Abstract
Bacteria, fungi, viruses, and protozoa are known to infect and induce diseases in the human central nervous system (CNS). Modeling the mechanisms of interaction between pathogens and the CNS microenvironment is essential to understand their pathophysiology and develop new treatments. Recent advancements in stem cell technologies have allowed for the creation of human brain organoids, which more closely resembles the human CNS microenvironment when compared to classical two-dimensional (2D) cultures. Now researchers can utilize these systems to investigate and reinvestigate questions related to CNS infection in a human-derived brain organoid system. Here in this review, we highlight several infectious diseases which have been tested in human brain organoids and compare similarities in response to these pathogens across different investigations. We also provide a brief overview of some recent advancements which can further enrich this model to develop new and better therapies to treat brain infections.
Introduction
The CNS is vulnerable to a variety of infectious diseases. Pathogens like zika-virus (ZIKV) and Mycobacterium tuberculosis (Mtb) can infiltrate past the brain’s barriers and inflict damage on neuronal cell populations. Studying pathogens of the human brain is technically challenging due to anatomical and ethical restraints, as well as the limited availability of human brain tissue to researchers. As a result, several in vitro systems have been developed to model many of the components of the human CNS. Recently, more advanced protocols to generate human three-dimensional (3D) brain organoids have been developed that now allow researchers to study not only the mechanisms of brain development, but also complex interactions between human brain cells and pathogens of interest. In the following sections, we will provide an in-depth summary of several studies which have already utilized brain organoids to investigate infectious CNS diseases, as well as some of the advancements and current limitations in the usage of brain organoids to the study of infectious diseases in the CNS.
Development of Brain Organoids
Early studies demonstrated that embryonic stem cell (ESC) aggregates called embryoid bodies could differentiate into neural lineages after exposure to fibroblast growth factor 2 (FGF2) creating 2D “neural rosettes”1. These neural rosettes shared many cellular features of the early stages of the developing brain, and neurons within these rosettes could be differentiated into different populations using inhibitors and activators of Wnt, Shh, FGF, and BMP pathways2,3. Particularly, it was found that dual inhibition of BMP and TGF-β/NODAL signaling (or dual SMAD inhibition) in human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) leads to more efficient induction of early anterior neuroectoderm fate4. However, these systems were still relatively simple and lacked many of the cyto-structural phenotypes of the human nervous system. Later, it was discovered that embedding hESCs or hiPSCs in Matrigel plays a crucial role in the formation of more complex organized spheroid 3D brain structures5,6. Lancaster et al., demonstrated with a relatively minimalistic unguided protocol the ability to create 3D cerebral organoids. Analysis of these first organoids revealed the formation of distinct progenitor zones characteristic of cortical development, as well as the number of small subregions that resembled developing forebrain, choroid plexus, midbrain, meninges, and retina6,7. Additionally, subsequent scRNAseq of these unguided or “self-patterning” organoids revealed that these systems produced diverse subpopulations of neurons, retinal cells, astrocytes, as well as precursors to oligodendrocytes8. These unguided systems were limited, as they could be highly heterogeneous and lack the ability to reliably target specific structural regions. As a result, almost instantaneously after the establishment of unguided organoids, researchers expanded upon culturing protocols to guide development of these organoids toward specific anatomical lineages, leading to several advancements relevant to the investigation of infectious disease in the CNS (see Advancements and Limitations section). The use of brain organoids has expanded dramatically in recent years both in the experimental complexity of these organoid systems, as well as in the number of diseases investigated. We will now expand on the recent experimental findings generated using these brain organoid systems across a wide variety of CNS infections.
Modeling Viral infections in Brain Organoids
Zika Virus
A member of the Flaviviridae family, ZIKV is a single-stranded RNA virus with a well-characterized role in CNS infection. The Flaviviridae family includes several pathologically significant disease-causing insect-borne viruses, such as yellow fever, West Nile, Japanese encephalitis, dengue, and tick-borne encephalitis9,10. ZIKV was initially identified in monkeys in Uganda in 1947 and re-discovered in the Aedes africanus mosquito species at the same site in 194811. According to the World Health Organization (WHO), the first human cases of ZIKV were discovered in Uganda and Tanzania in 195212. After that, human-ZIKV infection incidents were infrequently reported, even though the virus had spread to a few Asian pacific and African countries13. However, after a sudden outbreak occurred in late 2015 in South America, ZIKV emerged as a significant global healthcare threat, due to its rate of transmission and severe clinical manifestations in neonates and adults13–15. ZIKV is primarily transmitted to people by Aedes mosquitoes but can also be spread via numerous ways, such as blood transfusion and sexual contact14. According to previously published reports, ZIKV can be detected in body fluids (saliva, tears, urine, semen, and sweat) of infected patients. More importantly, ZIKV can be vertically transmitted from infected mother to fetus through the placenta during the pregnancy, resulting in congenital Zika syndromes, including severe microcephaly and other serious brain abnormalities16 . Microcephaly is a congenital brain malformation that significantly reduces the head circumference of infants mainly due to defects associated with proliferation and extensive cell death of cortical neural progenitor cells (NPC)9. Apart from causing microcephaly, ZIKV infection is also implicated in triggering Guillain-Barre syndrome (GBS) in certain individuals; however, acquiring the GBS is relatively low among the ZIKV positive population17–19.
To date, understanding of the pathogenesis of ZIKV, especially the mechanism of ZIKV mediated microcephaly in infants, remains unclear due to the unavailability of infected fetal tissue samples. As a result, identifying and testing novel drug treatments for ZIKV has severe limitations. Recently, several research groups have successfully utilized brain organoids as in vitro models for elucidating ZIKV mediated pathogenesis, and testing potential drug candidates to overcome many of the issues mentioned above. Several recent studies have shown that ZIKV efficiently infects hiPSCs-derived NPCs in 2D culture or neurospheres, promoting cell death and attenuating NPC growth20–24. Although hiPSC-derived 2D cultures of astrocytes and microglia-like cells can become infected with ZIKV, significant ZIKV-induced cytotoxicity was not detected22. Despite productive infectivity of ZIKV, the 2D NPC culture system is still limited in its ability to fully investigate the direct link between ZIKV and microcephaly since 2D systems do not resemble the characteristic organizational features of 3D brains such as cortical layer development. Thus, usage of brain organoids to model ZIKV infection provides another platform to determine specific cellular tropism of ZIKV within the different and complex neuronal cellular networks in 3D brain tissue.
The experiments performed with the 3D brain organoids revealed that ZIKV readily infects NPCs that specifically reside in the ventricular zone (VZ) over the intermediate NPCs or immature neurons24–32. In most cases, ZIKV positive cell number or viral copy number was significantly increased over the post-incubation time in infected brain organoids, suggesting ZIKV is efficiently hijacking host cell’s molecular machinery for viral replication26,32,33. Moreover, ZIKV infected brain organoids also had dramatically reduced neuronal layers, VZ thickness, and overall organoid size24,28,32. In addition to growth impairment of brain organoids, in-depth analysis after exposure to ZIKV manifested a drastic depletion of NPCs due to the two potential circumstances, suppression of NPC proliferation and induced cell death of both the ZIKV infected NPCs and bystander neuronal cells24,27,29,32. When compared with 2D NPC cultures, 3D brain organoids showed dramatic ZIKV associated cell death20,24,32. Another study reported that ZIKV infection induces premature differentiation of NPCs in human brain organoids resulting in thinner VZ formation potentially due to the loss of NPCs25. Intriguingly, ZIKV-associated neuropathology has also been addressed using human microglia-incorporated 3D brain organoids34. Microglia are considered specialized macrophage-like cells in the CNS, playing critical roles during brain development, including phagocytosis of apoptotic cells, synaptic organization, regulation of neurogenesis, and axonal growth, among many other functions35,36. Upon the ZIKV challenge, microglia-incorporated human brain organoids exhibited significant size reduction, macrophage activation, and up-regulation of inflammatory cytokines. Furthermore, these infected human brain organoid models which contained microglia exhibited increased synaptic elimination against ZIKV exposure, demonstrating the role of microglia response to viral infection in organoids34. Altogether, these observations strongly resemble many of the key characteristics of ZIKV-induced microcephaly at the cellular level.
