Abstract
PURPOSE
Immunoscore (IS) is prognostic in stage III colorectal cancer (CRC) and may predict benefit of duration (6 v 3 months) of adjuvant infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy. We sought to determine IS prognostic and predictive value in stage-III CRC treated with adjuvant FOLFOX or oral capecitabine and infusional oxaliplatin (CAPOX) in the SCOT and IDEA-HORG trials.
METHODS
Three thousand sixty-one cases had tumor samples, of which 2,643 (1,792 CAPOX) were eligible for IS testing. Predefined cutoffs (IS-Low and IS-High) were used to classify cases into two groups for analysis of disease-free survival (3-year DFS) and multivariable-adjusted hazard ratios (mvHRs) by Cox regression.
RESULTS
IS was determined in 2,608 (99.5%) eligible cases, with 877 (33.7%) samples classified as IS-Low. IS-Low tumors were more commonly high-risk (T4 and/or N2; 52.9% IS-Low v 42.2% IS-High; P < .001) and in younger patients (P = .024). Patients with IS-Low tumors had significantly shorter DFS in the CAPOX, FOLFOX, and combined cohorts (mvHR, 1.52 [95% CI, 1.28 to 1.82]; mvHR, 1.58 [95% CI, 1.22 to 2.04]; and mvHR, 1.55 [95% CI, 1.34 to 1.79], respectively; P < .001 all comparisons), regardless of sex, BMI, clinical risk group, tumor location, treatment duration, or chemotherapy regimen. IS prognostic value was greater in younger (≤65 years) than older (>65 years) patients in the CAPOX cohort (mvHR, 1.92 [95% CI, 1.50 to 2.46] v 1.28 [95% CI, 1.01 to 1.63], PINTERACTION = .026), and in DNA mismatch repair proficient than deficient mismatch repair disease (mvHR, 1.68 [95% CI, 1.41 to 2.00] v 0.67 [95% CI, 0.30 to 1.49], PINTERACTION = .03), although these exploratory analyses were uncorrected for multiple testing. Adding IS to a model containing all clinical variables significantly improved prediction of DFS (likelihood ratio test, P < .001) regardless of MMR status.
CONCLUSION
IS is prognostic in stage III CRC treated with FOLFOX or CAPOX, including within clinically relevant tumor subgroups. Possible variation in IS prognostic value by age and MMR status, and prediction of benefit from extended adjuvant therapy merit validation.
INTRODUCTION
Colorectal cancer (CRC) is the third most common malignancy globally, accounting for nearly 10% of all cancer diagnoses in 2020.1 For the three quarters of cases that are localized (stage I-III) at diagnosis,2 the standard of care is curative-intent surgical resection, with adjuvant chemotherapy recommended in stage III and high-risk stage II tumors.3 Adjuvant chemotherapy has evolved during the past 25 years. Addition of oxaliplatin to fluoropyrimidines has improved outcomes in stage III disease.4 Tolerability of treatment has improved after the demonstration that 3 months of oxaliplatin-based therapy is as effective as 6 months for most of these patients.5,6 However, the fact remains that existing risk stratification on the basis of patient and tumor factors is unable to reliably identify which patients require adjuvant chemotherapy, or what the optimum duration of treatment is for these individuals. Thus, there is a pressing unmet need for biomarkers to address these shortcomings.
CONTEXT
Key Objective
What is the prognostic and predictive value of the Immunoscore (IS) in stage III colorectal cancer (CRC) treated with adjuvant infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or oral capecitabine and infusional oxaliplatin (CAPOX)?
Knowledge Generated
IS is independently prognostic in stage III CRC treated with adjuvant FOLFOX or CAPOX, with prognostic value greater in younger (<65 years) patients treated with CAPOX, and in DNA mismatch repair proficient disease. IS did not predict benefit from extended duration of adjuvant chemotherapy, although our study was underpowered to detect this in the FOLFOX cohort.
Relevance (E.M. O'Reilly)
-
Combined analysis from these adjuvant studies endorses the prognostic value of IS in stage III colon cancer agnostic of specific cytotoxic therapy and further strengthens the value of this biomarker.*
*Relevance section written by JCO Associate Editor Eileen Mary O'Reilly, MD.
Immunoscore (IS) predicts recurrence risk in early-stage CRC by quantifying the host CD3+ and CD8+ T-cell response in the tumor core and invasive margin (IM).7 The prognostic value of IS has been shown in multiple studies, including an international validation cohort of 3,539 patients with stage I-III CRC,8 prompting the European Society for Medical Oncology guideline committee to recommend that IS should be considered as part of risk assessment for early-stage CRC.3 A recent analysis of the IDEA France trial, in which patients with stage III CRC received either 3 or 6 months of oxaliplatin-based (modified infusional fluorouracil, leucovorin, and oxaliplatin [mFOLFOX6]) adjuvant chemotherapy, confirmed IS prognostic value; exploratory analysis suggested that benefit of 6-month chemotherapy was restricted to patients with IS-Intermediate or IS-High tumors.9 We sought to confirm this finding and to define the prognostic value of IS in oral capecitabine and infusional oxaliplatin (CAPOX)–treated patients in the SCOT10 and IDEA HORG11 trials.
METHODS
Patient Selection for Biomarker Study
Details of the SCOT and HORG trials have been reported previously.10,11 Both compared the efficacy of 12 versus 24 weeks of oxaliplatin-based adjuvant chemotherapy (either mFOLFOX4/6 or CAPOX) after curative-intent resection of stage III or high-risk stage II (any of: pT4 primary, tumor obstruction, <10 lymph nodes harvested, grade 3 histology, perineural/extramural venous/lymphatic invasion) CRC. The SCOT trial randomly assigned 6,088 patients between March 2008 and November 2013. It met its primary end point, with the shorter course of chemotherapy confirmed to be noninferior (hazard ratio [HR], 1.01 [95% CI, 0.91 to 1.11], test for noninferiority P = .012)10 and associated with improved quality of life. The HORG study randomly assigned 1,121 patients between April 2009 and October 2015. It was designed to be analyzed as part of the pooled IDEA collaboration rather than alone, nevertheless standalone analysis revealed near-identical disease-free survival (DFS) between 3-month and 6-month groups (HR, 1.05 [95% CI, 0.61 to 1.55]; P = .647).11 After informed consent, a subset of participants in both studies donated tumor samples for research, of whom 3,031 had stage III disease (2,474 SCOT and 557 HORG) and were eligible for inclusion in this biomarker study. Demographic and clinicopathologic characteristics of these cases were similar to those of stage III cases in the study populations as a whole.
