Abstract
Objectives
Feline cardiomyopathies (CMs) represent a heterogeneous group of myocardial diseases. The most common CM is hypertrophic cardiomyopathy (HCM), followed by restrictive cardiomyopathy (RCM). Studies comparing survival and outcome for different types of CM are scant. Furthermore, little is known about the cardiovascular consequences of systemic diseases on survival. The aim of this retrospective study was to compare survival and prognostic factors in cats affected by HCM, RCM or secondary CM referred to our institution over a 10 year period.
Methods
The study included 94 cats with complete case records and echocardiographic examination. Fifty cats presented HCM, 14 RCM and 30 secondary CM.
Results
A statistically significant difference in survival time was identified for cats with HCM (median survival time of 865 days), RCM (273 days) and secondary CM (<50% cardiac death rate). In the overall population and in the primary CM group (HCM + RCM), risk factors in the multivariate analysis, regardless of the CM considered, were the presence of clinical signs, an increased left atrial to aortic root (LA/Ao) ratio and a hypercoagulable state.
Conclusions and relevance
Primary CMs in cats share some common features (ie, LA dimension and hypercoagulable state) linked to feline cardiovascular physiology, which influence survival greatly in end-stage CM. The presence of clinical signs has to be regarded as a marker of disease severity, regardless of the underlying CM. Secondary CMs are more benign conditions, but if the primary disease is not properly managed, the prognosis might also be poor in this group of patients.
Introduction
Cardiomyopathies (CMs) are a heterogeneous group of myocardial diseases that can primarily affect the heart (primary CM) or that are part of a generalised systemic illness (secondary CM).1,2
Hypertrophic cardiomyopathy (HCM) is the most common feline CM, and is characterised by a hypertrophied (>6 mm in diastole), non-dilated left ventricle (LV) in the absence of another systemic or cardiac disease able to mimic similar wall thickening.1 –5
HCM is characterised by a wide phenotypic spectrum, with diffuse hypertrophy (symmetrical and concentric hypertrophy of the LV), or asymmetric hypertrophy involving the interventricular septum (IVS) or the left ventricular free wall (LVFW) or isolated segments in the LV, including the papillary muscle.4 –8
The presence of a documented systemic illness that is responsible for LV wall thickening identifies a secondary CM.2,9 –11
Little is known about the prevalence and the consequences of cardiac abnormalities in cats affected by hyperthyroidism and systemic hypertension, and no study has investigated the outcome of these patients with a particular focus on the cardiovascular system.
Hyperthyroidism is a systemic disease that determines a ‘high output state’ as a consequence of a general decrease in peripheral vascular resistance and an increase in cardiac output associated with sinus tachycardia.9,11 Systemic hypertension can induce an increase in LV diastolic thickness by the increase in afterload associated with the disease. 10
Restrictive cardiomyopathy (RCM), which accounts for 20% of referred feline CMs, is defined by the presence of restrictive mitral inflow Doppler pattern, stiff, non-hypertrophied LV with biatrial enlargement. Two echocardiographic patterns can be identified: the myocardial form and the endomyocardial form.2,3,12
Since most of the published work in feline cardiology refers to HCM,6 –8,13 –16 with only one study 3 presenting the clinical and echocardiographic features of idiopathic feline CM, and no study focused on the effect of secondary CM on survival, the aim of our study is to provide data about population characteristics, survival time and prognostic factors in cats affected by the most common CMs in our referral institution: HCM, RCM and secondary CM.
Materials and methods
The clinical archive of the cardiology unit was reviewed for cats diagnosed with CM. Inclusion criteria were any patient with a complete case record (owner data, patient signalment and anamnesis, complete clinical findings and cardiac investigation), a follow-up available (telephone call or echocardiographic re-check at our institution) and a definitive diagnosis of HCM, RCM or secondary CM. Complete cardiac investigation included a detailed anamnesis and signalment, clinical findings and a complete echocardiography. Doppler technique was used to assess systemic blood pressure (BP) in all patients as recommended by the American College of Veterinary Internal Medicine guidelines. 17 When BP was >160 mmHg on serial repeated measurements, the cat was classified as affected by systemic hypertension. A complete blood count and biochemical blood analysis were performed, including thyroxine (T4), and treatment with ace inhibitor and/or calcium channel blocker was started as recommended. 17 All cats older than 10 years of age had their T4 levels tested. 18
If the patient presented with a clinical history or with clinical findings related to the presence of hyperthyroidism (polyphagia, progressive weight loss), T4 levels and a screening blood test were performed regardless of the patient’s age.
Cats diagnosed with dilated CM, arrhythmogenic CM or unclassified CM; congenital heart disease or infiltrative disease; or those with incomplete case records or no information regarding follow-up were excluded from the analysis.
