Abstract
Practical relevance:
Inappetence is a commonly encountered problem in feline medicine. Primary goals in managing the inappetent or anorectic cat are to diagnose and treat the underlying disease and reinstate adequate nutrition.
Rationale:
As cats are intolerant of prolonged periods of inadequate nutritional intake, especially given their propensity to develop hepatic lipidosis, their increased requirements for amino acids, and inability to slow their rate of gluconeogenesis, symptomatic therapy and nutritional support is often required during diagnostic investigations.
Clinical challenges:
Most cats presenting with reduced food intake will be suffering from an underlying systemic disease, and so the mechanism of action, pharmacokinetics and contraindications of appetite-stimulating medications will need to be considered in each case to ensure rational use of these agents. Pharmacological appetite stimulation should never replace monitoring and ensuring adequate caloric intake, and may not be appropriate in some cases, such as critically ill or severely malnourished patients.
Evidence base:
While there are no medications approved specifically for the treatment of anorexia in cats, some drugs have proven efficacious in the clinical field. Although several agents have been used historically for appetite stimulation, due to potential side effects and/or lack of efficacy or predictability only cyproheptadine and mirtazapine can currently be recommended for use.
Detrimental effects of prolonged inadequate nutrition
A reduced or absent appetite is a commonly encountered problem in feline medicine. Even healthy cats have the potential to be picky eaters. A cat that is unwell may present with inappetence or anorexia as one of the first clinical signs. 1 Cats have a higher protein and amino acid requirement than most other species and become protein malnourished quickly when anorexic. 2 Cats are dependent on protein for gluconeogenesis and are unable to downregulate production of aminotransferases and urea cycle enzymes in response to low protein intake. 3 Prolonged inadequate nutrition should be avoided as this may be more detrimental to the patient than the primary underlying disorder. Compromised immunity, delayed wound healing and altered hepatic metabolism are all possible sequelae.4,5
Cats are predisposed to accumulating triglycerides within hepatocytes and most systemically unwell cats develop some degree of hepatocellular fatty vacuolation. 6 Lipidosis occurs during periods of inadequate nutrition regardless of cause (eg, systemic illness, behavioural or environmental factors), with overconditioned individuals most at risk.6,7 This emphasises the importance of resuming and maintaining adequate food intake in cats that have been anorexic for longer than 3 days.7,8 While the ultimate goal for the clinician is to diagnose and treat underlying condition(s), symptomatic therapy during the diagnostic phase may be equally important to ensure a satisfactory outcome for the patient.
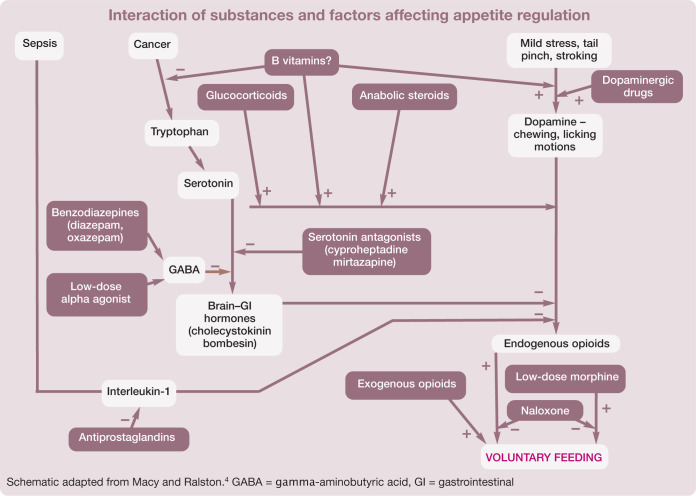
Appetite regulation
Ingestion of food is influenced by a complex system of interrelated metabolic, gastrointestinal and sensory signals. Taste, odour and texture of foods can all affect a patient’s motivation to eat, and dietary preferences are often dependent on previous feeding experiences. Mastication, distension of the gastrointestinal tract and postabsorptive changes in nutrient levels in the blood stimulate the release of hormones which impact the control of further food intake. For example, mastication results in dopamine release, which has a stimulatory effect on food intake, while most amino acids stimulate release of cholecystokinin, which inhibits appetite. 4 A number of other neurotransmitters such as catecholamines, serotonin and gamma-aminobutyric acid (GABA) also have a physiological role in the control of food intake, as described schematically in the box below.1,4
Noxious internal (nociception, nausea or ileus) or external (environmental) stimuli may override all other inputs. 9 All stimuli that influence feeding are integrated in the brain by hormonal and neuronal pathways. Important sites within the brain for control of hunger and satiety include the hypothalamus, especially the lateral and ventromedial nuclei,9,10 and the parabranchial nucleus in the caudal brainstem. 11 While the complexity of appetite regulation makes control of all components a challenge, it also offers multiple opportunities to manipulate appetite through management of external environmental and palatability factors, as well as pharmacological support of hunger cues.
