Abstract
PURPOSE
To compare giredestrant and physician's choice of endocrine monotherapy (PCET) for estrogen receptor–positive, HER2-negative, advanced breast cancer (BC) in the phase II acelERA BC study (ClinicalTrials.gov identifier: NCT04576455).
METHODS
Post-/pre-/perimenopausal women, or men, age 18 years or older with measurable disease/evaluable bone lesions, whose disease progressed after 1-2 lines of systemic therapy (≤1 targeted, ≤1 chemotherapy regimen, prior fulvestrant allowed) were randomly assigned 1:1 to giredestrant (30 mg oral once daily) or fulvestrant/aromatase inhibitor per local guidelines (+luteinizing hormone–releasing hormone agonist in pre-/perimenopausal women, and men) until disease progression/unacceptable toxicity. Stratification was by visceral versus nonvisceral disease, prior cyclin-dependent kinase 4/6 inhibitor, and prior fulvestrant. The primary end point was investigator-assessed progression-free survival (INV-PFS).
RESULTS
At clinical cutoff (February 18, 2022; median follow-up: 7.9 months; N = 303), the INV-PFS hazard ratio (HR) was 0.81 (95% CI, 0.60 to 1.10; P = .1757). In the prespecified secondary end point analysis of INV-PFS by ESR1 mutation (m) status in circulating tumor DNA–evaluable patients (n = 232), the HR in patients with a detectable ESR1m (n = 90) was 0.60 (95% CI, 0.35 to 1.03) versus 0.88 (95% CI, 0.54 to 1.42) in patients with no ESR1m detected (n = 142). Related grade 3-4 adverse events (AEs), serious AEs, and discontinuations due to AEs were balanced across arms.
CONCLUSION
Although the acelERA BC study did not reach statistical significance for its primary INV-PFS end point, there was a consistent treatment effect with giredestrant across most key subgroups and a trend toward favorable benefit among patients with ESR1-mutated tumors. Giredestrant was well tolerated, with a safety profile comparable to PCET and consistent with known endocrine therapy risks. Overall, these data support the continued investigation of giredestrant in other studies.
INTRODUCTION
Breast cancer (BC) is the most commonly diagnosed cancer in women, with an estimated 2.3 million new cases and 700,000 deaths in 2020.1 Sixty to seventy percent of BCs do not overexpress HER2 but express the estrogen receptor (ER),2 and most are dependent on the ER for growth and progression. ER-expressing BCs are treated with endocrine therapies (ETs) that suppress ER signaling, either by blocking estradiol synthesis (eg, aromatase inhibitors [AIs]), antagonizing the effects of estradiol via competitive binding of ERs (eg, selective ER modulators), or by fully antagonizing and degrading ERs (ie, selective ER antagonists and degraders [SERDs]).3,4 However, despite the effectiveness of available ETs, alone or combined with targeted agents such as cyclin-dependent kinase 4/6 inhibitors (CDK4/6is), advanced BC (aBC) remains incurable, with significant unmet needs in terms of reducing impact on quality of life (QoL) and delaying chemotherapy for as long as possible. In most patients with aBC, the tumor will ultimately progress because of primary or secondary ET resistance5; the optimal ET sequence in subsequent lines remains uncertain, and may depend on which agents were used previously in the early or advanced settings. Further considerations regarding the choice of therapy in the second line and beyond are duration of response (DoR) to previous ET (particularly for second-line ET), disease burden, patient preference, treatment availability, and mechanisms of resistance that may have arisen during previous treatments.6,7 Despite being refractory to prior ET, growth and survival of ER-positive (ER+) tumors may often remain dependent on ER signaling, and tumors can still respond to second- or third-line ET after prior progression.8 Continuing ET with agents not previously used in the advanced setting, either as monotherapy or combined with targeted agents, represents an option to delay chemotherapy initiation.9
CONTEXT
Key objective
Can the oral, selective estrogen receptor antagonist and degrader (SERD) giredestrant improve outcomes compared with physician's choice of endocrine therapy (PCET) in patients with pretreated, estrogen receptor–positive (ER+), HER2-negative, advanced breast cancer?
Knowledge Generated
Giredestrant did not show statistically significant superiority to PCET with regards to investigator-assessed progression-free survival. In patients with ESR1-mutated tumors, there was a trend toward favorable benefit with giredestrant. Giredestrant was well tolerated, with a safety profile comparable to PCET and consistent with known endocrine therapy risks.
Relevance (G. Fleming)
-
Hopeful results continue to emerge from studies of novel ER-targeting drugs, with a number appearing to show more activity than historically available agents in the presence of a tumor ESR1 mutation. However their significance in the treatment algorithm for women with ER+, HER2-negative breast cancer remains to be determined.*
*Relevance section written by JCO Associate Editor Gini Fleming, MD.
A common mechanism of acquired resistance to ET is the presence of an activating mutation (m) in the ligand binding domain of ESR1 that results in ligand-independent ER signaling.10 Although fulvestrant has some activity in ESR1m tumors, it has unfavorable bioavailability and pharmacokinetics requiring intramuscular injection; therefore, new oral SERDs with more potent activity against ESR1m tumors are needed.11,12
Giredestrant is a highly potent, nonsteroidal, oral SERD, with a similar mechanism of action to, but superior preclinical potency over, fulvestrant and other oral SERDs.12 Giredestrant is well tolerated, and has shown encouraging antitumor activity in aBC as a monotherapy and in combination with palbociclib, including in patients with ESR1m tumors and those who have received prior fulvestrant.12-18
The phase II acelERA BC study (ClinicalTrials.gov identifier: NCT04576455) compares the efficacy and safety of giredestrant with physician's choice of endocrine monotherapy (PCET) for ER+, HER2-negative (HER2–) aBC in the second or third line. We report the primary results.
METHODS
Study Design and Patients
Patients were postmenopausal or pre-/perimenopausal women, and men, age 18 years or older who had received one or two prior lines of systemic therapy for ER+, HER2– locally advanced or metastatic BC. One of the prior lines must have been ET for ≥6 months; ≤1 targeted agent (including, but not limited to, CDK4/6is) was allowed; and one of the lines may have included chemotherapy. Patients had measurable (per RECIST v1.119) or bone-only disease, which must have had ≥1 predominantly lytic bone lesion. Key exclusion criteria included prior treatment with an investigational SERD (prior fulvestrant was allowed, as long as it was terminated ≥28 days before random assignment); advanced, symptomatic, visceral spread that risked life-threatening complications in the short term; known active uncontrolled or symptomatic CNS metastases, carcinomatous meningitis, or leptomeningeal disease; and active cardiac disease or history of cardiac dysfunction.
Eligible patients were randomly assigned 1:1 using a permuted-block method to receive giredestrant or PCET. Random assignment was stratified by disease site (visceral [lung and/or liver involvement] v nonvisceral), prior CDK4/6i (yes v no), and prior fulvestrant (yes v no; Data Supplement, Fig S1, online only).
Study Oversight
acelERA BC was designed by the senior academic authors and representatives of the sponsor (F. Hoffmann-La Roche Ltd, Basel, Switzerland). Data were collected by the sponsor and analyzed in collaboration with the senior academic authors, who vouched for the completeness and accuracy of the data and analyses, and for the fidelity of the study to the protocol. acelERA BC was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Protocol approval was obtained from an independent ethics committee for each participating site. Every patient gave written informed consent. An internal monitoring committee comprising employees of the sponsor reviewed cumulative safety data periodically throughout.
Study Procedures/Assessments
Patients received once daily oral giredestrant 30 mg (F. Hoffmann-La Roche Ltd/Genentech, Inc, South San Francisco, CA) as part of 28-day cycles until disease progression or unacceptable toxicity, or PCET (fulvestrant or an AI per local guidelines). No dose reductions were allowed for giredestrant. Dose reductions for comparator drugs were allowed according to local prescribing information. Pre-/perimenopausal women, and men, also received luteinizing hormone–releasing hormone agonist. ER and HER2 status were assessed locally. ESR1 testing was performed centrally using the FoundationOne Liquid CDx assay (Foundation Medicine, Inc, Cambridge, MA) or the PredicineCARE assay (Huidu Shanghai Medical Sciences Ltd, Shanghai, China) on baseline plasma circulating tumor (ct)DNA, and mutations were defined as short nucleotide variants with known or likely impact on ER protein function based on Catalogue Of Somatic Mutations In Cancer (COSMIC) status. Tumors were assessed by computed tomography or magnetic resonance imaging according to RECIST v1.119 within 28 days before random assignment (baseline), every 8 weeks (±7 days) from random assignment for the first 18 months, and then every 12 weeks (±7 days) thereafter until disease progression (except bone scans, which were performed every 24 weeks ±7 days, or as clinically indicated). All radiologic data (eg, computed tomography scan, magnetic resonance imaging, bone scan), photographs of skin lesions, and any additional clinical information required were sent to a blinded, independent, core imaging laboratory (contracted by the sponsor) to facilitate a retrospective evaluation of disease response and progression for the full population by a blinded independent review committee (BIRC). Patient-reported outcome (PRO) assessments are described in the Data Supplement. Adverse events (AEs) were recorded and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) v5.0, and were evaluated at every patient visit from baseline until 30 days after study treatment discontinuation.
