Abstract
Objective:
To evaluate adverse events (AEs) of combination lenvatinib plus pembrolizumab for the treatment of recurrent endometrial cancer (EC) and to assess outcomes by lenvatinib starting dose.
Methods:
We retrospectively reviewed patients with recurrent EC treated with lenvatinib plus pembrolizumab at our institution between 10/1/2019–11/30/2021. Starting dose of lenvatinib was defined as standard (20mg) or reduced (10mg/14mg). AEs were manually extracted through chart review and graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. PFS, overall survival (OS), and duration of response (DOR) were analyzed.
Results:
Forty-three patients were identified; median age was 67 years (range, 54–85). The most common histologies were serous (35%), endometrioid (23%), and carcinosarcoma (21%). Starting lenvatinib doses were 10mg (n=10), 14mg (n=10), and 20mg (n=23). Median number of cycles received was 8 (range, 1–42). Twenty-four patients (56%) required ≥1 lenvatinib dose reduction; 3 (7%) discontinued lenvatinib, and 1 (2%) discontinued pembrolizumab for intolerance or AE. Thirty-six patients (84%) experienced grade ≥3 AEs; hypertension, weight loss, anemia, fatigue, and thrombocytopenia were most common. The standard dose group experienced significantly shorter observed PFS vs the reduced dose group (P=.02). There was no difference in DOR (P=.09) or OS (P=.27) between the groups.
Conclusion:
In clinical practice, AEs associated with combination lenvatinib plus pembrolizumab were common and comparable to Study 309/KEYNOTE-775 findings. AEs were similar regardless of starting lenvatinib dose. Further dose optimization studies of lenvatinib plus pembrolizumab may be indicated in recurrent EC. Clinical trial data remain the gold standard to guide starting lenvatinib dosing.
Keywords: adverse events, outcomes, lenvatinib, pembrolizumab, endometrial cancer, immunotherapy
Introduction
Endometrial cancer is the most common gynecologic malignancy in high-income countries, and both its incidence and disease-related mortality are rising in the United States (1, 2). Since the mid-2000s, the incidence of endometrial cancer in women over the age of 50 has increased approximately 1% per year, which is likely but not exclusively a consequence of the obesity epidemic (3). The management of advanced or recurrent endometrial cancer generally consists of systemic therapy (4); however, outcomes for advanced endometrial cancer have remained poor, with a 5-year overall survival (OS) of approximately 9 months in patients with distant or advanced disease (3). Platinum-based chemotherapy is the standard first-line treatment for advanced or recurrent endometrial cancer, and options for subsequent treatment have historically been limited.
In 2019, combination lenvatinib plus pembrolizumab (len/pem) was approved by the US Food and Drug Administration (FDA) for the treatment of patients with mismatch repair proficient (pMMR)/microsatellite stable (MSS) endometrial cancer following frontline treatment with paclitaxel and carboplatin (TC); this approval was based on findings of the Study 111/KEYNOTE-146 and Study 309/KEYNOTE-775 trials among patients with advanced endometrial cancer (5, 6). In KEYNOTE-146, a phase II trial, len/pem displayed an objective response rate of 39.8% (95% CI: 30.5%-49.7%) among 108 patients with previously treated endometrial cancer. Regardless of tumor microsatellite instability (MSI) status, the median duration of response (DOR) was 22.9 months (95% CI: 10.2 months-NE), median progression-free survival (PFS) was 7.4 months (95% CI: 5.2–8.7 months), and median OS was 17.7 months (95% CI: 15.5–25.8 months) (7, 8). Subsequently, the confirmatory phase III Study 309/KEYNOTE-775 trial demonstrated significantly longer PFS and OS among patients with endometrial cancer who were treated with len/pem compared to standard of care chemotherapy with physician’s choice weekly paclitaxel or doxorubicin. In the overall Study 309/KEYNOTE-775 population, PFS was 7.2 vs 3.8 months, respectively (HR: 0.56, 95% CI: 0.47–0.66, P<.001), and OS was 18.3 vs 11.4 months, respectively (HR: 0.62, 95% CI: 0.51–0.75, P<.001). In the Study 309/KEYNOTE-775 pMMR cohort, PFS was 6.8 vs 3.8 months, respectively (HR: 0.60, 95% CI: 0.50–0.72, P<.001), and OS was 17.4 vs 12.0 months, respectively (HR: 0.68, 95% CI: 0.56–0.84, P<.001) (5). Recently, clinical trial data from RUBY and NRG-GY018 have established a clinical benefit when adding pembrolizumab or dostarlimab to standard chemotherapy agents in the treatment of advanced or recurrent endometrial cancer (9, 10).
Although len/pem has demonstrated improvements in survival for patients with recurrent endometrial cancer, toxicities are common. While generally manageable with supportive care, these toxicities may be a barrier to effective treatment in clinical practice outside of the controlled setting of a clinical trial. In KEYNOTE-775, grade ≥3 AEs occurred in 88.9% of patients who received len/pem compared to 72.7% of patients who received chemotherapy (5). Although AEs of len/pem were consistent with other agents in these classes, 66.5% of patients required a lenvatinib dose reduction, 69.2% required a lenvatinib and/or pembrolizumab dose interruption, and 33% discontinued len/pem due to toxicity (5).
Two prior studies have evaluated outcomes for patients with endometrial cancer who were treated with len/pem. In a 2021 retrospective chart review, How et al. evaluated the toxicity and efficacy of len/pem at standard (20 mg) vs reduced (<20 mg) starting lenvatinib dosing among 70 patients with recurrent endometrial cancer treated at MD Anderson Cancer Center (MDACC) and affiliated sites. The investigators found that lenvatinib dose reductions were more frequent in the standard dose cohort (1.1 vs 0.4, P=.003); however, there were no differences in treatment-related hospitalizations, treatment discontinuation, or survival outcomes between the groups (11). Kim et al. conducted a similar single-institution retrospective study and noted a similar frequency of AEs but lower response rates compared to the KEYNOTE-775 trial (12).
Since the publication of Study 309/KEYNOTE-775 and the FDA’s approval of len/pem for the treatment of recurrent pMMR endometrial cancer, oncologists at Memorial Sloan Kettering Cancer Center (MSK) routinely have prescribed len/pem. To characterize our institution’s experience with this combination, we sought to evaluate the efficacy and AEs associated with len/pem among patients with endometrial cancer who are treated in routine clinical practice.
