Abstract
To establish a sensitive and specific antibody assay, potent antigenic proteins encoded by human herpesvirus 8 (HHV8) were studied. Fifteen recombinant HHV8-encoded proteins were produced as glutathione S-transferase fusion proteins. The sera from AIDS-associated Kaposi's sarcoma (KS) patients reacted with four proteins encoded by open reading frames (ORFs) K8.1, 59, 65, and 73 in a Western blot assay. An enzyme-linked immunosorbent assay (ELISA) using these four proteins as antigens (mixed-antigen ELISA) revealed that all 26 sera derived from KS patients (24 with and 2 without human immunodeficiency virus infection) became positive for anti-HHV8 antibodies. The presence of HHV8 was demonstrated in 14 (1.4%) of 1,004 sera from the Japanese general population and 10 (1.9%) of 527 sera from patients without HHV8-associated diseases. The presence of immunoglobulin G (IgG) and IgM antibodies against HHV8 examined further by the mixed-antigen ELISA and Western blotting revealed IgG antibody in all ELISA-positive sera, while IgM antibody against ORF K8.1 was absent. These data suggest that the ORF 73 and 65 proteins are potent antigens for a sensitive serological assay.
The human herpesvirus 8 (HHV8, Kaposi's sarcoma [KS]-associated herpesvirus) DNA sequence has been demonstrated in all forms of KS, primary effusion lymphomas (PEL, body cavity-based lymphomas [BCBL]), and a subset of patients with multicentric Castleman's disease (MCD) (4, 18, 19, 28). For a serological survey, PEL cell lines have been employed for immunofluorescence assay (IFA) and Western blot analysis to demonstrate antibodies against HHV8 in patients' sera (9, 13, 26). By Western blot analysis, sera from KS patients reacted with many proteins encoded by HHV8 open reading frames (ORFs) (1–3, 6, 9, 14, 20–23), of which more than 80 are known to be present in the HHV8 DNA (25). However, little information about the predominant antigen is available. By immunoscreening of a cDNA library from 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced BCBL-1 cells using a human immunodeficiency virus-positive (HIV+), KS+ serum, 12 ORF proteins (encoded by ORFs 6, 8, 9, 25, 26, 39, 59, 65, 68, 73, K8.1A, and K8.1B) were identified as the major proteins (3). Among these, the ORF65 protein (minor capsid protein) has been determined to be the most potent antigen for serological examination of KS sera (15, 20). The K8.1 protein has been cloned and characterized and has also been shown to be one of the predominant and useful antigens for serological analysis (2, 14, 21). Recently, it was shown that lytic (ORFs 65, K8.1A, and K8.1B) and latent (ORF73) proteins exhibited high reactivity with the sera from HHV8-seropositive individuals on Western blot analysis (30).
To date, anti-HHV8 antibodies in human sera have been detected by two methods, IFA and enzyme-linked immunosorbent assay (ELISA) (1, 5, 6, 8, 13, 16, 17, 20, 22, 24, 26). The target antigens of the two methods differ. Latency-associated nuclear antigen (LANA) derived from HHV8-encoded ORF73 and TPA-induced antigens in PEL cell lines have been employed for IFA, while the ORF65 and ORF26 proteins (possible minor capsid protein) or lysates of whole virus particles derived from KS-1 cells have been used as antigens for ELISA (1, 5, 6, 8, 13, 16, 17, 20, 22, 24, 26). The use of these different antigens often results in discrepancy between the data obtained from these serologic assays. Current HHV8 antibody testing, therefore, is of uncertain accuracy mainly in patients with asymptomatic HHV8 infection (22), and the definitive seroprevalence of HHV8 infection in specific populations cannot yet be determined clearly.
Despite the uncertain accuracy of serological tests for HHV8, several groups have reported the seroprevalence of antibodies to HHV8 in the general population. The positivity rates in these studies have varied from 0 to 53%, depending on the assay methods used and the countries examined (5, 8, 16, 29). Infection with HHV8 seems to be uncommon in the United States (0 to 25%) and Northern Europe (3 to 5.1%), more common in certain Mediterranean countries (4 to 12%), and widespread in countries such as Uganda in Africa (35 to 53%). The transmission modes of HHV8 have also not been clarified yet. In countries with endemic infection, horizontal transmission among children has been suggested to be prevalent (16) while sexual transmission appears to play an important role particularly among homosexual men in countries with nonendemic infection.
In this study, the predominant antigenic proteins encoded by HHV8 in AIDS-KS patients were investigated by the use of recombinant glutathione S-transferase (GST) fusion proteins and we have succeeded in developing a highly sensitive ELISA system for the detection of anti-HHV8 antibodies. In addition, HHV8 seroprevalence in the Japanese general population and among patients with various diseases was examined using this ELISA system.
MATERIALS AND METHODS
Recombinant HHV8 ORF proteins.
Fifteen sequences in the published HHV8 ORFs (25) amplified by PCR using the primers listed in Table 1 were cloned into bacterial expression vectors pGEX5X-2 (Pharmacia, Uppsala, Sweden) and pGEX2T (only ORF65; Pharmacia) at the two restriction enzyme sites. The selection of these proteins was based on our unpublished results of the immunoscreening of a cDNA library from TY-1 cells using sera of patients with AIDS-KS and on the published data of Chandran et al. (3, 10, 11). Among these proteins, K13, ORF72, and ORF73 are thought to be latent proteins and the others are thought to be lytic ones (7). The sequences of K2, ORF26, K8, K11, ORF65, K13, ORF72, and K14 were cloned almost in full length, whereas partial sequences of the proteins of ORF6, K10, ORF59, ORF64, and ORF73 were cloned for convenience of amplification and expression. For the cloning of ORF73, two partial sequences of the proteins, i.e., the amino- and carboxy-terminal parts, were cloned and these proteins were designated ORF73N and ORF73C, respectively. The constructs for protein expression formed fusion proteins consisting of GST and each of the ORF proteins. These were then expressed in Escherichia coli and affinity purified using glutathione-Sepharose as described previously (27). The purity and concentration of the eluted proteins were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the Bradford assay (Protein Assay; Bio-Rad, New York, N.Y.), respectively.
