Abstract
Purpose
To verify the effectiveness of embryo transfer (ET) using cryopreserved embryo as fertility preservation (FP).
Methods
This study was a questionnaire survey. The total number of embryo cryopreservation (EC) was investigated between 2014 and 2020. And for patients who underwent ET among study period, details of EC, outcome of ET, number of live births, and mortality were investigated.
Results
Of the 150 facilities, 114 responded (76.0%). A total of 1420 EC were performed during the study period; and ET was performed for 417 patients. Breast cancer was the most common primary disease. A total of 199 live births (including prospective) were obtained by ET; 1.7 EC and 2.2 ET were performed per patient, and live birth rate was 21.4% per ET (28.1% on 35–37‐year‐old patients). The number of EC and ET increased with age. The final birth rate, including pregnancies other than FP, was 51.8%. Ovarian stimulation with aromatase inhibitors was commonly used, although with no effect on live birth rates. Random start stimulation was also common, experienced by 36.3% of breast cancer patients.
Conclusion
Reproductive outcomes of ETs following EC as FP are acceptable. This research project was registered in the University Hospital Medical Information Network (UMIN000043664).
Keywords: breast cancer, embryo cryopreservation, embryo transfer, fertility preservation, oncofertility
Approximately half of patients who undergo embryo transfer following embryo cryopreservation as fertility preservation have live births. The overall live birth rate per embryo transfer was 21.4%.
1. INTRODUCTION
Currently, fertility preservation (FP) treatment is becoming more widespread in Japan, as in other parts of the world. The spread of FP in Japan started with the establishment of the Japan Society for Fertility Preservation in 2012, following the establishment of the Oncofertility Consortium in 2006 and International Society for Fertility Preservation in 2009. 1 , 2 Furthermore, from 2021, a national public subsidy system for FP was launched as a national research project, and the Japan Society of Obstetrics and Gynecology (JSOG) is involved in this in the form of facility certification for the subsidy system. In addition, a national FP registry system (Japan Oncofertility Registry [JOFR]) linked to the public subsidy system has been started, and it reportedly already has more than 7000 registrants as of January 2022. 3 However, the reproductive outcomes of FP in Japan are still unclear.
Embryo cryopreservation (EC) has been positioned as an “established” option which can be said to be a highly reliable FP option. 4 One reason for this is that it closely follows the procedures of regular infertility treatment. However, performing EC for female patients requires sexual maturity and a sperm‐providing partner. Therefore, EC is not suitable as an FP option for single women without a partner, or pediatric patients who have not reached sexual maturity, and oocyte cryopreservation and ovarian tissue cryopreservation are instead indicated for such patients. 5 Subsidy systems for FP treatment are currently in operation in various countries, with the procedure being covered by insurance in some countries. This trend is particularly strong in Europe, with the procedure provided for free in the UK, France, Denmark, Spain, and the Netherlands. 6 However, many countries in Asia, South America, and Africa do not receive sufficient economic support from the state. In Japan, a subsidy system has been started as part of a research project, and it is necessary to demonstrate the outcomes of FP to make this subsidy sustainable.
Therefore, as part of a national survey, we investigated the outcomes of EC in Japan. The outcome investigated in this study is the period before the start of the subsidy, which can be considered the start‐up phase of FP. The purpose of the present study was to clarify the efficacy of EC in Japan during the start‐up phase mentioned above and to demonstrate the significance of FP.
2. METHODS
2.1. The type of study
The present study was a questionnaire survey and was a cross‐sectional study.
2.2. Participants
This survey was conducted at 150 facilities certified by the JSOG as “Certified facilities for cryopreservation of embryo for medical indications” (September 2021). “Medical indication” as defined by JSOG refers to cases where emergency treatment of a disease may reduce fertility. These diseases include not only malignant disease but also benign hematological diseases and autoimmune diseases. Responses to the questionnaire were provided by the person in charge of the FP at each facility or someone nominated by them. However, respondents were anonymous, and only the name of the facility was recorded. There was no reward for participating in this survey, and the responses were voluntary. Additionally, respondents were given the option of responding electronically or in paper form, and all communications were conducted by mail.
2.3. Survey inclusion/exclusion
Surveys were excluded from analysis if the survey respondent failed to provide contact or identification information, or if the questionnaire was left blank. For those with blanks, the number of blanks are listed in the results section.
2.4. Survey on status of implementation of EC and ET
The survey included questions on EC performed from April 1, 2014, to December 31, 2020. In addition to the total number of EC cases during the survey period, the survey focused on patients who received embryo transfers (ETs). Evaluation of patient background characteristics (age at the time of FP, marital status, pregnancy and birth history, menstrual history, primary disease, comorbidities, presence or absence of prior treatment, etc.), and details of EC (oocyte collection results, presence or absence of complications due to EC), as well as pregnancy outcomes (number of pregnancies and live births, pregnancy rate (PR), live birth rate (LBR)) and patient prognosis were retrospectively investigated. In this study, “pregnancy” refers to clinical pregnancy and refers to a state in which a gestational sac is observed within the uterus. Among these survey items, the primary disease, comorbidities, complications due to FP, and ovarian stimulation methods were subject to wide variation, so a free entry format was used. Furthermore, we evaluated the results by age at time of EC for comparison. This classification was performed in accordance with the Centers for Disease Control and Prevention (CDC) of the United States' classification of reproductive medicine outcomes by age. In addition, in Japan, since the age limit for FP subsidies is <43 years, like the upper age limit for infertility treatment covered by insurance in Japan, results for patients over 43 years of age were examined separately. Also, we investigated the relationship between the use of aromatase inhibitors (AI) and pregnancy and mortality rates in breast cancer patients ≤40 years. Finally, by classifying patients who underwent ET according to their underlying disease and examining each patient's background and treatment results, we clarified disease‐related characteristics of EC.
2.5. Ethics approval and informed consent
The present study was approved by the institutional review board of our institution (approval no. 5180). And this research project was registered in the University Hospital Medical Information Network (UMIN000043664). All study protocols were performed in accordance with the Declaration of Helsinki. As mentioned in the explanation provided with the survey, participants were required to provide their written consent for participation in this survey at the start of the questionnaire. Additionally, at the time of survey explanation, subjects were informed that their response to the questionnaire would be considered as their consent to participate in the survey. In addition, we requested that each participating facility post a notice regarding the execution of this study, providing each patient with the opportunity to opt‐out from their data being used in this study.
2.6. Statistical analysis
Chi‐square tests using The JMP Pro version 16 program (SAS Institute Inc., Cary, NC, USA) were used to examine the effects of aromatase inhibitor use on PR, LBR, and mortality rate in breast cancer patients. A p‐value of <0.05 was considered significant. In principle, the mean value is shown with the standard deviation (SD), although the data in Figure 4 show the standard error (SE) for ease of viewing.
FIGURE 4.
