the presence of several independent B-cell LRT protectopes recently identified in the G2Na fragment (35).
In contrast, under the conditions described, BBG2Na Abs had no effect on URT infection after either extremely efficient mother-to-pup transfer or i.n. production by resident memory B cells. Indeed, unlike IgAs (18), and consistent with our results, IgGs to either of the two envelope glycoproteins of RSV were also found by others to have little or no effect on RSV infection in the nose after topical or intravenous administration (50, 51). This might be due to insufficient IgG Ab concentration obtained in the nasal tract or a lower avidity for virus of IgGs and a reduced resistance to proteolytic enzymes compared to secretory IgAs. Furthermore, RSV replication in the nasal tract may occur in a tissue which is less accessible to the transudated IgG than lung parenchyma.
In our experiments, using the i.p. route of immunization, RSV-A clearance from the URT was clearly dependent on the presence of T cells. CD8+ or CD4+ T cells were sufficient to ensure URT protection of RSV-A-immunized mice, while RSV-A clearance in the URT of BBG2Na-immunized mice relied exclusively on CD4+ T cells. The role of T cells, both CD4+ and especially CD8+ T cells, in clearing viral infections such as Sendai virus, influenza virus, and RSV from the lungs is well documented (12–14, 20). Adoptive cell transfers in previously infected nude or irradiated BALB/c mice demonstrated that RSV clearance is primarily mediated by CD8+ effector cells, although RSV-A-primed CD4+ T cells may also clear pulmonary RSV by an Ab-independent mechanism when transferred early after infection (2, 7). These observations are consistent with ours, in which accelerated RSV clearance in LRT and URT of SCID mice was observed after transfer of RSV-A or BBG2Na-primed splenic T cells and in the absence of B cells or Abs.
However, induction of RSV-specific cytotoxic CD8+ T cells following immunization with BBG2Na was unlikely since, in contrast to live RSV or F and 22K proteins, G protein fails to induce CD8+ and cytotoxic T-cell responses in BALB/c mice (32, 33). This probably explains the higher efficiency of T cells (including both RSV-primed CD8+ and CD4+ lymphocyte subsets) from mice immunized with RSV-A compared to BBG2Na in resolution of LRT and URT infection after adoptive transfer into SCID mice.
In contrast, the role of T cells in viral clearance from the URT is poorly understood. The high proportion of T lymphocytes (including both memory and naive cells) and elevated CD4+/CD8+ T-cell ratio in the NALT and the non-NALT lymphocytes of mouse nasal cavity compared with Peyer's patches and lymph nodes suggest that the URT is an important site for T-lymphocyte recirculation, where both inductive and effector functions of mucosal immune responses are generated (3, 53). Accordingly, cells from the cervical and mesenteric lymph nodes were shown to home more successfully to NALT than to Peyer's patches (22, 23). On the other hand, i.n. infection was recently shown to induce T-cell expansion in the periphery (15). In the present report, we demonstrate for the first time that i.p. administration of an adjuvated live virus or recombinant protein is an effective route for the priming of T cells that migrate to the URT and protect mice against a local viral infection.
RSV clearance from the URT by CD4+ T cells after immunization with BBG2Na is fully consistent with the importance of Cys173, Cys186, and amino acids 193 to 194 in the maintenance of URT protection. Three of these four residues overlap a domain of the G protein (residues 181 to 203) which contains a recently identified Th cell epitope and was implicated in lung eosinophilia induction in G protein-primed mice after RSV challenge (41, 47). Furthermore, none of the five major protective B-cell epitopes identified on G2Na and lacking the Th cell epitope was protective against URT infection of BALB/c mice after active immunization with appropriate peptides or passive transfer of specific Abs (35). Thus, these data provide the first evidence that different epitopes and separate immune mechanisms account for LRT and URT protection against RSV infection in mice previously immunized with a recombinant RSV G protein fragment.