The final diagnosis of CM was determined by echocardiography (M-mode, B-mode and Doppler echocardiography). Criteria for echocardiographic diagnosis were established as follows.
Primary HCM: Cats were diagnosed with HCM when diastolic left ventricular wall thickness was >6 mm in the absence of any other cardiac or systemic illness that might mimic the same echocardiographic feature. The measurement was obtained by M-mode and/or B-mode images, where available.
A detailed characterisation of LV hypertrophy was provided for each case: symmetrical (if both IVS and LVFW were >6 mm and IVS/LVFW ratio was 0.7–1.38,19) or asymmetrical (if the hypertrophied LV segment was localised at IVS, LVFW or at the papillary muscle only). The IVS asymmetric phenotype was characterised by a IVS/LVFW ratio of >1.3, while LVFW phenotype was associated with a IVS/LVFW ratio of <0.7.8,19 Papillary muscle hypertrophy was defined when no increase in IVS or LVFW in diastole was identified and when papillary muscle area 20 was >0.8 cm2.
Secondary CM: Cats were diagnosed with secondary CM when a complete echocardiography was performed in a patient with BP or T4 levels above the reference values and echocardiographic abnormalities were detected.
RCM: Echocardiographic diagnosis of RCM was based on the presence of a marked left atrial or biatrial dilation (left atrial to aortic root [LA/Ao] ratio >2) 4 without concomitant myocardial hypertrophy (LV wall thickness <6 mm), in the presence of a restrictive pattern on transmitral pulsed wave Doppler trace.
Left atrial enlargement was defined by an LA/Ao ratio on B-mode greater than 1.5. 4 Cats with an LA/Ao ratio between 1.5 and 2.0 were identified as having mild to moderate LA enlargement, while cats with a LA/Ao ratio >2.0 were considered to have severe LA enlargement. 4
Systolic anterior motion of the mitral valve (SAM) was defined as a midsystolic displacement of the anterior septal leaflet into the left ventricular outflow tract (LVOT) causing dynamic obstruction of blood flow, with an acceleration of LVOT flow and the presence of a jet of mitral regurgitation. 4
Echocardiographic signs of a hypercoagulable state included the presence of spontaneous echocardiographic contrast (‘smoke effect’) or the direct visualisation of intracardiac thrombi in the left atrium or auricle.
Owners or referring veterinarians were contacted in order to obtain information about long-term follow-up.
If the cat was alive, a re-check was offered. If the cat had died, an attempt was made to classify the event as cardiac related or not. Euthanasia due to worsening of heart failure was considered cardiac related if no other cause of death was obvious. Cats still alive or that had died or were euthanased for reasons unrelated to cardiac disease were censored in the statistical analysis. Subjects lost to follow-up were included in the survival analysis up until the last time point at which they were known to be alive and then were thereafter censored in the analysis.
Statistical analysis
Data were analysed using a computerised statistics software (SPSS Statistics for Windows v17), and in all cases P <0.05 was set to indicate statistical significance. Basic descriptive statistical analyses were performed using Microsoft Excel. The Shapiro–Wilk test was used to verify variables’ normal distribution. If the distribution was normal, a t-test was used to compare the means of two continuous variables; the Mann–Whitney U-test was used with non-normally distributed variables. Data with normal distribution are expressed as mean ± SD. For data not normally distributed, median and ranges are given.
Survival was calculated as the days between diagnosis and death or last visit/telephone contact.
The Kaplan–Meier method was used to estimate survival function and plot time to event curves in the different groups. A log-rank test with right censoring was used to determine whether a significant difference existed between groups.
Schoenfeld residuals and time dependent covariates were used to test the assumption of proportional hazards (the hazard in one group is a constant proportion of the hazard in the other group). Univariate and multivariate Cox proportional hazard analysis were performed in order to determine the effect of any variable on survival.
Variables were added to the multivariable model in a manual stepwise manner, including first all variables statistically significant in the univariate analysis, and then excluding those not reaching statistical significance one by one, until all the variables included were statistically significant. Univariate and multivariate Cox proportional hazard analysis were performed in the overall population (HCM, RCM and secondary CM) and in primary CM (HCM and RCM). Variables assessed for their effect on outcome in each group were CM, breed, sex, age at presentation, presence of clinical signs, presence of heart murmur, presence of SAM, echocardiographic hypertrophy pattern, indexed LA/Ao ratio, presence of echocardiographic signs of hypercoagulable state. Hazard ratio (HR) and 95% confidence intervals (CI) were calculated.
Results
From March 2003 to March 2013, 124 cats were diagnosed with HCM, RCM or secondary CM. Of these, 23 were lost to follow-up after the first visit, and seven were excluded for incomplete case record (n = 4) and incomplete BP and T4 in cats older than 10 years of age (n = 3).
