Abstract
Dysregulated intracellular pH (pHi) dynamics and an altered tumor microenvironment have emerged as drivers of cancer cell phenotypes. However, the molecular integration between the physical properties of the microenvironment and dynamic intracellular signaling responses remains unclear. Here, we use two metastatic cell models, one breast and one lung, to assess pHi response to varying extracellular matrix (ECM) stiffness. To experimentally model ECM stiffening, we use two tunable-stiffness hydrogel systems: Matrigel and hyaluronic acid (HA) gels, which mimic the increased protein secretion and crosslinking associated with ECM stiffening. We find that single-cell pHi decreases with increased ECM stiffness in both hydrogel systems and both metastatic cell types. We also observed that stiff ECM promotes vasculogenic mimicry (VM), a phenotype associated with metastasis and resistance. Importantly, we show that decreased pHi is both a necessary and sufficient mediator of VM, as raising pHi on stiff ECM reduces VM phenotypes and lowering pHi on soft ECM drives VM. We characterize β-catenin as a pH-dependent molecular mediator of pH-dependent VM, where stiffness-driven changes in β-catenin abundance can be overridden by increased pHi. We uncover a dynamic relationship between matrix stiffness and pHi, thus suggesting pHi dynamics can override mechanosensitive cell responses to the extracellular microenvironment.
Introduction
The extracellular matrix (ECM) is a protein-rich structure that becomes dysregulated in cancer, driving cancer cell adaptation and promotion of cancer cell phenotypes1. This increasingly rigid and dense tumor ECM has been shown to promote cancer cell invasion and vasculogenic mimicry, an adaptive cancer phenotype 2,3. In addition to the dysregulated extracellular environment, cancer cells also experience dysregulated pH dynamics4, with increased intracellular pH (pHi) (>7.4) and decreased extracellular pH (pHe) (<7.2) compared to normal epithelial cells (pHi 7.0–7.3; pHe 7.4)5. This reversal of the pH gradient is an early event in cellular transformation6 and has been directly linked to adaptive changes in cancer cell signaling, metabolism, proliferation, and evasion of apoptosis5.
Increased ECM stiffness promotes various cancer cell phenotypes including increased hypoxia7, vasculogenic mimicry8, cell durotaxis9, and selection for tumor initiating cell (TIC) or cancer stem-cell phenotypes4,10–13. Importantly, many equivalent or similar processes are also linked to dysregulated pHi dynamics including hypoxia11, cell invasion4, and maintenance of a stem-like phenotype in adult and embryonic stem cell models14. However, the molecular mechanisms that integrate the physical properties of the microenvironment with intracellular cancer cell signaling response are largely unknown.
While prior work has shown pHi dynamics can directly regulate normal mechanosensitive behaviors including focal adhesion remodeling15 and epithelial cell-cell contacts16,17, there are significant gaps in knowledge of the molecular crosstalk between ECM stiffening and pHi dynamics in cancer cells. One limitation is technical: it is challenging to develop mechanically tunable model systems that mimic physiological ECM dysregulation with suitable mechanical control. Previous studies have used synthetic ECM models, including Matrigel/Geltrex18 and Hyaluronan19 based systems. However, these studies have lacked the ability to decouple the contributions of ECM protein abundance and ECM crosslinking density as independent drivers of mechanosensitive cell responses.
Another limiting factor in characterizing molecular links between ECM stiffness and pHi is that most mechanistic studies of how pHi dynamics regulate cell behaviors are performed under non-physiological culture conditions and lack single-cell resolution. These limitations apply to most studies of pHi dynamics in biology, but are compounded when exploring effects of physical forces on cellular pHi dynamics and in the context of phenotypically heterogeneous cancer cells.
Here, we pair synthetic tunable-stiffness ECM models with live-cell pHi measurements and non-invasive pHi manipulation to elucidate how pHi dynamics respond to ECM stiffening. We further explore a mechanistic role of pHi in regulating a cancer-associated mechanosensitive phenotype called vasculogenic mimicry (VM). We use two unique synthetic matrix models to mimic ECM stiffening through increasing protein abundance (Matrigel/Geltrex) and crosslinking density (hyaluronic acid gels), and measure single-cell pHi in metastatic breast and lung cancer cells. We show that pHi decreases with increased stiffness using both matrix models. We also show that cells plated on stiff ECM acquire distinct VM phenotypes that can be modulated by dynamically altering pHi. Importantly, raising pHi in cells plated on stiff matrix reduces VM phenotypes while lowering pHi in cells plated on soft matrix induces acquisition of a stiffness-independent VM phenotype. We also investigate the pH dependence of two molecular regulators of VM that have been previously shown to be regulated by ECM stiffness (β-catenin and FOXC2). We show that β-catenin is a pH-dependent mediator of VM phenotype while FOXC2 activity is pHi insensitive in this system. This suggests β-catenin as a novel necessary regulator of pH-dependent vasculogenic mimicry. Overall, our work reveals a previously unidentified link between mechanosensing and pHi dynamics in cancer and further suggests low pHi as a necessary and sufficient mediator of VM, a phenotype associated with aggressive cancers.
Results
Stiffening extracellular matrix lowers pHi in metastatic human lung carcinoma
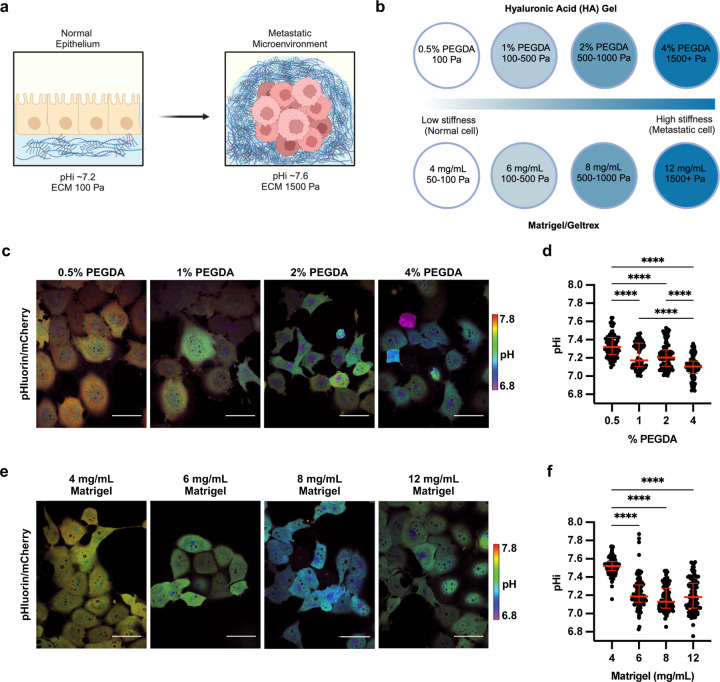
Increased tumor microenvironment (TME) stiffness can be caused by increased ECM protein deposition and increased crosslinking20 (Figure 1A). To investigate the central hypothesis of how a stiffening extracellular environment alters pHi, we used two tunable-stiffness hydrogel models to control ECM stiffness with high specificity and using two unique modes of mechanical modulation. To mimic the effects of ECM stiffness changes resulting from altered ECM protein crosslinking, we used a hyaluronic-acid (HA) gel system where variable crosslinking density tunes ECM stiffness independent of protein concentration and composition21,22. HA is a non-sulfated linear polysaccharide of (1-β-4)d-glucuronic acid and (1-β-3)N-acetyl-d-glucosamine, and is a ubiquitous component of the ECM19. HA is particularly abundant in the extracellular environment of the lung and brain19, and increased HA secretion is associated with cancers19 as well as fibrotic diseases of the liver and lung23.
Figure 1: Stiffening extracellular matrix lowers pHi in metastatic human lung carcinoma (H1299).
a) Schematic of increased pHi and ECM stiffening (via increased protein secretion and increased protein crosslinking) associated with tumorigenesis. b) Schematic of synthetic ECM models with tunable-stiffness (~50 Pa-1,500 Pa). The Matrigel (or Geltrex) model mimics increased ECM protein secretion while hyaluronic acid (HA) gel system mimics increased ECM protein crosslinking. c) Representative images of H1299 cells stably expressing mCherry-pHluorin pH biosensor plated on varying HA gel stiffnesses. Images show ratiometric display of pHluorin/mCherry fluorescence. Scale bars: 50 μm. d) Quantification of single-cell pHi data collected as shown in (c). (n=3 biological replicates; n=91 0.5% PEGDA, n=90 1% PEGDA, n=102 2% PEGDA, n=89 4% PEGDA. Red lines show medians ± IQR). e) Representative images of H1299 cells stably expressing mCherry-pHluorin pH biosensor plated on varying Matrigel stiffnesses. Images show ratiometric display of pHluorin/mCherry fluorescence. Scale bars: 50 μm. f) Quantification of single-cell pHi data collected as shown in (e). (n=3 biological replicates; n=93 4mg/mL, n=92 6mg/mL, n=102 8mg/mL, n=97 12mg/mL. Red lines show medians ± IQR). For (d) and (f), significance was determined by a Kruskal-Wallis test (****P<0.0001).