Tumor Sampling, IS, and Mismatch Repair Testing
Formalin-fixed, paraffin-embedded (FFPE) slides from tumor blocks were sectioned at 3 µm and sent in batches to accredited Veracyte laboratories in Marseille, France, and Richmond, VA, where IS testing was done blinded to clinical data. Testing was performed as previously reported9: after antigen retrieval, FFPE slides were stained with rabbit monoclonal antihuman CD3+ (clone HDx2; Veracyte SAS, Marseille, France) and mouse monoclonal antihuman CD8+ (clone HDx1; Veracyte SAS, Marseille, France), counterstained, and digitalized at ×10 magnification and 0.45 μm/pixel resolution by NanoZoomer-XR scanner (Hamamatsu, Japan). CD3+ and CD8+ cells in the tumor center IM were then quantified by image analysis software (Immunoscore Analyzer; Veracyte) and converted into IS using predefined cutoffs. Identification of deficient mismatch repair (dMMR) in SCOT cases was by AI-based image analysis of tumor slides immunohistochemically stained for MMR proteins (MLH1, PMS2, MSH2, and MSH6) and expert pathologist review (Data Supplement, Appendix A, online only, further details in preprint,12 manuscript under revision) and in the HORG cohort by expert pathologist review.
Statistical Analysis
Statistical analysis of this biomarker study was performed in accordance with a predefined statistical analysis plan (SAP), provided in the Data Supplement (Appendix B). The study primary objective was to assess the predictive value of IS, analyzed as two groups, for the duration of chemotherapy in patients treated with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy after curative-intent resection of stage III CRC. Secondary objectives included the evaluation of the prognostic value of IS in CAPOX-treated patients, within predefined subgroups, and against other known prognostic factors such as pT and N stage (Data Supplement, Appendix B). The study primary end point was DFS, defined as the time from random assignment to CRC recurrence or death from any cause, with patients who remained alive and disease-free during follow-up censored at the date of last contact. Survival curves were plotted using the Kaplan-Meier method and compared by the log-rank test. The relationship between IS and DFS was analyzed by Cox proportional hazards models by univariable analysis and multivariable analysis including prespecified covariables to derive univariable HRs (uvHRs) and multivariable-adjusted HRs (mvHRs); variable selection was not performed. Comparison between nested models was made by the likelihood ratio test, and variable contribution to model goodness of fit was determined by the chi-square statistic. Proportionality of hazards was confirmed by scaled Schoenfeld residuals. All statistical tests were two-sided, and statistical significance was accepted in event of P < .05, or P < .1 in case of interaction testing. A power calculation performed for the study primary end point revealed that 958 stage III patients were required to give power of ≥80% to detect a significant interaction (P < .1) between IS (High/Low) and duration of FOLFOX treatment (6 months v 3 months). All statistical analyses were performed in R version 4.2.2 (2022-10-31) using packages tidyverse, gtsummary, survival, survminer, rms, and webshot.
Ethical Approval and Consent to Participate
Ethical approval for patient recruitment and sample collection in the SCOT trial was approved centrally and at all recruiting centers. SCOT samples are stored at the NHSGGC Biorepository under ethics 22/WS/0020. Ethical approval for anonymized tumor molecular analysis was granted by Oxfordshire Research Ethics Committee B (Approval No. 05\Q1605\66).
RESULTS
Patient Characteristics and Determination of IS
The study flow diagram is provided in the Data Supplement (Fig S1). Two thousand four hundred eighty-eight of 4,922 patients with stage III CRC from the SCOT trial and 573 of 702 patients with stage III CRC from the IDEA-HORG trial had tumor samples available for analysis. Characteristics of stage III patients included in this biomarker study were similar to those not included, with maximum absolute difference of 7% in the frequency of any single variable (Data Supplement, Table S1). Of the total 3,061 cases, IS testing was successful in 2,608 (99.5%). Eight hundred thirty-four (32%) of these had been treated with mFOLFOX6 and 1,774 (68%) with CAPOX. Across the combined biomarker study population, 877 (33.6%) samples were classified as IS-Low, 1,387 (53.2%) IS-Intermediate (IS-Int), and 344 (13.2%) as IS-High. IS-Int and IS-High cases (1,731 cases; 66.4%) were combined for subsequent two-group comparisons (IS-Int/High), in accordance with the predefined SAP (Data Supplement, Appendix A); analyses of IS as three groups are explicitly reported as such. IS-Low tumors were more commonly high-risk (T4 and/or N2; 52.9% IS-Lo v 42.2% IS-Int/IS-Hi; P < .001) and in younger patients (P = .024), and to a lesser extent in higher BMI patients (P = .054); however, no significant association with sex or performance status was found (Data Supplement, Table S2). Patients with IS-Low tumors were also more commonly treated with CAPOX (74.2%) than patients with IS-High tumors (64.9%), P < .001, although the proportion treated with 6-month versus 3-month chemotherapy was similar between groups (Data Supplement, Table S2). Associations for IS as three groups were similar (Data Supplement, Table S3).
