Abstract
In this unselected population of women referred to a center specialized in gynecologic endocrinology for suspicion of PCOS, a minimum rate of misdiagnosed FHA patients of about 2% was found. It is necessary to evaluate reliable markers for the differential diagnosis between PCOS and FHA to avoid incorrect treatment, which might lead to negative long‐term effects in women with undiagnosed FHA.
Abstract
Purpose
To study how many women are misdiagnosed with polycystic ovary syndrome (PCOS) instead of functional hypothalamic amenorrhea (FHA), which is important to improve overall well‐being, long‐term health, and fertility issues.
Methods
The FHA prevalence in a cohort of 401 women previously diagnosed with PCOS (revised Rotterdam criteria) was estimated retrospectively based on experts and previous studies: luteinizing hormone (LH) <2 IU/mL, LH <5.36 IU/mL, sex hormone binding globulin (SHBG) >53.3 nmol/L, Testosterone <0.36 ng/mL, and the formula of Beitl et al. [(7.05*testosterone ng/mL) − (0.005*SHBG nmol/L) + (0.117*LH mIU/mL) − 2.463 < 0].
Results
The highest rate of women with suspicion of FHA in patients referred for PCOS was found when the SHBG cut‐off of ≥53.3 nmol/L was used (36.9%), followed by the use of the LH cut‐off of <5.36 IU/mL (12.5%). The minimal suspected rate was achieved with the LH cut‐off <2.0 IU/mL (1.7%). Women who fulfilled the criteria for PCOS phenotype D (ovulatory dysfunction and polycystic ovarian morphology) revealed the maximum rate for suspected FHA (up to 47.6%).
Conclusion
It is still necessary to evaluate reliable markers for the differential diagnosis between PCOS and FHA to avoid incorrect treatment, which might lead to negative long‐term effects in women with undiagnosed FHA.
1. INTRODUCTION
Secondary amenorrhea occurs in 3–5% of women of reproductive age and is most often caused by polycystic ovary syndrome (PCOS) or functional hypothalamic amenorrhea (FHA). 1 , 2 FHA is defined as a form of hypogonadotropic hypogonadism caused by hypothalamic dysregulation and typically presents with low estradiol levels due to reduced hypothalamic gonadotropin‐releasing hormone (GnRH) secretion. 3 Whilst both states present with distinct pathophysiologies and their diagnosis is maintained by guidelines, in practice, the differential diagnosis between these two groups continues to pose a clinical challenge to the physicians who treat it. Up to 50% of women with FHA present with polycystic ovarian morphology (PCOM) on ultrasound. 4 Thus, the greatest difficulty is the differentiation between women with FHA and PCOM (FHA‐PCOM) and PCOS without hyperandrogenism (phenotype D, PCOS‐D). 5 So far, no clear methods have been established to distinguish between PCOS and FHA. However, several criteria have been proposed and include body mass index (BMI), serum levels of luteinizing hormone (LH), androgens, insulin resistance, anti‐Mullerian‐hormone (AMH), and sex hormone binding globulin (SHBG). Furthermore, a negative progesterone withdrawal test as well as thin endometrial thickness at ultrasound (U/S) are also suggestive of FHA, with low sensitivity however. 4 , 6
PCOS is usually diagnosed by the revised Rotterdam criteria, namely by the presence of at least two of the following criteria: hyperandrogenism, oligo/amenorrhea, and PCOM on ultrasound. 7 It is of paramount importance to remind that these criteria should be applied after having excluded other causes of hyperandrogenism and/or oligo‐anovulation, such as FHA. 8 , 9 According to the Endocrine Society, the diagnosis of FHA is based on the following criteria: menstrual cycle length persistently >45 days or amenorrhea longer than 3 months, history of weight loss, excessive exercise, stress, in combination with hypogonadotropic hypo‐estrogenism. Furthermore, a negative progesterone withdrawal test and a normal MRI of the pituitary gland are also recommended. As for PCOS, other causes for menstrual disturbances must be excluded. 1 In a retrospective study, 86% of lean women with FHA would have met the Rotterdam definition of PCOS if FHA had not been identified. 10 Recent considerations suggest that a better distinction between FHA and PCOS is relevant to improve treatment strategies not only for anovulatory infertility but also for overall wellbeing. 11 , 12 Due to the significance of fertility treatment strategies, the World Health Organization (WHO) classification of anovulation differs between WHO group 1 anovulation including FHA and WHO group 2 anovulation, namely PCOS. Even after their reproductive needs have been fulfilled, long‐term health risks in patients with PCOS demand their general health be monitored with an emphasis on metabolic and cardiovascular issues. 13 It might be assumed that a misdiagnosis of FHA as PCOS may lead to more serious consequences for life‐long health and quality of life rather than vice versa, especially because of the effect on bone and cardiovascular health including the higher risk of stress fractures and lifelong low bone density for women suffering FHA. 14 , 15 , 16
Noteworthy, PCOS and FHA (with PCOM) may seem similar in some patients. However, they are definitively two distinct entities. Nonetheless, the rate of misdiagnosed FHA women in a population with suspected PCOS has not been evaluated yet. These data are of considerable value, since they could highlight the importance of this differential diagnosis. Thus, the aim of our retrospective study was to investigate the prevalence of FHA in a cohort of women diagnosed and referred to our center for PCOS.