Recent work has shown that HA can be functionalized to contain thiol-reactive cross-linkable regions, with increased crosslinking adding rigidity to the ECM allowing tunable stiffness24. Our HA gel tunable-stiffness model consists of a uniform mixture of gelatin and thiol-modified hyaluronan across stiffnesses, while stiffness is controlled by modulating amounts (%) of thiol-reactive PEGDA crosslinker (see methods for details). The HA gel model consists of four levels of crosslinking agent mimicking ECM stiffness changes induced by increased protein crosslinking and has a previously reported tunable stiffness range from ~100–1500 Pa25 (Figure 1B). This system allows us to modulate matrix stiffness by adjusting the extent of ECM protein crosslinking while maintaining a consistent concentration of matrix components (hyaluronan and gelatin) across all stiffness conditions. This ability to model ECM stiffness independent of matrix concentration is a unique feature which provides advantage over model systems which use natural hydrogels in decoupling individual drivers of ECM stiffening.
To mimic the effects of stiffness changes due to increased ECM protein secretion, we used a Matrigel- or Geltrex-based tunable-stiffness gel system. Matrigel and Geltrex are naturally-derived matrices that mimic the tumor microenvironment of stromal-rich tissues, such as breast, lung, and prostate26. The Matrigel and Geltrex commercial matrix mixtures are rich in laminin and collagen; EMC proteins that directly promote integrin signaling27. Varying the concentration of Matrigel and Geltrex effectively titrates ECM protein concentrations28, mimicking the increased secretion of ECM proteins associated with stiffening tumor microenvironment29. We used tunable-stiffness Matrigel/Geltrex models that consist of four Matrigel/Geltrex concentrations (4 mg/mL-12 mg/mL) with stiffness ranges of ~50–1,500 Pa30–32 (Figure 1B). For these stiffness determinations, the manufacturer reports an elastic modulus (G′) that can be converted to Young’s modulus (matrix stiffness) using the following equation E= 2G’(1 + v). Prior work has indicated that hydrogels can be assumed to be incompressible, such that their Poisson’s ratio (v) approaches 0.533, simplifying the equation to E=3G’ Importantly, in the Matrigel/Geltrex tunable-stiffness gel systems, as the ECM protein concentrations increase, so does the available ligand concentration for integrin-mediated interactions. This gel model allows us to assess effects of ECM stiffening on pHi when intracellular integrin signaling is also titrating.
With the two tunable-stiffness hydrogel systems established, we next selected cancer cell lines that originated from tissues with a relatively soft ECM, such as lung and breast, where tumorigenic ECM stiffening has been associated with both increased metastasis and invasion26. We have previously established and characterized single-cell pHi heterogeneity in a clonal metastatic lung cancer cell line (H1299) and a clonal breast cancer cell line (MDA-MB-231), all plated and imaged on glass34. We have engineered these cell lines to stably express a genetically-encoded ratiometric pH biosensor mCherry-pHluorin (mCh-pHl)34. This biosensor is a fusion of the fluorescent protein pHluorin (pKa 7.1) that is pH-sensitive in the physiological range, and the fluorescent protein mCherry, that is pH-insensitive in the physiological range35. For accurate pHi measurements in single cells, ratiometric imaging of pHluorin and mCherry fluorescence can be performed followed by single-cell standardization using isotonic buffers with a known pHi containing the protonophore Nigericin to equilibrate intracellular and extracellular (buffer) pH36. Single-cell standard curves are then generated, enabling back-calculation of pHi from pHluorin and mCherry fluorescence intensity ratios (Supplemental Figure 1, see methods for details). This biosensor has successfully been used in prior studies to measure single-cell spatiotemporal pHi dynamics in clonal cancer and normal epithelial cell populations without affecting cell morphology or behavior15,34,35.
To determine effects of altered ECM stiffness on pHi, we cultured H1299 cells expressing the mCh-pHl biosensor on matrix-coated imaging dishes for 48 hours. This incubation allowed for cells to adhere and respond to the varied stiffness of each matrix system. In cells plated on HA gels, single-cell pHi decreased with increasing stiffness (Figure 1D). Cells plated on the stiffest matrix (4% PEGDA) had a significantly decreased pHi (Figure 1D; 7.10±0.07; median±interquartile range (IQR)) compared to cells on the softest matrix (0.5% PEGDA) (Figure 1D; 7.32±0.10; median±IQR). We also observed that intermediate ECM stiffnesses (1% PEGDA and 2% PEGDA) produced intermediate effects on pHi, with a stepwise trend of decreasing pHi with increasing stiffness (Figure 1F; 2% PEGDA 7.20±0.10; 1% PEGDA 7.17±0.19; medians±IQR). The overall decrease in pHi of ~0.2 pH units between soft and stiff ECM is within the range of physiological pHi dynamics that have been shown to regulate normal cell behaviors including cell cycle progression34, differentiation13,37, and migration38. This result shows that stiffening of the ECM through changes in protein crosslinking drives significant decreases in single-cell pHi of clonal metastatic lung cancer cells. These data suggest that progressive changes in ECM stiffness within the physiological range of normal to metastatic mechanical stiffness environments can alter pHi in metastatic cancer cells, suggesting a potential role for pHi in mechanosensitive cancer cell signaling and behaviors.
We next determined whether the stiff ECM decreased pHi using the Matrigel tunable-stiffness models, where ECM protein concentration is the predominant driver of altered stiffness. In cells plated on varied Matrigel stiffnesses, single-cell pHi decreased with increasing stiffness (Figure 1E). Cells plated on the stiffest matrix (12 mg/mL) had a significantly decreased pHi (7.18±0.15; median±IQR) compared to the softest matrix (4 mg/mL; 7.52±0.49; median±IQR) (Figure 1F). The decrease in pHi of ~0.35 units between stiffest (~1,500 Pa) and softest (~50 Pa) ECM in this system is also consistent with the pHi changes we measured between stiffest and softest HA gel models. However, in the Matrigel tunable-stiffness model system, the pHi measured on intermediate stiffnesses (6 mg/mL Matrigel, 7.19±0.13; 8 mg/mL Matrigel, 7.13±0.14; medians±IQR) was not significantly different from the pHi of cells plated on a stiff matrix (Figure 1F). This result shows that ECM stiffening decreases pHi in metastatic cells via both increased ECM protein abundance and crosslinking, showing mechanism independent ECM stiffness driven pHi dynamics in metastatic cells.
We next confirmed that ECM stiffness leads to decreased pHi using metastatic breast epithelial cell model (MDA-MB-231) as another metastatic cell model derived from a stromal-rich environment. The pHi of MDA-MB-231 cells was decreased by ~0.2 units in cells plated on a stiff matrix compared to soft matrix in both the Matrigel (soft 7.40±0.14; stiff 7.20±0.13; median±IQR) and HA gel (soft 7.43±0.14; stiff 7.28±0.10; median±IQR) models (Supplemental Figure 1). Taken together, these data show that increases in ECM stiffness mediated by either increased crosslinking (HA gel model) or by increased ECM protein secretion (Matrigel model) both decrease pHi at the single-cell level. Our data also show that the stiffness-dependent decreases in pHi are not tissue specific as both breast and lung metastatic models exhibited a 0.2–0.35 decrease in pHi on stiff compared to soft matrices. In summary, these data show that there is an inverse relationship between ECM stiffening and pHi in these metastatic cancer cell models, and further suggests a role for pHi in regulating pH-sensitive molecular pathways to drive or reinforce stiffness-associated phenotypes.
Stiffness dependent vasculogenic mimicry is reduced in high pHi conditions in metastatic lung carcinoma
When performing the single-cell pHi measurements on tunable-stiffness ECM models, we also observed a distinct change in overall cancer cell morphology that correlated with increased ECM stiffness. Metastatic cancer cells plated on soft matrix grew in flat lawns of large rounded (H1299) or spindle-shaped cells (MDA-MB-231), forming a near-confluent sheet. However, on stiff matrix, the metastatic cancer cells grew in compact clusters of irregularly shaped cells, frequently exhibited 3D growth phenotypes, and formed connected bridges of significantly elongated spindle shaped cells between 3D “nodes” (Supplemental Figure 3). This change in cell morphology we observed on stiff matrices has been previously described as a vasculogenic mimicry (VM) phenotype. VM is an aggressive cancer phenotype observed both in vivo and in vitro, where tumor cells organize into vessel-like structures, allowing nutrients and oxygen access independent of traditional angiogenesis39. Previous studies have shown increased ECM stiffness can drive VM40 phenotypes and have also characterized 2D VM phenotypes as a pronounced growth pattern where cells form distinct networks of tightly packed cells with surrounding open space devoid of cell growth41.
