Key Points
Question
What are the incidence, outcome, and associated factors of pathological complete response (pCR) in patients with resected pancreatic adenocarcinoma after chemo(radio)therapy?
Findings
This cohort study of 1758 patients found a pCR rate of 4.8%, which was associated with longer overall survival compared with no pCR. Factors associated with pCR included preoperative (modified) FOLFIRINOX, preoperative radiotherapy (particularly stereotactic body radiation therapy), radiologic response, and normal(ized) serum carbohydrate antigen 19-9.
Meaning
Although pCR does not reflect cure, these findings suggest that it is associated with improved OS, and the identified factors associated with pCR may have implications for treatment strategies.
This cohort study analyzes the incidence, outcome, and factors associated with pathological complete response in patients with pancreatic adenocarcinoma who underwent preoperative chemo(radio)therapy followed by surgical resection.
Abstract
Importance
Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma, leading to pathological complete response (pCR) in a small subset of patients. However, multicenter studies with in-depth data about pCR are lacking.
Objective
To investigate the incidence, outcome, and risk factors of pCR after preoperative chemo(radio)therapy.
Design, Setting, and Participants
This observational, international, multicenter cohort study assessed all consecutive patients with pathology-proven localized pancreatic adenocarcinoma who underwent resection after 2 or more cycles of chemotherapy (with or without radiotherapy) in 19 centers from 8 countries (January 1, 2010, to December 31, 2018). Data collection was performed from February 1, 2020, to April 30, 2022, and analyses from January 1, 2022, to December 31, 2023. Median follow-up was 19 months.
Exposures
Preoperative chemotherapy (with or without radiotherapy) followed by resection.
Main Outcomes and Measures
The incidence of pCR (defined as absence of vital tumor cells in the sampled pancreas specimen after resection), its association with OS from surgery, and factors associated with pCR. Factors associated with overall survival (OS) and pCR were investigated with Cox proportional hazards and logistic regression models, respectively.
Results
Overall, 1758 patients (mean [SD] age, 64 [9] years; 879 [50.0%] male) were studied. The rate of pCR was 4.8% (n = 85), and pCR was associated with OS (hazard ratio, 0.46; 95% CI, 0.26-0.83). The 1-, 3-, and 5-year OS rates were 95%, 82%, and 63% in patients with pCR vs 80%, 46%, and 30% in patients without pCR, respectively (P < .001). Factors associated with pCR included preoperative multiagent chemotherapy other than (m)FOLFIRINOX ([modified] leucovorin calcium [folinic acid], fluorouracil, irinotecan hydrochloride, and oxaliplatin) (odds ratio [OR], 0.48; 95% CI, 0.26-0.87), preoperative conventional radiotherapy (OR, 2.03; 95% CI, 1.00-4.10), preoperative stereotactic body radiotherapy (OR, 8.91; 95% CI, 4.17-19.05), radiologic response (OR, 13.00; 95% CI, 7.02-24.08), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76; 95% CI, 1.79-7.89).
Conclusions and Relevance
This international, retrospective cohort study found that pCR occurred in 4.8% of patients with resected localized pancreatic adenocarcinoma after preoperative chemo(radio)therapy. Although pCR does not reflect cure, it is associated with improved OS, with a doubled 5-year OS of 63% compared with 30% in patients without pCR. Factors associated with pCR related to preoperative chemo(radio)therapy regimens and anatomical and biological disease response features may have implications for treatment strategies that require validation in prospective studies because they may not universally apply to all patients with pancreatic adenocarcinoma.
Introduction
The treatment of localized pancreatic adenocarcinoma has evolved during the past decade with the increasing use of preoperative chemo(radio)therapy, particularly multiagent chemotherapeutic regimens.1 Preoperative therapy provides the chance for improved disease control while selecting patients with more favorable tumor biology for surgical resection,2 leading to resection rates of 77%, 61%, and 22% among patients with primary resectable, borderline resectable, and locally advanced pancreatic cancer, respectively.3
Nevertheless, the interpretation of anatomical, biological, and conditional parameters for personalized restaging remains challenging,4,5,6 illustrated by high early recurrence rates.7,8 Histopathological residual tumor burden after resection following preoperative therapy is 1 of the biological parameters for disease response.9,10,11 The presence and extent of vital tumor burden are considered a surrogate marker for the tumor’s response on preoperative therapy, which could be used for prognostication and may guide the decision-making for adjuvant therapy.12 Pathological complete response (pCR) is the ultimate tumor response, with an estimated incidence of 4%.13
Radiation therapy and longer duration of preoperative chemotherapy have been suggested to be associated with pCR.14 Pathological complete response is associated with improved overall survival (OS), with a median up to 100 months,14,15,16,17 although up to half of patients with pCR develop disease recurrence.16,17,18 However, evidence regarding pCR is based on large national databases with limited granularity or small single-center series.13,14,15,16,19,20,21,22,23,24 Better insight into the outcomes and factors associated with pCR may contribute to the improvement of preoperative therapy in patients with pancreatic adenocarcinoma, improving selection for surgery and prognostication. Therefore, the current international, observational, multicenter cohort study aimed to perform an in-depth analysis on the incidence, outcome, and factors associated with pCR within a large cohort of consecutive patients with pancreatic adenocarcinoma who underwent preoperative chemo(radio)therapy followed by surgical resection.
Methods
This retrospective, observational, multicenter cohort study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.25 The study procedures were reviewed and approved by the Colorado Multiple Institutional Review Board at the University of Colorado. The need for informed consent was waived by the institutional review board because of the retrospective nature of this study.
Study Design and Patients
All consecutive adult patients (aged ≥18 years) were retrospectively included from institutional databases in 19 centers from 8 countries who underwent any type of pancreatic resection after preoperative chemo(radio)therapy (January 1, 2010, to December 31, 2018) for localized pancreatic adenocarcinoma. Exclusion criteria included fewer than 2 cycles of preoperative chemotherapy, unknown type of preoperative chemotherapy, and pCR cases without preoperative cytologic or histologic test results classified as “suspicious for malignancy” or “positive/malignant.”26 The arbitrary cutoff of fewer than 2 cycles of preoperative chemotherapy was selected to approach the daily clinical practice by reducing the selection. Because of the differently used tumor regression grading classification,27 the interobserver variability,28,29 and differences in sampling strategies12,30 among centers and pathologists around the world, the current study focused on comparing patients with pCR vs without pCR. Data on patient race and ethnicity were not collected because it was not considered during the design of this study.
Definitions
The American Society of Anesthesiologists Physical Status was used to indicate patients’ conditional status. Pancreatic adenocarcinoma was staged using the TNM Classification of Malignant Tumours (7th edition) and the National Comprehensive Cancer Network guideline, version 1.2019.31,32 If the tumor involved multiple anatomical locations in the pancreas (ie, head, body, and/or tail), the most proximal location was registered.
When a patient underwent a chemotherapy switch preoperatively, the dominant chemotherapeutic regimen was used, whereby other chemotherapy lines were registered as second-line chemotherapy. The following strategy was used to determine the dominant regimen. First, if both regimens were single-agent or multiagent chemotherapy but the number of administered cycles from both regimens was not available, the last regimen before surgery was considered as the dominant regimen. If the number of cycles from all regimens was available, the chemotherapeutic regimen with the most administered cycles was used as the dominant regimen. Second, if multiple lines were given, including a multiagent and single-agent chemotherapy, the multiagent chemotherapy was defined as the dominant regimen, regardless of the order or number of cycles. Third, systemic chemotherapy was considered superior to intraperitoneal-administered chemotherapy. Additional experimental drugs were not taken into account. The interval between the start of preoperative chemotherapy and surgery was used as a surrogate marker for the preoperative treatment duration. The duration was stratified into less than 4, 4 to less than 6, 6 or more to less than 12, and 12 or more months.
Radiological response evaluation was defined in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.33 A carbohydrate antigen 19-9 (CA 19-9) level of 37 U/mL or greater and a carcinoembryonic antigen (CEA) level greater than 5 ng/mL (to convert to micrograms per liter, multiply by 1) were considered elevated.
Type and extent of pancreatic surgery were defined using the International Study Group for Pancreatic Surgery definition.34 Major morbidity was defined as a Clavien-Dindo grade of IIIa or higher within 90 days after surgery.35 Radicality (R0 vs R1) was classified following the Royal College of Pathologist definition.36 Pathological complete response was defined as the absence of any vital tumor tissue in the sampled pancreatic resection specimen.
Recurrence-free survival (RFS) and OS were measured from the time of surgery. Additionally, the OS measured from the start of preoperative chemotherapy was provided. From the date of surgery, follow-up was measured until death or the most recent date alive. Data collection ranged from February 1, 2020, to April 30, 2022, and analyses were performed between January 1, 2022, and December 31, 2023.