Methods
Patient population
This retrospective study was approved by the Institutional Review Board at MSK. All patients with endometrial cancer who initiated treatment with len/pem at our institution between October 1, 2019, and November 30, 2021, were identified. Patients who were coded in the electronic medical record (EMR) as both having a diagnosis of endometrial cancer and having been administered len/pem were included. Inclusion criteria were as follows: age ≥18 years at diagnosis, pathologically confirmed endometrial cancer reviewed by an expert gynecologic pathologist, and treatment with at least one cycle of len/pem for recurrence. Exclusion criteria included receipt of any cycles of len/pem in the setting of a clinical trial, loss to follow-up after treatment with no documentation of AEs, and no documentation of toxicity or oncologic outcomes in the EMR (Supplementary Figure S1). All patients were prescribed len/pem by a gynecologic medical oncologist, with whom they followed while on treatment with regular office visits, physical exams, and imaging studies.
Clinical data
Clinicopathological characteristics were abstracted from the EMR and included age at diagnosis, race, Eastern Cooperative Oncology Group (ECOG) Performance Status, 2009 International Federation of Gynecology and Obstetrics (FIGO) stage at diagnosis, histological and molecular characteristics, treatment cycles of len/pem, prior treatment (systemic or radiotherapy), and AEs. Len/pem starting doses, dose holds, dose reductions, and discontinuations were collected from chemotherapy logs and physician documentation in the EMR. All AEs were collected from the EMR, specifically from physician follow-up documentation, urgent care visit documentation, review of laboratory values, and review of vital sign flowsheets. AEs were graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Radiographic evaluation
Baseline radiographic imaging prior to initiation of len/pem, which was most often a computed tomography (CT) scan of the chest, abdomen, and pelvis, was reviewed by a dedicated radiologist (WM) during the study. This radiologist also reviewed all subsequent imaging studies until disease progression, discontinuation of therapy, or death. Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (11) was used by the radiologist to determine complete response (CR), stable disease (SD), partial response (PR), or progression of disease (PD) during treatment with len/pem.
Statistical analysis
Overall response rate (ORR) was calculated as the proportion of patients with an evaluable response who demonstrated either a CR or PR. Clinical benefit rate (CBR) was calculated as the proportion of patients with an evaluable response who demonstrated a CR, PR, or SD. DOR was calculated from the date of PR until the date of PD, death, or last follow-up, whichever occurred first. PFS was calculated from the date of treatment initiation with len/pem to the date of progression, death, or last follow-up, whichever occurred first. OS was calculated from the date of treatment initiation with len/pem to the date of death or last follow-up. Median PFS and OS and 1-year PFS and OS rates were calculated using the Kaplan-Meier method, and the log-rank test was used to compare cohorts. All P values were calculated as two-sided, and a P<.05 was considered statistically significant. AEs were tabulated and stratified by grade. For select specific AEs, the time to onset from initiation of treatment with len/pem was calculated, excluding any AEs with an onset >100 days from the initiation of treatment, to ensure AEs were attributed to len/pem. All analyses were performed using R version 3.6.3 (https://www.R-project.org/).
Results
Clinicopathological characteristics
A total of 71 patients with endometrial cancer and initiation of treatment with len/pem at MSK were identified. Thirteen patients were excluded after manual review of the EMR demonstrated disease that was not uterine in origin, 13 were excluded who were treated with pembrolizumab alone, and 2 were excluded who received len/pem on a clinical trial. Overall, 43 patients were included in the final analysis (Supplementary Figure S1). The median age at diagnosis was 67 years (range, 54–85 years). ECOG status was 0 in 11 patients (28%), 1 in 28 patients (70%), 2 in 1 (3%) patient, and not designated in the EMR for 3 patients. Twenty-three patients (57%) were White, 12 (30%) were Black, and 5 (12%) were Asian or “other” race. FIGO stage at initial diagnosis was I/II for 13 patients (30%) and III/IV for 30 patients (70%). For histology, 15 patients (35%) had serous, 10 (23%) had endometrioid, 9 (21%) had carcinosarcoma, and 9 (21%) had other (carcinoma of mixed histology [n=7], dedifferentiated carcinoma [n=1], poorly differentiated carcinoma with yolk sac differentiation [n=1]). Mismatch repair (MMR) protein immunohistochemistry (IHC) was available for 41 patients, of whom 40 (93%) demonstrated retained expression and 1 indeterminate. P53 IHC was available for 36 patients, of whom 25 (69%) had aberrant expression. When classified by The Cancer Genome Atlas (TCGA) molecular subtype, 29 cases (71%) were copy number-high (CN-H), 10 (24%) were copy number-low (CN-L), and 2 (5%) were unable to be assigned; molecular information was not available for 2 patients (Table 1).
Table 1.
Clinicopathologic features and treatment characteristics of the entire cohort and by low vs high starting lenvatinib dose, compared to the KEYNOTE-775 cohort.