It is also essential to address ZIKV mediated microcephaly at a molecular level, to uncover vital genes and pathways involved in ZIKV-induced cell death, premature differentiation, and attenuation of cell proliferation, which will ultimately open the gateways to test potential therapeutic strategies. A recently published article by Li et al., demonstrated that the interaction of nonstructural protein 5 (NS5) produced by ZIKV with p53 of human NPCs could induce p53 mediated apoptosis of NPCs, implying its potential contribution to the ZIKV associated microcephaly37. Transcriptional analysis identified 531 differentially expressed genes (DEG) in ZIKV infected brain organoids28. The majority of up-regulated genes were engaged with antiviral defense, response to interferon (IFN), stress, and stimuli pathways, whereas most of the down-regulated DEGs associated with the gene ontology (GO) terms of cell cycle progression and cell division28. Moreover, in-depth analysis indicated that IFN-stimulated genes (ISGs) and IFN-I components such as IFN-α and IFN-β were differentially activated by ZIKV infection in brain organoids. Notably, more attenuated IFN-I responses were detected in 2D human NPC cultures than in 3D brain organoids after exposure to ZIKV, suggesting cell type-specific modulation of viral sensing28. In addition, RNA sequencing data of ZIKV infected organoids indicates that ZIKV infection, triggers host innate immune responses, leading to programmed cell death and neurodegeneration38. Consistent with these observations, Liu and colleagues also identified up-regulated GO terms related to the immune response to the virus and Type I IFN pathways27. However, they were unable to locate IFN mRNA induction, while all prominently up-regulated genes were categorized to the interferon-stimulated gene (ISG) family, indicating that acute antiviral responses and ZIKV-mediated neuropathy are elicited in an IFN-independent ISG overactivation manner27. Yoon et al discovered that the expression of a ZIKV encoded protein identified as nonstructural protein 2A (NS2A) is actively involved in the disruption of cortical neurogenesis and premature differentiation, attenuation of radial glial cell proliferation, and destabilization of adherens junction complex formation in human forebrain organoids39. Transcriptional profiling of ZIKV infected human cerebral organoids also demonstrated activation of Toll-like receptor 3 (TLR3)26. Further analysis revealed that ZIKV infection up-regulates pro-apoptotic pathways and down-regulates neurogenesis pathways in a TLR3-dependent manner, resulting from growth arrest of the organoids, leading to microcephaly-like phenotype26.
Findings of in vitro and in vivo experiments provide solid conclusions of efficient brain invasion of ZIKV, and its preferential tropism to NPCs, causing severe neurological complications. Scientists have made several attempts to discover the mechanisms that ZIKV utilizes to travel toward, and invade, the brain. However, there is still a central question to answer: how does ZIKV reach the CNS through the blood-brain barrier (BBB), an extremely tight endothelium providing protection to CNS from blood-borne pathogens and harmful substances? One possibility is that ZIKV may use circulating leukocytes with viral carrying capabilities, such as monocytes, as vehicles for transmigration through the BBB40,41. In agreement with this theory, Ayala-Nunez et al., identified ZIKV infected monocyte-derived cells in CNS of human fetuses diagnosed with microcephaly41. In addition, ZIKV exposure induced the expression of adhesion molecules in human monocytes and promoted their transmigrational ability across the endothelial layer41. In the same study, they demonstrated that ZIKV infected monocytes transmigrate through an in vitro, BBB-like endothelium and disseminate infection to 3D human cerebral organoids without affecting the permeability of the endothelial layer41. Additionally, ZIKV infected human macrophage/microglia also exhibited virus transmission capabilities to NPCs, inducing apoptosis of NPCs42. Consistent with this, Muffat et al, showed that ZIKV infected human microglia-like cells could migrate toward the 3D neuralized organoids and disseminate the infection to vulnerable cells in the organoids22.
It has been shown that human brain organoids are effective tools for screening therapeutic strategies for ZIKV-associated pathogenesis. For instance, Hippeastrine hydrobromide (HH) and amodiaquine dihydrochloride dihydrate (AQ) treatments inhibited ZIKV infection in 2D human NPC cultures43. Interestingly, only HH treatment abolished ZIKV induced differentiation and growth impairments in 3D human fatal-like forebrain organoids43. According to the study of Mesci et al, Sofosbuvir, an FDA-approved drug for Hepatitis C, efficiently blocked ZIKV infection in NPCs while rescuing the cells from apoptosis induced by the virus42. Data from a high content drug screening study demonstrated that Emricasan, a pan-caspase inhibitor, protects ZIKV infected human cortical NPCs in 2D cultures and 3D brain organoids by inhibiting ZIKV-induced caspase-3 activity44. Another study showed that treatments of antiviral compounds TH6744 and TH5487 rescued 3D brain organoids from ZIKV-induced neurotoxicity and morphological defects45. ZIKV infected human NPCs showed abundant production of virus-derived small interfering RNAs (vsiRNAs), leading to the promotion of host antiviral immunity30. Treatment with enoxacin, a well-known RNAi enhancer, completely abolished ZIKV infection and its consequences, such as ZIKV-induced microcephaly in human brain organoids30. Interestingly, IFN-β treatment successfully attenuated ZIKV RNA expression and ZIKV-mediated growth reduction in human brain organoids over the post-infection, suggesting its potent ability to prevent ZIKV-induced brain organoid defects28. Watanabe et al. identified expression of potential receptors including AXL tyrosine kinase, MER, AXL-related receptor tyrosine kinases TYRO3 and T cell immunoglobulin and mucin domain (TIM) family receptors that facilitate ZIKV entry into the cerebral organoids38. Moreover, they found that treatment with duramycin and ivermectin effectively blocks ZIKV entry receptors, thereby reducing virus propagation and ZIKV-induced cell death in infected human cerebral organoids38. Wells et al. demonstrated that genetic alteration of AXL did not protect human NPCs and cerebral organoids against ZIKV infection, questioning utilization of AXL inhibitors to prevent neuropathological effects orchestrated by the virus46.
SARS-CoV-2
Coronavirus disease 2019 (COVID 19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and as of early 2022 the virus has rapidly spread across the globe infecting over 450 million people, with over 6 million confirmed deaths (WHO, 2022). SARS-CoV-2 is a member of the Betacoronavirus genus in the coronaviridae family of positive-stranded RNA viruses47,48. According to genetic studies, SARS-CoV-2 likely originated in animals but identifying the original zoonotic origin and method of transmission into humans remains elusive49. Like other coronaviruses, SARS-CoV-2 is primarily transmitted through airborne droplets containing the virus and fomites during direct, indirect, or close contact between individuals50. SARS-CoV-2 primarily infects and causes adverse effects in cells of the respiratory system of infected patients51. In addition, virus infection may damage other organs, including the gastrointestinal (GI) tract, skeletal muscles, brain, lymph nodes, spleen, and heart48. Upon infection with SARS-CoV-2, patients may develop fever, lower respiratory tract complications, pneumonia, and fatal acute lung injury or acute respiratory distress syndrome48. Recent studies revealed rising cases of SARS-CoV-2 infected patients developing neurological manifestations, such as headache, seizure, ageusia, anosmia, encephalopathy, confusion, psychosis, meningitis, and encephalitis52–55. Intriguingly, detection of SARS-CoV-2 RNA in the brain or cerebrospinal fluid (CSF) samples of infected patients with associated neurological symptoms suggests a possible viral invasion to the human brain53,55–57. The entry receptor for SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2), has been identified to be in human brain cells, including neurons, astrocytes, oligodendrocytes, and endothelial cells further confirming susceptibility of the brain for infection by SARS-CoV-258. Interestingly, Chu et al. reported that SARS-CoV-2 can infect and subsequently replicate inside U251 neurons suggesting it has possible neuroinvasive capabilities in vivo59. However, it is hard to uncover insights into SARS-CoV-2 mediated neurological disorders using human clinical postmortem brain samples due to their variable quality, heterogeneous genetic background, and limited accessibility. Therefore, researchers have successfully developed hiPSC-derived brain organoids with regional specificity, including the cerebral cortex, hippocampus, choroid plexus (ChP), midbrain, and hypothalamus, to mimic infectious mechanisms and cellular tropism of SARS-CoV-2 in the human brain60,61.
To date, several studies have been conducted to model cellular tropism, route of infection, consequences of infection, and drug screening of treatments for SARS-CoV-2 using both 2D cell cultures and region-specific 3D brain organoids60–67. Studies conducted with hiPSC-derived 2D cultures of NPCs, neurospheres, neurons, and astrocytes showed their permissiveness to SARS-CoV-2 whereas, hiPSC-derived microglia did not become infected62,63,66,68. Interestingly, MAP2+ neurons co-cultured with GFAP+ astrocytes indicated significantly higher SARS-CoV-2 infectivity when compared to neuronal culture without astrocytes, suggesting astrocytes have the potential to boost the ability of SARS-CoV-2 infection in neurons62. Researchers have discovered that the e4 allele of apolipoprotein E (APOE4/4), which is involved in increased risk and acceleration of onset of Alzheimer’s disease (AD), has a positive correlation between risk for the severity of COVID1969. To determine the severity and cellular consequences of the ApoE4/4 genotype in SARS-CoV-2 infection, Wang and colleagues generated isogenic iPSCs carrying the ApoE3/3 genotype, which is associated with neutral risk of AD, from ApoE4/4 derived from an AD patient using CRISPR/Cas9 followed by differentiation into neurons and astrocytes62. This study found a significantly higher infection rate in ApoE4/4 neurons and astrocytes than in ApoE3/3. In addition, Astrocytes harboring ApoE4/4 genotypes exhibited more fragmented nuclei compared to ApoE3/3 upon SARS-CoV-2, suggesting a possible role of ApoE4/4 in COVID19 severity62.
