Abstract
Background
Metabolic abnormalities are closely tied to the development of ovarian cancer (OC), yet the relationship between anthropometric indicators as risk indicators for metabolic abnormalities and OC lacks consistency.
Method
The Mendelian randomization (MR) approach is a widely used methodology for determining causal relationships. Our study employed summary statistics from the genome-wide association studies (GWAS), and we used inverse variance weighting (IVW) together with MR-Egger and weighted median (WM) supplementary analyses to assess causal relationships between exposure and outcome. Furthermore, additional sensitivity studies, such as leave-one-out analyses and MR-PRESSO were used to assess the stability of the associations.
Result
The IVW findings demonstrated a causal associations between 10 metabolic factors and an increased risk of OC. Including “Basal metabolic rate” (OR= 1.24, P= 6.86×10-4); “Body fat percentage” (OR= 1.22, P= 8.20×10-3); “Hip circumference” (OR= 1.20, P= 5.92×10-4); “Trunk fat mass” (OR= 1.15, P= 1.03×10-2); “Trunk fat percentage” (OR= 1.25, P= 8.55×10-4); “Waist circumference” (OR= 1.23, P= 3.28×10-3); “Weight” (OR= 1.21, P= 9.82×10-4); “Whole body fat mass” (OR= 1.21, P= 4.90×10-4); “Whole body fat-free mass” (OR= 1.19, P= 4.11×10-3) and “Whole body water mass” (OR= 1.21, P= 1.85×10-3).
Conclusion
Several metabolic markers linked to altered fat accumulation and distribution are significantly associated with an increased risk of OC.
Keywords: ovarian cancer, metabolic factors, risk factors, Mendelian randomization, causal association
Introduction
Ovarian cancer (OC) is one of the leading causes of gynecological cancer-related death among females globally, ranking as the fifth most common reason for cancer-related mortality, with a five-year survival rate of less than 29% (1, 2). According to the most recent estimates from 2017, this condition affects around 224,940 females globally (3). Experts predict a 55% increase in OC prevalence and 67% increase in mortality rates by 2035 (4). Furthermore, more than 90% of the earliest malignant ovarian tumors are epithelial in origin (5). As a result, early detection of OC is critical in lowering the high fatality rate among women.
Metabolic processes play an important role in various diseases (6–10), and the investigation into the impact of metabolic and anthropometric factors on OC is becoming a progressively appealing research area (11–14). The bulk of OC is related to the development of ascites (15). Malignant ascites forms a distinct tumor microenvironment with several metabolic regulators, including growth factors, chemokines, and cytokines, which promote the invasion and resistance to medications in various types of cancers (3, 16–20). Furthermore, OC cells need adipocytes for energy, which might affect lipid metabolism and serum levels (21, 22). Multiple studies have indicated that overweight and obesity elevate the likelihood of developing OC (23–25). For instance, a meta-analysis and systematic review revealed that high body mass index (BMI) was associated with an increased risk of OC (14). Additionally, a prospective investigation found that for every 10-centimeter increase in waist circumference, the pooled relative risk (RR) for OC was 1.06 (26). However, one study showed no evidence connecting waist circumference with an increased chance of developing OC (27). Anthropometric measurements, such as hip circumference and waist-to-hip ratio, are considered risk factors for metabolic disorders, however evidence on their relationship with OC is inconsistent (27–30). Given the lack of reliable and consistent evidence, more research is needed to determine the overall risk and histological associations between numerous metabolic factors and OC.
We used Mendelian randomization (MR) to evaluate the causal relationship between numerous metabolic factors and OC. MR methods successfully reduce reverse causation and residual confounding by utilizing instrumental variables (IVs) that are closely related to the exposure (31). Our goal is to improve understanding of OC risk factors and develop new prevention strategies.
Methods
Study design
This study used IVs and MR analysis to emulate the randomization procedure in a randomized controlled experiment. It focused on several elements: 1) IVs were significantly associated with exposures; 2) The causal link between exposures and outcomes was assessed using inverse variance weighting (IVW), MR-Egger, and weighted median estimate (WM); 3) Sensitivity analyses were used to determine the robustness of the findings.
Data sources
This study used data solely from public databases. The research involved a group of European people. As exposure indicators, a complete collection of 23 anthropometric and metabolic parameters was used. Supplementary Table 1 provides further information on these 23 exposure variables. OC was identified as research outcome, and the information of genome-wide association study (GWAS) data is also given in Supplementary Table 1 .
Selection of IVs
The selection criteria included P < 5×10-8, which identified IVs with substantial relationships with the exposure variables. The clump=TRUE option was used to eliminate linkage disequilibrium (LD) and improved the accuracy of the IVs, as described in our previous publications (32–35). The parameters of r2 = 0.001 and kb=10000 represented the LD threshold and distance for clumping, respectively. To summarize, r2 = 1 implied a perfect LD association between two single nucleotide polymorphisms (SNPs), whereas r2 = 0 showed complete LD equilibrium, implying that the distribution of these two SNPs is random. The parameter kb denotes the length of the region evaluated for LD. In genetics, it is widely assumed that linked genetic loci on a chromosome are frequently inherited together. As a result, in our investigation, we utilize r2 = 0.001 and kb=10000 to achieve more accurate results while taking into consideration probable LD. We also calculated the F statistic: a high F statistic (greater than or equal to 10) indicated a strong IV (36).