TABLE 1.
PCR primers used in this study
| ORF | Full sequence
|
Amplified sequence
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sitea
|
Length
|
Size (kDa) | Strand | Putative function | Forward, reverse primer (5′–3′) | Enzyme | Sitea
|
Length
|
Size
|
Vs EBV
|
|||||||
| Start | Stop | DNA (bp) | Amino acids | Start | Stop | DNA (bp) | Amino acids | kDa | GST fusion protein (kDa) | Corresponding EBV ORF | % Homology | ||||||
| 6 | 3,210 | 6,611 | 3,402 | 1,133 | 126 | + | ssDNA binding proteinc | CTCggatccTAAACGCCCTGCACAAAACA | BamHI | 5,501 | 6,197 | 697 | 232 | 26 | 52 | BALF2 | 48 |
| CTCgaattcCCCAGAGTTGATGCCAACTAT | EcoRI | ||||||||||||||||
| K2 | 17,875 | 17,261 | 615 | 204 | 23 | − | vIL-6 | CTCggatccTGTGCTGGTTCAAGTTGTGG | BamHI | 17,875 | 17,261 | 615 | 205 | 23 | 49 | ||
| CTCctcgagTTACTTATCGTGGACGTCAGG | XhoI | ||||||||||||||||
| 26 | 46,933 | 47,850 | 918 | 305 | 34 | + | Capsid protein | CTCgaattcCACTCGACAAGAGTATAGTG | BamHI | 46,933 | 47,850 | 918 | 306 | 34 | 60 | BDLF1 | 27 |
| CTCctcgagCGTGGGGAATACCAACAGGA | XhoI | ||||||||||||||||
| K8 | 74,850 | 75,569 | 720 | 228 | 25 | + | CTCggatccCCAGAATGAAGGACATACCT | BamHI | 74,853 | 75,569 | 717 | 239 | 27 | 53 | BZLF1 | 18 | |
| CTCgaattcTATACCTGCTGCAGCTGTCT | EcoRI | ||||||||||||||||
| K8.1b | 75,915 | 76,692 | 720 | 240 | 30 | + | CTCggatccAGATTCGCACAGAAATCCCT | BamHI | 75928 | 76337 | 410 | 137 | 15.2 | 41 | |||
| CTCctcgagTGAAAAGGCTGATATTAAGGC | XhoI | ||||||||||||||||
| K10 | 88,164 | 86,074 | 2,091 | 697 | 77 | − | CTCggatccCATCTACGTCCCCGTGGATA | BamHI | 87,906 | 86,140 | 1,767 | 589 | 65 | 91 | |||
| CTCgaattcTGTAGATGCCGGGGATGCGC | EcoRI | ||||||||||||||||
| K11 | 93,367 | 91,964 | 1,404 | 467 | 52 | − | CGCggatccACAGTTTGTTTTTTGAAGAG | BamHI | 93,364 | 91,964 | 1,401 | 467 | 52 | 78 | |||
| CGCgaattcTTAGTCTCTGTGGTAAAATGG | EcoRI | ||||||||||||||||
| 59 | 96,739 | 95,549 | 1,191 | 396 | 44 | − | DNA replication protein | CTCggatccTACCTTTCCACGATCGGAT | BamHI | 96,289 | 95,549 | 741 | 247 | 27 | 53 | BMRF1 | 28 |
| CTCgaattcTCAAATCAGGGGGTTAAATG | EcoRI | ||||||||||||||||
| 64 | 104,000 | 111,907 | 7,908 | 2,636 | 293 | + | Tegument protein | CTCggattcAAAGGGACCTCAAAGAGGCT | BamHI | 107,251 | 107,984 | 734 | 245 | 27 | 53 | BPLF1 | 17 |
| CTCctcgagGACTTCATGTTGGAGGCGAT | XhoI | ||||||||||||||||
| 65 | 112,443 | 111,931 | 513 | 170 | 19 | − | Capsid protein | AAggatccATGTCCAACTTTAAGGTG | BamHI | 111,931 | 112,443 | 513 | 171 | 19 | 45 | BFRF3 | 26 |
| AAAAgctttcACTATTTCTTTTTGCC | HindIII | ||||||||||||||||
| K13 | 122,710 | 122,291 | 420 | 139 | 15 | − | vFLIP | CTCggatccTCCTAAACGTGTTCATACCT | BamHI | 122,638 | 122,291 | 348 | 116 | 13 | 39 | ||
| CTCgaattcTATGGTGTATGGCGATAGTG | EcoRI | ||||||||||||||||
| 72 | 123,566 | 122,793 | 774 | 257 | 29 | − | v-cyclinD | CTCggatccTATGTGAGGATCGGATCTTT | BamHI | 123,522 | 122,794 | 729 | 243 | 27 | 53 | ||
| TTCgaattcTTAATAGCTGTCCAGAATGCG | EcoRI | ||||||||||||||||
| 73N | 127,296 | 123,808 | 3,489 | 1,162 | 129 | − | LANA | CTCggatccTTGGCGATGACCTACATCTA | BamHI | 127,195 | 126,424 | 772 | 257 | 29 | 55 | ||
| CTCgaattcTGCAATCTCCGCAAGGAGCAC | EcoRI | ||||||||||||||||
| 73C | 127,296 | 123,808 | 3,489 | 1,162 | 129 | − | LANA | CTCggatccCTGTTGTTAGCACACATGAA | BamHI | 124441 | 123818 | 624 | 208 | 23.1 | 49 | ||
| CTCgaattcCTGTGGAGAGTCCCCAGGAC | EcoRI | ||||||||||||||||
| K14 | 127,883 | 128,929 | 1,047 | 349 | 39 | + | OX-2 homolog | CTCggatccTTTTTGATTGTCCCGGGCGC | BamHI | 127896 | 128845 | 950 | 317 | 35.2 | 61 | ||
| CTCgaattcCCAAATAGGCCCACCAGAGT | EcoRI | ||||||||||||||||
Western blot analysis and IFA.
