Abstract
All bacteria encode a multifunctional DNA-binding protein, DnaA, which initiates chromosomal replication. Despite having the most complex, segmented bacterial genome, little is known about Borrelia burgdorferi DnaA and its role in maintaining the spirochete’s physiology. In this work we utilized inducible CRISPR-interference and overexpression to modulate cellular levels of DnaA to better understand this essential protein. Dysregulation of DnaA, either up or down, increased or decreased cell lengths, respectively, while also significantly slowing replication rates. Using fluorescent microscopy, we found the DnaA CRISPRi mutants had increased numbers of chromosomes with irregular spacing patterns. DnaA-depleted spirochetes also exhibited a significant defect in helical morphology. RNA-seq of the conditional mutants showed significant changes in the levels of transcripts involved with flagellar synthesis, elongation, cell division, virulence, and other functions. These findings demonstrate that the DnaA plays a commanding role in maintaining borrelial growth dynamics and protein expression, which are essential for the survival of the Lyme disease spirochete.
Introduction.
Replication plays a significant role in the enzootic lifecycle of Borrelia burgdorferi, the Lyme disease spirochete. An extracellular bacterial parasite, B. burgdorferi exclusively colonizes Ixodid ticks and assorted vertebrates (1, 2). Naïve tick larvae acquire the Lyme bacteria when they feed on an infected host. After this single blood meal, the larvae drop off, overwinter, and molt to their nymphal stage. During this time, B. burgdorferi persists within the midgut of the nutrient-deplete tick. Once the nymph emerges and takes its blood meal, the spirochetes obtain the requisite materials to replicate, divide, and disseminate from the tick to the new vertebrate host (3, 4).
The highly conserved AAA+ family protein DnaA initiates the replication of bacterial chromosomes (5–7). DnaA monomers recognize the oriC locus and cooperatively multimerize to form a helical structure. The DnaA filament promotes the separation of AT-rich DNA elements, which recruits helicase and replication machinery. DnaA not only initiates chromosomal replication but also binds elsewhere in the genome to regulate gene expression (8, 9). We recently found that B. burgdorferi DnaA directly regulates the dnaX-ebfC operon, which codes for the Tau (τ) subunit of DNA polymerase III holoenzyme, DnaX, and a regulatory nucleoid-associated protein, EbfC (10). These genes are highly expressed during periods of rapid spirochete replication (10–12). At the tick-vertebrate interface, we hypothesize that B. burgdorferi DnaA not only commits to its replication initiation function but coordinates the expression of genes needed for vertebrate infection (10, 13, 14).
Replication of the B. burgdorferi linear chromosome proceeds bidirectionally from the centrally-located oriC (15). Outside of this, little is known about borrelial DNA replication, its regulation, or coordination with other cellular processes. Many of the genes involved in those pathways are essential, which has hampered their investigation. Historically, studying essential loci required the development or isolation of conditional mutants. The first studies into bacterial DnaA proteins utilized temperature-sensitive mutants (16). In the era of molecular genetics, many tools have been developed to produce conditional phenotypes more readily, such as inducible promoters. Overexpression has been a reliable means of elucidating a protein’s function in B. burgdorferi and other bacteria (14, 17, 18). Recently, inducible CRISPR interference (CRISPRi) systems have been developed and added to the repertoire of tools for studying borrelial biology (19, 20). This work will describe the consequence of CRISPRi-mediated knockdown of the essential replication initiator protein DnaA in B. burgdorferi. Using this approach, coupled with overexpression, we observed profound consequences of DnaA-dysregulation on spirochete morphology, chromosome partitioning, and gene expression.
RESULTS.
Construction and validation of plasmids to dysregulate levels of DnaA in B. burgdorferi.
DnaA is essential for initiating chromosomal replication, so deleting DnaA is lethal to bacteria. Thus, to gain insight into the impacts of DnaA on borrelial physiology, we produced IPTG-inducible constructs to reduce or elevate cellular levels of DnaA. Knockdown was carried out using the newly refined all-in-one CRISPR interference (CRISPRi) shuttle vector, which places the expression of both the sgRNA and a borrelial codon-optimized dCas9 gene under the control of the Lac repressor (20). We designed two CRISPRi constructs with sgRNAs targeting either the template (dnaAT1) or non-template (dnaANT1) DNA strands directly 5’ or within the dnaA gene (Fig. 1A). Overexpression was achieved using plasmid pACK121, which contains an inducible dnaA with an N-terminal 3xFLAG tag. All constructs were transformed into B. burgdorferi strain B31-e2, hereafter referred to as e2. The spirochetes tolerated each construct well, replicating at rates comparable to the parental strain without the inducer (Fig. 1B), with one exception. The dnaAT1 CRISPRi strain replicated slightly slower than the others, although it reached the same final density. This may suggest leakiness of the hybrid lac promoter that controls the sgRNA and dCas9 gene (B. Murphy & W. Zückert, personal communication).
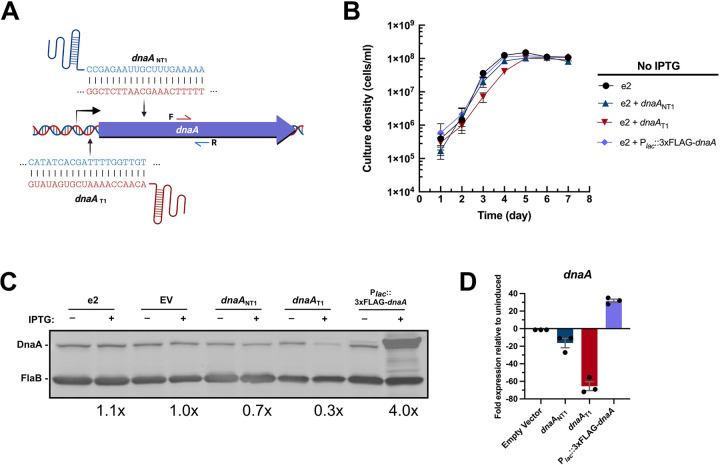
Figure 1. Validation of conditional dysregulation of DnaA.
(A) Two sgRNAs were designed to knock down borrelial dnaA transcription by CRISPRi. One sgRNA targeted the template strand directly upstream of the ORF (dnaAT1), while the other targeted the template strand within the ORF (dnaANT1). (B) The dnaA CRISPRi bacteria, CRISPRi empty vector bacteria, and the strain with the dnaA overexpression plasmid grew at the same rate as the parental e2. Adding IPTG to these strains resulted in appropriate knockdown or overexpression of DnaA protein (C) and transcript (D), as assessed by immunoblot and qRT-PCR. The overexpression shuttle vector encodes a DnaA with an N-terminal 3xFLAG moiety and thus migrates above the native protein. Error bars in (B) and (D) represent the standard error of the mean (SEM).
To assess the efficacy of these inducible constructs, spirochetes of each strain were grown to mid-exponential growth phase (3–5×107 cells/mL), then incubated overnight with 0.5 mM IPTG. The dnaA-targeting CRISPRi strains decreased the expression of DnaA, with the construct targeting the template strand, dnaAT1, consistently yielding the most effective gene silencing (Fig. 1C and D). The potency of the dnaAT1 construct likely explains the slowed spirochete growth seen in the absence of IPTG (Fig. 1B). Overexpression resulted in elevated quantities of DnaA protein and transcript (Fig. 1C and D). Neither the CRISPRi empty vector (EV) nor the parental e2 control strains yielded changes in DnaA levels when IPTG was added, demonstrating that we could specifically and effectively alter cellular DnaA concentrations.
Proper DnaA expression is essential for borrelial growth.
Given its essential nature for chromosomal replication, we first sought to evaluate the consequence of DnaA dysregulation on B. burgdorferi growth and cell division. To achieve this, all strains were grown to mid-exponential phase and passaged into fresh media with 0.5 mM of IPTG at a density of 1×105 cells/mL. Bacterial numbers of each culture were counted every day for seven days to generate growth curves.
CRISPRi knockdown of DnaA levels substantially reduced the generation time and carrying capacity of B. burgdorferi (Fig. 2A and B). The severity of these phenotypes was consistent with the knockdown efficacy of the two constructs, with dnaANT1 cultures reaching a maximum density that was one log lower and dnaAT1 cultures maxing out two logs lower than the controls. This suggests that reducing the cellular concentration of DnaA directly limits the number of division cycles, which is consistent with DnaA’s role as the chromosomal replication initiator.
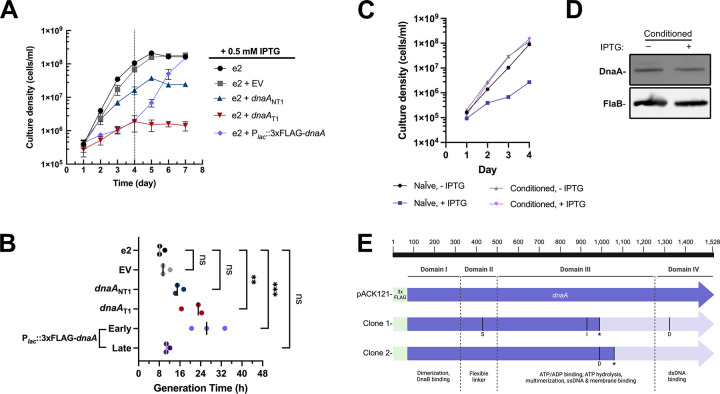
Figure 2. Appropriate expression of DnaA is required for B. burgdorferi replication.
(A) Growth curve studies were conducted on different B. burgdorferi strains incubated with 0.5 mM IPTG. Three independent cultures of each strain were measured for growth curve analysis. Error bars represent the SEM. The dashed line indicates when the overexpression strain resumed growth. (B) The generation time was determined from the growth curve analyses from three individual cultures of each strain. For the overexpression strain, the growth rate was determined before (early; 1–4 dpi) and after the resumption of growth (late; 4–7 dpi). The resumption of replication in that strain was evidently due to mutation of the overexpressed dnaA gene. The p-values are indicated as follows: **, p ≤ 0.01; ***, p ≤ 0.001; ns, not significant, p > 0.05. (C) IPTG-treated DnaA overexpression spirochetes that resumed growth (conditioned) lost sensitivity to IPTG when passaged to fresh media and did not overproduce 3xFLAG-DnaA as assessed by immunoblot (D), supporting the conclusion that accumulated mutations in the overexpression dnaA permitted replication. (E) Sequenced plasmid from these conditioned spirochetes showed unique mutations (S = substitution; I= Insertion; D = deletion) in the 3xFLAG-dnaA ORF that caused a frameshift and truncation of the full-length protein. The location of the truncation is indicated by *.