2. MATERIALS AND METHODS
The Clinical Division Of Gynecologic Endocrinology and Reproductive Medicine of the Medical University of Vienna, Austria, is the main referral center for women with gynecologic‐endocrinological problems in eastern Austria. In a retrospective data set, all the 470 women, who were referred to our center for PCOS from January 1, 2014 to December 31, 2019, were included. Of these, 66 women (14%) did only fulfill one Rotterdam criterion. 17 They were thus, excluded from the study as well as three patients who were diagnosed with non‐classical adrenal hyperplasia (0.6%). The remaining 401 women were included in the present analysis.
2.1. Parameters analyzed
The AKIM‐software (SAP‐based patient management system at the Medical University of Vienna) was used for data acquisition. For the definition of PCOS, the Rotterdam criteria were used 17 and the recent guidelines set forth by the International PCOS Network were followed. 18 Serological hyperandrogenism was defined as testosterone levels exceeding the in‐house cut‐off of >0.48 ng/mL. Hirsutism was rated as a clinical sign of hyperandrogenism. Oligomenorrhea was defined as a mean bleeding interval >35 days in the last three cycles before initial patient assessment, amenorrhea as an absence of bleeding of >3 months. In all patients, a transvaginal sonography was performed using an Aloka Prosound 6 ultrasound machine (Wiener Neudorf, Austria; frequency range 3.0–7.5 MHz) was used. Polycystic ovarian morphology (PCOM) was defined by a follicle number per ovary (FNPO) >12 and/or an ovarian volume ≥10 cm3 and/or an ovarian area ≥5.5 cm2, according to the recommendations of an international expert panel for ultrasound machine with frequency range less than 8 MHz. 19 Accordingly, the four common PCOS phenotypes were defined: A, PCOM, hyperandrogenism and ovulatory dysfunction, defined as oligo‐ or amenorrhea; B, hyperandrogenism and ovulatory dysfunction; C, hyperandrogenism and PCOM; and D, ovulatory dysfunction and PCOM. 20
The serum levels of AMH, LH, follicle‐stimulating hormone (FSH), total testosterone, SHBG, and estradiol were analyzed. Blood samples were obtained during the early follicular phase visit (cycle days 2–5). All serum parameters were determined at the Department of Laboratory Medicine, Medical University of Vienna, according to ISO 15189 quality standards. As reported previously, 21 , 22 Cobas electrochemiluminescence immunoassays (ECLIA) were performed on Cobas e 602 analyzers (Roche, Mannheim, Germany) for the determination of serum estradiol, FSH, LH, AMH, testosterone, and sex hormone binding globulin SHBG. The corresponding maximal coefficients of variation of these assays were 10.6, 4.5, 2.2, 3.5, 14.5, 2.7, 11.9 and 4.0%, respectively.
The HOMA‐IR (mg/dL) was determined and calculated as fasting insulin (mU/L) * fasting glucose (mg/dL)/405. A HOMA‐IR >2 mg/dL was considered suggestive for insulin resistance.