Statistical Analysis
Data analyses were performed using R software, version 4.2.2 (R Foundation for Statistical Computing).37 Statistical significance was determined using a 2-sided P < .05. Patients with and without pCR were compared using descriptive statistics. Bivariable statistics were estimated using χ2 or Fisher exact (when observed cell counts were <5) tests for categorical data. Normally distributed continuous variables were compared using the Welch independent 2-sample t test, and the Wilcoxon rank sum test was used for nonnormally distributed data.
The amount of missing data per variable ranged from 0% to more than 50%. Data appeared to be missing not at random for larger counts of missing data. Therefore, a missing data category was introduced for each categorical variable that was missing 2% or more; otherwise, patients with missing data for variables missing less than 2% were simply quantified and displayed as counts in bivariable comparisons. If a categorical variable had less than 2% missing data, the missing patients were not included in the overall proportions reported, and they were not included in the test of association because of a lack of power. If a categorical variable had missing data of 2% or more, these patients were classified as missing and included in the overall proportions and the test of association. For all continuous variables, the missing data were not included in the test of association.
Serum CA 19-9 was treated as a categorical variable because there were substantial missing data for this variable (so we needed to include a missing data category) and because the association between this variable and the outcomes was nonlinear. Furthermore, the groupings chosen for this variable were considered clinically meaningful. For the relative change in serum CA 19-9 between diagnosis and restaging, the area under the curve method was used to select the optimal threshold among patients with elevated serum CA 19-9 at diagnosis who showed any degree of reduction.
The median RFS and OS times with 95% CIs were calculated using the Kaplan-Meier method, and subgroups were compared using the log-rank test using the survival package in R.38,39 Univariable (unadjusted) and multivariable (adjusted) Cox proportional hazards regression models were used to assess the association between clinical parameters and OS measured from surgery using the survival package in R.38,39 The OS from surgery was used so that the findings can be used for prognostication immediately after surgery, because pCR is only known at that time. The results are presented in hazard ratios (HRs) with 95% CIs.
Univariable and multivariable logistic regression models were used to assess factors associated with pCR using the stats package in R.37 The results are presented as odds ratios (OR) with 95% CIs. For both the Cox proportional hazards and logistic regression models, the following strategy was used. The univariable models included all variables that were considered clinically relevant based on the literature and clinical experience. Independent variables with P < .25 were included in the multivariable models.
Due to collinearity among the 3 serum CA 19-9 variables, only the serum CA 19-9 parameter with the strongest bivariable statistical association was tested in the multivariable analysis, based on P value. Again, missing data were included as a category if they were missing for at least 2% of patients. Otherwise, missing data were excluded for variables missing less than 2% of the time. In the bivariable analyses, if the only significant comparison within a categorical variable was for the missing group, the variable was not included in the multivariable analysis.
Results
Overall, 1758 patients (mean [SD] age, 64 [9] years; 879 [50.0%] male and 879 [50.0%] female) underwent resection of pancreatic adenocarcinoma after chemo(radio)therapy and were included from 4 centers in the US (798 patients [45.4%]), 6 centers in Japan (366 patients [20.8%]), and 9 centers in Europe (594 patients [33.8%]). The number of patients per center ranged from 5 to 397. Of the 1758 included patients, 85 (4.8%) were diagnosed with pCR, with a median incidence of 3.8% (IQR, 0.3%-7.8%) per center. See eAppendix 1 in Supplement 1 for the incidences of pCR per center.
Clinicopathological Details
At time of diagnosis, pancreatic adenocarcinoma was staged as primary resectable (n = 429 [24.5%]), borderline resectable (n = 856 [48.9%]), or locally advanced (n = 465 [26.6%]). The primary tumor was mostly located in the pancreatic head (n = 1276 [72.6%]). The median (IQR) serum CA 19-9 level before preoperative therapy was 183 (46-626) U/mL, without a difference between patients with or without pCR. See Table 1 for baseline characteristics at time of diagnosis.
Table 1. Baseline Characteristics at Diagnosisa.
| Characteristic | Overall cohort (N = 1758) | pCR (n = 85) | No pCR (n = 1673) | P valueb |
|---|---|---|---|---|
| Age, mean (SD), y | 64 (9) | 62 (9) | 64 (9) | .01c |
| Sex | ||||
| Female | 879 (50.0) | 41 (48.2) | 838 (50.1) | .74d |
| Male | 879 (50.0) | 44 (51.8) | 835 (49.9) | |
| ASA-PS | ||||
| I-II | 1295 (74.0) | 45 (52.9) | 1250 (75.0) | <.001d |
| III-IV | 456 (26.0) | 40 (47.1) | 416 (25.0) | |
| Missing | 7 | 0 | 7 | |
| Resectability | ||||
| Primary resectable | 429 (24.5) | 6 (7.1) | 423 (25.4) | <.001d |
| Borderline resectable | 856 (48.9) | 43 (50.6) | 813 (48.8) | |
| Locally advanced | 465 (26.6) | 36 (42.4) | 429 (25.8) | |
| Missing | 8 | 0 | 8 | |
| Tumor location | ||||
| Pancreatic head | 1276 (72.6) | 71 (83.5) | 1205 (72.1) | .02d |
| Pancreatic body or tail | 481 (27.4) | 14 (16.5) | 467 (27.9) | |
| Missing | 1 | 0 | 1 | |
| Tumor size, mm | ||||
| ≤20 | 385 (22.2) | 9 (11.3) | 376 (22.7) | .005d |
| 21-40 | 1095 (63.1) | 51 (63.8) | 1044 (63.1) | |
| >40 | 255 (14.7) | 20 (25.0) | 235 (14.2) | |
| Missing | 23 | 5 | 18 | |
| cT stage | .10d | |||
| T1/T2 | 425 (24.2) | 27 (31.8) | 398 (23.8) | |
| T3/T4 | 1332 (75.8) | 58 (68.2) | 1274 (76.2) | |
| Missing | 1 | 0 | 1 | |
| CA 19-9, U/mL | ||||
| Median (IQR) | 183 (46-626) | 105 (31-475) | 185 (48-633) | .11e |
| <37 | 317 (18.0) | 16 (18.8) | 301 (18.0) | <.001d |
| ≥37 to <150 | 342 (19.5) | 17 (20.0) | 325 (19.4) | |
| ≥150 to <500 | 360 (20.5) | 8 (9.4) | 352 (21.0) | |
| ≥500 to <1000 | 156 (8.9) | 6 (7.1) | 150 (9.0) | |
| ≥1000 | 259 (14.7) | 8 (9.4) | 251 (15.0) | |
| Missing | 324 (18.4) | 30 (35.3) | 294 (17.6) | |
| CEA | ||||
| Median (IQR), ng/mL | 3.1 (2.1-5.3) | 2.6 (2.1-4.4) | 3.2 (2.1-5.4) | .41e |
| Normal | 633 (36.0) | 21 (24.7) | 612 (36.6) | <.001f |
| >5 to ≤20 | 204 (11.6) | 2 (2.4) | 202 (12.1) | |
| >20 | 34 (1.9) | 2 (2.4) | 32 (1.9) | |
| Missing | 887 (50.5) | 60 (70.6) | 827 (49.4) |
Abbreviations: ASA-PS, American Society of Anesthesiologists Performance Status; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; pCR, pathological complete response.
SI conversion factor: To convert CEA to micrograms per liter, multiply by 1.
Data are presented as number (percentage) of patients unless otherwise indicated. Categorical data with missing data for 2% or more of patients were included in a separate category and were therefore included in the overall proportions and in the test of association. Otherwise, for missing data less than 2%, the data are shown but were not included in the hypothesis tests. See eAppendix 4 in Supplement 1 for the presentation of these data using row percentages.
Comparison between patients with or without pCR.
Welch independent 2-sample t test.
χ2 test.
Wilcoxon rank sum test.
Fisher exact test.
Preoperative Therapy and Disease Response
Most patients were treated with preoperative (modified) leucovorin calcium (folinic acid), fluorouracil, irinotecan hydrochloride, and oxaliplatin ([m]FOLFIRINOX) (n = 797 [45.3%]) or gemcitabine and nab-paclitaxel (n = 501 [28.5%]). Patients with pCR were more frequently treated with preoperative (m)FOLFIRINOX compared with patients without pCR (n = 50 of 85 [58.8%] vs 747 of 1673 [44.7%]; P < .001). Concomitant radiotherapy was administered in 872 patients (50.0%). The rate of radiation therapy was higher among patients with pCR compared with patients without pCR (n = 68 of 85 [80.0%] vs 804 of 1658 [48.5%]; P < .001). See Table 2 for details regarding preoperative therapy and response evaluation.