Overproducing DnaA slowed growth during the first four days post-inoculation (dpi), growing at a rate similar to the dnaAT1 culture (Fig. 2A and B, early). After that time, however, cultures consistently resumed logarithmic growth, yielding generation times that were indistinguishable from the parental strain (Fig. 2B, late). We hypothesized this abrupt resumption of growth was due to mutations in the inducible dnaA plasmid that eliminated overexpression. To test this, we passaged spirochetes that had resumed growth into fresh media with IPTG to see if growth would still be perturbed. Consistent with our hypothesis, these “conditioned” bacteria immediately grew at rates that were similar to the control strain (Fig. 2C), and induction of the 3xFLAG tagged DnaA did not occur (Fig. 2D). To further test our hypothesis, we extracted the DnaA overexpression plasmid from the B. burgdorferi cultures and transformed them into E. coli. We sequenced purified plasmids from two colonies derived from the same induced borrelial culture. Each clone had unique mutations within the dnaA ORF that truncated the full-length protein (Fig. 2E). Notably, both mutations resulted in the complete loss of domain IV, the DNA-binding domain of DnaA. Plasmid isolated from transformed B. burgdorferi, which had never been exposed to IPTG, had no mutations within the inducible dnaA. These escape mutants, taken with the growth dynamics, highlight the stressful nature of dnaA overexpression, which has consistently been observed in other bacterial organisms (21–23).
Unlike the overexpression strain, we never observed a resumption of growth in the induced dnaA CRISPRi strains. Furthermore, normal growth was attainable from knocked-down cultures after passaging spirochetes into fresh media without IPTG. These collective data demonstrate that precisely controlled levels of DnaA are required for optimal borrelial growth.
DnaA affects spirochete cell division/elongation.
Chromosomal replication is intimately tied to cellular elongation and division (24–26). Having found that the conditional DnaA mutants had replication defects, we also sought to assess the impact of DnaA dysregulation on B. burgdorferi cell length. Over seven days, we imaged and measured spirochetes. In the parental e2 cultures, we observed a consistent pattern wherein median cell length peaked during early exponential phase, followed by a steady decrease, then stabilization once the cultures reached stationary phase (Fig. 3A, 3E, and 3I).
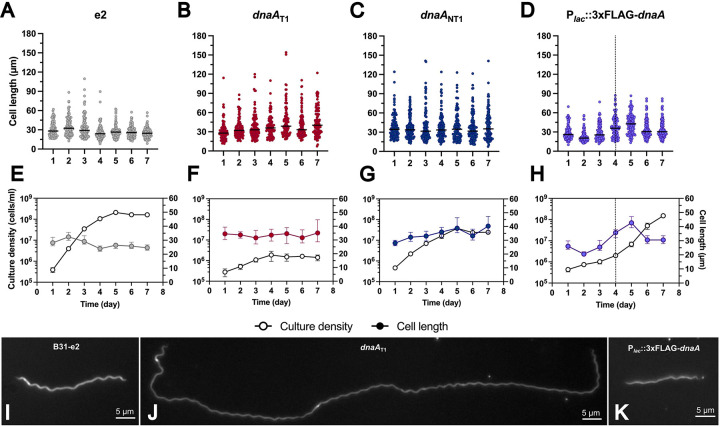
Figure 3. DnaA dysregulation affects spirochete cell length.
(A-D) Cell lengths of the 0.5 mM IPTG-treated B. burgdorferi B31-e2 strains measured over seven days (gray = e2; red = dnaAT1; dnaANT1 = blue; Plac::3xFLAG-dnaA = purple). The line represents the median cell length. (E-H) The median cell length (filled-in circle) with error bars representing the 95% CI superimposed against the corresponding growth curve (empty circle) from Fig. 2. The dashed line indicates when the overexpression strain resumed growth. (I-K) Representative images of observed phenotypes. (I) The e2 cell of approximate median length at 2 dpi. (J) DnaA-depleted cell with the characteristic elongation phenotype. (K) Median size of a DnaA overexpression spirochete at 2 dpi.
These findings sharply contrast with what we observed in the CRISPRi knockdown strains, where the spirochetes steadily maintained median lengths of 30–40 μm (Fig. 3B–C, 3F, 3H, and 3I); the parental e2 strain had median lengths that spanned from 24–32 μm. The ranges (maximum-minimum) of the cell lengths within these knockdown strains (147 μm, dnaAT1; 134 μm, dnaANT1) were greater than that of the overexpression (77 μm) and parental (101 μm) bacteria.
When DnaA was overexpressed, the median cell length decreased by 2 days post-induction (Fig. 3D, 3H, and 3K). As cultures accumulated mutations in the overexpressed dnaA, cell lengths gradually increased to a peak at day 5, then decreased. This oscillation in spirochete length aligned with the observed growth pattern of this strain, where slowed replication corresponded to cells of shorter size and rapid replication to longer. This suggests that high levels of DnaA can diminish borrelial cell length. These results demonstrate that DnaA plays a role in regulating B. burgdorferi cell elongation and/or division and that maintaining proper DnaA levels is critical to these processes.
Impact of DnaA on ploidy and partitioning.
B. burgdorferi is polyploid during exponential growth in vitro (27). As DnaA is the master initiator of chromosomal replication and affects B. burgdorferi morphology, we hypothesized that the ploidy and partitioning of chromosomes could be altered when DnaA levels are dysregulated. To test this, we transformed the parental e2 and dnaAT1 strains with a construct expressing the chromosomal ParB protein fused to mCherry (pBSV2G_P0826-mCherryBb-ParB), which has been demonstrated to bind near oriC (27). We first grew the two strains to mid-exponential phase without IPTG and measured the length and ParB-oriC puncta per cell. The parental bacteria were smaller than those of the uninduced dnaAT1, consistent with our prior observations. Yet, both strains had similar numbers of ParB-oriC (Fig. 4). After taking these measurements, the dnaAT1 culture was split in half, and IPTG was added to one of the cultures. The parental, uninduced, and induced strains were then incubated and examined for three more days.
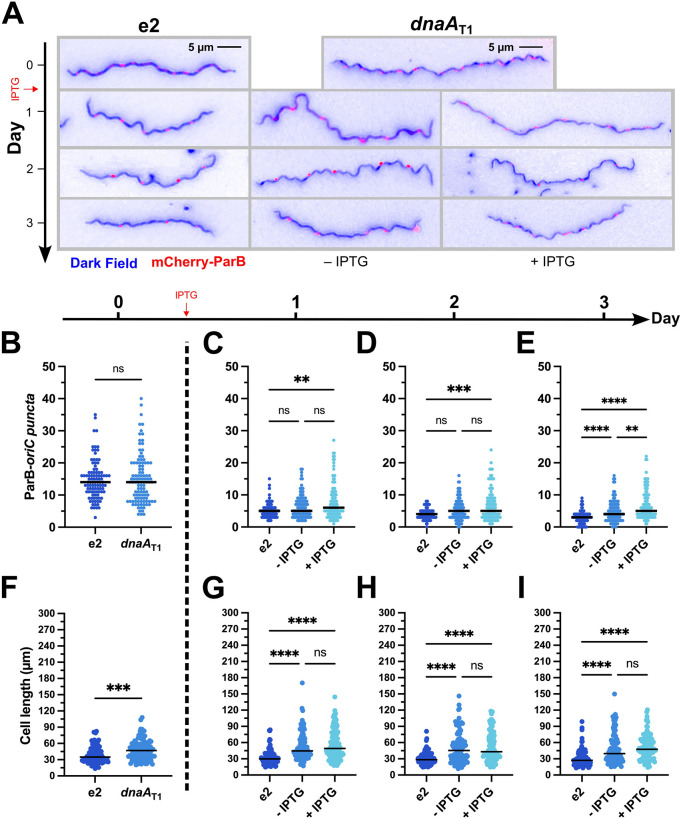
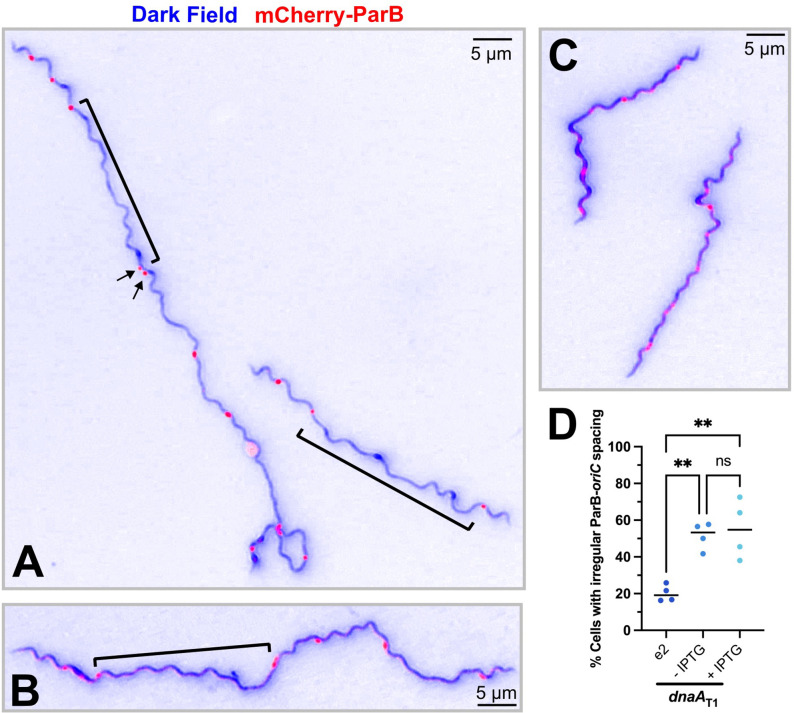
Figure 4. Knockdown of DnaA increases the number of chromosomes per spirochete.