Prognostic Value of IS in Pooled SCOT and HORG-IDEA Studies
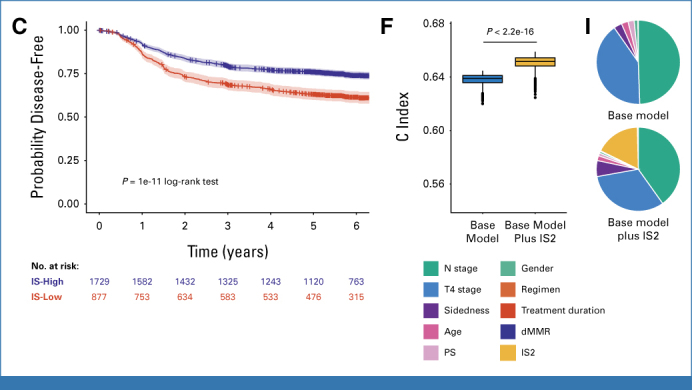
Univariable analysis of the combined study population after categorization of IS into two groups (IS2: IS-Low v IS-Int and IS-High) revealed significantly worse DFS of IS-Low tumors in the FOLFOX, CAPOX, and combined cohorts (uvHR, 1.83 [95% CI, 1.43 to 2.35]; uvHR, 1.55 [95% CI, 1.31 to 1.85]; and uvHR, 1.62 [95% CI, 1.41 to 1.86], respectively; P < .001 all comparisons; Figs 1A-1C, Table 1). Three-year DFS rates for IS-Low and IS-Int and IS-High tumors were 65.1% (95% CI, 58.5 to 71.0) versus 79.4% (95% CI, 76.0 to 82.5) in the FOLFOX group, 69.8% (95% CI, 66.1 to 73.2) and 79.4% (95% CI, 76.9 to 81.7) in the CAPOX group, and 68.6% (95% CI, 65.4 to 71.6) and 79.4% (95% CI, 77.4 to 81.3) in the combined groups, respectively. Multivariable analysis adjusted for prespecified prognostic factors demonstrated that IS2 was independently associated with DFS in the mFOLFOX, CAPOX, and combined groups (mvHR, 1.58 [95% CI, 1.22 to 2.04]; mvHR, 1.52 [95% CI, 1.28 to 1.82]; and mvHR, 1.55 [95% CI, 1.34 to 1.79], respectively; P < .001 all comparisons; Table 1). Other significantly prognostic factors in multivariable analyses included primary tumor and nodal stage and tumor sidedness (Data Supplement, Table S4-S6). Addition of IS to multivariable models significantly improved model discrimination and Akaike information criterion (Table 1, Figs 1D-1F); model χ2 statistics revealed that IS was a more important predictor than all factors with exception of pT and N stage in the combined models (Figs 1G-1I). Analysis of IS categorized into three groups in the combined cohort revealed comparable results (Data Supplement, Fig S2, Table S7). Further analysis of the 1,762 patients for whom MMR status was available (Data Supplement, Table S8) confirmed IS prognostic value, which was independent of, and stronger than, dMMR, which did not predict improved DFS in our cohort (Figs 1J and 1K, Data Supplement, Table S9).
FIG 1.
Prognostic value of IS as two groups by univariable and multivariable analyses in the SCOT-HORG trials. Kaplan-Meier plots showing DFS according to IS as two groups for (A) patients treated with FOLFOX, (B) patients treated with CAPOX, and (C) patients treated with either regimen in the pooled SCOT and IDEA-HORG population. (D-F) Harrell C indices obtained from multivariable Cox proportional hazards models of the patient groups shown in A-C including prespecified baseline and prognostic factors* before and after the addition of IS as two groups. Estimates were obtained by internal bootstrap (n = 1,000 samples). (G-I) Pie charts showing contribution of individual variables to goodness of model fit before and after the addition of IS as two groups. Statistical comparison between groups in A-C was made by using the log-rank test. Comparison of bootstrapped C-indices for base model and base model plus IS2 in D-F was by unpaired Wilcoxon test. *Variables included in Cox models include age, sex, PS, pT stage (pT1-3 v pT4), N stage (1 v 2), treatment regimen (in the case of combined population only), and chemotherapy duration. DFS, disease-free survival; dMMR, deficient mismatch repair; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IS, Immunoscore; MMR, mismatch repair; PS, performance status.
TABLE 1.
Prognostic Value of IS as Two Groups by Univariable and Multivariable Analyses in FOLFOX-Treated, CAPOX-Treated, and the Combined Cohort From the SCOT-HORG Trials
| Subgroup | Univariable | Multivariablea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases, No. | Events, No | 3-Year DFS (95% CI) | HR (95% CI) | P | Cases, No. | Events, No. | HR (95% CI) | P | |
| FOLFOX | |||||||||
| IS-High/IS-Int | 608 | 166 | 79.4 (76 to 82.5) | Ref | 599 | 164 | Ref | ||
| IS-Low | 226 | 99 | 65.1 (58.5 to 71) | 1.83 (1.43 to 2.35) | <.001 | 222 | 97 | 1.58 (1.22 to 2.04) | <.001 |
| CAPOX | |||||||||
| IS-High/IS-Int | 1,123 | 289 | 79.4 (76.9 to 81.7) | Ref | 1,093 | 276 | Ref | ||
| IS-Low | 651 | 239 | 69.8 (66.1 to 73.2) | 1.55 (1.31 to 1.85) | <.001 | 635 | 231 | 1.52 (1.28 to 1.82) | <.001 |
| Combined | |||||||||
| IS-High/IS-Int | 1,731 | 455 | 79.4 (77.4 to 81.3) | Ref | 1,692 | 440 | Ref | ||
| IS-Low | 877 | 338 | 68.6 (65.4 to 71.6) | 1.62 (1.41 to 1.86) | <.001 | 857 | 328 | 1.55 (1.34 to 1.79) | <.001 |
Abbreviations: AIC, Akaike information criterion; CAPOX, oral capecitabine and infusional oxaliplatin; DFS, disease-free survival; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; IS, Immunoscore; PS, performance status; Ref, reference.
Multivariable analyses are adjusted for age, sex, BMI, PS, tumor sidedness, primary tumor stage, and nodal stage (base model). Model discrimination: FOLFOX base model—C index = 0.637, AIC = 4,608.745; FOLFOX base model plus IS2—C index = 0.644, AIC = 3,312.377; CAPOX base model—C index = 0.633, AIC = 9,990.743; CAPOX base model plus IS2—C index = 0.649, AIC = 7,182.132; combined base model—C index = 0.635, AIC = 15,900.76; combined base model plus IS2—C index = 0.65, AIC = 11,471.33. Likelihood ratio test for comparison of nested models: FOLFOX base model versus base model plus IS P < .0001; CAPOX base model versus base model plus IS P < .0001; combined base model versus base model plus IS P < .0001.