MR analysis
The IVW approach was the principal analytic technique employed in this work. The WM technique is more resistant to some invalid IVs. Hence, it is employed as a complement to the IVW approach in this study (37). The MR-Egger method serves as an additional strategy, employing the intercept term to investigate potential pleiotropic effects (38).
Sensitivity analysis
LDlink was used to assess the relationship between all exposure-associated IVs and potential confounders (39, 40), and sensitivity analysis was performed after excluding these SNPs with external pleiotropy. The study used pleiotropy-corrected data from MR-PRESSO to indicate any likely deviants in the analysis. The Cochrane Q statistic was used to assess the heterogeneity present. Furthermore, a detailed sensitivity analysis using a leave-one-out technique was performed to check the reliability of the findings and to assess the influence of each IV on the causal relationship. Given the binary character of the outcome, the MR evaluation used odds ratios (ORs), as well as 95% confidence intervals (CIs), to present causal effect. To address the issue of multiple testing, the 5% false discovery rate (FDR) was set. All MR assessments were carried out utilizing the TwoSampleMR package in the R programming environment.
Results
The F-statistics of IVs
This research examined a total of 23 metabolic and anthropometric variables. 10 traits played a causal influence in OC. The F-statistic values for the associated IVs of these 10 variables were all more than 29, indicating good IVs ( Supplementary Table 2 ).
MR analysis results
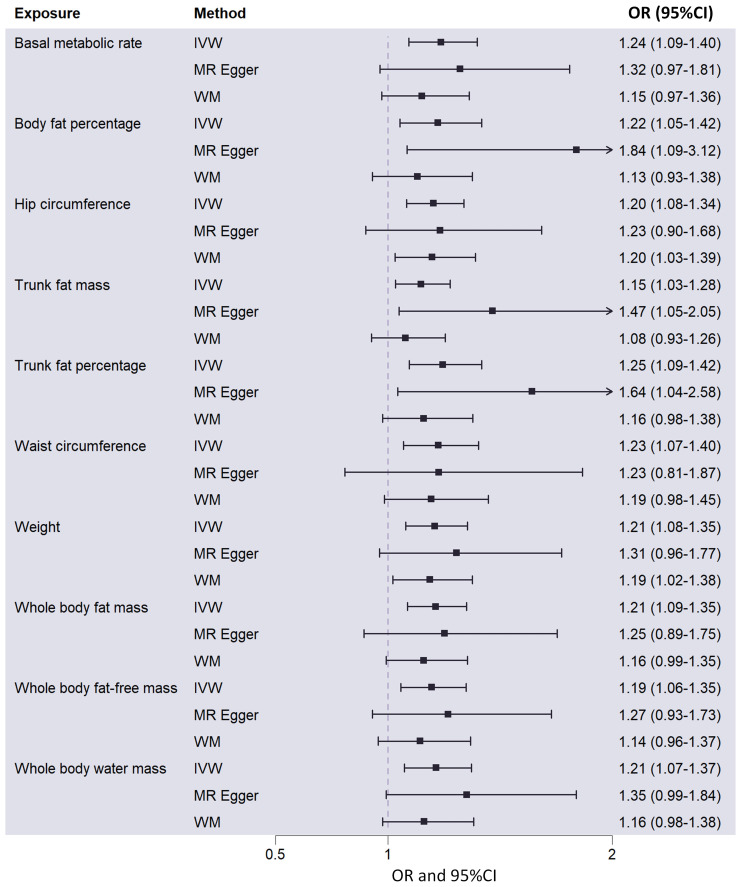
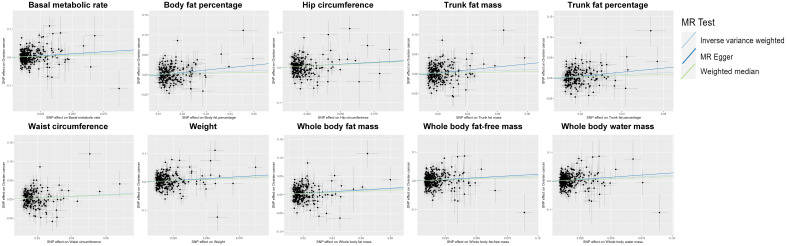
Among the 23 metabolic and anthropometric variables, 10 indicated a causative association with OC ( Figure 1 ). The IVW results of the MR analysis for each of the 10 traits were listed below: “Basal metabolic rate - OC” (OR: 1.24; 95% CI: 1.09,1.40), “Body fat percentage - OC” (OR: 1.22; 95% CI: 1.05,1.42), “Hip circumference - OC” (OR: 1.20; 95% CI: 1.08,1.34), “Trunk fat mass - OC” (OR: 1.15; 95% CI: 1.03,1.28), “Trunk fat percentage - OC” (OR: 1.25; 95% CI: 1.09,1.42), “Waist circumference - OC” (OR: 1.23; 95% CI: 1.07,1.40), “Weight - OC” (OR: 1.21; 95% CI: 1.08,1.35), “Whole body fat mass - OC” (OR: 1.21; 95% CI: 1.09,1.35), “Whole body fat-free mass - OC” (OR: 1.19; 95% CI: 1.06,1.35), “Whole body water mass - OC” (OR: 1.21; 95% CI: 1.07,1.37) ( Figure 2 ). In conclusion, all 10 metabolic factors were positively causally associated to OC( Figure 3 and Supplementary Table 3 ). After excluding IVs that were associated with potential confounders (e.g., smoking status and alcohol consumption), the majority of our analyses remained unchanged ( Supplementary Table 4 ). Reverse MR analysis of OC as the exposure and traits as outcomes revealed no evidence of reverse causality (all P > 0.05) (see Supplementary Table 5 ).
