Abstract
Background
Sarcoidosis is a systemic disease that can affect multiple organs. While pulmonary sarcoidosis is most commonly observed, renal sarcoidosis occurs less frequently. We herein report a case of sarcoidosis with an exceptionally rare distribution including renal lesions.
Case presentation
A 51-year-old Japanese female was referred because of bilateral parotid swelling and renal dysfunction. Computed tomography scan showed the swelling of bilateral kidneys, parotid glands, and uterus. Ga scintigraphy also showed remarkable accumulation in these organs. Renal biopsy and cytological evaluations of parotid gland and uterus were performed and she was diagnosed as sarcoidosis of these organs. Treatment was initiated with prednisolone 40 mg/day and then renal dysfunction subsequently improved. In addition, the swelling of parotid glands and uterus improved and Ga accumulation in each organ had disappeared.
Conclusion
This is a first case of renal sarcoidosis complicated by parotid glands and uterus lesions. Pathological findings and the reactivity observed in Ga scintigraphy indicated the presence of lesions in these organs.
Keywords: Acute kidney injury, Parotid glands, Sarcoidosis, Uterus
Background
Sarcoidosis is a multisystem disorder of unknown etiology characterized by the accumulation of T lymphocytes, mononuclear phagocytes and noncaseating granulomas in affected organs [1, 2]. In sarcoidosis, pulmonary involvement is the most prevalent, whereas the frequency of renal sarcoidosis remains unclear. In the previous study, 95% of 736 cases diagnosed with sarcoidosis showed pulmonary involvement, although only 0.7% cases showed renal sarcoidosis [3]. In addition, the prevalence of parotid gland sarcoidosis was 3.9% [3], and only a few cases have been reported in uterine sarcoidosis [4–6]. Herein, we report a first case of sarcoidosis in kidneys, parotid glands and uterus.
Case presentation
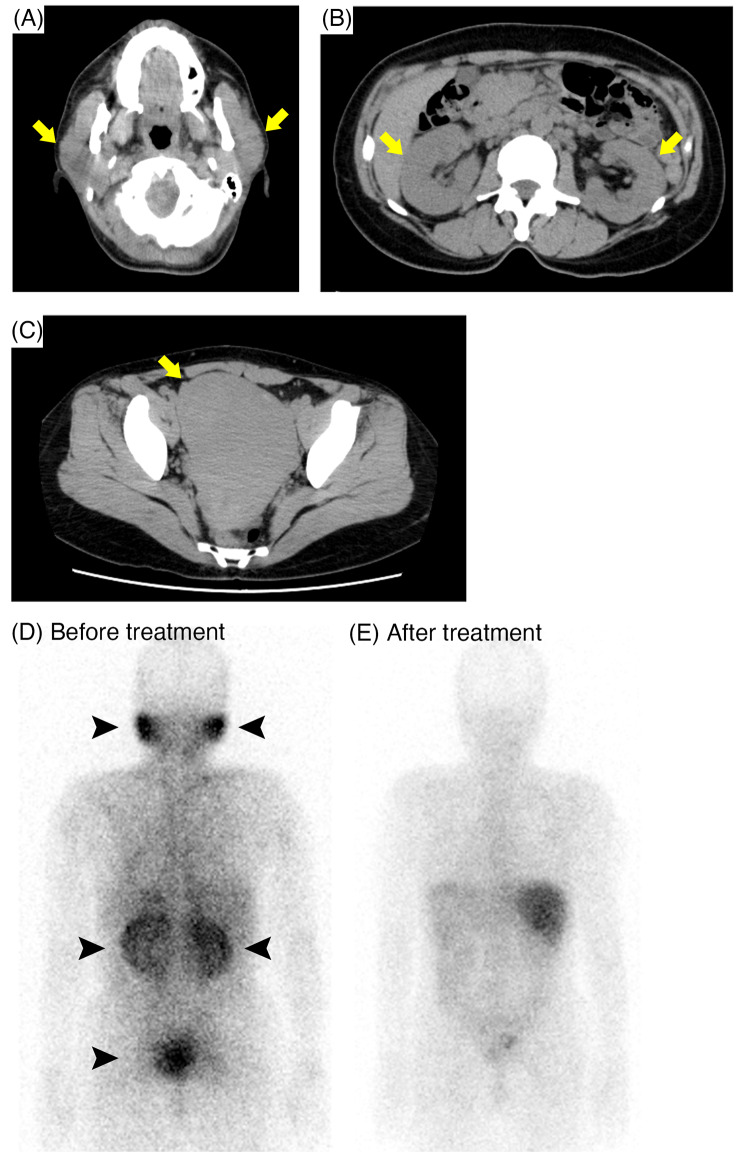
A 51-year-old Japanese female was referred to our hospital with bilateral parotid swelling and renal dysfunction. There was no previous medical history of renal dysfunction. Physical examination revealed bilateral parotid swelling and lower legs edema. She also presented hypermenorrhea. Laboratory findings showed elevated levels of serum creatinine 3.1 mg/dL, serum corrected calcium 10.6 mg/dL, angiotensin converting enzyme (ACE) 44.9 U/L (reference range 8.3–21.4 U/L), and lysozyme 75 µg/mL (reference range 5.0–10.2 µg/mL). The urinary β2-microglobulin (β2-MG) level was also elevated (Table 1). Computed tomography (CT) scan revealed swelling of bilateral kidneys, parotid glands, and uterus (Fig. 1A, B, C, respectively). Additionally, Ga scintigraphy showed remarkable accumulation in these organs (Fig. 1D). Ocular screening was performed but uveitis was not indicated.