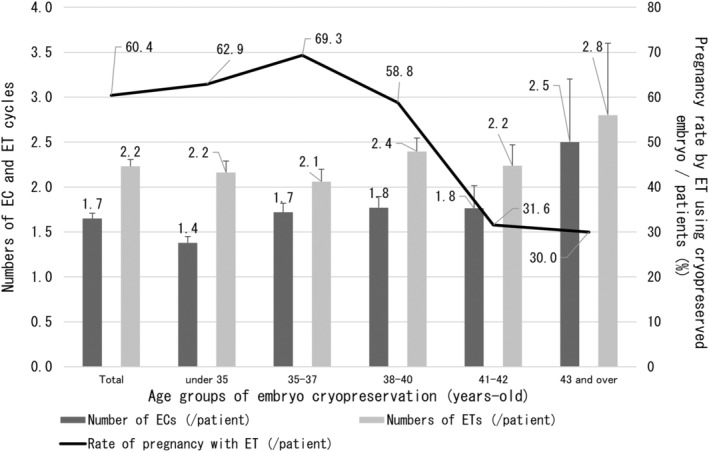
Number of embryo cryopreservation and embryo transfers, and trends in pregnancy rates per patient by age at embryo cryopreservation. Overall, each patient underwent 1.7 embryo cryopreservations and 2.2 embryo transfers, with increasing numbers with higher age. Furthermore, the pregnancy rate/patient with embryo transfers peaked at the age of 35–37 years. In the group whose embryos were frozen over 38 years of age, reproductive outcome decreased with age at the time of embryo cryopreservation. EC, embryo cryopreservation; ET, embryo transfer.
3. RESULTS
3.1. Number of EC implementations and characteristics of patients who underwent ET after EC for FP
Responses were received from 114 of the 150 facilities surveyed (response rate: 76.0%). EC was performed at all responding facilities. Of the 111 institutions for which the total number of EC was known (three facilities were left blank), the minimum number of ECs was 1, and the maximum was 180 (median 6, mean 12.8 ± 21.8). A total of 1420 ECs were performed during the study period, involving 417 patients whose embryos were thawed and led to ET (total 691 ECs and 930 ETs). The average age at the time of EC of the patients was 36.1 ± 4.0 years, 296 (71.0%) had never been pregnant, and 351 (84.2%) were nulliparous. In addition, 63 (15.1%) participants had undergone infertility treatment. 21 patients (5.0%) had received prior chemotherapy before EC, and 16 (3.8%) had comorbidities, such as leukopenia. Regarding prior chemotherapy, 12 patients had a blank, and regarding comorbidities, 23 patients had a blank, and 2 patients had an unknown. Figure 1 shows the age distribution at EC for patients who underwent ET following FP. Most patients were in their late 30s, with the most common age at EC being 38 years (52 patients, 12.5%). The youngest patient was 23 years (n = 1), and the oldest patient age was 45 years (n = 4). The primary diseases in these patients are shown in Figure 2. Breast cancer was the most common primary disease (324 patients, 77.1%), followed by ovarian cancer (18 patients, 4.3%) and malignant lymphoma (11 patients, 2.6%). Three of the patients had multiple cancers or two primary diseases that were eligible for FP.
FIGURE 1.
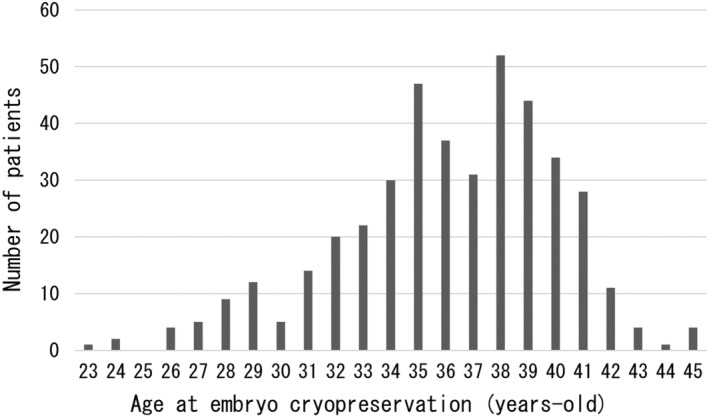
Age distribution at embryo cryopreservation for patients who underwent embryo transfer after fertility preservation. The age at the time of embryo cryopreservation increased rapidly after the age of 30, with the peak at 38 years. The youngest embryo frozen was 23 years old (n = 1), and the oldest was 45 years old (n = 4). Mean age at the time of embryo cryopreservation was 36.1 ± 4.0 years.
FIGURE 2.
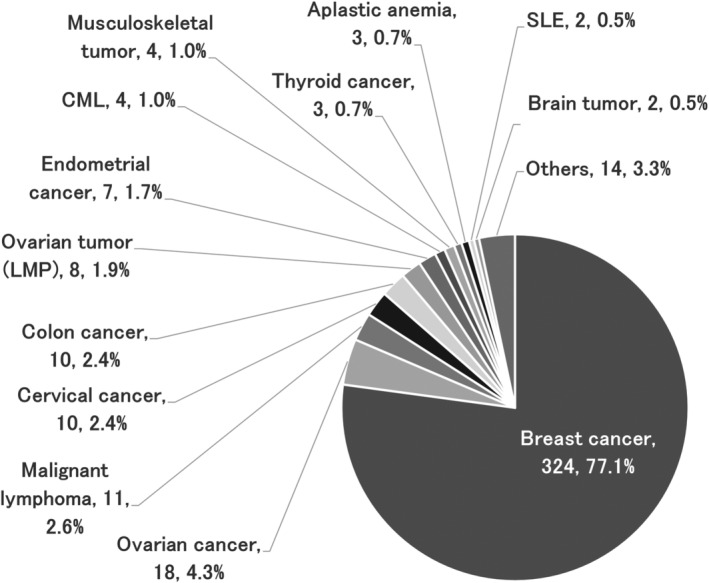
Primary disease of patients who underwent embryo transfer using cryopreserved embryos for fertility preservation. The most common disease was breast cancer (324 patients, 77.1%), followed by ovarian cancer (18 patients, 4.3%) and malignant lymphoma (11 patients, 2.6%). CML, chronic myeloid leukemia; LMP, low malignant potential; SLE, systemic lupus erythematosus.