The final study population comprised 94 patients: 50 patients with an echocardiographic diagnosis of HCM, 14 cats diagnosed with RCM and 30 cats with secondary CM due to systemic hypertension (n = 17) and hyperthyroidism (n = 13).
Breed population included mostly domestic shorthair cats (n = 60), followed by Persian (n = 20), longhair cats (n = 10; four Norwegian Forest Cats, three Maine Coons, two Ragdolls and one Birman) and four Siamese cats. Male cats were slightly predominant but not over-represented in the population, as 53% were male (n = 50) and 47% were female (n = 44).
Age at diagnosis varied widely, with a median age of 10 years (0.8–21 years). However, the median age for cats with HCM was statistically different (7.2 years, range 0.8–15 years, P <0.001) from cats with RCM (10.3 years, range 4–16 years) and cats with secondary CM (14.6 years, range 7–21 years).
Most cats in the study (n = 57) had a cardiac murmur at cardiac auscultation. Nevertheless, the presence of a heart murmur varied in the three groups of CM: 76% (n = 38) of cats with HCM had a cardiac murmur, while few cats (n = 5, 35%) in the RCM group had a cardiac murmur; 47% of cats with secondary CM had a cardiac murmur (Table 1).
Table 1.
Population characteristics at presentation
| Overall population | HCM | RCM | Secondary CM | |
|---|---|---|---|---|
| Age at diagnosis (years) | 10 (0.8–21) | 7.2 (0.8–15) | 10.3 (4–16) | 14.6 (7–21) |
| Sex | 50♂/44♀ | 32♂/18♀ | 4♂/10♀ | 14♂/16♀ |
| Breed | Domestic shorthair 60 (65%) Persian 20 (21%) Norwegian Forest Cat 4 (4%) Siamese 4 (4%) Maine Coon 3 (3%) Ragdoll 2 (2%) Birman 1 (1%) |
Domestic shorthair 25 (50%) Persian 16 (32%) Maine Coon 3 (6%) Norwegian Forest Cat 2 (4%) Ragdoll 2 (4%) Birman 1 (2%) Siamese 1 (2%) |
Domestic shorthair 8 (58%) Persian 3 (21%) Siamese 2 (14%) Norwegian Forest Cat 1 (7%) |
Domestic shorthair 27 (91%) Persian 1 (3%) Norwegian Forest Cat 1 (3%) Siamese 1 (3%) |
| Heart murmur | 37 (39%) | 38 (76%) | 5 (35%) | 14 (50%) |
| Arrhythmias during auscultation | 4 (4%) | 1 (2%) | 0 | 3 (10%) |
| Clinical signs | 53 (54%) | 29 (58%) | 14 (100%) | 10 (33%) |
| SAM | 40 (43%) | 31 (62%) | 0 (0) | 9 (30%) |
| Echocardiographic pattern of hypertrophy | Symmetric hypertrophy 30 (32%) Asymmetrical IVS 28 (30%) Asymmetrical LVFW 13 (14%) Asymmetrical PapMuscle 6 (6%) RCM phenotype 14 (15%) No hypertrophy 3 (3%) |
Symmetric hypertrophy 19 (38%) Asymmetrical IVS 21 (42%) Asymmetrical LVFW 5 (5%) Asymmetrical PapMuscle 5 (5%) RCM phenotype 0 (0) |
Symmetric hypertrophy 0 Asymmetrical IVS 0 Asymmetrical LVFW0 Asymmetrical PapMuscle 0 RCM phenotype 14 (100%) |
Symmetric hypertrophy 13 (41%) Asymmetrical IVS 7 (23%) Asymmetrical LVFW 8 (27%) Asymmetrical PapMuscle 1 (3%) No hypertrophy 2 (6%) |
| LA/Ao ratio | Normal 38 (40%) Mild to moderate 38 (40%) Severe 17 (20%) |
Normal 24 (48%) Mild to moderate 17 (34%) Severe 9 (18%) |
Normal 0 Mild to moderate 9 (64%) Severe 5 (36%) |
Normal 14 (50%) Mild to moderate 12 (40%) Severe 3 (10%) |
| Hypercoagulable state on echocardiography | 11 (12%) | 7 (14%) | 3 (27%) | 1 (3%) |
HCM = hypertrophic cardiomyopathy; RCM = restrictive cardiomyopathy; CM = cardiomyopathy; SAM = systolic anterior motion of the mitral valve; IVS = interventricular septum; LVFW = left ventricle free wall; LA/Ao = left atrial to aortic root; PapMuscle = papillary muscle
The majority of the overall population presented with one or more clinical signs (n = 57, Table 2). The presence of clinical signs varied between the three groups. All cats with RCM presented with clinical signs, cats with HCM showed a higher prevalence of symptomatic patients compared with asymptomatic ones, and cats with secondary CM were equally distributed in the two categories.