2.2. Criteria to distinguish FHA from PCOS
The main outcome parameter was whether FHA had been suspected by the experts in gynecologic endocrinology responsible for outpatient care (referred to as “expert opinion” in the results). This was usually based on the history of FHA‐typical causes (excessive exercise, weight loss, eating disorders, chronic psychosomatic stress), a negative progestogen challenge test, low estradiol levels, and low LH levels. However, the final diagnosis was up to the individual physician.
Several criteria were used to distinguish FHA from PCOS, based on previous studies 4 , 5 , 6 : the formula of Beitl et al. ((7.05*testosterone ng/mL) − (0.005*SHBG nmol/L) + (0.117*LH mIU/mL) − 2.463 < 0), 5 serum levels of LH <2, 4 serum levels of LH <4.7 IU/mL, 5 serum levels of LH <5.36 IU/mL, 6 a SHBG >53.3 nmol/L 6 and a serum testosterone <1.26 nmol/L = <0.36 ng/mL. 6 Last but not least, a negative progestogen challenge test with oral dydrogesterone 20 mg for 10–12 days was considered suggestive for FHA, 6 but was only available in women with amenorrhea.
2.3. Statistical analysis
Data are present as median and interquartile range (IQR) for numerical parameters and as numbers (frequencies) for categorical data. Differences between groups in categorical data were evaluated using Fisher's exact test. Sensitivity, specificity, positive (PPV), and negative predictive values (NPV) with their 95% confidence intervals (95% CI) were calculated using crosstabs. The IBM Statistical Package for Social Science software (SPSS 25.0) was used for all statistical tests. A p < 0.05 was considered statistically significant.
2.4. Ethics statement
The study was approved by the ethics committee of the Medical University of Vienna (IRB number 2255/2019).
3. RESULTS
Table 1 shows the basic patient characteristics of the whole study population included (n = 401). The median age was 25 years (IQR 22–28) and the median BMI was 26.6 kg/m2 (IQR 22.0–32.4).
TABLE 1.
General patient characteristics (n = 401).
| Age (years)a | 25 (22; 28) |
| BMI (kg/m2)a | 26.6 (22.0; 32.4) |
| Polycystic ovarian morphologyb | 348 (86.8) |
| Oligomenorrheab | 151 (37.6) |
| Amenorrheab | 157 (39.2) |
| Hyperandrogenismb | 361 (90.0) |
| Number of Rotterdam criteria fulfilleda | 3 (2; 3) |
| Insulin resistanceb | 159 (39.7) |
| LH (mIU/mL)a | 12.3 (7.8; 17.8) |
| FSH (mIU/mL)a | 5.6 (4.6; 6.8) |
| Estradiol (pg/mL)a | 54 (43; 77) |
| Testosterone (ng/mL)a | 0.58 (0.48; 0.73) |
| SHBG (nmol/L)a | 41.5 (25.3; 67.5) |
| AMH (ng/mL)a | 9.02 (6.02; 13.2) |
Note: Data are presented as amedian and interquartile range (IQR) for numerical parameters or as bnumbers (frequencies), n (%), for categorical data.
In 18 patients (4.5%), the experts in gynecologic endocrinology at our department had diagnosed FHA. The majority of these women presented with amenorrhea. Accordingly, the rate of FHA diagnosed by the experts was higher in amenorrheic patients (14/157, 8.9%). In accordance with the various methods listed above to differentiate between PCOS and FHA, Table 2 demonstrates the rates of women with suspicion of FHA in all 401 patients referred for PCOS. The highest rate was found when the SHBG cut‐off of ≥53.3 nmol/L was used (36.9%), followed by the use of the LH cut‐off of <5.36 IU/mL (12.5%). The minimal suspected rate was achieved with the LH cut‐off <2.0 IU/mL (1.7%).
TABLE 2.