Table 2. Preoperative Therapy and Disease Responsea.
| Variable | Patients, No. (%) | P valueb | ||
|---|---|---|---|---|
| Overall cohort (N = 1758) | pCR (n = 85) | No pCR (n = 1673) | ||
| Preoperative therapy | ||||
| Chemotherapy | ||||
| (m)FOLFIRINOX | 797 (45.3) | 50 (58.8) | 747 (44.7) | <.001c |
| Gemcitabine and nab-paclitaxel | 501 (28.5) | 11 (12.9) | 490 (29.3) | |
| Gemcitabine–S-1 | 100 (5.7) | 1 (1.2) | 99 (5.9) | |
| Gemcitabine-oxaliplatin | 101 (5.7) | 2 (2.4) | 99 (5.9) | |
| Other multiagent regimens | 90 (5.1) | 9 (10.6) | 81 (4.8) | |
| Gemcitabine | 97 (5.5) | 7 (8.2) | 90 (5.4) | |
| S-1 | 53 (3.0) | 2 (2.4) | 51 (3.0) | |
| Other single-agent regimens | 19 (1.1) | 3 (3.5) | 16 (1.0) | |
| Second-line chemotherapy | 216 (12.3) | 13 (15.3) | 203 (12.1) | .55c |
| Missing | 74 (4.2) | 2 (2.4) | 72 (4.3) | |
| Chemotherapy dose reduction | 250 (14.2) | 6 (7.1) | 244 (14.6) | <.001d |
| Missing | 660 (37.5) | 57 (67.1) | 603 (36.0) | |
| Radiotherapy | ||||
| No | 871 (50.0) | 17 (20.0) | 854 (51.5) | <.001d |
| Yes, conventional radiotherapy | 462 (26.5) | 37 (43.5) | 425 (25.6) | |
| Yes, SBRT | 410 (23.5) | 31 (36.5) | 379 (22.9) | |
| Missing | 15 | 0 | 15 | |
| Preoperative therapy duration, mo | ||||
| <4 | 475 (27.0) | 16 (18.8) | 459 (27.4) | <.001c |
| ≥4 to <6 | 469 (26.7) | 16 (18.8) | 453 (27.1) | |
| ≥6 to <12 | 637 (36.2) | 34 (40.0) | 603 (36.0) | |
| ≥12 | 118 (6.7) | 16 (18.8) | 102 (6.1) | |
| Missing | 59 (3.4) | 3 (3.5) | 56 (3.3) | |
| Response evaluation | ||||
| RECIST | ||||
| Complete response | 14 (0.8) | 4 (4.7) | 10 (0.6) | <.001c |
| Partial response | 506 (29.1) | 61 (71.8) | 445 (26.9) | |
| Stable disease | 1194 (68.7) | 19 (22.4) | 1175 (71.0) | |
| Progressive disease | 25 (1.4) | 1 (1.2) | 24 (1.5) | |
| Missing | 19 | 0 | 19 | |
| CA 19-9 | ||||
| Median (IQR), U/mL | 36 (15-92) | 19 (13-35) | 37 (15-95) | .003e |
| Normal | 773 (44.0) | 43 (50.6) | 730 (43.6) | <.001c |
| ≥37 to <150 U/mL | 493 (28.0) | 7 (8.2) | 486 (29.0) | |
| ≥150 to <500 U/mL | 170 (9.7) | 3 (3.5) | 167 (10.0) | |
| ≥500 to <1000 U/mL | 37 (2.1) | 1 (1.2) | 36 (2.2) | |
| ≥1000 U/mL | 40 (2.3) | 0 | 40 (2.4) | |
| Missing | 245 (13.9) | 31 (36.5) | 214 (12.8) | |
| CA 19-9 patterns | ||||
| Normal to normal | 281 (16.0) | 12 (14.1) | 269 (16.1) | <.001c |
| Normal to elevated | 17 (1.0) | 1 (1.2) | 16 (1.0) | |
| Elevated to normal | 415 (23.6) | 23 (27.1) | 392 (23.4) | |
| Elevated to elevated | 655 (37.3) | 10 (11.8) | 645 (38.6) | |
| Missing | 390 (22.2) | 39 (45.9) | 351 (21.0) | |
| Relative CA 19-9 change | ||||
| No change or increased | 93 (5.3) | 2 (2.4) | 91 (5.4) | <.001c |
| Decreased <87% | 538 (30.6) | 9 (10.6) | 529 (31.6) | |
| Decreased ≥87% | 439 (25.0) | 22 (25.9) | 417 (24.9) | |
| <37 U/mL at time of diagnosis | 317 (18.0) | 16 (18.8) | 301 (18.0) | |
| Missing | 371 (21.1) | 36 (42.4) | 335 (20.0) | |
| CEA | ||||
| Median (IQR), ng/mL | 2.9 (2.0-4.6) | 2.9 (1.8-4.2) | 2.9 (2.0-4.6) | .85e |
| Normal | 744 (42.3) | 21 (24.7) | 723 (43.2) | <.001c |
| >5 to ≤20 ng/mL | 189 (10.8) | 3 (3.5) | 186 (11.1) | |
| >20 ng/mL | 20 (1.1) | 0 | 20 (1.2) | |
| Missing | 805 (45.8) | 61 (71.8) | 744 (44.5) | |
| CEA patterns | ||||
| Normal to normal | 520 (29.6) | 13 (15.3) | 507 (30.3) | <.001c |
| Normal to elevated | 53 (3.0) | 1 (1.2) | 52 (3.1) | |
| Elevated to normal | 100 (5.7) | 2 (2.4) | 98 (5.9) | |
| Elevated to elevated | 110 (6.3) | 0 | 110 (6.6) | |
| Missing | 975 (55.5) | 69 (81.2) | 906 (54.2) | |
Abbreviations: CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; (m)FOLFIRINOX, (modified) leucovorin calcium (folinic acid), fluorouracil, irinotecan hydrochloride, and oxaliplatin; pCR, pathological complete response; RECIST, Response Evaluation Criteria in Solid Tumors; SBRT, stereotactic body radiotherapy.
SI conversion factor: To convert CEA to micrograms per liter, multiply by 1.
Data are presented as number (percentage) of patients unless otherwise indicated. Categorical data with missing data for 2% or more of patients were included in a separate category and were therefore included in the overall proportions and in the test of association. Otherwise, for missing less than 2%, the data are shown but were not included in the hypothesis tests. See eAppendix 4 in Supplement 1 for the presentation of these data using row percentages.
Comparison between patients with or without pCR.
Fisher exact test.
χ2 test.
Wilcoxon rank sum test.
Surgery Outcome
Pancreatoduodenectomy was the most commonly performed surgical procedure (n = 1262 [71.8%]). An extended resection was performed in 696 patients (39.6%). The rates of vascular resection among patients with a primary resectable, borderline resectable, and locally advanced tumor were 16.1% (n = 69 of 429), 39.6% (n = 339 of 855 [missing n = 1]), and 44.3% (n = 206 of 465), respectively. The 90-day mortality was 3.2% (n = 55). See eAppendix 2 in Supplement 1 for further details on surgical procedures and outcome and eAppendix 3 in Supplement 1 for histopathological outcomes. Patients with pCR received adjuvant chemotherapy (n = 30 of 85 [35.3%]) less frequently than patients without pCR (n = 1120 of 1673 [66.9%]) (P < .001).
Oncologic Outcome
Follow-up data were available from 1757 of the 1758 patients, of whom 817 patients (46.5%) died. The median (IQR) follow-up time was 19 (11-33) months. The median OS from the total cohort was 33 months (95% CI, 31-37 months) (Figure, A). The median OS outcomes of patients with a primary resectable, borderline resectable, or locally advanced tumor were 43 (95% CI, 36-49), 31 (95% CI, 27-35), and 31 (95% CI, 26-36) months (P = .19), respectively. The median OS was shorter in patients who underwent a vascular resection compared with no vascular resection: 28 (95% CI, 26-31) vs 38 (95% CI, 33-43) months (P < .001), respectively.
Figure. Kaplan-Meier Survival Curves.