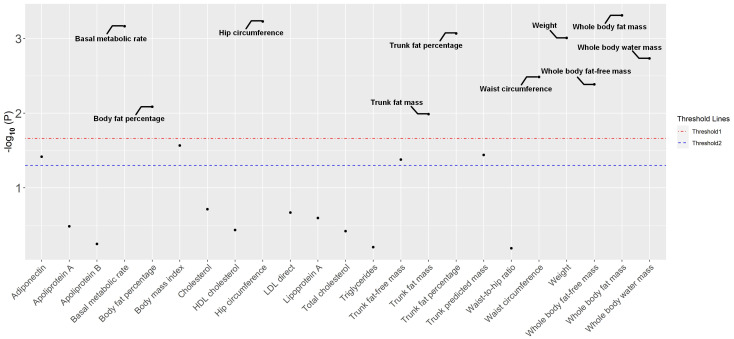
Figure 1.
The distribution of P-values for the associations between 23 metabolic factors related to anthropometric indicators and ovarian cancer in the MR analysis. Two thresholds were used to assess significance. One represented the threshold adjusted for false discovery rate (threshold line 1), while the other relied on a commonly used P-value (0.05). MR, Mendelian randomization.
Figure 2.
Causal effects of 10 metabolic factors on ovarian cancer in the MR analysis, showing odds ratios and corresponding 95% confidence intervals. MR, Mendelian randomization; IVW, inverse-variance weighted; WM, weighted median; OR, odd ratio; 95%CI, 95% confidence intervals.
Figure 3.
Scatter plot indicating the causal associations between 10 metabolic factors and ovarian cancer. SNP, single nucleotide polymorphism; MR, Mendelian randomization.
Results of the sensitivity analysis
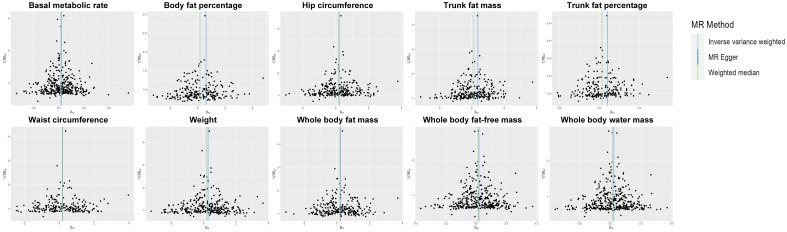
The Cochrane Q statistic was used to evaluate the existence of heterogeneity ( Supplementary Table 6 ), and the funnel plots demonstrated that the distribution of IVs exhibited symmetry ( Figure 4 ). The MR-Egger test showed no significant horizontal pleiotropy (all P >0.05), implying that the data are reliable (see Supplementary Table 7 ). Supplementary Table 8 shows the 10 traits remained associated with OC at suggestive evidence of significance after correcting for outliers (all P <0.05). Supplementary Figure 1 depicts the findings of the leave-one-out analysis, which showed that the majority of SNPs did not cross the null line after being removed, indicating that the study had low potential bias.
Figure 4.
Funnel plot of the IVs. IV, instrumental variable; SE, standard error; MR, Mendelian randomization
Discussion
Obesity and an increased risk of OC have been extensively studied (41, 42), but the impact of body measurement markers in multiple body areas on OC risk should also be investigated. Our MR analysis demonstrated that, after FDR correction, 10 metabolic parameters still had significant positive causal relationships with OC out of the total of 23 metabolic and anthropometric factors studied. These continuous variables are related to body fat distribution, including whole body fat mass, basal metabolic rate (BMR), whole body fat-free mass, and waist circumference.
Research indicates that varying body fat distribution might impact the progression of OC. A longitudinal research found that women with higher fat mass had a significantly increased chance of developing OC (43). An MR investigation found a connection between BMR and higher OC risk (44). Body composition is a better predictor of overall health status in OC patients than weight or BMI (45). Another MR study discovered that the proportion of trunk fat is a surrogate for abdominal adiposity, indicating a causal positive relationship with OC (46). In a study of normal-weight individuals (40-70 years old) in the UK Biobank cohort, researchers found no correlation between waist circumference, trunk fat mass index, trunk fat mass ratio, and waist-hip ratio with the risk of OC in women (47). The complicated influence of numerous anthropometric parameters on OC necessitates more exploration. Our findings may provide additional causal insights into the effect of fat distribution on OC.
