Abstract
PETERS, C. M., J. A. DEMPSEY, S. R. HOPKINS, and A. W. SHEEL. Is the Lung Built for Exercise? Advances and Unresolved Questions. Med. Sci. Sports Exerc., Vol. 55, No. 12, pp. 2143–2159, 2023. Nearly 40 yr ago, Professor Dempsey delivered the 1985 ACSM Joseph B. Wolffe Memorial Lecture titled: “Is the lung built for exercise?” Since then, much experimental work has been directed at enhancing our understanding of the functional capacity of the respiratory system by applying complex methodologies to the study of exercise. This review summarizes a symposium entitled: “Revisiting ‘Is the lung built for exercise?’” presented at the 2022 American College of Sports Medicine annual meeting, highlighting the progress made in the last three-plus decades and acknowledging new research questions that have arisen. We have chosen to subdivide our topic into four areas of active study: (i) the adaptability of lung structure to exercise training, (ii) the utilization of airway imaging to better understand how airway anatomy relates to exercising lung mechanics, (iii) measurement techniques of pulmonary gas exchange and their importance, and (iv) the interactions of the respiratory and cardiovascular system during exercise. Each of the four sections highlights gaps in our knowledge of the exercising lung. Addressing these areas that would benefit from further study will help us comprehend the intricacies of the lung that allow it to meet and adapt to the acute and chronic demands of exercise in health, aging, and disease.
Keywords: AIRWAY, EXERCISE TRAINING, GAS EXCHANGE, RESPIRATORY MUSCLE, VENTILATION
With dynamic exercise, there are increases in O2 extraction and CO2 production by the contracting musculature, and with heavy exercise, there are accompanying reductions in pH. Increases in ventilation during exercise replenish O2, eliminate CO2, and regulate acid–base balance. The respiratory system of most untrained and moderately trained healthy humans can respond to these physiological challenges and maintain arterial oxygenation homeostasis even in the face of heavy or prolonged exercise. That ventilation is well matched to the metabolic demands of exercise emphasizes the highly ordered structure and function of the lung parenchyma, airways, respiratory muscles, and respiratory control systems. Said differently, the architecture and regulation of the healthy respiratory system appear well suited to the demands of exercise, at least within the context of healthy populations with “normal” aerobic capacities.
Exercise has proved to be an excellent experimental tool to study the integrative aspects of whole-body function. There is a long tradition of studying exercising humans to understand better the capacity and role of the respiratory system, its mechanisms of control, and interaction(s) with other organ systems. Consider the 1913 seminal work of Krogh and Lindhard (1), which provided the basis for understanding how ventilation is initiated at the onset of exercise. The respiratory system provides the first step for O2 transport and attainment of maximal O2 uptake (). With regard to the other key determinants of O2 delivery and utilization (e.g., stroke volume, cardiac output, oxidative capacity of locomotor muscles), there is general consensus that each can respond positively to regular endurance training, and there is an accompanying increase in .
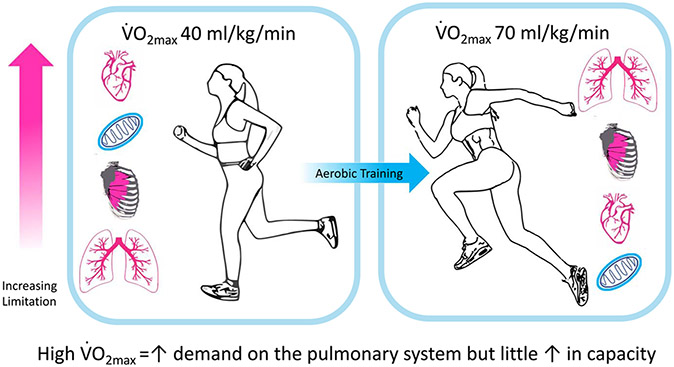
Professor Dempsey delivered the 1985 ACSM Joseph B. Wolffe Memorial Lecture, which was provocatively titled: “Is the lung built for exercise?” The lecture was later published in 1986 (2), and it serves as an exemplar of careful consideration of the complexities and unknowns concerning our understanding of the respiratory system. The following hypothesis was forwarded in the article: the healthy pulmonary system may become a limiting factor to O2 transport and utilization in the highly trained athlete performing near-maximal short-term exercise (see Fig. 1 and legend). The concept here is that chronic exercise training elicits functional and structural changes to those organ systems involved in O2 transport, but the respiratory system remains largely unchanged with training and in turn becomes a “weak link.” The article concludes with a challenge to scientists in the field: “given the long list of our still untested speculations, we could use a bit more data.” The lecture provided a call to further our understanding of the fundamentals of the respiratory system under the biologically relevant condition of dynamic exercise. Our knowledge of the capacity and function of the respiratory system during exercise has increased markedly since 1986, but several new questions have arisen.
FIGURE 1—
Hypothesis: in the untrained, the capacity for O2 transport by the pulmonary system (lungs and chest wall) far exceeds that of the cardiovascular system and the oxidative capacity of the limb locomotor muscles. The high in the highly trained athlete is accompanied by increased capacities of the left heart, locomotor muscle mitochondria and capillarity, and red cell mass, but with minimal alteration in lung structure between trained and untrained. Thus, eventually the capacity of the pulmonary system for O2 transport cannot meet the superior demands imposed by the limbs and cardiovascular systems; arterial blood gas and acid–base homeostasis fail, and the lungs become a significant limitation to performance capacity. Original caption from Dempsey (2).
The present article summarizes the proceedings of a scientific symposium entitled: “Revisiting ‘Is the lung built for exercise?’” presented at the 2022 American College of Sports Medicine annual meeting in San Diego, California. The session featured presentations from Drs. Jerome Dempsey, Carli Peters, Susan Hopkins, and William Sheel. A report dealing with the broad topic of the “respiratory physiology of exercise” must, by necessity, exclude several important areas, and there are limitations to the scope of our review. We have chosen to subdivide our topic into four areas of active study: (i) adaptation to the respiratory system, (ii) airway anatomy and lung mechanics, (iii) pulmonary gas exchange, and (iv) respiratory-cardiovascular interactions. In the spirit of Dr. Dempsey’s original lecture, we have provided questions within each subtheme that would, in our view, benefit from further attention and study.
IS THE LUNG ADAPTABLE?
Our nearly four-decade-old hypothesis suggested no or minimal adaptation to endurance training in the healthy lung, leaving the lung “behind” as cardiovascular, hematologic, and muscle metabolic adaptations drove and endurance performance to higher levels. What has the ensuing period taught us about this hypothesis concerning the young, aging, at risk, and diseased lungs?
Potential mechanisms for lung growth.
In humans, respiratory bronchioles in the very distal branches of the airways contain a unique secretory cell population that acts as progenitors for alveolar type 2 cells. These cells show remarkable phenotypic plasticity maintaining and repairing the tracheobronchial epithelium and in promoting angiogenesis in the pulmonary vasculature (3-5). In vitro, these cells also stimulate alveolar growth in a mouse lung injury model (6). Progenitor cell “exhaustion” in the lung is thought to occur secondary to chronic inflammation in response to excessive air pollution, cigarette smoke, or chronic obstructive pulmonary disease (COPD), rendering these cells “senescent” or nonresponsive to growth stimuli (7,8).
Growth of the lung’s alveolar–capillary surface and volume occurs in response to repeated, excessive stretch of lung parenchyma and vasculature and long-term hypoxia—the hypoxic effect mediated by activation of HIF-1α transcription. Thus, lung diffusing capacity is markedly increased in native and long-term human and canine high-altitude residents, allowing for highly effective gas exchange during exercise (9). Furthermore, after the resection of substantial portions of one lung, the remaining lung tissue, volume increased significantly because of its now excessive stretch within the chest cavity (10,11). Similarly, refeeding after long-term caloric restriction elicits lung growth in rodents (12).
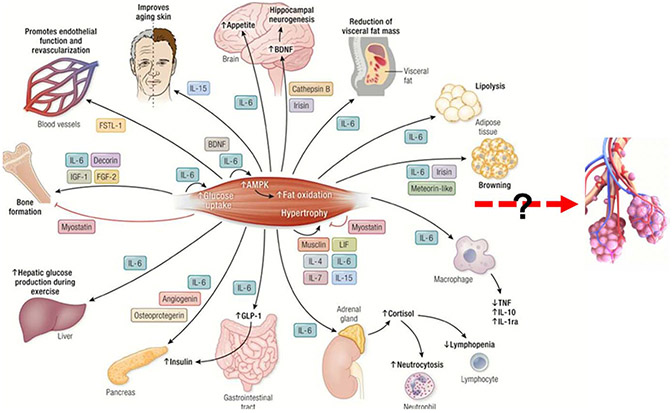
Exercise training might stimulate lung growth through the influence of myokines released from contracting skeletal muscle. To date, prototype myokines such as IL-6, IL-15, and irisin have been shown to be secreted from contracting muscle and to promote anti-inflammatory effects on such diverse organs as the pancreas, skin, liver, and brain (Fig. 2) (7,13,14). As discussed hereinafter, the lung as a potential target for these exercise-induced myokines has only begun to receive attention.
FIGURE 2—
Muscle Organ Xtalk: The “Exercise Factor.” As proposed two decades ago by Bente Pedersen and colleagues, contracting muscle operates as an endocrine organ producing and secreting hundreds of myokines, which exert their effects in autocrine, paracrine, or endocrine fashion. Xtalk effects of a select number of these myokines have been shown to occur between the skeletal muscle and several other organs as well as within the muscle itself. This review focuses on whether exercise training might grow, restore, or prevent loss of structure/function in the young, aging, or diseased lung and whether the metabolic and anti-inflammatory effects of the “exercise factor” might represent one avenue for this influence on the lung and specifically on respiratory bronchiolar secretory cells, as it does for other organs. Modified from Severinsen and Pedersen (13).
Lung adaptation to exercise training in health.
Extensive population studies in young and older adults show significant correlations of forced expired volume in 1 s (FEV1.0), forced vital capacity (FVC) and/or lung diffusing capacity of the lungs for carbon monoxide (), and pulmonary vascular volume with (15-17). These purely correlational data are interpreted to demonstrate that lung function is an important “determinant” of (16). However, experimental findings show this claim to be improbable in the untrained, healthy, young adult population who show little evidence of significant ventilatory, gas exchange, or respiratory muscle limitations during exercise (18). Alternatively, might the actual cause:effect interpretation of this association be that – as a surrogate for duration and intensity of habitual physical activity – determines lung function over long periods of time? We now address this question throughout the remainder of this section concerned with lung adaptation.
Short-term exercise training.
Weeks or months of endurance training in healthy humans or animals elicits no or only minor changes in lung function. Most notable evidence includes the following: a) rodent studies that use intense training beginning very early in life show no effect on the alveolar surface area or lung morphology to accompany considerable improvements in aerobic capacity (19,20); b) several weeks of bedrest in young adults caused a marked 28% reduction in followed by daily training, which increased by 33%. Neither of these changes was accompanied by variations in (or its components: pulmonary capillary blood volume and the rate of diffusion across tissue membrane and plasma barrier) at rest or exercise or by alterations in max flow rates or lung volumes (21); and c) aerobic training sufficient to significantly increase in males and females over several weeks at sea level and high-altitude has shown little or no coincident effect on or its components at rest or an equivalent cardiac output during exercise (22,23). On the other hand, swim training and/or respiratory muscle training elicits modest but significant increases in lung volumes and FVC (24-26).