3.2. Reproductive outcomes of ETs using cryopreserved embryos for FP
Table 1 shows the outcomes of oocyte retrieval for EC and outcomes of ET for the entire cohort and for each age group. Age groups were classified based on age at the time of EC. A total of 691 ECs were performed in the 417 patients, and 5336 oocytes were collected. Regarding the stimulation method, the most common method was the antagonist method with 371 (53.7%) out of 691 cycles (3 were blank), followed by the short method with 120 cycles (17.4%). Oocyte retrieval for EC in all patients resulted in retrieval of 7.7 oocytes/EC cycle and 12.8 oocytes/patient. Regarding the acquisition of mature oocytes, the total number was 5.8/EC cycle, with a decrease with increasing age. This result was consistent with the number of oocytes retrieved (Figure 3). The number of EC cycles in all patients was 1.7 times, although this tended to increase with age, with 2.5 EC cycles per patient over the age of 43 years. Interestingly, the number of retrieved oocytes and matured oocytes/patient was higher in patients aged ≥43 years than in patients aged 38–42 years. A total of 930 ETs were performed, with a rate of 2.2 times/patient, and a similar age‐related trend as for oocyte retrievals, with 2.8 ETs/patient for patients ≥43 years. Not surprisingly, the number of ETs per EC cycle decreased with age. And the average period from EC to ET was 2.2 years. However, the older the EC age, the shorter the period until ET (2.4 years for those under 35 years old, 2.4 years for those aged 35–37 years old, and 1.9 years for those aged 38–40 years old, 1.9 years for those aged 41 and 42 years old, and 1.1 years for those aged 43 and older). The total number of pregnancies resulting from ET was 252, with the majority occurring in young people ≤40 years of age. Interestingly, the overall PR per patient was high, at 60.4%, especially in the 35–37 year age group, reaching 69.3%. Furthermore, the ≥43 year group also had a good ET performance, with a PR of 30.0% per patients (Figure 4). The overall pregnancy outcome per ET was 27.1%, with the 35–37 year age group having the best outcomes, at 33.6%. Regarding live births, there were many patients who were still pregnant at the time of the survey. Considering these antepartum patients as live birth cases, the number of live births was 199 (156 already born and 43 antenatal). The overall LBR per ET was 21.4%, and as with PR, those aged 35 to 37 years had the best results, at 28.1%. Naturally, the LBR/ET decreased with age (Figure 5). As mentioned above, in each age group, patients who underwent two or more ETs showed a high live birth rate per patient. Patients under the age of 38 have a live birth rate of over 50% (54.8% for those under 35 and 57.9% for those between 35 and 37). Furthermore, as shown in Figure 5, the live birth rate per ET decreases markedly between the ages of 38 and 40, so increasing the number of ETs offsets the decrease in success rate (Figure 6).
TABLE 1.
Embryo transfer outcomes using cryopreserved embryos for fertility preservation.
| Group by age at EC | Total | Under 35 | 35–37 | 38–40 | 41–42 | 43 and over | |
|---|---|---|---|---|---|---|---|
| Number of patients (n) | 417 | 124 | 114 | 131 | 38 | 10 | |
| Number of EC cycles | Total | 691 | 171 | 196 | 232 | 67 | 25 |
| /Patient | 1.7 | 1.4 | 1.7 | 1.8 | 1.8 | 2.5 | |
| Number of retrieved oocytes | Total | 5336 | 1808 | 1663 | 1335 | 371 | 109 |
| /Cycle | 7.7 | 10.6 | 8.5 | 5.8 | 5.5 | 4.4 | |
| /Patient | 12.8 | 14.6 | 14.6 | 10.2 | 9.8 | 10.9 | |
| Number of mature oocytes | Total | 4003 | 1326 | 1225 | 1088 | 277 | 87 |
| /Cycle | 5.8 | 7.8 | 6.3 | 4.7 | 4.1 | 3.5 | |
| /Patient | 9.6 | 10.7 | 10.7 | 8.3 | 7.3 | 8.7 | |
| Maturation rate (%) | 75.0 | 73.3 | 73.7 | 81.5 | 74.7 | 79.8 | |
| Complications (n) | 11 | 5 | 1 | 4 | 1 | 0 | |
| Complication rate per cycle (%) | 1.6 | 2.9 | 0.5 | 1.7 | 1.5 | 0 | |
| Number of ET cycles | Total | 930 | 268 | 235 | 314 | 85 | 28 |
| /Patient | 2.2 | 2.2 | 2.1 | 2.4 | 2.2 | 2.8 | |
| /EC cycle | 1.3 | 1.6 | 1.2 | 1.4 | 1.3 | 1.1 | |
| Pregnancy through ET | Total | 252 | 78 | 79 | 77 | 12 | 3 |
| Ongoing pregnancy rate | /Patient (%) | 60.4 | 62.9 | 69.3 | 58.8 | 31.6 | 30.0 |
| /ET (%) | 27.1 | 29.1 | 33.6 | 24.5 | 14.1 | 10.7 | |
| Number of live births through ET | A | 156 | 52 | 51 | 45 | 7 | 1 |
| B | 43 | 16 | 15 | 10 | 2 | 0 | |
| c = a + b | 199 | 68 | 66 | 55 | 9 | 1 | |
| Live birth rate through ET | /Patient with c (%) | 47.7 | 54.8 | 57.9 | 42.0 | 23.7 | 10.0 |
| /ET cycle with c (%) | 21.4 | 25.4 | 28.1 | 17.5 | 10.6 | 3.6 | |
| /EC cycle with c (%) | 28.8 | 39.8 | 33.7 | 23.7 | 13.4 | 4.0 | |
| Number of pregnancies resulting from methods other than ET using cryopreserved embryo as FP | 34 | 13 | 5 | 15 | 1 | 0 | |
| d: Number of live births resulting from methods other than ET using cryopreserved embryo as FP | 17 | 7 | 1 | 9 | 0 | 0 | |
| Number of total births | e = c + d | 216 | 75 | 67 | 64 | 9 | 1 |
| Total live birth rate | /Patient with e (%) | 51.8 | 60.5 | 58.8 | 48.9 | 23.7 | 10.0 |
| Number of patients who died during the study period | 28 | 11 | 5 | 9 | 1 | 2 | |
| Mortality rate during the survey period (%) | 6.7 | 8.9 | 4.4 | 6.9 | 2.6 | 20.0 | |
Note: a, Already given birth; b, Pregnant at the time of the survey; c, Estimated total number of births through embryo transfer (a + b); d, Live births other than embryo transfer using cryopreserved embryos; e, Total number of live births among patients who underwent embryo cryopreservation (c + d). This includes not only births through embryo transfer but also natural conceptions and births resulting from infertility treatment after treatment of primary disease.
Abbreviations: EC, embryo cryopreservation; ET, embryo transfer.
FIGURE 3.