The Western blot analysis was carried out using sera from four AIDS-KS patients, one AIDS-PEL patient, one HIV-negative KS patient, one Epstein-Barr virus (EBV)-positive patient with infectious mononucleosis, and a healthy Japanese blood donor. All sera were diluted 1:200 in Block Ace (Snowbrand Milk Products, Tokyo, Japan). Cell lysates of a TPA-induced TY-1 cell line (HHV-8 positive, EBV negative) and recombinant HHV8 ORF proteins were used for the Western blot analysis (10). Briefly, TY-1 cells were grown in RPMI medium supplemented with 10% fetal calf serum. After the addition of TPA at 20 ng/ml to the medium, the TY-1 cells were cultured for 2 days. After being washed two times in phosphate-buffered saline (PBS), 107 TPA-induced TY-1 cells were lysed in 1 ml of sample buffer consisting of 25 mM Tris-HCl (pH 8.0), 5% glycerol, 1% sodium dodecyl sulfate, 1% 2-mercaptoethanol, and 0.05% bromophenol blue. For the cell lysate and recombinant HHV8 ORF proteins in the sampling buffer, 5 μl per lane, were applied to 10% polyacrylamide gels and then electrotransferred to membranes (Immobilon; Millipore, Bedford, Mass.). After blocking with Block Ace, the diluted serum samples were allowed to react for 60 min at room temperature. Goat anti-human immunoglobulin antibody conjugated with alkaline phosphatase (code no. AHI0705; Tago Immunologicals, Camarillo, Calif.) was used as the secondary antibody. To confirm the presence of GST fusion proteins on the membranes, a rabbit affinity-purified anti-GST polyclonal antibody and goat anti-rabbit immunoglobulin G (IgG) conjugated with alkaline phosphatase (Tago Immunologicals) were used as the primary and secondary antibodies, respectively. Furthermore, to identify IgG and IgM antibodies against HHV8 in the ELISA-positive sera, Western blot analysis using specific antibodies against human IgG or IgM (code no. 4600 and 2492; Tago Immunologicals) as the secondary antibodies was performed as described above.
The IFA was carried out as described previously (13, 26). In brief, TPA-induced TY-1 cells were spotted onto a slide (Erie Scientific Co., Erie, Colo.) after being washed two times in PBS and dried and then fixed in acetone for 10 min at room temperature. The patients' sera were diluted 1:40 in PBS–2% fetal calf serum and applied to the slide for 45 min at room temperature. Rabbit anti-human IgG conjugated with fluorescein isothiocyanate (Tago Immunologicals) was used as the secondary antibody. Between these steps, the slides were washed three times each in PBS for 5 min. The positive reaction of HHV8 antibody by IFA was determined by the presence of LANA, other nuclear antigens, and/or cytoplasmic antigens.
Sera.
Informed consent was obtained from all of the patients with HIV infection and other diseases, and from the healthy donors, before blood samples were obtained. Sera obtained from 21 HIV+ KS+ adult male patients and 17 HIV− KS− healthy donors were tested further to confirm the sensitivity and specificity of the various ELISAs. Moreover, 20 sera of healthy donors who were confirmed to have anti-cytomegalovirus (CMV) antibodies by CMV ELISA and 16 sera of patients with infectious mononucleosis who had been confirmed to be positive by both EBV ELISA and IFA were also tested by our mixed-antigen ELISA. We tested the sera of 1,004 healthy Japanese donors which were received from the World Health Organization and the National Serum Reference Bank/Tokyo, National Institute of Infectious Diseases (http://idsc.nih.go.jp/yosoku99/index-E.html). These sera were collected from all districts in Japan and from all generations equally in order to survey the prevalence of various infectious diseases. Sera of 527 patients with various diseases were also investigated (for the underlying diseases, see Table 3). All sera were stored at −20°C and heat inactivated at 56°C for 30 min before use.
TABLE 3.
HHV8 seroprevalence in general population and patients with various diseases
| Subject characteristics and diseasesa | No. of samples | No. positive | % Positive |
|---|---|---|---|
| Japanese general population (total) | 1,004 | 14 | 1.4 |
| Sex | |||
| Male | 510 | 9 | 1.8 |
| Female | 494 | 5 | 1.0 |
| Age (yrs) | |||
| 0–10 | 212 | 4 | 1.9 |
| 11–30 | 397 | 6 | 1.5 |
| 31–60 | 310 | 2 | 0.6 |
| 61+ | 85 | 2 | 2.4 |
| HHV-8-associated disease(s) | |||
| AIDS-KS | 24 | 24 | 100.0 |
| AIDS-PEL | 1 | 1 | 100.0 |
| KS (HIV negative) | 2 | 2 | 100.0 |
| MCD | 10 | 3 | 30.0 |
| HIV positive (sexual transmission, without KS) | 44 | 28 | 63.6 |
| Various diseases (total) | 527 | 10 | 1.9 |
| Viral infections | |||
| HIV positive (hemophilia) | 65 | 0 | 0.0 |
| Herpes zoster | 35 | 1 | 2.9 |
| CMV confirmed by ELISA | 22 | 0 | 0.0 |
| Infectious mononucleosis (EBV confirmed by ELISA) | 16 | 0 | 0.0 |
| Exanthema subitum | 57 | 0 | 0.0 |
| Hand-foot-mouth disease | 3 | 0 | 0.0 |
| Herpangina | 4 | 0 | 0.0 |
| Measles | 4 | 0 | 0.0 |
| Hematological diseases | |||
| Multiple myeloma | 11 | 0 | 0.0 |
| Malignant lymphoma | 10 | 0 | 0.0 |
| Leukemia | 4 | 0 | 0.0 |
| MDS | 9 | 0 | 0.0 |
| Aplastic anemia | 4 | 0 | 0.0 |
| Agammaglobulinemia | 4 | 0 | 0.0 |
| Pure red cell aplasia | 4 | 0 | 0.0 |
| Macroglobulinemia | 2 | 0 | 0.0 |
| ITP | 2 | 0 | 0.0 |
| Histiocytosis X | 2 | 0 | 0.0 |
| Neurological diseases | |||
| Alzheimer's disease | 31 | 1 | 3.2 |
| PSP | 6 | 0 | 0.0 |
| Cerebral infarction | 29 | 4 | 13.8 |
| Parkinson's disease | 4 | 0 | 0.0 |
| Myasthenia gravis | 1 | 0 | 0.0 |
| Malignancies | |||
| Cancers | |||
| Gastric | 28 | 1 | 3.6 |
| Colon | 15 | 0 | 0.0 |
| Liver | 16 | 0 | 0.0 |
| Lung | 25 | 1 | 4.0 |
| Prostate | 10 | 1 | 10.0 |
| Other | 18 | 0 | 0.0 |
| Other malignancy (melanoma, sarcoma, brain tumor) | 4 | 0 | 0.0 |
| Other | |||
| Uveitis | 10 | 0 | 0.0 |
| IDDM | 31 | 1 | 3.2 |
| Heart disease | 6 | 0 | 0.0 |
| Rheumatoid arthritis | 2 | 0 | 0.0 |
| MRSA | 2 | 0 | 0.0 |
| Pneumonia | 8 | 0 | 0.0 |
| Amyloidosis | 5 | 0 | 0.0 |
| MCLS | 18 | 0 | 0.0 |
MDS, myelodysplastic syndrome; ITP, ideopathic thrombocytopenic purpura; PSP, progressive supranuclear palsy; IDDM, insulin-dependent diabetes mellitus; MCLS, mucocutaneous lymph node syndrome; MRSA, methicillin-resistant Staphylococcus aureus.