| Characteristic | KEYNOTE-775 len/pem cohort, N= 411 | Overall cohort, N = 43 | Lenvatinib 10 mg/14 mg starting dose, n = 20 | Lenvatinib 20 mg starting dose, n = 23 | P value |
|---|---|---|---|---|---|
| Age at diagnosis, years (range) | 64 (30–82) | 67 (54–85) | 69 (61–85) | 64 (54–78) | .01 |
| Race | .74 | ||||
| White | 261 (63.5%) | 23 (57%) | 10 (53%) | 13 (62%) | |
| Black | 17 (4.1%) | 12 (30%) | 6 (32%) | 6 (29%) | |
| Asian/other | 85 (20.7%) | 5 (12%) | 3 (16%) | 2 (10%) | |
| Not available | - | 3 | 1 | 2 | |
| ECOG | .72 | ||||
| 0 | 246 (59.9%) | 11 (28%) | 4 (22%) | 7 (32%) | |
| 1 or 2 | 164 (39.9%) | 29 (72%) | 14 (78%) | 15 (68%) | |
| Missing | - | 3 | 2 | 1 | |
| Initial FIGO stage | .20 | ||||
| I/II | - | 13 (30%) | 4 (20%) | 9 (39%) | |
| III/IV | - | 30 (70%) | 16 (80%) | 14 (61%) | |
| Histology | .65 | ||||
| Serous | 103 (25.1%) | 15 (35%) | 6 (30%) | 9 (39%) | |
| Carcinosarcoma | 0 | 9 (21%) | 3 (15%) | 6 (26%) | |
| Endometrioid | 243 (59.1%) | 10 (23%) | 6 (30%) | 4 (17%) | |
| Other | 65 (15.8%) | 9 (21%) | 5 (25%) | 4 (17%) | |
| PDL-1 status | |||||
| Positive | - | 1 (20%) | 1 (33%) | 0 (0%) | |
| Negative | - | 4 (80%) | 2 (67%) | 2 (100%) | |
| Missing | - | 38 | 17 | 21 | |
| MMR status | |||||
| Retained | 346 (84.2%) | 40 (93%) | 20 (100%) | 20 (87%) | |
| Indeterminate | - | 1 (2%) | 0 (0%) | 1 (4%) | |
| Not available | - | 2 (5%) | 0 (0%) | 2 (9%) | |
| p53 status | .72 | ||||
| Wild type | - | 11 (31%) | 6 (35%) | 5 (26%) | |
| Aberrant | - | 25 (69%) | 11 (65%) | 14 (74%) | |
| Not available | - | 7 | 3 | 4 | |
| Molecular subtype | |||||
| CN-H | - | 29 (71%) | 13 (65%) | 16 (76%) | |
| CN-L | - | 10 (24%) | 6 (30%) | 4 (19%) | |
| Cannot assign | - | 2 (5%) | 1 (5%) | 1 (5%) | |
| Not available | - | 2 | 0 | 2 | |
| Total treatment cycles, median (range) | 10 | 8 (1–42) | 10 (2–42) | 6 (1–21) | .07 |
| Prior lines systemic therapy, median (range) | 2 (1–10) | 2 (1–10) | 2 (1–8) | .18 | |
| Prior lines platinum, median (range) | 1 (1–3) | 2 (1–3) | 1 (1–2) | .045 | |
| Prior lines taxane, median (range) | 1 (0–3) | 2 (0–3) | 1 (0–2) | .27 | |
| Prior hormonal therapy | >.99 | ||||
| Yes | 10 (23%) | 5 (25%) | 5 (22%) | ||
| No | 33 (77%) | 15 (75%) | 18 (78%) | ||
| Prior targeted therapy | .50 | ||||
| Yes | 11 (26%) | 4 (20%) | 7 (30%) | ||
| No | 32 (74%) | 16 (80%) | 16 (70%) | ||
| Prior anti-PDL-1 therapy | |||||
| Yes | 2 (5%) | 1 (5%) | 1 (4%) | ||
| No | 41 (95%) | 19 (95%) | 22 (96%) | ||
| Lenvatinib dose hold | .55 | ||||
| Yes | 17 (40%) | 9 (45%) | 8 (35%) | ||
| No | 26 (60%) | 11 (55%) | 15 (65%) | ||
| Lenvatinib dose reduction | >.99 | ||||
| Yes | 288 (70%) | 24 (56%) | 11 (55%) | 13 (57%) | |
| No | 123 (30%) | 19 (44%) | 9 (45%) | 10 (43%) | |
| Lenvatinib discontinued | 3 (7%) | 0 (0%) | 3 (13%) | ||
| Pembrolizumab dose hold | .74 | ||||
| Yes | 12 (28%) | 5 (25%) | 7 (30%) | ||
| No | 31 (72%) | 15 (75%) | 16 (70%) | ||
| Pembrolizumab discontinued | 1 (2%) | 0 (0%) | 1 (4%) | ||
| Prior curative surgery | |||||
| Yes | 39 (91%) | 18 (90%) | 21 (91%) | ||
| No | 4 (9%) | 2 (10%) | 2 (9%) | ||
| Prior radiotherapy | .21 | ||||
| Yes | 174 (42.3%) | 27 (63%) | 15 (75%) | 12 (52%) | |
| No | 237 (57.7%) | 16 (37%) | 5 (25%) | 11 (48%) |
Histology “other” category encompasses mixed, poorly differentiated, and dedifferentiated histologies.
CN-H, copy number high; CN-L, copy number low; ECOG, Eastern Cooperative Oncology Group; MMR, mismatch repair; PDL-1, programmed death ligand-1; FIGO= International Federation of Gynecology and Obstetrics
Treatment characteristics
Patients received a median of 2 prior lines of systemic cytotoxic chemotherapy (range, 1–10 lines), including a median of 1 prior line of platinum-based therapy (range, 1–3 lines). Ten patients (23%) received prior hormonal therapy, 11 (26%) received prior targeted therapy, and 2 (5%) received prior anti-programmed death-1 (PD-1) therapy (durvalumab [n=1], nivolumab [n=1]). Thirty-nine patients (91%) underwent prior surgery and 27 (63%) received prior radiotherapy. Len/pem was second-line treatment in 13 patients (30%), third-line treatment in 15 (35%), and fourth-line treatment and beyond in 15 (35%). Ten patients (23%) received a starting lenvatinib dose of 10 mg, 10 patients (23%) of 14 mg, and 23 patients (53%) of 20 mg. Among all patients, the median number of len/pem treatment cycles was 8 (range, 1–42 cycles) (Table 1).
Adverse events
AEs experienced by patients in the len/pem cohort are detailed in Table 2. The most common AEs were hypertension (n=41, 95%), weight loss (n=35, 81%), anemia (n=29, 67%), fatigue (n=28, 65%), and thrombocytopenia (n =24, 56%). The most common grade ≥3 AEs were hypertension (n=21, 49%), anemia (n=11, 26%), fatigue (n=6, 14%), thrombocytopenia (n=5, 12%), and creatinine elevation (n=5, 12%) (Table 2). There were no grade 5 AEs. The time of onset for select common AEs (fatigue, hypertension, anorexia, nausea, thrombocytopenia, anemia, weight loss, hypothyroidism) were comparable regardless of starting lenvatinib dose; these AEs presented 2 to 86 days after treatment initiation (Supplementary Table S1). Seventeen patients (40%) experienced ≥1 lenvatinib dose hold. Lenvatinib dose was reduced and discontinued for 24 patients (56%) and 3 patients (7%), respectively. There was no difference in rates of lenvatinib discontinuation between the standard dose (3 of 23, 13%) and reduced dose (0, 0%) groups (P=.24). Twelve patients (28%) experienced ≥1 pembrolizumab dose hold, and pembrolizumab was discontinued for 1 patient (2%) after development of an immune-related kidney injury (Table 1).
Table 2.