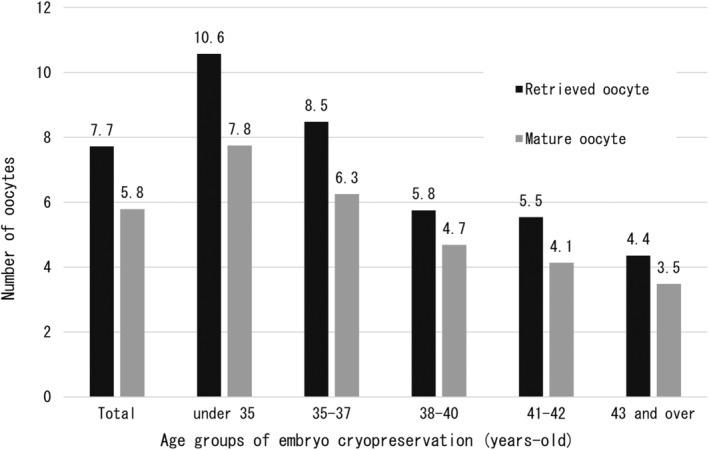
Number of retrieved oocytes and mature oocytes per embryo cryopreservation cycle. The number of retrieved oocytes and mature oocytes were 7.7 and 5.8, respectively, decreasing with age. However, this study did not examine the patients' ovarian reserve or the method of ovarian stimulation. In addition, 63 (15.1%) participants had undergone infertility treatment, 21 patients (5.0%) had received prior chemotherapy before embryo cryopreservation, and 16 (3.8%) had comorbidities such as leukopenia.
FIGURE 5.
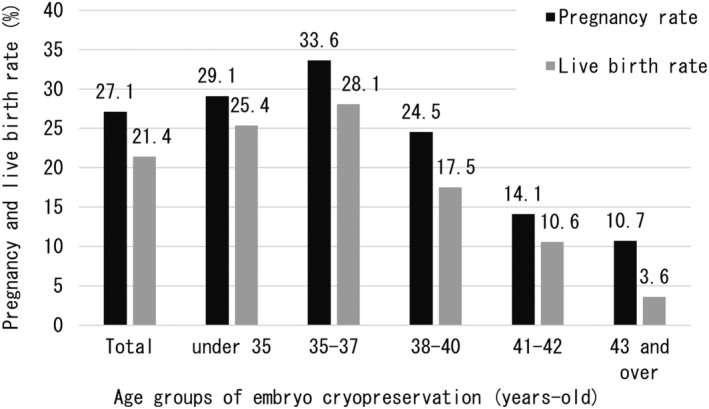
Reproductive outcomes of embryo transfer using cryopreserved embryos for fertility preservation. Pregnancy and live birth rates were calculated by dividing the number of pregnancies and births (including expected births) by the number of embryos transferred. Patients aged 35–37 years had the best outcomes, but those aged 35 and over worsened with age at embryo cryopreservation.
FIGURE 6.
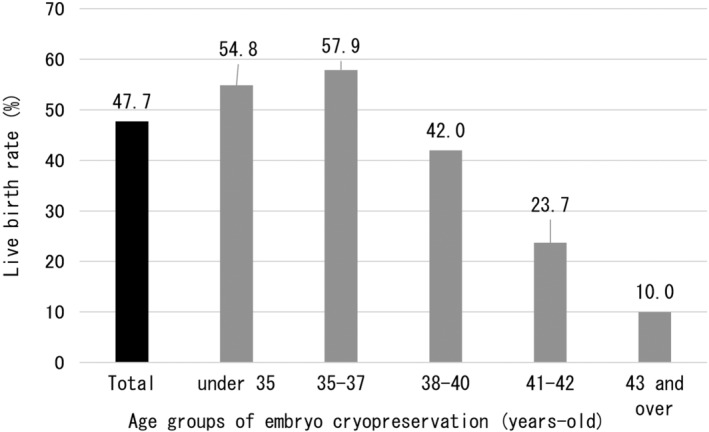
Live birth rate per patient by embryo transfer using fertility preservation embryos. In each age group, patients undergo two or more embryo transfers, and patients under 38 years of age have a greater than 50% of obtaining a live birth. Furthermore, in the age group of 38 to 40 years, when the live birth rate per embryo transfer decreases markedly, by increasing the number of embryo transfers, the decrease in success rate was offset, and a live birth rate of 42% was achieved.
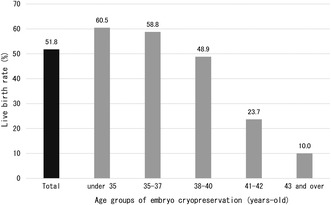
In addition, there were a total of 17 cases of live births without FP. All of them were patients ≤40 years of age, and among patients over 41 years, there was only one pregnancy by a method other than FP (no live births). The above results indicate that more than half of EC patients (51.8%) who underwent ET gave birth by “some method,” including around 60% of those who were ≤37 years of age, and around half of those between 38 and 40 years. Even among those between 41 and 42 years, 23.7% gave birth to live infants. Although the number of cases aged ≥43 years of age was small, 10% had given birth by the time of this survey (Figure 7). Note that “some method” here includes transplantation of embryos cryopreserved before the onset of the disease, in vitro fertilization or artificial insemination after disease treatment, natural pregnancy, etc.
FIGURE 7.
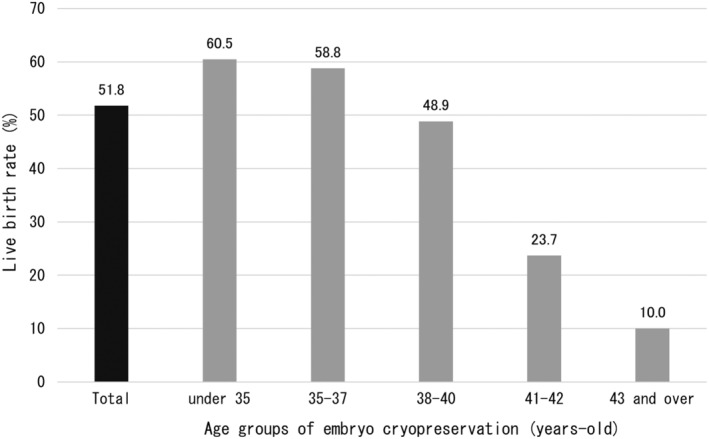
Final live birth rate of patients who underwent embryo transfer following embryo cryopreservation for fertility preservation (per patient). The overall pregnancy rate in the entire cohort was 51.8%, including pregnancies other than those with embryo transfer using cryopreserved embryos. The highest pregnancy rate was observed in those <35 years, which was thought to be because this was the age group with pregnancies other than due to fertility preservation.
3.3. Effects of aromatase inhibitors on embryo cryopreservation in breast cancer patients
We investigated pregnancy outcomes in each age group, and the use of AIs in breast cancer patients who underwent EC (Table 2). PR for the 38‐40 age group appeared to be better without AI, but the difference was not significant. Additionally, no significant difference was found in the number of pregnancies, live births with ET, or deaths depending on whether AI was used. Whether AI is used or not does not seem to affect pregnancy rates or live birth rates. Furthermore, no consistent trends were observed in the incidence of complications or the number of ETs per patient. The above results indicate that the use of AI has no negative effects on EC for FP in breast cancer patients.
TABLE 2.