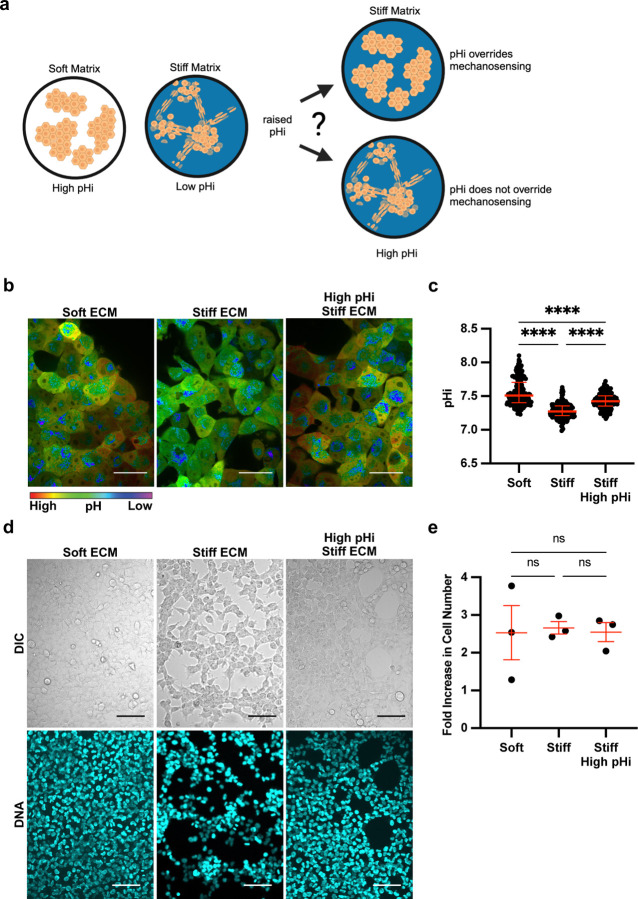
Our data showing single-cell pHi decreases in H1299 cells on stiff ECM led to the hypothesis that low pHi is a necessary mediator of VM and that raising pHi in H1299 cells plated on stiff matrix would reduce the VM phenotype (Figure 2A). To directly test this hypothesis, we established protocols to experimentally raise pHi in H1299 cells plated on stiff ECM. Prior work showed that 50 mM Sodium Bicarbonate supplemented into the media for 24 hours was sufficient to raise pHi in H1299 cells plated on glass34. We imaged single-cell pHi in H1299 cells plated on soft ECM, stiff ECM, and stiff ECM with bicarbonate supplementation (Figure 2B). We found that bicarbonate significantly increased pHi of cells plated on stiff ECM compared to untreated cells on stiff matrix (stiff 7.27±0.08; stiff + Bicarbonate 7.43±0.08; median±IQR) (Figure 2C). While the absolute pHi achieved with bicarbonate treatment on stiff HA gel matrix was lower than the matched pHi of control cells plated on soft ECM (Figure 2C), the bicarbonate treatment increased the pHi of cells plated on the stiffest ECM by approximately 0.2 pH units (Figure 2C), which is similar to the magnitude of pHi changes we observed between soft and stiff ECM across the various cell lines and gel systems.
Figure 2: Stiffness-dependent vasculogenic mimicry is reduced when pHi is increased on stiff ECM.
a) Schematic of vasculogenic mimicry (VM) in 2D on stiffening matrix. b) Representative images of H1299 cells stably expressing mCherry-pHluorin pH biosensor plated on soft (0.5% PEGDA) and stiff (4% PEGDA) HA gels and stiff (4% PEGDA) with raised pHi. Images show ratiometric display of pHluorin/mCherry fluorescence ratios. Scale bars: 50 μm. c) Quantification of single-cell pHi data collected as shown in (b) (n=3 biological replicates; n=201 soft, n=237 stiff, n=239 stiff high pHi. Red lines show medians ± IQR). d) Representative images of H1299 cells plated on soft (0.5% PEGDA) and stiff (4% PEGDA) HA gels. Images show differential interference contrast (DIC) and Hoechst stain (DNA, cyan). Scale bars: 100 μm. e) Quantification of cell proliferation across manipulation conditions. (n=3 biological replicates, n=9 per condition. Red lines show means ± SEM).
We next tested the effects of increased pHi on the stiffness-dependent vasculogenic mimicry phenotype. We found that H1299 cells acquired a vasculogenic mimicry phenotype on stiff matrix, and this VM phenotype was abrogated when pHi was increased on stiff matrix (Figure 2D). Cells plated on stiff matrix with bicarbonate-induced increases in pHi grew in a 2D cobblestone-like morphology similar to the morphology of cells grown on the soft ECM (Figure 2D, additional representative images in Supplemental Figure 4). To confirm that the observed pH-dependent change in cell morphology was not due to pH-dependent or stiffness-dependent differences in cell proliferation, we assayed proliferation rates in H1299 cells plated on soft and stiff ECM with and without increased pHi. Importantly, we did not observe any significant differences in proliferation rates across our experimental conditions (Figure 2E). Our data showing loss of VM networks when pHi is increased in cells plated on stiff ECM demonstrate that low pHi is necessary to maintain VM phenotypes on stiff ECM. Furthermore, these data show that high pHi can override stiffness-dependent vasculogenic mimicry in a metastatic cancer model.
To quantify the observed stiffness- and pHi-dependent changes in cell morphology, we used a cell membrane marker and quantitative image analysis (see methods for details) pipeline to assess cell area (Figure 3A). Notably, single-cell area of H1299 cells was significantly lower in cells plated on stiff ECM compared to soft ECM (Figure 3B; stiff 338.6 µm2±189; soft 406.5 µm2±224.6; median±IQR). This result demonstrates that cell area is a robust quantitative morphology indicator that decreases with acquisition of VM phenotype on stiff ECM. This allows us to quantitatively distinguish cell morphologies corresponding to low VM and high VM conditions. Importantly, we found that cell area significantly increased (Figure 3C; 374.3 µm2±210.6; median±IQR) when pHi was raised in H1299 cells plated on stiff ECM compared to control H1299 on stiff ECM (Figure 3B). This indicates that increased pHi attenuates the observed stiffness-dependent VM phenotype. The loss of VM networks and increased cell area when pHi is raised on stiff ECM demonstrates that low pHi is required for cells to acquire VM on stiff matrices. Together, our findings confirm previous literature characterizing vasculogenic mimicry as an ECM stiffness-mediated phenotype and further identifies decreased pHi as a previously unrecognized necessary regulator of VM.
Figure 3: Vasculogenic mimicry phenotype decreases cell area on stiff ECM, which is rescued by increasing pHi in metastatic lung carcinoma.
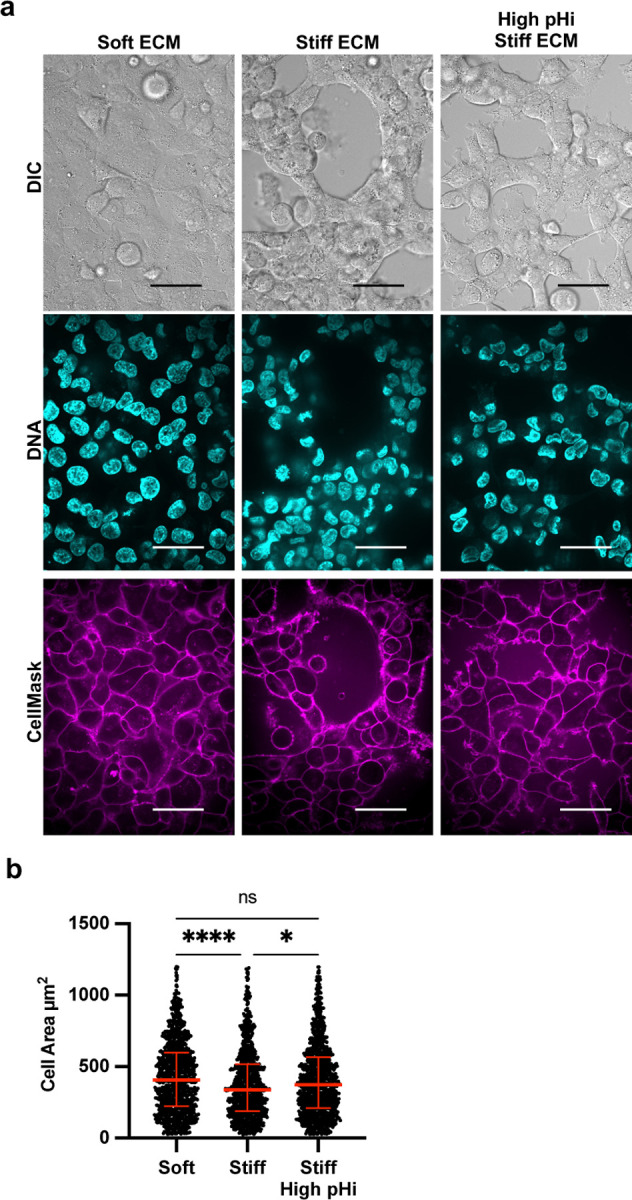
a) Representative images of H1299 cells plated on soft (0.5% PEGDA) and stiff (4% PEGDA) HA gels and stiff (4% PEGDA) with raised pHi. Images show differential interference contrast (DIC), Hoechst 33342 (DNA, cyan) and CellMask Deep Red membrane stain (Cy5, magenta). Scale bars: 50 μm. b) Quantification of single-cell area collected as shown in (a) (n=3 biological replicates, n=1061 soft, n=954 stiff, n=1078 stiff high pHi. Red lines show medians ± IQR).
β-catenin abundance is stiffness-dependent, pHi-dependent, and necessary for stiffness-dependent vasculogenic mimicry
We next investigated potential molecular drivers of pH-dependent regulation of VM. In epithelial cells, VM is regulated by several characterized mechanisms, including the activity and abundance of β-catenin42,43. β-catenin is a multifunctional protein involved in cell-cell adhesion and transcription. Previous work has shown that both whole cell abundance and transcriptional activity of β-catenin directly regulate VM42. A recent study using malignant melanoma cells showed that knockdown of β-catenin or silencing of its co-transcriptional activator transcription factor 4 (TCF4) disables VM phenotypes42. Additionally, previous work has shown that increased nuclear localization of β-catenin correlates with VM formation in colon cancer cells43 and is associated with a stiffening ECM in liver cancer cells44. Importantly, ECM stiffening has also been shown to increase whole-cell β-catenin abundance in some cell lines, including human mesenchymal stem cells45,46. While previous studies have demonstrated the role of β-catenin in regulating VM, these studies have not characterized the cellular cues by which a stiff ECM increases β-catenin abundance or nuclear localization.