Rate of women with suspicion of functional hypothalamic amenorrhea (FHA) according to various definitions in patients referred for polycystic ovary syndrome (PCOS).
| Expert opinion | 18 (4.5) |
| LH <2.0 IU/mL | 7 (1.7) |
| LH <5.36 IU/mL | 50 (12.5) |
| Testosterone <0.36 ng/mL | 45 (11.2) |
| SHBG ≥53.3 nmol/L | 148 (36.9) |
| FHA according to formula of Beitl et al. | 25 (6.2) |
Note: Data are presented as n (%).
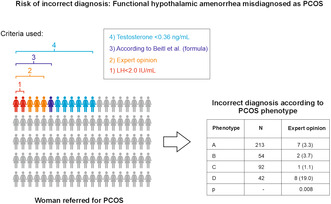
In the next step, the rates for suspected FHA were analyzed according to the PCOS phenotype (Table 3). Notably, the highest rates were found in women who fulfilled the criteria for the normoandrogenic PCOS phenotype D (up to 47.6%). The differences between phenotypes were statistically significant when the expert opinion (p = 0.008), the testosterone cut‐off <0.36 ng/mL (p < 0.001) and the formula for differential diagnosis published by Beitl et al. 5 (p < 0.001) were used.
TABLE 3.
Rate of women with suspicion of functional hypothalamic amenorrhea (FHA) according to the suspected polycystic ovary syndrome (PCOS) phenotype.
| Phenotype | N | Expert opinion | LH <2.0 IU/mL | LH <5.36 IU/mL | Testosterone <0.36 ng/mL | SHBG ≥53.3 nmol/L | FHA according to formula of Beitl et al. |
|---|---|---|---|---|---|---|---|
| A | 213 | 7 (3.3) | 2 (0.9) | 23 (10.8) | 18 (8.5) | 76 (35.7) | 9 (4.2) |
| B | 54 | 2 (3.7) | 2 (3.7) | 9 (16.7) | 5 (9.3) | 18 (33.3) | 3 (5.6) |
| C | 92 | 1 (1.1) | 2 (2.2) | 11 (12.0) | 4 (4.3) | 34 (37.0) | 2 (2.2) |
| D | 42 | 8 (19.0) | 1 (2.4) | 7 (16.7) | 18 (42.9) | 20 (47.6) | 11 (26.2) |
| p | – | 0.008 | 0.518 | 0.555 | <0.001 | 0.481 | <0.001 |
In a sub‐analysis, only women with amenorrhea were included (n = 157). These patients revealed a median age of 25 years (IQR 22–29) and a median BMI of 26.0 kg/m2 (IQR 22.1–31.6). A negative progestogen challenge test, a criterion that was only available in women with amenorrhea, was found in 6 women (3.8%). The various cut‐offs revealed suspicion of FHA in 4 (2.5%; LH <2 IU/mL), 32 (20.4%, LH <5.36 IU/mL), 23 (14.6%, testosterone <0.36 ng/mL), 35 (22.3%, SHBG ≥53.3 nmol/L), and 10 patients (6.4%, according to the formula of Beitl et al. 5 ).
Last but not least, the objective criteria were compared to the suspected diagnosis of the experts, which was used as the reference. This was done in the whole patient population as well as in women with amenorrhea only (Table 4). All criteria predicted FHA as diagnosed by the experts in a statistically significant manner (p < 0.05) in both groups apart from LH <2 IU/mL in the group of women with amenorrhea (LH levels <2 IU/mL were only found in 3 patients in this population). Sensitivity and specificity levels ranged from 11.1% to 66.7% and from 37.1% to 98.7%, respectively.
TABLE 4.
Prediction of functional hypothalamic amenorrhea (FHA) in a polycystic ovary syndrome (PCOS) population, based on the assessment of experts, according to various standard parameters.