Study End Points
The primary end point was progression-free survival (PFS), defined as the time from random assignment to the first occurrence of disease progression or death from any cause (whichever occurred first), as determined by the investigator (INV-PFS). PFS was also assessed by a BIRC as a sensitivity analysis. Secondary end points included overall survival (OS; time from random assignment to death from any cause), confirmed objective response rate (ORR; proportion of patients with a complete response [CR] or partial response [PR] on two consecutive occasions ≥4 weeks apart), DoR (time from first occurrence of a documented objective response to disease progression, or death from any cause [whichever occurred first]), clinical benefit rate (CBR; proportion of patients with stable disease for ≥24 weeks, or a CR or PR), INV-PFS in subgroups categorized by baseline ESR1m status, PROs, pharmacokinetics, and safety. Tumor assessments were done according to RECIST v1.1.
Statistical Analysis
The full analysis set comprised all patients assigned to treatment groups as randomly assigned; the safety population, all randomly assigned patients who received ≥1 dose of giredestrant or PCET; the ctDNA-evaluable population, all randomly assigned patients with evaluable plasma ctDNA at baseline (excluding 71 patients because of sample nonavailability or testing failure); and the PRO-evaluable population, all patients with a baseline and ≥1 postbaseline PRO assessment. Efficacy end points were assessed in the full analysis set (except PFS by ESR1m status, which was assessed in the ctDNA-evaluable population).
The planned sample size was 300 patients. The primary analysis was planned for when approximately 166 INV-PFS events from both arms had occurred; this enabled 80% power to detect a target INV-PFS hazard ratio (HR) of 0.647 (corresponding to an improvement in median INV-PFS from 5.5 to approximately 8.5 months) at a 5% (two-sided) level of significance. The largest HR determined to be statistically significant (minimal detectable difference) was approximately 0.738. Data for patients without disease progression or death at clinical cutoff were censored at the time of the last tumor assessment (or at the time of random assignment +1 day if no tumor assessment was performed after the baseline visit). INV-PFS was compared between arms using the stratified log-rank test, with the HR estimated using a stratified Cox proportional hazards model (stratified by disease site, prior CDK4/6i, and prior fulvestrant). Unstratified analyses were used for subgroups (excluding the secondary end point of INV-PFS in ESR1m tumors). For each treatment arm, Kaplan-Meier methodology was used to estimate median INV-PFS, and the Brookmeyer-Crowley method was used to construct the 95% CIs. OS was to be hierarchically tested using the same methodology if the primary end point was statistically significant at the primary analysis. For ORR analyses, patients without any postbaseline tumor assessment were considered nonresponders. An estimate of ORR and its 95% CI were calculated using the Clopper-Pearson method for each treatment arm. ORRs were compared between treatment arms using the stratified Cochran-Mantel-Haenszel test. The difference in ORR between treatment arms was calculated, and its 95% CI was calculated using the normal approximation to the binomial distribution. Analysis of DoR included only patients who had an objective response. The Kaplan-Meier approach was used to estimate the median DoR and the corresponding 95% CIs. CBR was analyzed using the same methods as those used for ORR. Safety analyses were descriptive.
RESULTS
Study Population
Between November 27, 2020, and October 27, 2021, 303 patients were enrolled (151 to giredestrant, 152 to PCET; Fig 1) across 85 sites in 17 countries. At clinical cutoff (February 18, 2022; median follow-up: 7.9 months), 106 patients (35.0%) were on treatment, 257 (84.8%) remained on study, and 46 (15.2%) had discontinued the study (Fig 1). In the PCET arm, 114 of 152 patients (75.0%) received fulvestrant and 38 (25.0%) received an AI.
FIG 1.
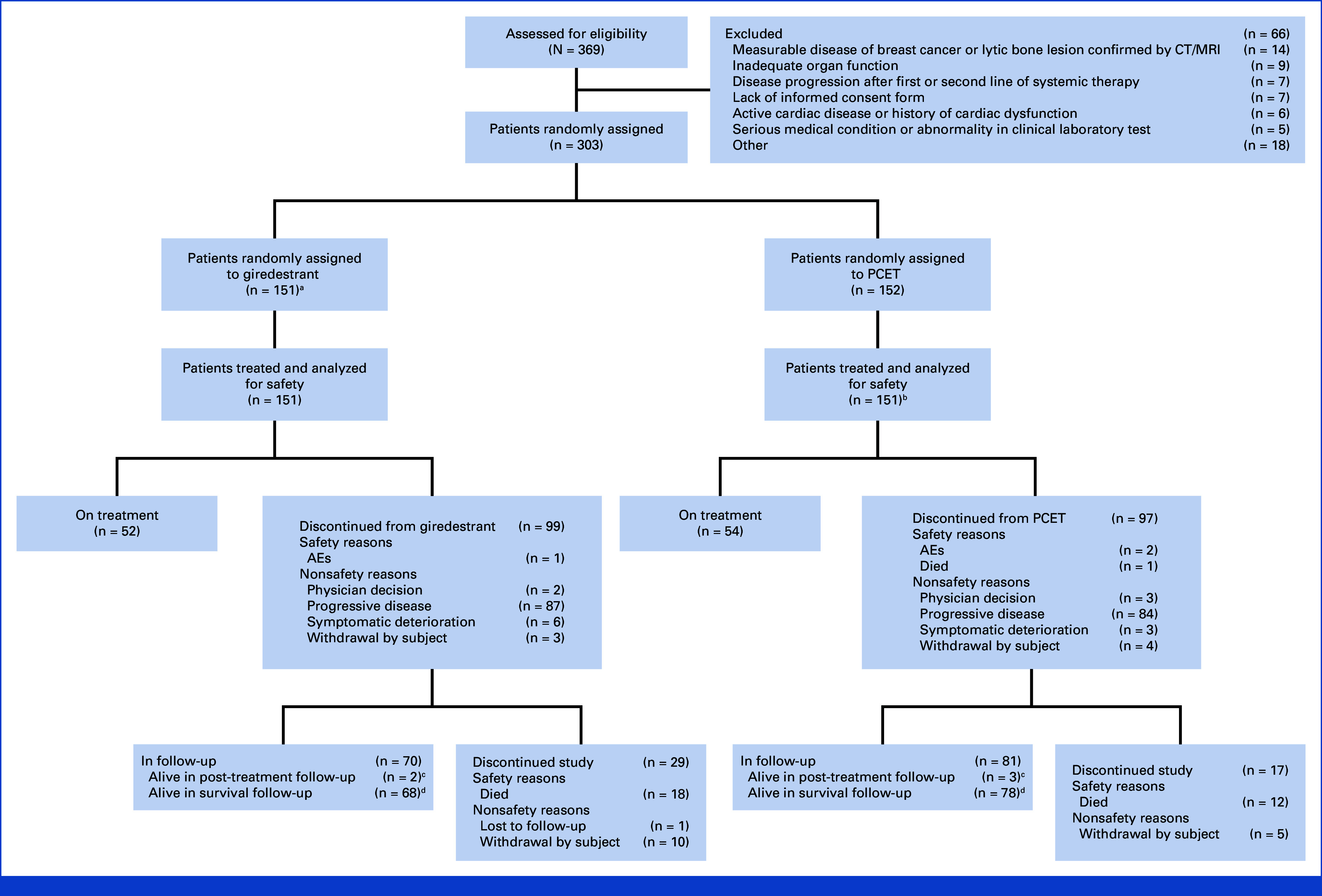
CONSORT diagram. aOne patient was randomly assigned to the giredestrant arm but received fulvestrant. bFulvestrant (n = 114), letrozole (n = 15), exemestane (n = 20), or anastrozole (n = 2). cPatients who discontinued treatment for reasons other than progressive disease. dPatients who discontinued treatment because of progressive disease. AE, adverse event; CT, computed tomography; MRI, magnetic resonance imaging; PCET, physician's choice of endocrine therapy.