The B. burgdorferi CRISPRi dnaAT1 and parent B31-e2 strains were transformed with a construct encoding a mCherry-tagged ParB protein, which binds near the oriC, to identify the locations of the chromosomes. The two strains were grown without inducer until mid-log (t = 0), and then the dnaAT1 strain was divided into two cultures, and one of those was induced with 0.5 mM IPTG. Cultures were tracked for three more days. (A) Representative merged micrographs (blue = dark field; red = mCherry-ParB) of spirochetes with the approximate median numbers of ParB-oriC puncta (B) and cell length (C). Significant differences in numbers of puncta and cell length at day 0 were determined by the Mann-Whitney U test. Differences between the three groups (e2, - IPTG, and + IPTG) on days 1–3 were tested for by the Kruskal-Wallis test and then Dunn’s test for multiple comparisons. Multiplicity-adjusted p-values are reported. **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; ns, not significant, p > 0.05. Approximately 50 spirochetes were imaged for each strain on each day. Data were gathered from two independent experiments.
On the first day post-induction, as the cultures entered stationary phase (≥1×108 cells/mL), all strains experienced a dramatic decline in the total ParB-oriC puncta per cell, consistent with prior observations (27). Over the next two days, the number of these continued to decrease in the parental and uninduced cultures, whereas the induced culture plateaued and ended with significantly more ParB-oriC puncta. Furthermore, the induced cell lengths, while longer than the parental, were the same as the uninduced cells, indicating the difference in puncta wasn’t simply due to differences in cell length. With this, we concluded that depleting DnaA impairs the completion of borrelial chromosomal replication and cell division.
In addition to differences in the number of ParB-oriC puncta in the dnaA-conditional mutant, we also noted an apparent difference in their spacing (Fig. 5A–B) relative to the parental strain (Fig. 5C). Some cells had wide gaps between the foci, while others had foci that were close together. We assessed the cultures two- and three-days post-induction and found about 23.7% of parental e2 cells had irregular puncta spacing. The dnaAT1 CRISPRi strains, in contrast, had significantly greater proportions of 53.8% and 55.3% of spirochetes with irregular spacing without and with added IPTG, respectively (Fig. 5D). Thus, knocking down DnaA also perturbs chromosomal partitioning.
Figure 5. Irregular spacing of oriC in dnaA knockdown B. burgdorferi.
(A-B) Representative images of spirochetes containing the dnaAT1 CRISPRi plasmid with irregular spacing of ParB-oriC puncta (red). DnaA-depleted cells had large regions where no foci were observed (brackets). Some cells also had foci that were close together (arrows). (C) Parental B31-e2 spirochetes with approximately the same number of ParB-oriC puncta as the mutant but exhibit regular oriC spacing. (D) The number of spirochetes with irregular ParB-oriC was quantified (e2, n = 184; uninduced dnaAT1, n = 198 cells; induced dnaAT1 n = 206). One-way ANOVA with Tukey’s post-hoc test was done to determine the significance and make comparisons. The multiplicity-adjusted p-values are reported **, p ≤ 0.01; ns, not significant, p > 0.05.
Dysregulation of DnaA disrupts flagellar homeostasis.
Depletion of DnaA also resulted in B. burgdorferi cells with aberrant helicity. Specifically, we observed spirochetes with a complete or partial loss of their characteristic corkscrew shape (Fig. 6A and B, respectively). Some of these tube-shaped cells were substantially elongated and showed evidence of incomplete division (Fig. 6C and Supplemental Video). About 13% of the spirochetes in the dnaAT1-induced cultures and 8.7% of the uninduced exhibited abnormal morphologies (Fig. 6D). The parental and empty vector strains, independent of IPTG, did not exhibit such elevated proportions of these cells, indicating that the observed helical abnormalities were due to targeted silencing of the dnaA gene. The high level of abnormal cells in the uninduced dnaAT1 cultures, relative to the controls, can again be explained by leaky expression of the dnaA sgRNA encoded in the CRISPRi construct.
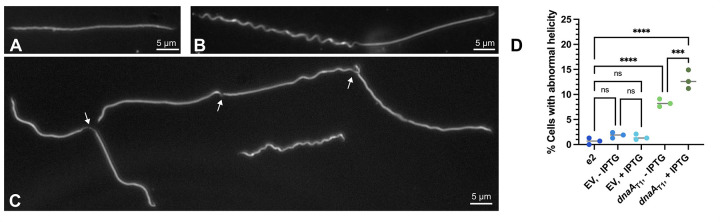
Figure 6. DnaA-depletion affects borrelial helicity.
Representative micrographs of observed phenotypes. When dnaA was knocked down, spirochetes with no (A) or asymmetrical helicity (B) were observed. (C) Some of the abnormal cells were elongated and showed division defects. (D) Spirochetes exhibiting similar defects were observed at a low level in the B31-e2 strain (0.7%, n = 1514). The empty vector (EV) control cultures had a similar proportion, independent of the addition of IPTG (uninduced: 2.1%, n = 1439; induced: 1.6%, n = 1608). The bacteria with the dnaAT1 CRISPRi plasmid had significantly more cells with perturbed helicity that was dependent on induction (uninduced: 8.7%, n = 1437; induced: 13%, n = 1472). Spirochetes were counted from three independent cultures. Statistical significance was determined by one-way ANOVA with a Holm-Šídák’s post-hoc test, and multiplicity-adjusted p-values are reported. ***, p ≤ 0.001, ****, p ≤ 0.0001; ns, not significant, p > 0.05.
The characteristic corkscrew appearance of B. burgdorferi is due to the 7–11 flagella found in the periplasm (28). The motors that direct the movement of the endoflagella are normally localized to the bacterium’s poles (29). The observed impacts on growth and partitioning suggest coordination between replication and flagellar assembly at the sites of division. This is not unheard of. In E. coli, for example, DnaA is known to be involved in flagellar regulation (30, 31).
DnaA is a global regulator of borrelial gene expression.
DnaA is well known to be a transcription factor in many other bacteria, with regulons driving specific phenotypes (9). In B. burgdorferi, we previously demonstrated that DnaA controls the expression of the dnaX-ebfC operon (10). Considering this and the dramatic impacts of DnaA dysregulation on the Lyme spirochete, we queried whether the myriad phenotypes of the conditional mutants are due to DnaA-dependent transcriptional effects. To address this question, we performed RNA-seq on three strains with different levels of DnaA enrichment: wild-type (e2), DnaA-up (e2 + pACK121), and DnaA-down (e2 + dnaAT1). All the strains had the plasmids cp26, lp17, lp54, cp32–1, cp32–3, and cp32–4, as assessed by PCR and whole genome sequencing. The bacteria for RNA-seq analysis were grown to mid-exponential phase, induced with 0.5 mM IPTG, and incubated overnight. We chose this strategy to mitigate the chance of escape-mutant development from confounding the results.
For the analyses of the RNA-seq data, comparisons were made between the three groups. We considered a gene to be differentially expressed if the false discovery rate (FDR) was less than or equal to 0.05 and had a log2FC (fold change) of ≥ 1 or ≤ −1. With these thresholds, 216 genes were noted as being differentially expressed between the wild-type and DnaA-down cultures (72 down, 144 up; Fig. 7A), 84 genes between WT and DnaA-up (40 down, 44 up; Fig. 7B), and 259 between DnaA-down and DnaA-up (105 down, 154 up; Fig. 7C). Principle component analysis showed that the replicates of each strain clustered together (Fig. 7D). Most of the genes that were differentially expressed are encoded on the chromosome: DnaA-down vs. e2: 69.9%; DnaA-up vs. e2: 72.6% (Fig. 7E). Of the plasmids, the linear lp54 replicon had the most impacted genes for the DnaA-down vs. e2 comparison (8.3%), while the circular cp32–1 had the most for the DnaA-up vs e2 comparison (7.1%; Fig. 7E).
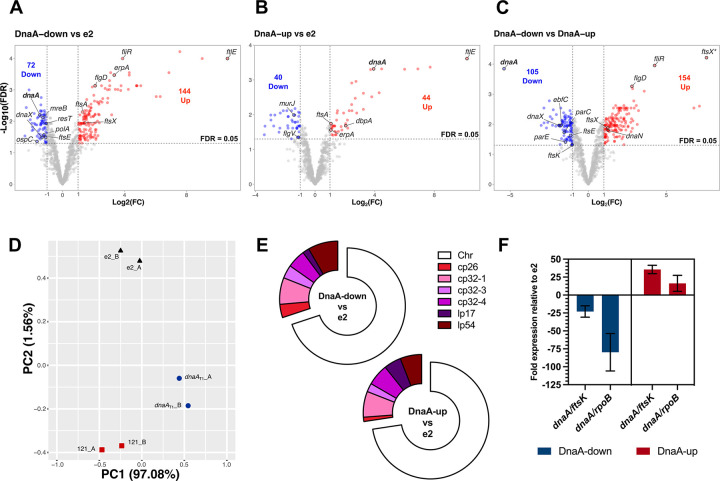
Figure 7. RNA-seq of DnaA-dysregulated B. burgdorferi.
The RNA from three B. burgdorferi strains with different cellular levels of DnaA were sequenced: B31-e2 (parent), CRISPRi dnaAT1 (DnaA-down), and overexpression pACK121 (DnaA-up). (A-C) Volcano plots plotting the fold change (log2FC) against each gene’s false discovery rate (−log10(FDR)). The threshold for significance was set at a log2FC ≥ 1 or ≤ − 1 (≥ 2-fold change) and an FDR of ≤ 0.05. (D) PCA plot showing the clustering of the replicate samples that were sequenced. (E) Donut charts showing the replicons on which the significantly affected genes are encoded. (F) qRT-PCR results of dnaA transcripts in the sequenced samples. Cq values were normalized to ftsK or rpoB (ΔCq) and then to the parental strain (ΔΔCq). Error bars represent the standard deviation (SD).
As expected, the RNA-seq showed that compared to the e2 parent, dnaA transcript was about 2.8-fold less abundant in the CRISPRi strain and 15-fold more abundant in the overexpression strain. Typically, we normalize transcript levels to ftsK, a gene previously observed to have stable expression across growth phases (32). RNA-seq showed that ftsK was impacted by dnaA dysregulation (discussed below). Given this, we performed validations normalizing to rpoB, which did not change under the tested conditions. Using this approach, dnaA transcript was 80-fold less abundant in the knockdown vs. the WT and 16-fold more abundant in the overexpression vs. the WT (Fig. 7E). DnaA protein levels were about half as abundant in the knockdown and 13 times more in overexpression than the WT (Fig. 10B).
