Abstract
Genetic and receptor interference data have indicated the presence of one or more cellular receptors for subgroup B, D, and E avian leukosis viruses (ALV) encoded by the s1 allele of the chicken tvb locus. Despite the prediction that these viruses use the same receptor, they exhibit a nonreciprocal receptor interference pattern: ALV-B and ALV-D can interfere with infection by all three viral subgroups, but ALV-E only interferes with infection by subgroup E viruses. We identified a tvbs1 cDNA clone which encodes a tumor necrosis factor receptor-related receptor for ALV-B, -D, and -E. The nonreciprocal receptor interference pattern was reconstituted in transfected human 293 cells by coexpressing the cloned receptor with the envelope (Env) proteins of either ALV-B or ALV-E. This pattern of interference was also observed when soluble ALV surface (SU)-immunoglobulin fusion proteins were bound to this cellular receptor before viral challenge. These data demonstrate that viral Env-receptor interactions can account for the nonreciprocal interference between ALV subgroups B, D, and E. Furthermore, they indicate that a single chicken gene located at tvbs1 encodes receptors for these three viral subgroups. The TVBS1 protein differs exclusively at residue 62 from the published subgroup B- and D-specific receptor, encoded by the s3 allele of tvb. Residue 62 is a cysteine in TVBS1 but is a serine in TVBS3, giving TVBS1 an even number of cysteines in the extracellular domain. We present evidence for a disulfide bond requirement in TVBS1 for ALV-E infection but not for ALV-B infection. Thus, ALV-B and ALV-E interact in fundamentally different ways with this shared receptor, a finding that may account for the observed biological differences between these two ALV subgroups.
Avian leukosis viruses (ALV) are divided into six well-characterized subgroups, A to E and J, based on receptor usage group and host range in chickens. The subgroup specificity of these viruses has been mapped to the viral surface (SU) domain of the envelope (Env) glycoprotein which is responsible for receptor binding (reviewed in reference 10). Several lines of evidence support the hypothesis that ALV-B, -D, and -E use a shared chicken receptor, including genetic analysis which defined several alleles of an ALV susceptibility locus, designated tvb. tvbs1 permits infection by all three viruses, tvbs3 permits infection only by ALV-B and -D, and the tvbr allele does not permit infection by any of these viral subgroups (reviewed in reference 23). Previously, we identified the TVBS3 receptor for ALV subgroups B and D, a member of the tumor necrosis factor receptor (TNFR) family (6, 21). ALV-E utilizes a turkey receptor (TVBT) that is highly homologous to TVBS3 (1), providing additional evidence that the chicken receptors for ALV-B, -D, and -E are probably related.
Receptor interference studies performed with chicken embryo fibroblasts (CEFs) that contain tvbs1 also indicated that these viruses may use related receptors. As expected for viruses that use the same receptor, preinfection of these cells with either ALV-B or ALV-D leads to a block to superinfection by viruses of each of the three subgroups. In contrast, if cells are preinfected by a subgroup E virus, there is a block to superinfection by ALV-E but not by subgroup B and D viruses (23). Because receptor interference is thought to occur as a consequence of interactions with newly synthesized Env proteins in the infected cell (23), the reason why viruses that use the same cellular receptor would exhibit nonreciprocal interference is unclear. However, several models have been proposed to explain this phenomenon, including one that suggests the existence of multiple receptors encoded by closely linked genes at tvb (e.g., one receptor for ALV-B, -D, and -E and another only for ALV-B and -D) (23).
In order to understand the biological characteristics of ALV-B, -D, and -E associated with their receptor usage, we have now isolated and characterized a tvbs1 cDNA clone. In this report, we present evidence that the protein encoded by this clone is a primary binding receptor for these viral subgroups and confers all of the properties of nonreciprocal interference when expressed in transfected human 293 cells. Thus, we conclude that a single chicken receptor gene for ALV-B, -D, and -E accounts for this interference pattern. In addition, we demonstrate that a single amino acid substitution, a cysteine in place of a serine, distinguishes the B, D, and E subgroup receptor encoded by tvbs1 from the B and D subgroup receptor encoded by tvbs3. Further characterization of TVBS1 indicates that ALV-E infection is strongly influenced by disulfide bonds involving cysteines located at the N-terminal region of the receptor, whereas ALV-B infection does not require these cysteine residues. These data argue that ALV-B and ALV-E interact in distinct ways with the common TVBS1 receptor.