Food aversion may be an important factor in feline inappetence, and can be associated with any food that is offered when a cat is feeling nauseous, vomiting, 1 painful or indisposed. 9 This aversion may persist even after recovery and highlights the importance of addressing these issues, not only to help improve appetite and hydration status, but also to help prevent continued rejection of certain foods after the patient returns home.12,13
Role of appetite stimulation
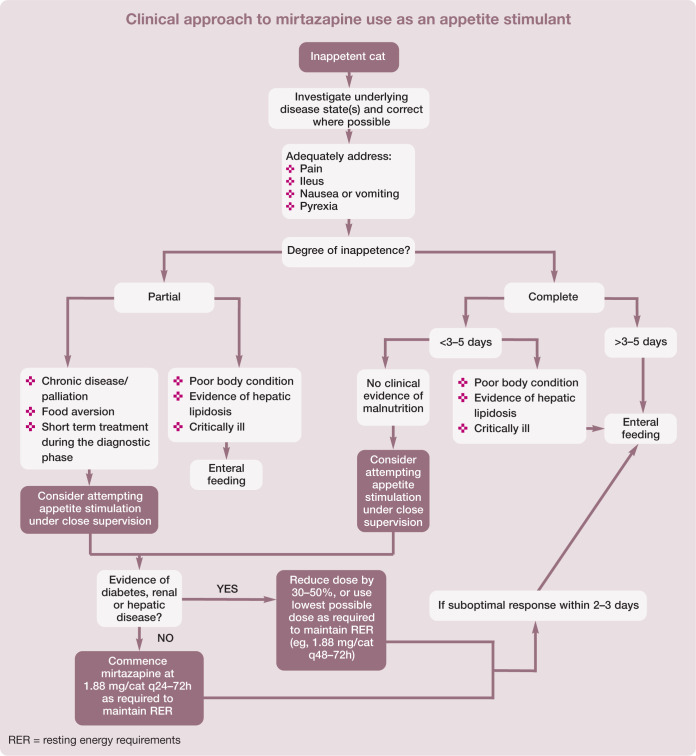
Appetite stimulation can play a valuable role in the management of anorectic and inappetent patients by inducing voluntary eating, thus improving their nutritional status and ability to recover from illness or injury. 4 It is important to be aware of the limitations and appropriate role of pharmacological appetite stimulants in clinical practice. Appetite stimulants should not be used as a substitute for accurate diagnosis and specific treatment of disease. It is important to ensure provision of adequate analgesia and address ileus, nausea and pyrexia before administering these medications. Appetite stimulants are not considered to be effective in very ill patients (Figure 1), 13 but can be useful in a number of situations (see below).
Figure 1.
Use of appetite stimulants is not recommended in critically ill cats. Enteral feeding is a more appropriate choice to ensure adequate nutrition in these patients
Figure 2.

Appetite stimulants can play a role in supporting adequate nutritional intake in chronically ill cats (such as patients with chronic kidney disease) or cats undergoing palliative cancer treatment once adequate analgesia is provided, and ileus, nausea and pyrexia are addressed
It is important to be aware that witnessing food ingestion does not necessarily indicate adequate caloric intake. Accurate measurement of food consumed and a comparison with the calculated resting energy requirements (RER) of the patient (eg, [30 x body weight (kg)] + 70) should be performed and used as a minimum baseline. The patient should be weighed daily using scales accurate for cats. Continued weight loss indicates an increased caloric requirement. If caloric intake remains insufficient after 2–3 days of using an appetite stimulant (or maximum 3–5 days since cessation of food intake), further measures, including enteral feeding, must be considered.8,9
Cats have a higher requirement for some B vitamins when compared with dogs. 19 Experimental depletion of B vitamins results in anorexia in other species. 20 Supplementation with B vitamins may prevent this occurring, although no evidence exists to confirm this. Still, provision of B vitamins is simple and should be considered in all inappetent cats.