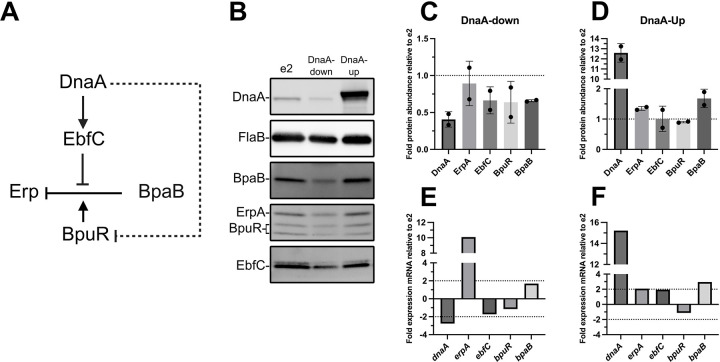
Figure. 10. Impacts of DnaA on the Erp regulatory network.
(A) Solid black lines indicate established interactions. Dashed lines indicate hypothesized interactions. Activation is denoted by lines with arrowheads, and inhibition by lines without arrowheads. Note that co-repressive or anti-repressive activities, i.e., BpuR and EbfC, respectively, are indicated by arrows directed at lines. (B) Representative immunoblots from samples of B. burgdorferi that were analyzed by RNA-seq. Quantitation of target proteins when DnaA was knocked down (C) or overexpressed (D). Band intensities for each replicate immunoblot were normalized to FlaB and then the parental e2 strain. (C-D) The dotted lines at 1 represent where the protein abundance is the same as e2. Bar graphs showing the fold change detected by RNA-seq in the DnaA-down/e2 (E) and DnaA-up comparisons (F). (E-F) The dotted lines indicate the 2-fold threshold for meaningful gene expression changes.
Transcript levels of many genes were most affected when dnaA levels were reduced. We initially hypothesized that transcriptomic profiles between the conditional mutants relative to the parental strain would reciprocally mirror each other. Only one gene fit this hypothesis, BB_0413 (2.2-fold increase for dnaAT1/e2; 2.3-fold decrease for pACK121/e2). Indeed, in many cases, transcript levels for a gene would experience the same degree of change regardless of whether DnaA was increased or decreased. Such instances suggested that some of the observed changes in gene expression were not due to DnaA itself but rather a shared cellular response to the changes in DnaA levels. Despite some of these complications, the RNA-seq data set offered insights that could explain the phenotypes observed in the conditional dnaA-mutant strains.
The earliest interrogations into borrelial DnaA focused on its function as a transcription factor (10, 14). Consistent with our prior in vitro studies, knocking down dnaA significantly decreased transcription of the dnaX-ebfC operon (10). This operon codes for the τ-subunit of the DNA polymerase III holoenzyme (DnaX) and the nucleoid-associated protein, EbfC. Other impacted replication genes included the DNA polymerase III β-clamp (dnaN), DNA polymerase I (polA), DNA topoisomerase IV (parEC), and telomere resolvase (resT). Except for dnaN, these genes were downregulated when dnaA levels were decreased. The upregulation of dnaN, seen when comparing DnaA-Up to DnaA-down conditions, suggests that DnaA represses the expression of this gene. This possibility is unsurprising given that the oriC is directly 5’ of the dnaN ORF, and we have shown that DnaA binds this region (10).
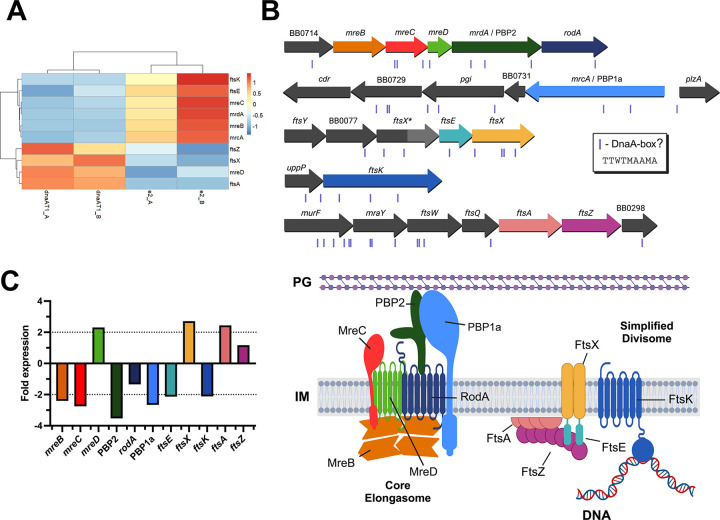
In the dnaA-knockdown B. burgdorferi, we observed cells that were considerably elongated. In our evaluation of the borrelial genome, we identified 22 homologs of genes that encode components of either the bacterial elongasome or divisome (Table 1). Of these, 39.1% were differentially expressed in the dnaAT1 CRISPRi strain (Fig. 8A). Among these were mreB, mreC, mreD, and mrdA, which constitute an apparent operon and encode core components of the bacterial elongasome (Fig. 8B). MreB is an actin-like cytoskeleton protein that polymerizes across the bacterial inner membrane to regulate the spatiotemporal peptidoglycan synthesis of growing cells (33–35). Knockdown of MreB in B. burgdorferi results in bulging and cell widening at the division sites (19). MreC and MreD are membrane-embedded proteins that interact and regulate the peptidoglycan crosslinking activity of PBP2 (penicillin-binding protein 2; MrdA) (36). The peptidoglycan polymerase RodA and PBP1a interact with PBP2, with the former stimulating its activity. Except for mreD, the genes in the apparent mre operon were downregulated in dnaA-deficient B. burgdorferi. A DNA sequence that is the same as those found in the oriC, which may constitute the borrelial DnaA-box, is located upstream of the mreB ORF, suggesting DnaA may directly regulate the expression of these genes. The decreased transcript levels for mreB, mreC, and mrdA would suggest decreased elongation, which is the opposite of what we observed (Fig. 3). This apparent contradiction suggests that filamentation may be due to defects in cell division.
Table 1.
Homologous elongation and division genes of B. burgdorferi.
| RNA-seq Differential Expression | ||||||
|---|---|---|---|---|---|---|
| Protein | Locus Tag | Elongasome | Divisome | DnaA-down vs e2 | DnaA-up vs e2 | DnaA-down vs DnaA-up |
| FtsE | BB_0080 | − | + | Down | NA | Down |
| FtsX | BB_0081 | − | + | Up | NA | Up |
| FtsI/PBP3 | BB_0136 | + | + | NA | NA | NA |
| MurE | BB_0201 | + | + | NA | NA | NA |
| FtsK | BB_0257 | − | + | Down | NA | Down |
| FtsZ | BB_0299 | − | + | NA | NA | NA |
| FtsA | BB_0300 | − | + | Up | Up | NA |
| FtsQ/DivlB | BB_0301 | − | + | NA | NA | NA |
| FtsW | BB_0302 | − | + | NA | NA | NA |
| MraY | BB_0303 | + | + | NA | NA | NA |
| MurF | BB_0304 | + | + | NA | NA | NA |
| MurD | BB_0585 | + | + | NA | NA | NA |
| MurB | BB_0598 | + | + | NA | NA | NA |
| Ami | BB_0666 | − | + | NA | NA | NA |
| MreB | BB_0715 | + | − | Down | NA | Down |
| MreC | BB_0716 | + | − | Down | NA | Down |
| MreD | BB_0717 | + | − | Up | NA | Up |
| PBP2 | BB_0718 | + | − | Down | NA | Down |
| RodA | BB_0719 | + | − | NA | NA | NA |
| PBP1a | BB_0732 | + | − | Down | NA | Down |
| MurG | BB_0767 | + | + | NA | NA | NA |
| MurJ | BB_0810 | + | + | NA | Down | Up |
| MurC | BB_0817 | + | + | NA | NA | NA |
Plus sign (+) indicates the protein is part of the complex, minus sign (−) indicates it is not
NA = Not affected
Figure 8. Impacts of DnaA on B. burgdorferi elongation and division genes.
(A) Heat map of the division and elongation genes that were significantly affected when DnaA was knocked down. (B) Top: Locus maps of the select elongation and division genes to scale. The sites of potential DnaA-boxes are denoted as purple boxes below the genes. Bottom: Simplified schema of the hypothesized borrelial elongasome and divisome containing the highlighted genes of interest. Informed by the work of Liu et al. and Hu et al. (36, 69) (C) Bar graph showing the fold change detected by RNA-seq of the elongation and division genes. The dashed lines indicate the 2-fold threshold for meaningful gene expression changes.
The transcripts of four important divisome proteins were impacted when dnaA was knocked down: FtsA, FtsK, FtsE, and FtsX (Fig. 8A and B). FtsA is the membrane anchor protein for the septation marker protein FtsZ (37, 38), and its transcript increased about 2-fold in the conditional dnaA mutant. Surprisingly, DnaA-overexpressed B. burgdorferi also experienced a 2-fold increase in ftsA. Transcripts for FtsK, the DNA translocase, declined 2-fold. FtsE and FtsX, encoded next to each other, had their transcripts decreased 2-fold and increased almost 3-fold, respectively. Interestingly, a second allele of ftsX (ftsX*), which is truncated, is located directly upstream of ftsEX, and transcript levels of this gene were substantially affected, with a 189-fold increase. Like the mre locus, DnaA could regulate these genes directly as potential DnaA-boxes are adjacent to these loci.
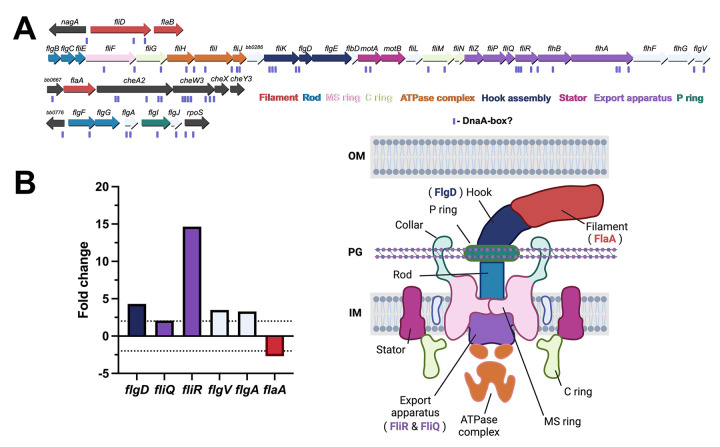
In the conditional dnaA-knockdown B. burgdorferi, we observed spirochetes that had lost their helical structure and hypothesized these cells had disrupted flagella. The B. burgdorferi genome encodes nearly three dozen flagellar genes (Fig. 9A). When dnaA was knocked down, six of these genes were significantly impacted: fliQ, fliR, flgD, flaA, flgV, and flgA (Fig. 9B). All these genes except for flaA and flgA are encoded in the flgB superoperon. FliQ and FliR are components of the flagellar export apparatus (39). FlgD is a scaffolding protein that is required for flagellar hook formation (40, 41). FlaA is the minor borrelial flagellar filament protein (42, 43). FlgV is a protein first characterized as a homolog of the RNA chaperone Hfq, but recent work has demonstrated the protein localizes with flagellar motors and modulates their assembly (44, 45). FlgA is a chaperone involved in the formation of the P-ring of the flagellar motor (46). The transcripts for fliR and flgD increased the most of these genes, about 15 and 4-fold, respectively, when DnaA levels were reduced. The changes in the expression of these genes could alter flagellar assembly homeostasis and explain the observed abnormalities in helicity in the dnaA-deficient spirochetes. It is also possible that defects in septation disrupted the localization and assembly of flagella.