Baseline demographics and disease characteristics are shown in Table 1. All patients had metastatic disease, and most were from Asia or Europe, were postmenopausal, and had visceral disease. Prior treatments for aBC included a CDK4/6i, chemotherapy, and fulvestrant. In the ctDNA-evaluable population, an ESR1m was detected in 90 of 232 patients (38.8%), with higher prevalence in the giredestrant (51 of 117 [43.6%]) than the PCET arm (39 of 115 [33.9%]).
TABLE 1.
Baseline Demographics, Disease Characteristics, and Prior Treatments in the FAS
| Patient Demographic, Disease Characteristic, or Prior Treatment | Giredestrant (n = 151) | PCET (n = 152) | All (N = 303) |
|---|---|---|---|
| Age, years | |||
| Median (range) | 60.0 (28-85) | 59.0 (32-93) | 60.0 (28-93) |
| ≥75, No. (%) | 16 (10.6) | 5 (3.3) | 21 (6.9) |
| Female, No. (%) | 151 (100) | 151 (99.3) | 302 (99.7) |
| Menopausal status, No. (%) | |||
| Pre-/perimenopausal | 23 (15.2) | 27 (17.9) | 50 (16.6) |
| Postmenopausal | 128 (84.8) | 124 (82.1) | 252 (83.4) |
| Race, No. (%) | |||
| American Indian or Alaska Native | 1 (0.7) | 0 | 1 (0.3) |
| Asian | 58 (38.4) | 66 (43.4) | 124 (40.9) |
| Black or African American | 2 (1.3) | 2 (1.3) | 4 (1.3) |
| White | 89 (58.9) | 81 (53.3) | 170 (56.1) |
| Unknown | 1 (0.7) | 2 (1.3) | 3 (1.0) |
| Multiple | 0 | 1 (0.7) | 1 (0.3) |
| Geographic region, No. (%) | |||
| Asia | 57 (37.7) | 65 (42.8) | 122 (40.3) |
| Europe | 57 (37.7) | 55 (36.2) | 112 (37.0) |
| Australia | 2 (1.3) | 5 (3.3) | 7 (2.3) |
| North America | 4 (2.6) | 1 (0.7) | 5 (1.7) |
| Africa | 3 (2.0) | 5 (3.3) | 8 (2.6) |
| South America | 28 (18.5) | 21 (13.8) | 49 (16.2) |
| ECOG performance status, No. (%) | |||
| 0 | 75 (49.7) | 82 (53.9) | 157 (51.8) |
| 1 | 76 (50.3) | 70 (46.1) | 146 (48.2) |
| Disease status, No. (%) | |||
| Viscerala | 104 (68.9) | 103 (67.8) | 207 (68.3) |
| Measurable | 141 (93.4) | 141 (92.8) | 282 (93.1) |
| CNS involvement | 3 (2.0) | 3 (2.0) | 6 (2.0) |
| Bone-only | 14 (9.3) | 14 (9.2) | 28 (9.2) |
| PgR-negative | 37 (24.5) | 32 (21.1) | 69 (22.8) |
| ESR1 status, No. (%) | n = 117 | n = 115 | n = 232b |
| Mutation detected | 51 (43.6) | 39 (33.9) | 90 (38.8) |
| No mutation detected | 66 (56.4) | 76 (66.1) | 142 (61.2) |
| Prior treatments for aBC, No. (%) | |||
| Prior lines | |||
| 1 | 103 (68.2) | 113 (74.3) | 216 (71.3) |
| 2 | 47 (31.1) | 38 (25.0) | 85 (28.1) |
| AI | 123 (81.5) | 111 (73.0) | 234 (77.2) |
| Last prior line | 102 (67.5) | 94 (61.8) | 196 (64.7) |
| Fulvestrant | 30 (19.9) | 28 (18.4) | 58 (19.1) |
| Last prior line | 24 (15.9) | 25 (16.4) | 49 (16.2) |
| Tamoxifen | 20 (13.2) | 32 (21.1) | 52 (17.2) |
| Last prior line | 15 (9.9) | 24 (15.8) | 39 (12.9) |
| Targeted | 72 (47.7) | 67 (44.1) | 139 (45.9) |
| Last prior line | 63 (41.7) | 58 (38.2) | 121 (39.9) |
| CDK4/6i | 65 (43.0) | 62 (40.8) | 127 (41.9) |
| Last prior line | 56 (37.1) | 53 (34.9) | 109 (36.0) |
| Chemotherapy | 47 (31.1) | 49 (32.2) | 96 (31.7) |
| Last prior line | 34 (22.5) | 40 (26.3) | 74 (24.4) |
Abbreviations: aBC, advanced breast cancer; AI, aromatase inhibitor; BIRC, blinded independent central review; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ctDNA, circulating tumor DNA; ECOG, Eastern Cooperative Oncology Status; FAS, full analysis set; PCET, physician's choice of endocrine therapy; PgR, progesterone receptor.
Visceral disease was defined as presence of liver and/or lung lesions. Looking specifically at liver involvement at baseline (by BIRC), there was a higher proportion of patients with at least one lesion in the liver in the giredestrant arm (37.1%) v the PCET arm (31.6%), which may have contributed to an observed imbalance in baseline AST (giredestrant: 9.3% v PCET: 0%) and ALT (giredestrant: 6.6% v PCET: 0.7%) levels, and might further be attributable to differences in tumor burden in the liver.
Seventy-one patients were excluded because of nonavailability or testing failure of ctDNA sample.
Efficacy
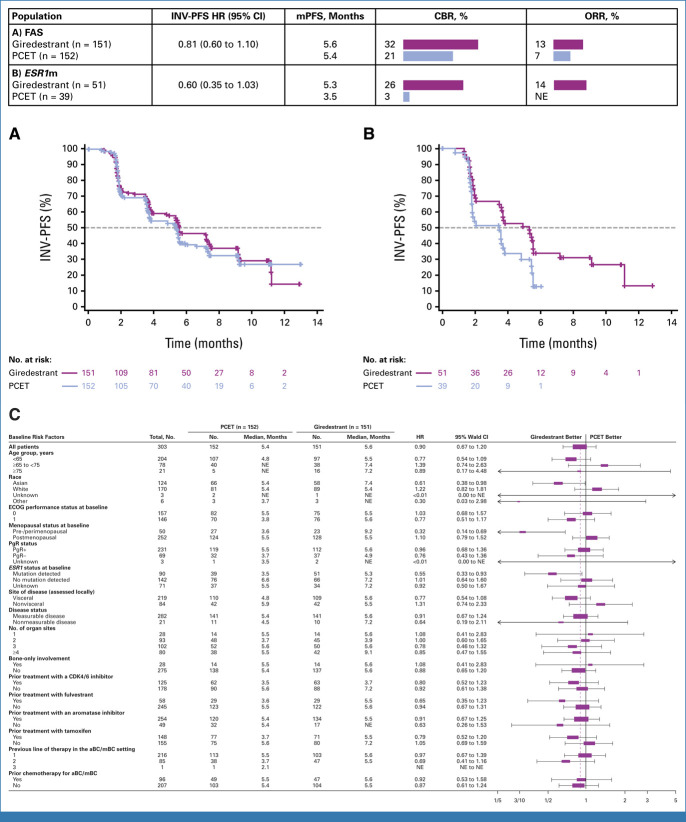
The INV-PFS HR was 0.81 (95% CI, 0.60 to 1.10; P = .1757), with medians of 5.6 months in the giredestrant arm and 5.4 months in the PCET arm (patients with an event: 90 [59.6%] and 92 [60.5%]; Fig 2A). The INV-PFS rates at 6 months were 46.8% and 39.6% in the giredestrant and PCET arms, respectively. In the BIRC-PFS sensitivity analysis, the HR was 0.92 (95% CI, 0.64 to 1.33). In the prespecified secondary end point analysis of INV-PFS by ESR1m status in the ctDNA-evaluable population, the HR in patients with a detectable ESR1m was 0.60 (95% CI, 0.35 to 1.03; Fig 2B), versus 0.88 (95% CI, 0.54 to 1.42) in patients with no ESR1m detected (Data Supplement, Fig S2A). Further exploratory subgroup analyses for PFS are shown in Figure 2C-E and the Data Supplement (Figs S2B-S2G).