Pharmacological options
A variety of agents have been used in cats for pharmacological appetite stimulation, and these are discussed in turn below, with dosage information provided in Table 1. Note, however, that only cyproheptadine and mirtazapine can currently be recommended for this purpose.
Table 1.
Appetite stimulants used in cats
| Drug | Class/mechanism of action | Dosage | Renal insufficiency dosing changes | Hepatic disease dosing changes | Other notes |
|---|---|---|---|---|---|
| Cyproheptadine | Serotonin antagonist antihistamine | • 1–4 mg/cat q12–24h4,13,15,17,31,32,36,43–45
• 0.2–1.0 mg/kg PO q12h1,27,46 |
1 mg/cat PO q12h 47 Elimination is reduced in renal failure 21 | Not recommended for use in presence of hepatic lipidosis 7 | Possible agitation, haemolysis |
| Diazepam* | Benzodiazepine | • 0.2 mg/kg slow IV once1,4,27,43,44 • 0.05–0.50 mg/kg slow IV once13,15,17,31,32,36 • 0.5–1.0 mg/kg IV once 48 |
Caution if renal disease 21 | Contraindicated in presence of hepatic dysfunction.21,27 Reduce dose by 25–50% if used with significant hepatic failure. 26 Not recommended for use in hepatic lipidosis 7 | Avoid oral dosing due to risk of acute hepatic necrosis |
| Megestrol acetate* | Synthetic progestin | • 1 mg/kg PO q12–24h4,13 • 2.5 mg q24h for 4 days, then q48–72h thereafter 17 • 0.25–0.50 mg/kg q24h PO for 3–5 days, then q48–72h31,46 |
No published reference dose alterations | Undergoes hepatic metabolism. Consider dosage reduction of 25–50% 21 | Used for palliation only. Not for prolonged use |
| Mirtazapine | Pre-synaptic α2-receptor antagonism resulting in norepinephrine increase. Also serotonin receptor antagonism | • 1.88 mg/cat PO q12–24h to 3.5 mg/cat PO q72h36,37,44,45 | 1.88 mg/cat q24–48h 18 to 3 mg/cat q72h.16,47 Renal impairment may reduce elimination by 30–50% 21 | Hepatic impairment may reduce clearance by up to 30% 21 | Mirtazapine has been associated with blood dyscrasias in humans, and subclinical ALT elevations in both feline and human patients. Do not use with cyproheptadine for appetite stimulation |
| Nandrolone* | Anabolic steroid | • 2.5 mg/kg IM every 2–3 weeks4,15 | Contraindicated in nephrotic stage of nephritis 21 | Contraindicated in presence of hepatic dysfunction 21 | Used for palliation only |
| Oxazepam* | Benzodiazepine | • 0.25–0.50 mg/kg PO q12–24h1,13,27,49 • 2.0–2.5 mg/cat PO q12h4,15,32,36,43,44 |
Caution in renal disease 21 | Use with caution in presence of hepatic dysfunction.21,27 Reduce dose by 25–50% if used with significant hepatic failure. 26 Not recommended for use in hepatic lipidosis 7 | Anecdotally associated with fulminant hepatic failure |
| Prednisolone* | Glucocorticoid | • 0.25–0.50 mg/kg PO q24–48h 4 | No published reference dose alterations | No published reference dose alterations | Used for palliation only |
Historically used appetite stimulant, no longer recommended
IM = intramuscular; IV = intravascular, PO = oral; ALT = alanine aminotransferase
Anabolic steroids
Nandrolone decanoate (Deca-Durabolin, Organon; Laurabolin, MSD Animal Health) and stanozolol (Winstrol; Winthrop-Breon) have historically been used as appetite stimulants.4,15 The positive nitrogen balance associated with the use of anabolic steroids in debilitated animals has been shown to be the mechanism of appetite-stimulating action. 4 The effects of anabolic steroids can be longer lived, but are less pronounced and less predictable, than those of glucocorticoids or benzodiazepines. 15
Anabolic steroids are associated with common and serious adverse effects such as sodium, calcium, potassium, water, chloride and phosphate retention, hepatotoxicity and androgenic behavioural changes. Stanozolol, in particular, has been associated with a high incidence of hepatotoxicity in cats. 21 Anabolic steroids are contraindicated in patients with hepatic dysfunction, hypercalcaemia, cardiovascular disease, pituitary insufficiency, prostate or mammary carcinomas, benign prostatic hypertrophy and during the nephrotic stage of nephritis. 21
Anabolic steroids are no longer recommended for appetite stimulation.