In addition, SARS-CoV-2 positive cells were detected in hiPSC-derived 3D brain organoids with regional-specific features including cortical, hypothalamic, midbrain, and hippocampal organoids60–67. In these organoids, NPCs, astrocytes, radial glia, and neurons were sparsely infected by SARS-CoV-2, exhibiting relatively higher tropism to neurons60,62–68. Surprisingly, predominant and productive SARS-CoV-2 infection was observed in ChP epithelium cells60,61. Researchers found abundant expression of ACE2 receptor and co-entry factor TMPRSS2, as well as a newly identified SARS-CoV-2 entry receptor, natriuretic peptide receptor 1 (NRP1), in ChP cells60,61,70. However, expression of those receptors was observed to be low in neurons and other cell types61,66. Furthermore, the Allen brain atlas recapitulates the validity of these in vitro findings, indicating that the highest level of ACE2 expression occurs in the ChP when compared to other brain regions analyzed61. Altogether, these findings revealed that prominent SARS-CoV-2 tropism to the ChP cells might occur through highest expressing ACE2 on cells of the ChP61.
Song et al. discovered increased cell death and viral titer in hiPSC-derived 2D cultures of NPCs after infection with SARS-CoV-265. In addition, SARS-CoV-2 induced neuronal cell death has also been demonstrated using 3D brain organoids65,66. Furthermore, upon exposure to SARS-CoV-2, increases in viral RNA in 3D brain organoids over the post-infection time suggests possible replication ability within neurons62,63. In contrast to these observations, Jacob et al. and McMahon et al. could not detect significant SARS-CoV-2 infection, viral propagation, and cell death in neurons of the brain organoids60,64. However, Song et al. further confirmed the neuroinvasive capabilities of SARS-CoV-2 by detecting the viral spike protein in autopsy brain samples from the patients who died from severe COVID-19 related complications65. The neuroinvasive capabilities of SARS-CoV-2 has raised interest in discovering a mechanism for the route of entry of the virus into the brain. Researchers postulate that the virus could enter the CNS via the olfactory bulb and the cribriform plate from the nasal cavity, since SARS-CoV-2 has a tropism for nasal epithelial cells71,72. Contrastingly, it is known that the blood-CSF barrier (B-CSF-B) and BBB play a critical role in protecting the CNS from pathogens, including viruses and harmful inflammatory agents61. Therefore, degradation of one of the barriers would allow for more permissive entry of immune cells, harmful inflammatory agents, and blood-borne pathogens to the CNS. It is thought that the B-CSF-B is a more vulnerable barrier for degradation due to the lower electrical resistance of tight junctions between cells when compared to the BBB73,74. Interestingly, Pellegrini et al. and Jacob et al. demonstrated predominant tropism and infection of SARS-CoV-2 in ChP epithelium cells, which maintain the integrity of B-CSF-B, using hiPSCs-derived 3D ChP organoids60,61. Infection of SARS-CoV-2 led to effective viral propagation and induced subsequent death of both infected and uninfected adjacent ChP cells in ChP organoids60. Furthermore, SARS-CoV-2 infection promoted tight junction disruption between the ChP cells. This additionally led to elevated leakage of previously internalized CSF-like fluid from the 3D ChP organoids, suggesting possible destruction of B-CSF-B integrity61. Overall, both of these studies provided substantial evidence toward the potency of SARS-CoV-2 infection on ChP epithelium cells and subsequent CNS invasion through B-CFS-B breakage. Additionally, using an in vitro 3D microfluidic model of the human BBB, Buzhdygan et al. found that the S1 subunit of SARS-CoV-2’s spike protein induces BBB destabilization, thus providing another possible explanation for neurological manifestation induced by SARS-CoV-275.
To dissect the impact of SARS-CoV-2 on the molecular level, scientists employed single cell or bulk RNA sequencing of SARS-CoV-2 infected human brain organoids. Single-cell RNA profiling of these infected human brain organoids further confirmed widespread infectious response of the virus in NPCs, radial glia, and neurons65. Upon SARS-CoV-2 exposure, virus-infected cells in the human brain organoids showed upregulated pathways related to the viral transcription and metabolic processes such as electron transport-coupled proton transport, NADH to ubiquinone, and cytochrome C to oxygen pathways65. On the other hand, SARS-CoV-2 negative cells exhibited a mitochondrial catabolic state with upregulation of cholesterol synthesis, alcohol metabolism, and cell death pathways65. Based on the single-cell RNA sequencing of SARS-CoV-2 infected organoids, Song et al. demonstrated the virus’s ability to compromise host neurons’ molecular machinery for replication65. In another study, 1721 upregulated and 1487 downregulated genes were detected by transcriptional analysis in the SARS-CoV-2 infected human ChP organoids60. Interestingly, top upregulated GO terms related to viral responses, response to cytokine, cytoskeletal rearrangement, RNA processing, and cell death. In-depth analysis revealed the up-regulation of inflammatory cytokines genes, including CCL7, IL32, CCL2, IL18, and IL8, which ultimately can lead to damaging inflammatory responses60. GO analysis of downregulated genes showed the enrichment of clusters corresponding to ion transport, transmembrane transport, cilium, and cell junctions. Furthermore, they identified the downregulation of genes involved in CSF secretory functions such as AQP1, AQP4, and SLC22A8 in ChP60. Collectively data from the transcriptional analysis suggest SARS-CoV-2 infected ChP cells undergo severe inflammatory responses followed by reduced integrity of the B-CFS-B and increased cell death60.
Moreover, a few researchers have utilized human brain organoids to screen therapeutic strategies against the SARS-CoV-2 infection. For instance, Song et al. demonstrated that blocking the ACE2 receptors in human brain organoids using anti-ACE2 antibodies and anti-spike antibodies collected from the CSF of COVID-19 patients efficiently inhibited SARS-CoV-2 neuroinvasion65. In addition, treatment with the antiviral drug Remdesivir, reduced SARS-CoV-2 infection and rescued virus-induced disease phenotypes in hiPSC-derived neurons and astrocytes62.
Human cytomegalovirus
Human cytomegalovirus (HCMV), a member of the Betaherpesvirinae subfamily, can cause lifelong infections in humans76. HCMV disease is globally distributed, and the estimated mean seroprevalence for the general population is 83%77. HCMV is a double-stranded DNA virus, mainly transmitted to people via direct contact with body fluid from an infected person. Sexual contact, breastfeeding, transplanted organs, and blood transfusion are common routes of infection78–82. The virus can also be vertically transmitted to the developing fetus from an infected mother during pregnancy, resulting in congenital CMV in newborn babies83. Congenital CMV is one of the leading causes of congenital disabilities in the US83. Approximately 10–15% of children born with congenital CMV show symptoms at birth, and at birth asymptomatic children have a probability of acquiring neurodevelopmental defects83. The majority of the symptomatic children for congenital CMV at birth may exhibit one or more neurodevelopmental disorders, including intellectual disability, microcephaly, and hearing loss83–85. Although cases of congenital CMV-associated births are higher than well-known childhood disabilities, including fetal alcohol syndrome, down syndrome, and spina bifida, CMV-induced neuropathology is poorly understood due to the limitation of a more complete human cell model83. However, with the development of human 3D organoids, researchers have a new opportunity to investigate the underlying mechanisms of CMV infiltration into the CNS, CMV-associated neurological impairments in developing brain, and finding novel treatment strategies for overcoming CMV-induced neuropathology.