| LH <2.0 IU/mL | LH <5.36 IU/mL | Testosterone <0.36 ng/mL | SHBG ≥53.3 nmol/L | FHA according to formula of Beitl et al. | |
|---|---|---|---|---|---|
| All patients (n = 401) | |||||
| Sensitivity | 11.1 (1.4; 34.7) | 61.1 (35.7; 82.7) | 50.0 (26.0; 74.0) | 66.7 (41.0; 86.7) | 44.4 (21.5; 69.2) |
| Specificity | 98.7 (97.0; 99.6) | 89.8 (86.3; 92.7) | 90.6 (87.2; 93.3) | 64.5 (59.5; 69.3) | 95.6 (93.0; 97.4) |
| PPV | 28.6 (3.7; 71.0) | 22.0 (11.5; 36.0) | 20.0 (9.6; 34.6) | 8.1 (4.3; 13.7) | 32.0 (15.0; 53.5) |
| NPV | 95.9 (93.5; 97.7) | 98.0 (95.9; 99.2) | 97.4 (95.3; 98.8) | 97.6 (94.9; 99.1) | 97.3 (95.2; 98.7) |
| True positives | 2 | 11 | 9 | 6 | 8 |
| True negatives | 378 | 344 | 347 | 136 | 366 |
| p | 0.035 | <0.001 | <0.001 | 0.011 | <0.001 |
| Amenorrhoeic patients (n = 157) | |||||
| Sensitivity | 7.1 (0.2; 33.9) | 57.1 (28.9; 82.3) | 57.1 (28.9; 82.3) | 28.6 (8.4; 58.1) | 50.0 (23.0; 77.0) |
| Specificity | 98.6 (95.3; 99.8) | 93.0 (87.5; 96.6) | 90.2 (84.1; 94.5) | 37.1 (29.1; 45.5) | 94.4 (89.3; 97.6) |
| PPV | 33.3 (0.8; 90.6) | 44.4 (21.5; 69.2) | 36.4 (17.2; 59.3) | 4.3 (1.2; 10.5) | 46.7 (21.3; 73.4) |
| NPV | 91.6 (86.0; 95.4) | 95.6 (90.8; 98.4) | 95.6 (90.6; 98.4) | 84.1 (72.7; 92.1) | 95.1 (90.1; 98.0) |
| True positives | 1 | 8 | 8 | 4 | 7 |
| True negatives | 141 | 133 | 129 | 53 | 135 |
| p | 0.246 | <0.001 | <0.001 | 0.020 | <0.001 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value.
4. DISCUSSION
Our retrospective study revealed a rate of 1.7–36.9% of women, who were probably misdiagnosed with and, thus, referred to our center for PCOS rather than FHA. It has been mentioned that FHA would still tend to be an under‐recognized and undertreated problem in clinical practice. 23
Several limitations must be considered for the present study: the retrospective design; and the fact that patients were included who had been referred for PCOS by general gynecologists, i.e. not experts in gynecologic endocrinology, to our department. Experts in gynecologic endocrinology might have made different initial diagnoses. This is obvious, since the experts in our team came to the diagnosis of FHA in 4.5% of all these women, who had been referred for PCOS. However, we believe that our study population reflects a more general PCOS population. Last but not least, as discussed above, there is a lack of clear criteria to distinguish between the two entities. In this context, it also needs to be stated that using the expert opinion as the diagnostic reference (Table 4) cannot be seen as reliable. However, we believe that the results presented should sensitize gynecologists to the problem of FHA and PCOS.