The distribution of body fat in humans might correlate with leptin levels, which play a crucial role in lipid metabolism (48). Research indicates that leptin plays a role in determining the resting metabolic rate in lean individuals (49). A prospective study in Sweden analyzed serum samples to examine leptin concentrations and their correlation with weight history (50). The study revealed that elevated leptin levels posed a risk for future weight gain in women aged 38 to 46 (50). Additionally, a four-year longitudinal study carried out in America identified that heightened plasma leptin levels in overweight males could signify leptin resistance and eventual weight gain (51). A longitudinal study involving African Americans revealed a distinct, independent association between heightened hip circumference, expanded waist circumference, and elevated serum leptin levels (52). Moreover, a recent study in young adults aged 20 to 21 demonstrated a positive correlation between serum leptin levels, waist circumference, and body fat percentage in both men and women (53). Furthermore, a study investigating the potential relationship between trunk fat levels and breast gene expression revealed that increased trunk fat levels were correlated with higher levels of leptin (54). These findings suggest that leptin may play a role in human fat distribution and endocrine metabolism. Leptin is synthesized and released by adipocytes to interact with its specific receptor, the leptin receptor (LEP-R), located in white adipose tissue (55). LEP-R facilitates the diverse effects of leptin and plays a vital role in regulating body weight. Leptin resistance is typified by diminished satiety, increased nutrient intake, and subsequent weight gain, ultimately contributing to the development of obesity (56).
Adipose tissue levels in the blood increase sensitivity to leptin expression, which might explain the link between obesity and the risk of OC (57). More than half of patients with epithelial ovarian cancer (EOC) in the Middle East demonstrated overexpression of leptin and its receptors, leading in a shorter overall survival (58). The advancement of OC under the influence of leptin is associated with the phosphorylation of STAT3 and the estrogen receptor (ER), as well as the synthesis of ER-responsive genes, which impacts the overall longevity of OC patients (59–61). Anomalously activated STAT3 promotes uncontrolled tumor cell growth and survival by a number of ways, including increased production of oncogenes like Myc proto-oncogene protein (c-myc) and cyclin D, as well as anti-apoptotic proteins like B-cell lymphoma-extra large (Bcl-XL) and Myeloid cell leukemia 1 (MCL-1) (62, 63). Furthermore, leptin boosts the circulating levels of follicle-stimulating hormone (FSH), which is essential for OC cell activation and proliferation (64). Hence, it is speculated that the 10 metabolic factors related to fat distribution identified in this study may contribute to the pathogenesis of OC through the overexpression of leptin and its receptor.
Hypoxia Inducible factor-1 alpha (HIF-1α), which can be induced by hypoxia and many other factors (65), regulates gene transcription in tumor cells and is corelated with the tolerance of chemotherapy (66–68). HIF-1α can be activated by hypoxia in obesity-related adipose tissue, and HIF-1α promotes the expression of collagen remodeling genes such Collagen type I alpha 1 chain (COL1A1) and Lysyl oxidase (LOX) in ovarian surface epithelial cells and activates Macrophage Type 2 (M2) macrophages (69–72). Surface epithelial cell collagen remodeling and M2 macrophages are associated with a bad outcome in OC patients (70). Adipocytes undergo phenotypic changes when they come into contact with tumor cells, becoming cancer-associated adipocytes (CAAs) and enduring delipidation at the leading edge of tumor invasion, adopting a fibroblast-like phenotype (73). CAAs can effectively halt the cell cycle, elevate the expression of genes linked to cell cycle arrest, and reduce the expression of genes that facilitate cell growth. Additionally, the transformation from regular adipocytes to CAAs may involve cellular aging (74). This process involves an increase in inflaming cytokine release (Interleukin-6 (IL-6) and Plasminogen activator inhibitor-1 (PAI-1)) and may result in the transformation of non-malignant stromal cell types (fibroblasts and macrophages) into cancer-related fibroblasts and macrophages (12, 41, 75, 76). Moreover, CAAs also secrete leptin, a hormone known to influence the immune system, potentially facilitating tumor metastasis and immune evasion (77). The role of a relationship between the development of non-malignant stromal cells and mature adipocytes is currently unknown and warrants more research.
Increased levels of androgens and estrogens, decreased progesterone, and control over the insulin-like growth factor (IGF) axis may be the molecular processes behind the link between fat accumulation and the risk of OC (78). Furthermore, obesity and fat redistribution, particularly in the central region, are known to enhance cancer susceptibility (79–83). Nonetheless, the mechanisms behind the relationship between body fat measurements and the risk of OC in adults of normal weight are still not fully understood.
Our research has various advantages. Our study examines the impact of many metabolic factors on OC by MR analysis, and the identified causal relationships are clinically significant. Furthermore, rigorous sensitivity studies confirmed the reliability and stability of our conclusions. Finally, by including genetic variants, we decreased confounding interference and so preserved the study’s validity.