Does lung function match in the endurance-trained athlete?.
The ideal example of “symmorphosis” among links in oxygen transport would be the prong-horned antelope in whom a several-fold superior —relative to sedentary counterparts—is matched by proportionate increases in the capacity of all links in the O2 transport cascade from lung surface area to muscle mitochondria (27). In contrast to this model of equitable organ system adaptations, in rodents selected for running endurance, increased substantially over seven generations of selection with no improvement in lung function (20). In human athletes, cross-sectional comparisons with the general population report that maximal expiratory flow/volume loops are commonly increased by 10%–20% in highly fit swimmers and rowers but not so consistently in other land-based, highly trained athletes (28,29). Resting trends higher in many endurance athletes, in most cases secondary to increased lung volume (30). Limited findings also suggest that pulmonary vascular pressure may be significantly reduced during exercise at a given cardiac output in the highly trained. In the absence of any known difference in pulmonary vascular structure, per se, this lower pulmonary arterial pressure is likely attributable to secondary effects of increased left ventricular compliance and reduced left atrial pressure during exercise in the highly trained (31,32).
There are several examples in the highly trained human of disproportionate increases in relative to the capability of lung function to meet these extraordinary demands.
Marked elevations in pulmonary artery pressures occur secondary to combinations of high cardiac output, increased left atrial pressure, and limited vasodilator reserve in the pulmonary vasculature. These high pressures elicit increased right ventricular wall stress and work. In turn, this leads acutely to right heart fatigability and possibly maximum stroke volume limitation and chronically to maladaptive right heart remodeling (33,34).
Ventilatory demand exceeds airway capacity for flow and volume, eliciting expiratory flow limitation with excessive intrathoracic pressures. Consequences of flow limitation include a constraint on left ventricular stroke volume and high flow rate–induced injury to airway epithelium with the long-term development of bronchial hyperresponsiveness.
The excessive work of breathing (Wb) at high ventilatory demand elicits metabolite accumulation and fatigue in inspiratory and expiratory muscles, precipitating sympathetic-induced reductions in locomotor muscle vascular conductance and blood flow (18,35,36).
Impaired gas exchange and exercise-induced arterial hypoxemia occasionally occur via nonuniformity in ventilation–perfusion () distribution and/or limited pulmonary capillary blood volume expansion relative to high cardiac output (18).
In these examples, the “underadapted” respiratory system presents significant limitations to maximal gas transport to locomotor muscles and to exercise performance in many highly trained humans. In the thoroughbred horse, the underadapted lung is the major limitation to performance (37,38). However, in human athletes, respiratory-induced exercise limitations, including the effects of arterial hypoxemia, excessive Wb, and pulmonary hypertension, are less consistent and smaller in magnitude in their impact on performance limitation than those presented by the cardiovascular system or locomotor muscles (18).
Demand versus capacity in the aging lung: training effects.
Beginning as early as the second decade of life and accelerating in the sixth decade, the healthy human lung loses elastic recoil due to reductions in the chest wall and pulmonary vascular compliance and in alveolar–capillary surface area. How does this decline in pulmonary system capacity compare with that of maximal demand as aging-induced cardiovascular and metabolic limitations reduce at approximately 10% per decade? Will a habitually active lifestyle ameliorate this potential imbalance? Sedentary aging is associated with a 40% reduction in at age 70 yr (vs 30 yr), so even the reduced lung function remains capable of meeting the falling demand for ventilation and gas exchange; limited findings report elevated pulmonary artery pressure during even moderate increases in cardiac output during exercise (18,39) in these individuals. However, in the highly fit elderly ( of 40–55 mL·kg−1·min−1 at age 70 yr), many experience flow limitation, high Wb, dyspnea, excessive pulmonary arterial pressures, and even hypoxemia—limitations that are not present in 30-yr-old lungs at the same absolute high-intensity workloads (40). These highly trained older subjects had lung function values that exceeded the general population; however, they experienced reductions in lung volumes and maximal flow rates when followed longitudinally from ages 67 to 73 yr, equivalent to those in the general population (41). Two large longitudinal aging studies assessed self-reported habitual physical activity levels vis-à-vis reductions in standard measures of resting lung function over a 10- to 25-yr period: a) a Finnish rural population showed an average 30% less decline in FEV1.0 and FVC in never-smokers and 20% less decline in smokers who were habitually active versus inactive (42), and b) a Copenhagen population showed a 25% less decline in FEV1.0 and FVC in active smokers but not in active nonsmokers (43). Thus, limited longitudinal aging data show mixed results—even in the nonsmoker or highly trained endurance athlete—on whether a habitually active lifestyle is protective against age-induced decrements in lung function.
Exercise training in chronic lung diseases.
Exercise performance is markedly impaired in obstructive and interstitial lung diseases via flow limitation, hyperinflation, dyspnea, arterial hypoxemia, and impaired blood flow and aerobic capacity of locomotor muscles (7,44). Exercise training as an integral part of pulmonary rehabilitation usually results in improved exercise tolerance, less dyspnea, and improved quality of life. Detailed measures of gas exchange and pulmonary vasculature are rare, and lung volumes measured only at rest commonly show little or no change (7,45,46). Thus, improvements in dyspnea and exercise tolerance were primarily attributable to improved locomotor muscle aerobic capacity, which would alleviate muscle metabolite accumulation and fatigue, with the following consequences: a) reduced inhibitory feedback on central drive to locomotor muscles (i.e., less “central fatigue”) (47,48), b) reduced afferent feedback drive to breathe from muscle afferents (45), and c) requiring less augmentation of central command to recruit motor units to maintain muscle force output, thereby also reducing the central respiratory drive to exercise hyperpnea.
Do these findings imply that cross-talk influences from contracting muscle on lung inflammation and senescent bronchiolar progenitor cells are not likely to occur with exercise training? Recently, Nymand et al. (7) argue to the contrary that the progressive loss of tissue in the COPD lung depends on a state of cellular senescence within the mesenchyme. Cellular substrates with a capacity for de novo tissue formation may be triggered by the mechanical forces and anti-inflammatory myokines attending exercise training. The following examples offer some promise in support of this postulate.
Mice exposed to cigarette smoke over several weeks show substantially less destruction of alveoli and lung vasculature when exercised (49,50). Similarly, in human smokers, high habitual activity levels protected against decrements in FVC over 10 yr (43).
In both healthy mice and those exposed to cigarette smoke, exercise training induced transcriptional changes in lung tissue, markedly reducing inflammation and increasing lung mitochondrial biogenesis and antioxidant capacity. Increased levels of myokines such as IL-6, IL-10, and Irisin appeared in lung tissue and bronchoalveolar lavage fluid (50-52). Patients with COPD showed significantly lowered systemic oxidative stress and raised locomotor muscle antioxidant capacity via exercise training (53,54).
In two observational studies of COPD patients, a 3- to 8-wk rehabilitation program that contained endurance and resistance exercise training reported measurable improvement in resting diffusing capacity for carbon monoxide () in a significant proportion of patients—especially in those with substantially depressed at baseline (55,56).
In experimental lung injury in mice, prior exercise training was shown to prevent mitochondrial dysfunction and ROS formation in the lung parenchyma and reduce lung edema formation and high airway resistance (57).
Moderate-intensity exercise training in murine models of human asthma prevented epithelial remodeling, lowered airway resistance, and increased epithelial expression of anti-inflammatory cytokines. These training-induced changes in the airways reduced the epithelial expression of primary proteins involved in the inflammatory asthma response (58,59).
Summary: Adaptations.
We propose the following concerning exercise training and the lung.
In the young, healthy adult, short-term exercise training has limited to no effect on the lung’s airways and volumes, pulmonary vasculature, or alveolar–capillary surface area.
In many endurance-trained athletes, especially in aquatic sports, some aspects of lung function are significantly enhanced relative to sedentary counterparts. Although some of these changes in lung function may result from prolonged exercise training, it is equally likely that larger lungs and diffusion capacities are inherent characteristics of these athletes. However, the flow–volume capacity of the airways, the reactivity of the airway epithelium, and the vasodilator capacity of the pulmonary vasculature often remain “underadapted” relative to the extraordinary demands imposed during maximal exercise by the trained cardiovascular system and locomotor muscles.
Aging influences on the healthy nonsmoking lung are substantial in sedentary and habitually active humans. Insufficient longitudinal data exist to judge whether habitual exercise training protects against aging-induced deterioration in lung function at rest and exercise. Hopefully, some of the comprehensive population studies, which include detailed assessments and imaging of lung gas exchange (see previous discussion), will continue to monitor their subjects during the aging process, perhaps even attempt a prolonged exercise training intervention in a subgroup.
Recent findings, mainly in rodent models but even in some humans, point to substantial positive influences of exercise training, especially via cross-organ anti-inflammatory influences on the compromised adult lung (i.e., COPD, asthma, and chronic smokers).
Unresolved questions.
To date, investigations of exercise training effects on the respiratory system in chronic disease have not been conducted with the same rigor and use of state-of-the-art methodology afforded training studies concerned with the cardiovascular system or locomotor muscles. Comprehensive, in-depth, long-term investigations of exercise training effects in chronic lung diseases and in humans at risk, such as chronic smokers or the elderly, are now required to build on the promising results obtained in recent years. They must incorporate state-of-the-art lung imaging and detailed measures of gas exchange and breathing mechanics at rest and during exercise over a broad range of disease severity. Special care should be taken to protect against the potential maladaptive influences of high flow rates in the airways and pulmonary vasculature by including a range of training intensities and durations. These recommendations for training studies apply especially to patients with asthma and pulmonary hypertension. Such studies are challenging to carry out, particularly in patients with symptom-limited exercise capabilities, and pose a moderately high risk of failure to uncover positive effects of training. On the other hand, they also provide the opportunity to reveal a potentially important, unique role for exercise training in preventing further deterioration and possibly restoring lung function and structure in individuals with chronic respiratory diseases.
IMAGING TO UNDERSTAND THE RELATIONSHIP BETWEEN AIRWAY ANATOMY AND THE MECHANICS OF BREATHING DURING EXERCISE IN HEALTH
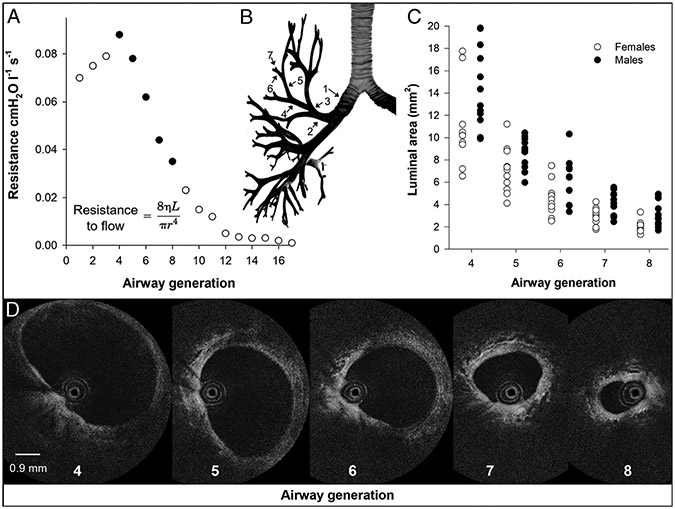
Airflow in the respiratory system depends on the driving pressure and airway resistance, with airway radius being the most crucial factor influencing airway resistance. Under laminar flow conditions, Poiseuille’s equation shows that airway resistance is proportional to the inverse of the airway radius raised to the fourth power. All other factors being equal, halving airway radius increases resistance to airflow 16-fold. In health, the small airways account for about 20% of airflow resistance, with the large airways responsible for the remaining 80% (60). Given the strong influence of airway radius, the increased resistance in the large airways seems paradoxical. However, based on Weibel’s model, human airways branch in a dichotomous manner, with each bifurcation representing one airway generation numbered from the trachea (generation 0) to the alveoli (generation 23) (61). The extensive branching of the airway tree leads to a large aggregate cross-sectional area above approximately generation 8, thus reducing resistance. The presumption can be made that when minute ventilation () and flow rates increase during exercise, individuals with smaller conducting airways would experience increased resistance to flow and mechanical constraints such as expiratory flow limitation.