Figure 9. Impacts of DnaA on B. burgdorferi flagellar genes.
(A) The borrelial flagellar motor is a complex molecular machine (bottom right) that is comprised of nearly three dozen proteins encoded in four separate loci on the chromosome (top). The schematic of the B. burgdorferi flagellar motor was informed from data by Zhao et al., Qin et al., and Chang et al. (98–100). (B) RNA-seq showed the transcripts for five flagellar genes increased when DnaA was knocked down. Two of these genes, FliQ and FliR (purple), are constituents of the flagellar export apparatus. The dashed lines indicate the 2-fold threshold for meaningful gene expression changes.
In addition to affecting genes for essential cellular processes, DnaA significantly impacted the expression of outer surface proteins that are involved in vertebrate infection and virulence, such as ErpA, decorin binding protein DbpA, and OspC (Fig. 7A–C and Fig. 10E–F). The Erps are multifunctional adhesins that bind vertebrate host factors such as laminin, plasminogen, and complement proteins (47–52). Transcripts for erpA increased in the dnaA knockdown and overexpression conditions (Fig. 10E–F). Interestingly, this increase in transcript didn’t correspond to increased ErpA protein (Fig. 10B–D).
The erp genes are encoded on the cp32 plasmids and have conserved operators that allow uniform regulation (53). The Erps are turned on when the tick begins to feed, a time of rapid borrelial replication, and remain on during vertebrate infection (54). Three proteins bind the erp operators and regulate their expression: BpaB (Borrelial cp32 ParB analog), BpuR, and EbfC. BpaB represses erp transcription along with the co-repressor BpuR (Fig. 10A) (55–57). EbfC is the antirepressor that antagonizes BpaB to allow for erp transcription (56). DnaA was previously hypothesized to regulate erp transcription by activating EbfC and repressing BpuR (10, 13, 14). EbfC transcript and protein levels decreased when dnaA was knocked down, consistent with that model (Fig. 10B and E). BpuR transcript, however, did not significantly change, but its protein levels appeared to decline with a decrease in cellular DnaA concentrations (Fig. 10B and E). BpuR alone does not repress erp expression, so this cannot account for the increase in ErpA transcript. Thus, we looked at the protein levels of the erp repressor. Despite the lack of a change in the transcript, immunoblots showed a decrease in BpaB protein levels in dnaA CRISPRi B. burgdorferi, which can explain the increase in erpA transcript (Fig. 10B, C, and E). BpaB levels were similar to those of the parental e2 strain during dnaA overexpression, and ErpA transcript and protein were unaffected, likely due to normal levels of the antirepressor EbfC. Overall, these results demonstrate that DnaA not only affects the expression of genes involved in basic cellular processes but also those involved in maintaining the Lyme spirochete’s infection cycle.
DISCUSSION
In this study, we sought to understand the roles of DnaA in controlling the physiology of B. burgdorferi. To do this, we utilized inducible CRISPRi and overexpression vectors. Overexpressing DnaA was toxic to the bacteria and was consistently overcome by mutations in the inducible dnaA. This, along with the difference in DnaA levels from overexpression vs. knockdown, 13-fold DnaA increase vs. 2-fold decrease, likely explains some of the results we encountered. Nevertheless, increasing DnaA levels yielded significantly different phenotypes, such as spirochete size. CRISPRi was the more reliable and effective means of producing conditional dnaA mutants in B. burgdorferi. This approach targeting dnaA has been successfully described in E. coli, Lactobacillus plantarum, and Streptococcus pyogenes (58–60). An attempt was made in Pseudomonas putida, but clones couldn’t be recovered (61).
We found that lowering the cellular levels of DnaA profoundly reduced cell division, consistent with the protein’s function as the chromosomal replication initiator. The reduction of DnaA also coincided with decreased expression of many essential replication genes. It is known that one of these genes, dnaX, is directly regulated by DnaA (10). This suggests that DnaA may directly regulate those other genes, or B. burgdorferi may have mechanisms to sense replication initiation and coordinate gene expression accordingly.
DnaA-deficient spirochetes also increased in length. The bacterial cell cycle is typically divided into three phases: B, C, and D (62). The B and D phases correspond to the birth and division of the bacteria, respectively. The C phase corresponds to everything in between, namely, chromosomal replication, along with elongation/growth. Replication is initiated by DnaA and then carried out by the replisome. Elongation is facilitated by the elongasome, which synthesizes peptidoglycan to allow for cell growth. The DnaA-depleted spirochetes, although considerably longer than the parental strain, had overall decreased expression of elongasome genes, the mre locus in particular. This suggested a division issue, but we cannot rule out the possibility that DnaA is required for cross-talk between replication and cell growth. Indeed, it is likely that replication, elongation, and division are all interconnected. Lyme and relapsing Borreliae elongate at three distinct zones along their length: 1/4, 1/2, and 3/4 (63). This localization pattern at the mid-cell suggests an interplay between elongation and division machinery. How or if DnaA directly plays into this delicate balance remains to be assessed.
Many bacterial cell division proteins are denoted “Fts” for their filamentous phenotypes caused by temperature-sensitive mutations (64). Knocking down DnaA affected the expression of ftsA, ftsEX, and ftsK. The dysregulation of these genes potentially explains both the elongated phenotype and the irregular spacing of the ParB-oriC puncta.
FtsA localizes to the inner leaflet of the inner membrane and binds FtsZ. Increased FtsA, seen when DnaA was knocked down, could decrease Z-ring assembly and, thus, cytokinesis. In E. coli, overexpression of FtsA causes cells to filament (65). Overexpressed DnaA spirochetes had elevated ftsA transcript, but no changes were observed in ftsEX. The FtsEX complex regulates the activity of peptidoglycan hydrolases and FtsA (66–69). Disruption of FtsE and FtsX levels could, therefore, prevent the breakdown of peptidoglycan and Z-ring formation at the site of division. The combined perturbation of ftsA and ftsEX expression in the dnaA CRISPRi strain potentially explains the observed elongated phenotype.
In addition to changes in spirochete length in the DnaA-depleted B. burgdorferi, we observed abnormal spacing of ParB-oriC puncta. These bacteria had decreased transcripts of the membrane-embedded DNA translocase FtsK, a core component of the divisome (70–72). FtsK, through its C-terminal αβγ-domain, resolves chromosome dimers and translocates DNA from the septum to allow for cytokinesis (73–76). A decrease in FtsK could thus result in the failure to separate sister chromosomes, leading to cells with abnormal ploidy. This could also explain why some oriC sites were located close together in the dnaA knockdown strain (Fig. 5). FtsK also recruits downstream divisome proteins through its N-terminal transmembrane domain (71, 77–79). Therefore, reducing FtsK may also limit the recruitment of divisome proteins to the septa.
Motility is perhaps the most important “virulence” factor that B. burgdorferi employs to infect and colonize its vertebrate hosts. By knocking down DnaA in the Lyme spirochete, we observed a substantial impact on the bacteria’s helicity. This corkscrew morphology is due to periplasmic flagella (28). Key genes, such as FlgD and FliR, were overexpressed when levels of DnaA were lowered. FliR is a component of the flagellar motor’s export apparatus, which transports flagellar filament substrates from the cytoplasm to the periplasm (39). Disrupting the balance of this subunit would very likely impair the overall function and structure of the flagellar motor and filament. By increasing FliR, it is possible that the export of the flagellar cargo to the periplasmic space would be affected, which in turn would prevent flagella formation. The flagellar motors are normally localized to the poles and developing septa of B. burgdorferi. This suggests that replication, elongation, or division processes may coordinate the formation of flagellar motors at the dividing mid-cell. In E. coli, DnaA regulates genes involved in flagellar assembly (30, 31, 80). Whether DnaA in B. burgdorferi acts through a similar mechanism is unknown.
In addition to the many morphological changes incurred by dysregulation of DnaA, there were also significant impacts on the expression of vertebrate infection-related outer surface proteins. Many of these antigenic proteins, such as OspC, DbpA, and the Erps are turned on during the tick blood meal, a period defined by rapid replication. Relative to these genes, levels of transcript for the alternative sigma factor RpoS were not significantly changed by manipulation of DnaA levels. We previously demonstrated that DnaA regulates the erp antirepressor EbfC and may regulate the erp co-repressor BpuR (10, 14). With our CRISPRi approach, we were able to validate our prior data on dnaX-ebfC regulation. While we could not detect any substantial differences in BpuR with DnaA manipulation, this might have been a consequence of assessing mid-exponential spirochetes grown at 34 °C; BpuR expression is highest when grown at 23 °C (14, 57). When DnaA levels were reduced, expression of ErpA increased. We suggest this could be due to decreased levels of erp repressor BpaB. While this was unexpected, it is logical that a partitioning protein be decreased when dnaA transcription is knocked down. This suggests that these extrachromosomal DNAs can sense changes in chromosomal replication. How this is mediated remains to be investigated.
We observed gene expression level changes in a vast number of regulatory networks. This led us to examine if dnaA knockdown or overexpression impacted any known regulatory factors. Among the known networks, we observed modest changes in gene expression levels of the following regulators: Hk1, Rrp1, and SpoVG. These changes did not meet the log fold change threshold but were significant (FDR ≤ 0.05) and had a log fold change of approximately 1.5. When comparing the dnaAT1 knockdown strain to the parental e2 strain, these genes were downregulated by a fold change of 1.52, 1.61, and 1.53, respectively. While not meeting our set threshold, it is possible the combined modest expression changes in these regulators considerably impacted the expression levels of the genes they modulate. For example, the nucleic acid binding protein SpoVG, the response regulator Hk1, and diguanylate cyclase Rrp1 are all known to regulate the glycerol metabolism (glpFKD) operon, which is essential for B. burgdorferi colonization and persistence in ticks (81–85). The downregulation of the glpFKD operon (~2.6 to 4-fold) is likely attributable to the decreased expression of spoVG, hk1, and rrp1. These observations link DnaA and/or DNA replication to crucial borrelial networks, including the c-di-GMP regulatory network (Hk1/Rrp1) critical to completing the B. burgdorferi enzootic life cycle.