Predictive Value of IS for Adjuvant Chemotherapy Duration
We sought to validate the association between IS and benefit from duration of mFOLFOX6 chemotherapy found in the IDEA France study.9 Of 834 mFOLFOX6-treated patients informative for analysis, 423 received 3 months therapy and 411 received 6 months therapy, with clinicopathologic characteristics similar between groups (Data Supplement, Table S10). Three-year DFS rates for patients with IS-High tumors were 79.4% (95% CI, 77.8 to 82.1) in the 3-month group and 79.4% (95% CI, 76.7 to 82.2) in the 6-month group (uvHR, 0.89 [95% CI, 0.66 to 1.21]; Fig 2A, Data Supplement, Fig S3). Corresponding 3-year DFS rates for patients with IS-Low tumors were 68.8% (95% CI, 64.5 to 73.4) and 68.4% (95% CI, 64.2 to 72.8), respectively (uvHR, 0.80 [95% CI, 0.54 to 1.20]; Fig 2B, Data Supplement, Fig S3). Formal testing for a IS × duration interaction was not significant (P = .71), although our sample size was less than the 958 patients required to detect this according to our power calculation (Methods). Corresponding analyses of IS predictive value for chemotherapy duration in 1,774 CAPOX-treated patients (Data Supplement, Table S11, Fig S4) and in the combined FOLFOX and CAPOX cohort (Data Supplement, Table S12, Fig S5) revealed similar lack of variation both in the total population and within clinical risk groups (Figs 2B and 2C and Data Supplement, Figs S4 and S5).
FIG 2.

Predictive value of IS for chemotherapy duration in the SCOT-HORG trials. Kaplan-Meier plots showing DFS according to chemotherapy duration (3 v 6 months) for (A) FOLFOX in IS-High group; (B) FOLFOX in IS-Low group; (C) CAPOX in IS-High group; and (D) CAPOX in IS-Low group. Statistical comparison between groups in A-C was made by using the log-rank test. DFS, disease-free survival; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IS, Immunoscore.
Analysis by Clinical Risk Strata and Within Subgroups
Prespecified analysis of IS2 within stage III clinical risk groups (pT1-3, N1 v pT4 or N2 disease stage) revealed similar prognostic value in patients treated with FOLFOX, CAPOX, or the combined cohort (Figs 3A-3C, Figs 4A and 4B, Fig S6). The combination of clinical risk group and IS stratified patients with markedly different prognoses: pT1-3, N1, IS-Int/IS-Hi patients had 3-year DFS of 86.7 (95% CI, 84.6 to 88.8), while pT4 and/or N2, IS-Low patients had 3-year DFS of 58.2 (95% CI, 53.9 to 62.9; Figs 3A-3C). Exploratory analysis of chemotherapy duration by clinical risk group and IS2 revealed a statistically significant interaction in favor of 6 months FOLFOX in patients with IS-High/IS-Int, low-risk tumors compared with those with high-risk tumors (PINT = .072; Data Supplement, Fig S3). Similar variation by risk group was not detected in FOLFOX patients with IS-low tumors, or in patients treated with CAPOX irrespective of IS group.
FIG 3.

Prognostic value of IS according to clinical risk group in the SCOT-HORG trials. Kaplan-Meier plots showing DFS according to clinical risk score and two-group IS for (A) patients treated with FOLFOX, (B) patients treated with CAPOX, and (C) patients treated with either regimen. Statistical comparison between groups was made by using the log-rank test. DFS, disease-free survival; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IS, Immunoscore.
FIG 4.

Prognostic value of IS within subgroups in the SCOT-HORG trials. Forest plots showing HR for DFS across patient and tumor subgroups according to two-group IS for (A) patients treated with FOLFOX and(B) patients treated with CAPOX. (C and D) Corresponding Kaplan-Meier plots of DFS according to patient age and two-group IS for respective cohorts. Interaction testing in A and B was obtained from the IS × subgroup cross-product term from univariable analysis. Statistical comparison between groups in C and D was made by using the log-rank test. DFS, disease-free survival; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; IS, Immunoscore.
We analyzed IS prognostic value in other subgroups in the whole cohort and in cases with MMR status. Analysis of FOLFOX-treated patients showed no obvious variation by age, BMI, sex, tumor location (right colon, left colon, or rectum), or study arm (Fig 4A). Corresponding analysis of CAPOX-treated patients revealed similar results, with the exception of age where IS held greater prognostic value in those age 65 years and younger (HR, 1.92 [95% CI, 1.50 to 2.46], P < .001) than in those older than 65 years (HR, 1.28 [95% CI, 1.01 to 1.63], P = .043; PINTERACTION = .026; Fig 4B). This discordance was reflected in differences in DFS: while younger (≤65 years) patients with IS-high tumors had the best outcome in both cohorts, among FOLFOX-treated patients, older patients (>65 years age) with IS-low tumors had the worst outcome (HR, 2.31 [95% CI, 1.59 to 3.34], P < .001); while in CAPOX-treated patients, younger patients (≤65 years) with IS-low tumors did worst (HR, 1.87 [95% CI, 1.45 to 2.41], P < .001; Figs 4C and 4D). This variation was not obviously due to imbalance in prognostic factors between groups in either cohort (Data Supplement, Tables S13 and S14). Subgroup analysis in the combined total cohort reflected the result observed in the individual treatment groups (Data Supplement, Fig S6). Analysis of the 1,762 cases with MMR status revealed a statistically significant interaction between IS and MMR status in both univariable and multivariable analyses (P = .025 and P = .062, respectively; Fig 5A). IS was strongly prognostic in mismatch repair proficient (pMMR) cases (mvHR, 1.68 [95% CI, 1.41 to 2.00], P < .001) but not in dMMR cases (mvHR, 0.67 [95% CI, 0.30 to 1.49], P = .57; Figs 5B and 5C, Data Supplement, Tables S15 and S16). Initial exploratory analysis by regimen suggested 3-year DFS for CAPOX-treated and FOLFOX-treated patients was similar for IS-High tumors but discordant for IS-low tumors; however, this did not persist after adjustment for confounders (Data Supplement, Fig S7).