Measuring airway size.
Early work by Green et al. (62) demonstrating a wide variation in maximal expiratory flow rates between individuals with similar lung size suggested no consistent association between lung and airway size. Following this work, the term “dysanapsis” has been used to describe the variation in airway and lung parenchyma growth within individuals. Using a measure sensitive to lung size (vital capacity) and a measure sensitive to airway size (maximal expiratory flow at 50% vital capacity divided by static recoil pressure at 50% vital capacity), termed the dysanapsis ratio, it has been shown that females have smaller airways relative to lung size than males (63). Tracheal area measurements made with acoustic reflectance confirm that, even after controlling for total lung capacity, tracheal areas in females are significantly smaller than in males (64). The aforementioned studies, although providing evidence for sex-based differences in airway size, are limited by relying on an index of airway size (dysanapsis ratio) or a quantitative measure of only the tracheal “region.” Computed tomography (CT) can consistently measure the airways down to the fourth generation, approximately 2 mm in diameter, but because of resolution limits, it is often unable to image deeper conducting airways (5th–7th generations) (65). Utilizing CT, multiple airways can be visualized simultaneously, and measures of luminal area, radius, and bifurcation angle can be obtained with readily available software. Several CT-based studies, collected in separate populations by different research groups, have found sex differences in airway size, with conducting airways consistently being ~14%–50% smaller in females than males (66-69). A limitation of most CT studies investigating airway size is their retrospective nature; the relatively high radiation exposure during imaging makes prospective studies on healthy individuals ethically problematic. A newer pulmonary imaging technique, optical coherence tomography (OCT), does not require ionizing radiation exposure, thus making it an appealing tool for studying the airways of healthy young individuals.
OCT is a catheter-based imaging modality that uses near-infrared light reflected from airway tissue to generate images of small airways. The fiberoptic OCT catheter is inserted into the small airways through the biopsy port of a bronchoscope. Imaging begins by advancing the catheter until it becomes wedged (this occurs when the catheter is the same size as the airway). The OCT camera spins in a circumferential manner collecting two-dimensional airway images while being pulled back from the smaller distal airways into the larger, more proximal airways. Combining the two-dimensional images generates a three-dimensional rendering of the airway. The catheter size makes OCT ideal for imaging smaller airways but less ideal for larger airways, as the near-infrared light does not penetrate well in airways with diameters larger than 2 cm (70). Multiple airway generations can be visualized in real-time during a single pullback with a resolution of 5–15 μm (71). Several measures of airway structure, including airway lumen area and airway wall area, can be obtained from OCT images (72). OCT-derived measures of human airways have been validated by comparison with pathology from the third- to the ninth-generation bronchi, suggesting that OCT can accurately measure airway anatomy (73).
The impact of airway size on respiratory mechanics during exercise.
The Wb can be divided into the work done by the respiratory muscles to overcome the elasticity of the lung during inspiration, the expiratory muscles to overcome the elastic recoil of the chest wall, and the work to overcome airflow resistance during both inspiration and expiration. Sex-based differences in the breakdown of the total Wb into its four components have been demonstrated in females and males across a wide range of aerobic fitness. When Wb is analyzed at absolute values of (beyond ~60 L·min−1), resistive Wb is consistently higher in females compared with males, and elastic Wb is similar between the sexes (74). A smaller dysanapsis ratio, regardless of sex, has been shown to be associated with a higher Wb and increased mechanical ventilatory constraints during exercise. When sex-based differences in vital capacity were accounted for, females had a smaller dysanapsis ratio than males, resulting in a higher resistive Wb (75). Although these findings helped define the interrelationships among sex, airway size, and the respiratory mechanics of exercise, important questions remained. First, does the dysanapsis ratio correspond to anatomical measures across multiple airway generations in females and males? Second, are in-vivo measures of airway luminal area related to respiratory mechanics during exercise?
We recently addressed these questions by making measures of airway anatomy and respiratory mechanics during exercise in the same group of healthy young females and males. In vivo measures of the 4th–8th airway generations of several airways in each subject were made using OCT during a bronchoscopy procedure. An index of airway size was calculated as the sum of the average luminal area of the 4th–8th generations for each subject. The total cross-sectional area for the 4th–8th generations was calculated based on the branching pattern modeled by Weibel (the number of airways in each generation was approximated by the number 2 raised to the power of generation number) (61). Similar to previous findings using CT (66,68,76), we found that females had significantly smaller airways than males (Fig. 3). In our cohort of healthy young females and males, the predicted dysanapsis ratio was not significantly related to either composite measure of airway size, suggesting that the functional estimate of airway size that the dysanapsis ratio captures may not equate to discrete measures of airway luminal area in healthy young people. The Wb and mechanical ventilatory constraint were then assessed during an incremental exercise test to exhaustion. As resistance highly depends on flow, we analyzed inspired resistance measures at the highest equivalent inspired flow rate achieved by all subjects (i.e., 4.1 ± 0.2 L·s−1). Inspired resistance at 4.1 ± 0.2 L·s−1 was significantly higher in females than males and significantly associated with both composite measures of airway size but not the dysanapsis ratio. Interestingly, logistic regression analysis showed that a small dysanapsis ratio, not composite measures of airway size, was the only statistically significant predictor of EFL. A relationship between EFL prevalence and the dysanapsis ratio in our cohort of females and males agrees with previous studies (75,78), suggesting that the dysanapsis ratio may better predict mechanical ventilatory constraint during exercise than discrete measures of airway size. The results from this study demonstrate the value of utilizing airway imaging in exercise physiology research and have furthered our understanding of the interrelationship between airway structure and function.
FIGURE 3—
The relationship between airway generation, airway resistance, and OCT measured luminal area. A, Airway resistance for a given airway generation. Airway generations measured using OCT in panels C and D are highlighted in black. Also included is Poiseuille’s equation to highlight the importance of airway radius. B, Airway tree highlighting how airway generations are numbered. C, Individual female and male luminal area data measured using OCT for airway generations 4–8. D, Fourth- to eighth-airway-generation OCT images of a representative female subject. The imaging probe, which is 0.9 mm in diameter, can be seen within the airway lumen in OCT images. OCT data in panels C and D are from Peters et al. (77).
Unresolved questions.
Several knowledge gaps remain in our understanding of the association between airway anatomy and exercise. For example, what are the exercise performance implications for differences in airway size between individuals? Future studies need to measure airway anatomy and exercise performance metrics in healthy young females and males to address this question. Researchers can also use novel imaging modalities to further our understanding of the impact of exercise-associated airway damage and remodeling. Athletes sustaining high ventilation during exercise in unfavorable environmental conditions (i.e., cold, dry air, or chlorinated air) are prone to repeated airway epithelium injury. The term airway remodeling describes structural changes in the airway wall caused by repeated injury and repair cycles. Bronchial biopsies have provided evidence for airway remodeling in elite cross-country skiers and swimmers (79). Future longitudinal studies can use OCT to monitor airway structure throughout elite athletes’ careers and into retirement, allowing researchers to address whether airway remodeling affects respiratory mechanics in elite athletes and whether or not remodeling is reversible when athletes retire.
PULMONARY GAS EXCHANGE DURING EXERCISE: INSIGHTS AND CONTROVERSIES, OLD AND NEW
Pulmonary gas exchange is accomplished by the process of ventilation, the delivery of fresh gas to the alveoli, accompanied by perfusion of the pulmonary capillaries with deoxygenated blood. Gas exchange then occurs with oxygen passively diffusing into, and carbon dioxide out of, the capillaries. A primary measure of the efficiency of the pulmonary gas exchange, the alveolar–arterial partial pressure difference for oxygen (AaDO2), reflects the difference between the partial pressure of oxygen in alveolar gas and what subsequently ends up in the arterial blood.
The AaDO2 depends on several factors affecting gas exchange: First, the delivery of fresh gas must be matched to the delivery of deoxygenated blood, ensuring that well-ventilated lung regions are also well perfused, termed ventilation–perfusion matching. When there is too much perfusion of a lung region relative to alveolar ventilation, blood will not oxygenate fully, increasing the AaDO2. The second contributor to the AaDO2 is shunt. This represents an extreme ventilation–perfusion mismatch where a region without ventilation is perfused. In this case, gas exchange does not occur, and pulmonary mixed venous blood is delivered into the arterial circulation. The third contributor to the AaDO2 is diffusion limitation—the time the blood is resident in the pulmonary capillaries and in contact with alveolar gas must be sufficient to allow passive diffusion of gases across the barrier between alveolus and capillary. If the barrier is thickened, contact duration in the capillaries shortened, or the characteristics of oxygen loading onto hemoglobin are altered alone, or in combination, oxygenation will not be complete. One factor that will also affect arterial oxygenation but not increase the AaDO2 is inadequate delivery of fresh gas by alveolar ventilation, termed hypoventilation. Hypoventilation will also impair the elimination of carbon dioxide and affect arterial oxygenation.
Exercise causes an increase in alveolar ventilation, which is adequate to maintain arterial CO2 and alveolar O2 close to preexercise levels during light and moderate exercise; AaDO2 remains relatively low. The net result is that PaO2 deviates little from preexercise levels up to moderate intensity exercise (reviewed in Ref. [80]). At rest, in young, healthy individuals, the AaDO2 is small, typically less than 10 mm Hg, but increases with age. The AaDO2 also increases with increasing exercise intensity up to . The magnitude of the increase is highly variable, but in general, the AaDO2 is higher in individuals with a higher and may reach greater than 50 Torr in some highly trained endurance athletes (80). As the exercise level becomes more intense, ventilation increases disproportionately to metabolic demand, partly because of metabolic acidosis. This increase in ventilation may not elevate alveolar PO2 sufficiently to overcome the increase in AaDO2, and in this case, arterial PO2 may decline.