Notably, an adequate diagnosis of FHA is not only a matter of academic interest, but inadequate treatment plans may result in life long implications for the individuals' health and quality of life. 11 If FHA persists, it may lead to severe consequences for metabolic, bone, cardiovascular, mental, and reproductive health. 14 Women correctly diagnosed with FHA may reach a regular menstrual cycle again after weight gain or reduction in physical activity, as the deficiency in the pulsatile secretion of GnRH is often associated with weight loss, excessive exercise, or psychological stress. Moreover, the strategies for fertility treatment differ between women with FHA and PCOS, as women with FHA may benefit from treatment with pulsatile GnRH or exogenous gonadotropins. 24 , 25 , 26 Empirically, pulsatile GnRH therapy is not that popular anymore. However, a retrospective study demonstrated that not only the ovulation rates but the pregnancy rates as well were significantly higher with GnRH substitution, either per initiated cycle (26.9% vs. 7.6%, p = 0.005) or per patient (65.8% vs. 23.5%, p = 0.007). 27 The authors concluded that GnRH treatment for ovulation induction was more successful and safer than fertility treatment with gonadotropins in FHA‐PCOM patients. 27 Besides the different approaches for fertility treatment, one might not forget that FHA comes along with low bone density and leads to a higher risk of stress or fragility fractures and failure to achieve peak bone mass in young women. 15 Even after recovery, the low bone density and increased fracture risk may persist lifelong. 16 , 28
However, the problem remains that the differential diagnosis is difficult. From a scientific point of view, we find ourselves in a vicious circle, since we would need criteria to clearly distinguish between the two patient groups in order to find these criteria. In a previous study, our team used stringent rules to define FHA by including only women with a negative progestogen challenge test and a strictly defined cause for FHA. 5 However, this approach does not rule out that some FHA patients with PCOM probably were included in the PCOS group. Thus, it is hard to say which criterion is the most reliable in the present study and, in consequence, what the true rate of misdiagnosed FHA in our population of women referred for PCOS was. While the rates using SHBG with its suggested cut‐off of ≥53.3 nmol/L 6 were high (36.9% in the whole population, 34.8% in women with amenorrhea) and considerably do not reflect the actual rate, a minimum rate of about 1.6–2.4% (Tables 2 and 3) must be assumed, at least when using the LH cut‐off of <2.0 IU/mL. One might hypothesize that the truth lies in between. Thus, it might be argued that the formula by Beitl et al., 5 which revealed an incorrect PCOS diagnosis in 6.2% of patients, could be the most reliable tool. However, despite the fact that the study that led to this formula used stringent definition criteria for FHA as stated above, the results might not be applicable for all women with suspicion of PCOS, since only PCOS phenotype D patients had been included. 5
Given the fact that women with FHA reveal estrogen deficiency and that this might be the most relevant characteristic in comparison to PCOS due to the negative effects on bone, cardiovascular health, 14 one could assume that the negative progesterone withdrawal test may be most important tool in the diagnostic process. Noteworthy, our experts also used to rely on this test. Thus, this important criterion was included in the reference method of this study.
That the patient population used for the analysis is of relevance, can also be seen when comparing the results in the whole patient population to those in women with amenorrhea. First, in all women referred for PCOS, the rate of FHA diagnosed by our experts was 4.5%, whereas it was 8.9% in amenorrhoeic patients. Consistently, the sensitivity and specificity levels were higher in the amenorrhea group for the majority of objective measures (Table 4). The special patient population analyzed herein (only women referred from PCOS) might also be the reason, why all tested cut‐offs revealed relevantly higher specificity than sensitivity. All in all, it can be stated that with none of these tools satisfactory PPVs could be achieved, which would also be important for both the patient and the physician. Notably, the formula of Beitl et al. was associated with the highest PPVs. Last but not least, it must be mentioned again that neither the expert opinion represents a perfect and reliable reference for FHA.
Although the actual rate of FHA patients misdiagnosed for PCOS remains unclear, our previous hypothesis that women with the normoandrogenic PCOS phenotype D might be most prone for such a misjudgment is supported by the data presented herein. This became especially clear, when the testosterone cut‐off of <0.36 ng/mL and the formula by Beitl et al. 5 was used (p < 0.001, Table 3). It seems somehow reasonable that in the absence of hyperandrogenism, the chance to be confronted with a FHA patient, who also has PCOM on ultrasound, is higher, especially given the fact that 40–50% of FHA patients reveal PCOM. 4 , 29
It is also possible that in some patients both FHA and PCOS co‐exist. In an older case series of 120 women, who had been initially diagnosed to suffer from primary or secondary hypothalamic amenorrhea and had been treated for ovulation induction with pulsatile GnRH administration, six patients developed an increase in LH and LH/FSH ratio as well as progressive rise in serum testosterone resulting in hyperandrogenemia during the treatment. 30 Although it remains a hypothetic question, whether these six women had been misdiagnosed with FHA instead of PCOS phenotype D, it must be assumed that some women with FHA and PCOM might also have underlying PCOS, since several PCOS‐specific characteristics were found in these women with FHA and PCOM in several studies. 2 , 4 , 29
In conclusion, in this unselected population of women referred to a center specialized in gynecologic endocrinology for suspicion of PCOS, a minimum rate misdiagnosed FHA patients of about 2% was found. Experts suspected FHA in 4.5% of all patients, whereby this rate was twice as high in women with amenorrhea (8.9%). Although this expert opinion might cannot be seen as an absolutely reliable parameter, it seemed necessary to include it due to the lack of clear criteria for the differentiation between FHA and PCOS. 6 The attention of the specialists in gynecologic endocrinology must be drawn to the possibility of FHA, especially when the pertinent markers are suggestive, and to also reconsider the treatment strategy for anovulation if clomiphene citrate and/or letrozole fail. Since no test can discriminate between the two conditions with high reliability, it is still necessary to evaluate reliable markers for the differential diagnosis between PCOS and FHA to avoid incorrect treatment, which might lead to negative long‐term effects in women with undiagnosed FHA. However, the problem with the initial definition of the two groups remains and must be solved in the future.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
JO received remuneration from Lenus Pharma for lecturing about the use of pulsatile GnRH treatment for FHA. All authors report no conflict of interest in relation to this work.