FIG 2.
INV-PFS, CBR, and ORR in (A) the FAS (primary end point) and in (B) patients with ESR1m tumors; (C) INV-PFS in subgroups of the FAS. aBC, advanced breast cancer; CBR, clinical benefit rate (complete or partial response, or stable disease for ≥6 months, calculated in the FAS); CDK4/6, cyclin-dependent kinase 4/6; ECOG, Eastern Cooperative Oncology Group; ESR1m, ESR1 mutation; ET, endocrine therapy; FAS, full analysis set; HR, hazard ratio (stratified); INV-PFS, investigator-assessed progression-free survival; mPFS, median progression-free survival; mBC, metastatic breast cancer; NE, not evaluable; ORR, objective response rate (confirmed complete or partial response, calculated in the FAS); PCET, physician's choice of endocrine therapy; PgR, progesterone receptor.
Secondary efficacy end points are shown in Table 2. Confirmed ORRs were all PRs, and OS data were immature. Most deaths were due to disease progression, with the imbalance between arms because of reasons classified as other, which were unrelated to study treatment and outside of the AE reporting period (COVID-19 [two patients], septic shock due to gastroenteritis, COVID-19 pneumonia, and septic shock in the giredestrant arm, and septicemia in the PCET arm [one patient each]; Table 2).
TABLE 2.
Secondary Efficacy End Points
| End Point | Giredestrant (n = 151) | PCET (n = 152) |
|---|---|---|
| Confirmed ORR, No. (%) | 19 (12.6) | 11 (7.2) |
| 95% CI | 7.75 to 18.95 | 3.67 to 12.58 |
| OR (95% CI) | 1.87 (0.86 to 4.07) | |
| DoR | n = 19 | n = 11 |
| Median, months (95% CI) | NR (5.55 to NE) | 7.39 (7.39 to NE) |
| Range, months | 2.0a to 8.9a | 2.8a to 9.3a |
| CBR, No. (%) | 48 (31.8) | 32 (21.1) |
| 95% CI | 24.46 to 39.85 | 14.87 to 28.40 |
| OR (95% CI) | 1.79 (1.06 to 3.04) | |
| OS: deaths, No. (%) | 18 (11.9) | 11 (7.2) |
| PD, No. | 12 | 9 |
| Grade 5 AE, No. | 1b | 1c |
| Other (post-treatment) | 5d | 1e |
Abbreviations: AE, adverse event; CBR, clinical benefit rate; DoR, duration of response; NE, nonestimable; NR, not reached; OR, odds ratio; ORR, objective response rate; OS, overall survival; PCET, physician's choice of endocrine therapy; PD, progressive disease.
Censored value.
Ischemic stroke.
Pulmonary embolism.
Unrelated–outside of AE reporting period (>30 days post-treatment discontinuation): three COVID-19–related, two septic shocks.
Unrelated–outside of AE reporting period (>30 days post-treatment discontinuation): septicemia.
Safety
The mean dose intensity was 96.98% (standard deviation [SD], 9.60) for giredestrant and 99.57% (SD, 2.19) for PCET; treatment duration is shown in the Data Supplement (Table S1).
The safety profile is shown in Table 3. The most common AEs (in ≥10% of patients in either arm) were AST increased, arthralgia, ALT increased, anemia, and nausea. The most common grade 3-4 AEs (in ≥3 patients in either arm) were anemia, hypertension, AST increased, bone pain, and blood bilirubin increased. Selected AEs are also shown in Table 3; hepatotoxicity-related selected AEs and shift tables for liver function tests are shown in the Data Supplement (Tables S2 and S3, respectively).
TABLE 3.
Overall Safety Profile, AEs Occurring in ≥10% of Patients in Either Arm, Most Common Grade 3-4 AEs in ≥3 Patients, and Selected AEs
| Safety Profile | Giredestrant (n = 150) | PCET (n = 152) |
|---|---|---|
| Overall Safety Profile | ||
| All-grade AEs | 127 (84.7) | 108 (71.1) |
| Treatment-related | 70 (46.7) | 43 (28.3) |
| Grade 3-4 AEs | 26 (17.3) | 18 (11.8) |
| Treatment-related | 6 (4.0) | 4 (2.6) |
| Serious AEs | 14 (9.3) | 12 (7.9) |
| Treatment-related | 3 (2.0) | 1 (0.7) |
| Grade 5 AEs | 1 (0.7)a | 1 (0.7)b |
| AEs leading to treatment discontinuation | 2 (1.3) | 3 (2.0) |
| AEs Occurring in ≥10% of Patients in Either Arm | ||
| AST increased | 22 (14.7) | 13 (8.6) |
| Arthralgia | 18 (12.0) | 12 (7.9) |
| ALT increased | 17 (11.3) | 11 (7.2) |
| Anemia | 15 (10.0) | 9 (5.9) |
| Nausea | 15 (10.0) | 11 (7.2) |
| Most Common Grade 3-4 AEs in ≥3 Patients | ||
| Anemia | 2 (1.3) | 5 (3.3) |
| Hypertension | 3 (2.0) | 3 (2.0) |
| AST increased | 4 (2.7) | 1 (0.7) |
| Bone pain | 3 (2.0) | 1 (0.7) |
| Blood bilirubin increased | 3 (2.0) | 0 |
| Selected AEs | ||
| Hepatotoxicity | ||
| Any grade | 35 (23.3) | 21 (13.8) |
| Grade 1-2 | 30 (20.0) | 19 (12.5) |
| Grade 3-4 | 5 (3.3) | 2 (1.3) |
| Musculoskeletal pain | ||
| Any grade | 22 (14.7) | 22 (14.5) |
| Grade 1-2 | 18 (12.0) | 21 (13.8) |
| Grade 3-4 | 4 (2.7) | 1 (0.7) |
| Fatigue | ||
| Any grade | 21 (14.0) | 12 (7.9) |
| Grade 1-2 | 20 (13.3) | 12 (7.9) |
| Grade 3-4 | 1 (0.7) | 0 |
| Arthralgia | ||
| Any grade | 18 (12.0) | 12 (7.9) |
| Grade 1-2 | 18 (12.0) | 12 (7.9) |
| Nausea | ||
| Any grade | 15 (10.0) | 11 (7.2) |
| Grade 1-2 | 15 (10.0) | 10 (6.6) |
| Grade 3-4 | 0 | 1 (0.7) |
| Diarrhea | ||
| Any grade | 13 (8.7) | 6 (3.9) |
| Grade 1-2 | 12 (8.0) | 6 (3.9) |
| Grade 3-4 | 1 (0.7) | 0 |
| Dizziness | ||
| Any grade | 9 (6.0) | 7 (4.6) |
| Grade 1-2 | 9 (6.0) | 7 (4.6) |
| Vomiting | ||
| Any grade | 13 (8.7) | 2 (1.3) |
| Grade 1-2 | 11 (7.3) | 2 (1.3) |
| Grade 3-4 | 2 (1.3) | 0 |
| Hot flushes | ||
| Any grade | 6 (4.0) | 7 (4.6) |
| Grade 1-2 | 6 (4.0)c | 7 (4.6)c |
| Bradycardia | ||
| Any grade | 5 (3.3) | 2 (1.3) |
| Grade 1-2 | 5 (3.3)c | 2 (1.3)c |
| Renal toxicity | ||
| Any grade | 4 (2.7) | 1 (0.7) |
| Grade 1-2 | 4 (2.7) | 0 |
| Grade 3-4 | 0 | 1 (0.7) |
| QT prolongation (preferred term: electrocardiogram QT prolonged) | ||
| Any grade | 1 (0.7) | 2 (1.3) |
| Grade 1-2 | 1 (0.7)c | 2 (1.3)c |
| VTE (preferred term: pulmonary embolism) | ||
| Any grade | 0 | 1 (0.7) |
| Grade 5 | 0 | 1 (0.7) |
NOTE: Safety population. Data are No. of patients with ≥1 AE (%). Multiple occurrences of the same AE in one individual are counted only once. Missed doses were counted as an AE (without being graded) in the PCET arm. Preferred terms coded using MedDRA v24.1 and AE severity graded according to NCI-CTCAE v5.0. Selected AEs were defined based on the known potential and identified risks of giredestrant (fatigue, nausea, dizziness, hot flush, arthralgia, vomiting, musculoskeletal pain, bradycardia, diarrhea, hepatotoxicity, renal dysfunction, VTEs, and QT prolongation). Preferred terms (MedDRA v24.1) occurring in each category were hepatotoxicity: AST increased (14.7% v 8.6% at any grade; 2.7% v 0.7% at grade 3-4), ALT increased (11.3% v 7.2%; 1.3% v 0.7%), blood bilirubin increased (6.0% v 0.7%; 2.0% v 0), gamma-glutamyl transferase increased (2.7% v 2.0%; 0 v 1.3%), ascites (1.3% v 0.7%; 0 v 0), hyperbilirubinemia (1.3% v 0; 0 v 0), AST abnormal (0 v 0.7%; 0 v 0),c bilirubin conjugated increased (0.7% v 0; 0 v 0), hepatic failure (0 v 0.7%; 0 v 0),c hepatic pain (0.7% v 0; 0 v 0), or hepatic steatosis (0.7% v 0; 0 v 0);c musculoskeletal pain: bone pain (6.0% v 6.6%; 2.0% v 0.7%), back pain (5.3% v 2.6%; 0 v 0), pain in extremity (2.7% v 4.6%; 0 v 0.7%), myalgia (3.3% v 2.0%; 0.7% v 0), or musculoskeletal pain (0 v 2.0%; 0 v 0); fatigue: fatigue (7.3% v 3.9%; 0 v 0)c or asthenia (6.7% v 3.9%; 0.7% v 0); bradycardia: sinus bradycardia (3.3% v 0; 0 v 0)c or bradycardia (0 v 1.3%; 0 v 0);c renal toxicity: blood creatinine increased (2.7% v 0.7%; 0 v 0), azotemia (0.7% v 0; 0 v 0), blood urea increased (0.7% v 0; 0 v 0),c or renal failure (0 v 0; 0 v 0.7%).d
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; NCI-CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events; PCET, physician's choice of endocrine therapy; VTE, venous thromboembolism.