Our prior work has shown that high pHi reduces stability of β-catenin in normal canine kidney (MDCK) epithelial cells, leading to loss of β-catenin from adherens junctions47. More recently, we have shown that low pHi stabilizes β-catenin and increases the transcriptional activity in MDCK epithelial cells17. Further, this study showed that β-catenin abundance and nuclear localization decreased when pHi was raised, suggesting pHi acts as a rheostat to modulate β-catenin abundance and adhesion and signaling functions17. However, our prior work did not assess pH-dependent β-catenin stability in non-epithelial models and did not characterize the functional consequences of pH-dependent β-catenin stability on cell behaviors. Thus, we next tested the hypothesis that stiffness-associated pHi dynamics modulate VM through regulation of β-catenin abundance in metastatic cancer cell lines.
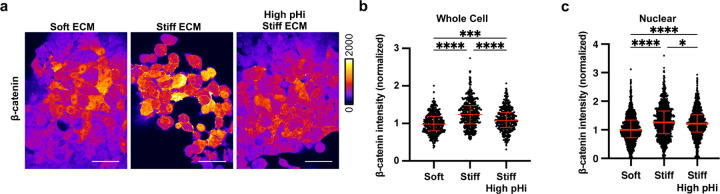
To determine the effect of ECM stiffening on β-catenin abundance in metastatic cancer cells, we performed immunofluorescent staining of β-catenin in H1299 cells plated on soft ECM and stiff ECM both with and without pHi manipulation (Figure 4A). In agreement with prior work46, we found that whole-cell abundance of β-catenin was significantly increased in cells plated on stiff ECM compared to cells plated on soft ECM (Figure 4B). Furthermore, we found that when we raised pHi in cells plated on stiff ECM, β-catenin abundance was significantly reduced compared to control cells plated on stiff ECM (Figure 4B). We also determined the effects of ECM stiffening and pHi modulation on nuclear localization of β-catenin. We quantified the intensity of β-catenin within single cell nuclei and found that β-catenin nuclear abundance was significantly increased on stiff compared to soft ECM (Figure 4C). When pHi was raised in cells plated on a stiff matrix, β-catenin nuclear intensity was significantly decreased compared to cells plated on a stiff matrix in the absence of pHi manipulation (Figure 4C). These data show that increased β-catenin abundance is correlated with low pHi in a human clonal metastatic cancer cell line and confirm our hypothesis that β-catenin is a pH-dependent regulator of stiffness-dependent vasculogenic mimicry. These data show that high pHi can override mechanosensing by decreasing β-catenin abundance, suggesting that low pHi functions as a necessary mediator of VM in cancer cells via stabilization of β-catenin abundance.
Figure 4: Increased pHi reduced β-catenin abundance and nuclear localization in stiff matrix conditions.
a) Representative images of H1299 cells plated on soft (0.5% PEGDA), stiff (4% PEGDA) and stiff with raised pHi (4% PEGDA) HA gels fixed and stained for β-catenin. β-catenin is pseudocolored according to scale. Scale bars: 50 μm. b) Quantification of whole cell β-catenin intensity collected as shown in (a). (n=3 biological replicates, n=452 soft, n=486 stiff, n=415 stiff high pHi. Red lines show medians ± IQR). c) Quantification of nuclear β-catenin intensity collected as described in (a). (n=3 biological replicates, n=1043 soft, n=975 stiff, n=1157 stiff high. Red lines show medians ± IQR). For (b) and (c), significance was determined by a Kruskal-Wallis test (*P<0.05; **P<0.01; ***P<0. 001; ****P<0.0001).
FOXC2 activity is stiffness-dependent, pHi-independent, and not sufficient for vasculogenic mimicry
It is possible that other molecular regulators of VM also exhibit pHi-sensitive activity and contribute to the abrogation of stiffness-associated VM phenotypes at high pHi. Increased expression and activity of the transcription factor FOXC2 has previously been shown to promote VM in ovarian cancer41 and breast cancer cells48 by upregulating expression of VM associated genes. However, while FOXC2 has been shown to be required for VM48, and sufficient to drive endothelial cell vascularization49, it is unclear whether FOXC2 abundance or activity is a sufficient driver of VM phenotypes. Furthermore, existing literature is conflicting as to whether FOXC2 and β-catenin are independent drivers of VM. For example, significant prior data suggests that β-catenin functions upstream of FOXC2 in VM, with β-catenin being shown to directly control expression of FOX transcription factors49. However, other data suggests that FOXC2 can directly induce Wnt signaling50,51 and rescues acquisition of vasculogenic mimicry when β-catenin levels are reduced49. Our data showing that β-catenin is a pH-dependent molecular mediator of VM allows us to explore both independence and crosstalk between FOXC2 and β-catenin in regulating stiffness- and pH-dependent VM phenotypes.
We first measured FOXC2 abundance and activity in our model of metastatic lung cancer cells that form VM phenotypes. We performed immunofluorescent staining of FOXC2 in H1299 cells plated on soft ECM and stiff ECM and found that whole-cell abundance of FOXC2 was the same in H1299 cells plated on soft vs. stiff matrix (Supplemental Figure 5A,B). This suggests that whole cell abundance of FOXC2 is not regulated by ECM stiffness in these cells. We next measured FOXC2 transcriptional activity in single cells. We performed single-cell analysis of FOXC2 transcriptional activity using a highly specific FOXC2-TAG-Puro reporter plasmid with FOXC2 specific tandem repeats flanking a core DNA binding element upstream of GFP (LipExoGen, see methods). Increased FOXC2 DNA binding and transcription drives increased GFP fluorescence (Supplemental Figure 5C). We performed these single-cell transcriptional assays in H1299 cells plated on soft ECM and on stiff ECM with and without increased pHi. We found that FOXC2 activity was significantly increased in cells plated on stiff ECM compared to cells plated on soft ECM, suggesting that ECM stiffening is sufficient to increase FOXC2 transcriptional activity (Supplemental Figure 3D, E). However, we found that FOXC2 transcriptional activity was not altered when pHi was increased on a stiff matrix (Supplemental Figure 3D,E). This result demonstrates that high pHi does not decrease FOXC2 activity in cells on a stiff matrix, suggesting that pHi dynamics do not override stiffness-driven increases in FOXC2 activity. Furthermore, our data show that high FOXC2 transcriptional activation is not a sufficient driver of VM, as high pHi abrogates VM phenotypes without altering FOXC2 transcriptional activity. Our data also suggest that β-catenin loss at high pHi overrides VM independently of FOXC2 activity, reducing VM phenotypes even while FOXC2 transcription remains high.
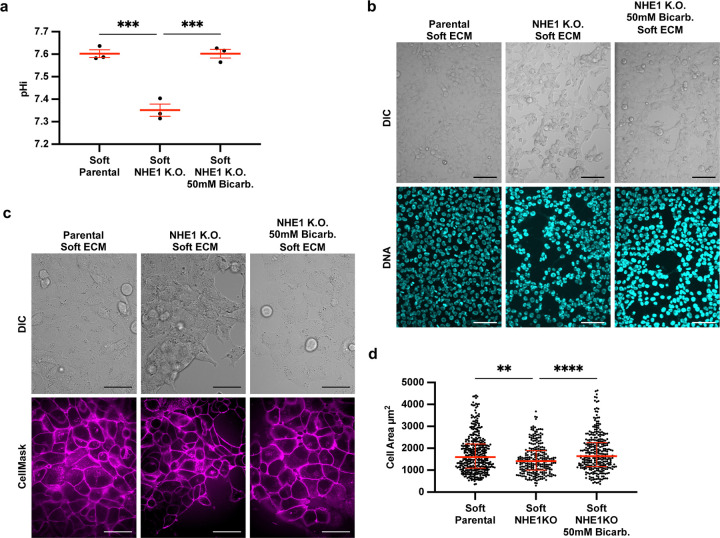
The prior results suggest that increased pHi can override stiffness-associated VM phenotypes. We next hypothesized that low pHi is a sufficient mediator of VM and that lowering pHi in H1299 cells plated on soft matrix would induce stiffness-independent acquisition of VM phenotypes. To directly test this hypothesis, we used an H1299 cell line that is deficient in the sodium proton exchanger (H1299-NHE1 K.O., see methods). This H1299-NHE1 K.O. cell line has significantly decreased pHi (7.35±0.04) compared to parental H1299 (7.60±0.03) (Figure 5A). Importantly, incubating the H1299-NHE1 K.O. cell line with bicarbonate raised pHi to the pHi of parental H1299 (7.62±0.04) Figure 5A). We performed an acid load recovery assay to confirm that the H1299-NHE1 K.O. cell line had no measurable NHE1 activity (Figure S6A). Using this experimental system, we tested the effects of decreased pHi on modulating VM phenotypes. We found that the H1299-NHE1 K.O. cells acquired a VM phenotype on soft matrix, suggesting low pHi is indeed a sufficient driver of VM in the absence of stiff ECM mechanical cues (Figure 5B). Importantly, the stiffness-independent VM phenotype observed in H1299-NHE1 K.O. cells on soft ECM was abrogated when pHi was increased in these cells on the soft matrix (Figure 5B). We again used cell area to quantify extent of VM phenotype and found that when pHi is lowered (H1299-NHE1 K.O.) in cells plated on soft ECM, single-cell area is significantly decreased compared to when the same cells plated on soft ECM but were manipulated to have a high pHi (Figure 5C). Our findings demonstrate that decreased pHi is sufficient to drive VM phenotype in the absence of stiffening ECM mechanical cues. Again, we confirm that increasing pHi is sufficient to override VM phenotypes, even when VM is aberrantly generated on soft ECM.