ELISA using recombinant HHV8 ORF proteins.
Purified recombinant GST fusion proteins (2 μg/ml, each protein) diluted in 100 mM carbonate buffer, pH 9.0, were used to coat the wells of ELISA plates (50 μl per well; Corning Coaster 3690 enzyme immunoassay-radioimmunoassay plate; Corning Glass Works, Corning, N.Y.) overnight at 4°C. After being washed in washing buffer (0.1 M PBS [pH 7.4], 0.02% Tween 20), the serum or plasma samples at various dilutions in the dilution buffer (Block Ace) were allowed to react for 30 min at 37°C with the recombinant proteins. To avoid nonspecific reactions with GST, the lysate of E. coli producing recombinant GST protein was added to the dilution buffer at a concentration of 500 μg/ml. Unbound serum was removed by the washing buffer. Mixed goat anti-human immunoglobulins (Igs, including IgG, IgA, IgM, and Ig light chains) conjugated with alkaline phosphatase (code no. AHI0705; Tago Immunologicals) were then added, and incubation was conducted for 30 min at 37°C. After washing, phosphate substrate tablets (5 mg per tablet; Sigma, St. Louis, Mo.) were used as the substrate to develop the color for 30 min. The absorbance (Ab) of the wells was measured at a wavelength of 405 nm. In the mixed-antigen ELISA, five recombinant proteins, i.e., K8.1, ORF59, ORF65, ORF73N, and ORF73C, were mixed at 2 μg/ml per protein and used to coat the wells at 50 μl per well. In an ELISA specific for IgG or IgM antibody, goat anti-human IgG or IgM conjugated with alkaline phosphatase (code no. 4600 and 2492; Tago Immunologicals) was used as the secondary antibody. The specificity of anti-human IgG and IgM was guaranteed by the supplier.
Based on the surveys of our negative control sera and sera from patients with AIDS-KS, an ELISA titer of 1:100 was considered to be positive. This dilution was chosen to evaluate the final results of the mixed-antigen ELISA by considering the positivity of these sera for LANA by IFA using HHV8-positive PEL cells. The cutoff value for the mixed-antigen ELISA was determined by the mean Ab plus 5 standard deviations (SD) for 43 normal serum samples. Different cutoff values were calculated among ELISAs for mixed Igs, IgG, and IgM. Only for the data shown in Table 2 was the cutoff value determined by the mean Ab plus 3 SD because it represents a comparison of proteins. To avoid different values between plates, two serum samples from an AIDS-KS patient and a healthy donor were placed as positive and negative controls on every plate. Moreover, all calculations were based on the values calculated as follows: (sample Ab − negative control Ab)/(positive control Ab − negative control Ab). For statistical analysis, a two-sample t test was employed.
TABLE 2.
Reactivities of KS and normal sera with recombinant HHV8 proteins
| Antigen(s) | Mean OD (SD)a
|
P valueb | No. of KS patients positive/total (%)c | |
|---|---|---|---|---|
| KS | Normal | |||
| GST | 0.265 (0.108) | 0.247 (0.102) | 0.299 | |
| K8.1 | 0.679 (0.538) | 0.262 (0.081) | <0.01 | 11/21 (52.4) |
| ORF59 | 0.307 (0.304) | 0.201 (0.052) | 0.066d | 4/21 (19.0) |
| ORF65 | 0.441 (0.351) | 0.184 (0.049) | <0.01 | 12/21 (57.1) |
| ORF73N | 1.066 (0.561) | 0.263 (0.098) | <0.01 | 17/21 (81.0) |
| ORF73C | 1.368 (0.614) | 0.266 (0.092) | <0.01 | 17/21 (81.0) |
| Mixturee | 1.307 (0.567) | 0.198 (0.049) | <0.01 | 21/21 (100) |
Results for 21 KS patients and 17 healthy controls are shown. OD, optical density.
Results of two-sample t test.
Cutoff value = mean ± 3 SD.
No significant difference.
The mixture contained K8.1, ORF59, ORF65, ORF73N, and ORF73C.
RESULTS
Different reaction patterns of sera from AIDS-KS patients.