FIG 5.

Prognostic value of IS within subgroups in patients with DNA MMR status in SCOT-HORG studies. (A) Forest plot showing HR for DFS across patient and tumor subgroups according to two-group IS for patients treated with FOLFOX or CAPOX with available tumor DNA MMR status. (B and C) Corresponding Kaplan-Meier plots of DFS according to two-group IS for cases with (B) pMMR and (C) dMMR. DFS, disease-free survival; dMMR, deficient mismatch repair; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; IS, Immunoscore; MMR, mismatch repair; pMMR, mismatch repair proficient.
DISCUSSION
This study confirms the IS prognostic value in patients with stage III CRC treated with FOLFOX and, to our knowledge, provides the first demonstration of IS prognostic value in patients treated with CAPOX. This value contrasted with MMR status, which was not prognostic in our cohort, possibly owing to its lack of prognostic value in N2 disease.13 IS was similarly prognostic across subgroups with the exception of dMMR cases, where it did not predict DFS, and age in CAPOX-treated patients, where its value appeared greater in patients younger than 65 years. Interestingly, we did not find any difference in IS value by tumor location, in contrast to a recent study that reported that low density of tumor-infiltrating lymphocytes was associated with worse outcome in right-sided but not left-sided colon cancers.14
Although interesting, the absence of IS prognostic value in dMMR tumors should be set in the context of previous studies that have found similar differences between IS-High and IS-Low cases regardless of MMR status.8,15 Thus, we suggest validation is required before conclusions on this can be drawn.
Similarly, apparent differences in IS prognostic value by age in CAPOX-treated patients should be interpreted very cautiously. Choice of chemotherapy regimen in the SCOT and HORG-IDEA studies was not randomized, but rather made by the treating clinician, and although we did not find obvious differences in risk factors between older and younger patients with IS-Low tumors to explain this result, it is plausible that this could reflect variation in unmeasured prognostic variables between groups. Furthermore, the international consensus IS study in stage III patients revealed no differences by age, with IS showing significant prognostic value regardless of age group.16 Again, validation is required. Despite including 834 informative cases, our study was underpowered to validate the predictive utility of IS for duration of FOLFOX, although improved DFS with extended therapy for low-risk, IS-high tumors was consistent with previous results.9 A definitive answer to this question also awaits the results of future analyses, which could also explore IS predictive value for oxaliplatin benefit.
Our study adds to the data supporting the value of clinical application of IS7-9,17 and underscores the importance of the antitumor immune response in CRC.18 Given that the usefulness of IS in prognostication in localized CRC is well established,19 there are several priorities for current and future research, of which we highlight three. First is the combination of IS with other promising methods for risk stratification, such as measurement of circulating free tumor DNA (ctDNA).20 Plasma samples were collected by several trials within the IDEA collaboration. Although it is currently unknown whether IS and ctDNA will provide nonredundant information, it is tempting to speculate that IS-High, ctDNA-negative patients and IS-Low, ctDNA-positive patients will have markedly different prognoses and be candidates for alternative management strategies. Indeed, a recent study in stage II CRC suggested such an association, and that IS prognostic value is retained in combination with ctDNA.21 Second is the evolution of IS to include other immune biomarkers. The Immunoscore Immune Checkpoint assay incorporates the density and spatial relationship of CD8 and PD-L1–positive cells in tumors. IS IC showed promise as a predictor of benefit from immune checkpoint blockade (ICB) in metastatic pMMR CRC22; it will be of interest to investigate similar predictive value in studies of ICB in early-stage disease. Third is the translation of IS to the preoperative setting, given recent evidence demonstrating feasibility and promising activity of neoadjuvant therapy in patients with localized CRC,23 and the utility of IS in predicting response to such treatment in rectal cancer.24
Our study has several strengths. These include the rigorous used for the IS, the large number of clinical trial cases with carefully curated clinical outcome data available for analysis, and our predefined SAP. It also has some limitations. Owing to lack of available samples, we only studied samples from two trials within the IDEA collaboration.10,11 Although our study is one of the largest biomarker analyses ever performed in CRC, pooled analysis including additional studies including TOSCA,25 CALGB 80702,26 and the previously published IDEA France analysis9 would increase power to identify and validate IS prognostic and predictive associations. Another limitation is the lack of molecular markers of known prognostic value beyond MMR such as KRAS and BRAF mutation,27 which were not available at the time of our report. Addition of these may further refine risk stratification and identify tumor subgroups for experimental intervention.
In summary, we show IS (1) is strongly prognostic in patients with stage III CRC treated with CAPOX or with FOLFOX; (2) holds similar prognostic value in both high- and low-risk, and in right- and left-sided tumors; and (3) with possible exception of low-risk tumors treated with FOLFOX does not appear to predict differential benefit of 3- versus 6-month chemotherapy in this patient group, although the study was underpowered for this objective. Clinical application of the IS could help stratify patients who do not require postoperative chemotherapy and others who are candidates for trials of more intensive therapy.
ACKNOWLEDGMENT
The authors are grateful to Alboukadel Kassambara (Veracyte) for statistical review, and to Alexia Dufrenne, Elisa Vongerichten, and Alexis Villalta (all Veracyte) for excellent technical assistance.
TransSCOT Group members are listed at Appendix 1 (online only).