Interestingly, and consistent with the presence of this T-cell epitope within G2Na, BBG2Na was shown to generate a predominant Th2-type T-cell response after immunization (11). Surprisingly, however, and in contrast to native G protein, no production or transcription of Th2-type cytokines was evident in blood or lung tissues following i.n. instillation of RSV-A. In addition, a transient transcription of Th1-type cytokine genes was evident in lungs (11, 36). These observations indicated that the Th2-type T-cell response induced upon immunization with BBG2Na was not recalled after RSV challenge and that transcription of gamma interferon (IFN-γ) and interleukin-2 (IL-2) was induced after RSV challenge as it would be in normal mice undergoing a primary RSV infection. Another possibility is that although they represent a minor fraction of BBG2Na-primed lymphocytes, memory CD4+ T cells producing Th1-type cytokines, and not Th2-type cytokines, were slightly recalled. This hypothesis is consistent with a recent study performed in RSV-G-sensitized mice which confirms that both Th1 (characterized by secretion of IL-2 and IFN-γ) and Th2 (IL-4 and IL-5) responses are elicited from memory CD4+ T cells specific for a single-peptide epitope located between amino acid residues 183 and 197 (42).
The mechanism by which CD4+ T cells clear RSV infection in the URT remains to be determined. However, it is conceivable that cytokines such as IFN-γ, tumor necrosis factor alpha, or transforming growth factor β which display a direct antiviral activity (29, 38) or may activate resident macrophages, NK cells, or mucosal γδ+ cytotoxic T cells (6, 8) are produced locally. This is consistent with RSV-A clearance in SCID mice, which lack both functional B and T cells (19). Ongoing experiments address the mechanism of viral clearance in the nasal tract after immunization with BBG2Na and question the effect of high-titered IgGs on the absence of the peripheral Th2-type T-cell recall response after RSV challenge.
Altogether, and in contrast to several previous reports in which RSV-specific CD4+ T cells have been implicated in adverse immunopathological responses (1, 2, 13, 17), our results indicate for the first time that such cells might play a positive and protective role in an RSV vaccine. These discrepancies reinforce the notion that there are many unknowns with regard to the factors responsible for the induction of immunoprotective versus immunopathogenic responses in relation to RSV vaccines. Consequently, much caution is required concerning recommendations of what should or should not constitute part of an RSV vaccine. For example, should protection of the URT also be observed in humans following BBG2Na vaccination, maintenance of the implicated Th epitope in this RSV vaccine candidate could represent a significant advantage for interruption of viral transmission.
ACKNOWLEDGMENTS
We thank Dominique Cyblat, Francis Derouet, Fabienne Damien, Jean-François Depoisier, Monika Berney, and Marco Cordova for expert technical help. We are grateful to S. Izui for providing GK1-5 and H35 17.2 hybridomas and to L. Goetsh for statistical analysis. We also thank D. Velin and J.-P. Aubry for advice and expertise as well as N. Corvaia for critically reviewing the manuscript.
REFERENCES
- 1.Alwan W H, Kozlowska W J, Openshaw P J M. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W H, Record F M, Openshaw P J. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma H, Thompson A H, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S-I. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 4.Bansal G P, Hatfield J, Young J F, et al. Efficacy of passively administered monoclonal antibodies against respiratory syncytial virus infection in cotton rats. In: Chanock R M, Ginsberg H S, Brown F, Lerner R A, editors. Vaccines—1991. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 283–8. [Google Scholar]
- 5.Brandt C, Power U F, Plotnicky-Gilquin H, Huss T, Nguyen T N, Lambert P H, Binz H, Siegrist C A. Protective immunity against respiratory syncytial virus in early life after murine maternal or neonatal vaccination with the recombinant G fusion protein BBG2Na. J Infect Dis. 1997;176:884–891. doi: 10.1086/516503. [DOI] [PubMed] [Google Scholar]
- 6.Bukowski J F, Morita C T, Brenner M B. Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol. 1994;153:5133–5140. [PubMed] [Google Scholar]
- 7.Cannon M J, Stott E J, Taylor G, Askonas B A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunol. 1987;62:133–138. [PMC free article] [PubMed] [Google Scholar]
- 8.Carding S R, Allan W, Kyes S, Hayday A, Bottomly K, Doherty P C. Late dominances of the inflammatory process in murine influenza by gamma-delta+ T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, P. L., K. McIntosh, and R. M. Chanock. Respiratory syncytial virus, p. 1313–1351. In B. N. Fields, D. N. Knipe, and P. M. Howley (ed.), Virology—1996. Lippincott-Raven, Philadelphia, Pa.