Table 1.
Laboratory Data on admission
| Complete blood count | Serology | ||||
| White blood cell | 4,900 | /µL | IgG | 1,649 | mg/dL |
| Red blood cell | 403 × 104 | /µL | IgA | 242 | mg/dL |
| Hemoglobin | 11.4 | g/dL | IgM | 170 | mg/dL |
| Platelet | 28.9 × 104 | /µL | IgG4 | 18.7 | mg/dL |
| Blood chemistry | ANA | <×40 | |||
| Total protein | 7.7 | g/dL | MPO-ANCA | < 1.0 | U/mL |
| Albumin | 4.2 | g/dL | PR3-ANCA | < 1.0 | U/mL |
| ALP | 66 | U/L | ACE | 44.9 | U/L |
| AST | 22 | U/L | Lysozyme | 75 | µg/mL |
| ALT | 16 | U/L | 25(OH)2Vit.D3 | 4.2 | ng/mL |
| LDH | 229 | U/L | 1–25(OH)2Vit.D3 | 135 | pg/mL |
| γ-GTP | 15 | U/L | intact-PTH | 9 | pg/mL |
| BUN | 33 | mg/dL | PTHrP | < 1.0 | pmol/L |
| Creatinine | 3.1 | mg/dL | sIL-2R | 7,650 | U/mL |
| eGFR | 13 | T-spot | (-) | ||
| Uric acid | 5.5 | mg/dL | |||
| Sodium | 137 | mEq/L | Urine analysis | ||
| Potassium | 4.1 | mEq/L | pH | 6.5 | |
| Chloride | 102 | mEq/L | Protein | + | |
| Corrected calcium | 10.6 | mg/dL | Occult blood | ± | |
| Phosphorus | 3.8 | mg/dL | Glucose | ± | |
| CRP | 0.34 | mg/dL | β2-MG | 101,202 | µg/L |
| Amylase | 167 | U/L | NAG | 12.8 | U/L |
Abbreviations: β2-MG, β2-microglobulin; γ-GTP, γ-glutamyl transpeptidase; ACE, angiotensin converting enzyme; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, anti-nuclear antibody; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; MPO-ANCA, Myeloperoxidase-antineutrophil cytoplasmic antibody; NAG, N-cetylglucosaminidase; PR3-ANCA, Proteinase 3-antineutrophil cytoplasmic antibody; PTH, parathyroid hormone; PTHrP, parathyroid hormone related peptide; sIL-2R, soluble interleukin-2 receptor
Fig. 1.
Findings of Computed tomography (CT) scan and Ga scintigraphy. (A, B, C) CT scan showed swelling of parotid glands (A), bilateral kidneys (B), and uterus (C, arrows). (D) Ga scintigraphy before treatment showed remarkable accumulation in these organs (arrowheads). (E) After the treatment, the accumulation has disappeared
After admission, the needle aspiration cytology of parotid gland, endometrial cytology and renal biopsy were performed, respectively. Both parotid gland and endometrial cytology showed the presence of epithelioid cells indicating sarcoidosis (Fig. 2A, B, respectively). In the renal biopsy, light microscopy showed tubulointerstitial nephritis with diffuse non-caseating epithelioid cell granulomas and presence of diffuse multinucleated giant cells (Fig. 2C and D). A significant infiltration of inflammatory cells was observed in the interstitial area and around the renal tubules, with positive staining for CD68 (Fig. 2E), a specific marker of macrophages. Due to the presence of hypercalcemia and increased lysozyme level, we also performed 1-α hydroxylase (CYP27B1) and lysozyme staining. Both staining showed remarkable positivity in multinucleated giant cells and infiltrating cells (Fig. 2F, G, respectively). No notable findings were observed in the glomeruli, and all immune-fluorescence staining were negative.