Figure 5: Low pHi is sufficient to induce vasculogenic mimicry on a soft ECM.
a) Quantification of pHi data from parental H1299 cells and H1299 cells where NHE1 knockout via CRISPR (H1299-NHE1 K.O.). with and without treatment with sodium bicarbonate (Bicarb.) (see methods) (n=3 biological replicates. n=9 parental, n=18 NHE1 K.O., n=18 NHE1 K.O. Bicarb. Red lines show means ± SEM). b) Representative images of H1299 cells plated on soft HA gels (0.5% PEGDA) with and without lowered pHi (H1299-NHE1 K.O.) and with or without increased pHi (H1299-NHE1 K.O. Bicarb.). Images show differential interference contrast (DIC) and Hoechst 33342 (DNA, cyan). Scale bars: 100 μm. c) Representative images of H1299 cells plated on soft HA gels (0.5% PEGDA) with and without lowered pHi (H1299-NHE1 K.O.) and with or without increased pHi (H1299-NHE1 K.O. Bicarb.). Images show differential interference contrast (DIC) and CellMask Deep Red membrane stain (Cy5, magenta). Scale bars: 50 μm. d) Quantification of single-cell area collected as shown in (a) (n=3 biological replicates, n=383 parental, n=267 NHE1 K.O., n=315 NHE1 K.O. Bicarb. Red lines show medians ± IQR).
Discussion
Our work identifies pHi dynamics as a previously unrecognized regulator of stiffness-dependent VM in metastatic cancer cell models. We combined physiologically relevant tunable-stiffness hydrogel systems with single-cell pHi imaging and quantitative microscopy approaches to reveal novel molecular integration of the extracellular mechanical environment and pHi. We show that increasing ECM stiffness, driven by either increased protein concentration or crosslinking, lowers the single-cell pHi of both lung and breast metastatic cell lines. Most previously described tumorigenic behaviors such as hyperplasia52, metastatic progression34,53, and drug resistance53 are associated with increased pHi. However, recent work suggests a potential role for comparatively low cancer cell pHi in regulating hypoxia response54 and modulating tumor initiating cell (or tumor stem cell) phenotypes14. Adding to these recent data, our new findings show that low pHi in cancer cells is both a necessary and sufficient driver of VM. When we raise pHi in cells plated on stiff ECM, we attenuate VM phenotypes, overriding mechanosensitive regulation of VM. More surprisingly, low pHi was sufficient to drive formation of VM phenotypes on soft ECM (in the absence of mechanical stiffening).
Our work characterizing pH-dependent molecular drivers of VM identified β-catenin as a pH-sensitive regulator of vasculogenic mimicry. We also show that another VM regulator, FOXC2 has stiffness-dependent, but pHi-independent, activity. Our molecular characterization of VM regulators using tunable-stiffness hydrogels in combination with pHi manipulation approaches also resolved conflicting data in the literature on the interdependence of β-catenin and FOXC2 in VM regulation. Importantly, our data suggest that β-catenin is a necessary regulator of stiffness-dependent VM and that FOXC2 transcriptional activation is not sufficient to drive VM in the absence of stabilized β-catenin.
Our data reveal that pHi is a master regulator of VM and can override mechanosensitive phenotypes in 2D, revealing an improved understanding of molecular mechanisms driving cancer cell adaptive behaviors in the context of a stiffening ECM. In this work, we limit our characterization to 2D ECM models and a few metastatic cell models and focus on just one tumorigenic mechanosensitive phenotype (VM). This approach enables us to combine single-cell pHi measurements and pHi manipulation with tunable-stiffness hydrogel systems that enable differentiation of contributions of stiffness and pHi in these complex cell morphology phenotypes. Our findings provide the groundwork for future experiments investigating pHi-dependent mechanosensitive behaviors in more complex 3D tumor spheroid models or even co-culture models with cancer associated fibroblasts or immune cells. Prior work has already independently shown that more complex 3D environments produce increased vasculogenic mimicry and phenotypic heterogeneity55 and pronounced pHi gradients56. Our current findings motivate expanding these studies to more complex mechanical and cellular environments to explore mechanistic roles for pHi dynamics in regulating other mechanosensitive tumorigenic behaviors such as durotaxis, invasion, and phenotypic plasticity (or dedifferentiation).
Methods
Cell Culture
H1299 (parental ATCC CRL-5803) or H1299-NHE1 K.O. (CRISPRed cell line was a gift from Dr. Diane Barber at the University of California, San Francisco) were grown in RPMI 1640 (Corning, 10–040-CV) supplemented with 10% Fetal Bovine Serum (FBS, Peak Serum, PS-FB2).
MDA-MB-231 (ATCC HTB-26) cells were grown in DMEM (Corning, MT10013CVV) supplemented with 10% FBS. All cells were maintained at 5% CO2 and 37°C in a humidified incubator. To increase pHi, cells were cultured under normal conditions for 24 hours before being treated for 24 hours with culture media supplemented to a final concentration of 50mM Sodium Bicarbonate (Sigma-Aldrich; S6297–250G).
Transient Expression and Stable Cell Line Generation
H1299 and MDA-MB-231 mCherry-pHluorin expressing cells were generated as previously described34. FOXC2-TAG-Puro (LipExoGen Biotech, SKU:LTV-0061) positive H1299 cells were generated using lentiviral article transduction. Briefly, H1299 cells were plated at 50% confluency in a 6 well tissue culture treated plate. After 24 hours, media was replaced with fresh media containing 10ug/mL of polybrene and 50uL/well of FOXC2-TAG-Puro lentiviral particles. Cells were incubated for 72 hours prior to selection with 0.8 mg/mL blasticidin (Thermo Fisher Scientific, BP264725). After 4 weeks of selection, GFP positive cells were sorted on a BD FACS ARIA III cell sorter using 488nm excitation with 515nm-545nm emission filter. These cells were collected into 1mL 1XPBS using high purity sort settings. Cells were then centrifuged and plated in complete RPMI media with 0.8 mg/mL blasticidin.
Preparation of tunable-stiffness hydrogels
Matrigel or Geltrex gel systems
Matrigel (Corning 356231, Lot 9035003) or Geltrex (Gibco, A14132–02, add LOT) coated plates were made in 35 mm diameter, 4-well (9.5 mm/well) glass bottom dishes (Matsunami, D141400). Stock Matrigel or Geltrex (12 or 16 mg/mL respectively) were diluted in cold complete media to concentrations of 4 mg/mL, 6 mg/mL, and 8 mg/mL which cover a range of stiffness from 50 Pa to ~1000 Pa30–32. Each well was coated with 2.6 µL matrix per mm of well surface area (25 µL/well for 9.5 mm 4-well plate). Matrix was allowed to solidify at 37°C for 20 minutes prior to cell plating. Cells were plated at 5,000 cells per well in 100 µL solution volume.
HA gel system
HyStem-C (Advanced BioMatrix GS313) gels are composed of thiol-modified hyaluronic acid (Glycosil, GS222F), thiol-modified gelatin (Gelin-S, GS231F), polyethylene glycol diacrylate (PEGDA, Extralink, GS3007F), and degassed, deionized water (DG Water)22,30. Basement matrix solution was made of 1:1 Glycosil and Gelin-S and varying final PEGDA percentages (0.5, 1, 2, and 4%) were prepared in degassed, deionized water. The basement matrix solution and respective percentage PEGDA were mixed in a 4:1 parts ratio immediately before plating. Each well was coated with 1.4 µL matrix per mm of well surface area (13.5 µL/well). Cells were plated on the pre-prepared synthetic ECM plates 48 hours prior to imaging at 5,000 (single-cell pHi measurements) or 75,000 (VM imaging/staining) cells/well in 100 µL solution volume. HA gels were pre-prepared a maximum of 3 days prior to plating of cells, and stored with Dulbecco’s phosphate buffered saline (DPBS) (Quality Biological, 114–057-101) in each well to maintain hydration at 4°C.
Microscope System
Confocal images were collected on a Nikon Ti-2 spinning disk confocal with a 10x (PLAN APO NA0.45) air objective, 40x (CFI PLAN FLUOR NA1.3) oil immersion objective, and 60x (PLAN APO NA1.4) oil immersion objective. The microscope is equipped with a stage-top incubator (Tokai Hit), a Yokogawa spinning disk confocal head (CSU-X1), four laser lines (405 nm (100 mW), 488 nm (100 mW), 561 nm (100 mW), 640 nm (75 mW)), a Ti2-S-SE motorized stage, multi-point perfect focus system, and an Orca Flash 4.0 CMOS camera. Images were acquired under the following settings: pHluorin (GFP) (488 laser excitation, 525/36 emission), mCherry (561 laser excitation, 630/75 emission), Cy5 (647 nm laser excitation, 705/72 nm emission), Hoechst 33342 Dye (405 nm laser excitation, 455/50 emission) and differential interference contrast (DIC) were used. Acquisition times for each fluorescence channel ranged from 50–600 milliseconds.