- 10.Connors M, Collins P L, Firestone C Y, Murphy B R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corvaia N, Tournier P, Nguyen T N, Haeuw J F, Power U F, Binz H, Andreoni C. Challenge of BALB/c mice with respiratory syncytial virus does not enhance the Th2 pathway induced after immunization with a recombinant G fusion protein, BBG2Na, in aluminum hydroxide. J Infect Dis. 1997;176:560–569. doi: 10.1086/514075. [DOI] [PubMed] [Google Scholar]
- 12.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty P C. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham B S, Bunton L A, Wright P F, Karzon D T. The role of T cell subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Investig. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham B S, Bunton L A, Wright P F, Karzon D T. Respiratory syncytial virus in anti-μ-treated mice. J Virol. 1991;65:4936–4942. doi: 10.1128/jvi.65.9.4936-4942.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haanen J B A G, Toebes M, Cordaro T A, Wolkers M C, Kruisbeek A M, Schuman T N M. Systemic T cell expension during localized viral infection. Eur J Immunol. 1999;29:1168–1174. doi: 10.1002/(SICI)1521-4141(199904)29:04<1168::AID-IMMU1168>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Hall C B, Powell K R, MacDonald N E, Gala C L, Menegus M E, Suffin S C, Cohen H J. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 17.Hancock G E, Speelman D J, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemming V G, Prince G A, London W T, Baron P A, Brown R, Chanock R M. Topically administered immunoglobulin reduces pulmonary respiratory syncytial virus shedding in owl monkeys. Antimicrob Agents Chemother. 1988;32:1269–1270. doi: 10.1128/aac.32.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzenberg L A, Stall A M, Lalor P A, Sidman C, Moore W A, Parks D R, Herzenberg L A. The LY-1 B lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 20.Hou S, Doherty P C, Zijlstra M, Jaenisch R, Katz J M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 21.Johnson P R, Collins P L. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol. 1988;69:2623–2628. doi: 10.1099/0022-1317-69-10-2623. [DOI] [PubMed] [Google Scholar]
- 22.Koornastra P J, De Jong F I C R S, Vlek L M K, Marres E H M A, Van Breda Vriesman P J C. The Waldeyer ring equivalent in the rat. Acta Otolaryngol (Stockholm) 1991;111:591–595. doi: 10.3109/00016489109138388. [DOI] [PubMed] [Google Scholar]
- 23.Kuper C F, Koornastra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, Van Breda Vriesman P J C, Sminnia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 24.Maurer R, Meyer B J, Ptashne M. Gene regulation of the right operator (CR) of bacteriophage lambda. I. CR3 and autogenous negative control by repressor. J Mol Biol. 1980;139:147–161. doi: 10.1016/0022-2836(80)90302-2. [DOI] [PubMed] [Google Scholar]
- 25.Mazanec M B, Lamm M E, Lyn D, Portner A, Nedrud J G. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992;23:1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- 26.McIntosh K, Masters H B, Orr I, Chao R K, Barkin R M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Mills J V, Van Kirk J A, Wright P F, Chanock R M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971;107:123–130. [PubMed] [Google Scholar]
- 28.Murby M, Samuelsson E, Nguyen T N, Mignard L, Power U, Binz H, Uhlen M, Stahl S. Hydrophobicity engineering to increase solubility and stability of a recombinant protein from respiratory syncytial virus. Eur J Biochem. 1995;230:38–44. doi: 10.1111/j.1432-1033.1995.tb20531.x. [DOI] [PubMed] [Google Scholar]
- 29.Neuzil K M, Tang Y W, Graham B S. Protective role of TNF alpha in respiratory syncytial virus infection in vitro and in vivo. Am J Med Sci. 1996;311:201–204. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen T N, Gourdon M H, Hansson M, Robert A, Samuelson P, Libon C, Andreoni C, Nygren P A, Binz H, Uhlen M. Hydrophobicity engineering to facilitate surface display of heterologous gene products in Staphylococcus xylosus. J Biotechnol. 1995;42:207–219. doi: 10.1016/0168-1656(95)00081-z. [DOI] [PubMed] [Google Scholar]