Adverse events for the entire cohort (N=43) compared to the KEYNOTE-775 cohort.
| Toxicity | Grade <3, N (%) | KEYNOTE-775, Grade ≥3, N (%) | Grade ≥3, N (%) | KEYNOTE-775, All grades, N (%) | All grades, N (%) |
|---|---|---|---|---|---|
|
| |||||
| Any adverse event | 6 (14) | 316 (77.8) | 36 (84) | 395 (97.3) | 42 (98) |
| Hypertension | 20 (47) | 154 (37.9) | 21 (49) | 260 (64.0) | 41 (95) |
| Weight loss | 33 (77) | 42 (10.3) | 2 (5) | 138 (34.0) | 35 (81) |
| Anemia | 18 (42) | 25 (6.2) | 11 (26) | 106 (26.1) | 29 (67) |
| Fatigue | 22 (51) | 21 (5.2) | 6 (14) | 134 (33.0) | 28 (65) |
| Thrombocytopenia | 19 (44) | 7 (1.7) | 5 (12) | 43 (10.6) | 24 (56) |
| Anorexia | 22 (51) | 32 (7.9) | 0 | 182 (44.8) | 22 (51) |
| Neutropenia | 18 (42) | 7 (1.7) | 1 (2) | 30 (7.4) | 19 (44) |
| Hypothyroidism | 17 (40) | 5 (1.2) | 0 | 233 (57.4) | 17 (40) |
| Nausea | 15 (35) | 14 (3.4) | 1 (2) | 201 (49.5) | 16 (37) |
| Creatinine elevation | 9 (21) | - | 5 (12) | - | 14 (33) |
| Diarrhea | 9 (21) | 31 (7.6) | 3 (7) | 220 (54.2) | 12 (28) |
| Hypomagnesemia | 12 (28) | - | 0 | - | 12 (28) |
| Oral mucositis | 7 (16) | 6 (1.5) | 3 (7) | 45 (11.1) | 10 (23) |
| Hyponatremia | 7 (16) | - | 1 (2) | - | 8 (19) |
| Constipation | 7 (16) | 3 (0.7) | 0 | 105 (25.9) | 7 (16) |
| Arthralgia | 4 (9) | 7 (1.7) | 2 (5) | 124 (30.5) | 6 (14) |
| Hyperkalemia | 6 (14) | - | 0 | - | 6 (14) |
| Hypokalemia | 1 (2) | - | 4 (9) | - | 5 (12) |
| Colitis | 3 (7) | - | 2 (5) | - | 5 (12) |
| PPE | 4 (9) | 11 (2.7) | 1 (2) | 84 (20.7) | 5 (12) |
| Hypernatremia | 5 (12) | - | 0 | - | 5 (12) |
| Vomiting | 4 (9) | 11 (2.7) | 0 | 149 (36.7) | 4 (9) |
| Dry skin | 4 (9) | - | 0 | - | 4 (9) |
| Rash | 2 (5) | 2 (0.5) | 1 (2) | 47 (11.6) | 3 (7) |
| Myalgia | 2 (5) | 3 (0.7) | 1 (2) | 54 (13.3) | 3 (7) |
| Dry mouth | 3 (7) | - | 0 | - | 3 (7) |
| Epistaxis | 3 (7) | - | 0 | - | 3 (7) |
| Weakness | 3 (7) | 17 (4.2) | 0 | 75 (18.5) | 3 (7) |
| Liver function test elevation | 3 (7) | 13 (3.2) | 0 | 63 (15.5) | 3 (7) |
| Immune hepatitis | 1 (2) | - | 1 (2) | - | 2 (5) |
| Thromboembolic event | 1 (2) | - | 1 (2) | - | 2 (5) |
| Cough | 1 (2) | - | 1 (2) | - | 2 (5) |
| Pain in extremity | 2 (5) | - | 0 | - | 2 (5) |
| Wound dehiscence | 2 (5) | - | 0 | - | 2 (5) |
| Headache | 2 (5) | 1 (0.2) | 0 | 53 (13.1) | 2 (5) |
| Hoarseness | 2 (5) | 0 | 0 | 76 (18.7) | 2 (5) |
| Hypophosphatemia | 2 (5) | - | 0 | - | 2 (5) |
| Gum infection abscess | 0 | - | 1 (2) | - | 1 (2) |
| Pneumonitis | 0 | - | 1 (2) | - | 1 (2) |
| Pruritus | 0 | - | 1 (2) | - | 1 (2) |
| Dehydration | 0 | - | 1 (2) | - | 1 (2) |
| Vasculitis | 0 | - | 1 (2) | - | 1 (2) |
| PRES | 0 | - | 1 (2) | - | 1 (2) |
| Pancreatitis | 0 | - | 1 (2) | - | 1 (2) |
| Encephalitis | 0 | - | 1 (2) | - | 1 (2) |
| Cellulitis | 0 | - | 1 (2) | - | 1 (2) |
| Intracranial hemorrhage | 0 | - | 1 (2) | - | 1 (2) |
| CKD | 0 | - | 1 (2) | - | 1 (2) |
| Appendicitis | 0 | - | 1 (2) | - | 1 (2) |
| Lower gastrointestinal hemorrhage | 1 (2) | - | 0 | - | 1 (2) |
| Abdominal pain | 1 (2) | - | 0 | - | 1 (2) |
| Neuropathy | 1 (2) | - | 0 | - | 1 (2) |
| Weight gain | 1 (2) | - | 0 | - | 1 (2) |
| Colonic perforation | 1 (2) | - | 0 | - | 1 (2) |
| Hyperglycemia | 1 (2) | - | 0 | - | 1 (2) |
| Blurred vision | 1 (2) | - | 0 | - | 1 (2) |
| Dyspepsia | 1 (2) | - | 0 | - | 1 (2) |
| Dizziness | 1 (2) | - | 0 | - | 1 (2) |
| Bronchopulmonary hemorrhage | 1 (2) | - | 0 | - | 1 (2) |
| Dyspnea | 1 (2) | - | 0 | - | 1 (2) |
| Hypermagnesemia | 1 (2) | - | 0 | - | 1 (2) |
Adverse events graded by Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. PPE=Palmar-plantar erythrodysesthesia; PRES=Posterior reversible leukoencephalopathy syndrome; CKD=chronic kidney disease.
Clinical response and survival
Of the 43 patients, 41 were evaluable for treatment response by RECIST 1.1 (13). Thirteen patients (32%) demonstrated a PR and 17 (41%) demonstrated SD (Supplementary Figure S2), with an ORR of 32% (95% CI: 18.1%-48.1%) and a CBR of 73% (95% CI: 57.1%-85.8%) (Table 3). The best overall response for each patient is shown in Figure 1. No patients demonstrated a CR. As of June 23, 2022, there were 36 progressions and 23 deaths among the entire cohort; 5 patients died without progression. The median follow-up for the 7 progression-free survivors was 22.6 months (range, 5.2–31.1 months), and the median follow-up for the 20 survivors was 18.3 months (range, 4.2–31.1 months). The median PFS was 6 months (95% CI: 4.2–8.9 months), the median OS was 18.3 months (95% CI: 9.3 months-NE), and the median DOR was 5.7 months (95% CI: 2.2 months-NE) (Table 4).