Benzodiazepines
Benzodiazepines are thought to cause direct appetite stimulation within the central nervous system. 13 They enhance inhibitory neurotransmission mediated by GABA, with receptors located on GABAA receptor proteins. The hyperphagia induced is likely mediated by the α2/α3 subtype, with the probable site of action being the parabranchial nucleus in the caudal brainstem. 11
Diazepam (Valium; Roche) has traditionally been the most frequently used benzodiazepine appetite stimulant, and is more effective when given intravenously than orally, making it a more appropriate choice for use in hospital than in the home environment. 17 It is important to remember, however, that appetite stimulation is rarely effective in critically ill cats. Oxazepam, available only in an oral form, may be a more powerful appetite stimulant than diazepam; however, oral administration tends to make it less effective clinically. 4 Feeding usually commences within 20 mins of administration, but is short-lived, and may not result in adequate daily intake. 15 Other drawbacks to the use of benzodiazepines include ataxia, sedation and unpredictable response to standard doses.4,13 These medications are metabolised in the liver, with the active metabolites eventually undergoing glucuronidation, and then excretion in the urine.
The use of oral benzodiazepines is controversial in cats due to multiple reports of serious, and almost invariably fatal, idiosyncratic hepatotoxicity observed 5–13 days after the use of oral diazepam.2,21 –25 These drugs are reportedly either contraindicated, or only to be used with caution/at reduced rates, in patients with hepatic dysfunction.1,21,26 Caution is also required in patients with renal disease. 21 As viable, effective alternatives exist, the use of these drugs has largely been superseded.
Cyproheptadine
Cyproheptadine (Periactin; Merck, Figure 3) is a serotonin (5-hydroxytryptamine, 5-HT) antagonist antihistamine that historically has been more commonly used as a longer term appetite stimulant in cats, although clinical effects may deteriorate over time.21,27 It is thought to stimulate appetite through antagonism of 5-HT2 receptors in the ventromedial hypothalamus. 28 Cyproheptadine may take a few days to reach effective therapeutic levels. 29 Adverse events may include sedation, paradoxical hyperexcitability or anticholinergic effects. It has also been shown to reduce seizure threshold in experimental mouse models. 30
Figure 3.
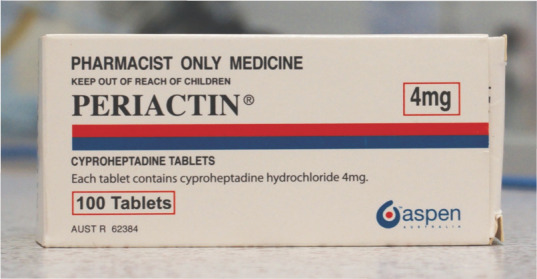
Cyproheptadine is a serotonin antagonist antihistamine which is thought to stimulate appetite through antagonism of 5-HT2 receptors in the ventromedial hypothalamus. As it is nearly completely metabolised in the liver and undergoes renal excretion, dose adjustments should be made for either renal or hepatic disease
Cyproheptadine is metabolised in the liver and undergoes renal excretion. Therefore, dose adjustments should be made in the presence of renal or hepatic insufficiency. Other conditions that require caution in the use of cyproheptadine include urinary or gastrointestinal obstruction and severe congestive heart failure. 21 Cyproheptadine has been reported to cause fulminant liver failure in individual cases, and is not recommended for use in the presence of hepatic lipidosis. 7
Glucocorticoids
Glucocorticoids have been used for non-specific appetite stimulation in veterinary patients for many years.4,10,31,32 The mechanism of action for appetite stimulation is unknown, but cortisol is thought to have a permissive action for other appetite-stimulating compounds such as opioids and epinephrine, and the ability of corticosteroids to stimulate metabolism has also been suggested to play a role.4,33 Corticosteroids have been demonstrated in clinical trials with human oncology patients to be effective in alleviating appetite loss, although the effect is noted to be short-lived (usually only a few weeks at best) and with a side effects profile that makes them a poor choice for prolonged use. 34 Caution should also be used in cats with diabetes mellitus (due to hyperglycaemic effects) or cardiovascular disease (due to hypertensive effects caused by vasoconstriction and increased blood volume). 21
Corticosteroids should generally only be used if other effects of these medications (eg, anti-inflammatory, immunosuppressive or antineoplastic) are desired.