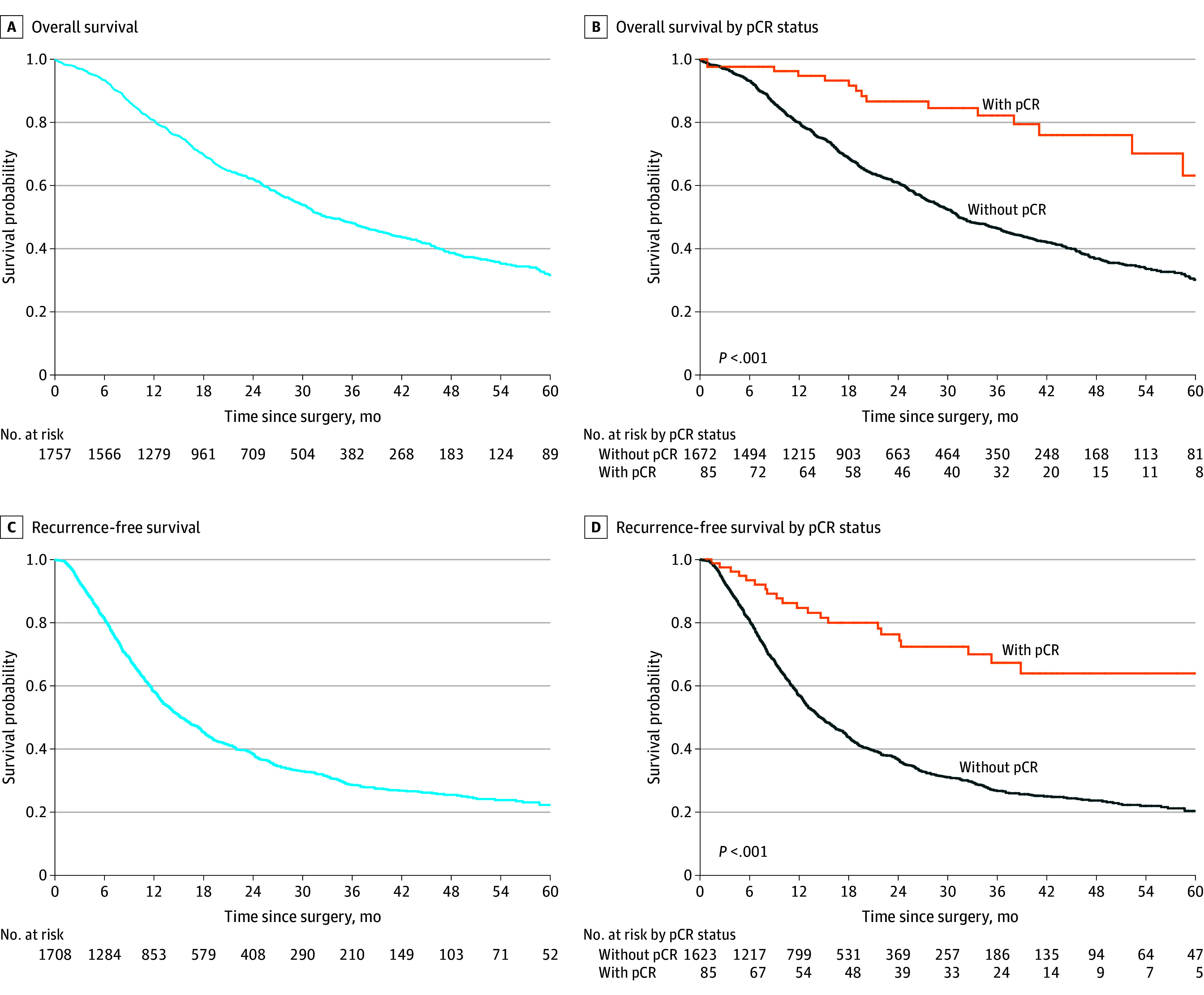
pCR indicates pathological complete response.
When comparing patients with and without pCR, the median OS was not reached in the pCR group, whereas the median OS was 31 (95% CI, 30-35) months in patients without pCR (P < .001). In patients with pCR, the 1-, 3-, and 5-year OS rates were 95% (95% CI, 90%-100%), 82% (95% CI, 73%-93%), and 63% (95% CI, 47%-86%), respectively. In patients without pCR, the 1-, 3-, and 5-year OS rates were 80% (95% CI, 78%-82%), 46% (95% CI, 44%-49%), and 30% (95% CI, 27%-34%), respectively (Figure, B). See eAppendix 5 in Supplement 1 for OS outcomes measured from the start of preoperative chemotherapy.
For the analysis on RFS, 50 patients had missing data on recurrence status (n = 18) and/or date of disease recurrence (n = 33). Therefore, these patients were excluded from this analysis. Among the 1708 patients with data about disease recurrence, 1036 (60.7%) developed disease recurrence. In the overall study cohort, the median RFS was 16 months (95% CI, 14-17 months) (Figure, C). The median RFS was not reached in the patients with pCR, whereas the median RFS was 15 months (95% CI, 14-16 months) in patients without pCR (P < .001) (Figure, D). See eAppendix 6 in Supplement 1 for the comparison of recurrence location between patients with or without pCR. After adjustment for potential confounders, pCR was associated with prolonged OS (HR, 0.46; 95% CI, 0.26-0.83). See Table 3 for the Cox proportional hazards regression analysis.
Table 3. Cox Proportional Hazards Regression Model for Estimating Associations Between Risk Factors and Mortality.
| Variable | No. of patients (N = 1677) | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | |||||
| Female | 842 | 1 [Reference] | NA | 1 [Reference] | NA |
| Male | 835 | 1.11 (0.96-1.28) | .15 | 1.09 (0.94-1.25) | .27 |
| ASA-PS | |||||
| I-II | 1242 | 1 [Reference] | NA | NA | NA |
| III-IV | 435 | 1.06 (0.90-1.25) | .49 | NA | NA |
| Tumor location | |||||
| Body or tail | 458 | 1 [Reference] | NA | NA | NA |
| Head | 1219 | 0.94 (0.81-1.10) | .47 | NA | NA |
| Tumor size at diagnosis, mm | |||||
| ≤20 | 374 | 1 [Reference] | NA | 1 [Reference] | NA |
| 21-40 | 1059 | 1.31 (1.09-1.58) | .004 | 1.08 (0.88-1.33) | .46 |
| >40 | 244 | 1.41 (1.11-1.80) | .006 | 0.79 (0.59-1.06) | .12 |
| Resectability at diagnosis | |||||
| Primary resectable | 417 | 1 [Reference] | NA | 1 [Reference] | NA |
| Borderline resectable | 819 | 1.14 (0.96-1.37) | .14 | 1.05 (0.86-1.28) | .62 |
| Locally advanced | 441 | 1.22 (1.00-1.49) | .05 | 1.16 (0.92-1.45) | .20 |
| CA 19-9 at diagnosis, U/mL | |||||
| <37 | 305 | 1 [Reference] | NA | NA | NA |
| ≥37 to <150 | 330 | 0.81 (0.64-1.02) | .07 | NA | NA |
| ≥150 to <500 | 340 | 1.09 (0.88-1.36) | .43 | NA | NA |
| ≥500 to <1000 | 146 | 0.95 (0.71-1.28) | .76 | NA | NA |
| ≥1000 | 249 | 1.21 (0.95-1.52) | .12 | NA | NA |
| Missing | 307 | 0.77 (0.60-0.98) | .03 | NA | NA |
| Preoperative chemotherapy | |||||
| (m)FOLFIRINOX | 764 | 1 [Reference] | NA | NA | NA |
| Other multiagent | 754 | 1.06 (0.92-1.24) | .42 | NA | NA |
| Single agent | 159 | 1.03 (0.81-1.31) | .80 | NA | NA |
| Preoperative radiotherapy | |||||
| None | 835 | 1 [Reference] | NA | 1 [Reference] | NA |
| Conventional radiotherapy | 446 | 0.79 (0.66-0.94) | .008 | 0.97 (0.79-1.19) | .75 |
| SBRT | 396 | 1.09 (0.91-1.29) | .34 | 1.27 (1.04-1.56) | .02 |
| Preoperative therapy duration, mo | |||||
| <4 | 452 | 1 [Reference] | NA | NA | NA |
| ≥4 to <6 | 452 | 0.88 (0.73-1.07) | .20 | NA | NA |
| ≥6 to <12 | 609 | 1.03 (0.86-1.22) | .76 | NA | NA |
| ≥12 | 111 | 0.91 (0.67-1.25) | .56 | NA | NA |
| Missing | 53 | 0.90 (0.55-1.45) | .65 | NA | NA |
| RECIST | |||||
| Stable | 1147 | 1 [Reference] | NA | 1 [Reference] | NA |
| Progressive disease | 24 | 1.81 (1.12-2.93) | .02 | 1.55 (0.93-2.56) | .09 |
| Partial or complete response | 506 | 0.72 (0.61-0.85) | <.001 | 0.80 (0.67-0.96) | .02 |
| CA 19-9 at restaging, U/mL | |||||
| ≥37 | 696 | 1 [Reference] | NA | 1 [Reference] | NA |
| <37 (normal[ization]) | 743 | 0.76 (0.65-0.88) | <.001 | 0.89 (0.76-1.04) | .15 |
| Missing | 238 | 0.82 (0.66-1.02) | .07 | 1.03 (0.80-1.33) | .80 |
| Relative CA 19-9 change | |||||
| No change/increased | 83 | 1 [Reference] | NA | NA | NA |
| Decreased <87% | 513 | 0.84 (0.61-1.16) | .29 | NA | NA |
| Decreased ≥87% | 424 | 0.70 (0.51-0.98) | .04 | NA | NA |
| <37 U/mL at diagnosis | 305 | 0.79 (0.57-1.11) | .18 | NA | NA |
| Missing | 352 | 0.64 (0.46-0.90) | .01 | NA | NA |
| pCR | |||||
| No | 1597 | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 80 | 0.31 (0.19-0.52) | <.001 | 0.46 (0.26-0.83) | .009 |
| Residual disease | |||||
| R0 | 1225 | 1 [Reference] | NA | 1 [Reference] | NA |
| R1-2 | 288 | 2.04 (1.72-2.41) | <.001 | 1.67 (1.38-2.01) | <.001 |
| Unknown | 164 | 1.28 (1.00-1.64) | .05 | 1.54 (1.10-2.15) | .01 |
| Tumor size in histopathology, mm | |||||
| ≤20 | 620 | 1 [Reference] | NA | 1 [Reference] | NA |
| 21-40 | 793 | 1.67 (1.42-1.97) | <.001 | 1.24 (1.03-1.49) | .03 |
| >40 | 216 | 2.24 (1.79-2.79) | <.001 | 1.61 (1.24-2.09) | <.001 |
| Missing | 48 | 1.90 (1.28-2.81) | .001 | 1.20 (0.79-1.83) | .40 |
| Lymphovascular invasion | |||||
| No | 867 | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 726 | 1.57 (1.36-1.82) | <.001 | 1.13 (0.95-1.33) | .16 |
| Missing | 84 | 1.33 (0.97-1.84) | .08 | 1.20 (0.77-1.86) | .42 |
| Perineural invasion | |||||
| No | 493 | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 1130 | 1.79 (1.51-2.13) | <.001 | 1.22 (1.01-1.48) | .04 |
| Missing | 54 | 1.31 (0.85-2.01) | .22 | 0.93 (0.52-1.68) | .81 |
| Tumor differentiation | |||||