- 31.Olmsted R A, Elango N, Prince G A, Murphy B R, Johnson P R, Moss B, Chanock R M, Collins P L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Openshaw P J M, Anderson K, Wertz G W, Askonas B A. The 22-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990;64:1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pemberton R M, Cannon M J, Openshaw P J M, Ball L A, Wertz G A, Askonas B A. Cytotoxic T-cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987;68:2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- 34.Piazza F M, Johnson S A, Ottolini M G, Schmidt H J, Darnell M E, Hemming V G, Prince G A. Immunotherapy of respiratory syncytial virus infection in cotton rats (Sigmodon fulviventer) using IgG in a small-particle aerosol. J Infect Dis. 1992;166:1422–1424. doi: 10.1093/infdis/166.6.1422. [DOI] [PubMed] [Google Scholar]
- 35.Plotnicky-Gilquin H, Goetsch L, Huss T, Champion T, Beck A, Haeuw J-F, Nguyen T N, Bonnefoy J-Y, Corvaïa N, Power U F. Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J Virol. 1999;73:5637–5645. doi: 10.1128/jvi.73.7.5637-5645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotnicky-Gilquin H, Huss T, Aubry J-P, Haeuw J-F, Beck A, Bonnefoy J-Y, Nguyen T N, Power U F. Absence of lung immunopathology following respiratory syncytial virus (RSV) challenge in mice immunized with a recombinant RSV G protein fragment. Virology. 1999;258:128–140. doi: 10.1006/viro.1999.9702. [DOI] [PubMed] [Google Scholar]
- 37.Power U F, Plotnicky-Gilquin H, Huss T, Robert A, Trudel M, Stahl S, Uhlen M, Nguyen T N, Binz H. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology. 1997;230:155–166. doi: 10.1006/viro.1997.8465. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay A J, Ruby J, Ramshaw I A. The case for cytokines as effector molecules in the resolution of virus infections. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 39.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 40.Routledge E G, Willcocks M M, Samson A C R, Morgan L, Scott R, Anderson J J, Toms G L. The purification of four respiratory syncytial virus proteins as protective agents against experimental infection in BALB/c mice. J Gen Virol. 1988;69:293–303. doi: 10.1099/0022-1317-69-2-293. [DOI] [PubMed] [Google Scholar]
- 41.Sparer T E, Matthews S, Hussell T, Rae A J, Garcia-Barreno B, Malero J A, Openshaw P J. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srikiatkhachorn A, Chang W, Braciale T J. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J Virol. 1999;73:6590–6597. doi: 10.1128/jvi.73.8.6590-6597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ståhl S, Sjölander A, Nygren P-Å, Berzins K, Perlmann P, Uhlén M. A dual expression system for the generation, analysis and purification of antibodies to a repeated sequence of the Plasmodium falciparum antigen Pf155/RESA. J Immunol Methods. 1989;124:43–52. doi: 10.1016/0022-1759(89)90184-1. [DOI] [PubMed] [Google Scholar]
- 44.Stott E J, Taylor G. Respiratory syncytial virus, brief review. Arch Virol. 1985;84:1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- 45.Stott E J, Taylor G, Ball L A, Young K K-Y, King A M Q, Wertz G W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor G, Stott E J, Bew M, Fernie B F, Cote P J, Collins A P, Hughes M, Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984;52:137–142. [PMC free article] [PubMed] [Google Scholar]
- 47.Tebbey P W, Hagen M, Hancock G E. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trudel M, Nadon F, Seguin C, Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991;185:749–757. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 49.Wagner D K, Muelenaer P, Henderson F W, Snyder M H, Reimer C B, Walsh E E, Anderson L J, Nelson D L, Murphy B R. Serum immunoglobulin G antibody subclass response to respiratory syncytial virus F and G glycoproteins after first, second and third infection. J Clin Microbiol. 1989;27:589–592. doi: 10.1128/jcm.27.3.589-592.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh E E, Hall C B, Briselli M, Brandriss M W, Schlesinger J J. Immunization with glycoprotein subunits of respiratory syncytial virus protect cotton rats against viral infection. J Infect Dis. 1987;155:1198–1204. doi: 10.1093/infdis/155.6.1198. [DOI] [PubMed] [Google Scholar]
- 51.Walsh E E, Schlesinger J J, Brandriss M W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984;43:756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watt P J, Robinson B S, Pringle C R, Tyrell D A. Determinants of susceptibility to challenge and the antibody response in adults volunteers given experimental respiratory syncytial virus vaccines. Vaccine. 1990;8:231–236. doi: 10.1016/0264-410x(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 53.Wu H-Y, Nikolova E B, Beagley K W, Russel M W. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]