Holzer I, Marculescu R, Begemann V, Haaser S, Dewailly D, Ott J. Prevalence of functional hypothalamic amenorrhea in a cohort of women referred because of polycystic ovary syndrome. Reprod Med Biol. 2024;23:e12591. 10.1002/rmb2.12591
DATA AVAILABILITY STATEMENT
The data are available from the corresponding author on reasonable request.
REFERENCES
- 1. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413–1439. [DOI] [PubMed] [Google Scholar]
- 2. Mayrhofer D, Dewailly D, Hager M, Marculescu R, Beitl K, Ott J. Functional hypothalamic amenorrhea with or without polycystic ovarian morphology: a retrospective cohort study about insulin resistance. Fertil Steril. 2022;118(6):1183–1185. [DOI] [PubMed] [Google Scholar]
- 3. Abou Sherif S, Newman R, Haboosh S, Al‐Sharefi A, Papanikolaou N, Dimakopoulou A, et al. Investigating the potential of clinical and biochemical markers to differentiate between functional hypothalamic amenorrhoea and polycystic ovarian syndrome: a retrospective observational study. Clin Endocrinol (Oxf). 2021;95(4):618–627. [DOI] [PubMed] [Google Scholar]
- 4. Makolle S, Catteau‐Jonard S, Robin G, Dewailly D. Revisiting the serum level of anti‐Mullerian hormone in patients with functional hypothalamic anovulation. Hum Reprod. 2021;36(4):1043–1051. [DOI] [PubMed] [Google Scholar]
- 5. Beitl K, Dewailly D, Seemann R, Hager M, Bunker J, Mayrhofer D, et al. Polycystic ovary syndrome phenotype D versus functional hypothalamic amenorrhea with polycystic ovarian morphology: a retrospective study about a frequent differential diagnosis. Front Endocrinol (Lausanne). 2022;13:904706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phylactou M, Clarke SA, Patel B, Baggaley C, Jayasena CN, Kelsey TW, et al. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2021;95(2):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guney G, Taskin MI, Sener N, Tolu E, Dodurga Y, Elmas L, et al. The role of ERK‐1 and ERK‐2 gene polymorphisms in PCOS pathogenesis. Reprod Biol Endocrinol. 2022;20(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tokmak A, Guzel AI, Guney G, Tasdemir U, Umit C, Yilmaz N. Effect of obesity on clinical parameters and pregnancy rates in women with polycystic ovary syndrome undergoing ovulation induction cycles. J Reprod Med. 2017;62(5–6):300–304. [PubMed] [Google Scholar]
- 10. Bradbury RA, Lee P, Smith HC. Elevated anti‐Mullerian hormone in lean women may not indicate polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol. 2017;57(5):552–557. [DOI] [PubMed] [Google Scholar]
- 11. Lauritsen MP, Pinborg A, Loft A, Petersen JH, Mikkelsen AL, Bjerge MR, et al. Revised criteria for PCOS in WHO Group II anovulatory infertility ‐ a revival of hypothalamic amenorrhoea? Clin Endocrinol (Oxf). 2015;82(4):584–591. [DOI] [PubMed] [Google Scholar]
- 12. Wang JG, Lobo RA. The complex relationship between hypothalamic amenorrhea and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(4):1394–1397. [DOI] [PubMed] [Google Scholar]
- 13. Group ECW . Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update. 2012;18(5):586–599. [DOI] [PubMed] [Google Scholar]
- 14. Sophie Gibson ME, Fleming N, Zuijdwijk C, Dumont T. Where have the periods gone? The evaluation and management of functional hypothalamic amenorrhea. J Clin Res Pediatr Endocrinol. 2020;12(Suppl 1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Indirli R, Lanzi V, Mantovani G, Arosio M, Ferrante E. Bone health in functional hypothalamic amenorrhea: what the endocrinologist needs to know. Front Endocrinol (Lausanne). 2022;13:946695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucas AR, Melton LJ 3rd, Crowson CS, O'Fallon WM. Long‐term fracture risk among women with anorexia nervosa: a population‐based cohort study. Mayo Clin Proc. 1999;74(10):972–977. [DOI] [PubMed] [Google Scholar]