MATERIALS AND METHODS
Cells, lines, and viruses.
Line 15B1 primary CEFs were a generous gift of Connie Cepko. Human 293 cells and CEFs were grown as previously described (4, 26, 27). Subgroup B-specific (RCASH-B) and subgroup E-specific (RCASH-E) ALV-based retroviral vectors containing the hygromycin B phosphotransferase gene were described previously (6). Pseudotyped murine leukemia virus (MLV) virions with ALV Env proteins, MLV-lacZ (EnvB) or MLV-lacZ (EnvE), were generated with a tripartite transfection system (14) in which 293 cells were transfected with 15 μg of plasmid pMD.old.gag.pol, 20 μg of plasmid pMMP-nlslacZ, and 5 μg of either plasmid pAB7 encoding ALV-B Env or pAB9 encoding ALV-E Env (5). Plasmid pMD.old.gag.pol encoding MLV Gag and Pol proteins and plasmid pMMP-nlslacZ, an MLV vector encoding β-galactosidase, were a generous gift of Richard Mulligan at Children's Hospital, Boston, Mass. Extracellular supernatants containing virus were then collected at 48 and 60 h posttransfection and pooled.
cDNA cloning and sequencing.
Total RNA was isolated from CEFs as described previously (6). Approximately 5 μg of polyadenylated mRNA, isolated from 125 μg of total RNA with the RNA Isolation Kit (Stratagene), was reverse transcribed to generate cDNA with a commercially available kit (ZAP cDNA synthesis kit; Stratagene) and introduced into the λZAP Express vector (Stratagene). The cDNA library of approximately 80,000 clones was screened, as described previously, using a tvbs3 cDNA probe (1). Using a standard phagemid excision protocol (Stratagene), the plasmid pBK3-1 was then isolated, and this cDNA clone was subsequently sequenced using dideoxy terminator cycle sequencing on a Perkin-Elmer ABI 377 DNA sequencer by the core DNA sequencing facility in the Department of Microbiology and Molecular Genetics at Harvard Medical School.
Mutant construction.
An altered TVBS1 protein was generated by PCR mutagenesis of the pBK3-1 clone to generate plasmid pHA1. The altered TVBS1 protein, TVBS1(ΔDD), was truncated at amino acid 280 in the cytoplasmic tail and a FLAG epitope (DYKDDDDK) was added to the C terminus. The mutations of Cys-46, Cys-59, Cys-62, and Cys-77 to serines were introduced in pHA1 with overlapping PCR primers to create plasmids pHA12[TVBS1(ΔDD)-C46S], pHA13[TVBS1(ΔDD)-C46S/C62S], pHA14[TVBS1(ΔDD)-C59S], pHA15[TVBS1(ΔDD)-C59S/C77S], pHA16[TVBS1(ΔDD)-C62S/C77S], pHA25[TVBS1(ΔDD)-C77S], pHA26[TVBS1(ΔDD)-C46S/C62S/C77S], and pHA27[TVBS1(ΔDD)-C46S/C59S/C62S/C77S]. All constructs were sequenced to confirm their open reading frames. PCR primer sequences used in making these constructs are available upon request.
Transfections and infections.
Human 293 cells, plated at approximately 20% confluency on 100-mm tissue culture plates, were transfected with 10 μg of plasmid DNA. Cells were split into six-well tissue culture dishes 60 h after transfection and incubated with 2 ml of medium containing 100 μl of RCASH-B or RCASH-E viruses or 1 to 10 μl of MLV-lacZ (EnvB) or MLV-lacZ (EnvE). In the case of RCASH virus infections, cells were placed under selection in medium containing 300 μg of hygromycin B per ml 2 days after viral challenge, and after approximately 2 weeks of selection, colonies of infected cells were then stained with a methylene blue solution (20% isopropanol, 5% acetic acid, 1% methylene blue) and counted. MLV-lacZ-challenged transfectants were fixed 2 days after infection in 1% formaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline and then stained with a 0.1% 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal) (Gibco)–phosphate-buffered saline solution containing 2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1% dimethyl formamide in order to detect the virus-encoded β-galactosidase protein. Infection was quantified by counting blue colonies.