Comparison of embryo transfer outcomes with and without aromatase inhibitor usage on breast cancer patients.
| Group by age at EC | Under 35 | 35–37 | 38–40 | ||||
|---|---|---|---|---|---|---|---|
| Number of patients | AI+ | AI‐ | AI+ | AI‐ | AI+ | AI‐ | |
| 44 | 33 | 57 | 31 | 62 | 51 | ||
| Age of patients at EC (years‐old) | 32.0 ± (2.0) | 31.8 ± (2.6) | 35.8 ± (0.8) | 36.0 ± (0.8) | 38.8 ± (0.8) | 38.9 ± (0.8) | |
| Number of EC cycles | (n) | 57 | 42 | 105 | 49 | 115 | 77 |
| /Pt | 1.3 ± (0.6) | 1.3 ± (0.5) | 1.8 ± (1.3) | 1.6 ± (0.6) | 1.9 ± (1.2) | 1.5 ± (0.8) | |
| Number of retrieved oocytes (mean) | /EC cycle | 12 | 11.2 | 8.2 | 9.6 | 6.6 | 5.9 |
| Number of mature oocytes (mean) | /EC cycle | 8.6 | 8.6 | 6.0 | 7.2 | 4.9 | 4.8 |
| Complications | (n) | 2 | 0 | 0 | 0 | 3 | 1 |
| /EC (%) | 3.5 | 0 | 0 | 0 | 2.6 | 1.3 | |
| Number of ET cycles | (n) | 83 | 57 | 112 | 68 | 142 | 133 |
| /Pti | 1.9 ± (1.2) | 1.7 ± (1.2) | 2.0 ± (1.4) | 2.3 ± (1.5) | 2.3 ± (1.5) | 2.6 ± (1.9) | |
| Pregnancy through ET | (n) | 29 | 22 | 40 | 23 | 33 | 31 |
| p | 0.94 | 0.69 | 0.09 | ||||
| /Pt (%) | 65.9 | 66.7 | 70.2 | 74.2 | 53.2 | 60.8 | |
| /ET (%) | 34.9 | 38.6 | 35.7 | 33.8 | 23.2 | 23.3 | |
| Number of live birth through ET | a | 18 | 16 | 28 | 15 | 20 | 16 |
| b | 8 | 5 | 6 | 4 | 7 | 2 | |
| c = a + b | 26 | 21 | 34 | 19 | 27 | 18 | |
| p | 0.69 | 0.89 | 0.30 | ||||
| Live birth rate through ET | /Pt with c (%) | 59.1 | 63.6 | 59.6 | 61.3 | 43.5 | 35.3 |
| /ET cycle with c (%) | 31.3 | 36.8 | 30.4 | 27.9 | 19.0 | 13.5 | |
| Number of live births without FP | d | 2 | 1 | 1 | 0 | 3 | 5 |
| Total live births | e (n) | 28 | 22 | 35 | 19 | 30 | 23 |
| /Pt with e (%) | 63.6 | 66.7 | 61.4 | 61.3 | 48.4 | 45.1 | |
| Number of patients who died during the study period | 2 | 2 | 4 | 1 | 3 | 4 | |
| p = 0.77 | p = 0.44 | p = 0.80 | |||||
| Mortality rate during the survey period | 4.5 | 6.1 | 7.0 | 3.2 | 4.8 | 7.8 | |
Abbreviations: a, Already given birth; b, Pregnant at the time of the survey; c, Estimation of the total number of births through embryo transfer (a + b); d, Live births other than embryo transfer using cryopreserved embryos; e, Total number of live births among patients who underwent embryo cryopreservation (c + d). This includes not only births through embryo transfer but also natural conceptions and births resulting from infertility treatment after treatment of primary disease.
Abbreviations: AI, aromatase inhibitor; EC, embryo cryopreservation; ET, embryo transfer; FP, fertility preservation; Pt, patient.
3.4. Implementation status and outcome of ET using embryo which cryopreserved as FP on each primary disease
Table 3 shows the implementation status and ET outcome by disease. Only patients who underwent EC for benign hematological diseases were in their 20s (median age 27.5 years). Furthermore, although the grace period for FP tended to be relatively long for all diseases, the grace period was particularly long for benign diseases. Regarding leukemia, there were four cases of chronic myeloid leukemia (CML) and one case of acute myeloid leukemia (AML) with long grace periods. However, 101 out of 417 (24.2%) respondents left blank regarding the grace period, indicating low reliability of the data. As can be seen from Table 3, most patients undergoing ET after EC as FP in Japan have breast cancer, and the PR per patient is 60.9%, and the LBR per patient is 49.1%. Benign hematological diseases performed poorly in terms of PRs and LBRs per ET, with PRs and LBRs of 8.3%. Additionally, the random start method was commonly used, with 36.3% of breast cancer patients experiencing random start ovarian stimulation. The random start method was also actively used in other diseases. For musculoskeletal tumors where the grace period for FP was short, 75% of patients received random start ovarian stimulation. In addition, for most diseases except breast cancer, pregnancies other than FP were rare, with only a few non‐FP pregnancies being observed in patients with cervical and colorectal cancers and ovarian tumors. Regarding the mortality rate, musculoskeletal tumor patients had a high mortality rate, with 75% dying. Other cancers, such as ovarian cancer and ovarian with low malignant potential (LMP) tumors, and cervical cancer had mortality rates of over 10%.
TABLE 3.
Embryo transfer outcomes using cryopreserved embryos for fertility preservation in patients with various underlying diseases.