Ischemic stroke.
Pulmonary embolism.
Grade 1 only.
Grade 4 only.
In general, laboratory shifts from baseline for AST and ALT were consistent between the study arms, with a slightly higher number of patients in the giredestrant arm experiencing bilirubin shifts versus PCET.
PROs
For key PROs, mean scores generally remained stable; however, differences between arms were observed for time to deterioration in pain presence and interference (HR, 0.55 [95% CI, 0.35 to 0.87]), pain severity (HR, 0.84 [95% CI, 0.51 to 1.38]), physical functioning (HR, 0.61 [95% CI, 0.36 to 1.05]), and global health status/QoL (HR, 0.67 [95% CI, 0.39 to 1.16]), though not in role functioning (HR, 0.96 [95% CI, 0.60 to 1.54]), and without medians being reached (Data Supplement, Fig S3).
A comparison of selected AEs via PRO-CTCAE versus clinician-reported AEs is shown in the Data Supplement (Table S4). Fatigue, nausea, diarrhea, and vomiting were mostly mild/moderate or occurred rarely/infrequently; similarly, AEs were reported as being mostly grade 1-2 by clinicians.
DISCUSSION
Although the phase II acelERA BC study did not reach statistical significance for its primary INV-PFS end point, giredestrant showed a numerical improvement versus PCET, with an approximately 20% relative reduction in the risk of progression or death in the overall population, and favorable results for the secondary efficacy end points CBR and ORR.
Other randomized studies of new oral SERDs versus single-agent ET in the pretreated aBC setting have shown conflicting efficacy results. Although the phase III EMERALD trial of elacestrant met its coprimary end points of PFS in the intention-to-treat and ESR1m populations (elacestrant was approved by the US Food and Drug Administration in 2023 in the ESR1m population only20),21 and the phase II SERENA-2 multidose trial demonstrated a statistically significant (two-sided alpha 10%) benefit of camizestrant versus fulvestrant,22 the phase II AMEERA-3 study of amcenestrant did not show superiority or numerical improvements in the intention-to-treat population.23 Cross-trial comparisons are challenging because of marked differences in patient populations (eg, prior CDK4/6i, ESR1m prevalence), sample sizes, comparator choice, and end point assessment (BICR v INV).23 Nevertheless, the limited efficacy gains over available single-agent ET observed with novel oral SERDs in later-line aBC questions the clinical usefulness as monotherapy in this setting. In particular, in an unselected population, combination treatment approaches might be warranted, as in the ongoing evERA BC study with giredestrant plus everolimus.24
In acelERA BC, giredestrant trended toward a favorable benefit among patients with ESR1m tumors, consistent with findings in EMERALD21 and SERENA-2,22 indicating that giredestrant, a potent antagonist of mutant ER-reversing progesterone hypersensitivity,25 can target this mechanism of endocrine resistance more effectively than an AI or fulvestrant. In later lines, particularly in patients without ESR1m tumors, additional mechanisms of resistance emerge that progressively decrease the dependence on the ER pathway,26,27 as evidenced by the limited efficacy of single-agent ET in the aforementioned studies in patients without ESR1m tumors. However, the efficacy of giredestrant is not only linked to its activity in ESR1m tumors. In earlier lines or ET-naive settings, where ESR1m is infrequent and tumors are more dependent on ER signaling,28 superiority over a standard-of-care AI (anastrozole) was demonstrated with giredestrant in the coopERA BC study in terms of reduction of Ki67.29,30 Giredestrant thus has the potential to improve on AIs and fulvestrant given its mechanism of action, which targets both estrogen-dependent and -independent (eg, ESR1m) ER activity, with superior potency.
Other subgroups, such as Asian patients and those who were pre-/perimenopausal, appeared to show a benefit of giredestrant; however, there were important confounders (eg, imbalances in baseline characteristics, small numbers of patients), which mean results should be interpreted with caution.
Giredestrant exhibited manageable toxicities, the vast majority being grade 1 or 2, and its safety profile, including cardiac safety, appeared comparable overall to the PCET control and was consistent with known ET risks and previous experience.17,31 Related grade 3-4 AEs, serious AEs, and discontinuations due to AEs were balanced across arms. Higher hepatotoxicity was reported with giredestrant (Table 3 and Data Supplement, Table S2); however, shifts in laboratory values of AST/ALT elevations were comparable across treatment arms (Data Supplement, Table S3), suggesting that liver abnormalities were similar across arms. Factors likely contributing to this were imbalances in proportions of patients with abnormal baseline AST/ALT levels (Table 1), as well as differential reporting between arms. GI toxicities occurred at a lower incidence and grades with giredestrant than reported with other oral SERDs.21,23
Patient-reported selected AEs showed, overall, no meaningful differences between arms compared with those reported by clinicians.
This phase II study has a number of limitations that may have impacted the results. The population was markedly heterogeneous, and, driven by the geographic footprint of the study (that included regions where CDK4/6is are not standard of care), included a majority of CDK4/6i-naive patients, with lower prevalence of ESR1m and higher use of first-line chemotherapy, and in whom efficacy versus PCET appeared worse than in those with prior CDK4/6i therapy. The relevance of endocrine monotherapy as a comparator for second-/third-line treatment in ER+, HER2– aBC, while commonly used, might be argued in some regions where combination therapy is preferred, such as the United States (especially with fit patients).6,32
Inherent to the open-label design, which was motivated by ethical concerns regarding intramuscular placebo administration, is the risk of investigator bias. For efficacy, there was a notable discordance between INV and BIRC assessments, and for safety, a trend toward underreporting of AEs in the PCET arm emerged in comparison with the expected rates from previous experience, likely related to the well-known safety profile of comparator ETs. The opposite trend was observed for the giredestrant arm, as evidenced by the comparison of selected common AEs reported by investigators versus by patients. With regard to the ESR1m assessment, of note were the approximately 25% sample attrition and imbalances in ESR1m prevalence between treatment arms (mutations were approximately 10% more frequent in the giredestrant arm), as baseline ESR1m status was not a random assignment stratification factor. ESR1m is a complex net effect, as it is negatively prognostic but positively predictive. However, the biologic plausibility and external replication with other agents of the oral SERD class21,22 provides reassurance regarding the findings of a larger magnitude of benefit of giredestrant versus PCET, and specifically versus fulvestrant, in patients with ESR1m tumors.
Giredestrant is currently being investigated in two ongoing phase III trials in ER+, HER2– aBC: persevERA BC versus letrozole as an add-on to palbociclib in the first-line setting33; and evERA BC with everolimus in the postCDK4/6i setting.24 In addition, lidERA BC34 is a large, currently recruiting adjuvant study in medium-to-high risk ER+, HER2– early BC; the rationale for which is supported by the positive results of the randomized phase II neoadjuvant coopERA BC study.29,30,35 The target populations in these studies include patients with tumors with expected high dependence on ER signaling and likelihood of benefit with giredestrant.