Table 2.
Clinical signs at presentation
| Reason for presentation | Overall population | HCM | RCM | Secondary CM |
|---|---|---|---|---|
| Dyspnoea/CHF | 38 | 19 | 12 | 7 |
| Arterial thromboembolism | 8 | 5 | 2 | 1 |
| Syncope | 7 | 5 | 0 | 2 |
| Other (arrhythmias with no syncopal episodes) | 4 | 1 | 0 | 3 |
| Total | 57 (61%) | 30 (60%) | 14 (100%) | 13 (43%) |
CHF = congestive heart failure; HCM = hypertrophic cardiomyopathy; RCM = restrictive cardiomyopathy; CM = cardiomyopathy
On echocardiographic evaluation, five patterns of LV morphology were identified (Table 1). All cats with HCM had a variable degree of LV hypertrophy. Most of the cats presented with an asymmetric echocardiographic pattern (52% of cats with HCM), localised in most patients at the IVS (n = 21) and to a lesser extent to the LVFW (n = 5). A concentric symmetrical pattern was identified in 38% of patients (n = 19), while only 10% of patients had hypertrophied papillary muscles but no evidence of increased wall thicknesses in diastole.
All cats with RCM showed IVS and LVFW <6 mm, atrial enlargement and a restrictive pattern at pulsed wave transmitral Doppler flow.
Cats with secondary CM presented with a wide spectrum of echocardiographic pattern: 41% (n = 9) of cats had symmetrical hypertrophy, 27% (n = 8) and 23% (n = 7) had asymmetrical hypertrophy of the LVFW and IVS, respectively, two cats presented absence of hypertrophy with LV dilation (6%) and only one cat (3%) presented with isolated papillary muscle hypertrophy.
Most of the cats with hypertensive heart disease showed concentric (n = 8, 47%) and IVS hypertrophy (n = 5, 30%), while few of them presented with isolated LVFW (n = 3, 17%) and papillary muscle hypertrophy (n = 1, 6%). Cats with hyperthyroidism showed a more variable echocardiographic pattern, with four (32%) cats with concentric and seven cats with asymmetrical LV hypertrophy (53%, most LVFW hypertrophy). Two cats (15%) presented no hypertrophy but LV dilation with preserved fractional shortening and ejection fraction.
SAM was identified in 40 patients. It was more common in cats with HCM (n = 31) compared with secondary CM, equally distributed between hypertensive and hyperthyroid cats (n = 9). No cat with RCM had SAM.
In the overall population, left atrial dimensions were normal in 40% of patients and were above the reference interval in 60% of patients. LA/Ao ratio was mildly to moderately enlarged in 40% of patients, while only 20% of patients had severe LA enlargement.
Most of the cats with HCM and secondary CM had normal and mild to moderate left atrial enlargement, while most of the cats with RCM showed severe left atrial enlargement (Table 1).
Echocardiographic signs of a hypercoagulable state (smoke effect or direct thrombi visualisation in the left auricle or in the left atrium) were identified in 12% of patients, mainly cats with HCM and RCM (Table 1).
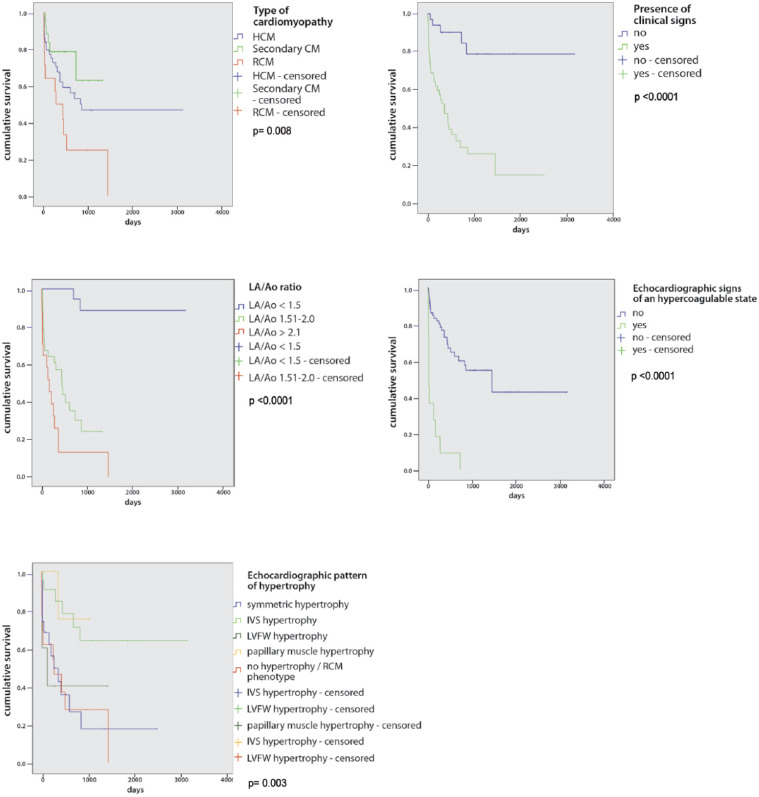
Univariate and multivariate analysis was performed in the overall population and in patients with primary CM (Table 3). Cardiac-related death occurred in 43% of cats, with different death rates depending on the CM: most of the cats with RCM died of cardiac related death (86%), 44% of cats with HCM and only 20% of cats with secondary CM died of cardiac related death (Figure 1).