| Gx | 178 | 1 [Reference] | NA | 1 [Reference] | NA |
| G1-G2 | 1030 | 1.16 (0.90-1.49) | .25 | 1.09 (0.83-1.44) | .54 |
| G3-G4 | 313 | 1.87 (1.42-2.47) | <.001 | 1.60 (1.19-2.16) | .002 |
| Missing | 156 | 1.32 (0.94-1.84) | .11 | 1.35 (0.94-1.94) | .10 |
| Lymph node status | |||||
| ypN0 | 895 | 1 [Reference] | NA | 1 [Reference] | NA |
| ypN1-2 | 782 | 1.90 (1.64-2.19) | <.001 | 1.54 (1.30-1.81) | <.001 |
| Metastatic disease | |||||
| M0 | 1191 | 1 [Reference] | NA | 1 [Reference] | NA |
| M1 | 40 | 1.59 (1.04-2.43) | .03 | 1.26 (0.82-1.94) | .30 |
| Mx | 252 | 0.81 (0.65-1.01) | .06 | 1.07 (0.81-1.41) | .63 |
| Missing | 194 | 0.77 (0.61-0.99) | .04 | 0.68 (0.51-0.91) | .008 |
| Major morbidity | |||||
| No | 1160 | 1 [Reference] | NA | 1 [Reference] | |
| Yes | 250 | 1.43 (1.19-1.73) | <.001 | 1.28 (1.05-1.56) | .01 |
| Missing | 267 | 0.87 (0.71-1.07) | .19 | 0.65 (0.48-0.88) | .005 |
| Adjuvant chemotherapy | |||||
| No | 430 | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 1098 | 0.60 (0.51-0.70) | <.001 | 0.50 (0.42-0.59) | <.001 |
| Missing | 149 | 0.79 (0.59-1.06) | .12 | 0.69 (0.50-0.95) | .02 |
Abbreviations: ASA-PS, American Society of Anesthesiologists Performance Status; CA 19-9, carbohydrate antigen 19-9; HR, hazard ratio; (m)FOLFIRINOX, (modified) leucovorin calcium (folinic acid), fluorouracil, irinotecan hydrochloride, and oxaliplatin; NA, not applicable; pCR, pathological complete response; RECIST, Response Evaluation Criteria in Solid Tumors; SBRT, stereotactic body radiotherapy.
Factors Associated With pCR
Tumors located in the pancreatic head (OR, 2.51; 95% CI, 1.25-5.06), tumor size greater than 40 mm (vs ≤20 mm) on cross-sectional imaging at diagnosis (OR, 2.58; 95% CI, 1.03-6.48), conventional radiotherapy (vs no radiotherapy) (OR, 2.03; 95% CI, 1.00-4.10), stereotactic body radiation therapy (SBRT) (vs no radiotherapy) (OR, 8.91; 95% CI, 4.17-19.05), partial or complete radiologic response (vs stable disease) (OR, 13.00; 95% CI, 7.02-24.08), and serum CA 19-9 normal(ization) (vs CA 19-9 ≥37 U/mL) (OR, 3.76; 95% CI, 1.79-7.89) were associated with pCR. In contrast, preoperative multiagent chemotherapy other than (m)FOLFIRINOX (vs [m]FOLFIRINOX) (OR, 0.48; 95% CI, 0.26-0.87) was associated with not achieving pCR. See Table 4 for the logistic regression analysis.
Table 4. Logistic Regression Model for Estimating Associations Between Risk Factors and Pathological Complete Response.
| Variable | No. of patients (N = 1695) | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Gender | |||||
| Female | 850 | 1 [Reference] | NA | NA | NA |
| Male | 845 | 1.12 (0.71-1.75) | .63 | NA | NA |
| Tumor location | |||||
| Body or tail | 462 | 1 [Reference] | NA | 1 [Reference] | NA |
| Head | 1233 | 1.98 (1.08-3.63) | .03 | 2.51 (1.25-5.06) | .01 |
| Tumor size at diagnosis, mm | |||||
| ≤20 | 376 | 1 [Reference] | NA | 1 [Reference] | NA |
| 21-40 | 1073 | 2.03 (0.99-4.17) | .05 | 1.52 (0.69-3.33) | .29 |
| >40 | 246 | 3.61 (1.62-8.06) | .002 | 2.58 (1.03-6.48) | .04 |
| Resectability at diagnosis | |||||
| Primary resectable | 424 | 1 [Reference] | NA | 1 [Reference] | NA |
| Borderline resectable | 827 | 3.54 (1.49-8.42) | .004 | 1.63 (0.60-4.40) | .34 |
| Locally advanced | 444 | 5.78 (2.40-13.91) | <.001 | 2.31 (0.84-6.36) | .10 |
| CA 19-9 at diagnosis, U/mL | |||||
| <37 | 307 | 1 [Reference] | NA | NA | NA |
| ≥37 to <150 | 331 | 0.99 (0.48-2.04) | .98 | NA | NA |
| ≥150 to <500 | 348 | 0.40 (0.16-0.99) | .05 | NA | NA |
| ≥500 to <1000 | 148 | 0.82 (0.31-2.16) | .69 | NA | NA |
| ≥1000 | 252 | 0.64 (0.27-1.53) | .31 | NA | NA |
| Missing | 309 | 1.94 (1.01-3.71) | .05 | NA | NA |
| Preoperative chemotherapy | |||||
| (m)FOLFIRINOX | 771 | 1 [Reference] | NA | 1 [Reference] | NA |
| Other multiagent | 765 | 0.38 (0.22-0.64) | <.001 | 0.48 (0.26-0.87) | .02 |
| Single agent | 159 | 1.20 (0.62-2.32) | .58 | 2.42 (0.99-5.92) | .05 |
| Preoperative radiotherapy | |||||
| None | 845 | 1 [Reference] | NA | 1 [Reference] | NA |
| Conventional radiotherapy | 448 | 3.75 (2.06-6.83) | <.001 | 2.03 (1.00-4.10) | .05 |
| SBRT | 402 | 4.07 (2.22-7.45) | <.001 | 8.91 (4.17-19.05) | <.001 |
| Preoperative therapy duration, mo | |||||
| <4 | 456 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥4 to <6 | 455 | 1.15 (0.55-2.39) | .71 | 0.59 (0.24-1.46) | .25 |
| ≥6 to <12 | 616 | 1.67 (0.88-3.18) | .12 | 0.63 (0.27-2.45) | .27 |
| ≥12 | 113 | 5.21 (2.46-11.03) | <.001 | 2.13 (0.83-5.43) | .12 |
| Missing | 55 | 1.82 (0.51-6.55) | .36 | 0.99 (0.23-4.23) | .98 |
| RECIST | |||||
| Stable disease | 1165 | 1 [Reference] | NA | 1 [Reference] | NA |
| Progressive disease | 24 | 2.94 (0.37-23.00) | .31 | 1.84 (0.20-17.30) | .59 |
| Partial or complete response | 506 | 9.43 (5.45-16.31) | <.001 | 13.00 (7.02-24.08) | <.001 |
| CA 19-9 at restaging, U/mL | |||||
| ≥37 | 709 | 1 [Reference] | NA | 1 [Reference] | NA |
| <37 (normal[ization]) | 748 | 3.59 (1.82-7.04) | <.001 | 3.76 (1.79-7.89) | <.001 |
| Missing | 238 | 8.80 (4.32-17.92) | <.001 | 10.89 (4.73-25.06) | <.001 |
| Relative CA 19-9 change | |||||
| No change or increased | 86 | 1 [Reference] | NA | NA | NA |
| Decreased <87% | 521 | 0.74 (0.16-3.48) | .70 | NA | NA |
| Decreased ≥87% | 427 | 2.17 (0.50-9.44) | .30 | NA | NA |
| <37 U/mL at diagnosis | 307 | 2.16 (0.48-9.62) | .31 | NA | NA |
| Missing | 354 | 4.32 (1.02-18.36) | .05 | NA | NA |
Abbreviations: CA 19-9, carbohydrate antigen 19-9; (m)FOLFIRINOX, (modified) leucovorin calcium (folinic acid), fluorouracil, irinotecan hydrochloride, and oxaliplatin; NA, not applicable; OR, odds ratio; RECIST, Response Evaluation Criteria in Solid Tumors; SBRT, stereotactic body radiotherapy.