When the decrease in arterial oxygenation is substantial, exercise-induced arterial hypoxemia may arise (see Refs. [80-82] for a review). It is important to note that EIAH can only be accurately identified in a directly measured sample of arterial blood, with the results corrected for the measured increase in body temperature that occurs with exercise. This condition is not common but affects some highly trained athletes exercising near maximal. EIAH severity is categorized by either the magnitude of decline in arterial oxygen saturation of hemoglobin or the extent of the increase AaDO2 (see Ref. [82] for a review). The development of EIAH may have significant effects on exercise performance. For example, even a small decline in oxygen saturation is enough to reduce , (83,84), with every 1% decline in saturation resulting in a drop of ~2% in (85). Similarly, inducing moderate hypoxemia in exercising athletes results in a decrease in the total amount of work during a times exercise task (86) and increasing locomotor muscle fatigue (87).
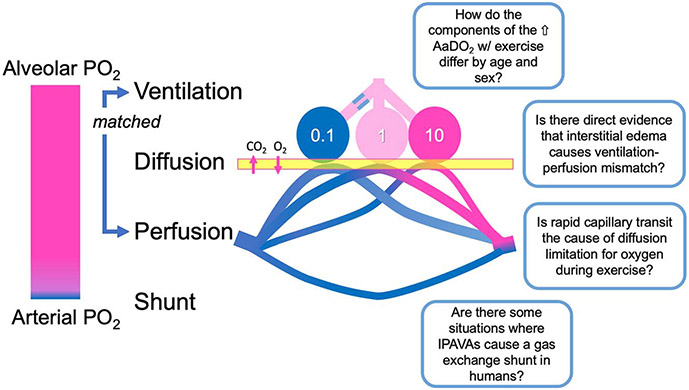
Although EIAH is usually observed at intensities close to and is more common in individuals with a high aerobic capacity, it is occasionally observed during more moderate intensities and in less aerobically gifted individuals (84,88). Typically, it is only when is greater than ~55 mL·kg−1·min−1 that mean AaDO2 exceeds 25 mm Hg, and the PaO2 less than 90 mm Hg—which are the limits of normal response (80,82). An open question is whether EIAH, particularly during submaximal exercise, is more common in females than males (reviewed in Refs. (89,90). Although several studies have evaluated EIAH in females, few have measured arterial blood gases directly. In addition, there are open questions about how to compare between sexes, because in general, females are smaller and is less, both mass-specific and absolute. Hereinafter, we identify some unresolved research questions related to the three causes of the increased AaDO2: ventilation–perfusion mismatch, diffusion limitation, and shunt (Fig. 4).
FIGURE 4—
Research questions related to pulmonary gas exchange illustrated in a schematic diagram of a three-compartment lung. In order for efficient gas exchange to occur, there must be delivery of fresh gas to the alveoli that is well matched to perfusion of the pulmonary capillaries. Here, low (0.1, in this case because of airway obstruction), optimal (1), and high (10, in this case because of reduced perfusion) ventilation–perfusion ratios are shown. When ventilation–perfusion ratio is not close to 1, gas exchange is less efficient. Oxygen and carbon dioxide diffuse passively down their concentration gradients, and diffusion may be incomplete in some circumstances. Blood that bypasses gas exchange is termed a shunt. Alone or in combination, ventilation–perfusion mismatch, diffusion limitation, and/or shunt decreases pulmonary gas exchange efficiency and causes hypoxemia. This is reflected in the AaDO2. Some unresolved research questions regarding pulmonary gas exchange impairments with exercise relate to the following: 1) differences in the AaDO2 by sex and with aging, 2) direct evidence for exercise-induced interstitial edema affecting ventilation–perfusion mismatch, 3) mechanisms of exercise-induced diffusion limitation for oxygen, and 4) possible circumstances where intrapulmonary arteriovenous anastomoses affect gas exchange in humans.
Ventilation–perfusion mismatch
A great deal of what we know about the contribution of ventilation–perfusion mismatch to pulmonary gas exchange has come from the multiple inert gas elimination technique (91-93), MIGET, which can be used to evaluate gas exchange in health and disease both at rest and during exercise. MIGET uses trace amounts of marker gases dissolved in saline that are infused intravenously and the relationships between arterial, expired, and mixed venous concentrations used to solve for the distribution of ventilation–perfusion ratio in multiple gas exchange units. The contributions of ventilation–perfusion mismatch and intrapulmonary shunt to the AaDO2 can be quantified (94,95), and diffusion limitation for oxygen assessed indirectly as the amount of the A-aDO2 not already allocated. During exercise, ventilation–perfusion mismatch accounts for almost all of the AaDO2 until is greater than ~40 mL·kg−1·min−1, where the contribution of diffusion limitation becomes more common in some individuals. Only one study compares the components of the AaDO2 between males and females, and suggests that the increase in ventilation–perfusion mismatch with exercise may be less pronounced in females (96). However, this is not established and is an avenue for further exploration.
There are several lines of indirect evidence that the increase in ventilation–perfusion mismatch with exercise is related to the development of interstitial pulmonary edema acting to distort the nearby alveoli and capillary network and/or altering local compliance affecting distribution of ventilation and blood flow (97). These include 1) changes that persist in recovery after ventilation and cardiac output return to preexercise levels (98), consistent with transient functional changes; 2) the finding that ventilation–perfusion mismatch is increased during normobaric hypoxic exercise (which increases pulmonary arterial pressure and thus the driving pressure for fluid efflux) and reduced by breathing 100% oxygen (94); 3) the observation that increasing exercise duration, potentially exposing the pulmonary circulation to higher pressures for longer, increases ventilation–perfusion mismatch (97); 4) data showing that prolonged exercise increases spatial heterogeneity of pulmonary blood flow consistent with edema compressing blood vessels, and the changes are correlated with ventilation–perfusion mismatch measured by MIGET (99); and 5) evidence that changes with exercise occur in anatomic portions of the lung, which are expected to have greater fluid efflux (100). Despite the aforementioned findings, there is still ongoing debate (101), largely because of limited direct evidence for the presence of pulmonary edema after exercise using various techniques (80). Although this is likely because of limitations of clinical imaging techniques as well as delays between exercise cessation and measurements, there have been no studies that systematically compare anatomical evidence of edema with impairments in pulmonary gas exchange (e.g., by MIGET or arterial blood gas measurement).
Diffusion Limitation
There are two components to diffusion limitation: a gas-phase diffusion limitation related to mixing of alveolar air and an alveolar–capillary membrane diffusion limitation with incomplete equilibrium of gases across the alveolar wall. In humans, gas phase diffusion limitation during exercise is probably small because high gas flow rates during exercise likely improve gas mixing (80). However, alveolar–capillary membrane diffusion limitation for oxygen is possible. With exercise, increases in cardiac output and increased vascular pressures recruit and distend capillaries. This acts to increase capillary blood volume and the area for gas diffusion and diffusing capacity (102), and helps to buffer the effect that the increased cardiac output has on red blood cell capillary transit time. At rest at sea level, there is no evidence that diffusion limitation contributes to the AaDO2 in healthy humans. However, MIGET studies suggest that diffusion limitation occurs during exercise in those capable of oxygen consumptions greater than ~40 mL·kg−1·min−1 at sea level and is increased at higher exercise intensity (80) and while breathing moderate hypoxia (103,104). The extent of diffusion limitation is greater in elite athletes who develop EIAH than those who do not (81,104).
Although the mechanism of diffusion limitation is thought to be inadequate time for oxygen equilibration, secondary to rapid red blood cell transit (105), this is poorly studied. One study did not find evidence for mean transit times below the time required for full equilibration (106), but importantly, mean transit time may be misleading because only a portion of the red cells needs to traverse too rapidly to affect gas exchange. In addition, the exercise intensity evaluated was not maximal, and the contribution of diffusion limitation to the AaDO2 was not assessed. Another study evaluated whole lung (not capillary) transit times and demonstrated a relationship between transit time and diffusion limitation using MIGET (107), but again, this is not definitive proof.
Subjects who develop diffusion limitation often do at submaximal levels of exercise (104), arguing against rapid red cell transit as a mechanism. However, it is the ratio of diffusional conductance (DL) to perfusional conductance (βQ) that determines the completeness of end-capillary equilibration. Cardiac output and thus βQ may be recruited more quickly than diffusional conductance resulting in diffusion limitation. There is some support for this because a lower DL/βQ is seen in those with EIAH compared with those without (104).
Thickening of the blood gas barrier, perhaps due to pulmonary edema, is an unlikely but possible cause of diffusion limitation. In part, this is because interstitial edema accumulates away from the site of gas exchange (108). However, pulmonary edema has not been definitively ruled out as a cause. Although changes in the diffusing capacity for carbon monoxide () are documented after exercise (80), a relationship between postexercise changes and pulmonary gas exchange during exercise (104) is not seen, and although the decreases, the O2 diffusing capacity calculated from the inert gases (8) does not. Thus, these changes in may be related to decreased pulmonary capillary volume after exercise (109).
Shunt
In gas exchange terms, a shunt is defined as blood that passes from the venous system to the arterial system without contacting ventilated lung areas. Shunts occur 1) outside the lungs, such as communications between atria or ventricles (intracardiac); 2) after pulmonary arising from the bronchial or the coronary circulations, both of which drain blood into the left side of the heart; or 3) within the lungs (intrapulmonary). All will increase AaDO2 because deoxygenated blood enters the systemic circulation. It is generally thought that extrapulmonary shunting contributes a minimal amount to the overall A-aDO2 in normal subjects as postpulmonary shunt likely comprises a relatively small amount of cardiac output (94,110,111), and intracardiac shunts are not considered normal.
However, there has been considerable investigation and controversy over the role of the contribution of intrapulmonary shunt to the AaDO2 during exercise, particularly that arising from anatomical arteriovenous anastomoses in the lung (112). These anastomoses between pulmonary arteries and veins are larger than the normal capillary and are seen in isolated human and dog lungs perfused with physiologic pressures (113,114). They are documented by microspheres in dogs where they are recruited during exercise (114) and thus have to potential to contribute significantly to the AaDO2. Agitated saline contrast echocardiography, a nonquantitative technique, has suggested that exercise recruits these pathways in hypoxia, exercise, and with certain pharmacologic interventions (80,115). Although studies demonstrating these pathways during exercise using microspheres of a fixed diameter are lacking in humans, several authors have suggested that they may contribute significantly to the AaDO2. The controversy has arisen because none of the MIGET studies have documented significant (>1% of cardiac output) shunt of any type during exercise under any circumstance (94,97,103,110,116), similar to results using 100% oxygen to assess shunt ([117] and reviewed in Ref. [80]).
Recent work in animals (118) has resolved some of this controversy by comparing anatomical intrapulmonary/intracardiac arteriovenous connections evaluated by microspheres and gas exchange shunt measured by MIGET to agitated saline contrast echocardiography. The work confirmed the presence of anatomical arteriovenous anastomoses in the lung. Anatomical shunt, measured by microspheres, paralleled MIGET gas exchange shunt, with an overestimate by MIGET, which is expected because of MIGET’s detection of shunts arising from unventilated lung as well as anatomic/cardiac shunts. However, agitated saline contrast was very sensitive but almost entirely nonspecific with contrast bubbles visualized in the left ventricle when only a very small (or absent) anatomic shunt was measured using microspheres. In addition, the gas exchange abnormality associated with anatomic intrapulmonary connections detected by contrast echocardiography was often small and largely insignificant for gas exchange. Thus, these tools should not be used to assess the magnitude of anatomical arteriovenous anastomoses in the lung. Studies comparing MIGET assessment of gas exchange with a rigorous assessment of anatomical arteriovenous anastomoses in humans are lacking.