Another regulatory network impacted was the Hk2/Rrp2 pathway, whose expression levels were increased by 8.2 and 1.5, respectively. The Hk2/Rrp2 two-component system is a known activator of the RpoN/RpoS alternative sigma factor cascade, which regulates essential virulence factors like OspC (86–88). Deletion of rrp2 is lethal, and investigations into rrp2 regulation have relied on conditional mutants (18). Although neither rpoN nor rpoS levels changed significantly, we note that rpoS transcription can be activated by the housekeeping sigma factor RpoD in addition to RpoN (89). This further provides strong evidence that the Hk2/Rrp2 pathway has impacts outside of rpoS regulation potentially mediated by proper dnaA levels and/or associated with DNA replication. In addition, this data supports our past data showing that ospC regulation, through the repressor Gac, can occur independently of RpoS regulation (90). Additional data from our lab indicates that DnaA is a positive activator of OspC (unpublished results). This supports the RNA-seq data in this study, showing that knockdown of DnaA levels reduces ospC transcript.
Organisms have evolved complex and varied molecular circuits to regulate cellular homeostasis in ever-changing environments. Bacterial pathogens, especially, have developed such networks in their tussle against host defenses. During the tick blood meal, B. burgdorferi must take advantage of the nutrients to propagate its numbers, reprogram its transcriptome, and disseminate to the vertebrate. Failure to do so means death, as B. burgdorferi cannot be transmitted vertically to tick offspring (91). In B. burgdorferi, DnaA, the master regulator of replication initiation, appears to form a complex regulatory network to coordinate these essential processes (Fig. 11). Future studies need to be conducted to determine which of the DnaA-dysregulated effects described here are due to DnaA itself or a consequence of a lack of initiation of chromosomal replication.
Figure 11. Roles and impacts of DnaA dysregulation on borrelial physiology.
The DnaA of B. burgdorferi functions as the chromosome replication initiator and a transcription factor. Altering levels of this essential protein modulate aspects of DNA replication and bacterial physiology. The data presented here point to crosstalk between the systems controlling replication and bacterial morphology. Future studies will need to be conducted to ascertain the direct roles DnaA plays in maintaining this delicate balance.
METHODS AND MATERIALS.
Bacterial strains, plasmids, and genetic manipulations.
Studies were conducted using the readily transformable B. burgdorferi clonal strain B31-e2 (92). Spirochetes were grown in liquid Barbour-Stoener-Kelly II (BSK-II) medium supplemented with 6% rabbit serum (v/v) at 35 °C. For all experiments, B. burgdorferi was diluted from mid-exponential phase (3–5 × 107 cells/mL) into fresh BSK-II medium. Cultures were only used if they were at least two passages away and no more than three from the initial −80°C stock. Borrelial culture densities were enumerated using a Petroff-Hauser counting chamber and dark-field microscopy.
The dnaA overexpression (pACK121) and CRISPRi plasmids (pACK136, dnaAT1; pACK138, dnaANT1) were generated by GenScript. The pACK121 plasmid is a derivative of pJSB268 (17). The plasmid was designed to replace the luc gene with the B. burgdorferi dnaA gene containing an N-terminal 3xFLAG epitope. The pACK136 (dnaAT1) and pACK137 (dnaANT1) plasmids are derivatives of pJJW101 (20). These plasmids were created by inserting the desired sgRNA sequence between the BsaI sequences. The sgRNA targets used in this study were chosen using CRISPy-web (https://crispy.secondarymetabolites.org) and B31 chromosome contig (NC_001318). Whole-plasmid sequencing was performed by Plasmidsaurus using Oxford Nanopore Technology with custom analysis and annotation.
Growth curve analysis.
Growth curves for each strain were generated from three independent experiments. Cultures were seeded at the same initial density (1 or 5 × 105 cells/mL) and enumerated over seven days. Generation times were calculated in Microsoft Excel by fitting an exponential curve to the data points denoting the beginning and end of logarithmic growth. Statistically significant differences were determined by one-way ANOVA.
Immunoblot analyses.
Polyclonal DnaA antibodies were generated by Thermo Scientific in rabbits using recombinant GST-DnaA generated in-house (10). The serum from the final bleed was purified by affinity purification using MBP-DnaA on an amylose column. Murine monoclonal anti-FlaB antibodies were used to assess the even loading of SDS-PAGE gels (93). Other primary antibodies were generated previously (56, 57, 94). Cells for immunoblot were pelleted and washed three times with 1x PBS, pH 7.4. Approximately 107 spirochetes were loaded per lane for SDS-PAGE. Goat anti-Rabbit Alexa Fluor 488 (1:10, 000, Thermo Scientific) and Goat anti-Mouse IRDye800 (1:5,000, Licor) were used as secondary antibodies. Densitometric analyses were performed using ImageLab software (BioRad). The intensities of the bands of interest were normalized to the corresponding loading control.
Quantitative reverse transcription-PCR (qRT-PCR).
Mid-exponential phase (3–5 × 107 cells/mL) bacteria were washed three times with PBS (pH 7.4) before RNA extraction using Qiagen Mini RNA kits. Genomic DNA was cleared by on-column DNase treatment. RNA was reverse transcribed using iScript cDNA synthesis kits (Bio-Rad). Quantitative PCR was conducted using iTaq Universal SYBR Green Supermix (Bio-Rad) and a Bio-Rad CFX96 Touch Real-time PCR thermocycler. The primer sets used for this study were designed using the IDT Primer Quest Tool (https://www.idtdna.com/PrimerQuest/Home/Index). Cq values were normalized to ftsK or rpoB (ΔCq) and then to the parental strain or uninduced control (ΔΔCq). Fold expression was determined using the function 2−ΔΔCq. If ΔΔCq > 0, then the function −2ΔΔCq was used.
Cell length analysis.
Wet mounts of live bacteria were visualized by dark-field with an Olympus Bx51 microscope at 400x total magnification. Micrographs were taken using a C-mounted Accu-scope Excelis HD camera. Cell lengths were determined using Captavision+ software. A conversion factor of 13.80 pixels/μm was used for all measurements. For each replicate and time point, approximately 50 spirochetes were measured.
Fluorescence microscopy and image analysis.
Spirochetes were pelleted by centrifugation, washed twice with PBS (pH 7.4), and diluted to a final concentration of 1 × 105 cells/mL. Ten microliters of cells were mounted on a glass slide. Cells were visualized using an Olympus Bx51 microscope with a Cool LED p-E300 illumination system. Micrographs of dark-field and red fluorescence were captured for each field of view using a C-mounted Accu-scope Excelis HD camera. Micrographs were merged in ImageJ, and the background was inverted using EZreverse (https://amsterdamstudygroup.shinyapps.io/ezreverse/) with the HSL color space option (95).
Whole Genome Sequencing (WGS) and analysis.
The parental B. burgdorferi B31-e2, dnaAT1 CRISPRi, and dnaA-overexpression strains were sequenced to verify plasmid content and determine if mutations occurred in the dnaA sequence of the generated strains. Briefly, the strains were grown in duplicate to mid-exponential phase (3–5 × 107 cells/mL). Two mLs of cells were pelleted and washed twice with PBS (pH 7.4) and frozen at −80 °C. DNA extraction and whole genome sequencing were performed by SeqCenter. Briefly, the cells were sent to the SeqCenter facility. DNA was extracted from the pellets using the ZymoBIOMICS™ DNA Miniprep Kit according to the manufacturer’s protocol. DNA concentrations were determined by Qubit.
Sequencing libraries were prepared via the tagmentation-based and PCR-based Illumina DNA Prep kit and custom IDT 10 bp unique dual indices (UDI) with a target insert size of 320 bp. The sequencing was performed either in one or more multiplexed flow-cell runs on an Illumina NovaSeq 6000 sequencer resulting in 2×151 bp paired-end reads. Quality control steps including demultiplexing and adapter trimming were performed with bcl-convert (v4.1.5). Reads were aligned to the B. burgdorferi B31 genome assembly (GCF_000008685.2_ASM868v2). Variant calling was performed using BreSeq (v. 0.38.1) under default settings.
RNA sequencing (RNA-seq) and analysis.
The same cultures of the parental B. burgdorferi B31-e2, dnaAT1 CRISPRi, and dnaA-overexpression strains used for WGS were used for RNA-seq. All strains were grown in duplicate to mid-exponential growth phase (3–5 × 107 cells/mL) and induced with 0.5 mM IPTG overnight at 35 °C. Nine mLs of the cells were pelleted and washed two times with PBS (pH 7.4) and frozen at −80 °C. The cells were sent to SeqCenter for RNA extraction and sequencing using their intermediate RNA analysis with replicates (Prokaryotic) service. Briefly, their method consisted of quality control and adapter trimming by bclconvert (version 4.1.5) and mapping reads using HISAT2 (version 2.2.0) to the RefSeq version of the B. burgdorferi B31 genome assembly (GCF_000008685.2_ASM868v2). The read counts were uploaded into R (version 4.0.2) and normalized using the edgeR Trimmed Mean of M values (TMM) algorithm (1.14.5). Differential gene expression analysis was performed with edgeR using the normalized TMM values. Only plasmids that were confirmed to be present were included in the analyses. We further filtered the data set by setting the thresholds for differential expression at log2FC ≥ 1 or ≤ −1 and an FDR ≤ 0.05. Differentially expressed genes (DEGs) were visualized via volcano plots made using VolcaNoseR (96). DEGs belonging to the elongasome and divisome were visualized by heatmap. The heatmap was generated in R (v 4.4.1) using the pheatmap package, with the log2 transformed normalized counts per million (CPMs) (97). The clustering method used for the heatmap was the default method of complete linkage, while the distance measure used was correlation.
To identify flagellar, cell division, and elongation homologs, the genome was directly searched for annotations, or the sequences of characterized proteins from other bacteria were aligned by BLAST against the B. burgdorferi genome.
Table 2.