APPENDIX 1. TransSCOT Group
The TransSCOT Trial Management Group includes (alphabetical order):
David Church (Cancer Genomics and Immunology Group, The Wellcome Center for Human Genetics, University of Oxford, United Kingdom), Enric Domingo (Department of Oncology, University of Oxford, United Kingdom), Joanne Edwards (School of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom), Bengt Glimelius (Uppsala University, Uppsala, Sweden), Ismail Gogenur (Center for Surgical Science, Zealand University Hospital, Denmark), Andrea Harkin (CRUK Glasgow Clinical Trials Unit, University of Glasgow, Glasgow, United Kingdom), Jen Hay (Glasgow Tissue Research Facility, University of Glasgow, Queen Elizabeth University Hospital, Glasgow, United Kingdom), Timothy Iveson (University of Southampton, Southampton, United Kingdom), Emma Jaeger (Department of Oncology, University of Oxford, United Kingdom), Caroline Kelly (CRUK Glasgow Clinical Trials Unit, University of Glasgow, Glasgow, United Kingdom), Rachel Kerr (Department of Oncology, University of Oxford, United Kingdom), Noori Maka (Glasgow Tissue Research Facility, University of Glasgow, Queen Elizabeth University Hospital, Glasgow, United Kingdom), Hannah Morgan (Glasgow Tissue Research Facility, University of Glasgow, Queen Elizabeth University Hospital, Glasgow, United Kingdom), Karin Oien (Glasgow Tissue Research Facility, University of Glasgow, Queen Elizabeth University Hospital, Glasgow, United Kingdom), Clare Orange (NHS Greater Glasgow and Clyde Biorepository, Glasgow, United Kingdom), Claire Palles (University of Birmingham, Birmingham, United Kingdom), Campbell Roxburgh (School of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom), Owen Sansom (CRUK Beatson Institute of Cancer Research, Garscube Estate, Glasgow, United Kingdom), Mark Saunders (The Christie NHS Foundation Trust, Manchester, United Kingdom), and Ian Tomlinson (Department of Oncology, University of Oxford, United Kingdom)
Owen Sansom
Research Funding: Novartis, AstraZeneca, Boehringer Ingelheim, Cancer research technology
Karin Oien
Research Funding: BioClavis Limited (Inst)
Tim Iveson
Honoraria: Servier, Amgen
Consulting or Advisory Role: Servier, Bristol Myers Squibb, MSD Oncology, Bayer, Amgen
Mark Saunders
Honoraria: Servier, Merck Serono, Takeda, Bayer
Travel, Accommodations, Expenses: Servier
Ian Tomlinson
Honoraria: Pfizer
Patents, Royalties, Other Intellectual Property: Molecular testing panel for 5-fluorouracil toxicity
Joanne Edwards
Research Funding: Varian Medical Systems
Viktor Koelzer
Employment: University Hospital of Zurich
Consulting or Advisory Role: V.K. is on an advisory board of Takeda (unrelated to the content of this study) and member of the European Key Opinion Leader Network in Digital Pathology supported by Roche
Speakers' Bureau: V.K. has acted as an invited speaker for Sharing Progress in Cancer Care (a non-profit educational organization) and Indica Labs unrelated to the content of this study.
Research Funding: V.K. holds sponsored research agreements with Roche and IAG unrelated to the content of this study
Patents, Royalties, Other Intellectual Property: V.K. is participant of a patent application co-owned by the Netherlands Cancer Institute (NKI-AVL) and the University of Basel on Methods for Predicting Treatment Outcome and/or for Selecting a Subject Suitable for Immune Checkpoint Therapy (Filing No. NL73455NL), unrelated to the current study. V.K. is participant of a patent application co-owned by the Leiden University Medical Center (LUMC) and the University of Zurich on Methods for training and using a machine learning model for predicting a likelihood of an event occurring in a patient having a malignant tumour by computational pathology (Application No. 23315438.4), unrelated to the current study
Travel, Accommodations, Expenses: V.K. is on an advisory board of Takeda (unrelated to the content of this study) and member of the European Key Opinion Leader Network in Digital Pathology supported by Roche
Alistair Easton
Consulting or Advisory Role: Immunocore, Scancell (Inst)
Ioannis Boukovinas
Employment: Pierre Fabre
Honoraria: Roche, MSD, Bristol Myers Squibb, Pfizer, Novartis, Merck, AstraZeneca, LEO Pharma, Servier
Consulting or Advisory Role: Roche, Sanofi, AstraZeneca, Bristol Myers Squibb, LEO Pharma, MSD, Novartis, Ipsen, Genesis Pharma
Research Funding: Roche, Novartis, Bristol Myers Squibb, MSD, Regeneron, Boehringer Ingelheim, Lilly, Pfizer
Travel, Accommodations, Expenses: MSD, Roche, Pfizer, Bristol Myers Squibb, Servier, Ipsen
Franck Pagès
Honoraria: BMS, Gilead Sciences, Sanofi
Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Gilead Sciences, HalioDx
Speakers' Bureau: Gilead Sciences
Research Funding: HalioDx, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Patents associated with the immune prognostic markers filled by Inserm
Travel, Accommodations, Expenses: HalioDX
Fanny Arnoux
Stock and Other Ownership Interests: Veracyte
Christelle Lautard
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Yoann Lovera
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Isabelle Boquet
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Aurélie Catteau
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Jérôme Galon
Employment: Veracyte
Leadership: HalioDx
Stock and Other Ownership Interests: Veracyte
Consulting or Advisory Role: Northwest Biotherapeutics, Catalym, Lunaphore Technologies, Georgiamune
Research Funding: Ultivue (Inst), ImCheck therapeutics (Inst), Veracyte (Inst)
Patents, Royalties, Other Intellectual Property: Inserm (French NIH)
Travel, Accommodations, Expenses: Veracyte
Ioannis Souglakos
Consulting or Advisory Role: Roche (Inst), Servier (Inst), Ipsen (Inst), Pierre Fabre, MSD, Bristol-Myers Squibb-Ono Pharmaceutical
Speakers' Bureau: Sanofi, Merck KGaA (Inst), Roche (Inst), MSD, GlaxoSmithKline (Inst)
Research Funding: Amgen (Inst), Sanofi (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Merck Serono, Amgen, Sanofi, Roche, Servier
David N. Church
Employment: Amgen (I)
Stock and Other Ownership Interests: Amgen (I)
Consulting or Advisory Role: MSD
Research Funding: HalioDx (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
PRIOR PRESENTATION
Presented in part at the 2022 ASCO GI cancer symposium, San Francisco, CA, January 20-22, 2022; the 2022 European Society for Medical Oncology Annual Meeting, Paris, France, September 9-13, 2022.