Unresolved Questions
The aforementioned review has briefly focused on some of the questions and controversies in our understanding of pulmonary gas exchange during exercise. Although considerable progress has been made, there remain a great many unresolved questions involving the following aspects.
Sex- and age-based differences in pulmonary gas exchange.
Although many studies have evaluated differences in lung mechanics between sexes, fewer have comprehensively addressed pulmonary gas exchange. How do the various contributions to the AaDO2 differ between females and males? How the components of the AaDO2 change with healthy aging is also unknown. Answering these questions will require thoughtful consideration of the appropriate way to compare between sexes/subjects of different ages and exercise capacity and moving beyond pulse oximeter studies to directly assess pulmonary gas exchange and the components of the AaDO2.
Interstitial pulmonary edema during exercise.
Is interstitial pulmonary edema the cause of ventilation–perfusion mismatch? Does it affect diffusion limitation for oxygen? Answering this question will require the development of new and more sensitive imaging techniques that allow intravascular lung water (i.e., pulmonary blood volume) to be distinguished from extravascular water (i.e., edema fluid) and combined with comprehensive gas exchange measurements to assess the various components of the AaDO2.
Capillary transit time and diffusion limitation.
Is the mechanism of diffusion limitation of oxygen rapid red blood cell transit time through the pulmonary circulation? Answering this question will require measurement of pulmonary capillary transit time, consideration of heterogeneity of transit times, and gas exchange measurements to assess the extent of diffusion limitation.
Anatomical arteriovenous anastomoses.
Although anatomical arteriovenous anastomoses are well documented, the contribution of shunt to exercising gas exchange in healthy humans is minimal. Are there some circumstances where anatomical arteriovenous anastomoses significantly affect gas exchange in humans? Answering this question will require comparing detailed gas exchange measurements to a careful and fully quantitative assessment of anatomical arteriovenous anastomoses. Agitated saline contrast echocardiography is not adequate for this task. Quantifying anatomical arteriovenous anastomoses may require the development of a microsphere that is suitable for use in humans and can be injected intravenously and sampled in arterial blood; current imaging-based techniques such as technetium-99 macroaggregated albumin are not sufficiently accurate.
RESPIRATORY INFLUENCES ON CARDIOVASCULAR CONTROL DURING EXERCISE
Demands Placed on the Respiratory Muscle during Exercise
In healthy humans, the architecture of ventilation changes in response to exercise in a predictable fashion. The rise in with low levels of exercise is accomplished by increasing both the frequency of breathing () and tidal volume (). With mild to moderate exercise, the dominant ventilatory response is that expands into both inspiratory and expiratory reserve volumes with relatively smaller increases in . During heavier work, once reaches 50%–60% of vital capacity, plateaus, and further increases in are primarily accomplished by elevations in . With increasing work intensity, can reach approximately 50% of vital capacity and fb may increase to 40–50 breaths per minute, although there is considerable between-person variation. The respiratory musculature must contract rhythmically and in a highly coordinated fashion to increase ventilation such that it is well matched to exercise-induced increases in . The large ventilations accompanying heavy exercise cost a considerable amount of energy, and it has been suggested that breathing may be regarded as a form of muscular exercise in and of itself (119). With this in mind, it is important to consider: what constitutes a “respiratory muscle”? The answer is not easily addressable and is dependent on the definition used. As a useful start point, consider that there are approximately 63 muscles with a documented role in breathing (i.e., assists lung inflation or deflation) across a range of physiological states (120), and that in healthy humans, the respiratory muscle mass is estimated to be in the order of 4–6 kg (121).
Under conditions of heavy exercise, the primary and accessory muscles of inspiration are recruited to generate negative intrathoracic pressure and airflow. It is equally important to consider that the increased ventilatory demand of exercise requires the use of accessory expiratory muscles, notably those of the abdomen, to increase the rate of expiration above that produced by elastic recoil alone. Given the high demands placed on the respiratory musculature, what are the associated physiological costs? The Wb can be determined by measuring the total energy required by the respiratory muscles or the work done by them (if both are measured simultaneously mechanical efficiency can be calculated). The total energy required, or O2 cost, of ventilation can account for approximately 7%–10% of whole-body in healthy but untrained male subjects (122,123) and 15%–16% in highly trained males (122). Moreover, recent work shows that the O2 cost of ventilation is higher in females relative to fitness-matched males (124).
Given the aforementioned brief summary, we now turn our attention to the following. Owing to the close relationship between O2 uptake and blood flow (i.e., Fick Principle), do the high demands of both muscle groups—limb versus respiratory—influence one another? And if so, what might the consequences be to the integrated physiology of exercise? In addressing these questions, we have focused, in part, on a series of studies that unloaded the inspiratory muscles during exercise using a proportional assist ventilator (PAV) to prevent most of the normally occurring exercise-induced increases in respiratory muscle work. By necessity, we also draw upon those investigations that have used exercising animal models to address questions that cannot be answered in exercising humans.
Blood Flow Distribution
The blood flow requirements of the respiratory musculature in exercising humans are difficult to quantify owing to methodological and anatomical constraints. Estimates from the exercising equine suggest that the blood flow to the “inspiratory and expiratory” muscles amount to 15%–16% of total cardiac output (125), which is consistent with other species that have high respiratory muscle blood flows during exercise including the dog (126) and rodent (127). Harms et al. (128) were the first to investigate the effect of respiratory muscle work on the distribution of cardiac output in humans. In this investigation, highly trained cyclists performed heavy exercise (>85% ) while leg blood flow was measured using thermodilution techniques and the Wb was artificially lowered with a PAV by 50%. With a reduced Wb, there was a commensurate increase in limb blood flow (~7%) and vascular conductance (~10%). An important consideration is that the changes to leg blood flow that accompany PAV-induced reductions in the Wb are not present during submaximal exercise (129). This can be interpreted to mean that the load on (or when removed from) the respiratory muscles is sufficiently small during submaximal exercise to alter the sympathetic nervous system’s control of blood flow distribution (see discussion hereinafter: Autonomic Control of the Circulation).
Based on the available evidence, it can be concluded that a substantial portion of the total cardiac output and total VO2 (~10%–16%) is devoted to inspiratory and expiratory muscle work during heavy exercise. However, these estimates do not reflect blood flow measures to the respiratory muscles, per se. When unloading the Wb during exercise, is the “extra” blood flow diverted away from the respiratory muscles to the working limb muscle? Methodological advances now permit the infusion of indocyanine green dye coupled with near-infrared spectroscopy to allow quantification of blood flow to accessory muscle of breathing. For example, Guenette et al. (130,131) have shown that, with increasing ventilation during exercise or voluntary hyperpnea, there is a corresponding increase of blood flow to the muscular region of the seventh intercostal space and the sternocleidomastoid. More recently, Dominelli et al. (132) used a similar approach to simultaneously assess blood flow to both limb and respiratory muscle during heavy exercise (90% peak work rate) when the Wb was lowered with a PAV. With a reduction in the Wb, there was a corresponding reduction in respiratory muscle (sternocleidomastoid; −49% ± 26% of control) blood flow and an increase in locomotor muscle (vastus lateralis) blood flow with some between-participant variation. These findings provide supporting evidence to the concept that respiratory muscle work significantly influences the distribution of blood flow to both respiratory and locomotor muscles under conditions of heavy exercise.
Autonomic Control of the Circulation
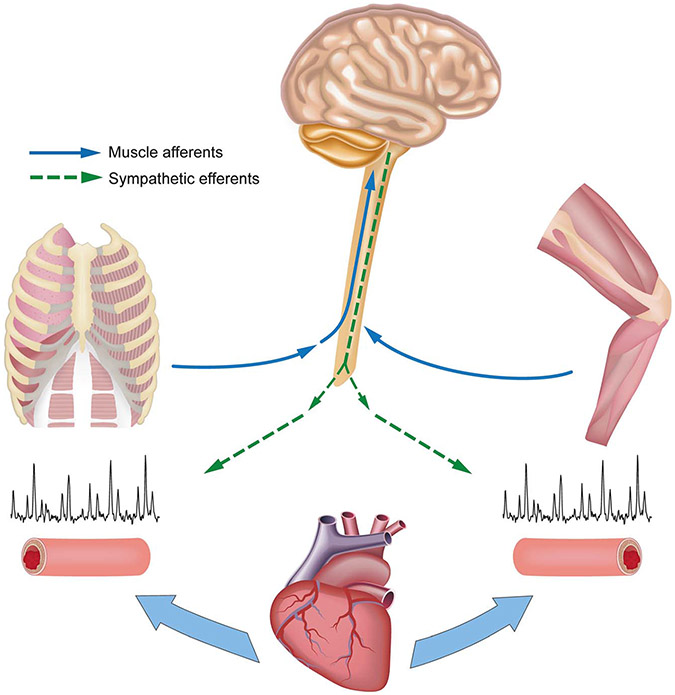
The regulation of blood flow to contracting skeletal muscle is a balance between local vasodilation and sympathetic vasoconstriction where the cardiac output that is available is “sent” to the contracting musculature most in demand of O2. The regulation of blood pressure is also required to ensure that there is appropriate perfusion to all organs. An important consideration is that skeletal muscle has an enormous capacity to vasodilate during exercise, whereas the pumping capacity of the human heart is limited (133), which in turn means that, during heavy exercise, perfusion of the contracting muscles must be limited or mean arterial pressure will fall. There is a complex interplay between sympathetic vasoconstriction and metabolic vasodilation that limits blood flow to contracting muscles to maintain mean arterial pressure. There are several lines of evidence in support of significant sympathetic vasoconstrictor influences over local vasodilation in exercising muscle in healthy humans (35). Can high levels of respiratory muscle work activate sympathetically mediated vasoconstriction in the limb as has been demonstrated for limb locomotor skeletal muscles? The hypothesis is that, as with limb muscle, intense and repeated muscle contractions and accumulation of metabolites in the respiratory muscles activate phrenic afferents, which in turn increases sympathetic vasoconstrictor activity via a supraspinal reflex (Fig. 5). Here we present three examples to illustrate that the high Wb or “metabolic conditions” of the respiratory musculature have an effect on the autonomic control of blood flow to the exercising limb.
FIGURE 5—
Schematic of a “two-way street” of sympathetic vasoconstrictor activity emanating from both limb and respiratory muscle metaboreceptors during exercise, which serves to constrain blood flow and O2 transport. High-intensity contractions of both sets of muscles cause increased group III and IV afferent activity leading to a sympathetically mediated vasoconstriction of respiratory and limb locomotor muscle vasculatures, thereby contributing to their mutual fatigue during whole-body exercise. Figure and original caption from Sheel et al. (35).