Fig. 2.
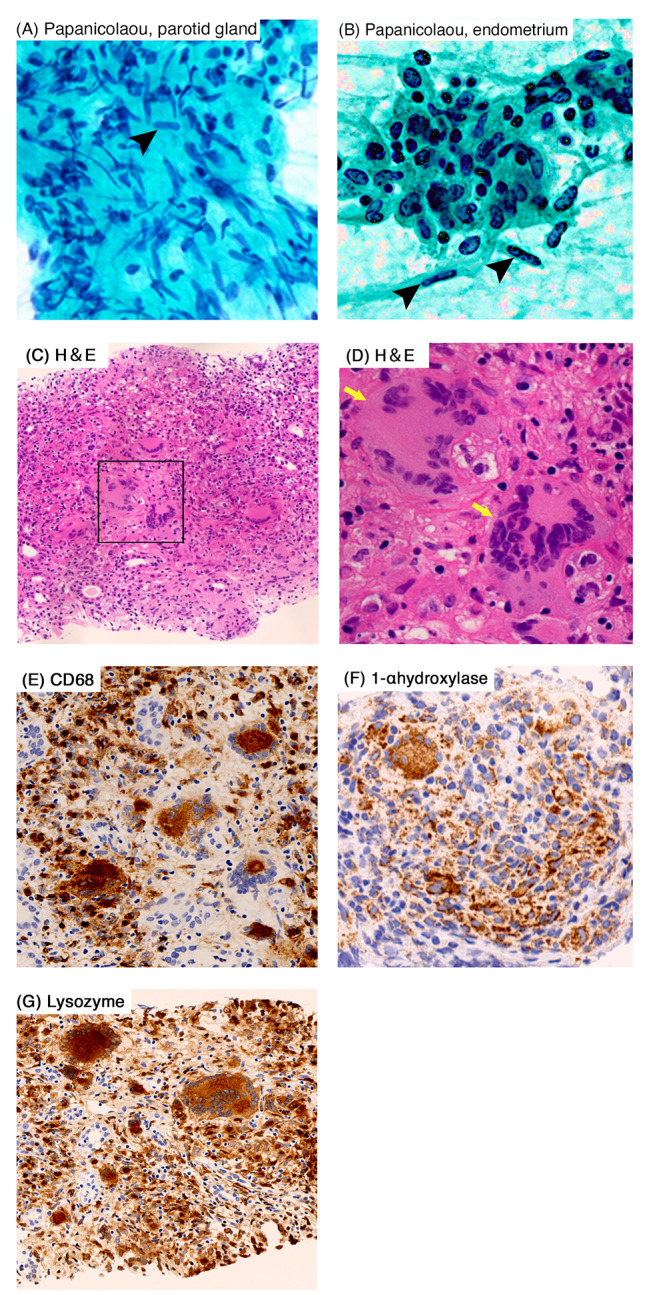
Cytologic findings of parotid gland and endometrium, and light microscopic findings of the renal biopsy. (A and B) Papanicolaou staining showed the presence of epithelioid cells in parotid gland (A) and endometrium (B). (C and D) Hematoxylin and eosin staining (H&E) showed the tubulointerstitial nephritis with diffuse non-caseating epithelioid cell granulomas and diffuse multinucleated giant cells (arrows). (E) Inflammatory cells and multinucleated giant cells showed positive in CD68 staining. (F and G) 1-α hydroxylase (F) and lysozyme staining (G) were positive in inflammatory cells and multinucleated giant cells. Original magnification, ×400 (A, B, D), x100 (C), ×200 (E, F, G), respectively
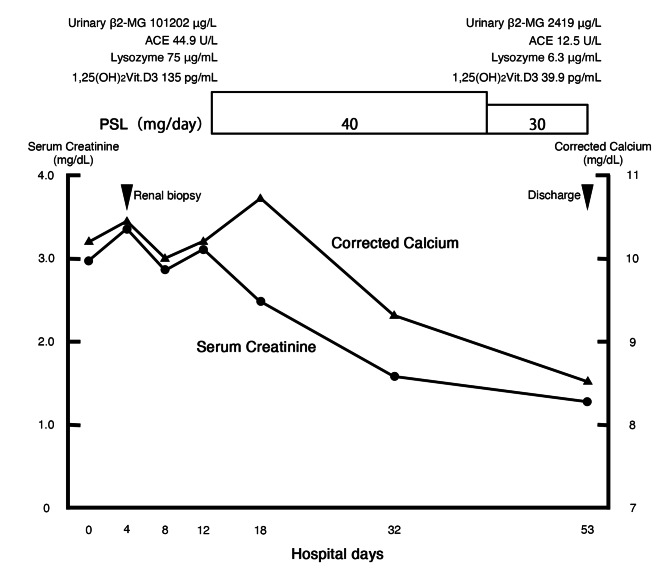
Based on the pathological findings, we diagnosed as renal sarcoidosis complicated by parotid glands and uterine lesions. As a treatment, prednisolone was initiated at a dose of 40 mg/day (0.8 mg/kg/day) since the 11th day, resulting in rapid improvement of renal function and hypercalcemia (Fig. 3). Furthermore, levels of urinary β2-MG, serum ACE, serum lysozyme, and serum 1,25(OH)2 vitamin D3 decreased. After the treatment, second Ga scintigraphy was performed, and the accumulation in the kidneys, parotid glands and uterus was disappeared (Fig. 1E). On the 53th day, the patient discharged.
Fig. 3.
Clinical course after admission. ACE, angiotensin converting enzyme; β2-MG, β2-microglobulin; PSL, prednisolone
After the discharge, the patient’s condition is stable without the apparent recurrence and serum ACE levels are also maintained within the normal range from 7.2 to 18.4 U/L for at least 30 months.
Discussion
Sarcoidosis is a multisystem disorder of unknown etiology characterized by the infiltration of T lymphocytes and mononuclear phagocytes, and the formation of noncaseating granulomas in involved tissues [1, 2]. Sarcoidosis can affect a variety of organs, although pulmonary sarcoidosis is the most common organ involved. Baughman et al. [3]. reported that 95% cases exhibited pulmonary involvement, while only 0.9% cases exhibited renal involvement among 736 patients diagnosed as sarcoidosis. Parotid gland sarcoidosis has been reported to occur in approximately 5% of patients with sarcoidosis [3, 7]. On the other hand, there were only a few case reports in uterine sarcoidosis [4–6]. Therefore, the present case exhibited a very rare distribution of lesions as sarcoidosis. To the best of our knowledge, this is the first report of sarcoidosis in kidneys, parotid glands and uterus, although its relevance developed simultaneously in these organs is unknown.