Nonetheless, our study has limitations. To begin, the risk of selection bias cannot be fully ruled out. Furthermore, While the MR-Egger intercept test did not indicate the presence of horizontal pleiotropy, it is important to note that the possibility of its existence cannot be entirely ruled out. Moreover, because our study only included Europeans, the applicability of our findings to other ethnic groups may be restricted. Future research in diverse ethnic populations, including Asians and Africans, is warranted. Finally, the effect of metabolic factors on OC may not be a simple linear relationship, which cannot be evaluated by our study.
Conclusion
We conducted a comprehensive investigation of the relationships between metabolic indicators and OC risk. We discovered that fat accumulation and distribution contribute to metabolic alterations that have a deleterious impact on OC. The results will also potentially accelerate the identification of indicators and assessment methods, thus enhancing early intervention and treatment strategies for OC. Additional comprehensive studies are necessary to clarify the underlying mechanisms of our observations.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
LH: Writing – original draft. SX: Writing – original draft. DZ: Writing – original draft. RC: Writing – original draft. YD: Writing – original draft. MZ: Writing – original draft. MB: Writing – review & editing. BH: Writing – review & editing. SL: Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (Grant No. 82100040), the Natural Science Foundation of Hunan Province (Grant No. 2024JJ5054), the Scientific Research Foundation of Hunan Provincial Education Department (Grant No. 23A0666), and the ESI Special Project of Changsha Medical University (Grant No. 2022CYY012).
Abbreviations
OC, Ovarian cancer; MR, Mendelian randomization; GWAS, Genome-wide association study; IVW, Inverse-variance weighted; WM, Weighted median; BMI, Body mass index; RR, Relative risk; IVs, Instrumental variables; LD, linkage disequilibrium; SNPs, Single nucleotide polymorphism; ORs, Odds ratios; CIs, Confidence intervals; FDR, False discovery rate; BMR, Basal metabolic rate; LEP-R, leptin receptor; EOC, Epithelial ovarian cancer; ER, Estrogen receptor; STAT3,Signal Transducer and Activator of Transcription; c-myc, Myc proto-oncogene protein; Bcl-XL, B-cell lymphoma-extra large; MCL-1, Myeloid cell leukemia 1; FSH, follicle-stimulating hormone; HIF-1α, Hypoxia Inducible factor-1 alpha; COL1A1, Collagen type I alpha 1 chain; LOX, Lysyl oxidase; M2, Macrophage Type 2; CAAs, Cancer-associated adipocytes; IL-6, Interleukin-6; PAI-1, Plasminogen activator inhibitor-1; IGF, insulin-like growth factor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1401648/full#supplementary-material
References
- 1. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. (2021) 1875:188503. doi: 10.1016/j.bbcan.2021.188503 [DOI] [PubMed] [Google Scholar]
- 2. Tang L, Li J, Bao M, Xiang J, Chen Y, Wang Y. Genetic association between HER2 and ESR2 polymorphisms and ovarian cancer: a meta-analysis. Onco Targets Ther. (2018) 11:1055–66. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ji Z, Shen Y, Feng X, Kong Y, Shao Y, Meng J, et al. Deregulation of lipid metabolism: The critical factors in ovarian cancer. Front Oncol. (2020) 10:593017. doi: 10.3389/fonc.2020.593017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barua A, Bahr JM. Ovarian cancer: Applications of chickens to humans. Annu Rev Anim Biosci. (2022) 10:241–57. doi: 10.1146/annurev-animal-021419-084001 [DOI] [PubMed] [Google Scholar]
- 5. Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. (2009) 4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bao MH, Luo HQ, Chen LH, Tang L, Ma KF, Xiang J, et al. Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(-/-) mice. Sci Rep. (2016) 6:34161. doi: 10.1038/srep34161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Luo J, Liu L, Fu H, Tang L. The genetic association between apolipoprotein E gene polymorphism and Parkinson disease: A meta-Analysis of 47 studies. Med (Baltimore). (2018) 97:e12884. doi: 10.1097/MD.0000000000012884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiong T, Li Z, Huang X, Lu K, Xie W, Zhou Z, et al. TO901317 inhibits the development of hepatocellular carcinoma by LXRα/Glut1 decreasing glycometabolism. Am J Physiol Gastrointest Liver Physiol. (2019) 316:G598–g607. doi: 10.1152/ajpgi.00061.2018 [DOI] [PubMed] [Google Scholar]