First, during heavy exercise when cardiac output is finite, the addition of arm exercise to leg exercise results in a reduction in leg blood flow to allow perfusion of the upper limb (134). The analogous paradigm is when respiratory muscle work is altered and blood flow to the limb and respiratory muscles is changed in a reciprocal manner (see Refs. (132,135,136) and the aforementioned description). Lowering the Wb with heavy exercise is accompanied by a significant increase in norepinephrine spillover across the working muscle (135). More recent and direct evidence comes from studies that have lowered the normally occurring Wb during exercise and found commensurate reductions in muscle sympathetic nerve activity (median nerve) of the resting upper limb (137). Collectively, these observations provide evidence of a sympathetically mediated vasoconstrictor effect emanating from respiratory muscles during exercise. Second, Rodman et al. (138) transiently infused lactic acid into the diaphragm via the phrenic artery of exercising dogs and observed a reduction in hind-limb blood flow and vascular conductance, which is consistent with metaboreflex activation as previously reported for limb muscles metaboreflex. As such, the metabolite production (or accumulation) associated with high levels of respiratory muscle work may contribute to the increased sympathetic tone and redistribution of blood. Additional support for this idea comes from Hussain et al. (139), who showed that chemical stimulation of thin fiber phrenic afferents resulted in systemic vasoconstriction. Third, with high levels of voluntary efforts against loads—and presumably metabolite accumulation—in resting humans, there is a time-dependent increase in limb muscle sympathetic nerve activity (140,141) and reduction in vascular conductance and blood flow to the resting limb (142).
Respiratory Muscle Fatigue
With heavy exercise (>85% ), diaphragm fatigue occurs as defined by a reduction in transdiaphragmatic twitch pressure (Pdi,tw) in response to either electrical or magnetic stimulation of the phrenic nerves stimulation (143). Diaphragmatic fatigue occurs relatively early during high-intensity endurance exercise and is related to the amount of pressure and work generated by respiratory muscles (144). There is also a strong relationship between the force output of the diaphragm and the magnitude of fatigue observed (145). By unloading the respiratory muscles, fatigue of the diaphragm is prevented (146). These aggregate findings are important as they illustrate that the workload performed by the respiratory muscles is a critical determinant of exercise-induced diaphragmatic fatigue. It should also be noted that the abdominal expiratory muscles also incur significant decrements in pressure-generating capacity with heavy-intensity exercise (147).
As described previously, the diaphragm (and other respiratory muscles) commands a significant fraction of cardiac output during heavy exercise. These observations are important when considering fatigue of the respiratory musculature. For example, blood flow has been shown to be a primary determinant of diaphragm fatigue where it can be attenuated by increasing phrenic artery blood flow in dogs (148). Although this is highly relevant, it does not necessarily speak to the conditions of whole-body, high-intensity exercise where there is acidification of blood, accumulation of metabolites within the diaphragm, and finite cardiac output along with global sympathetic vasomotor outflow. Said differently, do the conditions of exercise themselves (rather than simply high respiratory work) influence blood flow distribution and facilitate fatigue of the diaphragm? Babcock et al. (145) indirectly addressed this by having subjects voluntarily replicate the fatiguing high work of exercise ventilation under resting conditions and found minimal evidence of diaphragm fatigue, and in those subjects where small-degree fatigue was present, recovery was hastened relative to the exercise condition. Moreover, only when voluntary work was 60%–80% in excess of that produced during exercise did diaphragm fatigue occur. These observations can be taken to indicate that there is a critical influence of blood flow distribution to the diaphragm during exercise that contributes to diaphragm fatigue. Do high levels of sympathetic vasoconstrictor activity originating from the exercising limb place constraint on blood flow and O2 transport in the diaphragm, thereby promoting diaphragm fatigue (Fig. 5; “two way street of sympathetic vasoconstrictor activity”)? The aforementioned work of Babcock et al. would indirectly support this hypothesis. Additional indirect evidence to support the idea of “preferential” redistribution of blood flow to the diaphragm versus limb muscles during exercise is seen in the rodent heart failure model (149). Heart failure is often characterized by a high level of sympathetic vasoconstrictor activity and a high Wb, and at least in the rodent model, conductance and blood flow to the diaphragm appear prioritized at the expense of conductance and flow to the exercising limb.
Unresolved Questions
Do respiratory muscles have a “priority” over locomotor muscles for blood flow distribution during exercise in health?.
There is a blunted response to α1-adrenergic constriction in diaphragm versus locomotor arterioles in rodents (150), which may contribute to nonuniform muscle blood flow seen under exercising conditions. Evidence for the argument of prioritization in humans is indirect, and further experiments and methodological advances and approaches are needed. For example, valid and reliable measures of blood flow and conductance to both respiratory and locomotor muscles are required. An ability to assess the responsiveness and densities of adrenergic receptors across different muscle groups would help to shed light on the question.
Clinical relevance.
Recent work has highlighted that respiratory muscle unloading improves limb blood flow and conductance, reduces limb fatigue, and improves exercise tolerance in heart failure and COPD (151,152). Importantly, many of these effects are seen during submaximal exercise, which differs from that seen with higher intensities in health. A relevant question here is whether or not chronic training of limb or respiratory muscles alters reflexive blood flow distribution and muscular fatigue. Based on initial evidence and our summary, there is rationale to pursue further training interventions that determine the role of afferents in both sets of muscles and the associated sympathetic responses that may be altered. A greater understanding of the underlying mechanism(s) of training-induced changes may lead to a more targeted basis for exercise prescription and the attenuation of disease progression.
SUMMARY: THE FUTURE
The past four decades have yielded key advances and also raised exciting new challenges concerning our questions of the lung’s suitability and adaptability in meeting the demands posed by exercise. Major advances that further underscore and explain the near ideal respiratory system response to exercise in the healthy, untrained young adult include quantification of distribution and diffusion contributions to alveolar to arterial O2 transport and identification of key elements of airway mechanics and respiratory muscle recruitment to ensure minimal respiratory muscle work and O2 cost. Some further mechanistic insights into the long-held mystery of exercise hyperpnea have even been gained. In addition, new examples of an underadapted respiratory system in many highly trained include exercise-induced hypoxemia in the female endurance athlete, airways susceptible to flow limitation, epithelial injury and remodeling at high ventilatory demand, a pulmonary vasculature and right heart underbuilt for the demands imposed by high cardiac output, and fatigable respiratory muscles triggering sympathetically mediated blood flow redistribution. The substantial negative influences of aging on airway, chest wall, and pulmonary vascular structures and the narrowed intrathoracic airways in the adult female have been documented as additional potential sources of exercise limitation, especially in the highly fit. Long-term adaptability of lung structure, especially at the alveolar–capillary interface, is an exciting emerging area of discovery that includes the presence of an abundant progenitor/stem cell population with an intrinsic capacity for de novo lung tissue formation in response to chronic stressors. We questioned if these growth factors would respond to exercise training. To date, the available evidence confirms traditional concepts of a minimal, if any, adaptive short-term training response in the healthy young adult lung. However, other evidence in animal models primarily but even in some humans strongly suggests that the compromised lung as occurs in chronic smokers, asthma, lung diseases, and even aging may respond to the exercise training stimulus via de novo growth or prevention of further deterioration of lung parenchyma. Bold new innovative investigations as suggested throughout this review for several key problems in exercise pulmonary physiology and pathophysiology are now needed to build on these new understandings.
Acknowledgments
Many thanks to Professor Barbara Morgan for her assistance and insight and to Professor Michael Stickland for sharing his expertise.
C. M. P.: Canadian Lung Association—research fellowship. J. A. D.: original work reported in this publication was supported by the National Heart, Lung, and Blood Institute. S. R. H.: research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1R56HL159710. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A. W. S.: research reported in the publication was supported by the Natural Sciences and Engineering Research Council of Canada under Award Number RGPIN-2022-02977.
Footnotes
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constituted endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47(1–2):112–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey JA. J.B. Wolffe memorial lecture. Is the lung built for exercise? Med Sci Sports Exerc. 1986;18(2):143–55. [PubMed] [Google Scholar]
- 3.Liu M, Zhang L, Marsboom G, et al. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat Commun. 2019;10(1):2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekh KR, Nawroth J, Pai A, et al. Stem cells and lung regeneration. Am J Physiol Cell Physiol. 2020;319(4):C675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basil MC, Cardenas-Diaz FL, Kathiriya JJ, et al. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature. 2022;604(7904):120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Zhao Y, Li D, et al. Intrapulmonary distal airway stem cell transplantation repairs lung injury in chronic obstructive pulmonary disease. Cell Prolif. 2021;54(6):e13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nymand SB, Hartmann JP, Ryrso CK, et al. Exercise adaptations in COPD: the pulmonary perspective. Am J Physiol Lung Cell Mol Physiol. 2022;323(6):L659–66. [DOI] [PubMed] [Google Scholar]
- 8.Ad hoc Statement Committee, American Thoracic Society. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170(3):319–43. [DOI] [PubMed] [Google Scholar]
- 9.Cerny FC, Dempsey JA, Reddan WG. Pulmonary gas exchange in nonnative residents of high altitude. J Clin Invest. 1973;52(12):2993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlin JI, Hsia CC, Cassidy SS, et al. Recruitment of lung diffusing capacity with exercise before and after pneumonectomy in dogs. J Appl Physiol (1985). 1991;70(1):135–42. [DOI] [PubMed] [Google Scholar]
- 11.Butler JP, Loring SH, Patz S, et al. Evidence for adult lung growth in humans. N Engl J Med. 2012;367(3):244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massaro D, Alexander E, Reiland K, et al. Rapid onset of gene expression in lung, supportive of formation of alveolar septa, induced by refeeding mice after calorie restriction. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1313–26. [DOI] [PubMed] [Google Scholar]