Table 3.
Univariate and multivariate analysis in the overall population and in cats with hypertrophic cardiomyopathy (HCM) and restrictive cardiomyopathy (RCM)
| Univariate analysis | Confidence intervals | P value | Multivariate analysis | Confidence intervals | P value | |
|---|---|---|---|---|---|---|
| Overall population | ||||||
| Presence of clinical signs | 2.61 | 1.632–4.183 | <0.001 | 2.38 | 1.163–3.187 | 0.003 |
| LA/Ao ratio | 37.03 (severe LA enlargement vs normal LA) 1.92 (severe LA vs moderate LA) |
8.400–166.660 0.988–3.770 |
<0.001 | 3.65 (LA enlargement vs normal LA) |
1.601–4.210 | <0.001 |
| Presence of echocardiographic signs of hypercoagulable state | 2.00 | 1.572–2.549 | <0.001 | 1.81 | 1.279–2.137 | <0.001 |
| Cardiomyopathy | 2.28 (HCM vs secondary CM) 3.84 (RCM vs secondary CM) |
1.127–4.629 1.434–10.309 |
0.014 | NS | ||
| Presence of heart murmur | 0.72 | 0.524–0.988 | 0.042 | NS | ||
| Echocardiographic pattern of hypertrophy | 2.80 (asymmetrical vs symmetrical HCM) 7.14 (RCM vs asymmetrical HCM) |
1.915–12.500 0.926–55.555 |
0.09 | NS | ||
| HCM+RCM | ||||||
| LA/Ao ratio | 27.78 (severe LA enlargement vs normal) 1.49 (severe vs moderate LA enlargement) |
6.289–125.000 0.727–3.105 |
<0.001 | 5.35 (enlargement vs normal LA) |
1.813–6.250 | <0.001 |
| Echocardiographic signs of hypercoagulable state | 2.31 | 1.714–3.109 | <0.001 | 2.01 | 1.348–2.524 | <0.001 |
| Echocardiographic pattern of hypertrophy | 4.60 (symmetrical vs asymmetrical HCM) 6.40 (RCM vs asymmetrical HCM ) |
1.692–12.500 0.822–50.000 |
0.013 | NS | ||
| Cardiomyopathy | 0.76 (HCM vs RCM) | 0.602–0.964 | 0.024 | NS | ||
| Presence of heart murmur | 0.58 | 0.412–0.820 | 0.002 | NS | ||
| Clinical signs | 2.74 | 1.509–4.964 | 0.001 | NS | ||
| Presence of SAM | 0.70 | 0.498–0.997 | 0.048 | NS |
LA/Ao = left atrial to aortic root; LA = left atrium; CM = cardiomyopathy; SAM = systolic anterior motion of the mitral valve; NS = not statistically significant
Figure 1.
Kaplan–Meier survival curves in the overall population regarding type of cardiomyopathy, presence of clinical signs, left atrial to aortic root (LA/Ao) ratio and echocardiographic signs of a hypercoagulable state; a different survival time was identified in every category, as depicted. In the hypertrophic cardiomyopathy (HCM) and restrictive cardiomyopathy (RCM) group, a different survival time was observed depending on the echocardiographic pattern of hypertrophy. Log-rank P value is shown in each graph. CM = cardiomyopathy; IVS = interventricular septum; LVFW = left ventricular free wall
The poorest survival was found in cats with RCM (median survival 273 days), followed by cats with HCM (median survival 865 days). Cats with secondary CM showed the best survival time, not reaching the final endpoint (cardiac death) in around 80% of the total. Most of the cats died of non-cardiac-related causes, and a few of them were alive at last follow-up.
The presence of clinical signs was associated with decreased survival both in the overall population and in the primary CM group (Figure 1). Left atrial enlargement was associated with decreased survival regardless of the underlined CM (Figure 1).
Echocardiographic signs of hypercoagulable state were shown to decrease markedly survival (Figure 1). Cats with the RCM phenotype (median survival time 273 days), with symmetrical concentric hypertrophy (median survival time 273 days) and LVFW hypertrophy (median survival time 127 days) showed poorer survival compared with cats with asymmetrical IVS or papillary muscle hypertrophy (both groups had <50% of patients reaching the final endpoint; Figure 1).