It has been previously shown that the HCMV is permissive to human NPCs and induces apoptosis, inhibits NPC proliferation and neuronal differentiation using 2D cell cultures86–88. In addition, HCMV infection also resulted in a loss of purinergic and voltage gated calcium signaling in 2D culture of NPCs89. Studies performed using 3D human brain organoids demonstrated that HCMV can effectively infect the cells of organoids89–91. Interestingly, Sun et al. observed extensive growth attenuation in 3D human brain organoids after exposure to the HCMV strain TB40/E, which has the clinical features of CMV, and induced a microcephaly-like phenotype in the organoid model91. In the same study, they found HCMV primarily targeted NPCs in the subventricular zone (SVZ) of human brain organoids leading to the disruption of SVZ organization and impaired cortical layer formation91. In support of these results, Brown et al. and Sison et al. also reported HCMV-induced impairments of cortical layer development and the overall organization of brain organoids89,90. Additionally, exposure to HCMV led to induction of apoptosis or necrosis, disruption of neuronal differentiation, and reduction of proliferating cells in brain organoids89–91. More importantly, researchers confirmed epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor alpha (PDGFRα) are the primary entry receptors for HCMV into the human brain organoids. RNA sequence analysis revealed that top-down regulatory genes such as BNIP3, ENO2, and PDK1 which relate to calcium signaling were downregulated. Researchers then identified significantly down-regulated GO terms, including the calcium-mediated exocytosis of neurotransmitters, and regulation of calcium-dependent exocytosis, brain, astrocyte, and hippocampal development. Most of the upregulated GO terms were associated with immune responses and inflammatory responses in HCMV infected brain organoids91. Consistent with the RNA sequencing data, signatures related to disorganized calcium signaling and neural network activity were also measured in brain organoids infected with HCMV91. Similarly, Sison et al. also found that impairment of calcium activity in HCMV infected cortical organoids89. Researchers then tested the possible treatment strategies for inhibiting virus entry and overcoming HCMV-associated neuropathogenesis. Treatment with the antiviral compound maribavir limited HCMV spread within the brain organoid and allowed relatively normal calcium function in HCMV uninfected cells when compared to infected cells89. In addition, neutralization antibody 1B2 effectively rescued brain organoids from HCMV-induced growth defects, abnormal cortical layer formation, and impairments of neural network activities91. Collectively, these findings suggest that brain organoids can provide essential insights into HCMV-associated neurological impairments, which will lead to developing novel therapeutic strategies in the future.
Human herpes simplex virus-1
Human herpes simplex virus-1 (HSV-1) is a double-stranded DNA virus, and is the causative agent of genital and oral herpes92. HSV-1 is one of the most prevalent human viruses in the world and is capable of establishing lifelong infections93. Notably, the HSV-1 is also considered a neurotropic virus, reaching the CNS and triggering severe pathological manifestations such as herpes simplex encephalitis (HSE)94,95. However, there are some obstacles to the full understanding of HSV-1-associated neurodevelopmental disorders due to the limited accessibility to the post-mortem human samples and the lack of proper experimental models which resemble the in vivo neural microenvironments. To overcome this hurdle, researchers have recently attempted to use 3D brain organoids to characterize HSV-1-induced neurological manifestations in CNS. Previous 2D cultures of hiPSC-derived neural stem cells (NSC), and neurons showed permissiveness to HSV-196–98, and upon HSV-1 infection, elevation of apoptotic cells and impaired neural differentiation were observed98. Later investigations using hiPSC-derived 3D brain organoids revealed that HSV-1 could also efficiently infect the model system, exhibiting prominent tropism towards neurons28,96–98. Consequently, HSV-1 infected 3D brain organoids demonstrated excessive accumulation of apoptotic cells, growth attenuation, disrupted cortical layer formation, impairment of neurogenesis, and neuronal differentiation28,96,98. Interestingly, Qiao et al. demonstrated that abnormal microglia activation and proliferation are accompanied by induced expression of pro-inflammatory cytokines (TNF-α and IL-6) and inflammatory cytokines (IL-10 and IL-4) after exposure to HSV-1 in 3D human brain organoids with incorporated microglia98. In another study, 423 DEGs were identified in HSV-1 infected brain organoids by bulk RNA sequencing analysis28. Furthermore, GO terms analysis of genes upregulated in HSV-1 infected brain organoids revealed enrichment in the regulation of developmental, cellular process, and cell death-related pathways28. Surprisingly, HSV-1 failed to extensively induce type-1 interferon pathways in brain organoids. More importantly, IFNα2 treatment efficiently prevented HSV-1 infection progression and virus-induced brain organoid growth defects, thereby restoring organoid architecture28. These studies suggest that brain organoids can be vital tools to studying HSV-1-associated neuropathology.
Human Immunodeficiency Virus
Chronic infection of Human Immunodeficiency Virus (HIV-1) has been shown to result in progressive deterioration of cognition, behavior, motor function, and memory in more than 50% of HIV-1 positive people despite the use of antiviral treatments99. These neurological manifestation terms are commonly referred to as HIV-1-associated neurocognitive disorders (HAND) and are thought to occur as a byproduct of neuroinflammation and glial activation100. Unfortunately, there is no cure available for HAND and studying these disorders remains difficult. Recently, researchers developed a 3D human brain model containing neurons, astrocytes, and HIV-infected microglia, to investigate HIV-1 associated neuropathogenesis99. In this study, researchers identified uninfected, and HIV-1 infected human microglia could infiltrate the 3D human brain organoids mimicking migration of peripheral microglial precursor cells into the developing CNS99. In addition, HIV-1 infected primary microglia also engaged with 3D human brain organoids and led to viral replication and production of pro-inflammatory molecules including TNF-α and IL-1β in infected organoids. This upregulation of pro-inflammatory molecules closely mimics the microenvironment of patients suffering from HIV-1-induced neuroinflammation. Furthermore, HIV-1-induced cytotoxicity, neuronal loss, astrocyte damage, and loss of synaptic integrity were clearly detected in 3D human brain organoids with microglia and resembled HIV-1-associated CNS pathology signatures99. This multicellular 3D brain organoid system provided a platform for a better understanding of host-pathogen interaction and delineating the molecular mechanism of neuropathogenesis associated with HIV.
Modeling Non-Viral infections in Brain Organoids
Toxoplasma gondii infection
Toxoplasmosis is an infection caused by a single-cell protozoan parasite called Toxoplasma gondii. This disease is found worldwide and it is estimated that approximately 25–30% of the world population is positive for the infection, with most cases being asymptomatic101. The infection can be acquired congenitally or postnatally. Congenital transmission of T. gondii to the fetus from an infected mother may cause intellectual disabilities, epilepsy, blindness, and death102. Furthermore, acute infection or reactivation of latent infection of T. gondii may result in severe encephalitis conditions in immunocompromised people102. Recently, Seo and colleagues developed an in vitro model to screen for T. gondii infection using human brain organoids103. Infection was conducted with two T. gondii strains and resulted in a productive infection of both strains in human brain organoids. Both T. gondii strains exhibited higher tropism to TUJ1+ neurons, GFAP+ astrocytes, and O1+ oligodendrocytes. Moreover, T. gondii formed cyst-like structures in TUJ-1 positive cells suggesting parasites can replicate inside the human brain organoids103. According to the RNA sequencing analysis, 786 DEGs were identified after T. gondii infection in human brain organoids103. In-depth analysis of RNA sequencing data revealed several interactions related to the host-pathogen responses of T. gondii103. More importantly, Type-1 interferon responses were activated against T. gondii infection, suggesting possible immune responses against the parasite invasion. Altogether, results indicate that human brain organoids can also be utilized to model CNS neuropathology elicited by parasitic infection.
Human cerebral malaria
Malaria is a parasitic disease caused by the protozoan pathogen in the genus Plasmodium spp, particularly the Plasmodium vivax and Plasmodium falciparum species. Malaria is transmitted to people through the bites of infected female mosquitoes of the Anopheles genus104. Human cerebral malaria (HCM) is one of the deadliest malaria-related complications and is associated with severe neuropathological complications such as altered consciousness, coma, and seizures105,106. Especially, some CNS pathogens, including P. falciparum, do not have intracellular characteristics in the CNS. Their pathogenic effects on cells in CNS are primarily elicited by cytotoxic factors derived from the pathogen107. One consequence of HCM is the presence of increased systemic free heme levels, which can lead to BBB damage and neuronal injury in the brain108,109. Harbuzariu et al. discovered disrupted structural organization and elevated necrosis in heme-treated human brain cortical organoids108. In addition, elevated brain injury-related biomarkers, including CXCL10, CXCR3, brain-derived neurotrophic factor (BDNF), and decreased levels of receptor tyrosine-protein kinase ERBB4, were detected in human brain cortical organoids upon heme treatment. Interestingly, neuregulin-1 (NRG-1) treatment rescued human brain cortical organoids from neuropathological events elicited by heme108.
Mycobacterium tuberculosis
Mtb is the most common bacterial infection of the CNS. Despite the fact that Mtb responses have been shown to be heterogeneous across different model species which restricts interpretation of non-human studies, there is still no definitive human model for brain tuberculosis. To accomplish this, our lab has seeded 21 days old human brain organoids with Mtb infected phagocytes and measured the transcriptional effects on neuronal populations. The brain organoids used in this study have been derived from the H1 hESC-derived NPCs (Provided by J. Thompson, University of Wisconsin-Madison, WI, USA) with conditions optimized by Stem Pharm Inc, Madison, WI, USA. Our study is currently under review but data suggests that many of the gene responses induced by viral infected brain organoids are also activated by Mtb infection. It is our hope that this model will contribute to understanding the pathogenesis of CNS tuberculosis.