- 17. Rotterdam EA‐SPcwg . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 18. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, et al. Definition and significance of polycystic ovarian morphology: a task force report from the androgen excess and polycystic ovary syndrome society. Hum Reprod Update. 2014;20(3):334–352. [DOI] [PubMed] [Google Scholar]
- 20. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova‐Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. [DOI] [PubMed] [Google Scholar]
- 21. Hager M, Wenzl R, Riesenhuber S, Marschalek J, Kuessel L, Mayrhofer D, et al. The prevalence of incidental endometriosis in women undergoing laparoscopic ovarian drilling for clomiphene‐resistant polycystic ovary syndrome: a retrospective cohort study and meta‐analysis. J Clin Med. 2019;8(8):1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beitl K, Rosta K, Poetsch N, Seifried M, Mayrhofer D, Soliman B, et al. Autoimmunological serum parameters and bone mass density in premature ovarian insufficiency: a retrospective cohort study. Arch Gynecol Obstet. 2021;303(4):1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morrison AE, Fleming S, Levy MJ. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin Endocrinol (Oxf). 2021;95(2):229–238. [DOI] [PubMed] [Google Scholar]
- 24. Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363(4):365–371. [DOI] [PubMed] [Google Scholar]
- 25. Dumont A, Dewailly D, Plouvier P, Catteau‐Jonard S, Robin G. Does polycystic ovarian morphology influence the response to treatment with pulsatile GnRH in functional hypothalamic amenorrhea? Reprod Biol Endocrinol. 2016;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christou F, Pitteloud N, Gomez F. The induction of ovulation by pulsatile administration of GnRH: an appropriate method in hypothalamic amenorrhea. Gynecol Endocrinol. 2017;33(8):598–601. [DOI] [PubMed] [Google Scholar]
- 27. Dumont A, Dewailly D, Plouvier P, Catteau‐Jonard S, Robin G. Comparison between pulsatile GnRH therapy and gonadotropins for ovulation induction in women with both functional hypothalamic amenorrhea and polycystic ovarian morphology. Gynecol Endocrinol. 2016;32(12):999–1004. [DOI] [PubMed] [Google Scholar]
- 28. Southmayd EA, Hellmers AC, De Souza MJ. Food versus pharmacy: assessment of nutritional and pharmacological strategies to improve bone health in energy‐deficient exercising women. Curr Osteoporos Rep. 2017;15(5):459–472. [DOI] [PubMed] [Google Scholar]
- 29. Hager M, Ott J, Marschalek J, Marschalek ML, Kinsky C, Marculescu R, et al. Basal and dynamic relationships between serum anti‐Mullerian hormone and gonadotropins in patients with functional hypothalamic amenorrhea, with or without polycystic ovarian morphology. Reprod Biol Endocrinol. 2022;20(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mattle V, Bilgyicildirim A, Hadziomerovic D, Ott HW, Zervomanolakis I, Leyendecker G, et al. Polycystic ovarian disease unmasked by pulsatile GnRH therapy in a subgroup of women with hypothalamic amenorrhea. Fertil Steril. 2008;89(2):404–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.