The presence of clinical signs at presentation, left atrial enlargement and the echocardiographic identification of an hypercoagulable state were all negative prognostic factors in the multivariate analysis in the overall population (Table 3).
When considering primary CM only, the presence of left atrial enlargement and the identification of a hypercoagulable state were negative prognostic factors in the multivariate analysis
Discussion
CMs affecting feline patients include a heterogeneous group of myocardial disease with wide phenotypical variability. Since the last study on idiopathic CMs was published, 3 population characteristics and disease epidemiology have changed, with a strong decrease in dilated CM.
Furthermore, there is limited information about cardiovascular abnormalities in cats with systemic hypertension and hyperthyroidism, with most of the published work dating back 10 years ago.21 –24
The present study is the first to present data from a feline referral population in Italy. HCM is the most common CM diagnosed at our institution (53% of the population included), followed by secondary CM (32% of the population included) and RCM (15%).
The main cat breed is the domestic shorthair cat, which is the most common cat breed in Italy. A more pronounced breed variability was found in the HCM group, where half of the cats were pedigree cats, with a high prevalence of Persian cats and some longhair breed cats (Maine Coon, Norwegian Forest Cat, Ragdoll and Birman), while most of the cats with RCM and secondary CM were domestic shorthairs.
Age at diagnosis in the overall population and in cats with HCM, RCM and secondary CM was higher than previously reported (mean age for HCM cats 7.2 years vs 4.8–6.5 years in previous studies3,13 –15). The population of cats referred to our institution and diagnosed with HCM is older than previous studies because not so many Maine Coon and Ragdoll cats were present in our population. It has been highlighted by different studies that Maine Coon and Ragdoll cats have an early onset HCM, with cats of 1–2 years of age affected by aggressive HCM forms that eventually lead to death.15,25
In contrast, Persian and domestic shorthair cats have an older onset HCM, and this might reflect the results in our population. 8
Sex distribution is different between CMs. Cats with HCM are predominantly males (64%), while cats with RCM show female predisposition (71%). These findings agree with previous HCM studies, which found a male predisposition,3,13 –15 while sex predisposition for RCM cats was similar to our results in the study by Ferasin et al. 3 No sex predisposition was found for secondary CM.
A different survival rate was identified in cats affected by CM, with the worse outcome associated with cats diagnosed with RCM, followed by HCM, while few cats died of cardiac-related death in the secondary CM group.
HCM is still the most commonly diagnosed primary CM, with wide echocardiographic appearance, as previously identified by some clinical and anatomopathological studies.2,6 –8
Hypertrophy distribution was variable in cats with HCM, as the asymmetrical IVS form was the most commonly encountered (42% of all cats with HCM), followed by the concentric symmetrical form (38%). Few cats presented with LVFW or papillary muscle hypertrophy (10% each). Our results identify a different hypertrophy distribution compared with other clinical studies, where the concentric symmetrical form was the most frequently encountered (Brizard et al 7 61% and Trehiou-Sechi 8 34%). Although this finding was statistically significant only in the univariate analysis, the degree and the localisation of hypertrophy affects survival, as cats with asymmetrical IVS or papillary muscle HCM live longer than those with concentric symmetrical hypertrophy and asymmetrical LVFW hypertrophy.
Interestingly, the worst outcome was identified for asymmetrical LVFW HCM. This finding might be explained by the fact that hypertrophy of LVFW might be associated with an absence of murmur (only one cat with LVFW hypertrophy had a heart murmur) and delayed diagnosis associated with the onset of clinical signs (all cats had one or more clinical signs). Unfortunately, this category was limited to only a few patients, and this could be associated with a selection bias. Hence, further studies are needed to support this hypothesis.
Symmetric HCM is associated with diffuse myocardial LV hypertrophy, and this could determine more severe diastolic dysfunction, which could favour the onset of clinical signs and early myocardial impairment.
The lack of hypertrophy is not a protective factor in cats with CM either, as cats with an RCM echocardiographic phenotype have poor survival. The same might be hypothesised for cats with HCM that show the ‘burnt out’ echocardiographic morphology, where myocardial thinning is related to severe myocardial ischemia and myocardial death. 4 However, no burnt out morphology was identified in our case series.
The protective effect of asymmetrical IVS hypertrophy has also been related to SAM pathogenesis and to the association of a heart murmur, thus providing clinical tools to perform early diagnosis. 15 As a matter of fact, most of the cats with IVS hypertrophy presented with a heart murmur (81%).
SAM can be considered a protective factor only if primary CMs are analysed, as well as the presence of a heart murmur. Both these findings can be considered protective, because they could favour early diagnosis in an asymptomatic patient.