In addition to its rarity of the distribution, several interesting findings were obtained in this case. First, inflammatory cells infiltrating into the renal tissue were positive for 1-α hydroxylase (CYP27B1) staining (Fig. 2F). Since these inflammatory cells were positive for CD68 (Fig. 2E), a specific marker for macrophages, these results indicated that 1-α hydroxylase might be originated from infiltrated macrophages into the renal tissue. In sarcoidosis, hypercalcemia has been reported to occur up to 10% cases and is caused by 1-α hydroxylase production from infiltrating macrophages into the affected organ resulting in the excessive formation of 1,25(OH)2 vitamin D3 [8]. This mechanism of hypercalcemia is primarily investigated in pulmonary sarcoidosis, and glucocorticoid therapy inhibits 1-α hydroxylase production in macrophages, leading to improvement of hypercalcemia [9, 10]. On the other hand, there are few reports investigating its mechanism of hypercalcemia in the other affected organs [11], while 1-α hydroxylase strongly stained infiltrating macrophages into the renal tissue and hypercalcemia was improved after glucocorticoid therapy in our case. Therefore, our case is highly suggestive to indicate that the same mechanism of hypercalcemia exists in renal sarcoidosis as that in pulmonary sarcoidosis. Second, lysozyme staining was positive in the renal tissue of this case. Sanada et al. [12] previously reported the significance of lysozyme staining in renal sarcoidosis. They actually performed lysozyme staining in various cases of tubulointerstitial nephritis from different etiologies and only cases of sarcoidosis consistently showed positive for lysozyme. Since lysozyme was also produced from infiltrating macrophages into the affected tissue of sarcoidosis same as 1-α hydroxylase [13, 14], the positivity of lysozyme staining could potentially serve as a novel supportive finding for the diagnosis of sarcoidosis. On the other hand, further studies should be needed to identify its diagnostic value.