Prion diseases
Creutzfeldt–Jakob Disease (CJD) is a rare progressive neurodegenerative disorder that is incurable110. The causative agents of CJD are prions, which are abnormally refolded protease resistant forms of mammalian protein which spread their misfolded shape to other proteins. Abnormal refolding of the prion protein can occur in three ways, including sporadic manifestation, inherited genetic mutation, and acquired by infection111. Accumulation of abnormally refolded prion proteins results in neuronal death, astrocytosis, brain damage, dementia, and movement disorders112. In order to model prion-induced neuropathogenesis and disease progression, researchers infected human cerebral organoids with brain homogenates from two sporadic CJD (sCJD) subtypes113. Cerebral organoids were able to uptake and propagate prion infection from sCJD inoculums. Moreover, inoculum-specific physiological manifestations were observed in different sCJD infected cerebral organoids113. Interestingly, treatment with the anti-prion compound pentosan polysulfate (PPS) in sCJD infected human cerebral organoids effectively reduced prion propagation112. Collectively, these findings suggest that human brain organoids can be used to mimic many key features of prion disease.
Commonalities of Brain Organoid Infection Models
The recent development of the hiPSC-derived 3D brain organoid technology has provided a versatile platform for understanding host-pathogen interactions and gives new insight into the underlying molecular mechanism of the human brain. As we have seen across a wide variety of studies, many aspects of CNS infection can be investigated with brain organoids, including neuroinflammation, cell entry/tropism, transcriptional alterations, cell fate, routes of dissemination, and treatment screening (Figure 1). Interestingly, a comparison of all the recently conducted studies in human 3D brain organoids revealed some key consequences of infections with many similarities and differences across each disease model (see Table 1). For example, all the infectious agents discussed here exhibit some level of efficient infectivity to the 3D brain organoids but some showed distinct preference for tropism in specific subpopulations of the model. In the case of ZIKV, SARS-CoV-2, and T. gondii, each showed higher tropism in either NPCs, ChP epithelium cells, or neurons respectively, whereas the other pathogens exhibited more variable cellular tropism with less specificity. In nearly all the pathogens discussed in this review, productive propagation of pathogens and induced cell death, apoptosis, or necrosis were observed after infection in the human brain organoids. ZIKV and HCMV are the main causative agents responsible for significantly reducing NPC proliferation in human 3D brain organoids. More broadly, the impairment of growth and disrupted structural organization of the organoids is another typical pathological feature associated with both viral and non-viral pathogenic agents. Intriguingly, activation of inflammatory or type-1 interferon responses could be observed in brain organoids after exposure to all the pathogenic agents described, indicating a common innate capacity of inducing proinflammatory responses in neural elements as a reaction to infection.
Figure 1. Applications of Human Brain Organoids in Infectious Disease Research.
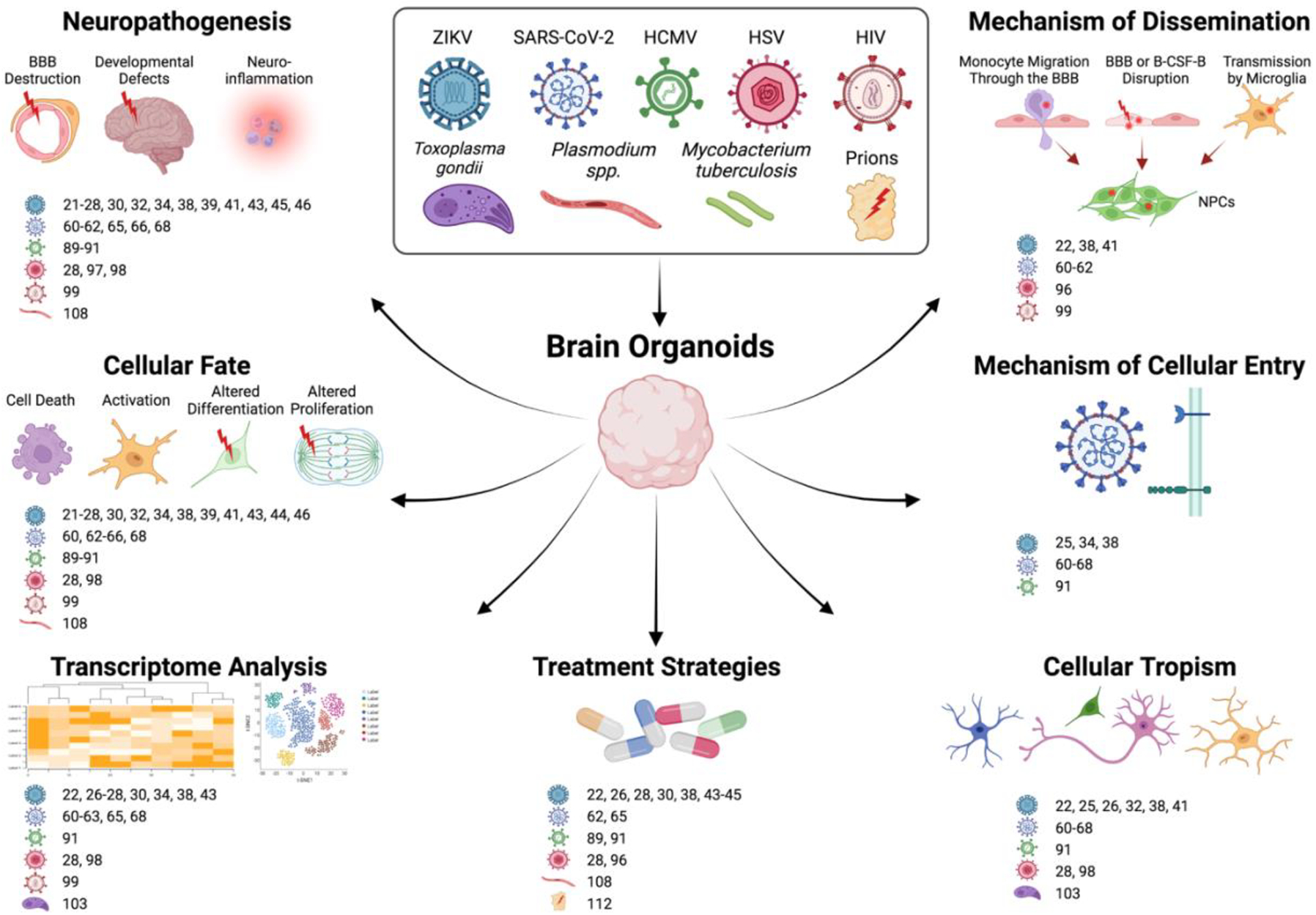
Various types of responses can be used to study through the use of brain organoid models. References for each disease model are given next to the respective icons of diseases causing agents. Created with BioRender.com.
Table 1.
Characterization of CNS infectious diseases using human brain organoids and reported treatment strategies
| Disease-causing agent | Major target cells in organoids | Main cellular consequences in infected organoids | Reported treatment strategies | Major limitations | References |
|---|---|---|---|---|---|
| Zika virus (ZIKV) |
|
|
|
|
Li et al.152
Krenn et al.28 Qian et al.32 Zhou et al.43 Xu et al.30 Xu et al.44 Gabriel et al.25 Liu et al.27 Watanabe et al.38 Liang et al.23 Dang et al.26 Pettke et al.45 |
| SARS-CoV-2 |
|
|
|
|
Jacob et al.60
Pellegrini et al.61 Wang et al.62 McMahon et al.64 Song et al.65 Ramani et al.66 Yi et al.67 |
| HCMV |
|
|
|
|
Sison et al.89
Sun et al.91 Brown et al.90 |
| HSV-1 |
|
|
|
|
Krenn et al.28
Qiao et al.98 D’Aiuto et al.96 Abrahamson et al. 97 |
| HIV-1 |
|
|
|
|
Dos Reis99 |
| T. gondii |
|
|
|
|
Seo et al.103 |
| Malaria induced heme |
|
|
|
|
Harbuzanariu et al.108 |
| Prion |
|
|
|
|
Groveman et al.113 |
Advancements and Limitations to Brain Organoids in Infectious Disease
Region specificity and lack of cytoarchitectural information in brain organoids
Much of the more recent advancements in brain organoid technology have hinged on utilizing a diverse repertoire of patterning factors to steer human pluripotent stem cells (hPSCs) towards novel lineages for more targeted investigations. The primary example of this is the proliferation of brain region-specific organoids across many fields of investigation, which have emerged to help replicate CNS cytoarchitecture of key areas of the brain. Recent publications have demonstrated the ability to promote the guided development of a variety of CNS structures, including the cerebral cortex114, hippocampus115, midbrain116, hindbrain117, hypothalamus32, and choroid plexus115,118. Factors that steer regional organoid development include activators and inhibitors of Wnt, BMP, SHH, and TGF-β signaling pathways among several others; all of which can influence the organizational axis of the developing brain119,120. For example, exposure to a combination of SHH and Wnt3a can steer organoids toward more hypothalamic fate32. As a result, this methodology of guided self-organization is a powerful tool in the context of studying infectious disease in the CNS, where exposure of the infectious agent could reveal region-specific responses to infection. Additionally, the combination of these region-specific brain organoids with relevant immune components, genetic manipulations, or potential treatments can allow researchers to create increasingly specific and dynamic systems more closely resembling in vivo conditions. As mentioned in the previous sections, recently published papers in ZIKV32 and SARS-CoV-260 research have already shown promise in utilizing these region-specific models, but usage will likely expand far beyond these two infectious diseases in the years to come.