Discussion
This retrospective, international cohort study of 1758 patients who underwent resection of pancreatic adenocarcinoma after preoperative chemo(radio)therapy demonstrated that pCR occurs in 4.8% of patients and is associated with better OS compared with patients without pCR. This finding is illustrated by the 3-fold higher 5-year RFS (64% vs 20%) and doubled 5-year OS (63% vs 30%) compared with patients without pCR. Factors associated with pCR included a tumor located in the pancreatic head, larger tumors, (m)FOLFIRINOX chemotherapy compared with other multiagent regimens, preoperative conventional radiotherapy and SBRT, partial or complete radiologic response, and normal(ized) serum CA 19-9 at restaging.
The 4.8% rate of pCR in the current international study is somewhat higher than the 4% reported by a systematic review comprising 27 prospective studies including 1129 patients with localized pancreatic adenocarcinoma.13 This finding could be explained by the more recent study period of our study because preoperative (m)FOLFIRINOX and modern radiotherapeutic modalities are probably more often used in recent years. Both (m)FOLFIRINOX and radiotherapy were associated with pCR after adjustment, whereby SBRT had a stronger association compared with conventional radiotherapy. This hypothesis is strengthened by the increasing incidence of pCR in the period 2004 to 2016, according to the National Cancer Database.14
This is the first study, to our knowledge, investigating the association between pCR and preoperative treatment strategies (ie, chemotherapy regimen and radiotherapeutic modalities) and serum CA 19-9. Previous reports have queried databases with small sample sizes or large databases with limited information about preoperative therapy and clinicopathological characteristics.10,11,14,16,20,21,40 Cloyd et al14 studied the National Cancer Database (pCR in 244 of 7902 patients [3.0%] diagnosed with localized pancreatic cancer [2004-2016]) and demonstrated that preoperative multiagent vs single-agent chemotherapy was not associated with pCR. In the current study, however, the odds of developing pCR were greater after (m)FOLFIRINOX compared with other multiagent chemotherapies. Remarkably, no difference was seen between (m)FOLFIRINOX and single-agent chemotherapy in our study. In contrast to the findings in our study, Cloyd et al11 demonstrated that the duration of preoperative therapy was associated with pCR. The absence of this association in the current study could be explained by probably a higher rate of modern, more potent preoperative therapies (eg, [m]FOLFIRINOX and SBRT). Some recent literature suggests the potential value of total neoadjuvant therapy (ie, chemotherapy followed by chemoradiotherapy) compared with chemotherapy followed by radiotherapy, chemoradiotherapy, or chemotherapy alone, possibly associated with higher rate of pCR and/or OS.17,19,41 In general, preoperative chemotherapy with radiation is associated with improved pathological outcomes (eg, higher rates of R0, negative lymph nodes, and tumor response) compared with chemotherapy alone, but this rarely translates into prolonged OS42,43 and might even be considered harmful when an arterial resection or divestment is needed during surgery.43,44 The lack of OS benefit was also seen in the current study, in which preoperative radiotherapy did not improve OS and SBRT was even independently associated with impaired OS. However, SBRT was associated with pCR. This finding seems conflicting because pCR is associated with longer OS (ie, median OS not reached; 63% 5-year OS), as reported by previous studies.14,15,17 This divergence suggests that pCR might not always reflect an optimal disease response and does not guarantee cure,45 illustrated by the 5-year RFS rate of 64% in this study. Nevertheless, the lower serum CA 19-9 level at restaging among patients with pCR and the association of normal(ized) serum CA 19-9 with pCR suggest that pCR represents both local and systemic disease responses in a substantial group of patients, leading to prolonged OS. Of note, the association of SBRT with shorter OS should be interpreted with caution because this finding is derived solely from patients who underwent a resection. Randomized clinical trials are necessary to determine the value of additional radiotherapy.46
The 5-year OS was doubled in patients with pCR compared with those without pCR in this study (63% vs 30%). Therefore, it is clear that pCR cannot be interpreted as synonymous with cure, also considering the 5-year RFS of 64%. Serum CA 19-9 response for prognostication and clinical decision-making in patients with pCR seems crucial because it might be a surrogate marker for the systemic disease response.47,48 Only 35% of patients with pCR received adjuvant chemotherapy compared with 67% of patients without pCR. Certain patients with pCR may benefit from additional systemic chemotherapy. However, the remaining micrometastases might have different genetic or molecular characteristics compared with the primary tumor, which might be responsible for other chemosensitivity or chemotherapy resistance.49 Better tumor markers are needed to detect the presence of remaining systemic disease.46
Reliable pathological assessment is a major concern. Recently, the International Study Group of Pancreatic Pathologists12 found moderate correlations of 0.66 and 0.71 for tumor regression grading among 23 world-leading pancreatic pathologists, using the College of American Pathologists and MD Anderson Cancer Center classification systems.28 Eight of 50 patients were classified as having pCR by at least 1 pathologist, but consensus was reached in none of the cases.28 Furthermore, variation exists among pathologists in the sampling strategy, varying from complete specimen sampling to macroscopy-based tumor sampling.30 Because pancreatic adenocarcinoma is characterized by irregular distribution of tumor cells embedded in stroma and fibrosis (ie, intratumor heterogeneity), vital tumor cells can be easily missed when the specimen is not fully sampled, particularly when the distance between vital cells further increases due to preoperative chemotherapy.30 These limitations affect the reliability of the diagnosis of pCR and could have contributed to the variability of pCR incidence per center in this study (median [IQR], 3.8% [0.3%-7.8%]). Unfortunately, the retrospective nature of the current study prohibited reliable data collection regarding the sampling strategies. Even though it is not unlikely that the pCR group in this study also contains patients with vital tumor cells left in the resected pancreas specimen, the associated prolonged OS suggests the presence of at least extensive tumor response.
Strengths and Limitations
This study has several strengths. The major strength is the large number of patients originating from multiple countries and continents (see eAppendix 7 in Supplement 1 for region-specific data) and having detailed data on treatment and disease characteristics. Furthermore, most patients were treated with modern preoperative regimens, reflecting the current clinical practices. Of note, one-quarter of the included patients were diagnosed with a primary resectable tumor, possibly treated with neoadjuvant therapy in the setting of clinical trials because neoadjuvant therapy was generally not the standard of care during the study period.50,51,52
Future research should focus on improving tumor response scoring systems. Artificial intelligence models have the potential to accurately and objectively determine residual tumor burden.53 Such models need to be further developed and validated. For now, pancreatic pathologists should strive for a uniform strategy for sampling and response evaluation. Nevertheless, the patient does not die because of what the surgeon takes out but rather what is left behind. Therefore, there is an urgent need for better markers (eg, solid, liquid, and imaging based),54,55,56 allowing more adequate response evaluation, patient selection for surgery, and postoperative clinical decision-making for adjuvant therapy.46 Fluorodeoxyglucose positron emission tomography with computed tomography or magnetic resonance imaging is a promising tool to identify biological tumor response after preoperative therapy, including pCR. However, this method needs to be investigated in all-comers instead of solely surgical cohorts.57,58
The results of this study should be interpreted in light of some limitations. First, it was considered infeasible to perform a Cox proportional hazards regression analysis on OS within the cohort of patients with pCR because of the sample size and the limited number of events. Information about associated factors might elucidate in which patients pCR means a cure or requires adjuvant therapy and whether there is a difference in prognosis for pCR after preoperative chemotherapy with or without radiotherapy. Comparative subanalyses were underpowered by the small subgroups and small number of events. Second, a more detailed stratification for preoperative chemotherapy regimens was not feasible due to the number of patients with pCR. Third, a Cox proportional hazards regression analysis investigating potential factors associated with RFS was not performed because of heterogeneity in local follow-up strategies among centers. Fourth, information about patients’ race and presence of BRCA germline mutations were not collected or available, whereas these factors seem to be of relevance for the chance to achieve major pathological response.59,60 Fifth, the number of patients with R1 was relatively low (17%), which could be explained by different local protocols for which not all specimen surfaces were assessed. Sixth, serum bilirubin levels were not collected; therefore, serum CA 19-9 levels might be reactively elevated in some patients due to hyperbilirubinemia. Seventh, patients with pCR were included when preoperative pathology was suggestive of or conclusive for malignancy22 but without central review. Therefore, the diagnosis of (pancreatic) adenocarcinoma might not always have been certain.61
Conclusions
In this international, observational, multicenter cohort study, pCR was found in 4.8% of patients with resected pancreatic adenocarcinoma after chemo(radio)therapy. Although pCR does not reflect cure, it is associated with better OS. Factors associated with pCR included preoperative chemotherapy regimens, radiation, and anatomical and biological disease response, which may have implications for treatment strategies. This finding should be confirmed in prospective studies because these factors may not universally apply to all patients with pancreatic adenocarcinoma, as illustrated by the association of SBRT with impaired OS, whereas SBRT was also associated with pCR.