Megestrol acetate
Megestrol acetate (Ovaban, Schering-Plough; Ovarid, Virbac) is a synthetic progestin. Although less consistently observed than with other agents, it does have appetite-stimulating properties, the mechanism of action of which is poorly defined. 4 Megestrol acetate is associated with serious and relatively common adverse effects including adrenocortical suppression, diabetes mellitus, increased risk of mammary adenocarcinomas, and hepatotoxicity,21,35 making it a difficult choice to justify for all but short-term palliative cases. Contraindications include pregnancy, uterine disease, diabetes mellitus and mammary neoplasia. 21 Megestrol acetate should be avoided in intact females or cats already receiving glucocorticoids.
Due to the widespread availability of agents with a higher efficacy and lower risk of side effects, it is difficult to justify the use of megestrol acetate.
Mirtazapine
Mirtazapine (Avanza; MSD, Figure 4) is a serotonin receptor antagonist that also antagonises pre-synaptic α2-receptors. The net increase in norepinephrine that results likely contributes most to its appetite-stimulating effects as norepinephrine acts at other α-receptors to increase appetite. 21 Mirtazapine also antagonises 5-HT2 receptors, which can result in appetite stimulation via nuclei within the hypothalamus. Although mainly used as an antidepressant in humans, it has the added benefit of anti-nausea and antiemetic properties mediated primarily through antagonism of the 5-HT3 receptor. 16
Figure 4.
Although mirtazapine was originally designed as a human antidepressant medication, it has also been reported to have both anti-nausea and appetite-stimulating properties. Recent clinical trials have provided evidence for mirtazapine’s efficacy in select groups of patients and have supported the use of lower and extended dosing regimens, especially in cats with renal disease
Administration of mirtazapine in cats was first reported in 2006; however, pharmacological studies were not performed. 8 Two subsequent, related studies16,37 have demonstrated a significant increase in food consumed after administration of mirtazapine to healthy young cats, with minimal side effects observed when a low dose was used (1.88 mg/cat). These studies identified a 9 h half-life of mirtazapine in cats at this dose, suggesting daily dosing in healthy cats that only show a response for 24 h may be most appropriate, with no accumulation detected after 6 days of administration.
Cats with chronic kidney disease have been shown to exhibit reduced mirtazapine clearance, necessitating an extended (q48h) dosing regimen in these patients. 16 A placebo-controlled crossover clinical trial demonstrated a statistically significant increase in appetite and weight gain in cats with stable chronic kidney disease given mirtazapine q48h over a 3 week period. 18 Although the studies from Quimby et al found the increase in appetite to occur within 8 h of administration, other authors have reported that it may take up to 36 h for a response to be seen. 36 Adverse effects may include muscle twitching, hyperactivity, vocalisation and behavioural changes (eg, increased affection). These effects are less likely to be observed at lower doses.21,37 A subclinical elevation in alanine aminotransferase has been reported in feline and human patients, which resolved upon discontinuation of mirtazapine.18,38
Metabolism of mirtazapine occurs via multiple pathways, including glucuronidation, and the human literature documents elimination via urine (75%) and faeces (25%). 39 Renal impairment may reduce elimination by 30–50%, and hepatic impairment may reduce clearance by up to 30%, indicating the need to adjust dosing accordingly.21,39 Caution should be used in patients with renal or hepatic insufficiency, and in patients with diabetes mellitus due to indirect effects on blood glucose regulation. 21 Although extremely rare, mirtazapine has also been associated with blood dyscrasias in humans. 21
Mirtazapine should not be used in patients receiving monoamine oxidase inhibitors (eg, selegiline) or tramadol due to an increased risk of serotonin syndrome. Serotonin syndrome is characterised by three types of neuro-excitatory symptoms including: a) neuromuscular hyperactivity (tremor, clonus, hyperreflexia, pyramidal rigidity); b) autonomic hyperactivity (pyrexia, tachycardia, tachypnoea); and c) altered mental status (agitation, excitement).40,41 In the event of serotonin syndrome occurring, cyproheptadine (a non-selective serotonin antagonist) has been suggested as an antidote.21,41,42 Therefore, it is not recommended that cyproheptadine and mirtazapine be administered together for appetite stimulation, as their effects may be negated by each other. As feline patients require smaller doses than the available human preparation, utilising a compounding pharmacy for accurate dosing may make the use of mirtazapine easier for clients.