Oligonucleotides used in this study.
| Target | Name | Sequence | Purpose | Source |
|---|---|---|---|---|
|
| ||||
| dnaA ORF | qPCR dnaA F | CATGTGACCGATCTCCTTCTG | qPCR | (10) |
| qPCR dnaA R | CGACAATAGCTGCTCTGAGTT | |||
| ftsK ORF | qPCR ftsK F | GACCTTCTGATGAGCCAATGT | qPCR | (10) |
| qPCR ftsk R | GCTGCTCTGTTGTAACCTATCT | |||
| rpoB ORF | qPCR rpoB F | GTTCATCTGGGACAAGGAAGAG | qPCR | This study |
| qPCR rpoB R | CAGACTCAAGGCCCTCATTAAG | |||
IMPORTANCE.
Lyme disease is the most prevalent tick-borne infection in the Northern Hemisphere. Borrelia burgdorferi, the causative spirochete bacteria, has been maintained in nature for millennia in a consistent enzootic cycle between Ixodes ticks and various small vertebrate hosts. During the tick’s blood meal, B. burgdorferi substantially increases its replication rate, alters its repertoire of outer surface proteins, and disseminates into the new vertebrate host. Across eubacteria, DnaA is the master regulatory protein that initiates chromosomal replication and acts as a transcription factor to regulate specific pathways. Here, we describe the roles that B. burgdorferi DnaA has on the physiology and gene expression of this medically important pathogen.
Acknowledgments
This research was funded by NIH grant R21 AI147139. We thank Dr. Wolfram Zückert and Bryan Murphy for their help designing and troubleshooting the CRISPRi shuttle vectors and Dr. Jon Blevins for providing the pJSB268 shuttle vector. BioRender was used to make the schematics in Figures 1, 2, 8, 9, and 10.
Works Cited.
- 1.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Silva AM, Fikrig E. 1995. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg 53:397–404. [DOI] [PubMed] [Google Scholar]
- 4.Piesman J, Oliver JR, Sinsky RJ. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg 42:352–7. [DOI] [PubMed] [Google Scholar]
- 5.Speck C, Messer W. 2001. Mechanism of origin unwinding: sequential binding of DnaA to double- and single-stranded DNA. EMBO J 20:1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur G, Vora MP, Czerwonka CA, Rozgaja TA, Grimwade JE, Leonard AC. 2014. Building the bacterial orisome: high-affinity DnaA recognition plays a role in setting the conformation of oriC DNA. Mol Microbiol 91:1148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozaki S, Katayama T. 2012. Highly organized DnaA-oriC complexes recruit the single-stranded DNA for replication initiation. Nucleic Acids Res 40:1648–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messer W, Weigel C. 1997. DnaA initiator--also a transcription factor. Mol Microbiol 24:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Menikpurage IP, Woo K, Mera PE. 2021. Transcriptional Activity of the Bacterial Replication Initiator DnaA. Front Microbiol 12:662317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krusenstjerna AC, Saylor TC, Arnold WK, Tucker JS, Stevenson B. 2023. Borrelia burgdorferi DnaA and the Nucleoid-Associated Protein EbfC Coordinate Expression of the dnaX-ebfC Operon. J Bacteriol 205:e0039622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medrano MS, Ding Y, Wang X-G, Lu P, Coburn J, Hu LT. 2007. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. Journal of bacteriology 189:2653–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutras BL, Bowman A, Brissette CA, Adams CA, Verma A, Chenail AM, Stevenson B. 2012. EbfC (YbaB) is a new type of bacterial nucleoid-associated protein and a global regulator of gene expression in the Lyme disease spirochete. J Bacteriol 194:3395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson B, Krusenstjerna AC, Castro-Padovani TN, Savage CR, Jutras BL, Saylor TC. 2022. The Consistent Tick-Vertebrate Infectious Cycle of the Lyme Disease Spirochete Enables Borrelia burgdorferi To Control Protein Expression by Monitoring Its Physiological Status. J Bacteriol 204:e0060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jutras BL, Savage CR, Arnold WK, Lethbridge KG, Carroll DW, Tilly K, Bestor A, Zhu H, Seshu J, Zuckert WR, Stewart PE, Rosa PA, Brissette CA, Stevenson B. 2019. The Lyme disease spirochete’s BpuR DNA/RNA-binding protein is differentially expressed during the mammal-tick infectious cycle, which affects translation of the SodA superoxide dismutase. Mol Microbiol 112:973–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picardeau M, Lobry JR, Hinnebusch BJ. 1999. Physical mapping of an origin of bidirectional replication at the centre of the Borrelia burgdorferi linear chromosome. Mol Microbiol 32:437–45. [DOI] [PubMed] [Google Scholar]
- 16.Hirota Y, Ryter A, Jacob F. 1968. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol 33:677–93. [DOI] [PubMed] [Google Scholar]
- 17.Blevins JS, Revel AT, Smith AH, Bachlani GN, Norgard MV. 2007. Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi. Appl Environ Microbiol 73:1501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groshong AM, Gibbons NE, Yang XF, Blevins JS. 2012. Rrp2, a prokaryotic enhancer-like binding protein, is essential for viability of Borrelia burgdorferi. J Bacteriol 194:3336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takacs CN, Scott M, Chang Y, Kloos ZA, Irnov I, Rosa PA, Liu J, Jacobs-Wagner C. 2020. A CRISPR interference platform for selective downregulation of gene expression in Borrelia burgdorferi. Appl Environ Microbiol doi: 10.1128/AEM.02519-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy BT, Wiepen JJ, He H, Pramanik AS, Peters JM, Stevenson B, Zuckert WR. 2023. Inducible CRISPRi-Based Operon Silencing and Selective in Trans Gene Complementation in Borrelia burgdorferi. J Bacteriol 205:e0046822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W, Li Z, Skarstad K, Crooke E. 2001. Mutations in DnaA protein suppress the growth arrest of acidic phospholipid-deficient Escherichia coli cells. EMBO J 20:1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonas K, Chen YE, Laub MT. 2011. Modularity of the bacterial cell cycle enables independent spatial and temporal control of DNA replication. Curr Biol 21:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson ME, Smith JL, Grossman AD. 2022. Multiple mechanisms for overcoming lethal over-initiation of DNA replication. Mol Microbiol 118:426–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper S, Helmstetter CE. 1968. Chromosome replication and the division cycle of Escherichia coli Br. Journal of Molecular Biology 31:519–540. [DOI] [PubMed] [Google Scholar]
- 25.Donachie WD. 1968. Relationship between Cell Size and Time of Initiation of DNA Replication. Nature 219:1077–1079. [DOI] [PubMed] [Google Scholar]
- 26.Wallden M, Fange D, Lundius EG, Baltekin Ö, Elf J. 2016. The Synchronization of Replication and Division Cycles in Individual E. coli Cells. Cell 166:729–739. [DOI] [PubMed] [Google Scholar]
- 27.Takacs CN, Wachter J, Xiang Y, Ren Z, Karaboja X, Scott M, Stoner MR, Irnov I, Jannetty N, Rosa PA, Wang X, Jacobs-Wagner C. 2022. Polyploidy, regular patterning of genome copies, and unusual control of DNA partitioning in the Lyme disease spirochete. Nat Commun 13:7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, Charon NW. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci U S A 97:10899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Bakker RG, Motaleb MA, Sartakova ML, Cabello FC, Charon NW. 2002. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc Natl Acad Sci U S A 99:6169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, Baigalmaa L, Yao Y, Li G, Su M, Fan L, Morigen. 2021. The Escherichia coli QseB/QseC signaling is required for correct timing of replication initiation and cell motility. Gene 773:145374. [DOI] [PubMed] [Google Scholar]
- 31.Mizushima T, Tomura A, Shinpuku T, Miki T, Sekimizu K. 1994. Loss of flagellation in dnaA mutants of Escherichia coli. J Bacteriol 176:5544–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold WK, Savage CR, Brissette CA, Seshu J, Livny J, Stevenson B. 2016. RNA-Seq of Borrelia burgdorferi in Multiple Phases of Growth Reveals Insights into the Dynamics of Gene Expression, Transcriptome Architecture, and Noncoding RNAs. PLoS One 11:e0164165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones LJ, Carballido-Lopez R, Errington J. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913–22. [DOI] [PubMed] [Google Scholar]
- 34.Kruse T, Bork-Jensen J, Gerdes K. 2005. The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Mol Microbiol 55:78–89. [DOI] [PubMed] [Google Scholar]
- 35.Figge RM, Divakaruni AV, Gober JW. 2004. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol 51:1321–32. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Biboy J, Consoli E, Vollmer W, den Blaauwen T. 2021. MreC and MreD balance the interaction between the elongasome proteins PBP2 and RodA. PLOS Genetics 16:e1009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pichoff S, Lutkenhaus J. 2005. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol Microbiol 55:1722–34. [DOI] [PubMed] [Google Scholar]
- 38.Pichoff S, Lutkenhaus J. 2002. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. Embo j 21:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minamino T, Macnab RM. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol 181:1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutsukake K, Doi H. 1994. Nucleotide sequence of the flgD gene of Salmonella typhimurium which is essential for flagellar hook formation. Biochim Biophys Acta 1218:443–6. [DOI] [PubMed] [Google Scholar]
- 41.Ohnishi K, Ohto Y, Aizawa S, Macnab RM, Iino T. 1994. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol 176:2272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge Y, Li C, Corum L, Slaughter CA, Charon NW. 1998. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J Bacteriol 180:2418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motaleb MA, Sal MS, Charon NW. 2004. The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. J Bacteriol 186:3703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lybecker MC, Abel CA, Feig AL, Samuels DS. 2010. Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 78:622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamba-Campero M, Soliman D, Yu H, Lasseter AG, Chang Y-Y, Liu J, Aravind L, Jewett MW, Storz G, Adams PP. 2024. Broadly conserved FlgV controls flagellar assembly and Borrelia burgdorferi dissemination in mice. bioRxiv doi: 10.1101/2024.01.09.574855:2024.01.09.574855. [DOI] [PubMed] [Google Scholar]