Table 3.
Response rates and adverse events by starting lenvatinib dose.
| Endpoint | KEYNOTE-775 len/pem, N= 411 | Lenvatinib 10 mg starting dose, n = 10 | Lenvatinib 14 mg starting dose, n = 10 | Lenvatinib 20 mg starting dose, n = 23 |
|---|---|---|---|---|
|
| ||||
| Overall response rate | 131 (31.9%) | 3 (30%) | 3 (30%) | 7 (30%) |
| Clinical benefit rate | 297 (72%) | 7 (70%) | 7 (70%) | 16 (70%) |
| Best response | ||||
| Partial response | 104 (24.3%) | 3 (30%) | 3 (30%) | 7 (33%) |
| Stable disease | 193 (47%) | 4 (40%) | 4 (40%) | 9 (43%) |
| Progression of disease | 61 (14.8%) | 3 (30%) | 3 (30%) | 5 (24%) |
| Missing | - | 0 | 0 | 2 |
| Time to response, months (range) Adverse events | 2.1 (1.5–16.3) | 2.5 (2.0–2.6) | 2.8 (2.6–3.9) | 2.8 (1.8–5.2) |
| Grade <3 | - | 1 (10%) | 1 (10%) | 4 (18%) |
| Grade ≥3 | 361 (88.9%) | 9 (90%) | 9 (90%) | 18 (82%) |
Adverse events graded by Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Figure 1.
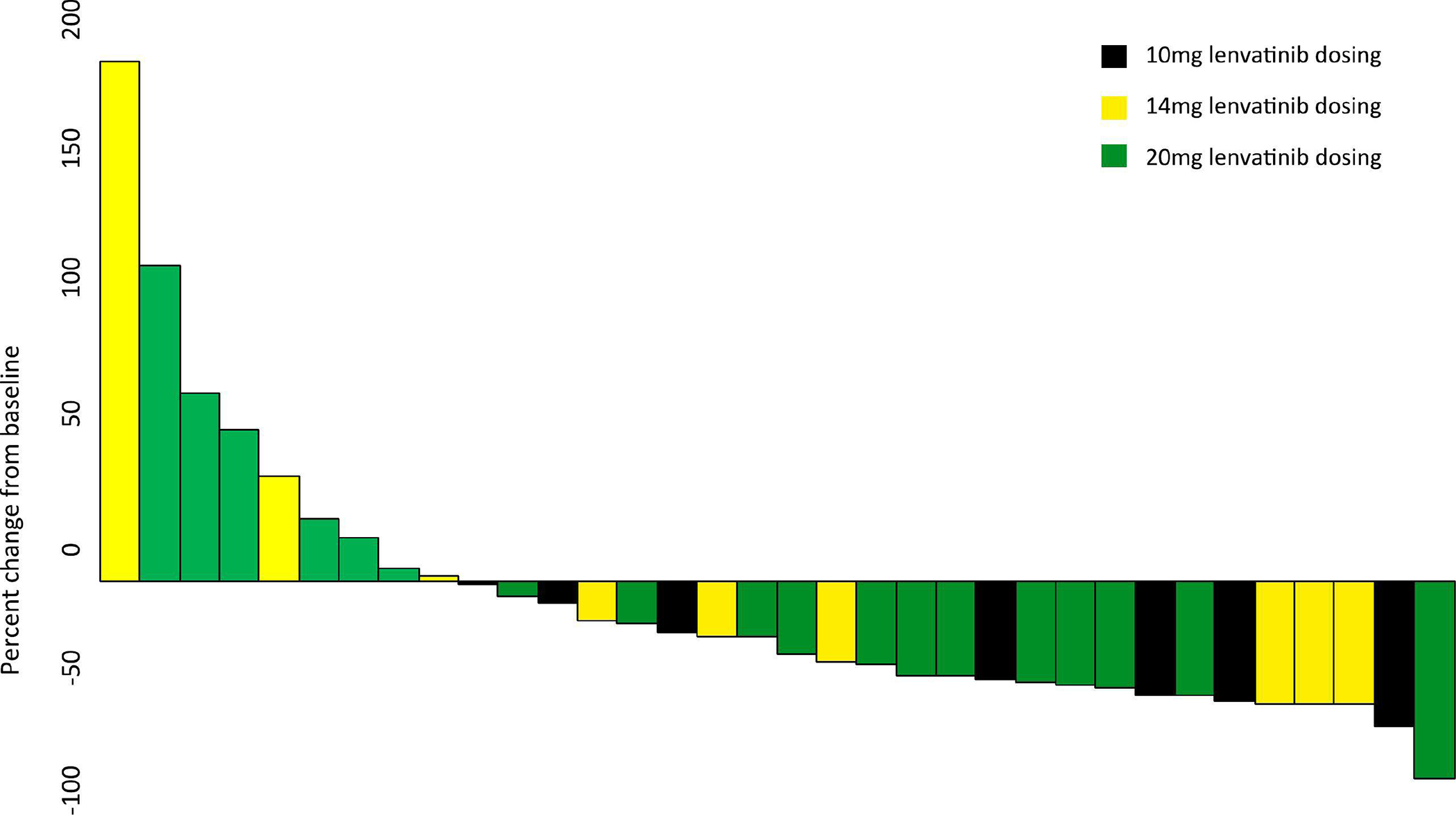
Percent change in radiologic tumor size from baseline at time of best response. Each bar represents one patient.
Table 4.