- 13.Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41(4):594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat Rev Rheumatol. 2015;11(2):86–97. [DOI] [PubMed] [Google Scholar]
- 15.Hassel E, Stensvold D, Halvorsen T, et al. Association between pulmonary function and peak oxygen uptake in elderly: the generation 100 study. Respir Res. 2015;16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeill J, Chernofsky A, Nayor M, et al. The association of lung function and pulmonary vasculature volume with cardiorespiratory fitness in the community. Eur Respir J. 2022;60(2):2101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasch-Halvorsen Ø, Hassel E, Langhammer A, Brumpton BM, Steinshamn S. The association between dynamic lung volume and peak oxygen uptake in a healthy general population: the HUNT study. BMC Pulm Med. 2019;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempsey JA, La Gerche A, Hull JH. Is the healthy respiratory system built just right, overbuilt, or underbuilt to meet the demands imposed by exercise? J Appl Physiol (1985). 2020;129(6):1235–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross KA, Thurlbeck WM. Lung growth in newborn guinea pigs: effects of endurance exercise. Respir Physiol. 1992;89(3):353–64. [DOI] [PubMed] [Google Scholar]
- 20.Kirkton SD, Howlett RA, Gonzalez NC, et al. Continued artificial selection for running endurance in rats is associated with improved lung function. J Appl Physiol (1985). 2009;106(6):1810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saltin B, Blomqvist G, Mitchell JH, et al. Response to exercise after bed rest and after training. Circulation. 1968;38(5 Suppl):VII1–78. [PubMed] [Google Scholar]
- 22.Flaherty JM, Smoliga JM, Zavorsky GS. The effect of increased physical activity on pulmonary diffusing capacity in unfit women. Exp Physiol. 2014;99(3):562–70. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey JA, Gledhill N, Reddan WG, et al. Pulmonary adaptation to exercise: effects of exercise type and duration, chronic hypoxia and physical training. Ann N Y Acad Sci. 1977;301:243–61. [DOI] [PubMed] [Google Scholar]
- 24.Clanton TL, Dixon GF, Drake J, Gadek JE. Effects of swim training on lung volumes and inspiratory muscle conditioning. J Appl Physiol (1985). 1987;62(1):39–46. [DOI] [PubMed] [Google Scholar]
- 25.Mickleborough TD, Stager JM, Chatham K, Lindley MR, Ionescu AA. Pulmonary adaptations to swim and inspiratory muscle training. Eur J Appl Physiol. 2008;103(6):635–46. [DOI] [PubMed] [Google Scholar]
- 26.Leith DE, Bradley M. Ventilatory muscle strength and endurance training. J Appl Physiol. 1976;41(4):508–16. [DOI] [PubMed] [Google Scholar]
- 27.Taylor CR. Structural and functional limits to oxidative metabolism: insights from scaling. Annu Rev Physiol. 1987;49:135–46. [DOI] [PubMed] [Google Scholar]
- 28.Armour J, Donnelly PM, Bye PT. The large lungs of elite swimmers: an increased alveolar number? Eur Respir J. 1993;6(2):237–47. [PubMed] [Google Scholar]
- 29.Mazic S, Lazovic B, Djelic M, et al. Respiratory parameters in elite athletes—does sport have an influence? Rev Port Pneumol (2006). 2015;21(4):192–7. [DOI] [PubMed] [Google Scholar]
- 30.Zavorsky GS, Smoliga JM. The association between cardiorespiratory fitness and pulmonary diffusing capacity. Respir Physiol Neurobiol. 2017;241:28–35. [DOI] [PubMed] [Google Scholar]
- 31.Lalande S, Yerly P, Faoro V, Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol. 2012;590(17):4279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tedjasaputra V, Bouwsema MM, Stickland MK. Effect of aerobic fitness on capillary blood volume and diffusing membrane capacity responses to exercise. J Physiol. 2016;594(15):4359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claessen G, Claus P, Ghysels S, et al. Right ventricular fatigue developing during endurance exercise: an exercise cardiac magnetic resonance study. Med Sci Sports Exerc. 2014;46(9):1717–26. [DOI] [PubMed] [Google Scholar]
- 34.La Gerche A, Rakhit DJ, Claessen G. Exercise and the right ventricle: a potential Achilles' heel. Cardiovasc Res. 2017;113(12):1499–508. [DOI] [PubMed] [Google Scholar]
- 35.Sheel AW, Boushel R, Dempsey JA. Competition for blood flow distribution between respiratory and locomotor muscles: implications for muscle fatigue. J Appl Physiol (1985). 2018;125(3):820–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kippelen P, Anderson SD. Airway injury during high-level exercise. Br J Sports Med. 2012;46(6):385–90. [DOI] [PubMed] [Google Scholar]
- 37.Bayly WM, Hodgson DR, Schulz DA, Dempsey JA, Gollnick PD. Exercise-induced hypercapnia in the horse. J Appl Physiol (1985). 1989;67(5):1958–66. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Erickson HH. Highly athletic terrestrial mammals: horses and dogs. Compr Physiol. 2011;1(1):1–37. [DOI] [PubMed] [Google Scholar]
- 39.Reeves J, Dempsey J, Grover R. n: Weir E, Reeves J, editiors. Pulmonary Vascular Physiology and Pathophysiology. New York (NY): Marcel Dekker, 1989, p. 107–33. [Google Scholar]
- 40.Johnson BD, Badr MS, Dempsey JA. Impact of the aging pulmonary system on the response to exercise. Clin Chest Med. 1994;15(2):229–46. [PubMed] [Google Scholar]
- 41.McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol (1985). 1995;78(5):1957–68. [DOI] [PubMed] [Google Scholar]
- 42.Pelkonen M, Notkola IL, Lakka T, et al. Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med. 2003;168(4):494–9. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–63. [DOI] [PubMed] [Google Scholar]
- 44.Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stav D, Raz M, Shpirer I. Three years of pulmonary rehabilitation: inhibit the decline in airflow obstruction, improves exercise endurance time, and body-mass index, in chronic obstructive pulmonary disease. BMC Pulm Med. 2009;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aakerøy L, Nørstebø EA, Thomas KM, et al. High-intensity interval training and pulmonary hemodynamics in COPD with hypoxemia. Eur Clin Respir J. 2021;8(1):1984642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586(1):161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gagnon P, Bussieres JS, Ribeiro F, et al. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(7):606–15. [DOI] [PubMed] [Google Scholar]
- 49.Hassel E, Berre AM, Skjulsvik AJ, Steinshamn S. Effects of exercise training on pulmonary vessel muscularization and right ventricular function in an animal model of COPD. Respir Res. 2014;15(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toledo-Arruda AC, Vieira RP, Guarnier FA, et al. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J Appl Physiol (1985). 2017;123(3):674–83. [DOI] [PubMed] [Google Scholar]
- 51.Tang K, Xia FC, Wagner PD, Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol. 2010;170(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jarmuszkiewicz W, Dominiak K, Galganski L, et al. Lung mitochondria adaptation to endurance training in rats. Free Radic Biol Med. 2020;161:163–74. [DOI] [PubMed] [Google Scholar]
- 53.Mercken EM, Hageman GJ, Schols AM, et al. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(8):994–1001. [DOI] [PubMed] [Google Scholar]
- 54.Ryrsø CK, Thaning P, Siebenmann C, et al. Effect of endurance versus resistance training on local muscle and systemic inflammation and oxidative stress in COPD. Scand J Med Sci Sports. 2018;28(11):2339–48. [DOI] [PubMed] [Google Scholar]
- 55.Sahin H, Naz I, Varol Y, et al. COPD patients with severe diffusion defect in carbon monoxide diffusing capacity predict a better outcome for pulmonary rehabilitation. Rev Port Pneumol (2006). 2016;22(6):323–30. [DOI] [PubMed] [Google Scholar]
- 56.Santus P, Radovanovic D, Balzano G, et al. Improvements in lung diffusion capacity following pulmonary rehabilitation in COPD with and without ventilation inhomogeneity. Respiration. 2016;92(5):295–307. [DOI] [PubMed] [Google Scholar]
- 57.da Cunha MJ, da Cunha AA, Scherer EBS, et al. Experimental lung injury promotes alterations in energy metabolism and respiratory mechanics in the lungs of rats: prevention by exercise. Mol Cell Biochem. 2014;389(1–2):229–38. [DOI] [PubMed] [Google Scholar]
- 58.Vieira RP, de Toledo AC, Ferreira SC, et al. Airway epithelium mediates the anti-inflammatory effects of exercise on asthma. Respir Physiol Neurobiol. 2011;175(3):383–9. [DOI] [PubMed] [Google Scholar]
- 59.Silva RA, Vieira RP, Duarte ACS, et al. Aerobic training reverses airway inflammation and remodelling in an asthma murine model. Eur Respir J. 2010;35(5):994–1002. [DOI] [PubMed] [Google Scholar]
- 60.Hogg JC, Pare PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97(2):529–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weibel ER. Why measure lung structure? Am J Respir Crit Care Med. 2001;163(2):314–5. [DOI] [PubMed] [Google Scholar]
- 62.Green M, Mead J, Turner JM. Variability of maximum expiratory flow–volume curves. J Appl Physiol. 1974;37(1):67–74. [DOI] [PubMed] [Google Scholar]
- 63.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121(2):339–42. [DOI] [PubMed] [Google Scholar]
- 64.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol (1985). 1987;63(5):2042–7. [DOI] [PubMed] [Google Scholar]
- 65.Vasilescu DM, Phillion AB, Kinose D, et al. Comprehensive stereological assessment of the human lung using multiresolution computed tomography. J Appl Physiol (1985). 2020;128(6):1604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheel AW, Guenette JA, Yuan R, et al. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985). 2009;107(5):1622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christou S, Chatziathanasiou T, Angeli S, et al. Anatomical variability in the upper tracheobronchial tree: sex-based differences and implications for personalized inhalation therapies. J Appl Physiol (1985). 2021;130(3):678–707. [DOI] [PubMed] [Google Scholar]
- 68.Dominelli PB, Ripoll JG, Cross TJ, et al. Sex differences in large conducting airway anatomy. J Appl Physiol (1985). 2018;125(3):960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ripoll JG, Guo W, Andersen KJ, et al. Sex differences in paediatric airway anatomy. Exp Physiol. 2020;105(4):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paré PD, Nagano T, Coxson HO. Airway imaging in disease: gimmick or useful tool? J Appl Physiol (1985). 2012;113(4):636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wijmans L, d'Hooghe JN, Bonta PI, Annema JT. Optical coherence tomography and confocal laser endomicroscopy in pulmonary diseases. Curr Opin Pulm Med. 2017;23(3):275–83. [DOI] [PubMed] [Google Scholar]
- 72.Kirby M, Ohtani K, Nickens T, et al. Reproducibility of optical coherence tomography airway imaging. Biomed Opt Express. 2015;6(11):4365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Y, Ding M, Guan WJ, et al. Validation of human small airway measurements using endobronchial optical coherence tomography. Respir Med. 2015;109(11):1446–53. [DOI] [PubMed] [Google Scholar]
- 74.Guenette JA, Querido JS, Eves ND, Chua R, Sheel AW. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R166–75. [DOI] [PubMed] [Google Scholar]
- 75.Dominelli PB, Molgat-Seon Y, Bingham D, et al. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol (1985). 2015;119(10):1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vameghestahbanati M, Kirby M, Tanabe N, et al. Central airway tree dysanapsis extends to the peripheral airways. Am J Respir Crit Care Med. 2021;203(3):378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters CM, Leahy MG, Hohert G, et al. Airway luminal area and the resistive work of breathing during exercise in healthy young females and males. J Appl Physiol (1985). 2021;131(6):1750–61. [DOI] [PubMed] [Google Scholar]
- 78.Smith JR, Rosenkranz SK, Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol. 2014;198:25–31. [DOI] [PubMed] [Google Scholar]
- 79.Kippelen P, Fitch KD, Anderson SD, et al. Respiratory health of elite athletes—preventing airway injury: a critical review. Br J Sports Med. 2012;46(7):471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stickland MK, Lindinger MI, Olfert IM, Heigenhauser GJ, Hopkins SR. Pulmonary gas exchange and acid–base balance during exercise. Compr Physiol. 2013;3(2):693–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins SR. Exercise induced arterial hypoxemia: the role of ventilation–perfusion inequality and pulmonary diffusion limitation. Adv Exp Med Biol. 2006;588:17–30. [DOI] [PubMed] [Google Scholar]