- 9. Wang K, Ma J, Li Y, Han Q, Yin Z, Zhou M, et al. Effects of essential oil extracted from Artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front Nutr. (2022) 9:1024722. doi: 10.3389/fnut.2022.1024722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai XN, Zhou BQ, Ning N, Pan T, Xu F, He SH, et al. Effects of lifestyle intervention on adults with metabolic associated fatty liver disease: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1081096. doi: 10.3389/fendo.2023.1081096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baczewska M, Bojczuk K, Kołakowski A, Dobroch J, Guzik P, Knapp P. Obesity and energy substrate transporters in ovarian cancer—Review. Molecules. (2021) 26(6):1659. doi: 10.3390/molecules26061659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duarte Mendes A, Freitas AR, Vicente R, Vitorino M, Vaz Batista M, Silva M, et al. Adipocyte microenvironment in ovarian cancer: A critical contributor? Int J Mol Sci. (2023) 24(23):16589. doi: 10.3390/ijms242316589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stelten S, Schofield C, Hartman YAW, Lopez P, Kenter GG, Newton RU, et al. Association between energy balance-related factors and clinical outcomes in patients with ovarian cancer: A systematic review and meta-analysis. Cancers. (2022) 14(19):4567. doi: 10.3390/cancers14194567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whelan E, Kalliala I, Semertzidou A, Raglan O, Bowden S, Kechagias K, et al. Risk factors for ovarian cancer: An umbrella review of the literature. Cancers. (2022) 14(11):2708. doi: 10.3390/cancers14112708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ford CE, Werner B, Hacker NF, Warton K. The untapped potential of ascites in ovarian cancer research and treatment. Br J Cancer. (2020) 123:9–16. doi: 10.1038/s41416-020-0875-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monavarian M, Elhaw AT, Tang PW, Javed Z, Shonibare Z, Scalise CB, et al. Emerging perspectives on growth factor metabolic relationships in the ovarian cancer ascites environment. Semin Cancer Biol. (2022) 86:709–19. doi: 10.1016/j.semcancer.2022.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen S, Chen Y, Yu L, Hu X. Overexpression of SOCS4 inhibits proliferation and migration of cervical cancer cells by regulating JAK1/STAT3 signaling pathway. Eur J Gynaecological Oncol. (2021) 42:554–60. doi: 10.31083/j.ejgo.2021.03.2416 [DOI] [Google Scholar]
- 18. Shen F, Long D, Yu T, Chen X, Liao Y, Wu Y, et al. Vinblastine differs from Taxol as it inhibits the Malignant phenotypes of NSCLC cells by increasing the phosphorylation of Op18/stathmin. Oncol Rep. (2017) 37:2481–9. doi: 10.3892/or.2017.5469 [DOI] [PubMed] [Google Scholar]
- 19. Chen S, Zhao Y, Shen F, Long D, Yu T, Lin X. Introduction of exogenous wild−type p53 mediates the regulation of oncoprotein 18/stathmin signaling via nuclear factor−κB in non−small cell lung cancer NCI−H1299 cells. Oncol Rep. (2019) 41:2051–9. doi: 10.3892/or [DOI] [PubMed] [Google Scholar]
- 20. Li L, Wang S, Zhou W. Balance cell apoptosis and pyroptosis of caspase-3-activating chemotherapy for better antitumor therapy. Cancers (Basel). (2022) 15(1):26. doi: 10.3390/cancers15010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khanlarkhani N, Azizi E, Amidi F, Khodarahmian M, Salehi E, Pazhohan A, et al. Metabolic risk factors of ovarian cancer: a review. JBRA Assist Reprod. (2022) 26:335–47. doi: 10.5935/1518-0557.20210067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tania M, Khan MA, Song Y. Association of lipid metabolism with ovarian cancer. Curr Oncol. (2010) 17:6–11. doi: 10.3747/co.v17i5.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 24. Ellwanger B, Schüler-Toprak S, Jochem C, Leitzmann MF, Baurecht H. Anthropometric factors and the risk of ovarian cancer: A systematic review and meta-analysis. Cancer Rep (Hoboken). (2022) 5:e1618. doi: 10.1002/cnr2.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang J, Chan WC, Ngai CH, Lok V, Zhang L, Lucero-Prisno DE, 3rd, et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: A global study. Cancers (Basel). (2022) 14(9):2230. doi: 10.3390/cancers14092230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aune D, Navarro Rosenblatt DA, Chan DS, Abar L, Vingeliene S, Vieira AR, et al. Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer. (2015) 136:1888–98. doi: 10.1002/ijc.29207 [DOI] [PubMed] [Google Scholar]
- 27. Kotsopoulos J, Baer HJ, Tworoger SS. Anthropometric measures and risk of epithelial ovarian cancer: results from the nurses' health study. Obes (Silver Spring). (2010) 18:1625–31. doi: 10.1038/oby.2009.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mink PJ, Folsom AR, Sellers TA, Kushi LH. Physical activity, waist-to-hip ratio, and other risk factors for ovarian cancer: a follow-up study of older women. Epidemiology. (1996) 7:38–45. doi: 10.1097/00001648-199601000-00008 [DOI] [PubMed] [Google Scholar]
- 29. Tworoger SS, Huang T. Obesity and ovarian cancer. Recent Results Cancer Res. (2016) 208:155–76. doi: 10.1007/978-3-319-42542-9_9 [DOI] [PubMed] [Google Scholar]
- 30. Mota JF, Rinaldi AE, Pereira AF, Orsatti FL, Burini RC. [Anthropometric indicators as risk markers for metabolic abnormalities]. Cien Saude Colet. (2011) 16:3901–8. doi: 10.1590/S1413-81232011001000026 [DOI] [PubMed] [Google Scholar]