For broad infectious disease research, regions like the choroid plexus are of significant interest as their involvement as a site of bacterial and viral infiltration into the CNS is well-documented74. Interfacing directly with the peripheral blood, interactions between pathogens and choroid plexus epithelial cells can facilitate the hematogenous spread of infectious disease into the brain121. Although region specific brain organoids with choroid plexus-like structures lack fenestrated capillaries as observed in in vivo choroid plexuses, these models still provide a unique opportunity to test the underlying dynamics of CNS infiltration on human choroid plexus-like epithelial cell populations. Still, while infectious pathogens within the choroid plexus have previously been investigated using in vitro methods122–124, the complete mosaic of their influence on human choroid plexus cells is not fully characterized in a number of neuropathological infections. To date SARS-CoV-2 is the only infectious disease to be investigated specifically in a choroid plexus brain organoid model60,118. However, it should be noted that Watanabe et al., did identify the presence of ZIKV tropism within sporadically forming AQP1+ SOX2− PAX6− epithelial regions of their cerebral organoid model suggesting infiltration of choroid plexus epithelial cells is likely38. Other diseases like CMV, Hepatitis E virus (HEV), HIV, and Mtb have all been implicated to have choroid plexus involvement and are prime candidates for further investigation125–128. Additionally, choroid plexus brain organoids have also been documented to contain a fluid with high similarity to cerebral spinal fluid (CSF), highlighting their functional similarity to in vivo structures. This is important as hydrocephalus remains to be one of the most common complications of CNS infections129 and with the choroid plexus representing the primary site of CSF production in the body, investigating the expressional and functional consequences of infection on choroid plexus brain organoids could provide new insights into how CSF regulation is altered by disease.
Other brain region-specific organoids, like those which attempt to replicate components of the cerebral cortex can also yield clinically relevant results in infection models. Several subregions of interest are present in these cortical organoids, including a prominent ventricular zone, as well as inner and outer SVZs. Additionally, Paşca et al. reported electrically active deep and superficial cortical neurons in their organoid culture. Together these structural and functional features, can allow researchers the ability to test the consequences of different infections on cortical electrophysiology and development114. This is relevant as brain infections like ZIKV can induce pathologies that directly target the process of cortical development, and several landmark ZIKV studies utilize region specific cerebral cortex organoids. Additionally, region specificity of cortical layers is highly relevant to potentially study a number of diseases like cytomegalovirus (CMV) which is known to influence cortical regions and development130 or even HIV which is associated with reduction in cortical thickness131,132.
Overall, while the generation of brain organoids with region specificity unlocks new possibilities of more targeted studies and assays, unguided methods will continue to play an important role in brain organoid research. Unguided protocols produce heterogenous cell types of all lineages which can be leveraged to generate hypotheses for infectious disease research. Additionally, unguided protocols are typically more cost effective than guided methodologies. Together, both methodologies can be utilized to screen for broad infectious cell responses with unguided cultures and then utilizing region-specificity for analysis of cell types of interest with more guided methods.
Modeling Neuroinflammation
The neuroimmune environment is an important aspect when modeling cellular responses to pathogens. While it is likely impossible to model the full extent of the brain’s immune system inside an organoid model, recent advances and protocols have attempted to more closely replicate the immunoregulatory environment of the CNS. As it is discussed in earlier sections, neural progenitors and neurons can themselves activate innate proinflammatory pathways in response to pathogens. An example of this is the activation of type I IFN pathways which induce a complex array of genes. As a result, even without the explicit addition of specific immune cell populations into brain organoid models, it is important to understand how neuronal responses can be affected by these pathways even if they are not infected.
With that said, it is also an important quest to incorporate microglia, the brain’s innate immune cell into these organoid systems. Proper incorporation of microglia into brain organoids is not only important to recapitulate key mechanisms of neuroinflammation, but microglia have been demonstrated to serve as important mediators of brain development and synapse formation133. Original studies of unguided brain organoids reveal a considerable lack of any definitive microglia populations, but Quadrato et al. did highlight the presence of mesodermal progenitors8. A later study indicated that these mesodermal progenitors could in fact be differentiated into microglial cells by reducing heparin levels in the culture medium134. These differentiated microglial cells displayed traditional microglial ramifications and expressed several transcription factors of a microglial phenotype: SPI1, AIF1, CSF1R, and TLR4. Additionally, the overexpression of the myeloid-specific transcription factor PU.1 within brain organoids has been shown to reliably induce microglia populations in a more targeted manner135.
While differentiation of microglia within an organoid system is highly useful, many researchers still elect to use a co-culture system using independently derived microglia for many studies, often in an effort to more closely control microglial concentration ratio34 or pathogen exposure procedures99. Microglial populations have been generated for organoid co-culture from hiPSC populations in a number of studies136,137. In Xu et al. researchers differentiated microglia from yolk sac macrophages progenitors, which are known to populate the CNS in vivo, and co-cultured with region specific forebrain organoids34. Here microglia concentrations can be reliably controlled and titrated to physiologically relevant percentages. Additionally, the developmental stage of these microglia populations is important for consideration. For example, Dos Reis et al. utilized the immortalized microglial cell line HMC3 to mimic adult brains populations during HIV infection, as well as primary human brain microglia collected post-mortem99. Interestingly, it has also been reported that pathogen exposure alone can result in the induction of microglial proliferation in unguided protocols which do not typically display prominent microglial populations, as reported by Qiao et al. in HSV-1 infected cerebral organoids98.
Finally, microglia represent only a single component of the immune system and many infectious diseases of the CNS are associated with the infiltration of peripheral immune cells to the site of infection. As a result, incorporation of a wide variety of immune cells will need to be appropriately utilized to more holistically replicate the complex neuroimmune environment of the CNS. For example, co-culture with patient derived peripheral mononuclear cells which contain lymphocytes and monocytes can replicate not only the inflammatory peripheral immune response in the brain, but also test mechanisms of pathogen infiltration. This is relevant as there is mounting evidence that indicates several viruses and bacteria can directly infect immune cells and transverse the BBB138. Recently this “trojan horse” strategy of CNS infection was tested using a cerebral brain organoid model with co-cultured ZIKV infected monocytes41
In summary, a more complete incorporation of immune cells is necessary to test not only the damaging immune response to CNS infection, but to properly recapitulate the microglial-dependent pathways of brain development and mechanisms of pathogen infiltration. Additionally, there is increasing evidence that neuro-immune interactions are bi-directional, meaning immune cells influence neurons and neuronal factors can regulate nearby inflammatory cells. Brain organoids co-cultured with inflammatory cells provide an exceptional approach to further characterize these complex neuro-immune circuits. However, further work is needed to ensure that the application of these incorporated immune cells is done so in a physiologically relevant manner, which can be highly variable depending on the disease model.
Vascularization and Myelination
Another disadvantage is the lack of vascularization of brain organoids, but attempts have been made to incorporate novel workarounds. Vascularization is thought to be one of the main limiting factors in further organoid growth and the presence of relevant endothelial structures is likely essential in understanding the full impact of many CNS diseases. Perhaps most striking was the model outlined by Mansour et al., in which researchers were able to vascularize human brain organoids by implanting them in vivo into a mouse brain139. Here the organoid showed indications of host mediated vascularization, and functional integration of neuronal circuitry. It should be noted however that these implantation models utilize immune-deficient NOD-SCID mice to prevent host immune response toward the organoid graft. As a result, applicability of these transplant systems in the realm of CNS infection research will be severely inhibited. A later study by Cakir et al., modified hESCs to express human ETS variant 2 (ETV2) where the now ETV2-expressing cells were then able to form “microvasculature-like” structures in human cortical organoids140. Additionally, while these vascular structures lack traditional blood oxygenation capabilities, improved diffusion of oxygen and cell survival was observed in these organoids. In another interesting model, Song et al., was able to investigate neural-vascular interactions using a tri-culture system of fused spheroid of cortical neural progenitors, endothelial cells, and mesenchymal stem cells. Here CD31+ and Nestin+ cell-cell interactions could be studied in a controlled cortical microenvironment.