Progression-free survival (PFS), overall survival (OS), and duration of response (DOR) for the entire cohort and by starting lenvatinib dose.
| Characteristic | N | Progression # | Median PFS, months (95% CI) | 1-year PFS rate (95% CI) | HR (95% CI) | P value |
|---|---|---|---|---|---|---|
|
| ||||||
| Entire cohort | 43 | 36 | 6 (4.2–8.9) | 21% (10.1%-34.5%) | ||
| Reduced dose | 20 | 15 | 9.1 (4.1–12.6) | 35% (15.7%-55.2%) | 1 | .02 |
| Standard dose | 23 | 21 | 5.2 (3.1–6.7) | 6.3% (0.5%-24%) | 2.23 (1.11–4.47) | |
| Characteristic | N | Death # | Median OS, months (95% CI) | 1-year OS rate (95% CI) | HR (95% CI) | P value |
|
| ||||||
| Entire cohort | 43 | 23 | 18.3 (9.3-NE) | 61.7% (45.3%-74.6%) | ||
| Reduced dose | 20 | 9 | Not Reached | 69.1% (43.6%-84.8%) | 1 | .27 |
| Standard dose | 23 | 14 | 14.1 (5.7–27.3) | 55.4% (33%-73.1%) | 1.6 (0.69-3.71) | |
| Characteristic | PR | Progression/Death # | Median DOR, months (95% CI) | 1-year DOR rate (95% CI) | HR (95% CI) | P value |
|
| ||||||
| Entire cohort | 13 | 9 | 5.7 (2.2-NE) | 28.8% (7.9%-54.5%) | ||
| Reduced dose | 6 | 3 | 8.3 (1.9-NE) | 50% (11.1%-80.4%) | .09 | |
| Standard dose | 7 | 6 | 3.5 (1.3–6.1) | Not Reached | 3.64 (0.72-18.29) | |
P values obtained using log-rank test.
Reduced dose: 10 mg or 14 mg starting lenvatinib dosage; standard dose: 20 mg starting lenvatinib dose. PFS=progression-free survival; OS=overall survival; DOR=duration of response; CI=confidence interval; HR=hazard ratio; PR=partial response.
Clinical response and survival in Black patients
Of the 43 study patients, 12 (28%) identified as Black. Black patients demonstrated an ORR of 41.7%, with no difference between standard and reduced dose lenvatinib (P=.56). Black patients demonstrated a CBR of 75%, with no difference between standard and reduced dose lenvatinib (P=.50). The median PFS for Black patients was 5.9 months (95% CI: 2.9–16.5 months) compared to 8 months (95% CI: 4.2–9.7 months) for all other races (P=.7). The median OS for Black patients was 20.2 months (95% CI: 4.8-NE) compared to 18.3 months (95% CI: 8.9-NE) for all other races (P=.86).
Reduced vs standard lenvatinib starting dose
Twenty patients (47%) received a primary dose reduction for lenvatinib, defined as a starting dose less than the 20 mg standard dose; 10 patients received a starting dose of 14 mg and 10 received a starting dose of 10 mg. Patients who received a primary dose reduction were significantly older than those who received the standard starting dose, with a median age of 69 years vs 64 years, respectively (P=.01). No differences in race, ECOG status, stage at diagnosis, histology, or p53 status were observed between the reduced and standard dose groups (Table 1). Those who received a primary dose reduction had a median of 2 prior lines of platinum therapy compared to a median of 1 prior line among those who received the standard starting dose (P=.045). There was no difference in total cycles received or treatment interruptions, reductions, or discontinuations between the reduced and standard dose groups (Table 1). Toxicities were common in both groups; 18 (90%) of 20 patients with a starting dose of 10 mg or 14 mg compared to 18 (82%) of 23 patients in the standard dose group experienced a grade ≥3 AE (Table 3).
ORR and CBR were similar across starting dose groups (30% and 70%, respectively, for both the 10 mg and 14 mg groups; and 30% and 70%, respectively, for 20 mg group). The standard dose group experienced a statistically significantly shorter PFS compared to the reduced dose group (HR: 2.23, 95% CI: 1.11–4.47, P=.02) (Figure 2A); however, there was no difference in OS (HR: 1.6, 95% CI: 0.69–3.71, P=.27) or DOR (HR: 3.64, 95% CI: 0.72–18.29, P=.09) between the two groups (Figure 2B, 2C).
Figure 2.
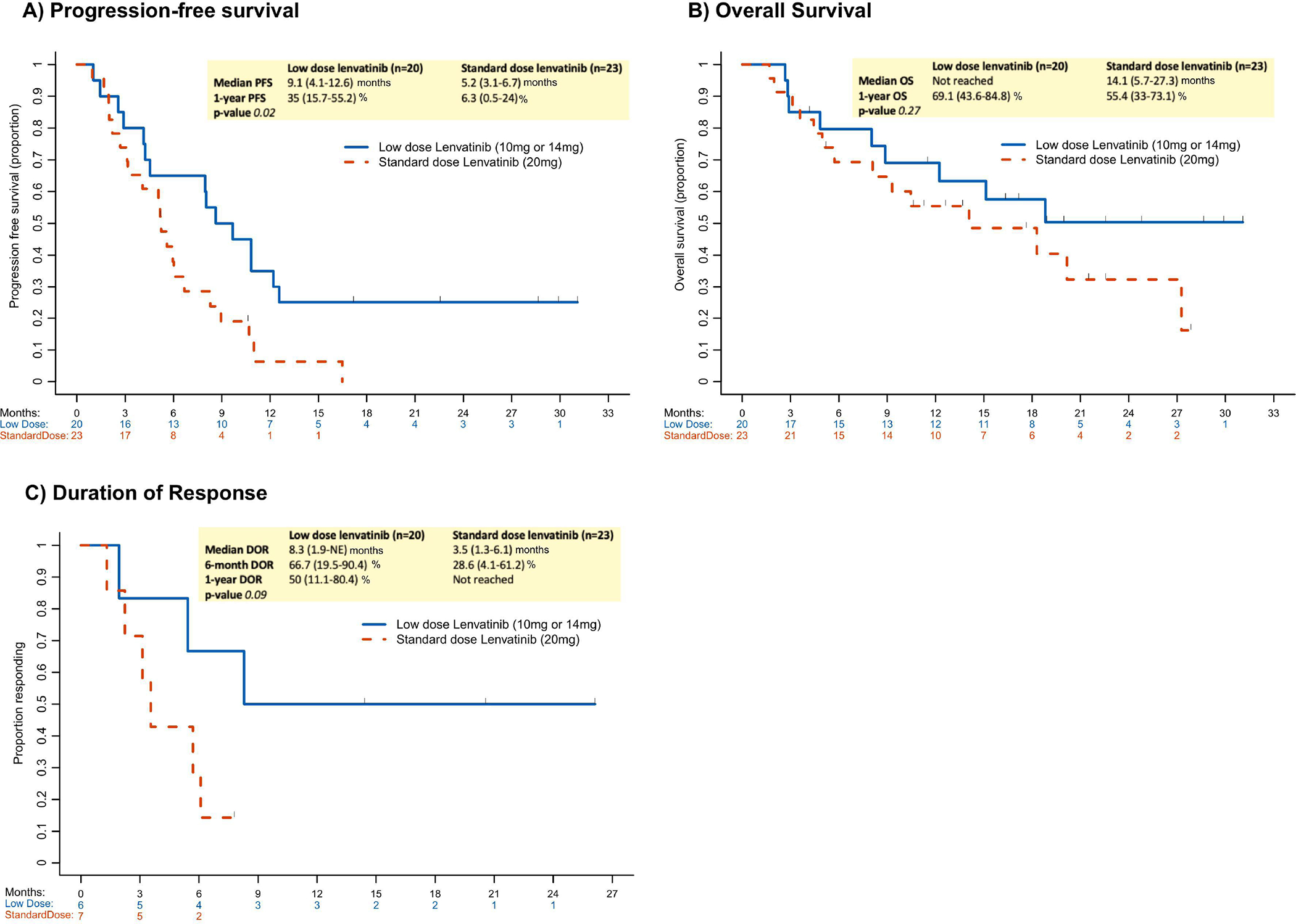
Oncologic outcomes by starting lenvatinib dose (standard vs low dose). A) rogression-free survival; B) Overall survival; and C) Duration of response.