Immunoprecipitations, immunoblotting, and flow cytometry.
Plasmid pKZ452, which encodes subgroup B-specific immunoglobin (Ig) fusion protein in the SUB-rIgG immunoadhesin, and plasmid pCIE-rIgG, encoding an immunoadhesin containing a subgroup E-specific SU protein (SUE-rIgG), were described previously (1, 6). The SUB-rIgG and SUE-rIgG proteins were produced in the extracellular supernatants of transiently transfected 293 cells as described elsewhere (6, 27).
For the immunoprecipitations, human 293 cells were transfected with 10 μg of plasmid pBK3-1 or with no DNA, and the procedures used were as described previously (1). Briefly, transfected cells were lysed at 60 h posttransfection in NP-40 lysis buffer. Approximately 1 mg of total-cell lysate was incubated for 1 h at 4°C with protein A-Sepharose beads preloaded with SUB-rIgG or SUE-rIgG. The immunoprecipitates were then washed in lysis buffer, boiled in sodium dodecyl sulfate sample buffer, and electrophoresed on a reducing sodium dodecyl sulfate-polyacrylamide gel. The precipitated protein was detected by immunoblotting with the IQS579 polyclonal antibody raised against a TVB peptide sequence (1).
For flow cytometry, human 293 cells were transfected as described above with 10 μg of plasmid pHA1. Approximately 60 h after transfection, cells were prepared for flow cytometry as described previously (1). Cells were incubated with 1 ml of medium (mock) or with 1 ml of extracellular supernatants containing approximately equal amounts of either SUB-rIgG or SUE-rIgG as assessed by immunoblotting with a horseradish peroxidase-conjugated anti-rabbit antibody as a probe (data not shown). Samples of 5,000 cells were then analyzed on a Coulter Epics XL flow cytometer in the Department of Hematologic Oncology at the Dana Farber Cancer Institute.
Viral interference assays.
Human 293 cells were transiently transfected with 1 μg of the plasmid pBK3-1, and 48 h posttransfection cells were split into six-well plates as described above. Cells were incubated for 1 h at 37°C in a total volume of 3.5 ml of medium containing approximately equal amounts of SUB-rIgG or SUE-rIgG, before addition of 100 μl of either RCASH-B or RCASH-E viruses. Two days after the viral challenge, cells were placed under selection in medium containing 300 μg of hygromycin B per ml, and then colonies of infected cells were stained and counted after approximately 2 weeks.
In order to generate TVBS1-Env coexpressing cell lines (designated as either EnvB-S1 or EnvE-S1 cells), plasmids encoding TVBS1(ΔDD) (pHA1) and either subgroup B-Env (pAB7) or E-Env (pAB9) were cotransfected with 1 μg of a plasmid expressing a puromycin resistance gene (pPUR) in a ratio of 2 or 5 μg of pHA1 to 1 μg of pAB7 or pAB9. After approximately 2 weeks of selection in medium containing 100 μg of puromycin per ml, drug-resistant colonies were isolated and expanded. The resulting EnvB-S1 and EnvE-S1 clonal cell lines were then screened for expression of TVBS1(ΔDD) by immunoblotting: whole-cell lysates were screened for TVBS1 expression by probing with SUB-rIgG and for expression of EnvB and EnvE by immunoblotting with TVBS1-rIgG and TVBT-rIgG under nonreducing conditions (data not shown). The TVB-rIgG proteins are comprised of a rabbit immunoglobulin constant region (Fc) that is fused to the C terminus of the extracellular domain of TVBS1 or TVBT, respectively. Cell lines which were shown to express both the receptor and the appropriate Env protein were then screened by infection with MLV-lacZ (EnvB) or MLV-lacZ (EnvE) to assay for viral interference. A control cell line (S1-5) expressing only TVBS1(ΔDD) was generated in a similar manner by cotransfection of plasmid pHA1 with pPUR.
Nucleotide sequence accession number.
The nucleotide sequence of the tvbs1 cDNA clone has been submitted to GenBank under accession no. AF 161713.
RESULTS
Identification of a tvbs1 cDNA clone which encodes a receptor for ALV-B, -D, and -E.