Single-cell pHi measurements
Prior to imaging, stage top incubator and microscope objectives were pre heated to 37°C and kept at 5% CO2/95% air. Single-cell pHi measurements were performed as previously described34. Briefly, initial fields of view (FOV) were collected on the cells in their respective media. Two isotonic buffers (25 mM HEPES, 105 mM KCl, 1 mM MgCl2) were prepared and supplemented with 10 μM nigericin (Thermo Fisher Scientific, N1495). For standardization, isotonic buffers were pre-warmed to 37°C and pH of the “Nigericin buffers” was adjusted to ∼6.7 and ∼7.7 (with 1M KOH) (recorded for each biological replicate to the hundredths place). For each standardization point, cells were washed three times consecutively with no waiting time with appropriate Nigericin buffer followed by a 5–7 minute equilibration prior to image acquisition. All required buffer exchanges were carried out on the stage incubator to preserve XY positioning. Multiple Z-planes were collected with the center focal plane maintained using the Perfect Focus System (PFS).
pHi Image Quantification
NIS Analysis Software was used to quantify pHi. All images were background subtracted using a region of interest (ROI) drawn on glass coverslip (determined by DIC). Individual ROIs were drawn for each cell in each condition (initial, high pH nigericin, and low pH nigericin). For each cell ROI, mean pHluorin and mCherry pixel intensities were quantified and pHluorin/mCherry ratios calculated in Microsoft Excel. For each cell, the nigericin standard fluorescence intensity values were used to generate single-cell standard curves where single-cell pHi was back-calculated based on nigericin buffer pH values reported to the hundredths.
Proliferation Assay
H1299 cells were plated at 1,000 cells/well in a 24 well tissue-culture treated plate on pre-prepared matrix (65 µL/well) (see Preparation of tunable-stiffness hydrogels). After 24 and 48 hours of culture, cells were lifted via trypsinization (0.25%, Corning, 25–0530Cl) for 20 minutes and counted by hemocytometer.
Immunofluorescence Staining
Fixed Cell Staining
H1299 cells were plated at 75,000 cells/well in 100 µL solution volume on the pre-prepared synthetic ECM plates. After 48 hours, the media was removed and a 3.7% Formaldehyde (Alfa Aesar, 50–000) solution in DPBS was added to each well and allowed to fix at room temperature for 10 minutes. Cells were washed 3x2 minutes with DPBS before a permeabilization solution (0.1% Triton-X (Fisher Scientific, 9002–93-1) in DPBS) was added to each well for ten minutes at room temperature (RT). The Triton-X permeabilization solution was removed and cells were washed 3x2 minutes with DPBS at RT before a blocking solution (1% BSA (Fisher Scientific, BP1600–100) was added to cells for one hour at RT with rocking. The blocking solution was removed and cells were washed 3x2 minutes with DPBS before primary antibody solutions were added to each well and incubated with rocking at 4°C overnight. Primary antibodies were prepared in 1% BSA with 0.1% Triton-X at 1:50 dilutions. Primary antibodies used were: β-catenin mouse (BD Biosciences, BDB610154) and FOXC2 rabbit (Cell Signaling Technology, 12974S). The following day, primary antibody solutions were removed and cells were washed 3x2 minutes with DPBS before secondary antibodies (Goat anti-mouse IgG (H+L) Cross-Absorbed Secondary Antibody, Alexa Fluor 488; Invitrogen; A-11001, Goat anti-rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 488; Invitrogen; A-11008) were added at 1:1,000 in solution of 1% BSA, 0.1% Triton-X, and Hoechst 33342 (DAPI; Thermo Scientific, cat: 62249 were added to each well (1:20,000) in DPBS and incubated with rocking at RT for one hour. Cells were washed 3x2 minutes with DPBS just prior to imaging on the Nikon Ti-2 spinning disk confocal with a 40x oil immersion objective. Images were captured with multiple Z planes to allow visualization of labeled protein colocalization. After acquisition, IMARIS Software (Bitplane, Oxford Instruments, version 9.5.1) and Nikon Elements Analysis software were used to quantify stained proteins. Nuclear pools of proteins were identified using IMARIS software by generating surfaces based on the DAPI channel that represent individual cell nuclei. Mean intensities for all channels within each nuclear surface were exported and analyzed for statistical significance using GraphPad Prism software. Whole cell protein abundance was determined by drawing regions of interest in Nikon Elements Analysis software of single-cells. Mean intensities for all channels were exported and analyzed for statistical significance using GraphPad Prism software.
Live Cell Staining
H1299 cells were plated on the pre-prepared synthetic ECM plates 48 hours prior to imaging at 75,000 cells/well in 100 µL solution volume. Images were acquired as outlined in the above sections. Cell nuclei and cell membranes were visualized via Hoechst dye (DAPI; Thermo Scientific, cat: 62249; 1:10,000) and CellMask Deep Red (Thermo Fisher, C10046; 1:20,000), respectively, incubated for 15 minutes at 37⁰ C in complete media. Fields of view were selected by visualizing nuclei (DAPI) and images were collected in the DAPI (30% laser power, 600 ms), GFP (30% laser power, 600 ms), Cy5 (30% laser power, 600 ms), and DIC (32.6 DIA, 50 ms) channels. Individual cells were analyzed by IMARIS software by generating cells based on the CellMask channel that represents cell membranes. Cell areas were exported and analyzed for statistical significance using GraphPad Prism software.
Single-cell FOXC2 transcriptional activity assay using live-cell microscopy
FOXC2-TAG-Puro expressing H1299 cells were plated on the pre-prepared synthetic ECM plates 48 hours prior to imaging at 75,000 cells/well in 100 µL solution volume. Images were acquired as outlined in the above sections. Cell nuclei and cell membranes were visualized via Hoechst dye (DAPI; Thermo Scientific, cat: 62249; 1:10,000) and CellMask Deep Red (Thermo Fisher, C10046; 1:20,000), respectively, incubated for 15 minutes at 37⁰ C in complete media. Fields of view were selected by visualizing nuclei (DAPI) and images were collected in the DAPI (30% laser power, 600 ms), GFP (30% laser power, 600 ms), Cy5 (30% laser power, 600 ms), and DIC (32.6 DIA, 50 ms) channels. Whole-cell regions of interest (ROIs) were drawn within individual cells using cell mask as a membrane marker and the average GFP intensity for individual cells were exported to Excel. Single-cell intensities were imported to GraphPad Prism for statistical analysis and visualization.
BCECF plate reader assays
Cells were plated at 4.0×105–8.0×105 cells/well in a 24-well plate and incubated overnight. Cells were treated with 2 μM 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM; VWR, 89139–244) for 20 min at 37°C and 5% CO2. H1299 parental and NHE1 K.O. cells were washed three times for 5 min each time with a pre-warmed (37°C) HEPES-based wash buffer (30 mM HEPES pH 7.4, 145 mM NaCl, 5 mM KCl, 10 mM glucose, 1 mM MgSO4, 1 mM KHPO4, 2 mM CaCl2, pH 7.4) to match their low bicarbonate medium (RPMI) and NHE1 K.O. Bicarb. cells were washed three times for 5 min each time with a pre-warmed (37°C) HEPES-based wash buffer (30 mM HEPES pH 7.4, 95 mM NaCl, 5 mM KCl, 10 mM glucose, 1 mM MgSO4, 1 mM KHPO4, 2 mM CaCl2, pH 7.4) to match sodium bicarbonate treatment. For standardization, three calibration buffers (25 mM HEPES, 105 mM KCl, 1 mM MgCl2) were supplemented with 10 μM nigericin (Thermo Fisher Scientific, N1495), pH was adjusted to ∼6.7, ~7.0, and ∼7.7, and were pre-warmed to 37°C. Fluorescence was read (excitation of 440 and 490 nm, both with emission at 535 nm) on a Cytation 5 (BioTek) plate reader incubated at 37°C with 5% CO2. Kinetic reads were taken at 15-s intervals for 5 min, using a protocol established within BioTek Gen5 software. After the initial pHi read, the HEPES/bicarbonate wash was aspirated and replaced with one of the nigericin buffer standards, and cells were incubated at 37°C with 5% CO2 for 7 min. BCECF fluorescence was read by the plate reader as above. This process was repeated with the second nigericin standard. As it takes significant time to equilibrate CO2 in the plate reader, we did not measure nigericin standardizations without CO2. The mean intensity ratio (490/440 values) was derived from each read. Measurements were calculated from a nigericin linear regression using exact nigericin buffer pH to two decimal places (Grillo-Hill et al., 2014).
NHE1 Recovery Assay
40,000 cells were plated in the first two rows of a 24-well plate two days prior to transfection (one row of H1299 parental, the other H1299 NHE1 K.O.). Cells were loaded with 10uM SNARF in serum free media and incubated in the dark at 37° C for 30min. Each well was washed three times at 37°C for 5 minutes with a HEPES buffer (30mM HEPES pH-7.4, 115mM NaCl, 5mM KCl, 10mM glucose, 1mM MgSO4, 1mM KHPO4, 2mM CaCl2). The cells were imaged using a BioTek Cytation5 in imager mode. The SNARF was imaged with SNARF cube (531x/586m) and TexasRed cube (586x/647m). Images were taken approximately every two minutes which was the shortest interval allowed by the imager mode software for two rows of a 24-well plate. Initial baseline images were taken in the HEPES buffer at pH 7.4 at 3 time points (approx. 6 mins total). Next, cells were loaded with ammonium chloride using a HEPES-based ammonium chloride buffer (30mM HEPES pH-7.4, 30mM NH4Cl, 115mM NaCl, 5mM KCl, 10mM glucose, 1mM MgSO4, 1mM KHPO4, 2mM CaCl2) and cells were imaged for 3 time points (approx. 6min). An acid load was induced by removing the ammonium chloride buffer and replacing it with the HEPES buffer (no NH4Cl) with or without NHE1 inhibitor (10 µM EIPA (5-(N-ethyl-N-isopropyl) amiloride), Chemscene, CS-7935). Cells were imaged while they recovered (7 time points, approx. 14 minutes). A calibration curve was then obtained by imaging the cells in nigericin containing buffers at the various pH’s, around 7.5 (6 time points, approx. 12min), 7.0 (4 time points, approx. 8min), and 6.5 (4 time points, approx. 8min). The standard curve was then used to back calculate the pHi of the cells during the experiment. The data was normalized to the initial point of the recovery period to look at the recovery rate.