The clinical approach to mirtazapine use as an appetite stimulant is described on page 754.
Key points
The complexity of appetite regulation makes control of all components a challenge, but also offers multiple opportunities to manipulate appetite through management of external environmental and palatability factors, as well as pharmacological support of hunger cues.
While not indicated for every inappetent cat, appetite stimulants do have a role in supporting nutrition for a select group of feline patients.
A careful history, physical examination and knowledge or suspicion of underlying disease states can help direct the selection and use of these medications.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors declare that there is no conflict of interest.
References
- 1. Marks S. Diagnostic and therapeutic approach to the anorectic cat. Proceedings of the World Small Animal Veterinary Association World Congress; 2001 Aug 8–11; Vancouver, Canada. http://www.vin.com/Members/proceedings/Proceedings.plx?CID=WSAVA2001&Category=&PID=168&O=VIN. [Google Scholar]
- 2. MacDonald M, Rogers Q, Morris J. Nutrition of the domestic cat, a mammalian carnivore. Annu Rev Nutr 1984; 4: 521–562. [DOI] [PubMed] [Google Scholar]
- 3. Witzel AL, Bartges J, Kirk C, Hamper B, Murphy M, Raditic D. Nutrition for the normal cat. In: Little SE. (ed). The cat. St Louis, MO: WB Saunders, 2012, pp 243–247. [Google Scholar]
- 4. Macy DW, Ralston SL. Cause and control of decreased appetite. In: Kirk RW. (ed). Current veterinary therapy X: small animal practice. Philadelphia: WB Saunders, 1989, pp 18–24. [Google Scholar]
- 5. Freitag KA, Saker KE, Thomas E, Kalnitsky J. Acute starvation and subsequent refeeding affect lymphocyte subsets and proliferation in cats. J Nutr 2000; 130: 2444–2449. [DOI] [PubMed] [Google Scholar]
- 6. Scherk M, Center SA. Toxic, metabolic, infectious and neoplastic liver diseases. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. St Louis, MO: Saunders Elsevier, 2010, pp 1672–1689. [Google Scholar]
- 7. Armstrong PJ, Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract 2009; 39: 599–616. [DOI] [PubMed] [Google Scholar]
- 8. Chan DL. The inappetent hospitalised cat: clinical approach to maximising nutritional support. J Feline Med Surg 2009; 11: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michel KE. Management of anorexia in the cat. J Feline Med Surg 2001; 3: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hackett T. Anorexia. In: Wingfield WE. (ed). Veterinary emergency medicine secrets. 2nd ed. Philadelphia: Hanley & Belfus, 2001, pp 121–124. [Google Scholar]
- 11. Cooper SJ. Palatability-dependent appetite and benzodiazepines: new directions from the pharmacology of GABA(A) receptor subtypes. Appetite 2005; 44: 133–150. [DOI] [PubMed] [Google Scholar]
- 12. Michel KE. Management of anorexia. Proceedings of the Atlantic Coast Veterinary Conference. 2002. Oct 15–17; Atlantic City, NJ, USA. http://www.vin.com/Members/proceedings/Proceedings.plx?CID=ACVC2002&Category=&PID=2421&O=VIN. [Google Scholar]
- 13. Sparkes AH. Assessing and tempting the ‘finicky’ cat. Proceedings of the Western Veterinary Conference. 2005 Feb 20–24; Las Vegas, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=WVC2005&Category=&PID=7840&O=VIN. [Google Scholar]
- 14. Forman MA. Anorexia. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, MO: Saunders Elsevier, 2010, pp 172–173. [Google Scholar]
- 15. Hartke JA, Rojko JL, Mathes LE. Cachexia associated with cancer and immunodeficiency in cats. In: Kirk RW, Bonagura JD. (eds). Current veterinary therapy XI: small animal practice. Philadelphia: WB Saunders, 1992, pp 438–441. [Google Scholar]