eAppendix 1. Incidence of pCR per Center
eAppendix 2. Surgical Details, Surgical Outcome, and Adjuvant Therapy
eAppendix 3. Histopathology
eAppendix 4. Clinicopathological and Treatment Characteristics (Row Percentages)
eAppendix 5. Overall Survival From Starting Preoperative Chemotherapy
eAppendix 6. Site of Disease Recurrence in Patients With pCR vs Without pCR
eAppendix 7. Region-Specific Data
Data Sharing Statement
References
- 1.Springfeld C, Ferrone CR, Katz MHG, et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20(5):318-337. doi: 10.1038/s41571-023-00746-1 [DOI] [PubMed] [Google Scholar]
- 2.Bratlie SO, Wennerblom J, Vilhav C, Persson J, Rangelova E. Resectable, borderline, and locally advanced pancreatic cancer: “the good, the bad, and the ugly” candidates for surgery? J Gastrointest Oncol. 2021;12(5):2450-2460. doi: 10.21037/jgo-2020-slapc-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown ZJ, Heh V, Labiner HE, et al. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: meta-analysis. Br J Surg. 2022;110(1):34-42. doi: 10.1093/bjs/znac354 [DOI] [PubMed] [Google Scholar]
- 4.Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2-11. doi: 10.1016/j.pan.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 5.Oba A, Del Chiaro M, Satoi S, et al. New criteria of resectability for pancreatic cancer: a position paper by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). J Hepatobiliary Pancreat Sci. 2022;29(7):725-731. doi: 10.1002/jhbp.1049 [DOI] [PubMed] [Google Scholar]
- 6.Dekker EN, van Dam JL, Janssen QP, et al. ; Trans-Atlantic Pancreatic Surgery (TAPS) Consortium . Improved clinical staging system for localized pancreatic cancer using the ABC factors: a TAPS Consortium study. J Clin Oncol. 2024;42(12):1357-1367. doi: 10.1200/JCO.23.01311 [DOI] [PubMed] [Google Scholar]
- 7.Seelen LWF, Floortje van Oosten A, Brada LJH, et al. Early recurrence after resection of locally advanced pancreatic cancer following induction therapy: an international multicenter study. Ann Surg. 2023;278(1):118-126. doi: 10.1097/SLA.0000000000005666 [DOI] [PubMed] [Google Scholar]
- 8.Schorn S, Demir IE, Samm N, et al. Meta-analysis of the impact of neoadjuvant therapy on patterns of recurrence in pancreatic ductal adenocarcinoma. BJS Open. 2018;2(2):52-61. doi: 10.1002/bjs5.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273(2):341-349. doi: 10.1097/SLA.0000000000003284 [DOI] [PubMed] [Google Scholar]
- 10.Maeda S, Mederos MA, Chawla A, et al. Pathological treatment response has different prognostic implications for pancreatic cancer patients treated with neoadjuvant chemotherapy or chemoradiotherapy. Surgery. 2022;171(5):1379-1387. doi: 10.1016/j.surg.2021.10.015 [DOI] [PubMed] [Google Scholar]
- 11.Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152(11):1048-1056. doi: 10.1001/jamasurg.2017.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen BV, Tutucu F, van Roessel S, et al. ; International Study Group of Pancreatic Pathologists (ISGPP) . Amsterdam International Consensus Meeting: tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod Pathol. 2021;34(1):4-12. doi: 10.1038/s41379-020-00683-9 [DOI] [PubMed] [Google Scholar]
- 13.Antolino L, Cinquepalmi M, Moschetta G, et al. Is complete pathologic response in pancreatic cancer overestimated? a systematic review of prospective studies. J Gastrointest Surg. 2020;24(10):2336-2348. doi: 10.1007/s11605-020-04697-1 [DOI] [PubMed] [Google Scholar]
- 14.Cloyd JM, Ejaz A, Shen C, et al. Pathologic complete response following neoadjuvant therapy for pancreatic ductal adenocarcinoma: defining the incidence, predictors, and outcomes. HPB (Oxford). 2020;22(11):1569-1576. doi: 10.1016/j.hpb.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 15.He J, Blair AB, Groot VP, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268(1):1-8. doi: 10.1097/SLA.0000000000002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellon EA, Jin WH, Frakes JM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol. 2017;56(3):391-397. doi: 10.1080/0284186X.2016.1256497 [DOI] [PubMed] [Google Scholar]
- 17.Villano AM, O’Halloran E, Goel N, et al. Total neoadjuvant therapy is associated with improved overall survival and pathologic response in pancreatic adenocarcinoma. J Surg Oncol. 2022;126(3):502-512. doi: 10.1002/jso.26906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blair AB, Yin LD, Pu N, et al. Recurrence in patients achieving pathological complete response after neoadjuvant treatment for advanced pancreatic cancer. Ann Surg. 2021;274(1):162-169. doi: 10.1097/SLA.0000000000003570 [DOI] [PubMed] [Google Scholar]
- 19.Barrak D, Villano AM, Villafane-Ferriol N, et al. Total neoadjuvant therapy for pancreatic adenocarcinoma increases probability for a complete pathologic response. Eur J Surg Oncol. 2022;48(6):1356-1361. doi: 10.1016/j.ejso.2021.12.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sell NM, Lee GC, Fernández-Del Castillo C, et al. Evaluation of pathologic response on overall survival after neoadjuvant therapy in pancreatic ductal adenocarcinoma. Pancreas. 2020;49(7):897-903. doi: 10.1097/MPA.0000000000001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittmann D, Hall WA, Christians KK, et al. Impact of neoadjuvant chemoradiation on pathologic response in patients with localized pancreatic cancer. Front Oncol. 2020;10:460. doi: 10.3389/fonc.2020.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donisi G, Nappo G, Pacilli M, et al. Pathologic tumor response to neoadjuvant therapy in resected pancreatic cancer: does it affect prognosis? Updates Surg. 2023;75(6):1497-1508. doi: 10.1007/s13304-023-01628-y [DOI] [PubMed] [Google Scholar]
- 23.Bao QR, Frigerio I, Tripepi M, et al. Prognostic value of major pathological response following neoadjuvant therapy for non resectable pancreatic ductal adenocarcinoma. Pancreatology. 2023;23(3):266-274. doi: 10.1016/j.pan.2023.02.005 [DOI] [PubMed] [Google Scholar]
- 24.Servin-Rojas M, Fong ZV, Fernandez-Del Castillo C, et al. Tumor size reduction and serum carbohydrate antigen 19-9 kinetics after neoadjuvant FOLFIRINOX in patients with pancreatic ductal adenocarcinoma. Surgery. 2024;175(2):471-476. doi: 10.1016/j.surg.2023.09.041 [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 26.Pitman MB, Centeno BA, Ali SZ, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11(suppl 1):3. doi: 10.4103/1742-6413.133343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Roessel S, Janssen BV, Soer EC, et al. Scoring of tumour response after neoadjuvant therapy in resected pancreatic cancer: systematic review. Br J Surg. 2021;108(2):119-127. doi: 10.1093/bjs/znaa031 [DOI] [PubMed] [Google Scholar]
- 28.Janssen BV, van Roessel S, van Dieren S, et al. ; International Study Group of Pancreatic Pathologists (ISGPP) . Histopathological tumour response scoring in resected pancreatic cancer following neoadjuvant therapy: international interobserver study (ISGPP-1). Br J Surg. 2022;110(1):67-75. doi: 10.1093/bjs/znac350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cacciato Insilla A, Vivaldi C, Giordano M, et al. Tumor regression grading assessment in locally advanced pancreatic cancer after neoadjuvant FOLFIRINOX: interobserver agreement and prognostic implications. Front Oncol. 2020;10:64. doi: 10.3389/fonc.2020.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbeke C, Löhr M, Karlsson JS, Del Chiaro M. Pathology reporting of pancreatic cancer following neoadjuvant therapy: challenges and uncertainties. Cancer Treat Rev. 2015;41(1):17-26. doi: 10.1016/j.ctrv.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 31.Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):202-210. doi: 10.6004/jnccn.2019.0014 [DOI] [PubMed] [Google Scholar]