Different pathophysiological mechanisms are responsible for myocardial hypertrophy in cats with secondary CM compared with primary HCM. Systemic hypertension induces an increase in LV thickness, mainly as a result of an attempt of the LV to normalise LV wall stress and cope with the increase in chronically elevated afterload. 10 LV hypertrophy and mass are prognostic markers in hypertensive human patients and antihypertensive therapies reduce LV mass, thus reducing the risk for cardiovascular events and death. 10 The increase in thyroid hormone concentration induces a positive chronotropic effect, an increase in β-adrenergic response and a reduction in systemic vascular resistance, which will in turn determine a so-called ‘high output state’ of the heart. 11
The echocardiographic appearance of cats with systemic hypertension showed a predominant concentric and IVS hypertrophy pattern, as also identified in previous studies,22 –24 while cats with hyperthyroidism did not show a prevalent echocardiographic pattern, with a similar number of cats showing symmetrical, asymmetrical IVS or LVFW hypertrophy.
Because there is no clear distinction between the echocardiographic appearance of cats with primary or secondary hypertrophy, the importance of excluding concomitant systemic illnesses during diagnostic workout should be emphasised, as in most cases its recognition determines regression of cardiac disease and/or reduced risk for cardiovascular events.
In a few select cases, however, cardiac remodelling secondary to a systemic illness that is not properly managed can still lead to cardiac worsening and eventually death. It is not clear whether these patients have a primary CM associated with a systemic illness that exacerbates primary CM or whether cats with a secondary CM die of cardiac-related death as a consequence of unsuccessful response to systemic therapy and clinical worsening.
RCM is a less commonly encountered CM and is thought to be similar to human RCM for its echocardiographic phenotypical appearance. However, in veterinary studies,2,12 infiltrative disease was not reported to be as common as it is in human patients (50% of RCM cases in human medicine 1 ). So our knowledge concerning the etiopathogenesis of RCM in cats still appears to be unclear.
RCM is the second most common primary CM in cats, although its frequency is much lower than HCM and secondary CM combined (15% of the population studied). The prognosis is poor, with most of the cats dying of cardiac-related death less than 1 year after first diagnosis. Nevertheless, our case series showed longer median survival time compared with previous studies, with a median survival time of 7 months.
As all the cats in the RCM group were symptomatic, this finding might explain poor survival and does not help to clarify the etiopathogenesis, as no subclinical phase was identified in our case series or in other studies.2,3,12 What might determine fibrosis and LV stiffness exactly remains unclear. Further studies are needed to categorise this condition better and to slow the progression of the disease.
Independently from the underlined CM, negative prognostic markers in the overall population are the presence of clinical signs, left atrial enlargement and echocardiographic signs of an hypercoagulable state. The presence of clinical signs is considered to be associated with the presence of congestive heart failure (CHF) and thus supports the hypothesis that cats with CHF do not live as long as a consequence of severe myocardial impairment. Asymptomatic cats failed to reach a survival probability of <50% by the end of the study. Hence, the absence of clinical signs is also a protective factor in our study.
Left atrial enlargement has always been considered as a poor prognostic factor in all previous HCM studies,3,13 –16 and is also confirmed in our study, regardless of CM classification. Left atrial enlargement might be a marker of long-standing, progressing CM and thus might explain the onset of clinical signs and might be responsible for the increased risk of hypercoagulable state. LA enlargement might thus be considered as a marker of disease severity, regardless of the underlined CM.
A hypercoagulable state was not common in our population, but proved fatal in most of the cats by markedly reducing survival (median survival time 7 days). The majority of cats with a hypercoagulable state had arterial thromboembolism or a direct echocardiographic visualisation of LA thrombi. Those presenting with smoke effect but no thrombi showed a better outcome with longer survival times: up to 732 days after diagnosis compared with 123 days for cats with echocardiographic confirmation of LA thrombi. Arterial thromboembolism is therefore a marker of severe cardiovascular impairment.4,26
Limitations of this study were mainly related to its retrospective nature. No systematic treatment protocols were performed, some clinical (T4 measurement) and echocardiographic data (ie, transmitral pulsed wave Doppler pattern) were not systematically assessed in our archive. The distribution of CMs might reflect some bias related to the referral centre where the study was carried out, and the possibility that some cats classified as affected by secondary CM might have a primary CM as well, exacerbated by systemic disease. Finally, owner-related information could have biased the results due to misinterpretation of clinical signs or failure to recognise cardiac-related death.
Conclusions
HCM and RCM are the most commonly diagnosed primary CMs in our cohort of patients. Secondary CMs are commonly reported as a cause for cardiac investigation due to the presence of clinical signs or clinical abnormalities during general clinical examination and echocardiographic study. The present survival study showed an overall risk of death in cats with clinical signs, LA enlargement and echocardiographic signs of a hypercoagulable state, regardless of the underlying CM. Secondary CMs are associated with few cases of cardiac death. Asymptomatic HCM patients showed longer survival times. Cats with RCM generally have a poor prognosis in the short and long term.