- 46.Juhas M, Ajioka JW. 2015. Identification and validation of novel chromosomal integration and expression loci in Escherichia coli flagellar region 1. PLoS One 10:e0123007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson B, Brissette CA. 2023. Erp and Rev Adhesins of the Lyme Disease Spirochete’s Ubiquitous cp32 Prophages Assist the Bacterium during Vertebrate Infection. Infect Immun 91:e0025022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun 70:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brissette CA, Haupt K, Barthel D, Cooley AE, Bowman A, Skerka C, Wallich R, Zipfel PF, Kraiczy P, Stevenson B. 2009. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect Immun 77:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. 2009. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology (Reading) 155:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrigues RJ, Thomas S, Leong JM, Garcia BL. 2022. Outer surface lipoproteins from the Lyme disease spirochete exploit the molecular switch mechanism of the complement protease C1s. J Biol Chem 298:102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pereira MJ, Wager B, Garrigues RJ, Gerlach E, Quinn JD, Dowdell AS, Osburne MS, Zückert WR, Kraiczy P, Garcia BL, Leong JM. 2022. Lipoproteome screening of the Lyme disease agent identifies inhibitors of antibody-mediated complement killing. Proc Natl Acad Sci U S A 119:e2117770119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babb K, McAlister JD, Miller JC, Stevenson B. 2004. Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J Bacteriol 186:2745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller JC, von Lackum K, Babb K, McAlister JD, Stevenson B. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect Immun 71:6943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burns LH, Adams CA, Riley SP, Jutras BL, Bowman A, Chenail AM, Cooley AE, Haselhorst LA, Moore AM, Babb K, Fried MG, Stevenson B. 2010. BpaB, a novel protein encoded by the Lyme disease spirochete’s cp32 prophages, binds to erp Operator 2 DNA. Nucleic Acids Res 38:5443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jutras BL, Verma A, Adams CA, Brissette CA, Burns LH, Whetstine CR, Bowman A, Chenail AM, Zuckert WR, Stevenson B. 2012. BpaB and EbfC DNA-binding proteins regulate production of the Lyme disease spirochete’s infection-associated Erp surface proteins. J Bacteriol 194:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jutras BL, Chenail AM, Carroll DW, Miller MC, Zhu H, Bowman A, Stevenson B. 2013. Bpur, the Lyme disease spirochete’s PUR domain protein: identification as a transcriptional modulator and characterization of nucleic acid interactions. J Biol Chem 288:26220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Si F, Li D, Cox SE, Sauls JT, Azizi O, Sou C, Schwartz AB, Erickstad MJ, Jun Y, Li X, Jun S. 2017. Invariance of Initiation Mass and Predictability of Cell Size in Escherichia coli. Curr Biol 27:1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myrbraten IS, Wiull K, Salehian Z, Havarstein LS, Straume D, Mathiesen G, Kjos M. 2019. CRISPR Interference for Rapid Knockdown of Essential Cell Cycle Genes in Lactobacillus plantarum. mSphere 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bjånes E, Stream A, Gibson PS, Bravo AM, Dahesh S, Baker JL, Varble A, Nizet V, Veening J-W. 2024. An efficient in vivo-inducible CRISPR interference system for group A Streptococcus genetic analysis and pathogenesis studies. bioRxiv doi: 10.1101/2024.02.22.581527:2024.02.22.581527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenster JA, Werner AZ, Tay JW, Gillen M, Schirokauer L, Hill NC, Watson A, Ramirez KJ, Johnson CW, Beckham GT, Cameron JC, Eckert CA. 2022. Dynamic and single cell characterization of a CRISPR-interference toolset in Pseudomonas putida KT2440 for β-ketoadipate production from p-coumarate. Metab Eng Commun 15:e00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meunier A, Cornet F, Campos M. 2021. Bacterial cell proliferation: from molecules to cells. FEMS Microbiol Rev 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jutras BL, Scott M, Parry B, Biboy J, Gray J, Vollmer W, Jacobs-Wagner C. 2016. Lyme disease and relapsing fever Borrelia elongate through zones of peptidoglycan synthesis that mark division sites of daughter cells. Proc Natl Acad Sci U S A 113:9162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van De Putte P, Van D, Roersch A. 1964. The selection of mutants of Escherichia coli with impaired cells division at elevated temperature. Mutat Res 106:121–8. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Henk MC, Gayda RC. 1993. Overexpression of ftsA induces large bulges at the septal regions in Escherichia coli. Current Microbiology 26:175–181. [Google Scholar]
- 66.Meisner J, Montero Llopis P, Sham LT, Garner E, Bernhardt TG, Rudner DZ. 2013. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Mol Microbiol 89:1069–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Xu X, Shi J, Hermoso JA, Sham L-T, Luo M. 2023. Regulation of the cell division hydrolase RipC by the FtsEX system in Mycobacterium tuberculosis. Nature Communications 14:7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu X, Li J, Chua W-Z, Pages MA, Shi J, Hermoso JA, Bernhardt T, Sham L-T, Luo M. 2023. Mechanistic insights into the regulation of cell wall hydrolysis by FtsEX and EnvC at the bacterial division site. Proceedings of the National Academy of Sciences 120:e2301897120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du S, Pichoff S, Lutkenhaus J. 2016. FtsEX acts on FtsA to regulate divisome assembly and activity. Proceedings of the National Academy of Sciences 113:E5052–E5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diez AA, Farewell A, Nannmark U, Nyström T. 1997. A mutation in the ftsK gene of Escherichia coli affects cell-cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J Bacteriol 179:5878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu G, Draper GC, Donachie WD. 1998. FtsK is a bifunctional protein involved in cell division and chromosome localization in Escherichia coli. Mol Microbiol 29:893–903. [DOI] [PubMed] [Google Scholar]
- 72.Steiner W, Liu G, Donachie WD, Kuempel P. 1999. The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol 31:579–83. [DOI] [PubMed] [Google Scholar]
- 73.Crozat E, Rousseau P, Fournes F, Cornet F. 2014. The FtsK family of DNA translocases finds the ends of circles. J Mol Microbiol Biotechnol 24:396–408. [DOI] [PubMed] [Google Scholar]
- 74.Yu XC, Weihe EK, Margolin W. 1998. Role of the C terminus of FtsK in Escherichia coli chromosome segregation. J Bacteriol 180:6424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Capiaux H, Lesterlin C, Pérals K, Louarn JM, Cornet F. 2002. A dual role for the FtsK protein in Escherichia coli chromosome segregation. EMBO Rep 3:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aussel L, Barre FX, Aroyo M, Stasiak A, Stasiak AZ, Sherratt D. 2002. FtsK Is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell 108:195–205. [DOI] [PubMed] [Google Scholar]
- 77.Begg KJ, Dewar SJ, Donachie WD. 1995. A new Escherichia coli cell division gene, ftsK. J Bacteriol 177:6211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grenga L, Luzi G, Paolozzi L, Ghelardini P. 2008. The Escherichia coli FtsK functional domains involved in its interaction with its divisome protein partners. FEMS microbiology letters 287:163–167. [DOI] [PubMed] [Google Scholar]
- 79.Chen JC, Beckwith J. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Molecular microbiology 42:395–413. [DOI] [PubMed] [Google Scholar]
- 80.Sperandio V, Torres AG, Kaper JB. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43:809–21. [DOI] [PubMed] [Google Scholar]
- 81.Savage CR, Jutras BL, Bestor A, Tilly K, Rosa PA, Tourand Y, Stewart PE, Brissette CA, Stevenson B. 2018. Borrelia burgdorferi SpoVG DNA- and RNA-Binding Protein Modulates the Physiology of the Lyme Disease Spirochete. J Bacteriol 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saylor TC, Savage CR, Krusenstjerna AC, Jusufovic N, Zuckert WR, Brissette CA, Motaleb M, Schlax PJ, Stevenson B. 2023. Quantitative analyses of interactions between SpoVG and RNA/DNA. Biochem Biophys Res Commun 654:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. 2011. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog 7:e1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. 2011. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS pathogens 7:e1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. 2015. Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun 83:3043–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blevins JS, Xu H, He M, Norgard MV, Reitzer L, Yang XF. 2009. Rrp2, a sigma54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J Bacteriol 191:2902–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Q, Shi Y, Dadhwal P, Liang FT. 2012. RpoS regulates essential virulence factors remaining to be identified in Borrelia burgdorferi. PLoS One 7:e53212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ouyang Z, Zhou J, Norgard MV. 2014. Synthesis of RpoS is dependent on a putative enhancer binding protein Rrp2 in Borrelia burgdorferi. PLoS One 9:e96917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Samuels DS. 2011. Gene regulation in Borrelia burgdorferi. Annual review of microbiology 65:479–99. [DOI] [PubMed] [Google Scholar]
- 90.Castro-Padovani TN, Saylor TC, Husted OT, Krusenstjerna AC, Jusufovic N, Stevenson B. 2023. Gac Is a Transcriptional Repressor of the Lyme Disease Spirochete’s OspC Virulence-Associated Surface Protein. J Bacteriol doi: 10.1128/jb.00440-22:e0044022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rollend L, Fish D, Childs JE. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis 4:46–51. [DOI] [PubMed] [Google Scholar]
- 92.Casjens S, van Vugt R, Tilly K, Rosa PA, Stevenson B. 1997. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol 179:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barbour AG, Hayes SF, Heiland RA, Schrumpf ME, Tessier SL. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun 52:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El-Hage N, Babb K, Carroll JA, Lindstrom N, Fischer ER, Miller JC, Gilmore RD Jr., Mbow ML, Stevenson B. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology (Reading) 147:821–830. [DOI] [PubMed] [Google Scholar]
- 95.Song X, Goedhart J. 2024. EzReverse – a Web Application for Background Adjustment of Color Images. bioRxiv doi: 10.1101/2024.05.27.594095:2024.05.27.594095. [DOI] [Google Scholar]
- 96.Goedhart J, Luijsterburg MS. 2020. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Scientific Reports 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kolde R, Kolde MR. 2015. Package ‘pheatmap’. R package 1:790. [Google Scholar]
- 98.Zhao X, Zhang K, Boquoi T, Hu B, Motaleb MA, Miller KA, James ME, Charon NW, Manson MD, Norris SJ, Li C, Liu J. 2013. Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proc Natl Acad Sci U S A 110:14390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin Z, Tu J, Lin T, Norris SJ, Li C, Motaleb MA, Liu J. 2018. Cryo-electron tomography of periplasmic flagella in Borrelia burgdorferi reveals a distinct cytoplasmic ATPase complex. PLoS Biol 16:e3000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang Y, Moon KH, Zhao X, Norris SJ, Motaleb MA, Liu J. 2019. Structural insights into flagellar stator–rotor interactions. eLife 8:e48979. [DOI] [PMC free article] [PubMed] [Google Scholar]