| Malignant disease | Benign disease | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | Ovarian tumor | Ovarian cancer | Ovarian tumor (LMP) | Cervical cancer | Endometrial cancer | Malignant lymphoma | Leukemia | Colorectal cancer | Musculoskeletal tumor | Thyroid cancer | Brain cancer | Hematological disease | SLE | Neurological diseases | |
| Number of patients | 324 | 26 | 18 | 8 | 10 | 7 | 11 | 5 | 10 | 4 | 3 | 2 | 4 | 2 | 2 |
| Age of patients at EC (years‐old) | 36.7 ± 3.6 | 32.5 ± 5.1 | 33.3 ± 5.1 | 30.6 ± 4.8 | 35.4 ± 3.3 | 30.6 ± 4.8 | 32.2 ± 2.8 | 38.2 ± 5.3 | 35.7 ± 4.5 | 35.3 ± 3.6 | 36.3 ± 1.5 | 34.0 ± 1.4 | 29.0 ± 4.2 | 36.5 ± 2.1 | 36.5 ± 2.1 |
| Median age at EC (years‐old) | 37 | 33.5 | 34 | 29 | 35 | 34 | 32 | 40 | 36.5 | 34.5 | 36 | 34 | 27.5 | 36.5 | 36.5 |
| Grace period for FP(days) | 85 ± 154 | 61 ± 89 | 70 ± 99 | 30 ± 2 | 51 ± 34 | 60 ± 35 | 48 ± 43 | 131 ± 202 | 38 ± 20 | 45 ± 13 | 88 ± 55 | 69 ± 55 | 171 ± 168 | 180 | 135 ± 64 |
| Median (min‐max) (days) | 57.5 (7–1400) | 31 (14–365) | 34 (14–365) | 30 (28–32) | 40 (24–100) | 60 (30–90) | 35 (16–148) | 15 (14–365) | 40 (8–60) | 44.5 (30–60) | 63 (50–150) | 69 (30–108) | 90 (60–365) | 180 | 135 (90–180) |
| Chemotherapy before FP (n, %) | 12 (3.7) | 5 (19.2) | 4 (22.2) | 1 (12.5) | 0 | 2 (28.6) | 1 (9.1) | 2 (40) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comorbidities (n, %) | 8 (2.5) | 0 | 0 | 0 | 1 (10.0) | 0 | 0 | 0 | 0 | 2 (50.0) | 0 | 0 | 2 (50.0) | 1 (50.0) | 2 (100) |
| Experience of fertility treatment (n, %) | 40 (12.3) | 6 (23.1) | 4 (22.2) | 2 (25.0) | 2 (20.0) | 1 (14.3) | 2 (18.2) | 0 | 3 (30.0) | 1 (25.0) | 2 (66.7) | 1 (50.0) | 0 | 1 (50.0) | 0 |
| Number of EC cycles total | 523 | 47 | 33 | 14 | 20 | 28 | 12 | 14 | 14 | 4 | 8 | 2 | 8 | 2 | 4 |
| /Patient | 1.6 ± 1.0 | 1.8 ± 1.2 | 1.8 ± 1.2 | 1.8 ± 1.4 | 2.0 ± 1.6 | 1.1 ± 0.3 | 1.1 ± 0.3 | 2.8 ± 3.0 | 1.4 ± 0.7 | 1 | 2.6 ± 2.1 | 1 | 2.0 ± 0.8 | 1 | 2.0 ± 1.4 |
| Number of ET cycles total | 707 | 59 | 37 | 22 | 26 | 28 | 21 | 7 | 24 | 6 | 5 | 5 | 12 | 4 | 2 |
| /Patient | 2.2 ± 1.5 | 2.3 ± 1.2 | 2.1 ± 1.1 | 2.8 ± 1.4 | 2.6 ± 1.8 | 4.0 ± 2.3 | 1.9 ± 1.3 | 1.4 ± 0.5 | 2.4 ± 1.8 | 1.5 ± 1.0 | 1.7 ± 1.2 | 2.5 ± 0.7 | 3.0 ± 1.4 | 2.0 ± 1.4 | 1 |
| Comorbidities (n, %) | 6 (1.8) | 0 | 0 | 0 | 2 (20) | 0 | 3 (27.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Random start (n, %) | 118 (36.3) | 6 (23.1) | 4 (22.2) | 2 (25) | 1 (10.0) | 1 (14.3) | 3 (27.2) | 0 | 5 (50.0) | 3 (75.0) | 0 | 1 (50.0) | 1 (25.0) | 0 | 0 |
| Pregnancy through ET total | 198 | 13 | 9 | 4 | 6 | 5 | 9 | 3 | 3 | 2 | 3 | 1 | 1 | 1 | 2 |
| Pregnancy rate through ET/patient (%) | 60.9 | 50.0 | 50.0 | 50.0 | 60.0 | 71.4 | 81.8 | 60.0 | 30.0 | 50.0 | 100 | 50.0 | 25.0 | 50.0 | 100 |
| /ET (%) | 28.0 | 22.0 | 24.3 | 18.2 | 23.1 | 17.9 | 42.9 | 42.9 | 12.5 | 33.3 | 60.0 | 20.0 | 8.3 | 25.0 | 100 |
| Number of live birth through ET | |||||||||||||||
| a | 123 | 8 | 5 | 3 | 4 | 2 | 7 | 2 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| b | 36 | 4 | 3 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Live birth rate through ET with a (%) | 17.4 | 13.6 | 13.5 | 13.6 | 15.4 | 7.1 | 33.3 | 28.6 | 4.2 | 16.7 | 60 | 20 | 8.3 | 25 | 100 |
| Live birth rate through ET with c (%) | 22.5 | 20.3 | 21.6 | 18.2 | 19.2 | 10.7 | 38.1 | 28.6 | 8.3 | 33.3 | 60 | 20 | 8.3 | 25 | 100 |
| /patient with c (%) | 49.1 | 46.2 | 44.4 | 50.0 | 50.0 | 42.9 | 72.7 | 40.0 | 20.0 | 50.0 | 100 | 50.0 | 25.0 | 50.0 | 100 |
| Number of live births without FP d | 19 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total live birth rate /patient (%) e | 54.9 | 50.0 | 50.0 | 50.0 | 60.0 | 42.9 | 72.7 | 40.0 | 30.0 | 50.0 | 100.0 | 50.0 | 25.0 | 50.0 | 100.0 |
| Number of patients who died during the study period | 19 | 4 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| Mortality rate during the survey period (%) | 5.9 | 15.4 | 16.7 | 12.5 | 10.0 | 0 | 0 | 0 | 10.0 | 75.0 | 0 | 0 | 0 | 0 | 0 |
Note: a, Already given birth; b, Pregnant at the time of the survey; c, Estimation of the total number of births thorough embryo transfer (a + b); d, Live births other than embryo transfer using cryopreserved embryos; e, Total number of live births among patients who underwent embryo cryopreservation (c + d). This includes not only births through embryo transfer but also natural conceptions and births resulting from infertility treatment after treatment of primary disease.
Abbreviations: CML, chronic myeloid leukemia; EC, embryo cryopreservation; ET, embryo transfer; FP, fertility preservation; LMP, low malignant potential; SLE, systemic lupus erythematosus.
4. DISCUSSION
In this survey, 1400 cases of EC were performed in Japan in the 6 years since 2014. According to previous reports that investigated the implementation status of EC for FP in Japan, 627 cases of EC were performed in Japan between 2011 and 2015, and 1246 cases were performed between 2016 and 2019. 7 , 8 Therefore, more than 1800 cases of EC as FP have been performed since 2011, but those studies covered a wider range of facilities than those covered by this survey. These clinical studies targeted approximately 600 facilities certified as facilities for ART (assisted reproductive technologies) by JSOG. The present study targeted facilities certified as FP facilities for medically indication (of course, these are certified by JSOG as ART facilities), and the number of such facilities was only 150 (114 responded). Despite this, 1400 ECs were implemented in 6 years, indicating that the number of FP implementations is rapidly increasing in Japan. And previous systematic reviews and meta‐analyses have investigated the outcomes of EC for FP in other countries, 9 , 10 the number of cases investigated is not large. Therefore, the present study is the clinical study with the largest number of cases to date. Also, the present study is unique in that it investigated ET outcome by age and disease. Furthermore, in this survey, breast cancer was the most common primary disease, and it is thought that the distribution of patient age influenced by breast cancer patients. Many of the existing reports on EC involve breast cancer patients, as well as gynecological and hematological disease patients. 9 , 10 But the results of this study were not only consistent for the common primary diseases but also included other diseases, such as colorectal cancer and brain cancer, as well as systemic lupus erythematosus (SLE) and neurological diseases.