Acknowledgments
The authors would like to thank Marco Colombo for technical image assistance and all the referring vets and cat owners that made this study possible.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 6 May 2015
References
- 1. Elliot P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2006; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 2. Ferasin L. Feline myocardial disease part 1: classification, pathophysiology and clinical presentation. J Feline Med Surg 2009; 11: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferasin L, Sturgess CP, Cannon MJ. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg 2003; 5: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coté E, Macdonald K, Meurs K, et al. Feline cardiology. 1st ed. Somerset, NJ: Wiley-Blackwell, 2011. [Google Scholar]
- 5. Boon J. Veterinary echocardiography. 2nd ed. Chichester: Wiley-Blackwell, 2011, pp 37–255. [Google Scholar]
- 6. Peterson EN, Moise NS, Brown CA. Heterogeneity of hypertrophy in feline hypertrophic heart disease. J Vet Intern Med 1993; 7: 183–189. [DOI] [PubMed] [Google Scholar]
- 7. Brizard D, Amberger C, Hartnack S, et al. Phenotypes and echocardiographic characteristics of an European population of domestic shorthair cat with idiopathic hypertrophic cardiomyopathy. Schweiz Arch Tierheilkd 2009; 151: 529–538. [DOI] [PubMed] [Google Scholar]
- 8. Trehiou-Sechi E, Tissier R, Gouni R, et al. Comparative echocardiographic and clinical features of hypertrophic cardiomyopathy in 5 breeds of cats: a retrospective analysis of 344 cases (2001–2011). J Vet Intern Med 2012; 26: 532–543. [DOI] [PubMed] [Google Scholar]
- 9. Syme HM. Cardiovascular and renal manifestations of hyperthyroidism. Vet Clin North Am Small Anim Pract 2007; 37: 723–743. [DOI] [PubMed] [Google Scholar]
- 10. Santos M, Shah AM. Alterations in cardiac structure and function in hypertension. Curr Hypertens Rep 2014; 16: 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344: 501–509. [DOI] [PubMed] [Google Scholar]
- 12. Fox P, Basso C, Thiene G, et al. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in the domestic cats: a new animal model of human disease. Cardiovasc Pathol 2014; 23: 28–34. [DOI] [PubMed] [Google Scholar]
- 13. Atkins CE, Gallo AM, Kurzman ID, et al. Risk factors, clinical signs and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989). J Am Vet Med Assoc 1992; 201: 613–618. [PubMed] [Google Scholar]
- 14. Rush JE, Freeman LM, Fenollosa LK, et al. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002; 220: 202–207. [DOI] [PubMed] [Google Scholar]
- 15. Payne JR, Luis Fuentes V, Boswood A, et al. Population characteristic and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010; 51: 540–547. [DOI] [PubMed] [Google Scholar]
- 16. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013; 27: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 17. Brown S, Atkins C, Bagley R, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 18. Pittari J, Rodan I, Beekman G, et al. American Association of Feline Practitioners: senior care guidelines. J Feline Med Surg 2009; 11: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation 2008; 117: 429–439. [DOI] [PubMed] [Google Scholar]
- 20. Adin DB, Diley-Poston L. Papillary muscle measurements in cats with normal echocardiograms and cats with concentric left ventricular hypertrophy. J Vet Intern Med 2007; 21: 737–741. [DOI] [PubMed] [Google Scholar]
- 21. Weichselbaum R, Feeney D, Jessen C. Relationship between selected echocardiographic variables before and after radioiodine treatment in 91 hyperthyroid cats. Vet Radiol Ultrasound 2005; 46: 506–513. [DOI] [PubMed] [Google Scholar]
- 22. Chetboul V, Lefebre HP, Pinhas C, et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities and survival rate. J Vet Internal Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 23. Snyder P, Salek D, Jones GL. Effect of amlodipine on echocardiographic variables in cats with systemic hypertension. J Vet Internal Med 2001; 15: 52–56. [DOI] [PubMed] [Google Scholar]
- 24. Nelson L, Reidesel E, Ware W, et al. Echocardiographic and radiographic changes associated with systemic hypertension in cats. J Vet Intern Med 2002; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 25. Lefbom BK, Rosenthal SL, Tyrrell WDJ, et al. Severe hypertrophic cardiomyopathy in 10 young Ragdoll cats. J Vet Intern Med 2001; 15: 308. [Google Scholar]
- 26. Luis Fuentes V. Arterial thromboembolism: risks, realities and a rational first-line approach. J Feline Med Surg 2012; 14: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]