Statistical analysis
GraphPad Prism was used to prepare graphs and perform statistical analyses. All data sets were subject to normality tests (D’Angostino & Pearson, Anderson-Darling, Shapiro-Wilk, and Kolmogorov-Smirnov) and outlier analyses using the ROUT method (Q=1%). For non-normally distributed data, a Kruskal–Wallis test with Dunn’s multiple comparisons correction was used (Figures 1–5) For fold increase in cell number and population pHi data, one-way ANOVA was used (Figure 2E, 5D). All significance was indicated in figures by the following: *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Supplementary Material
Acknowledgements:
We like to thank members of the White lab for their helpful conversations and feedback during figure and manuscript preparation. We also thank Dr. Diane Barber for her generous gift of the H1299-NHE1 K.O. cell line. The spinning disk confocal microscope used in this work is a part of the Notre Dame Integrated Imaging Facility (NDIIF).
Funding:
This work was supported by the Walther Cancer Foundation Interdisciplinary Interface Training Project (to LML), the Henry Luce Foundation (to KAW), Harper Cancer Research Institute (to KAW & DH-P), an NIH Director’s New Innovator Award (1DP2CA260416-01) (to KAW), American Cancer Society Institutional Research Grant (IRG-17-182-04 to DH-P), American Heart Association Career Development Award (19-CDA-34630012 to DH-P), National Institutes of Health (1R35-GM-143055 to DH-P).
Funding Statement
This work was supported by the Walther Cancer Foundation Interdisciplinary Interface Training Project (to LML), the Henry Luce Foundation (to KAW), Harper Cancer Research Institute (to KAW & DH-P), an NIH Director’s New Innovator Award (1DP2CA260416-01) (to KAW), American Cancer Society Institutional Research Grant (IRG-17-182-04 to DH-P), American Heart Association Career Development Award (19-CDA-34630012 to DH-P), National Institutes of Health (1R35-GM-143055 to DH-P).
Footnotes
References
- (1).Walker C.; Mojares E.; del Río Hernández A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19 (10), 3028. 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Carey S. P.; Martin K. E.; Reinhart-King C. A. Three-Dimensional Collagen Matrix Induces a Mechanosensitive Invasive Epithelial Phenotype. Sci. Rep. 2017, 7 (1), 42088. 10.1038/srep42088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wechman S. L.; Emdad L.; Sarkar D.; Das S. K.; Fisher P. B. Vascular Mimicry: Triggers, Molecular Interactions and in Vivo Models. Adv. Cancer Res. 2020, 148, 27–67. 10.1016/bs.acr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Webb B. A.; Chimenti M.; Jacobson M. P.; Barber D. L. Dysregulated pH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11 (9), 671–677. 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- (5).White K. A.; Grillo-Hill B. K.; Barber D. L. Cancer Cell Behaviors Mediated by Dysregulated pH Dynamics at a Glance. J. Cell Sci. 2017, 130 (4), 663–669. 10.1242/jcs.195297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Reshkin S. J.; Greco M. R.; Cardone R. A. Role of pHi, and Proton Transporters in Oncogene-Driven Neoplastic Transformation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369 (1638), 20130100. 10.1098/rstb.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gilkes D. M.; Semenza G. L.; Wirtz D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat. Rev. Cancer 2014, 14 (6), 430–439. 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Bordeleau F.; Mason B. N.; Lollis E. M.; Mazzola M.; Zanotelli M. R.; Somasegar S.; Califano J. P.; Montague C.; LaValley D. J.; Huynh J.; Mencia-Trinchant N.; Negrón Abril Y. L.; Hassane D. C.; Bonassar L. J.; Butcher J. T.; Weiss R. S.; Reinhart-King C. A. Matrix Stiffening Promotes a Tumor Vasculature Phenotype. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (3), 492–497. 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).DuChez B. J.; Doyle A. D.; Dimitriadis E. K.; Yamada K. M. Durotaxis by Human Cancer Cells. Biophys. J. 2019, 116 (4), 670–683. 10.1016/j.bpj.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Nallanthighal S.; Heiserman J. P.; Cheon D.-J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. 10.3389/fcell.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bevensee M. O.; Boron W. F. EFFECTS OF ACUTE HYPOXIA ON INTRACELLULAR-pH REGULATION IN ASTROCYTES CULTURED FROM RAT HIPPOCAMPUS. Brain Res. 2008, 1193, 143–152. 10.1016/j.brainres.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Burbridge M. F.; West D. C.; Atassi G.; Tucker G. C. The Effect of Extracellular pH on Angiogenesis in Vitro. Angiogenesis 1999, 3 (3), 281–288. 10.1023/a:1009092511894. [DOI] [PubMed] [Google Scholar]
- (13).Ulmschneider B.; Grillo-Hill B. K.; Benitez M.; Azimova D. R.; Barber D. L.; Nystul T. G. Increased Intracellular pH Is Necessary for Adult Epithelial and Embryonic Stem Cell Differentiation. J. Cell Biol. 2016, 215 (3), 345–355. 10.1083/jcb.201606042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Liu Y.; White K. A.; Barber D. L. Intracellular pH Regulates Cancer and Stem Cell Behaviors: A Protein Dynamics Perspective. Front. Oncol. 2020, 10, 1401. 10.3389/fonc.2020.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Choi C.-H.; Webb B. A.; Chimenti M. S.; Jacobson M. P.; Barber D. L. pH Sensing by FAK-His58 Regulates Focal Adhesion Remodeling. J. Cell Biol. 2013, 202 (6), 849–859. 10.1083/jcb.201302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Czowski B. J.; Romero-Moreno R.; Trull K. J.; White K. A. Cancer and pH Dynamics: Transcriptional Regulation, Proteostasis, and the Need for New Molecular Tools. Cancers 2020, 12 (10), 2760. 10.3390/cancers12102760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Czowski B. J.; White K. A. Intracellular pH Regulates β-Catenin with Low pHi Increasing Adhesion and Signaling Functions. March 27, 2024. 10.1101/2024.03.22.586349. [DOI] [Google Scholar]
- (18).Benton G.; Arnaoutova I.; George J.; Kleinman H. K.; Koblinski J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- (19).Kim P. K.; Halbrook C. J.; Kerk S. A.; Radyk M.; Wisner S.; Kremer D. M.; Sajjakulnukit P.; Andren A.; Hou S. W.; Trivedi A.; Thurston G.; Anand A.; Yan L.; Salamanca-Cardona L.; Welling S. D.; Zhang L.; Pratt M. R.; Keshari K. R.; Ying H.; Lyssiotis C. A. Hyaluronic Acid Fuels Pancreatic Cancer Cell Growth. eLife 10, e62645. 10.7554/eLife.62645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Deng B.; Zhao Z.; Kong W.; Han C.; Shen X.; Zhou C. Biological Role of Matrix Stiffness in Tumor Growth and Treatment. J. Transl. Med. 2022, 20 (1), 540. 10.1186/s12967-022-03768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Fan F.; Su B.; Kolodychak A.; Ekwueme E.; Alderfer L.; Saha S.; Webber M. J.; Hanjaya-Putra D. Hyaluronic Acid Hydrogels with Phototunable Supramolecular Cross-Linking for Spatially Controlled Lymphatic Tube Formation. ACS Appl. Mater. Interfaces 2023, 15 (50), 58181–58195. 10.1021/acsami.3c12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hanjaya-Putra D.; Yee J.; Ceci D.; Truitt R.; Yee D.; Gerecht S. Vascular Endothelial Growth Factor and Substrate Mechanics Regulate in Vitro Tubulogenesis of Endothelial Progenitor Cells. J. Cell. Mol. Med. 2010, 14 (10), 2436–2447. 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Albeiroti S.; Soroosh A.; de la Motte C. A. Hyaluronan’s Role in Fibrosis: A Pathogenic Factor or a Passive Player? BioMed Res. Int. 2015, 2015, e790203. 10.1155/2015/790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Nemec S.; Ganda S.; Al Taief K.; Kopecky C.; Kuchel R.; Lebhar H.; Marquis C. P.; Thordarson P.; Kilian K. A. A Tunable Tumor Microenvironment through Recombinant Bacterial Collagen-Hyaluronic Acid Hydrogels. ACS Appl. Bio Mater. 2022. 10.1021/acsabm.2c00186. [DOI] [PubMed] [Google Scholar]