In this study, we showed the results of EC for FP by age group. Evaluation showed that the PR for each ET was 27.1%, and the LBR was 21.4% in the entire cohort. To date, although there have not been many reports on the results of ET using EC for FP, a previous study reported a PR of ET due to EC of 49.0%, and an LBR of 35.3%. 10 This discrepancy between ours and the previous study might be due to the age difference of the patients involved, although we cannot rule out that it could be due to the insufficiency of FP techniques in Japan. However, when considering the entire cohort, since the LBR/patient was 47.7% even when considering patients of all age groups, we consider that EC for FP in Japan might provide acceptable results. Additionally, in each study, the number of embryos used for ET was unknown (including the present study), so this may also be a factor that differentiates our results from other studies.
In this study, as shown in Figure 4, the PR was 60.4%/patient, decreasing with increasing age. On the other hand, the number of EC cycles and number of ETs per patient increased with age. There could be several reasons for the observed age‐related differences in numbers of EC and ET. Although subsidy systems existed for each region in Japan even before public subsidies were introduced, regional subsidies varied and were weaker than the current public subsidy system. Additionally, the period covered by this study was before the start of the public subsidy system, and costs varied widely depending on the FP facility. Therefore, relatively affluent middle‐aged patients might have been able to undergo EC or ET more often than younger patients from an economic point of view. Additionally, younger patients might have been considered as still being fertile, with the possibility of conceiving even after chemotherapy and/or radiation, and hence might not have been recommended multiple times of ECs and ETs. Therefore, it is possible that the number of ECs and ETs in younger patients in this study was small based on discussion between patients and FP doctors. In older patients, on the other hand, the FP doctor might have recommended multiple ECs and ETs to ensure a sufficient chance of childbirth considering the low LBR of aged women. Although this was only among patients who underwent ET, it was found that there were 17 live births by methods other than FP. These 17 cases were in young patients. This fact indicates that FP is not necessary for all young patients. Needless to say, it is necessary to discuss the treatment protocol and the severity of the disease with a doctor who specializes in FP. However, the authors also believe that the idea of receiving FP as one of procedure just in case cannot be ruled out. On the other hand, although it is possible to suggest that there are other methods of pregnancy other than FP for aged patients too, the fact remains that FP may be a better option.
The indications for EC, whether for FP or for regular infertility treatment, might also affect its reproductive outcomes. Thus, we need to verify whether EC for FP is equivalent to EC as infertility treatment. In 2020, a total of 211 042 ETs using cryopreserved embryos were performed in Japan, leading to 75 981 pregnancies (36.0%) and 53 891 births (25.5%). 11 Although the statistical method for handling age in that study was different from that in our study, the mean age of patients seeking ART at registered infertility treatment facilities was 37.8 ± 4.8 years, which was slightly older than in this study. 11 Furthermore, according to European ART statistics for 2018, the PR was 33.4% and LBR was 24.2% for 279 948 cycles of frozen ET. 12 Based on these reports, the results of the present study tend to be lower than the results of existing ARTs. There are several reasons for this, including the limitations of this research. First, in the case of emergency EC performed to preserve fertility, an adequate treatment plan might not be possible, resulting in incomplete ART. Second, the cancer itself may have affected the quality of the embryos. However, there are currently no reports that cancer affects oocyte quality. The third, if the patient is suffering from a disease, including cancer, the performance of oocyte retrieval and EC might be inadequate. This could be related to the fact that the PR and LBR for patients younger than 35 years are lower than those for patients aged 35–37 years, as shown in Figure 5. This is because younger people are more prone to systemic diseases such as blood cancer, and unlike solid cancers, it is assumed that they are more likely to experience symptoms such as fatigue. Furthermore, in this study, we were unable to confirm what grade of embryo was used for ET, whether it was a cleavage embryo or blastocyst, and whether multiple or single embryos were transferred. Therefore, the results of this study are limited in that they do not fully capture the results of ET. Although the results of EC for FP have been previously reported, they vary widely, with LBRs ranging from 12.5% to 50.0%. 13 , 14 , 15 , 16 Since this study is the largest reported study on the topic to date, it could potentially provide useful information. Also, the final LBR which including by methods other than FP (Figure 7), approximately half of the patients had a live birth. This would be very encouraging information for young female patients who hope to have children.
During the observation period, there were 28 deaths (6.7%). This was particularly high among young people (11 (8.9%)) and two (20.0%) among those aged 43 years and over. These results are for cases that resulted in ET, so the overall mortality rate is unknown. We cannot deny the possibility that the mortality rate is even higher in patients who do not undergo ET. Furthermore, if the observation period is lengthened, it can be expected that the mortality rate will naturally increase.
AI inhibits the conversion of androgens to estrogen, suppressing the increase in estrogen levels during ovarian stimulation for EC in estrogen‐sensitive breast cancer patients. The effectiveness of ovarian stimulation combined with AI on the outcome of oocyte retrieval has been previously demonstrated. 17 , 18 Although ovarian stimulation for EC was not verified in detail, it is safe to assume that the use of AI did not cause a clear deterioration in EC outcomes (Table 2). Recent randomized control studies have also shown no difference in the AI use group compared with the control group, including in terms of clinical PR. 19 , 20 The present study showed no differences in PR and LBR in each age group between AI‐treated and non‐treated groups. Additionally, there were no differences in mortality rates depending on whether AI was used. Hence, although AI might reduce mortality rates in breast cancer patients through the accumulation of cases. And we consider that further major randomized clinical control studies are needed to confirm the effect of AI for PR and LBR. Because the quality of FP might have been lower in the group where AI was not used, which might have led to bias.
The novelty of the present study lies in the fact that reproductive outcomes of EC were evaluated for a wide range of diseases, while, in the past, reproductive outcomes of EC have only been reported for breast cancer and hematological diseases. 9 , 21 However, since there were not enough cases for some of the primary diseases, further validation of our results using national registry systems are required.