SUPPORT
Supported by the Medical Research Council (transferred to NETSCC—Efficacy and Mechanism Evaluation; Grant Ref: G0601705), NIHR Health Technology Assessment (Grant Ref: 14/140/84), Cancer Research UK Core CTU Glasgow Funding (Funding Ref: C6716/A9894), Cancer Research UK funding to the Cancer Research UK Glasgow Centre (Grant Ref: A25142) and the Swedish Cancer Society. The TransSCOT sample collection was funded by a Cancer Research UK Clinical Trials Awards and Advisory Committee—Sample Collection (Grant Ref: C6716/A13941). The IDEA-HORG trial was funded by the HORG foundation. This biomarker study was funded by an award to the TransSCOT and HORG consortia from HalioDx/Veracyte, the Oxford NIHR Comprehensive Biomedical Research Centre (BRC) and CRUK award A25142 to the CRUK Glasgow Centre. VHK acknowledges funding by the Promedica Foundation (F-87701-41-01). DNC is funded by a Cancer Research UK (CRUK) Advanced Clinician Scientist Fellowship (C26642/A27963).
I.S. and D.N.C. contributed equally to this work.
Contributor Information
Collaborators: David Church, Enric Domingo, Joanne Edwards, Bengt Glimelius, Ismail Gogenur, Andrea Harkin, Jen Hay, Timothy Iveson, Emma Jaeger, Caroline Kelly, Rachel Kerr, Noori Maka, Hannah Morgan, Karin Oien, Clare Orange, Claire Palles, Campbell Roxburgh, Owen Sansom, Mark Saunders, and Ian Tomlinson
DATA SHARING STATEMENT
The data sets pertaining to the SCOT trial used during the current study are available from the TransSCOT on reasonable request. Applications for analysis of TransSCOT samples are welcome and should be addressed to JH: Jennifer.Hay@glasgow.ac.uk.
AUTHOR CONTRIBUTIONS
Conception and design: Enric Domingo, Caroline Kelly Jennifer Hay, Owen Sansom, Tim Iveson, Rachel Kerr, Marta Nowak, Ioannis Boukovinas, Isabelle Boquet, Aurélie Catteau, Jérôme Galon, Ioannis Souglakos, David N. Church
Financial support: Owen Sansom, David N. Church
Administrative support: Caroline Kelly Jennifer Hay, Andrea Harkin, Ioannis Souglakos, Andrea Harkin
Provision of study materials or patients: Tim Iveson, Joanne Edwards, Andrea Harkin, Viktor Koelzer, Ioannis Boukovinas, Ippokratis Messaritakis, Michaela Karagianni, Franck Pagès, Christelle Lautard, Ioannis Souglakos, David N. Church
Collection and assembly of data: Enric Domingo, Noori Maka, Karin Oien, Tim Iveson, Rachel Kerr, Ian Tomlinson, Joanne Edwards, Andrea Harkin, Marta Nowak, Viktor Koelzer, Ioannis Boukovinas, Eleni Moustou, Ippokratis Messaritakis, Maria Chondrozoumaki, Michaela Karagianni, Fanny Arnoux, Christelle Lautard, Yoann Lovera, Isabelle Boquet, Ioannis Souglakos, David N. Church
Data analysis and interpretation: Enric Domingo, Caroline Kelly Jennifer Hay, Tim Iveson, Mark Saunders, Marta Nowak, Viktor Koelzer, Alistair Easton, Ioannis Boukovinas, Franck Pagès, Christelle Lautard, Jérôme Galon, David N. Church
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognostic and Predictive Value of Immunoscore in Stage III Colorectal Cancer: Pooled Analysis of Cases From the SCOT and IDEA-HORG Studies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Owen Sansom
Research Funding: Novartis, AstraZeneca, Boehringer Ingelheim, Cancer research technology
Karin Oien
Research Funding: BioClavis Limited (Inst)
Tim Iveson
Honoraria: Servier, Amgen
Consulting or Advisory Role: Servier, Bristol Myers Squibb, MSD Oncology, Bayer, Amgen
Mark Saunders
Honoraria: Servier, Merck Serono, Takeda, Bayer
Travel, Accommodations, Expenses: Servier
Ian Tomlinson
Honoraria: Pfizer
Patents, Royalties, Other Intellectual Property: Molecular testing panel for 5-fluorouracil toxicity
Joanne Edwards
Research Funding: Varian Medical Systems
Viktor Koelzer
Employment: University Hospital of Zurich
Consulting or Advisory Role: V.K. is on an advisory board of Takeda (unrelated to the content of this study) and member of the European Key Opinion Leader Network in Digital Pathology supported by Roche
Speakers' Bureau: V.K. has acted as an invited speaker for Sharing Progress in Cancer Care (a non-profit educational organization) and Indica Labs unrelated to the content of this study.