- 31. Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M, et al. Assessing causal associations of hyperparathyroidism with blood counts and biochemical indicators: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1295040. doi: 10.3389/fendo.2023.1295040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen R, Xu S, Ding Y, Li L, Huang C, Bao M, et al. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1307468. doi: 10.3389/fendo.2023.1307468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu Q, Chen R, Ding Y, Xu S, Huang C, He B, et al. Sodium intake and the risk of various types of cardiovascular diseases: a Mendelian randomization study. Front Nutr. (2023) 10. doi: 10.3389/fnut.2023.1250509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu Q, Chen R, Xu S, Ding Y, Huang C, He B, et al. Assessment of potential risk factors associated with gestational diabetes mellitus: evidence from a Mendelian randomization study. Front Endocrinology. (2024) 14. doi: 10.3389/fendo.2023.1276836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M, et al. Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: A Mendelian randomization study. Front Endocrinol (Lausanne). (2024) 14. doi: 10.3389/fendo.2023.1321576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 37. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin SH, Brown DW, Machiela MJ. LDtrait: an online tool for identifying published phenotype associations in linkage disequilibrium. Cancer Res. (2020) 80:3443–6. doi: 10.1158/0008-5472.CAN-20-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. (2015) 31:3555–7. doi: 10.1093/bioinformatics/btv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. (2014) 10:455–65. doi: 10.1038/nrendo.2014.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. (2007) 43:690–709. doi: 10.1016/j.ejca.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 43. Chionh F, Baglietto L, Krishnan K, English DR, MacInnis RJ, Gertig DM, et al. Physical activity, body size and composition, and risk of ovarian cancer. Cancer Causes Control. (2010) 21:2183–94. doi: 10.1007/s10552-010-9638-y [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Qiu J, Meng F, Shu X. Insight into the causality between basal metabolic rate and endometrial and ovarian cancers: Analysis utilizing systematic Mendelian randomization and genetic association data from over 331,000 UK biobank participants. Eur J Clin Invest. (2023) 53:e13971. doi: 10.1111/eci.13971 [DOI] [PubMed] [Google Scholar]
- 45. Purcell SA, Elliott SA, Kroenke CH, Sawyer MB, Prado CM. Impact of body weight and body composition on ovarian cancer prognosis. Curr Oncol Rep. (2016) 18:8. doi: 10.1007/s11912-015-0488-3 [DOI] [PubMed] [Google Scholar]
- 46. Freuer D, Linseisen J, O'Mara TA, Leitzmann M, Baurecht H, Baumeister SE, et al. Body fat distribution and risk of breast, endometrial, and ovarian cancer: A two-sample mendelian randomization study. Cancers (Basel). (2021) 13(20):5053. doi: 10.3390/cancers13205053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arthur RS, Dannenberg AJ, Kim M, Rohan TE. The association of body fat composition with risk of breast, endometrial, ovarian and colorectal cancers among normal weight participants in the UK Biobank. Br J Cancer. (2021) 124:1592–605. doi: 10.1038/s41416-020-01210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Su M, Hu R, Tang T, Tang W, Huang C. Review of the correlation between Chinese medicine and intestinal microbiota on the efficacy of diabetes mellitus. Front Endocrinol (Lausanne). (2022) 13:1085092. doi: 10.3389/fendo.2022.1085092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bi X, Loo YT, Henry CJ. Does circulating leptin play a role in energy expenditure? Nutrition. (2019) 60:6–10. doi: 10.1016/j.nut.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 50. Lissner L, Karlsson C, Lindroos AK, Sjöström L, Carlsson B, Carlsson L, et al. Birth weight, adulthood BMI, and subsequent weight gain in relation to leptin levels in Swedish women. Obes Res. (1999) 7:150–4. doi: 10.1002/j.1550-8528.1999.tb00696.x [DOI] [PubMed] [Google Scholar]
- 51. Chu NF, Spiegelman D, Yu J, Rifai N, Hotamisligil GS, Rimm EB. Plasma leptin concentrations and four-year weight gain among US men. Int J Obes. (2001) 25:346–53. doi: 10.1038/sj.ijo.0801549 [DOI] [PubMed] [Google Scholar]
- 52. Caffo O, Ralston PA, Lemacks JL, Young-Clark I, Wickrama K, Ilich JZ. Sex and body circumferences associated with serum leptin in African American adults. J Womens Health (Larchmt). (2021) 30:1769–77. doi: 10.1089/jwh.2020.8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khwanchuea R, Punsawad C. Associations between body composition, leptin, and vitamin D varied by the body fat percentage in adolescents. Front Endocrinol (Lausanne). (2022) 13:876231. doi: 10.3389/fendo.2022.876231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cho BA, Iyengar NM, Zhou XK, Mendieta H, Winston L, Falcone DJ, et al. Increased trunk fat is associated with altered gene expression in breast tissue of normal weight women. NPJ Breast Cancer. (2022) 8:15. doi: 10.1038/s41523-021-00369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. (2006) 393:7–20. doi: 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: Role and clinical implication. Front Endocrinol (Lausanne). (2021) 12:585887. doi: 10.3389/fendo.2021.585887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ahima RS, Flier JS. Leptin. Annu Rev Physiol. (2000) 62:413–37. doi: 10.1146/annurev.physiol.62.1.413 [DOI] [PubMed] [Google Scholar]