- (25).Alderfer L.; Russo E.; Archilla A.; Coe B.; Hanjaya-Putra D. Matrix Stiffness Primes Lymphatic Tube Formation Directed by Vascular Endothelial Growth Factor-C. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35 (5), e21498. 10.1096/fj.202002426RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Piersma B.; Hayward M.-K.; Weaver V. M. Fibrosis and Cancer: A Strained Relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873 (2), 188356. 10.1016/j.bbcan.2020.188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cooper J.; Giancotti F. G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35 (3), 347–367. 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gan Z.; Qin X.; Liu H.; Liu J.; Qin J. Recent Advances in Defined Hydrogels in Organoid Research. Bioact. Mater. 2023, 28, 386–401. 10.1016/j.bioactmat.2023.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Pickup M. W.; Mouw J. K.; Weaver V. M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014, 15 (12), 1243–1253. 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Vanderhooft J. L.; Alcoutlabi M.; Magda J. J.; Prestwich G. D. Rheological Properties of Cross-Linked Hyaluronan-Gelatin Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9 (1), 20–28. 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mammoto A.; Connor K. M.; Mammoto T.; Yung C. W.; Huh D.; Aderman C. M.; Mostoslavsky G.; Smith L. E. H.; Ingber D. E. A Mechanosensitive Transcriptional Mechanism That Controls Angiogenesis. Nature 2009, 457 (7233), 1103–1108. 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Slater K.; Partridge J.; Nandivada H.; Usa M. Tuning the Elastic Moduli of Corning® Matrigel® and Collagen I 3D Matrices by Varying the Protein Concentration. [Google Scholar]
- (33).Soofi S. S.; Last J. A.; Liliensiek S. J.; Nealey P. F.; Murphy C. J. The Elastic Modulus of Matrigel™ as Determined by Atomic Force Microscopy. J. Struct. Biol. 2009, 167 (3), 216–219. 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Spear J. S.; White K. A. Single-Cell Intracellular pH Dynamics Regulate the Cell Cycle by Timing the G1 Exit and G2 Transition. J. Cell Sci. 2023, 136 (10), jcs260458. 10.1242/jcs.260458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Koivusalo M.; Welch C.; Hayashi H.; Scott C. C.; Kim M.; Alexander T.; Touret N.; Hahn K. M.; Grinstein S. Amiloride Inhibits Macropinocytosis by Lowering Submembranous pH and Preventing Rac1 and Cdc42 Signaling. J. Cell Biol. 2010, 188 (4), 547–563. 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Grillo-Hill B. K.; Webb B. A.; Barber D. L. Ratiometric Imaging of pH Probes. Methods Cell Biol. 2014, 123, 429–448. 10.1016/B978-0-12-420138-5.00023-9. [DOI] [PubMed] [Google Scholar]
- (37).Liu Y.; Reyes E.; Castillo-Azofeifa D.; Klein O. D.; Nystul T.; Barber D. L. Intracellular pH Dynamics Regulates Intestinal Stem Cell Lineage Specification. Nat. Commun. 2023, 14 (1), 3745. 10.1038/s41467-023-39312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Martin C.; Pedersen S. F.; Schwab A.; Stock C. Intracellular pH Gradients in Migrating Cells. Am. J. Physiol.-Cell Physiol. 2011, 300 (3), C490–C495. 10.1152/ajpcell.00280.2010. [DOI] [PubMed] [Google Scholar]
- (39).Williamson S. C.; Metcalf R. L.; Trapani F.; Mohan S.; Antonello J.; Abbott B.; Leong H. S.; Chester C. P. E.; Simms N.; Polanski R.; Nonaka D.; Priest L.; Fusi A.; Carlsson F.; Carlsson A.; Hendrix M. J. C.; Seftor R. E. B.; Seftor E. A.; Rothwell D. G.; Hughes A.; Hicks J.; Miller C.; Kuhn P.; Brady G.; Simpson K. L.; Blackhall F. H.; Dive C. Vasculogenic Mimicry in Small Cell Lung Cancer. Nat. Commun. 2016, 7, 13322. 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Racordon D.; Valdivia A.; Mingo G.; Erices R.; Aravena R.; Santoro F.; Bravo M. L.; Ramirez C.; Gonzalez P.; Sandoval A.; González A.; Retamal C.; Kogan M. J.; Kato S.; Cuello M. A.; Osorio G.; Nualart F.; Alvares P.; Gago-Arias A.; Fabri D.; Espinoza I.; Sanchez B.; Corvalán A. H.; Pinto M. P.; Owen G. I. Structural and Functional Identification of Vasculogenic Mimicry in Vitro. Sci. Rep. 2017, 7 (1), 6985. 10.1038/s41598-017-07622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Schnellmann R.; Ntekoumes D.; Choudhury M. I.; Sun S.; Wei Z.; Gerecht S. Stiffening Matrix Induces Age‐Mediated Microvascular Phenotype Through Increased Cell Contractility and Destabilization of Adherens Junctions. Adv. Sci. 2022, 9 (22), 2201483. 10.1002/advs.202201483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Delgado-Bellido D.; Zamudio-Martínez E.; Fernández-Cortés M.; Herrera-Campos A. B.; Olmedo-Pelayo J.; Perez C. J.; Expósito J.; de Álava E.; Amaral A. T.; Valle F. O.; Diaz A. G.; Oliver F. J. VE-Cadherin Modulates β-Catenin/TCF-4 to Enhance Vasculogenic Mimicry. Cell Death Dis. 2023, 14 (2), 135. 10.1038/s41419-023-05666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Qi L.; Song W.; Liu Z.; Zhao X.; Cao W.; Sun B. Wnt3a Promotes the Vasculogenic Mimicry Formation of Colon Cancer via Wnt/β-Catenin Signaling. Int. J. Mol. Sci. 2015, 16 (8), 18564–18579. 10.3390/ijms160818564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Xu X.; Zhang Y.; Wang X.; Li S.; Tang L. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int. J. Mol. Sci. 2021, 22 (21), 12066. 10.3390/ijms222112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Astudillo P. Extracellular Matrix Stiffness and Wnt/β-Catenin Signaling in Physiology and Disease. Biochem. Soc. Trans. 2020, 48 (3), 1187–1198. 10.1042/BST20200026. [DOI] [PubMed] [Google Scholar]
- (46).Sun M.; Chi G.; Xu J.; Tan Y.; Xu J.; Lv S.; Xu Z.; Xia Y.; Li L.; Li Y. Extracellular Matrix Stiffness Controls Osteogenic Differentiation of Mesenchymal Stem Cells Mediated by Integrin Α5. Stem Cell Res. Ther. 2018, 9 (1), 52. 10.1186/s13287-018-0798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).White K. A.; Grillo-Hill B. K.; Esquivel M.; Peralta J.; Bui V. N.; Chire I.; Barber D. L. β-Catenin Is a pH Sensor with Decreased Stability at Higher Intracellular pH. J. Cell Biol. 2018, 217 (11), 3965–3976. 10.1083/jcb.201712041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Cannell I. G.; Sawicka K.; Pearsall I.; Wild S. A.; Deighton L.; Pearsall S. M.; Lerda G.; Joud F.; Khan S.; Bruna A.; Simpson K. L.; Mulvey C. M.; Nugent F.; Qosaj F.; Bressan D.; Dive C.; Caldas C.; Hannon G. J. FOXC2 Promotes Vasculogenic Mimicry and Resistance to Anti-Angiogenic Therapy. Cell Rep. 2023, 42 (8). 10.1016/j.celrep.2023.112791. [DOI] [PubMed] [Google Scholar]
- (49).Cha B.; Geng X.; Mahamud M. R.; Fu J.; Mukherjee A.; Kim Y.; Jho E.-H.; Kim T. H.; Kahn M. L.; Xia L.; Dixon J. B.; Chen H.; Srinivasan R. S. Mechanotransduction Activates Canonical Wnt/β-Catenin Signaling to Promote Lymphatic Vascular Patterning and the Development of Lymphatic and Lymphovenous Valves. Genes Dev. 2016, 30 (12), 1454–1469. 10.1101/gad.282400.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Koch S. Regulation of Wnt Signaling by FOX Transcription Factors in Cancer. Cancers 2021, 13 (14), 3446. 10.3390/cancers13143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Gozo M. C.; Aspuria P.-J.; Cheon D.-J.; Walts A. E.; Berel D.; Miura N.; Karlan B. Y.; Orsulic S. Foxc2 Induces Wnt4 and Bmp4 Expression during Muscle Regeneration and Osteogenesis. Cell Death Differ. 2013, 20 (8), 1031–1042. 10.1038/cdd.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Grillo-Hill B. K.; Choi C.; Jimenez-Vidal M.; Barber D. L. Increased H+ Efflux Is Sufficient to Induce Dysplasia and Necessary for Viability with Oncogene Expression. eLife 2015, 4, e03270. 10.7554/eLife.03270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Amith S. R.; Wilkinson J. M.; Fliegel L. Assessing Na+/H+ Exchange and Cell Effector Functionality in Metastatic Breast Cancer. Biochim. Open 2016, 2, 16–23. 10.1016/j.biopen.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Hulikova A.; Harris A. L.; Vaughan-Jones R. D.; Swietach P. Regulation of Intracellular pH in Cancer Cell Lines under Normoxia and Hypoxia. J. Cell. Physiol. 2013, 228 (4), 743–752. 10.1002/jcp.24221. [DOI] [PubMed] [Google Scholar]
- (55).Konen J.; Summerbell E.; Dwivedi B.; Galior K.; Hou Y.; Rusnak L.; Chen A.; Saltz J.; Zhou W.; Boise L. H.; Vertino P.; Cooper L.; Salaita K.; Kowalski J.; Marcus A. I. Image-Guided Genomics of Phenotypically Heterogeneous Populations Reveals Vascular Signalling during Symbiotic Collective Cancer Invasion. Nat. Commun. 2017, 8, 15078. 10.1038/ncomms15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Andersen A. P.; Flinck M.; Oernbo E. K.; Pedersen N. B.; Viuff B. M.; Pedersen S. F. Roles of Acid-Extruding Ion Transporters in Regulation of Breast Cancer Cell Growth in a 3-Dimensional Microenvironment. Mol. Cancer 2016, 15 (1), 45. 10.1186/s12943-016-0528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.