In conclusions, we report a first case of renal sarcoidosis complicated by parotid glands and uterine lesions. The distribution of lesions in our case was exceptionally rare for sarcoidosis. Furthermore, both 1-α hydroxylase and lysozyme staining were positive for infiltrating cells, possibly macrophages in renal granulomatous lesions. 1-α hydroxylase in these cells could lead to hypercalcemia and positivity of lysozyme might contribute to a supporting finding for the diagnosis of sarcoidosis in the future.
Acknowledgements
We are grateful to Akira Shimizu (Department of Analytic Human Pathology, Nippon Medical School, Tokyo, Japan) for advice on pathological analysis.
Non-standard abbreviations
- β2-MG
β2-microglobulin
- γ-GTP
γ-glutamyl transpeptidase
- ACE
angiotensin converting enzyme
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- ANA
anti-nuclear antibody
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- eGFR
estimated glomerular filtration rate
- LDH
lactate dehydrogenase
- MPO-ANCA
Myeloperoxidase-antineutrophil cytoplasmic antibody
- NAG
N-cetylglucosaminidase
- PR3-ANCA
Proteinase 3-antineutrophil cytoplasmic antibody
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone related peptide
- sIL-2R
soluble interleukin-2 receptor
Author contributions
SK, MK, KO, TM, RF, and HF diagnosed and treated the patient. SK and MK drafted HY and HF critically revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Data availability
The datasets used and/or analyzed are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patients and their parents for the publication of this case report. A copy of the written consent is available for review by the editor of this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuhei Kitamoto and Mai Kaneko contributed equally to this work.
References
- 1.Baughman RP, Lower EE, du Bois RM. Sarcoidosis Lancet. 2003;361(9363):1111–8. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PD, Hunninghake GW. Current concepts of the pathogenesis of sarcoidosis. Am Rev Respir Dis. 1987;135(3):747–60. doi: 10.1164/arrd.1987.135.3.747. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Teirstein AS, Judson MA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 4.Menzin AW, You TT, Deger RB, Brooks JS, King SA. Sarcoidosis in a uterine leiomyoma. Int J Gynaecol Obstet. 1995;48(1):79–84. doi: 10.1016/0020-7292(94)02240-2. [DOI] [PubMed] [Google Scholar]
- 5.Zurkova M, Turkova M, Tichy T, et al. Sarcoidosis of female reproductive organs in a postmenopausal woman: a case report and review of the literature: is there a potential for hormone therapy? Menopause. 2015;22(5):549–53. doi: 10.1097/GME.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 6.Neumann G, Rasmussen KL, Olesen H. Premenopausal metrorrhagia as a symptom of sarcoidosis. Eur J Obstet Gynecol Reprod Biol. 2002;104(2):171–3. doi: 10.1016/S0301-2115(02)00112-4. [DOI] [PubMed] [Google Scholar]
- 7.James DG, Sharma OP. Parotid gland sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17(1):27–32. [PubMed] [Google Scholar]
- 8.Tebben PJ, Kumar R, Vitamin D-M, Hypercalcemia Mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37(5):521–47. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JS, Singer FR, Gacad MA, et al. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 1985;60(5):960–6. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- 10.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72(5):1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toriu N, Sumida K, Oguro M, et al. Increase of 1,25 dihydroxyvitamin D in sarcoidosis patients with renal dysfunction. Clin Exp Nephrol. 2019;23(10):1202–10. doi: 10.1007/s10157-019-01760-3. [DOI] [PubMed] [Google Scholar]
- 12.Sanada S, Yoda S, Sato T. Pathological value of lysozyme staining for renal sarcoidosis. Nephrol Dial Transpl. 2020;35(9):1638–41. doi: 10.1093/ndt/gfaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prokop S, Stenzel W. M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis. Am J Pathol. 2011;178(3):1279–86. doi: 10.1016/j.ajpath.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergantini L, Bargagli E. Prognostic biomarkers of sarcoidosis: a comparative study of serum chitotriosidase, ACE, Lysozyme, and KL-6. Dis Markers. 2019;2019:8565423. doi: 10.1155/2019/8565423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed are available from the corresponding author upon reasonable request.