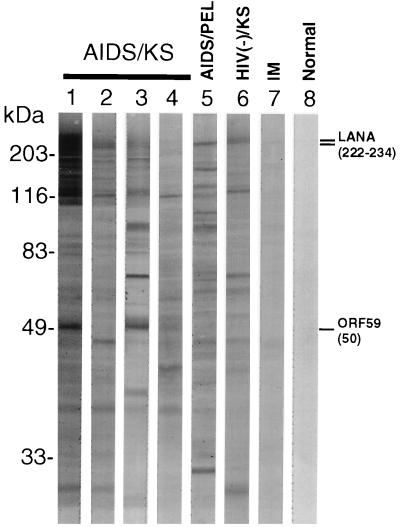
In order to confirm the sizes of antigenic proteins encoded by HHV8, the reactivity of the sera of AIDS-KS patients with lysates of the HHV8-infected PEL cell line TY-1 was first examined by Western blot analysis. Several bands, including the 222- and 234-kDa bands of LANA and the 50-kDa band of ORF59, were noted (Fig. 1), and the reaction patterns varied, but in general, the patterns were similar to those reported before (23). The variation of these patterns depended on the sera examined, and no band was found for the sera of patients with infectious mononucleosis and healthy individuals. These data showed clearly that many antigenic proteins encoded by HHV8 existed in the lysate of the TPA-induced TY-1 cell line and that every serum sample recognized these in different patterns.
FIG. 1.
Western blot analysis using TPA-induced TY-1 cell lysates revealed that the sera of AIDS-KS or PEL patients reacted with many bands and that the reaction patterns varied depending on the individual sera (lanes 1 to 6). No band was detected in the lanes containing the sera from a patient with infectious mononucleosis (IM) and a healthy blood donor (lanes 7 and 8). The reported sizes of the LANA and ORF59 bands are indicated on the right in kilodaltons.
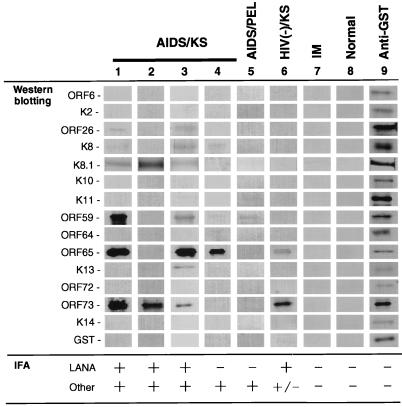
To clarify the antigenic proteins encoded by HHV8, we prepared 15 recombinant proteins using the GST fusion protein system, i.e., for ORF6, K2, ORF26, K8, K8.1, K10, K11, ORF59, ORF64, ORF65, K13, ORF72, ORF73N, ORF73C, and K14. Western blot analysis indicated that the sera from patients with KS or PEL reacted with some of these recombinant proteins, i.e., the K8.1, ORF59, ORF65, ORF73N, and ORF73C proteins; however, the reaction patterns differed for each serum sample (Fig. 2). The patterns were classified into the following three categories; (i) strong reaction with both lytic (ORF26, ORF59, and ORF65, etc.) and latent (ORF73) proteins (sera 1 and 2 in Fig. 2), (ii) strong reaction with lytic proteins but weak reaction with latent proteins (sera 3 and 4), and (iii) weak reaction with lytic proteins but strong reaction with latent proteins (serum 6). No antigen was found to react with all of the sera examined. None of the sera recognized the ORF6, K2, K8, K10, ORF64, K13, ORF72, or K14 protein.
FIG. 2.
Western blot analysis. Some of the recombinant GST fusion proteins were recognized by AIDS-KS or PEL patients' sera. The sera in lanes 1 to 8 are the same as those in Fig. 1. Only the recombinant protein bands in the membrane, whose sizes are listed in Table 1, are shown. IFA results are indicated at the bottom. The K8.1, ORF59, ORF65, and ORF73 proteins were recognized by some sera. Reactions with other proteins were weak. Lane 9 indicates that each protein was present on the membrane. IM, infectious mononucleosis.
Identification of antigenic proteins for ELISA.
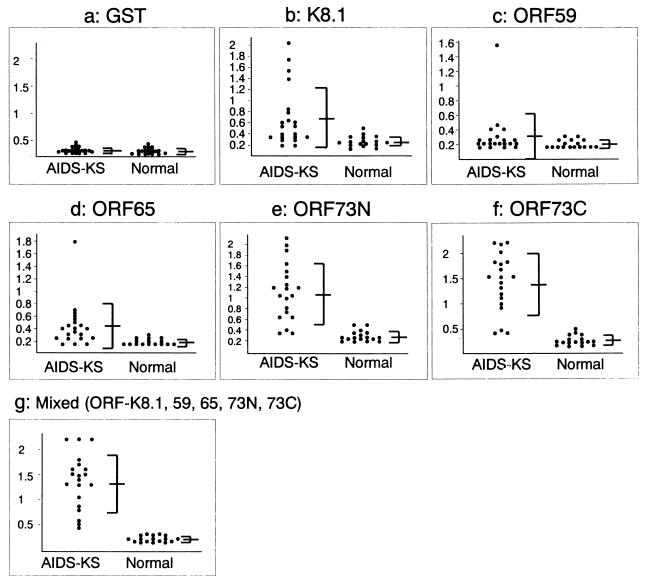
Considering the variable reaction patterns among the sera in the Western blot analysis, the K8.1, ORF59, ORF65, ORF73N, and ORF73C proteins were selected as candidate antigens for establishing an ELISA system to detect anti-HHV8 antibodies. Twenty-one AIDS-KS and 17 normal serum samples were tested by ELISA using each of the proteins as the antigen. All of these 21 AIDS-KS serum samples were confirmed to be positive by IFA. As shown in Fig. 3 and Table 2, the sera from some of these AIDS-KS patients reacted with the K8.1, ORF59, ORF65, ORF73N, and ORF73C proteins in the ELISA. However, each ELISA system using only one protein as the antigen missed some of the anti-HHV8 antibodies present in the AIDS-KS sera. Both ORF73 proteins reacted with over 80% of the AIDS-KS serum samples. Three serum samples found to be negative by ELISA using the ORF73 protein as the antigen reacted with the K8.1 or ORF65 protein. In the ELISA using ORF59, there was no significant difference between the optical density values of AIDS-KS and normal sera overall but four serum samples reacted with the ORF59 protein and also reacted strongly with both lytic and latent proteins. All AIDS-KS serum samples were finally found to be positive by ELISA using all of these five recombinant proteins as mixed antigens. Therefore, we established a highly sensitive ELISA system for detecting anti-HHV8 antibodies using mixed antigens comprising the ORF59, ORF65, K8.1, ORF73N, and ORF73C proteins and designated it a mixed-antigen ELISA.