In addition to the lack of vascularization, lack of physiologically comparable myelination also presents an issue in studying many components of infectious response. Oligodendrocytes can represent a direct target for certain infectious diseases like human polyomavirus JC (JCV)141, and white matter damage is common by product of infectious diseases particularly in the secondary immune response driven from the infection142–144. Standard brain organoid protocols appear to produce some oligodendrocyte populations, but specifying these cells is highly variable and cells are likely too immature for translational relevance for many infectious diseases8. Pioneering work published by Madhavan et al.145, and later modified by others146–148 demonstrated that structurally intact cultures with myelination can in fact occur, allowing for more mature oligodendrocyte populations and the potential study of demyelination, viral tropism, or bacterial infiltration in these organoid systems. It should be noted however that rates of compact myelination using these protocols are relatively low, so further refinement is likely needed. Together, more efficient vascularization and myelination protocols, can enhance the organoid model to study more complex effects of infectious agents.
Maturation of Neurodevelopmental Features
The advancements in brain organoid technology in the past decade have been robust but many limitations still remain, particularly for researchers investigating infectious disease in the adult CNS. One obvious limitation is that the developmental lineage of the brain organoid model systems consists mainly of immature neuronal populations. While these early developmental neuronal phenotypes are perhaps ideal for research in diseases that affect early embryonic development like ZIKV, many infectious diseases like SARS-CoV-2 highly impact adult and elderly populations so interpretation of these studies is more difficult. Furthermore, extended maturation protocols of human brain organoids do exist, but can be highly time consuming (~250–300 days) and still only yield early post-natal neuronal phenotypes and structures149. As a result, any interpretations into the cellular response to an experimental pathogen within a brain organoid system should take into consideration the early developmental stages of these models. However, this fundamental immaturity of brain organoids has not stopped researchers from studying diseases of adult neurodegeneration in these systems150. Finally, since maturation typically requires longer and more robust culturing procedures, costs remain prohibitive to many researchers. However, techniques to lower cost and increase feasibility of organoid generation and maintenance continue to improve. For example, recent publications outlining the use of more refined spinning bioreactors to facilitate the culturing process have demonstrated success in improving cost-efficiency151.
Conclusion:
Brain organoids represent a novel advancement to study not only brain development, but a wide variety of CNS disease states in human cell populations. While there are a number of limitations when utilizing a brain organoid model to study infections in the CNS, these systems have already proved useful in improving our understanding of a number of diseases. In summary, brain organoids have been useful to discover common pathways in different infectious diseases. Our understanding of common pathways for CNS dissemination, cellular tropism, cellular entry and fate of infected CNS cells, and pathophysiological manifestations of these diseases have all been improved by brain organoids (Figure 1). Additionally, recent improvements in region specificity, maturation, and other culturing protocols continue to unlock an ever-growing toolkit for researchers to target specific questions. Together in combination with in vivo models and other in vitro systems, brain organoids can continue to help us understand the complex neuropathology of a wide number of diseases and potentially aid in the development of new and better treatments for CNS infectious diseases.
Acknowledgment
This work was supported by National Institutes of Health (NIH). CL was supported by AHA grant 915125 and in part by NIH/NINDS T32 NS105602. Authors do not declare any conflicts of interest. All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest.
References
- 1.Zhang SC, Wernig M, Duncan ID, Brüstle O & Thomson JA In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol 19, 1129–1133 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Watanabe K et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci 8, 288–296 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Danjo T et al. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J. Neurosci 31, 1919–1933 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers SM et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol 27, 275–280 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadoshima T et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. U. S. A 110, 20284–20289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster MA & Knoblich JA Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc 9, 2329–2340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quadrato G et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ming G-L, Tang H & Song H Advances in Zika Virus Research: Stem Cell Models, Challenges, and Opportunities. Cell Stem Cell 19, 690–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driggers RW et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med 374, 2142–2151 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Dick GWA, Kitchen SF & Haddow AJ Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg 46, 509–520 (1952). [DOI] [PubMed] [Google Scholar]
- 12.Kindhauser MK, Allen T, Frank V, Santhana RS & Dye C Zika: the origin and spread of a mosquito-borne virus. Bull. World Health Organ 94, 675–686C (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver SC et al. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 130, 69–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittal R et al. Zika Virus: An Emerging Global Health Threat. Front. Cell. Infect. Microbiol 7, 486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymann DL et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet 387, 719–721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen SA, Jamieson DJ, Honein MA & Petersen LR Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N. Engl. J. Med 374, 1981–1987 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Araujo LM, Ferreira MLB & Nascimento OJ Guillain-Barré syndrome associated with the Zika virus outbreak in Brazil. Arq. Neuropsiquiatr 74, 253–255 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Cao-Lormeau V-M et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387, 1531–1539 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soto-Hernández JL et al. Guillain-Barré Syndrome Associated With Zika Virus Infection: A Prospective Case Series From Mexico. Front. Neurol 10, 435 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang H et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 18, 587–590 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcez PP et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Muffat J et al. Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to Zika and dengue infections. Proc. Natl. Acad. Sci. U. S. A 115, 7117–7122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Q et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 19, 663–671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cugola FR et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabriel E et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 20, 397–406.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Dang J et al. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 19, 258–265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L et al. Protection of ZIKV infection-induced neuropathy by abrogation of acute antiviral response in human neural progenitors. Cell Death Differ. 26, 2607–2621 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krenn V et al. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 28, 1362–1379.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salick MR, Wells MF, Eggan K & Kaykas A Modelling Zika Virus Infection of the Developing Human Brain In Vitro Using Stem Cell Derived Cerebral Organoids. J. Vis. Exp (2017) doi: 10.3791/56404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y-P et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 29, 265–273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desole G et al. Modelling Neurotropic Flavivirus Infection in Human Induced Pluripotent Stem Cell-Derived Systems. Int. J. Mol. Sci 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian X et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonucci J & Gehrke L Cerebral Organoid Models for Neurotropic Viruses. ACS Infect Dis 5, 1976–1979 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu R et al. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Reports 16, 1923–1937 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachiller S et al. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci 12, 488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casano AM & Peri F Microglia: multitasking specialists of the brain. Dev. Cell 32, 469–477 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Li P et al. Non-Structural Protein 5 of Zika Virus Interacts with p53 in Human Neural Progenitor Cells and Induces p53-Mediated Apoptosis. Virol. Sin 36, 1411–1420 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe M et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 21, 517–532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon K-J et al. Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins. Cell Stem Cell 21, 349–358.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerhardt T & Ley K Monocyte trafficking across the vessel wall. Cardiovasc. Res 107, 321–330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayala-Nunez NV et al. Zika virus enhances monocyte adhesion and transmigration favoring viral dissemination to neural cells. Nat. Commun 10, 4430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesci P et al. Modeling neuro-immune interactions during Zika virus infection. Hum. Mol. Genet 27, 41–52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou T et al. High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain. Cell Stem Cell 21, 274–283.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu M et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med 22, 1101–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettke A et al. Broadly Active Antiviral Compounds Disturb Zika Virus Progeny Release Rescuing Virus-Induced Toxicity in Brain Organoids. Viruses 13, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells MF et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell 19, 703–708 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Torres J et al. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 16, 2065–2071 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelrahman Z, Li M & Wang X Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol 11, 552909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu B, Guo H, Zhou P & Shi Z-L Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol 19, 141–154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M-Y et al. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol 10, 587269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulay A et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. bioRxiv (2020) doi: 10.1101/2020.06.29.174623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao L et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. (2020) doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriguchi T et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis 94, 55–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varatharaj A et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7, 875–882 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helms J et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med 382, 2268–2270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puelles VG et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med 383, 590–592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solomon IH et al. Neuropathological Features of Covid-19. N. Engl. J. Med 383, 989–992 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen R et al. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol 11, 573095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu H et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe 1, e14–e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacob F et al. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 27, 937–950.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pellegrini L et al. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 27, 951–961.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang C et al. ApoE-Isoform-Dependent SARS-CoV-2 Neurotropism and Cellular Response. Cell Stem Cell 28, 331–342.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang BZ et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Research (2020) doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMahon CL, Staples H, Gazi M, Carrion R & Hsieh J SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Reports 16, 1156–1164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song E et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med (2021) doi: 10.1084/JEM.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramani A et al. SARS ‐CoV‐2 targets neurons of 3D human brain organoids. EMBO J (2020) doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yi SA et al. Infection of Brain Organoids and 2D Cortical Neurons with SARS-CoV-2 Pseudovirus. Viruses 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tiwari SK, Wang S, Smith D, Carlin AF & Rana TM Revealing Tissue-Specific SARS-CoV-2 Infection and Host Responses using Human Stem Cell-Derived Lung and Cerebral Organoids. Stem Cell Reports 16, 437–445 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo C-L et al. APOE e4 Genotype Predicts Severe COVID-19 in the UK Biobank Community Cohort. J. Gerontol. A Biol. Sci. Med. Sci 75, 2231–2232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cantuti-Castelvetri L et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sungnak W et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med 26, 681–687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montalvan V, Lee J, Bueso T, De Toledo J & Rivas K Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg 194, 105921 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Redzic Z Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8, 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwerk C, Tenenbaum T, Kim KS & Schroten H The choroid plexus-a multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci 9, 80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buzhdygan TP et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis 146, 105131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffiths P & Reeves M Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol 19, 759–773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuhair M et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol 29, e2034 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Handsfield HH et al. Cytomegalovirus infection in sex partners: evidence for sexual transmission. J. Infect. Dis 151, 344–348 (1985). [DOI] [PubMed] [Google Scholar]