DOR=duration of response; NE=not evaluable; OS=overall survival; PFS=progression-free survival.
Discussion
The landmark Study 309/KEYNOTE-775 trial established len/pem as the standard treatment for advanced pMMR/MSS endometrial cancer after failure of platinum-based chemotherapy (5, 14). This trial demonstrated an improvement in PFS and OS of 3.4 months and 6.9 months, respectively, among patients treated with len/pem vs physician’s choice chemotherapy (5). Given the toxicities of len/pem may hinder its success in clinical practice, we sought to characterize the treatment experience with len/pem at our institution since the FDA’s approval for patients with endometrial cancer in 2019.
Compared to KEYNOTE-775, our population was older (median age, 67 vs 64 years) and less fit (ECOG status of 0 in 28% vs 60%), which is consistent with prior studies comparing real-world and clinical trial populations (15, 16). Furthermore, our study population had a higher proportion of serous carcinomas (35% vs 25%), and we included carcinosarcoma and poorly differentiated histotypes. In our study, 63% of patients had undergone prior pelvic irradiation compared to only 42% in the KEYNOTE-775 cohort. Despite these differences, AEs associated with len/pem in our population were comparable to the experience in KEYNOTE-775, suggesting that toxicities with len/pem are not considerably worse in routine clinical practice. In our study, 36 (84%) of 43 patients experienced a grade ≥3 AE, which is comparable to 316 (77%) of 411 patients on the KEYNOTE-775 trial.
In our population, the percentage of patients with grade ≥3 AEs may have been attenuated by the high proportion of lenvatinib primary dose reductions. We found that 47% of patients received a primary dose reduction, with an initial starting dose of 14 mg or 10 mg, as opposed to the standard 20 mg dose. These patients were older and received more lines of treatment compared to those who received the standard dose, which may signify the clinician identified higher toxicity risks or frailty in these patients; however, reasons for primary dose reductions may not have been captured in our study. It is important to note that the physicians of patients who underwent primary dose reductions likely proactively reduced the lenvatinib dose to limit significant toxicity, reduce dose holds, and maximize benefit. Grade ≥3 AEs were somewhat more common in the reduced dose vs standard starting dose group (90% vs 82%). Dose reductions, holds, and discontinuations, however, were similar. The similar outcomes and frequency of AEs found in our cohort and the KEYNOTE-775 cohort suggests that patients can continue therapy and AEs can be mitigated in a routine clinical setting (ie, outside of the parameters of a clinical trial). While clinical trial data are the gold standard to inform dosing of lenvatinib, our findings suggest that primary dose reductions for older or more frail patients may not negatively impact outcomes. Notably, the standard dose group had a significantly worse 1-year PFS compared to the reduced dose group (HR: 2.23, 95% CI: 1.11–4.47, P=.02). While the cause of this difference in PFS is uncertain, it did not translate into a difference in OS (HR: 1.6, 95% CI: 0.69–3.71, P=.27) or DOR (HR: 3.64, 95% CI: 0.72–18.29, P=.09) between the groups. When comparing Black patients to those who identified as another race (White/Asian/Other), there was no difference in PFS or OS. This finding is notable, as only 2.5% of patients in KEYNOTE-775 identified as Black, while 28% of patients in our study identified as Black.
Two prior studies have evaluated experience with len/pem for the treatment of endometrial cancer outside of a clinical trial setting. In the MDACC study, AEs were only designated when identified as the cause of lenvatinib dose reduction; thus, it is difficult to ascertain the overall AEs and toxicity experienced by this cohort. The MDACC cohort underwent more lenvatinib dose interruptions, fewer lenvatinib dose reductions, and more treatment discontinuation due to toxicity compared to our cohort (11). Compared to our cohort, the MDACC cohort was healthier (ECOG status of 0 in 44% vs 28% of patients) and had similar tumor pathology; although, patients in the MDACC cohort were more heavily pretreated (median prior lines platinum therapy, 2 vs 1) (11). Standard (20 mg) vs reduced (<20 mg) starting lenvatinib dosing in the MDACC study was associated with a higher mean number of lenvatinib dose reductions (1.1 vs 0.4, respectively; P=.003) and a shorter median time to treatment toxicity (1.3 vs 3.7 days, respectively; P=.0001). In contrast, there was no difference in treatment cycles, dose reductions or holds of lenvatinib or pembrolizumab, or treatment discontinuation rates between the standard and reduced groups in our study. We demonstrated a shorter median PFS in the standard dose (5.2 months, 95% CI: 3.1–6.7) vs reduced dose (9.1 months, 95% CI: 4.1–12.6) groups, and no difference in median OS between the standard dose (14.1 months, 95% CI: 5.7–27.3) and reduced dose (median OS, not reached) groups. The MDACC study, however, found no difference in median PFS for the standard dose (3.2 months, 95% CI: 2.3–6.4) vs reduced dose (5.5 months, 95% CI: 3.8–6.6) groups, as well as no difference in median OS for the standard dose (8.6 months, 95% CI: 5.0-NR) vs reduced dose (9.4 months, 95% CI: 7.6–12.2) groups (11).