- 32.Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell; 2010. [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 34.Hartwig W, Vollmer CM, Fingerhut A, et al. ; International Study Group on Pancreatic Surgery . Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014;156(1):1-14. doi: 10.1016/j.surg.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 35.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell F, Foulis A, Verbeke C. Dataset for the Histopathological Reporting of Carcinomas of the Pancreas, Ampulla of Vater and Common Bile Duct. Royal College of Pathologists; 2010. [Google Scholar]
- 37.R Computing Team . A language and environment for statistical computing. Accessed May 11, 2023. https://www.R-project.org/
- 38.Therneau T. A Package for Survival Analysis in R_. R package version 3.5-8. Accessed April 4, 2024. https://CRAN.R-project.org/package=survival
- 39.Therneau TM, Grambsch P. M. Modeling Survival Data: Extending the Cox Model. Springer; 2000. doi: 10.1007/978-1-4757-3294-8 [DOI] [Google Scholar]
- 40.Yamada S, Yokoyama Y, Sonohara F, et al. Tumor marker recovery rather than major pathological response is a preferable prognostic factor in patients with pancreatic ductal adenocarcinoma with preoperative therapy. J Hepatobiliary Pancreat Sci. 2020;27(8):487-495. doi: 10.1002/jhbp.748 [DOI] [PubMed] [Google Scholar]
- 41.Kim RY, Christians KK, Aldakkak M, et al. Total neoadjuvant therapy for operable pancreatic cancer. Ann Surg Oncol. 2021;28(4):2246-2256. doi: 10.1245/s10434-020-09149-3 [DOI] [PubMed] [Google Scholar]
- 42.Katz MHG, Shi Q, Meyers J, et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022;8(9):1263-1270. doi: 10.1001/jamaoncol.2022.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oba A, Wu YHA, Colborn KL, et al. Comparing neoadjuvant chemotherapy with or without radiation therapy for pancreatic ductal adenocarcinoma: national Cancer Database cohort analysis. Br J Surg. 2022;109(5):450-454. doi: 10.1093/bjs/znac002 [DOI] [PubMed] [Google Scholar]
- 44.Del Chiaro M, Schulick RD. Use of total pancreatectomy and preoperative radiotherapy in patients undergoing pancreatectomy with artery resection. J Am Coll Surg. 2019;228(1):131. doi: 10.1016/j.jamcollsurg.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Liao S, You J. Pathological complete response after neoadjuvant therapy for pancreatic ductal adenocarcinoma does not equal cure. ANZ J Surg. 2021;91(5):E254-E259. doi: 10.1111/ans.16665 [DOI] [PubMed] [Google Scholar]
- 46.Stoop TF, Theijse RT, Seelen LWF, et al. ; International Collaborative Group on Locally Advanced Pancreatic Cancer . Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21(2):101-124. doi: 10.1038/s41575-023-00856-2 [DOI] [PubMed] [Google Scholar]
- 47.Newhook TE, Vreeland TJ, Griffin JF, et al. Prognosis Associated with CA19-9 response dynamics and normalization during neoadjuvant therapy in resected pancreatic adenocarcinoma. Ann Surg. 2023;277(3):484-490. doi: 10.1097/SLA.0000000000005184 [DOI] [PubMed] [Google Scholar]
- 48.Seelen LWF, Doppenberg D, Stoop TF, et al. ; Dutch Pancreatic Cancer Group . Minimum and optimal CA19-9 response after two months induction chemotherapy in patients with locally advanced pancreatic cancer: a nationwide multicenter study. Ann Surg. 2024;279(5):832-841. doi: 10.1097/SLA.0000000000006021 [DOI] [PubMed] [Google Scholar]
- 49.Connor AA, Gallinger S. Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat Rev Cancer. 2022;22(3):131-142. doi: 10.1038/s41568-021-00418-1 [DOI] [PubMed] [Google Scholar]
- 50.Ducreux M, Cuhna AS, Caramella C, et al. ; ESMO Guidelines Committee . Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56-v68. doi: 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 51.Tempero MA, Arnoletti JP, Behrman SW, et al. ; National Comprehensive Cancer Networks . Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10(6):703-713. doi: 10.6004/jnccn.2012.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaguchi K, Okusaka T, Shimizu K, et al. ; Committee for revision of clinical guidelines for pancreatic cancer of Japan Pancreas Society . EBM-based Clinical Guidelines for Pancreatic Cancer (2013) issued by the Japan Pancreas Society: a synopsis. Jpn J Clin Oncol. 2014;44(10):883-888. doi: 10.1093/jjco/hyu127 [DOI] [PubMed] [Google Scholar]
- 53.Janssen BV, Theijse R, van Roessel S, et al. Artificial intelligence-based segmentation of residual tumor in histopathology of pancreatic cancer after neoadjuvant treatment. Cancers (Basel). 2021;13(20):5089. doi: 10.3390/cancers13205089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinny-Köster B, Habib JR, Wolfgang CL, He J, Javed AA. Favorable tumor biology in locally advanced pancreatic cancer-beyond CA19-9. J Gastrointest Oncol. 2021;12(5):2484-2494. doi: 10.21037/jgo-20-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen BV, Verhoef S, Wesdorp NJ, et al. Imaging-based machine-learning models to predict clinical outcomes and identify biomarkers in pancreatic cancer: a scoping review. Ann Surg. 2022;275(3):560-567. doi: 10.1097/SLA.0000000000005349 [DOI] [PubMed] [Google Scholar]
- 56.Doppenberg D, Stoop TF, van Dieren S, et al. ; Trans-Atlantic Pancreatic Surgery (TAPS) Consortium . Serum CEA as prognostic marker for overall survival in patients with localized pancreatic adenocarcinoma and non-elevated CA19-9 levels treated with FOLFIRINOX as initial treatment: a TAPS consortium study. Ann Surg Oncol. 2024;31(3):1919-1932. doi: 10.1245/s10434-023-14680-0 [DOI] [PubMed] [Google Scholar]
- 57.Abdelrahman AM, Goenka AH, Alva-Ruiz R, et al. FDG-PET predicts neoadjuvant therapy response and survival in borderline resectable/locally advanced pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2022;20(9):1023-1032.e3. doi: 10.6004/jnccn.2022.7041 [DOI] [PubMed] [Google Scholar]
- 58.de Jong TL, Koopman D, van der Worp CAJ, et al. Added value of digital FDG-PET/CT in disease staging and restaging in patients with resectable or borderline resectable pancreatic cancer. Surg Oncol. 2023;47:101909. doi: 10.1016/j.suronc.2023.101909 [DOI] [PubMed] [Google Scholar]
- 59.Ogobuiro I, Collier AL, Khan K, et al. Racial disparity in pathologic response following neoadjuvant chemotherapy in resected pancreatic cancer: a multi-institutional analysis from the Central Pancreatic Consortium. Ann Surg Oncol. 2023;30(3):1485-1494. doi: 10.1245/s10434-022-12741-4 [DOI] [PubMed] [Google Scholar]
- 60.Golan T, Barenboim A, Lahat G, et al. Increased rate of complete pathologic response after neoadjuvant FOLFIRINOX for BRCA mutation carriers with borderline resectable pancreatic cancer. Ann Surg Oncol. 2020;27(10):3963-3970. doi: 10.1245/s10434-020-08469-8 [DOI] [PubMed] [Google Scholar]
- 61.Janssen QP, Quispel R, Besselink MG, et al. ; Dutch Pancreatic Cancer Group . Diagnostic performance of endoscopic tissue acquisition for pancreatic ductal adenocarcinoma in the PREOPANC and PREOPANC-2 trials. HPB (Oxford). 2023;25(10):1161-1168. doi: 10.1016/j.hpb.2023.04.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Incidence of pCR per Center
eAppendix 2. Surgical Details, Surgical Outcome, and Adjuvant Therapy
eAppendix 3. Histopathology
eAppendix 4. Clinicopathological and Treatment Characteristics (Row Percentages)
eAppendix 5. Overall Survival From Starting Preoperative Chemotherapy
eAppendix 6. Site of Disease Recurrence in Patients With pCR vs Without pCR
eAppendix 7. Region-Specific Data
Data Sharing Statement