- 79.Barbara JA & Tegtmeier GE Cytomegalovirus and blood transfusion. Blood Rev. 1, 207–211 (1987). [DOI] [PubMed] [Google Scholar]
- 80.Bardanzellu F, Fanos V & Reali A Human Breast Milk-acquired Cytomegalovirus Infection: Certainties, Doubts and Perspectives. Curr. Pediatr. Rev 15, 30–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannon MJ, Hyde TB & Schmid DS Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol 21, 240–255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics 70, 515–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheeran MC-J, Lokensgard JR & Schleiss MR Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin. Microbiol. Rev 22, 99–126, Table of Contents (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Zhang X, Bialek S & Cannon MJ Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin. Infect. Dis 52, e11–3 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Teissier N et al. Cytomegalovirus-induced brain malformations in fetuses. J. Neuropathol. Exp. Neurol 73, 143–158 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Odeberg J et al. Late human cytomegalovirus (HCMV) proteins inhibit differentiation of human neural precursor cells into astrocytes. J. Neurosci. Res 85, 583–593 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Odeberg J et al. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J. Virol 80, 8929–8939 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan X et al. Later passages of neural progenitor cells from neonatal brain are more permissive for human cytomegalovirus infection. J. Virol 87, 10968–10979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sison SL et al. Human Cytomegalovirus Disruption of Calcium Signaling in Neural Progenitor Cells and Organoids. J. Virol 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown RM et al. Human Cytomegalovirus Compromises Development of Cerebral Organoids. J. Virol 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun G et al. Modeling Human Cytomegalovirus-Induced Microcephaly in Human Ipsc-Derived Brain Organoids. Cell Rep Med 1, 100002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiao X et al. Complete Genome Sequence of Herpes Simplex Virus 1 Strain McKrae. Microbiol Resour Announc 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doll JR, Thompson RL & Sawtell NM Infectious Herpes Simplex Virus in the Brain Stem Is Correlated with Reactivation in the Trigeminal Ganglia. J. Virol 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duarte LF et al. Herpes Simplex Virus Type 1 Infection of the Central Nervous System: Insights Into Proposed Interrelationships With Neurodegenerative Disorders. Front. Cell. Neurosci 13, 46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marcocci ME et al. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 28, 808–820 (2020). [DOI] [PubMed] [Google Scholar]
- 96.D’Aiuto L et al. Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells. J. Virol 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abrahamson EE et al. Modeling Aβ42 Accumulation in Response to Herpes Simplex Virus 1 Infection: 2D or 3D? J. Virol (2020) doi: 10.1128/JVI.02219-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiao H et al. Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog. 16, e1008899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dos Reis RS, Sant S, Keeney H, Wagner MCE & Ayyavoo V Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci. Rep 10, 15209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zayyad Z & Spudich S Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr. HIV/AIDS Rep 12, 16–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matta SK, Rinkenberger N, Dunay IR & Sibley LD Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol 19, 467–480 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Jones JL et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am. J. Epidemiol 154, 357–365 (2001). [DOI] [PubMed] [Google Scholar]
- 103.Seo H-H et al. Modelling Toxoplasma gondii infection in human cerebral organoids. Emerg. Microbes Infect 9, 1943–1954 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Phillips MA et al. Malaria. Nat Rev Dis Primers 3, 17050 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Gordon EB et al. Targeting glutamine metabolism rescues mice from late-stage cerebral malaria. Proc. Natl. Acad. Sci. U. S. A 112, 13075–13080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghazanfari N, Mueller SN & Heath WR Cerebral Malaria in Mouse and Man. Front. Immunol 9, 2016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Idro R, Marsh K, John CC & Newton CRJ Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res 68, 267–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harbuzariu A et al. Modelling heme-mediated brain injury associated with cerebral malaria in human brain cortical organoids. Sci. Rep 9, 19162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu M, Dickinson-Copeland C, Hassana S & Stiles JK Plasmodium-infected erythrocytes (pRBC) induce endothelial cell apoptosis via a heme-mediated signaling pathway. Drug Des. Devel. Ther 10, 1009–1018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uttley L, Carroll C, Wong R, Hilton DA & Stevenson M Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect. Dis 20, e2–e10 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Prusiner SB, Scott MR, DeArmond SJ & Cohen FE Prion protein biology. Cell 93, 337–348 (1998). [DOI] [PubMed] [Google Scholar]
- 112.Groveman BR et al. Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt-Jakob disease. Sci. Rep 11, 5165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groveman BR et al. Sporadic Creutzfeldt-Jakob disease prion infection of human cerebral organoids. Acta Neuropathol Commun 7, 90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paşca AM et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sakaguchi H et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun 6, 8896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jo J et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 19, 248–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muguruma K, Nishiyama A, Kawakami H, Hashimoto K & Sasai Y Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Pellegrini L et al. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eiraku M et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Brafman D & Willert K Wnt/β-catenin signaling during early vertebrate neural development. Dev. Neurobiol 77, 1239–1259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dando SJ et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev 27, 691–726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strazielle N, Belin M-F & Ghersi-Egea J-F Choroid plexus controls brain availability of anti-HIV nucleoside analogs via pharmacologically inhibitable organic anion transporters. AIDS 17, 1473–1485 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Figueiredo TC & de Oliveira JRM Reconsidering the association between the major histocompatibility complex and bipolar disorder. J. Mol. Neurosci 47, 26–30 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Schwerk C et al. Polar invasion and translocation of Neisseria meningitidis and Streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLoS One 7, e30069 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kawasaki H et al. Cytomegalovirus initiates infection selectively from high-level β1 integrin-expressing cells in the brain. Am. J. Pathol 185, 1304–1323 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Shi R et al. Evidence of Hepatitis E virus breaking through the blood-brain barrier and replicating in the central nervous system. J. Viral Hepat 23, 930–939 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Tumbarello M et al. HIV-associated bacteremia: how it has changed in the highly active antiretroviral therapy (HAART) era. J. Acquir. Immune Defic. Syndr 23, 145–151 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Bhattacharyya A et al. Involvement of the Choroid Plexus in Neurotuberculosis: MR Findings in Six Cases. Neuroradiol. J 23, 590–595 (2010). [DOI] [PubMed] [Google Scholar]
- 129.Warf BC Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J. Neurosurg 102, 1–15 (2005). [DOI] [PubMed] [Google Scholar]
- 130.Barkovich AJ & Lindan CE Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am. J. Neuroradiol 15, 703–715 (1994). [PMC free article] [PubMed] [Google Scholar]
- 131.Sanford R et al. Regionally Specific Brain Volumetric and Cortical Thickness Changes in HIV-Infected Patients in the HAART Era. J. Acquir. Immune Defic. Syndr 74, 563–570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanford R et al. Longitudinal Trajectories of Brain Volume and Cortical Thickness in Treated and Untreated Primary Human Immunodeficiency Virus Infection. Clin. Infect. Dis 67, 1697–1704 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parkhurst CN et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ormel PR et al. Microglia innately develop within cerebral organoids. Nat. Commun 9, 4167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cakir B et al. Expression of the transcription factor PU.1 induces the generation of microglia-like cells in human cortical organoids. Nat. Commun 13, 430 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abud EM et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94, 278–293.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abreu CM et al. Microglia Increase Inflammatory Responses in iPSC-Derived Human BrainSpheres. Front. Microbiol 9, 2766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim KS Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol 6, 625–634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mansour AA et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol 36, 432–441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cakir B et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Elphick GF et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306, 1380–1383 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Tan CS & Koralnik IJ Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 9, 425–437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schultz V et al. Oligodendrocytes are susceptible to Zika virus infection in a mouse model of perinatal exposure: Implications for CNS complications. Glia 69, 2023–2036 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Linden JR et al. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. MBio 6, e02513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Madhavan M et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 15, 700–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim H et al. Pluripotent Stem Cell-Derived Cerebral Organoids Reveal Human Oligodendrogenesis with Dorsal and Ventral Origins. Stem Cell Reports 12, 890–905 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaker MR et al. Rapid and Efficient Generation of Myelinating Human Oligodendrocytes in Organoids. Front. Cell. Neurosci 15, 631548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marton RM et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci 22, 484–491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gordon A et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci 24, 331–342 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grenier K, Kao J & Diamandis P Three-dimensional modeling of human neurodegeneration: brain organoids coming of age. Mol. Psychiatry 25, 254–274 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Qian X et al. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat. Protoc 13, 565–580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li C et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 46, 446–456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