FIG. 3.
Group scatter diagrams of ELISAs using the recombinant proteins. The scatter diagrams show reactions with various recombinant proteins in each ELISA. Compared with normal sera (right), AIDS-KS sera (left) reacted with the K8.1, ORF59, ORF65, ORF73N, ORF73C, and mixed proteins in ELISAs. The vertical axis indicates Ab, and the means and SD are shown on the right.
To check the specificity of the mixed-antigen ELISA, the reactivity and titers of the sera used in a previous experiment were tested again by IFA and Western blot analysis using HHV8-positive cell lines (TY-1 and BCBL-1; data not shown). The results of IFA and Western blot analysis were in accordance with those of the mixed-antigen ELISA. Moreover, 20 serum samples from CMV-infected individuals and 16 from patients with infectious mononucleosis were all found to be negative by the mixed-antigen ELISA. These data strongly suggested that no cross-reactions with CMV and EBV had occurred in this ELISA (Table 3). Thus, it was concluded that the mixed-antigen ELISA was specific for HHV8.
Seroprevalence of anti-HHV8 antibodies in the Japanese general population and in patients with various diseases.
Using this mixed-antigen ELISA system, the seroprevalence of anti-HHV8 antibodies in the Japanese general population and among patients with various diseases was investigated (Table 3). The antibodies were found in 14 (1.4%) of 1,004 serum samples from the general population. All of the mixed-antigen ELISA-positive sera were confirmed to be positive by IFA and Western blot analysis using the recombinant proteins (data not shown). The 14 HHV8 antibody-positive sera were from nine (64.3%) males and five (35.7%) females (mean age, 26 years; age range, 0 to 66 years). Moreover, 5 of the 14 mixed-antigen ELISA-positive serum samples from young individuals (mean age, 12 years; age range, 0 to 29 years; three males and two females), were positive for IgM antibodies by the mixed-antigen ELISA using anti-human IgM antibody as the secondary antibody, while the remaining 9 were positive for only IgG antibodies to HHV8 (data not shown). The reactivity of IgM was confirmed by Western blot analysis as described in the next section.
Our mixed-antigen ELISA also revealed that all of the 24 serum samples from AIDS-KS patients were positive for anti-HHV8 antibodies. The sera of patients with MCD and AIDS patients who were homosexual exhibited high positivity rates (30.0 and 63.6%, respectively). On the other hand, 10 (1.9%) of the 527 sera from patients with various diseases other than the HHV8-associated diseases were positive. All of these sera were also confirmed to be positive by IFA and Western blot analysis using recombinant proteins (data not shown). No significant association between the underlying diseases and HHV8 infection was found. The mixed-antigen ELISA specific for the IgM or IgG antibody revealed that 7 of the 24 AIDS-KS sera were positive for both IgM and IgG antibodies, while the rest were positive for only the IgG antibody (data not shown). All of the 10 positive sera from patients with various diseases were negative for the IgM antibody.
Reactivity of ELISA-positive sera with the K8.1, ORF59, ORF65, and ORF73 proteins on Western blot analysis.
The Western blot analysis for IgG or IgM antibody to HHV8 revealed that the IgG antibody was present in all of the mixed-antigen ELISA-positive sera, while the IgM antibody was found in 7 (30%) of the 23 sera from patients with KS (six HIV+ and one HIV−) and 5 (36%) of the 14 from the general population (0, 2, 6, 23, and 29 years old, three males and two females; Table 4). All of the ELISA-positive sera reacted mainly with the recombinant ORF65 and ORF73N proteins, while the ORF59 and K8.1 proteins were recognized by some sera. Interestingly, the K8.1 protein was recognized by the IgG, but not the IgM, antibody. In addition, this protein reacted predominantly with the sera from KS patients and rarely with the sera from the general population or from patients with various diseases other than the HHV8-associated diseases.
TABLE 4.
Western blot analysis for ELISA-positive sera
| Secondary antibody and serum sources | Total no. | No. (%) positive for:
|
|||
|---|---|---|---|---|---|
| ORF K8.1 | ORF 59 | ORF 65 | ORF 73N | ||
| IgG | |||||
| KS patients | 23 | 11 (48) | 2 (9) | 21 (91) | 23 (100) |
| General population | 14 | 2 (14) | 2 (14) | 9 (64) | 14 (100) |
| Patients with other diseases | 16 | 2 (13) | 7 (44) | 9 (56) | 16 (100) |
| IgM | |||||
| KS patients | 7 | 0 (0) | 3 (42) | 5 (71) | 7 (100) |
| General population | 5 | 0 (0) | 1 (20) | 4 (80) | 5 (100) |
DISCUSSION
The present study revealed that (i) the reaction patterns of AIDS-KS sera with HHV8-encoded proteins varied; (ii) the ORF59, ORF65, K8.1, and ORF73 proteins were the most strongly antigenic for AIDS-KS patients; (iii) the seroprevalence of HHV8 was 1.4% in the general population and 1.9% among patients with various diseases in Japan; and (iv) the K8.1 proteins were recognized predominantly by sera from KS patients. A highly sensitive ELISA for detection of anti-HHV8 antibodies was also established.