In order to characterize tvbs1, a cDNA library was prepared from CEFs derived from line 15B1 chickens which are genetically homozygous for this tvb allele (2). The library was screened with a tvbs3 cDNA probe as described previously (1). Individual cDNA clones which hybridized with this probe were identified, and because tvb is a single-copy gene in chicken cells (21), we reasoned that any cross-hybridizing clone must be derived from tvbs1. Clones greater than 2 kb in size were isolated in a plasmid-based mammalian expression vector and then tested for their ability to confer susceptibility to infection by ALV-B and ALV-E vectors when expressed in transfected human 293 cells.
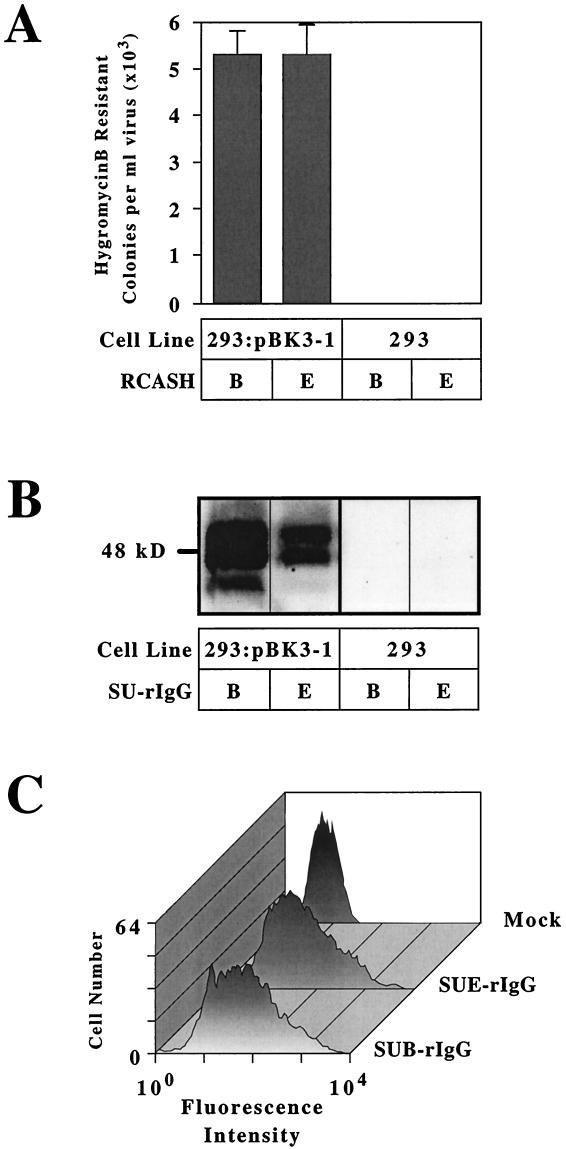
The transfected cells were challenged with ALV vectors containing the hygromycin B phosphotransferase gene and the envelope proteins derived from either RCASH-B or RCASH-E viruses (6). Following viral infection, cells were selected in medium containing hygromycin B, and colonies of infected cells were then counted. A 2.3-kb cDNA clone, designated pBK3-1, conferred susceptibility to both RCASH-B and RCASH-E when expressed in human 293 cells (Fig. 1A), an activity expected of a cDNA encoding TVBS1.
FIG. 1.
The pBK3-1 clone encodes a receptor for ALV-B and ALV-E. (A) Human 293 cells were transfected with plasmid pBK3-1 (293:pBK3-1) or no DNA (293) and were then challenged with subgroup B-specific (RCASH-B) or subgroup E-specific (RCASH-E) ALV vectors encoding hygromycin B phosphotransferase. The resultant hygromycin B-resistant colonies were counted, and the data shown represent the average numbers obtained in three independent experiments. (B) Human 293 cells transfected with plasmid pBK3-1 or with no DNA were lysed in NP-40 lysis buffer and subjected to immunoprecipitation with subgroup B-specific (SUB-rIgG) or subgroup E-specific (SUE-rIgG) SU-rabbit Ig fusion proteins. The immunoprecipitated proteins were then subjected to immunoblotting using an antiserum specific for TVB (1) and visualized by enhanced chemiluminescence. (C) The death domain of TVBS1 is not required for cell surface expression or binding to ALV-B or ALV-E SU. Transfected 293 cells expressing TVBS1(ΔDD), a truncated receptor lacking the cytoplasmic death domain, were incubated with SUB-rIgG or SUE-rIgG and with a fluorescein isothiocyanate-conjugated antibody specific for rabbit Igs. The cells were then analyzed by flow cytometry.