In conclusion, while the phase II acelERA BC study did not reach statistical significance for its primary INV-PFS end point, there was a consistent treatment effect with giredestrant across most key subgroups and a trend toward favorable benefit among patients with ESR1m tumors. Secondary efficacy end points numerically favored giredestrant in terms of CBR and ORR; while DoR and OS were still immature. Giredestrant was well tolerated, with a safety profile comparable to that of PCET and consistent with known ET risks. Grade 3-4 treatment-related AEs, serious AEs, and discontinuations due to AEs were balanced across arms. Overall, these data support the continued investigation of giredestrant in other studies.
ACKNOWLEDGMENT
The authors thank the patients and their families, participating study investigators, and clinical sites. Research support in the form of third-party medical writing assistance, including the initial draft, was provided by Daniel Clyde, PhD (Nucleus Global, an Inizio company, Manchester, United Kingdom), funded by F. Hoffmann-La Roche Ltd. A list of the acelERA Breast Cancer Study investigators can be found in the Appendix (online only).
APPENDIX. LIST OF INVESTIGATORS
The following investigators participated in the acelERA Breast Cancer study:
Argentina—G. Aguil, M. Alfie, V. Caceres, G. Lerzo, S. Ostoich
Australia—F. Boyle, E. Lim, H. Martin, C. Oakman
Brazil—F.M. Cruz, F.A. Franke, A. Mattar, E.H. Silva, K. Tiscoski
China—W. Chen, W. Li, Z. Tong, J. Wang, S. Wang, X. Wang, J. Wu, X. Wu, J. Yang, Q. Zhang
Germany—T.-O. Emde, G. Gaffunder, C. Hielscher, M. Lux, C. Schem, M. Welslau, C. Schumacher
Israel—I. Kuchuk, T. Peretz, L. Ryvo, R. Yerushalmi
Republic of Korea—H. Chae, Y. S. Chae, S.-A. Im, H. J. Kim, J. H. Kim, S.-B. Kim, J. E. Lee, Y. H. Park, J. Sohn
Poland—M. Jarząb, M. Nowaczyk, Z. Nowecki, T. Pienkowski, M. Wojtukiewicz, P. Wysocki
Russia—E. Fomin, I. Ganshina, N. Kislov, M. Kopp, N. Kovalenko, Y. Makarova, M. Matrosova, R. Orlova, A. Poltoratsky, R. Safin, R. Zukov
Singapore—A. Wong, Y.S. Yap
South Africa—M. Coccia-Portugal, N. Fourie, R. Khanyile, L. Schoeman
Taiwan—T.-C. Chao, S.-T. Chen, W.-P. Chung, Y.-H. Feng, Y.-C. Lin
Thailand—T. Dejthevaporn, N. Parinyanitikul, C. Sathitruangsak, A. Somwangprasert, P. Tienchaianada
Turkey—A. Alacacioglu, E. Algin, D. Cabuk, C. Demir, U. Demirci, D. Erdem, Ş. Gündüz, M.A. Kaplan, M.E. Yildirim
UK—S. Khan, P. Schmid, I. Sandri, O. Oikonomidou, T. Ansari, A. Konstantis
Ukraine—S. Hrybach, A. Krochkin, O. Lipetska, D. Osinskii
USA—J.C. Andersen, M. Cairo, P. Cobb, V. Konala, S.L. McCune, A.J. Montero, D.A. Patt, I. Sanchez-Rivera, S. Strain, K. Wendell
Miguel Martín
Honoraria: Roche/Genentech, Lilly, Pfizer, Novartis, Pierre Fabre, Seagen
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Lilly, AstraZeneca, Daiichi-Sankyo
Speakers' Bureau: Lilly/ImClone, Lilly/ImClone, Roche/Genentech, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Puma Biotechnology (Inst)
Travel, Accommodations, Expenses: Daiichi-Sankyo
Other Relationship: Roche, Novartis
Elgene Lim
Consulting or Advisory Role: Novartis (Inst), Lilly (Inst), Pfizer (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), MSD Australia (Inst)
Speakers' Bureau: Limbic Australia
Research Funding: Novartis (Inst), Pfizer (Inst), Ellipses Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737
Travel, Accommodations, Expenses: Gilead Sciences, Novartis
Other Relationship: Roche/Genentech, Lilly, Novartis
Mariana Chavez-MacGregor
Employment: MD Anderson Physician's Network
Consulting or Advisory Role: Exact Sciences, AstraZeneca/Daiichi Sankyo, Pfizer, Abbott Laboratories, Exact Sciences, Pfizer, Lilly, AstraZeneca/Daiichi Sankyo, Exact Sciences, Roche/Genentech, Adium Pharma, Merck
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Lilly
Expert Testimony: Lilly
Travel, Accommodations, Expenses: AstraZeneca, Exact Sciences, Zodiac Pharma
Uncompensated Relationships: Legacy Healthcare Services, The Hope Foundation
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Merck, Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Sanofi, Daiichi Sankyo/Astra Zeneca, Lilly, Lilly (Inst), Gilead Sciences, Gilead Sciences (Inst), Menarini, Menarini (Inst), Mersana
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
Jiong Wu
Leadership: Chinese Breast Cancer Society (Chairman), Breast Surgeon Committee (Vice Chairman), Chinese College of Surgeons
Honararia: Lilly, F. Hoffmann-La Roche Ltd, AstraZeneca, Pfizer
Research Funding: National Natural Science Foundation (Inst), F. Hoffmann-La Roche Ltd (Inst)
Patents, Royalties, Other Intellectual Property: Fudan University Shanghai Cancer Center (Microvascular anastomosis support dilator; degradable breast-conserving stent)
Qingyuan Zhang
Research Funding: F. Hoffmann-La Roche Ltd (Inst)
Zbigniew Nowecki
Travel, Accommodations, Expenses: F. Hoffmann-La Roche Ltd/Genentech, Inc
Research Funding: F. Hoffmann-La Roche Ltd (Inst)
Felipe Melo Cruz
Consulting or Advisory Role: Pfizer
Travel, Accommodations, Expenses: Janssen Oncology, Novartis
Rustem Safin
Speakers’ Bureaus: F. Hoffmann-La Roche Ltd
Research Funding: F. Hoffmann-La Roche Ltd (Inst)
Sung-Bae Kim
Stock and Other Ownership Interests: Genopeaks
Honoraria: DAEHWA Pharmaceutical, LegoChem Biosciences, Kalbe Farma
Consulting or Advisory Role: Lilly (Inst), AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis, BeiGene, Daiichi Sankyo/Astra Zeneca, OBI Pharma
Research Funding: Novartis (Inst), Dongkook Pharma (Inst), Genzyme (Inst)
Christian Schem
Honoraria: Roche, Lilly, AstraZeneca, MSD Oncology, Exact Sciences, Novartis
Consulting or Advisory Role: Novartis, AstraZeneca, Roche
Speakers' Bureau: Roche, AstraZeneca, Novartis
Research Funding: Roche (Inst), Daiichi Sankyo Europe GmbH (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca, Novartis, Gilead Sciences
Alberto J. Montero
Honoraria: Celgene, AstraZeneca, OncoSec
Consulting or Advisory Role: New Century Health, Welwaze, Paragon healthcare
Research Funding: F. Hoffmann-La Roche Ltd, Basel, Switzerland
Uncompensated Relationships: Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/618396
Sarah Khan
Honoraria: Novartis Pharmaceuticals UK Ltd, Roche/Genentech
Consulting or Advisory Role: Lilly
Travel, Accommodations, Expenses: Novartis Pharmaceuticals UK Ltd
Reeti Bandyopadhyay
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Heather M. Moore
Employment: Genentech, Pfizer (I)
Stock and Other Ownership Interests: Roche, Pfizer (I)
Mahesh Shivhare
Employment: Roche
Stock and Other Ownership Interests: Roche
Monika Patre
Employment: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: US20190030181A1 Methods for treating HER2 breast cancer
Jorge Martinalbo
Employment: Roche, Novartis, Inhibrx
Stock and Other Ownership Interests: Roche
Laura Roncoroni
Employment: Roche, AstraZeneca
Stock and Other Ownership Interests: Roche
Pablo Diego Pérez-Moreno
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech, Merck, Zymeworks, Johnson & Johnson/Janssen, Johnson & Johnson/Janssen
Joohyuk Sohn
Stock and Other Ownership Interests: Daiichi Sankyo
Research Funding: MSD (Inst), Roche (Inst), Novartis (Inst), Lilly (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Seagan (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2021 ASCO Virtual Congress, virtual, June 4-8, 2021; the 2022 European Society for Medical Oncology Congress, Paris, France, September 9-13, 2022; the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2022; and the 2023 ASCO Congress, Chicago, IL, June 2-6, 2023.