In Korea, Kim et al. performed a multicenter retrospective cohort study to evaluate 48 patients treated with len/pem for recurrent endometrial cancer (12). Most patients (83%) received standard starting lenvatinib dosing (20 mg), and overall, 34 (71%) of 48 patients experienced a treatment-related AE (any grade); this is lower than what was reported in KEYNOTE-775, the MDACC retrospective cohort study, and the current study. In the Korean study, the ORR was 24% (95% CI: 11.9%-38.1%) and the median PFS for the entire cohort was 5.3 months (95% CI: 3.9–6.6 months) (12). This contrasts with our findings and the MDACC study, which demonstrated comparable ORR, PFS, and OS in the overall population to the Study 309/KEYNOTE-775 trial. It is important to note that the pharmacogenomics of lenvatinib are not fully understood in regard to endometrial cancer but have been described in other cancer types (17, 18).
There are several limitations to our study. Given the retrospective study design, it is possible that not all AEs were captured. However, the EMR was used to identify AEs in physician documentation, vital sign flowsheets, and laboratory records. Oncologists may have reserved reduced dose lenvatinib for patients they deemed to have a lower performance status or ability to tolerate therapy; however, the ECOG Performance Status and other clinicodemographic features were similar between the two groups. Although MSK is a high-volume center, our study does have a limited sample size, which makes it difficult to compare oncologic outcomes between groups. Nonetheless, this study provides valuable and granular insights regarding our experience with len/pem and demonstrates the regimen is tolerable and provides oncologic benefit.
This study evaluated AEs and oncologic outcomes of patients with recurrent endometrial cancer who received len/pem and found similar results to the Study-309/KEYNOTE-775 trial. AEs were comparable when stratified by lenvatinib starting dose, and OS was similar between patients treated with standard vs reduced dose lenvatinib. The most common AEs presented during the first 4 weeks of treatment, which demonstrates that frequent monitoring by oncologists can manage toxicities in routine clinical settings, allowing patients to achieve the full potential of this combination therapy. Although there was a statistically significantly worse PFS in the standard dose group, OS was similar between patients treated with standard compared to reduced starting lenvatinib doses. The similar OS between groups may be due to a successful change to a subsequent effective treatment, and this information could not be captured within our current study design. Primary dose reductions may be reasonable for patients at high risk for toxicity; however, clinical trial data should remain the gold standard to guide starting lenvatinib dose when using len/pem to treat recurrent endometrial cancer. Further dose optimization studies of len/pem may be indicated and would likely require collaboration between multiple institutions and the US FDA.
Supplementary Material
Highlights.
Adverse events associated with combination lenvatinib plus pembrolizumab for recurrent endometrial cancer are common
Adverse events with lenvatinib plus pembrolizumab observed in this study were similar to those in Study 309/KEYNOTE-775
The most common grade ≥3 adverse events were hypertension, anemia, weight loss, fatigue, and thrombocytopenia
Overall survival was similar between patients treated with the standard starting lenvatinib dose compared to a reduced dose
Funding:
This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of Interest: Outside the current study, A. Iasonos reports consulting fees from Mylan. N.R. Abu-Rustum reports honoraria from Klinik Essen Germany and NCCN; and GRAIL grants paid to the institution. V. Makker reports meeting/travel support by Eisai and Merck; participation on Data Safety Monitoring or Advisory Board of Duality, Merck, Karyopharm, Exelexis, Eisai, Karyopharm, BMS, Clovis, Faeth Immunocore, Morphosys, AstraZeneca, Novartis, GSK, Bayer (all unpaid), and study support to the institution by Merck, Eisai, AztraZeneca, Faeth, Karyopharm, Zymeworks, Duality, Clovis, Bayer and Takeda. C. Aghajanian reports clinical trial funding paid to the institution from AstraZeneca; consulting fees (advisory board) from Eisai/Merck, Roche/Genentech, Abbvie, AstraZeneca/Merck, and Repare Therapeutics; advisory board participation (no fee) for Blueprint Medicine; and leadership/fiduciary roles for the GOG Foundation Board of Directors (travel cost reimbursement) and NRG Oncology Board of Directors (unpaid). M. Rubinstein reports research funding from Merk, Zentalis, and AstraZeneca. A.K. Green reports research funding from Eli Lilly, Mereo Biopharma, and the American Society for Clinical Oncology (ASCO), and personal fees from Merck. All other authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–28. [DOI] [PubMed] [Google Scholar]
- 2.Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol Oncol. 2021;161(2):573–80. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 4.Gordhandas S, Zammarrelli WA, Rios-Doria EV, Green AK, Makker V. Current Evidence-Based Systemic Therapy for Advanced and Recurrent Endometrial Cancer. J Natl Compr Canc Netw. 2023;21(2):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makker V, Colombo N, Casado Herraez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386(5):437–48. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22(7):946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020;38(26):2981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makker V, Aghajanian C, Cohn AL, Romeo M, Bratos R, Brose MS, et al. A Phase Ib/II Study of Lenvatinib and Pembrolizumab in Advanced Endometrial Carcinoma (Study 111/KEYNOTE-146): Long-Term Efficacy and Safety Update. J Clin Oncol. 2023;41(5):974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med. 2023;388(23):2159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novak Z, Black D, et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med. 2023. [DOI] [PubMed] [Google Scholar]
- 11.How JA, Patel S, Fellman B, Lu KH, Hwu P, Ramondetta LM, et al. Toxicity and efficacy of the combination of pembrolizumab with recommended or reduced starting doses of lenvatinib for treatment of recurrent endometrial cancer. Gynecol Oncol. 2021;162(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Noh JJ, Lee TK, Kim SI, Lee JY, Lee JW, et al. Real-world experience of pembrolizumab and lenvatinib in recurrent endometrial cancer: A multicenter study in Korea. Gynecol Oncol. 2022;165(2):369–75. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 14.van Halteren HK, Bennouna J, Brasiuniene B, Tomas AJC, Trinidad AMG, Indini A, et al. Twelve ESMO Congress 2022 breakthroughs: practicing oncologists’ perceptions and potential application on presented data. ESMO Open. 2023;8(1):100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green AK, Curry M, Trivedi N, Bach PB, Mailankody S. Assessment of Outcomes Associated With the Use of Newly Approved Oncology Drugs in Medicare Beneficiaries. JAMA Netw Open. 2021;4(2):e210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarey J, Kao SC, Clarke SJ, Vardy J. The eligibility of advanced non-small-cell lung cancer patients for targeted therapy clinical trials. Ann Oncol. 2012;23(5):1229–33. [DOI] [PubMed] [Google Scholar]
- 17.Ozeki T, Nagahama M, Fujita K, Suzuki A, Sugino K, Ito K, et al. Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci Rep. 2019;9(1):5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Mattia E, Cecchin E, Guardascione M, Foltran L, Di Raimo T, Angelini F, et al. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol. 2019;25(29):3870–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