Western blot analysis revealed that the reaction patterns of the examined sera with the lysates of HHV8-infected cells were different. We speculate that the variation in the reaction patterns arose from the viral replication status, immune response, and the disease(s) of each patient. In the present study, we selected the K8.1, ORF59, ORF65, and ORF73 proteins as the antigens for the mixed-antigen ELISA system. This selection was considered to be appropriate based on the evidence shown in Fig. 2 and 3 and Table 2. This protein panel may not be sufficient for detection of the entire spectrum of anti-HHV8 antibodies, because some of the sera also reacted with other bands, as illustrated in Fig. 1. However, we concluded that these proteins are sufficient for the detection of anti-HHV8 antibodies because of the high positivity rate obtained with the AIDS-KS sera examined. Our selection of these proteins partially corresponded to that reported by Zhu et al. (30).
Among these proteins, ORF73 (LANA) is known to be a latent protein and ORF65 and K8.1 are known to be lytic proteins (21, 23). The effective antigenicity of ORF65 and K8.1 for antibody assay has also been reported (1, 2, 6, 14, 20, 21, 22). Our K8.1 and ORF65 positivity rates were lower than those previously reported (2, 14, 15, 20, 21, 22). We speculate that there are two reasons for this. One is that we employed the first exon of K8.1β protein, and another reason is the difference in the samples. It may be important that the antigens used for an anti-HHV8 antibody assay contain both latent and lytic proteins, because some of the sera in this study reacted only with either latent or lytic proteins. Our ELISA data (Fig. 3 and Table 2) suggest that LANA is recognized by most of the sera from HHV8-infected individuals. Figure 3 and Table 2 also indicate that some of the sera had antibodies only against latent proteins. Taken together, these data suggest that antibodies against latent proteins are the major components of anti-HHV8 antibodies in human sera and that LANA exhibited stronger antigenicity than lytic proteins in the antibody assay. In addition, we recently reported that most of the spindle cells in KS express LANA in their nuclei (12). Therefore, previous ELISA systems employing one of the HHV8-encoded lytic proteins as an antigen were probably insufficient. Likewise, the ELISA system using whole virus lysate as the antigens may miss the antibodies against latent proteins. In fact, we observed that some of the sera positive by our mixed-antigen ELISA were negative by an ELISA using whole virus lysate as the antigens (Advanced Biotechnologies Inc.) (data not shown; 5). Thus, it is essential for the effective detection of anti-HHV8 antibodies in sera to employ both latent and lytic proteins as antigens.
There are two reports on the prevalence of anti-HHV8 antibodies in Japanese subjects. Fujii et al. reported finding that the seroprevalence of HHV8 infection among healthy Japanese blood donors was 0.2% using IFA to detect LANA (8), and in our previous study, we reported that 2.2% of HIV-negative and KS-negative Japanese patients had antibodies against HHV8 as determined by ORF59 ELISA (11). Chatlynne et al. demonstrated that 11% of sera from blood donors in the United States were positive for HHV8 antibodies by a whole-virus lysate ELISA (5). This frequency is much higher than that in the Japanese general population, and the discrepancy may be attributed to the difference in race. The seropositivity rate of anti-HHV8 antibodies was also found to be higher in the elderly than in other age groups (Table 3). However, further analyses are required to determine the precise positivity rate in each generation because the number of positive sera was limited in this study.
Our mixed-antigen ELISA system revealed that the positivity rate was slightly higher in sera from patients with various diseases than in those from the Japanese general population. This result suggests the possibility that underlying diseases and as yet unknown factors may activate HHV8 infection. However, we could not find any definite association between HHV8 infection and any underlying disease. Sera from patients with cerebral infarction (mean age, 83.6 years; age range, 71 to 98 years) showed a relatively high positivity rate for HHV8 antibodies (13.8%); however, this is unlikely to indicate any association of HHV8 infection with cerebral infarction because of the small number of samples examined.
Our data from Western blot analysis using anti-human IgG or IgM antibody as the secondary antibody indicate that the K8.1 protein reacts with only the IgG antibody from KS patients, suggesting an association of the K8.1 protein with the pathogenesis of KS, although further studies are necessary to verify the association. In the present study, IgM antibodies in the sera from HHV8-infected individuals were noted, for the first time, to recognize mainly the ORF73 and ORF65 proteins.
In conclusion, we demonstrated the antigenicity of HHV8-encoded proteins in AIDS-KS patients and have developed a new sensitive ELISA system. We believe that our mixed-antigen ELISA system will be a useful tool in the clinical diagnosis of HHV8-related diseases and in screening for and monitoring of antibodies against HHV8 in AIDS patients and organ transplant recipients.
ACKNOWLEDGMENTS
We are very grateful to Y. Matsunaga and S. Inoue, Infectious Disease Surveillance Center, National Institute of Infectious Diseases; M. Goto, Department of Infectious Diseases, Institute of Medical Science, University of Tokyo; N. Tachikawa and S. Oka, AIDS Clinical Center, International Medical Center; G. Masuda, Department of Infectious Diseases, Komagome Metropolitan Hospital; T. Kumasaka, Department of Pathology, Juntendo University School of Medicine; H. Mizoguchi, Department of Hematology, and K. Yoda, Department of Otorhinolaryngology, Tokyo Women Medical University; T. Minematsu, Department of Microbiology, Miyazaki Medical College; N. Suematsu, Department of Pathology, Yokufukai Hospital; A. Maeda, Department of Pediatrics, Kochi Medical College; R. Muraki, Department of Dermatology, National Kasumigaura Hospital; H. Harada, Department of Dermatology, St. Lukes International Hospital; A. Urabe, Division of Hematology, Kanto-Teishin Hospital; M. Ito, Department of Pediatrics, Mie University School of Medicine; and T. Ihara, Department of Pediatrics, Mie National Hospital, for providing the serum samples. We thank B. Herndier, Department of Pathology, University of California, San Francisco, for supplying the BCBL-1 cell line.