Research Funding: V.K. holds sponsored research agreements with Roche and IAG unrelated to the content of this study
Patents, Royalties, Other Intellectual Property: V.K. is participant of a patent application co-owned by the Netherlands Cancer Institute (NKI-AVL) and the University of Basel on Methods for Predicting Treatment Outcome and/or for Selecting a Subject Suitable for Immune Checkpoint Therapy (Filing No. NL73455NL), unrelated to the current study. V.K. is participant of a patent application co-owned by the Leiden University Medical Center (LUMC) and the University of Zurich on Methods for training and using a machine learning model for predicting a likelihood of an event occurring in a patient having a malignant tumour by computational pathology (Application No. 23315438.4), unrelated to the current study
Travel, Accommodations, Expenses: V.K. is on an advisory board of Takeda (unrelated to the content of this study) and member of the European Key Opinion Leader Network in Digital Pathology supported by Roche
Alistair Easton
Consulting or Advisory Role: Immunocore, Scancell (Inst)
Ioannis Boukovinas
Employment: Pierre Fabre
Honoraria: Roche, MSD, Bristol Myers Squibb, Pfizer, Novartis, Merck, AstraZeneca, LEO Pharma, Servier
Consulting or Advisory Role: Roche, Sanofi, AstraZeneca, Bristol Myers Squibb, LEO Pharma, MSD, Novartis, Ipsen, Genesis Pharma
Research Funding: Roche, Novartis, Bristol Myers Squibb, MSD, Regeneron, Boehringer Ingelheim, Lilly, Pfizer
Travel, Accommodations, Expenses: MSD, Roche, Pfizer, Bristol Myers Squibb, Servier, Ipsen
Franck Pagès
Honoraria: BMS, Gilead Sciences, Sanofi
Consulting or Advisory Role: Bristol Myers Squibb, Janssen, Gilead Sciences, HalioDx
Speakers' Bureau: Gilead Sciences
Research Funding: HalioDx, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Patents associated with the immune prognostic markers filled by Inserm
Travel, Accommodations, Expenses: HalioDX
Fanny Arnoux
Stock and Other Ownership Interests: Veracyte
Christelle Lautard
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Yoann Lovera
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Isabelle Boquet
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Aurélie Catteau
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Jérôme Galon
Employment: Veracyte
Leadership: HalioDx
Stock and Other Ownership Interests: Veracyte
Consulting or Advisory Role: Northwest Biotherapeutics, Catalym, Lunaphore Technologies, Georgiamune
Research Funding: Ultivue (Inst), ImCheck therapeutics (Inst), Veracyte (Inst)
Patents, Royalties, Other Intellectual Property: Inserm (French NIH)
Travel, Accommodations, Expenses: Veracyte
Ioannis Souglakos
Consulting or Advisory Role: Roche (Inst), Servier (Inst), Ipsen (Inst), Pierre Fabre, MSD, Bristol-Myers Squibb-Ono Pharmaceutical
Speakers' Bureau: Sanofi, Merck KGaA (Inst), Roche (Inst), MSD, GlaxoSmithKline (Inst)
Research Funding: Amgen (Inst), Sanofi (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Merck Serono, Amgen, Sanofi, Roche, Servier
David N. Church
Employment: Amgen (I)
Stock and Other Ownership Interests: Amgen (I)
Consulting or Advisory Role: MSD
Research Funding: HalioDx (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Cardoso R, Guo F, Heisser T, et al. : Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol 22:1002-1013, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Argilés G, Tabernero J, Labianca R, et al. : Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1291-1305, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Andre T, Boni C, Mounedji-Boudiaf L, et al. : Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343-2351, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Sobrero AF, Shields AF, et al. : Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378:1177-1188, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André T, Meyerhardt J, Iveson T, et al. : Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): Final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol 21:1620-1629, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galon J, Mlecnik B, Bindea G, et al. : Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 232:199-209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagès F, Mlecnik B, Marliot F, et al. : International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 391:2128-2139, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Pagès F, André T, Taieb J, et al. : Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol 31:921-929, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Iveson TJ, Kerr RS, Saunders MP, et al. : 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): An international, randomised, phase 3, non-inferiority trial. Lancet Oncol 19:562-578, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souglakos J, Boukovinas I, Kakolyris S, et al. : Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: The efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann Oncol 30:1304-1310, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Nowak M, Jabbar F, Rodewald A-K, et al. : Single cell AI-based detection of DNA mismatch repair deficiency in 1,988 colorectal cancers reveals prognostic and predictive value in the SCOT trial. medRxiv 10.1101/2023.12.18.23300137 [DOI]
- 13.Cohen R, Taieb J, Fiskum J, et al. : Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An ACCENT pooled analysis of 12 adjuvant trials. J Clin Oncol 39:642-651, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saberzadeh-Ardestani B, Foster NR, Lee HE, et al. : Association of tumor-infiltrating lymphocytes with survival depends on primary tumor sidedness in stage III colon cancers (NCCTG N0147) [Alliance]. Ann Oncol 33:1159-1167, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlecnik B, Bindea G, Angell HK, et al. : Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44:698-711, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Mlecnik B, Bifulco C, Bindea G, et al. : Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol 38:3638-3651, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galon J, Mlecnik B, Marliot F, et al. : Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients. J Clin Oncol 34, 2016. (suppl 15; abstr 3500) [Google Scholar]
- 18.Galon J, Bruni D: Tumor immunology and tumor evolution: Intertwined histories. Immunity 52:55-81, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Bruni D, Angell HK, Galon J: The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer 20:662-680, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Tie J, Cohen JD, Lahouel K, et al. : Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 386:2261-2272, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Lu S, Cao D, et al. : Prognostic and predictive value of Immunoscore and its correlation with ctDNA in stage II colorectal cancer. Oncoimmunology 12:2161167, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoniotti C, Rossini D, Pietrantonio F, et al. : Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 23:876-887, 2022 [DOI] [PubMed] [Google Scholar]
- 23.Morton D, Seymour M, Magill L, et al. : Preoperative chemotherapy for operable colon cancer: Mature results of an international randomized controlled trial. J Clin Oncol 41:1541-1552, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Sissy CA-O, Kirilovsky AA-O, Van den Eynde M, et al. A diagnostic biopsy-adapted Immunoscore predicts response to neoadjuvant treatment and selects patients with rectal cancer eligible for a watch-and-Wait strategy. Clin Cancer Res 26:5198-5207, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Sobrero A, Lonardi S, Rosati G, et al. : FOLFOX or CAPOX in stage II to III colon cancer: Efficacy results of the Italian three or six colon adjuvant trial. J Clin Oncol 36:1478-1485, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Shi Q, Fuchs CS, et al. : Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: The CALGB/SWOG 80702 (Alliance) randomized clinical trial. Jama 325:1277-1286, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Formica V, Sera F, Cremolini C, et al. : KRAS and BRAF mutations in stage II and III colon cancer: A systematic review and meta-analysis. J Natl Cancer Inst 114:517-527, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets pertaining to the SCOT trial used during the current study are available from the TransSCOT on reasonable request. Applications for analysis of TransSCOT samples are welcome and should be addressed to JH: Jennifer.Hay@glasgow.ac.uk.