SUPPORT
Supported by F. Hoffmann-La Roche Ltd.
CLINICAL TRIAL INFORMATION
NCT04576455 (acelERA Breast Cancer)
Contributor Information
Collaborators: G. Aguil, M. Alfie, V. Caceres, G. Lerzo, S. Ostoich, F. Boyle, E. Lim, H. Martin, C. Oakman, F.M. Cruz, F.A. Franke, A. Mattar, E.H. Silva, K. Tiscoski, W. Chen, W. Li, Z. Tong, J. Wang, S. Wang, X. Wang, J. Wu, X. Wu, J. Yang, Q. Zhang, T.-O. Emde, G. Gaffunder, C. Hielscher, M. Lux, C. Schem, M. Welslau, C. Schumacher, I. Kuchuk, T. Peretz, L. Ryvo, R. Yerushalmi, H. Chae, Y. S. Chae, S.-A. Im, H. J. Kim, J. H. Kim, S.-B. Kim, J. E. Lee, Y. H. Park, J. Sohn, M. Jarząb, M. Nowaczyk, Z. Nowecki, T. Pienkowski, M. Wojtukiewicz, P. Wysocki, E. Fomin, I. Ganshina, N. Kislov, M. Kopp, N. Kovalenko, Y. Makarova, M. Matrosova, R. Orlova, A. Poltoratsky, R. Safin, R. Zukov, A. Wong, Y.S. Yap, M. Coccia-Portugal, N. Fourie, R. Khanyile, L. Schoeman, T.-C. Chao, S.-T. Chen, W.-P. Chung, Y.-H. Feng, Y.-C. Lin, T. Dejthevaporn, N. Parinyanitikul, C. Sathitruangsak, A. Somwangprasert, P. Tienchaianada, A. Alacacioglu, E. Algin, D. Cabuk, C. Demir, U. Demirci, D. Erdem, Ş. Gündüz, M.A. Kaplan, M.E. Yildirim, S. Khan, P. Schmid, I. Sandri, O. Oikonomidou, T. Ansari, A. Konstantis, S. Hrybach, A. Krochkin, O. Lipetska, D. Osinskii, S. Hrybach, A. Krochkin, O. Lipetska, D. Osinskii, J.C. Andersen, M. Cairo, P. Cobb, V. Konala, S.L. McCune, A.J. Montero, D.A. Patt, I. Sanchez-Rivera, S. Strain, and K. Wendell
DATA SHARING STATEMENT
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli: https://vivli.org/ourmember/roche/. For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient reidentification.
AUTHOR CONTRIBUTIONS
Conception and design: Miguel Martín, Heather M. Moore, Mahesh Shivhare, Monika Patre, Laura Roncoroni, Pablo Diego Pérez-Moreno
Provision of study materials or patients: Jiong Wu, Qingyuan Zhang, Rustem Safin, Sung-Bae Kim, Christian Schem, Alberto J. Montero, Joohyuk Sohn
Collection and assembly of data: Elgene Lim, Qingyuan Zhang, Zbigniew Nowecki, Felipe Melo Cruz, Rustem Safin, Sarah Khan, Reeti Bandyopadhyay, Heather M. Moore, Jorge Martinalbo, Laura Roncoroni, Pablo Diego Pérez-Moreno, Joohyuk Sohn
Data analysis and interpretation: Miguel Martín, Elgene Lim, Mariana Chavez-MacGregor, Aditya Bardia, Jiong Wu, Zbigniew Nowecki, Felipe Melo Cruz, Sung-Bae Kim, Christian Schem, Alberto J. Montero, Sarah Khan, Reeti Bandyopadhyay, Heather M. Moore, Mahesh Shivhare, Jorge Martinalbo, Laura Roncoroni, Pablo Diego Pérez-Moreno
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Giredestrant for Estrogen Receptor–Positive, HER2-Negative, Previously Treated Advanced Breast Cancer: Results From the Randomized, Phase II acelERA Breast Cancer Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Miguel Martín
Honoraria: Roche/Genentech, Lilly, Pfizer, Novartis, Pierre Fabre, Seagen
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Lilly, AstraZeneca, Daiichi-Sankyo
Speakers' Bureau: Lilly/ImClone, Lilly/ImClone, Roche/Genentech, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Puma Biotechnology (Inst)
Travel, Accommodations, Expenses: Daiichi-Sankyo
Other Relationship: Roche, Novartis
Elgene Lim
Consulting or Advisory Role: Novartis (Inst), Lilly (Inst), Pfizer (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), MSD Australia (Inst)
Speakers' Bureau: Limbic Australia
Research Funding: Novartis (Inst), Pfizer (Inst), Ellipses Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737
Travel, Accommodations, Expenses: Gilead Sciences, Novartis
Other Relationship: Roche/Genentech, Lilly, Novartis
Mariana Chavez-MacGregor
Employment: MD Anderson Physician's Network
Consulting or Advisory Role: Exact Sciences, AstraZeneca/Daiichi Sankyo, Pfizer, Abbott Laboratories, Exact Sciences, Pfizer, Lilly, AstraZeneca/Daiichi Sankyo, Exact Sciences, Roche/Genentech, Adium Pharma, Merck
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Lilly
Expert Testimony: Lilly
Travel, Accommodations, Expenses: AstraZeneca, Exact Sciences, Zodiac Pharma
Uncompensated Relationships: Legacy Healthcare Services, The Hope Foundation
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Merck, Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Sanofi, Daiichi Sankyo/Astra Zeneca, Lilly, Lilly (Inst), Gilead Sciences, Gilead Sciences (Inst), Menarini, Menarini (Inst), Mersana
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
Jiong Wu
Leadership: Chinese Breast Cancer Society (Chairman), Breast Surgeon Committee (Vice Chairman), Chinese College of Surgeons
Honararia: Lilly, F. Hoffmann-La Roche Ltd, AstraZeneca, Pfizer
Research Funding: National Natural Science Foundation (Inst), F. Hoffmann-La Roche Ltd (Inst)
Patents, Royalties, Other Intellectual Property: Fudan University Shanghai Cancer Center (Microvascular anastomosis support dilator; degradable breast-conserving stent)
Qingyuan Zhang
Research Funding: F. Hoffmann-La Roche Ltd (Inst)
Zbigniew Nowecki
Travel, Accommodations, Expenses: F. Hoffmann-La Roche Ltd/Genentech, Inc
Research Funding: F. Hoffmann-La Roche Ltd (Inst)
Felipe Melo Cruz
Consulting or Advisory Role: Pfizer
Travel, Accommodations, Expenses: Janssen Oncology, Novartis
Rustem Safin
Speakers’ Bureaus: F. Hoffmann-La Roche Ltd
Research Funding: F. Hoffmann-La Roche Ltd (Inst)
Sung-Bae Kim
Stock and Other Ownership Interests: Genopeaks
Honoraria: DAEHWA Pharmaceutical, LegoChem Biosciences, Kalbe Farma
Consulting or Advisory Role: Lilly (Inst), AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis, BeiGene, Daiichi Sankyo/Astra Zeneca, OBI Pharma
Research Funding: Novartis (Inst), Dongkook Pharma (Inst), Genzyme (Inst)
Christian Schem
Honoraria: Roche, Lilly, AstraZeneca, MSD Oncology, Exact Sciences, Novartis
Consulting or Advisory Role: Novartis, AstraZeneca, Roche
Speakers' Bureau: Roche, AstraZeneca, Novartis
Research Funding: Roche (Inst), Daiichi Sankyo Europe GmbH (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca, Novartis, Gilead Sciences
Alberto J. Montero
Honoraria: Celgene, AstraZeneca, OncoSec
Consulting or Advisory Role: New Century Health, Welwaze, Paragon healthcare
Research Funding: F. Hoffmann-La Roche Ltd, Basel, Switzerland
Uncompensated Relationships: Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/618396
Sarah Khan
Honoraria: Novartis Pharmaceuticals UK Ltd, Roche/Genentech
Consulting or Advisory Role: Lilly
Travel, Accommodations, Expenses: Novartis Pharmaceuticals UK Ltd
Reeti Bandyopadhyay
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Heather M. Moore
Employment: Genentech, Pfizer (I)
Stock and Other Ownership Interests: Roche, Pfizer (I)
Mahesh Shivhare
Employment: Roche
Stock and Other Ownership Interests: Roche
Monika Patre
Employment: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: US20190030181A1 Methods for treating HER2 breast cancer
Jorge Martinalbo
Employment: Roche, Novartis, Inhibrx
Stock and Other Ownership Interests: Roche
Laura Roncoroni
Employment: Roche, AstraZeneca
Stock and Other Ownership Interests: Roche
Pablo Diego Pérez-Moreno
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech, Merck, Zymeworks, Johnson & Johnson/Janssen, Johnson & Johnson/Janssen
Joohyuk Sohn
Stock and Other Ownership Interests: Daiichi Sankyo
Research Funding: MSD (Inst), Roche (Inst), Novartis (Inst), Lilly (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Seagan (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Gaudet MM, et al. : Breast cancer statistics, 2019. CA Cancer J Clin 69:438-451, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe C, Friedman LS, Hager JH: Hormone-targeted therapy and resistance. Annu Rev Cancer Biol 2:291-312, 2018 [Google Scholar]