In order to test whether the protein encoded by the pBK3-1 clone was a receptor that can bind to ALV SU proteins, binding experiments were performed using subgroups B and E SU-Ig fusion proteins, SUB-rIgG and SUE-rIgG, respectively (1, 6). Several protein species were precipitated specifically from pBK3-1-transfected cells, but not from nontransfected cells, by both SU-rIgG proteins (Fig. 1B). These proteins presumably represent differently glycosylated forms of TVBS1, similar to those described previously for TVBS3 (6). In addition, a TVBS1-rIgG fusion protein, comprising the extracellular domain of TVBS1 fused to a rabbit Fc chain, specifically bound to transfected 293 cells expressing subgroup D Env, as assessed with a flow cytometry-based assay (data not shown). Thus, through Env-binding and viral infection experiments, we have confirmed that the TVBS1 protein encoded by the pBK3-1 cDNA clone is a primary binding receptor for subgroup B, D, and E ALV.
In order to avoid potential toxicity problems associated with expression of a death receptor, several of the subsequent experiments were performed with truncated TVB proteins lacking the cytoplasmic death domain but retaining the first 63 amino acids of the cytoplamic tail. This protein, TVBS1(ΔDD), was expressed on the surfaces of transfected 293 cells and bound to both SUB-rIgG and SUE-rIgG, as detected by flow cytometry (Fig. 1C). In addition, TVBS1(ΔDD) permitted both subgroup B and subgroup E viral infection: the titer of each virus on these cells was approximately 105 infectious units/ml (Table 1). This result confirms that this region of the cytoplasmic tail is not necessary for surface expression of the protein or for infection by subgroup B and E viruses.
TABLE 1.
The effect of cysteine mutations in TVBS1 upon subgroup B and E viral entrya
| TVBS1(ΔDD) construct | Residue
|
Mean (SD) colonies/ml of virus
|
||||
|---|---|---|---|---|---|---|
| 46 | 59 | 62 | 77 | MLV-lacZ (EnvB) | MLV-lacZ (EnvE) | |
| WT | C | C | C | C | 2.2 × 105 (4.9 × 104) | 1.9 × 105 (3.6 × 104) |
| C46S | S | C | C | C | 6.3 × 105 (5.1 × 103) | 24.3 (5.1) |
| C46S/C62S | S | C | S | C | 5.3 × 105 (3.1 × 104) | 4.4 (2) |
| C59S | C | S | C | C | 4.9 × 105 (2.4 × 105) | 2.2 (2.7) |
| C59S/C77S | C | S | C | S | 2.3 × 105 (1.8 × 104) | 1.1 (1.9) |
| C62S/C77S | C | C | S | S | 4.0 × 105 (1.3 × 105) | 2.8 × 103 (8.9 × 102) |
| C77S | C | C | C | S | 1.6 × 105 (2.6 × 104) | 33 (58) |
| C46S/C62S/C77S | S | C | S | S | 8.7 × 104 (3.5 × 103) | 0 |
| C46S/C59S/C62S/C77S | S | S | S | S | 1.1 × 105 (2.1 × 104) | 0 |
Wild-type (WT) or mutant forms of TVBS1(ΔDD) were tested for their ability to mediate entry of MLV-LacZ (EnvB) and MLV-LacZ (EnvE). The averages from three independent experiments are shown with standard deviations in parentheses. C, cysteine; S, serine.
Reconstitution of nonreciprocal interference with TVBS1.