- 16. Quimby JM, Gustafson DL, Lunn KF. The pharmacokinetics of mirtazapine in cats with chronic kidney disease and in age-matched control cats. J Vet Intern Med 2011; 25: 985–989. [DOI] [PubMed] [Google Scholar]
- 17. Ogilvie GK. Nutrition and cancer: new keys for cure and control. Proceedings of the World Small Animal Veterinary Association World Congress. 2003 Oct 24–27; Bangkok, Thailand. http://www.vin.com/Members/proceedings/Proceedings.plx?CID=WSAVA2003&Category=&PID=6569&O=VIN. [Google Scholar]
- 18. Quimby JM, Lunn KF. Mirtazapine as an appetite stimulant and anti-emetic in cats with chronic kidney disease: a masked placebo-controlled crossover clinical trial. Vet J 2013; 197: 651–655. [DOI] [PubMed] [Google Scholar]
- 19. Zoran DL. The carnivore connection to nutrition in cats. J Am Vet Med Assoc 2002; 221: 1559–1567. [DOI] [PubMed] [Google Scholar]
- 20. Kirchgessner M, Kösters WW. Influence of vitamin B6 deficiency in early weaned piglets on the digestibility and conversion of protein and energy [Article in German]. Arch Tierernahr 1977; 27: 299–308. [DOI] [PubMed] [Google Scholar]
- 21. Plumb DC. Plumb’s veterinary drug handbook. 7th ed. Ames: Wiley-Blackwell, 2011. [Google Scholar]
- 22. Chan DL. Critical care nutrition. In: August JR. (ed). Consultations in feline internal medicine. St Louis, MO: Elsevier, 2010, pp 116–126. [Google Scholar]
- 23. Park FM. Successful treatment of hepatic failure secondary to diazepam administration in a cat. J Feline Med Surg 2012; 14: 158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hughes D, Moreau RE, Overall KL, Winkle TJV. Acute hepatic necrosis and liver failure associated with benzodiazepine therapy in six cats, 1986–1995. J Vet Emerg Crit Care 1996; 6: 13–20. [Google Scholar]
- 25. Center S, Elston T, Rowland P, Rosen DK, Reitz BL, Brunt JE, et al. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J Am Vet Med Assoc 1996; 209: 618–625. [PubMed] [Google Scholar]
- 26. Trepanier L. Case presentations: drug dose adjustments. Proceedings of the ACVIM. 2008 June 4–7; San Antonio, TX, USA. http://www.vin.com/Members/proceedings/Proceedings.plx?CID=ACVIM2008&Category=&PID=22789&O=VIN. [Google Scholar]
- 27. Marks S. Demystifying the anorectic cat. Proceedings of the ACVIM. 1998 May 22–25; San Diego, California. http://www.vin.com/Members/CMS/document/default.aspx?id=2992247&pid=26&catid=137&said=1. pp 62–64. [Google Scholar]
- 28. Çalka Ö, Metin A, Dülger H, Erkoç R. Effect of cyproheptadine on serum leptin levels. Adv Ther 2005; 22: 424–428. [DOI] [PubMed] [Google Scholar]
- 29. Norris CR, Boothe DM, Esparza T, Gray C, Ragsdale M. Disposition of cyproheptadine in cats after intravenous or oral administration of a single dose. Am J Vet Res 1998; 59: 79–81. [PubMed] [Google Scholar]
- 30. Singh D, Goel RK. Proconvulsant potential of cyproheptadine in experimental animal models. Fundam Clin Pharmacol 2010; 24: 451–455. [DOI] [PubMed] [Google Scholar]
- 31. Smith AN. Special concerns in cat chemotherapy. Proceedings of the ACVIM. 2003 June 4–7; Charlotte, NC, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=ACVIM2003&Category=&PID=4081&O=VIN. [Google Scholar]
- 32. Brown MR, Rogers KS. Supportive medical care and pain management in feline cancer patients. In: August JR. (ed). Consultations in feline internal medicine. St Louis, MO: Elsevier Saunders, 2006, pp 665–672. [Google Scholar]
- 33. Wade BJ. Corticosteroids. 2001. Accessed 13 January 2014. http://www.vin.com/members/cms/document/default.aspx?id=2992989&pid=&catid=&said=1.