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Andre S, Schatz O, Bogner J R, Zeichhardt H, Stoffler M M, Jahn H U, Ullrich R, Sonntag A K, Kehm R, Haas J. Detection of antibodies against viral capsid proteins of human herpesvirus 8 in AIDS-associated Kaposi's sarcoma. J Mol Med. 1997;75:145–152. doi: 10.1007/s001090050099. [DOI] [PubMed] [Google Scholar]
- 2.Chandran B, Bloomer C, Chan S R, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 3.Chandran B, Smith M S, Koelle D M, Corey L, Horvat R, Goldstein E. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology. 1998;243:208–217. doi: 10.1006/viro.1998.9055. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 5.Chatlynne L G, Lapps W, Handy M, Huang Y Q, Masood R, Hamilton A S, Said J W, Koeffler H P, Kaplan M H, Friedman K A, Gill P S, Whitman J E, Ablashi D V. Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi's sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood. 1998;92:53–58. [PubMed] [Google Scholar]
- 6.Davis D A, Humphrey R W, Newcomb F M, O'Brien T R, Goedert J J, Straus S E, Yarchoan R. Detection of serum antibodies to a Kaposi's sarcoma-associated herpesvirus-specific peptide. J Infect Dis. 1997;175:1071–1079. doi: 10.1086/516444. [DOI] [PubMed] [Google Scholar]
- 7.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii T, Taguchi H, Katano H, Mori S, Nakamura T, Nojiri N, Nakajima K, Tadokoro K, Juji T, Iwamoto A. Seroprevalence of HHV-8 in HIV-1 positive and negative populations in Japan. J Med Virol. 1998;57:159–162. doi: 10.1002/(sici)1096-9071(199902)57:2<159::aid-jmv12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 10.Katano H, Hoshino Y, Morishita Y, Nakamura T, Satoh H, Iwamoto A, Mori S. Establishing and characterizing a CD30-positive cell line harboring HHV-8 from primary effusion lymphoma. J Med Virol. 1999;58:394–401. [PubMed] [Google Scholar]
- 11.Katano H, Sata T, Suda T, Nakamura T, Tachikawa N, Nishizumi H, Sakurada S, Hayashi Y, Koike M, Iwamoto A, Kurata T, Mori S. Expression and antigenicity of human herpesvirus 8 encoded ORF59 protein in AIDS-associated Kaposi's sarcoma. J Med Virol. 1999;59:346–355. doi: 10.1002/(sici)1096-9071(199911)59:3<346::aid-jmv15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Katano H, Sato Y, Kurata T, Mori S, Sata T. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi's sarcoma. Am J Pathol. 1999;155:47–52. doi: 10.1016/S0002-9440(10)65097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedes D H, Ganem D, Ameli N, Bacchetti P, Greenblatt R. The prevalence of serum antibody to human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA. 1997;277:478–481. [PubMed] [Google Scholar]
- 14.Li M, MacKey J, Czajak S C, Desrosiers R C, Lackner A A, Jung J U. Identification and characterization of Kaposi's sarcoma-associated herpesvirus K8.1 virion glycoprotein. J Virol. 1999;73:1341–1349. doi: 10.1128/jvi.73.2.1341-1349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin S F, Sun R, Heston L, Gradoville L, Shedd D, Haglund K, Rigsby M, Miller G. Identification, expression, and immunogenicity of Kaposi's sarcoma-associated herpesvirus-encoded small viral capsid antigen. J Virol. 1997;71:3069–3076. doi: 10.1128/jvi.71.4.3069-3076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayama S, Cuevas L E, Sheldon J, Omar O H, Smith D H, Okong P, Silvel B, Hart C A, Schulz T F. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817–820. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Melbye M, Cook P M, Hjalgrim H, Begtrup K, Simpson G R, Biggar R J, Ebbesen P, Schulz T F. Risk factors for Kaposi's-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–548. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 19.Nador R G, Cesarman E, Chadburn A, Dawson D B, Ansari M Q, Said J, Knowles D M. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 20.Pau C P, Lam L L, Spira T J, Black J B, Stewart J A, Pellett P E, Respess R A. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65-encoded protein. J Clin Microbiol. 1998;36:1574–1577. doi: 10.1128/jcm.36.6.1574-1577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raab M S, Albrecht J C, Birkmann A, Yaguboglu S, Lang D, Fleckenstein B, Neipel F. The immunogenic glycoprotein gp35-37 of human herpesvirus 8 is encoded by open reading frame K8.1. J Virol. 1998;72:6725–6731. doi: 10.1128/jvi.72.8.6725-6731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabkin C S, Schulz T F, Whitby D, Lennette E T, Magpantay L I, Chatlynne L, Biggar R J. Interassay correlation of human herpesvirus 8 serologic tests. HHV-8 Interlaboratory Collaborative Group. J Infect Dis. 1998;178:304–309. doi: 10.1086/515649. [DOI] [PubMed] [Google Scholar]
- 23.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S-J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regamey N, Cathomas G, Schwager M, Wernli M, Harr T, Erb P. High human herpesvirus 8 seroprevalence in the homosexual population in Switzerland. J Clin Microbiol. 1998;36:1784–1786. doi: 10.1128/jcm.36.6.1784-1786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson G R, Schulz T F, Whitby D, Cook P M, Boshoff C, Rainbow L, Howard M R, Gao S J, Bohenzky R A, Simmonds P, Lee C, De R A, Hatzakis A, Tedder R S, Weller I V, Weiss R A, Moore P S. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 27.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 28.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M F, Clauvel J P, Raphael M, Degos L, Sigaux F. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 29.Tedeschi R, De P P, Schulz T F, Dillner J. Human serum antibodies to a major defined epitope of human herpesvirus 8 small viral capsid antigen. J Infect Dis. 1999;179:1016–1020. doi: 10.1086/314657. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L, Wang R, Sweat A, Goldstein E, Horvat R, Chandran B. Comparison of human sera reactivities in immunoblots with recombinant human herpesvirus (HHV)-8 proteins associated with the latent (ORF73) and lytic (ORFs 65, K8.1A, and K8.1B) replicative cycles and in immunofluorescence assays with HHV-8-infected BCBL-1 cells. Virology. 1999;256:381–392. doi: 10.1006/viro.1999.9674. [DOI] [PubMed] [Google Scholar]