- 58. Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, et al. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer. (2009) 8:74. doi: 10.1186/1476-4598-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana AW, Changou CA, et al. Leptin OB3 peptide suppresses leptin-induced signaling and progression in ovarian cancer cells. J BioMed Sci. (2017) 24:51. doi: 10.1186/s12929-017-0356-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schüler-Toprak S, Weber F, Skrzypczak M, Ortmann O, Treeck O. Expression of estrogen-related receptors in ovarian cancer and impact on survival. J Cancer Res Clin Oncol. (2021) 147:2555–67. doi: 10.1007/s00432-021-03673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cunat S, Hoffmann P, Pujol P. Estrogens and epithelial ovarian cancer. Gynecol Oncol. (2004) 94:25–32. doi: 10.1016/j.ygyno.2004.03.026 [DOI] [PubMed] [Google Scholar]
- 62. Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. (2006) 12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861 [DOI] [PubMed] [Google Scholar]
- 63. Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer. (2004) 4:97–105. doi: 10.1038/nrc1275 [DOI] [PubMed] [Google Scholar]
- 64. Tessitore L, Vizio B, Pesola D, Cecchini F, Mussa A, Argiles JM, et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int J Oncol. (2004) 24:1529–35. [PubMed] [Google Scholar]
- 65. Wang M, Xie Z, Xu J, Feng Z. TWEAK/Fn14 axis in respiratory diseases. Clin Chim Acta. (2020) 509:139–48. doi: 10.1016/j.cca.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 66. Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in Malignancy and ischemia. Yale J Biol Med. (2007) 80:51–60. [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu LM, Zeng D, Lei XC, Huang J, Deng YF, Ji YB, et al. KLF2 regulates neutrophil migration by modulating CXCR1 and CXCR2 in asthma. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165920. doi: 10.1016/j.bbadis.2020.165920 [DOI] [PubMed] [Google Scholar]
- 68. He Z, Yue C, Chen X, Li X, Zhang L, Tan S, et al. Integrative analysis identified CD38 as a key node that correlates highly with immunophenotype, chemoradiotherapy resistance, and prognosis of head and neck cancer. J Cancer. (2023) 14:72–87. doi: 10.7150/jca.59730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. (2011) 300:E877–85. doi: 10.1152/ajpendo.00626.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang X, Du ZW, Xu TM, Wang XJ, Li W, Gao JL, et al. HIF-1α Is a rational target for future ovarian cancer therapies. Front Oncol. (2021) 11:785111. doi: 10.3389/fonc.2021.785111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, et al. A collagen-remodeling gene signature regulated by TGF-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res. (2014) 20:711–23. doi: 10.1158/1078-0432.CCR-13-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Natarajan S, Foreman KM, Soriano MI, Rossen NS, Shehade H, Fregoso DR, et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res. (2019) 79:2271–84. doi: 10.1158/0008-5472.CAN-18-2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yao H, He S. Multi−faceted role of cancer−associated adipocytes in the tumor microenvironment (Review). Mol Med Rep. (2021) 24(6):886. doi: 10.3892/mmr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ishay-Ronen D, Diepenbruck M, Kalathur RKR, Sugiyama N, Tiede S, Ivanek R, et al. Gain fat-lose metastasis: converting invasive breast cancer cells into adipocytes inhibits cancer metastasis. Cancer Cell. (2019) 35:17–32.e6. doi: 10.1016/j.ccell.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 75. Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. (2005) 65:10862–71. doi: 10.1158/0008-5472.CAN-05-1231 [DOI] [PubMed] [Google Scholar]
- 76. Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. (2011) 71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323 [DOI] [PubMed] [Google Scholar]
- 77. Naylor C, Petri WA, Jr. Leptin regulation of immune responses. Trends Mol Med. (2016) 22:88–98. doi: 10.1016/j.molmed.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 78. Lahmann PH, Cust AE, Friedenreich CM, Schulz M, Lukanova A, Kaaks R, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. (2010) 126:2404–15. doi: 10.1002/ijc.24952 [DOI] [PubMed] [Google Scholar]
- 79. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. (2012) 3:13. doi: 10.1186/2042-6410-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agalliu I, Lin WJ, Zhang JS, Jacobson JS, Rohan TE, Adusei B, et al. Overall and central obesity and prostate cancer risk in African men. Cancer Causes Control. (2022) 33:223–39. doi: 10.1007/s10552-021-01515-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yu D, Zheng W, Johansson M, Lan Q, Park Y, White E, et al. Overall and central obesity and risk of lung cancer: A pooled analysis. J Natl Cancer Inst. (2018) 110:831–42. doi: 10.1093/jnci/djx286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev. (2016) 17:1167–77. doi: 10.1111/obr.12443 [DOI] [PubMed] [Google Scholar]
- 83. Genkinger JM, Kitahara CM, Bernstein L, Berrington de Gonzalez A, Brotzman M, Elena JW, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol. (2015) 26:2257–66. doi: 10.1093/annonc/mdv355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.