- 34. Behl D, Jatoi A. Pharmacological options for advanced cancer patients with loss of appetite and weight. Expert Opin Pharmacother 2007; 8: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 35. Mauldin GE. Supportive care for the cancer patient. In: Withrow SJ, Vail DM. (eds). Withrow and MacEwan’s small animal clinical oncology. 4th ed. St Louis: Saunders Elsevier, 2007, pp 307–326. [Google Scholar]
- 36. Saker KE, Remillard RL. Critical care nutrition and enteral-assisted feeding. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Novotny BJ. (eds). Small animal clinical nutrition. 5th ed. Topeka: Mark Morris Institute, 2010, pp 439–476. [Google Scholar]
- 37. Quimby JM, Gustafson DL, Samber BJ, Lunn KF. Studies on the pharmacokinetics and pharmacodynamics of mirtazapine in healthy young cats. J Vet Pharmacol Ther 2011; 34: 388–396. [DOI] [PubMed] [Google Scholar]
- 38. Adetunji B, Basil B, Mathews M, Osinowo T. Mirtazapine-associated dose-dependent and asymptomatic elevation of hepatic enzymes. Ann Pharmacother 2007; 41: 359. [DOI] [PubMed] [Google Scholar]
- 39. Delbressine LPC, Moonen MEG, Kaspersen FM, Wagenaars GN, Jacobs PL, Timmer CJ, et al. Pharmacokinetics and biotransformation of mirtazapine in human volunteers. Clin Drug Investig 1998; 15: 45–55. [DOI] [PubMed] [Google Scholar]
- 40. Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. B J Anaesth 2005; 95: 434–441. [DOI] [PubMed] [Google Scholar]
- 41. Indrawirawan Y, McAlees T. Tramadol toxicity in a cat: case report and literature review of serotonin syndrome. J Feline Med Surg 2014; 16: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16: 615–619. [DOI] [PubMed] [Google Scholar]
- 43. Rand J. The cat with upper respiratory signs. In: Rand J. (ed). Problem-based feline medicine. Toronto: Saunders Elsevier, 2006, pp 5–18. [Google Scholar]
- 44. Churchill JA. Pleasing geriatric palates – nutritional management of the finicky senior cat. Proceedings of the American Association of Feline Practitioners Fall 2006 Meeting. Toronto, Canada: http://www.vin.com/Members/proceedings/Proceedings.plx?CID=AAFP2006F&Category=&PID=14991&O=VIN. [Google Scholar]
- 45. Armstrong PJ, Williams DA. Pancreatitis in cats. Top Companion Anim Med 2012; 27: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore AS. Practical chemotherapy. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine: diseases of the dog and cat. St Louis, MO: Saunders Elsevier, 2010, pp 2126–2133. [Google Scholar]
- 47. Scherk M. What’s new pussycat? An update on feline renal insufficiency. Proceedings of the Western Veterinary Conference. 2008 Feb 17–21; Las Vegas, USA. http://www.vin.com/Members/proceedings/Proceedings.plx?CID=WVC2008&Category=&PID=19346&O=VIN. [Google Scholar]
- 48. Ramsey I. Small animal formulary. 7th ed. Quedgeley, Gloucester: British Small Animal Veterinary Association, 2011. [Google Scholar]
- 49. Bartges J. Rusty plumbing: chronic renal failure. Proceedings of the Western Veterinary Conference. 2002 Feb 11–14; Las Vegas, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=WVC2002&Category=&PID=992&O=VIN. [Google Scholar]