Another limitation is that patients who underwent EC but did not undergo ET were not included. Of course, this includes patients who became pregnant without requiring ET at all, patients who did not receive ET due to illness, and patients who did not receive ET due to divorce, bereavement, etc. In this respect, the PR and LBR shown in this study might not be completely accurate. Additionally, since 63 (15.1%) of the 417 patients were already undergoing infertility treatment, we cannot rule out the possibility that they would have had more difficulty conceiving than the normal population. It has been pointed out that PR and LBR might be reduced in patients with a history of cancer treatment. 22 Although this is mainly seen in patients who have received pelvic radiation, reportedly, PR might also be lower in patients who receive chemotherapy. 23
5. CONCLUSIONS
This study demonstrated the efficacy and safety of EC for FP. Although fertility‐preserving EC is particularly effective in patients ≤42 years of age, our results also showed its efficacy in patients' ≥43 years of age with multiple EC cycles. This suggests that the reproductive outcomes of ET following EC for FP in Japan are acceptable, and the practice of multiple ECs might contribute to improve live birth rates. To that end, we should continue to devise ways to implement EC, such as the random start method, and should improve the reproductive outcome of EC for FP. In the future, it is possible that reproductive outcome might improve as we learn more about FP techniques.
CONFLICT OF INTEREST STATEMENT
None of the authors have any commercial or financial involvement in connection with this work that represents or appears to represent any conflicts of interest.
APPROVAL BY ETHICS COMMITTEE
The research protocol of this study was approved by the institutional review board of St. Marianna University School of Medicine (approval no. 5180) and was registered in the University Hospital Medical Information Network (UMIN000043664).
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1964 and its later amendments. Information about the clinical trial was posted as an opt‐out at each hospital, giving eligible patients the opportunity to choose not to participate in the clinical study.
ACKNOWLEDGMENTS
We thank all the clinicians at the JSOG‐certified facilities who participated in this study. The study was supported by a Ministry of Health, Labour and Welfare, Japan, grant to S.N. (grant no. 19EA1015).
Takae S, Harada M, Nakamura K, Furuyama S, Ono M, Osuga Y, et al. Reproductive outcomes of embryo cryopreservation and transfer at the start‐up phase of fertility preservation in Japan. Reprod Med Biol. 2024;23:e12581. 10.1002/rmb2.12581
REFERENCES
- 1. von Wolff M, Andersen CY, Woodruff TK, Nawroth F. FertiPROTEKT, Oncofertility consortium and the Danish fertility‐preservation networks ‐ what can we learn from their experiences? Clin Med Insights Reprod Health. 2019;13:1179558119845865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takae S, Lee JR, Mahajan N, Wiweko B, Sukcharoen N, Novero V, et al. Fertility preservation for child and adolescent cancer patients in Asian countries. Front Endocrinol. 2019;10:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shigematsu K, Shimizu C, Furui T, Kataoka S, Kawai K, Kishida T, et al. Current status and issues of the Japan Oncofertility registry. J Adolesc Young Adult Oncol. 2023;12(4):584–591. [DOI] [PubMed] [Google Scholar]
- 4. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. [DOI] [PubMed] [Google Scholar]
- 5. Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rashedi AS, de Roo SF, Ataman LM, Edmonds ME, Silva AA, Scarella A, et al. Survey of fertility preservation options available to patients with cancer around the globe. JCO glob Oncologia. 2020;6:331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanada Y, Harada M, Kunitomi C, Kanatani M, Izumi G, Hirata T, et al. A Japanese nationwide survey on the cryopreservation of embryos, oocytes and ovarian tissue for cancer patients. J Obstet Gynaecol Res. 2019;45(10):2021–2028. [DOI] [PubMed] [Google Scholar]
- 8. Kunitomi C, Harada M, Sanada Y, Kusamoto A, Takai Y, Furui T, et al. The possible effects of the Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 on the practice of fertility preservation in female cancer patients in Japan. Reprod Med Biol. 2022;21(1):e12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Z, Ibrahim S, Burdett S, Rydzewska L, Al Wattar BH, Davies MC. Long term pregnancy outcomes of women with cancer following fertility preservation: a systematic review and meta‐analysis. Eur J Obstet Gynecol Reprod Biol. 2023;281:41–48. [DOI] [PubMed] [Google Scholar]
- 10. Ni Dhonnabhain B, Elfaki N, Fraser K, Petrie A, Jones BP, Saso S, et al. A comparison of fertility preservation outcomes in patients who froze oocytes, embryos, or ovarian tissue for medically indicated circumstances: a systematic review and meta‐analysis. Fertil Steril. 2022;117(6):1266–1276. [DOI] [PubMed] [Google Scholar]
- 11. Katagiri Y, Jwa SC, Kuwahara A, Iwasa T, Ono M, Kato K, et al. Assisted reproductive technology in Japan: a summary report for 2020 by the ethics Committee of the Japan Society of obstetrics and gynecology. Reprod Med Biol. 2023;22(1):e12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Ivf Monitoring Consortium ftESoHR , Embryology WC, De Geyter C, Calhaz‐Jorge C, Kupka MS, et al. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod. 2022;2022(3):hoac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayeur A, Puy V, Windal V, Hesters L, Gallot V, Benoit A, et al. Live birth rate after use of cryopreserved oocytes or embryos at the time of cancer diagnosis in female survivors: a retrospective study of ten years of experience. J Assist Reprod Genet. 2021;38(7):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michaan N, Ben‐David G, Ben‐Yosef D, Almog B, Many A, Pauzner D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):175–177. [DOI] [PubMed] [Google Scholar]
- 15. Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33(22):2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolmans MM, Hollanders de Ouderaen S, Demylle D, Pirard C. Utilization rates and results of long‐term embryo cryopreservation before gonadotoxic treatment. J Assist Reprod Genet. 2015;32(8):1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–4353. [DOI] [PubMed] [Google Scholar]
- 18. Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98(6):1363–1369. [DOI] [PubMed] [Google Scholar]
- 19. Bulow NS, Warzecha AK, Nielsen MV, Andersen CY, Holt MD, Petersen MR, et al. Impact of letrozole co‐treatment during ovarian stimulation on oocyte yield, embryo development, and live birth rate in women with normal ovarian reserve: secondary outcomes from the RIOT trial. Hum Reprod. 2023;38:2154–2165. [DOI] [PubMed] [Google Scholar]
- 20. Letourneau J, Juarez‐Hernandez F, Wald K, Ribeiro S, Wang A, McCulloch CE, et al. Concomitant tamoxifen or letrozole for optimal oocyte yield during fertility preservation for breast cancer: the TAmoxifen or Letrozole in estrogen sensitive tumors (TALES) randomized clinical trial. J Assist Reprod Genet. 2021;38(9):2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraison E, Huberlant S, Labrune E, Cavalieri M, Montagut M, Brugnon F, et al. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: a systematic review and meta‐analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum Reprod. 2023;38(3):489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meernik C, Poole C, Engel SM, Rauh‐Hain JA, Luke B, Nichols HB. Outcomes after assisted reproductive technology in women with cancer: a systematic review and meta‐analysis. Hum Reprod. 2023;38(1):30–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Griffiths MJ, Winship AL, Hutt KJ. Do cancer therapies damage the uterus and compromise fertility? Hum Reprod Update. 2020;26(2):161–173. [DOI] [PubMed] [Google Scholar]