- 82.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol (1985). 1999;87(6):1997–2006. [DOI] [PubMed] [Google Scholar]
- 83.Powers SK, Lawler J, Dempsey JA, Dodd S, Landry G. Effects of incomplete pulmonary gas exchange on VO2 max. J Appl Physiol (1985). 1989;66(6):2491–5. [DOI] [PubMed] [Google Scholar]
- 84.Harms CA, McClaran SR, Nickele GA, et al. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998;507(Pt 2):619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harms CA, McClaran SR, Nickele GA, et al. Effect of exercise-induced arterial O2 desaturation on VO2max in women. Med Sci Sports Exerc. 2000;32(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 86.Koskolou MD, McKenzie DC. Arterial hypoxemia and performance during intense exercise. Eur J Appl Physiol. 1994;68(1):80–6. [DOI] [PubMed] [Google Scholar]
- 87.Amann M, Eldridge MW, Lovering AT, et al. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575(Pt 3):937–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rice AJ, Scroop GC, Gore CJ, et al. Exercise-induced hypoxaemia in highly trained cyclists at 40% peak oxygen uptake. Eur J Appl Physiol. 1999;79(4):353–9. [DOI] [PubMed] [Google Scholar]
- 89.Dominelli PB, Molgat-Seon Y, Sheel AW. Sex differences in the pulmonary system influence the integrative response to exercise. Exerc Sport Sci Rev. 2019;47(3):142–50. [DOI] [PubMed] [Google Scholar]
- 90.Hopkins SR, Harms CA. Gender and pulmonary gas exchange during exercise. Exerc Sport Sci Rev. 2004;32(2):50–6. [DOI] [PubMed] [Google Scholar]
- 91.Evans JW, Wagner PD, West JB. Conditions for reduction of pulmonary gas transfer by ventilation–perfusion inequality. J Appl Physiol. 1974;36(5):533–7. [DOI] [PubMed] [Google Scholar]
- 92.Wagner PD, Naumann PF, Laravuso RB. Simultaneous measurement of eight foreign gases in blood by gas chromatography. J Appl Physiol. 1974;36(5):600–5. [DOI] [PubMed] [Google Scholar]
- 93.Wagner PD. The multiple inert gas elimination technique (MIGET). Intensive Care Med. 2008;34(6):994–1001. [DOI] [PubMed] [Google Scholar]
- 94.Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during exercise at sea level. J Appl Physiol (1985). 1986;60(5):1590–8. [DOI] [PubMed] [Google Scholar]
- 95.Hammond MD, Hempleman SC. Oxygen diffusing capacity estimates derived from measured VA/Q distributions in man. Respir Physiol. 1987;69(2):129–47. [DOI] [PubMed] [Google Scholar]
- 96.Olfert IM, Balouch J, Kleinsasser A, et al. Does gender affect human pulmonary gas exchange during exercise? J Physiol. 2004;557(Pt 2):529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hopkins SR, Gavin TP, Siafakas NM, et al. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J Appl Physiol (1985). 1998;85(4):1523–32. [DOI] [PubMed] [Google Scholar]
- 98.Schaffartzik W, Poole DC, Derion T, et al. VA/Q distribution during heavy exercise and recovery in humans: implications for pulmonary edema. J Appl Physiol (1985). 1992;72(5):1657–67. [DOI] [PubMed] [Google Scholar]
- 99.Burnham KJ, Arai TJ, Dubowitz DJ, et al. Pulmonary perfusion heterogeneity is increased by sustained, heavy exercise in humans. J Appl Physiol (1985). 2009;107(5):1559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tedjasaputra V, Sá RC, Anderson KM, Prisk GK, Hopkins SR. Heavy upright exercise increases ventilation–perfusion mismatch in the basal lung: indirect evidence for interstitial pulmonary edema. J Appl Physiol (1985). 2019;127(2):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hopkins SR. Point: Pulmonary edema does occur in human athletes performing heavy sea-level exercise. J Appl Physiol (1985). 2010;109(4):1270–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson RL Jr., Spicer WS, Bishop JM, Forster RE. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol. 1960;15:893–902. [DOI] [PubMed] [Google Scholar]
- 103.Bebout DE, Story D, Roca J, et al. Effects of altitude acclimatization on pulmonary gas exchange during exercise. J Appl Physiol (1985). 1989;67(6):2286–95. [DOI] [PubMed] [Google Scholar]
- 104.Rice AJ, Thornton AT, Gore CJ, et al. Pulmonary gas exchange during exercise in highly trained cyclists with arterial hypoxemia. J Appl Physiol (1985). 1999;87(5):1802–12. [DOI] [PubMed] [Google Scholar]
- 105.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol. 1984;355:161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warren GL, Cureton KJ, Middendorf WF, Ray CA, Warren JA. Red blood cell pulmonary capillary transit time during exercise in athletes. Med Sci Sports Exerc. 1991;23(12):1353–61. [PubMed] [Google Scholar]
- 107.Hopkins SR, Belzberg AS, Wiggs BR, McKenzie DC. Pulmonary transit time and diffusion limitation during heavy exercise in athletes. Respir Physiol. 1996;103(1):67–73. [DOI] [PubMed] [Google Scholar]
- 108.Staub NC, Nagano H, Pearce M. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol. 1967;22(2):227–40. [DOI] [PubMed] [Google Scholar]
- 109.Hanel B. Pulmonary function after exercise with special emphasis on diffusion capacity. Dan Med Bull. 2000;47(2):196–217. [PubMed] [Google Scholar]
- 110.Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE, Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol (1985). 1985;58(3):989–95. [DOI] [PubMed] [Google Scholar]
- 111.Wagner PD, Gale GE, Moon RE, et al. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol (1985). 1986;61(1):260–70. [DOI] [PubMed] [Google Scholar]
- 112.Hopkins SR, Olfert IM, Wagner PD, et al. Last word on point: counterpoint “exercise-induced intrapulmonary shunting is imaginary vs. real”. J Appl Physiol (1985). 2009;107(3):1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-microm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol. 2007;292(4):H1777–81. [DOI] [PubMed] [Google Scholar]
- 114.Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med. 2007;176(3):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bryan TL, van Diepen S, Bhutani M, et al. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol (1985). 2012;113(4):541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hopkins SR, McKenzie DC, Schoene RB, Glenny RW, Robertson HT. Pulmonary gas exchange during exercise in athletes. I. Ventilation–perfusion mismatch and diffusion limitation. J Appl Physiol (1985). 1994;77(2):912–7. [DOI] [PubMed] [Google Scholar]
- 117.Vogiatzis I, Zakynthinos S, Boushel R, et al. The contribution of intrapulmonary shunts to the alveolar-to-arterial oxygen difference during exercise is very small. J Physiol. 2008;586(9):2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stickland MK, Tedjasaputra V, Seaman C, et al. Intra-pulmonary arteriovenous anastomoses and pulmonary gas exchange: evaluation by microspheres, contrast echocardiography and inert gas elimination. J Physiol. 2019;597(22):5365–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rankin J, Dempsey JA. Respiratory muscles and the mechanisms of breathing. Am J Phys Med. 1967;46(1):198–244. [PubMed] [Google Scholar]
- 120.Pilarski JQ, Leiter JC, Fregosi RF. Muscles of breathing: development, function, and patterns of activation. Compr Physiol. 2019;9(3):1025–80. [DOI] [PubMed] [Google Scholar]
- 121.Roussos C, Macklem PT. The respiratory muscles. N Engl J Med. 1982;307(13):786–97. [DOI] [PubMed] [Google Scholar]
- 122.Aaron EA, Johnson BD, Seow CK, Dempsey JA. Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol (1985). 1992;72(5): 1810–7. [DOI] [PubMed] [Google Scholar]
- 123.Shephard RJ. The oxygen cost of breathing during vigorous exercise. Q J Exp Physiol Cogn Med Sci. 1966;51(4):336–50. [DOI] [PubMed] [Google Scholar]
- 124.Dominelli PB, Render JN, Molgat-Seon Y, et al. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol. 2015;593(8):1965–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manohar M. Inspiratory and expiratory muscle perfusion in maximally exercised ponies. J Appl Physiol (1985). 1990;68(2):544–8. [DOI] [PubMed] [Google Scholar]
- 126.Musch TI, Friedman DB, Pitetti KH, et al. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol (1985). 1987;63(6):2269–77. [DOI] [PubMed] [Google Scholar]
- 127.Poole DC, Sexton WL, Behnke BJ, et al. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol (1985). 2000;88(1):186–94. [DOI] [PubMed] [Google Scholar]
- 128.Harms CA, Wetter TJ, McClaran SR, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985). 1998;85(2):609–18. [DOI] [PubMed] [Google Scholar]
- 129.Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on VO(2) and leg blood flow during submaximal exercise. J Appl Physiol (1985). 1999;87(2):643–51. [DOI] [PubMed] [Google Scholar]
- 130.Guenette JA, Henderson WR, Dominelli PB, et al. Blood flow index using near-infrared spectroscopy and indocyanine green as a minimally invasive tool to assess respiratory muscle blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2011;300(4):R984–92. [DOI] [PubMed] [Google Scholar]
- 131.Guenette JA, Vogiatzis I, Zakynthinos S, et al. Human respiratory muscle blood flow measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol (1985). 2008;104(4):1202–10. [DOI] [PubMed] [Google Scholar]
- 132.Dominelli PB, Archiza B, Ramsook AH, et al. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol. 2017;102(11):1535–47. [DOI] [PubMed] [Google Scholar]
- 133.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95(2):549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand. 1977;100(3):288–97. [DOI] [PubMed] [Google Scholar]
- 135.Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985). 1997;82(5):1573–83. [DOI] [PubMed] [Google Scholar]
- 136.Katayama K, Goto K, Shimizu K, et al. Effect of increased inspiratory muscle work on blood flow to inactive and active limbs during submaximal dynamic exercise. Exp Physiol. 2019;104(2):180–8. [DOI] [PubMed] [Google Scholar]
- 137.Dominelli PB, Katayama K, Vermeulen TD, et al. Work of breathing influences muscle sympathetic nerve activity during semi-recumbent cycle exercise. Acta Physiol (Oxf). 2019;225(4):e13212. [DOI] [PubMed] [Google Scholar]
- 138.Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol (1985). 2003;95(3):1159–69. [DOI] [PubMed] [Google Scholar]
- 139.Hussain SN, Chatillon A, Comtois A, Roussos C, Magder S. Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. J Appl Physiol (1985). 1991;70(1):77–86. [DOI] [PubMed] [Google Scholar]
- 140.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529(Pt 2):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol (1985). 2002;92(4):1539–52. [DOI] [PubMed] [Google Scholar]
- 142.Sheel AW, Derchak PA, Morgan BJ, et al. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537(Pt 1):277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Archiza B, Welch JF, Geary CM, et al. Temporal characteristics of exercise-induced diaphragmatic fatigue. J Appl Physiol (1985). 2018;124(4):906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol (1985). 1995;78(5):1710–9. [DOI] [PubMed] [Google Scholar]
- 146.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol (1985). 2002;93(1):201–6. [DOI] [PubMed] [Google Scholar]
- 147.Taylor BJ, How SC, Romer LM. Exercise-induced abdominal muscle fatigue in healthy humans. J Appl Physiol (1985). 2006;100(5):1554–62. [DOI] [PubMed] [Google Scholar]
- 148.Supinski G, DiMarco A, Ketai L, Hussein F, Altose M. Reversibility of diaphragm fatigue by mechanical hyperperfusion. Am Rev Respir Dis. 1988;138(3):604–9. [DOI] [PubMed] [Google Scholar]
- 149.Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am J Physiol. 1993;265(5 Pt 2):H1721–6. [DOI] [PubMed] [Google Scholar]
- 150.Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to alpha1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol (1985). 2002;92(5):1808–16. [DOI] [PubMed] [Google Scholar]
- 151.Olson TP, Joyner MJ, Dietz NM, et al. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol. 2010;588(Pt 13):2487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amann M, Regan MS, Kobitary M, et al. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]