- 4.Hanker AB, Sudhan DR, Arteaga CL: Overcoming endocrine resistance in breast cancer. Cancer Cell 37:496-513, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso F, Paluch-Shimon S, Senkus E, et al. : 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623-1649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein HJ, Somerfield MR, Barton DL, et al. : Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol 39:3959-3977, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gennari A, André F, Barrios CH, et al. : ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 32:1475-1495, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Di Leo A, Jerusalem G, Petruzelka L, et al. : Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28:4594-4600, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Burstein HJ: Systemic therapy for estrogen receptor–positive, HER2-negative breast cancer. N Engl J Med 383:2557-2570, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Jeselsohn R, Buchwalter G, De Angelis C, et al. : ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 12:573-583, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel HK, Bihani T: Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther 186:1-24, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Yu J, Metcalfe C, et al. : Latest generation estrogen receptor degraders for the treatment of hormone receptor-positive breast cancer. Expert Opin Investig Drugs 31:515-529, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Jhaveri KL, Boni V, Sohn J, et al. : Safety and activity of single-agent giredestrant (GDC-9545) from a phase Ia/b study in patients (pts) with estrogen receptor-positive (ER+), HER2-negative locally advanced/metastatic breast cancer (LA/mBC). J Clin Oncol 39, 2021. (suppl 15; abstr 1017) [Google Scholar]
- 14.Liang J, Zbieg JR, Blake RA, et al. : GDC-9545 (giredestrant): A potent and orally bioavailable selective estrogen receptor antagonist and degrader with an exceptional preclinical profile for ER+ breast cancer. J Med Chem 64:11841-11856, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Jhaveri K, Winer EP, Lim E, et al. : A first-in-human phase I study to evaluate the oral selective estrogen receptor degrader (SERD), GDC-9545, in postmenopausal women with estrogen receptor-positive (ER+) HER2-negative (HER2–) metastatic breast cancer. Cancer Res 80, 2020. (abstr PD7-05) [Google Scholar]
- 16.Metcalfe C, Ingalla E, Blake R, et al. : GDC-9545: A novel ER antagonist and clinical candidate that combines desirable mechanistic and pre-clinical DMPK attributes. Cancer Res 79, 2019. (suppl 4; abstr P5-04-07) [Google Scholar]
- 17.Lim E, Jhaveri KL, Perez-Fidalgo JA, et al. : A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC-9545 alone or combined with palbociclib in metastatic ER-positive HER2-negative breast cancer. J Clin Oncol 38, 2020. (suppl 15; abstr 1023) [Google Scholar]
- 18.Turner NC, Loi S, Moore HM, et al. : Activity and biomarker analyses from a phase Ia/b study of giredestrant (GDC-9545; G) with or without palbociclib (palbo) in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer (ER+/HER2- LA/mBC). Cancer Res 82, 2022. (abstr PD13-07) [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration (FDA) : FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer
- 21.Bidard FC, Kaklamani VG, Neven P, et al. : Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the randomized phase III EMERALD trial. J Clin Oncol 40:3246-3256, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira M, Pominchuck D, Nowecki Z, et al. : Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose phase 2 SERENA-2 trial. Cancer Res 83, 2023. (suppl 5; abstr GS3-02) [Google Scholar]
- 23.Tolaney SM, Chan A, Petrakova K, et al. : AMEERA-3: Randomized phase II study of amcenestrant (oral selective estrogen receptor degrader) versus standard endocrine monotherapy in estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. J Clin Oncol 41:4014-4024, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer EL, Tolaney S, Brufsky AM, et al. : evERA Breast Cancer: A phase III study of giredestrant (GDC-9545) + everolimus vs exemestane + everolimus in patients with estrogen receptor+, HER2– locally advanced or metastatic breast cancer. Cancer Res 83, 2023. (suppl 5; abstr OT2-01-07) [Google Scholar]
- 25.Liang J, Ingalla ER, Yao X, et al. : Giredestrant reverses progesterone hypersensitivity driven by estrogen receptor mutations in breast cancer. Sci Transl Med 14:eabo5959, 2022 [DOI] [PubMed] [Google Scholar]
- 26.Razavi P, Chang MT, Xu G, et al. : The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34:427-438.e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertucci F, Ng CKY, Patsouris A, et al. : Genomic characterization of metastatic breast cancers. Nature 569:560-564, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Kinslow CJ, Tang A, Chaudhary KR, et al. : Prevalence of estrogen receptor alpha (ESR1) somatic mutations in breast cancer. JNCI Cancer Spectr 6:pkac060, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fasching PA, Bardia A, Quiroga V, et al. : Neoadjuvant giredestrant (GDC-9545) plus palbociclib (P) versus anastrozole (A) plus P in postmenopausal women with estrogen receptor–positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Final analysis of the randomized, open-label, international phase 2 coopERA BC study. J Clin Oncol 40, 2022. (suppl 16; abstr 589) [Google Scholar]
- 30.Hurvitz SA, Quiroga V, Park YH, et al. : Neoadjuvant giredestrant (GDC-9545) + palbociclib versus anastrozole + palbociclib in postmenopausal women with estrogen receptor-positive, HER2-negative, untreated early breast cancer: Primary analysis of the randomized, open-label, phase II coopERA breast cancer study. Cancer Res 82, 2022. (abstr PD13-06) [Google Scholar]
- 31.Neilan TG, Villanueva-Vázquez R, Bellet M, et al. : Heart rate changes, cardiac safety, and exercise tolerance from a phase Ia/b study of giredestrant (GDC-9545) ± palbociclib in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer. Clin Cancer Res 82, 2022. (abstr P5-18-P07) [Google Scholar]
- 32.Choong GM, Liddell S, Ferre RAL, et al. : Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: A retrospective single-institution study. Breast Cancer Res Treat 196:229-237, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner NC, Jhaveri KL, Bardia A, et al. : persevERA Breast Cancer (BC): phase III study evaluating the efficacy and safety of giredestrant (GDC-9545) + palbociclib versus letrozole + palbociclib in patients (pts) with estrogen-receptor-positive, HER2-negative locally advanced or metastatic BC (ER+/HER2– LA/mBC). J Clin Oncol 39, 2021. (suppl 15; abstr TPS1103) [Google Scholar]
- 34.Bardia A, Schmid P, Harbeck N, et al. : Lidera breast cancer: A phase III adjuvant study of giredestrant (GDC-9545) vs physician’s choice of endocrine therapy (ET) in patients (pts) with estrogen receptor-positive, HER2-negative early breast cancer (ER+/HER2- EBC). Cancer Res 82, 2022. (suppl 4; abstr OT2-11-09) [Google Scholar]
- 35.Bardia A, Fernando TM, Fasching PA, et al. : Neoadjuvant giredestrant (GDC-9545) + palbociclib (P) vs anastrozole (A) + P in postmenopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Biomarker subgroup analysis of the randomised, phase II coopERA BC study. Ann Oncol 33:S605-S606, 2022. (suppl 7; abstr 144P) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli: https://vivli.org/ourmember/roche/. For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient reidentification.