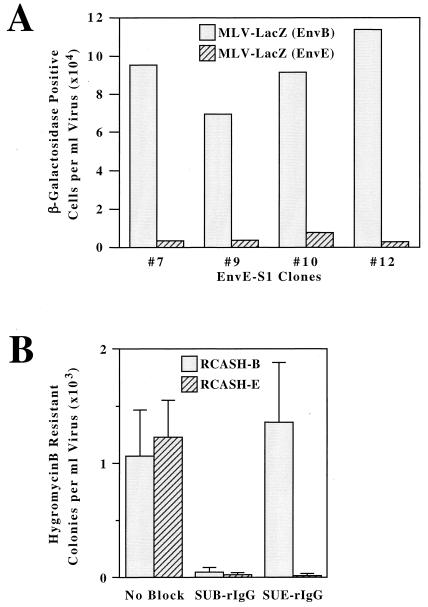
With the identification of TVBS1 as a cellular receptor for ALV-B, -D, and -E, we were interested in investigating whether this protein could account for the nonreciprocal interference observed between these viruses in CEFs. In order to recapitulate this interference pattern, TVBS1(ΔDD) and either subgroup B or subgroup E ALV Env proteins were coexpressed in stably transfected human 293 cell lines that were designated as EnvB-S1 and EnvE-S1 cells, respectively. These cells were characterized for expression of Env and TVBS1 proteins (as described in Materials and Methods) and were challenged with 103 to 104 infectious units of MLV vectors containing the gene for β-galactosidase, pseudotyped with either ALV-B Env [MLV-lacZ (EnvB)] or ALV-E Env [MLV-lacZ (EnvE)]. Four independent clonal lines of EnvE-S1 cells were identified as being fully susceptible to subgroup B viral infection but highly resistant to subgroup E viral infection (Fig. 2A). By contrast, six independent clones of EnvB-S1 cells were almost completely resistant to infection by both the subgroup B and subgroup E viruses: in each case, viral titers of less than 10 infectious units/ml were obtained (data not shown). It should be noted that other transfected cells that did not express ALV Env proteins or instead expressed disproportionately higher levels of TVBS1 were susceptible to infection by both subgroup B and E viruses (data not shown), as would be expected if receptor interference results from Env-receptor interactions. Taken together, these data demonstrate that the nonreciprocal receptor interference seen between subgroup B and E viruses can be explained by properties of the cloned TVBS1 receptor, indicating that this protein is the only receptor for these viruses in chicken cells.
FIG. 2.
Reconstitution of nonreciprocal interference between ALV-B and ALV-E with the cloned TVBS1 protein. (A) Four independent lines of human 293 cells stably expressing TVBS1(ΔDD) and EnvE (EnvE-S1 cells) were challenged with 10 μl (approximately 103 infectious units) of MLV-lacZ (EnvB) or with 100 μl (approximately 104 infectious units) of MLV-lacZ (EnvE). The numbers of infected β-galactosidase-positive cells obtained in a representative experiment were determined, and these numbers were corrected so that they represent those that would be obtained per milliliter of each original virus stock. (B) Human 293 cells transiently expressing the full-length TVBS1 protein were incubated with extracellular supernatants containing SUB-rIgG or SUE-rIgG or with a control supernatant lacking any SU-rIgG protein (no block) for 1 h at 37°C prior to the addition of either RCASH-B or RCASH-E viruses. After approximately 2 weeks of selection in medium containing hygromycin B, the drug-resistant colonies were counted. The data shown represent the average number of colonies obtained in three independent experiments.
Because receptor interfence can be reconstituted with coexpression of cloned receptor and Env proteins, the question arises whether interference is occurring through direct receptor-Env interactions and whether it can occur at the cell surface. Specifically, we asked what effect blocking either the subgroup B receptor sites on TVBS1 with SUB-rIgG or subgroup E receptor sites with SUE-rIgG would have upon subsequent ALV-B and ALV-E entry. SUB-rIgG effectively blocked infection by both subgroup B and subgroup E ALV vectors when incubated with 293 cells expressing wild-type TVBS1 (Fig. 2B) by presumably binding to, and interfering with the function of, all of the cell surface receptors for these viruses. However, SUE-rIgG blocked ALV-E infection but was unable to prevent subgroup B virus entry (Fig. 2B). Similar results were obtained after blocking endogenously expressed TVBS1 receptors on line 15B1 CEFs with SUB-rIgG and SUE-rIgG (data not shown). Therefore, the binding activities of the soluble SU fusion proteins reconstitute the nonreciprocal interference originally observed in virus-infected CEFs. Thus, we can conclude that interference can occur by direct receptor-Env interactions and, furthermore, that it can occur at the cell surface.
Residue Cys-62 of TVBS1 is a critical determinant for subgroup E viral infection.
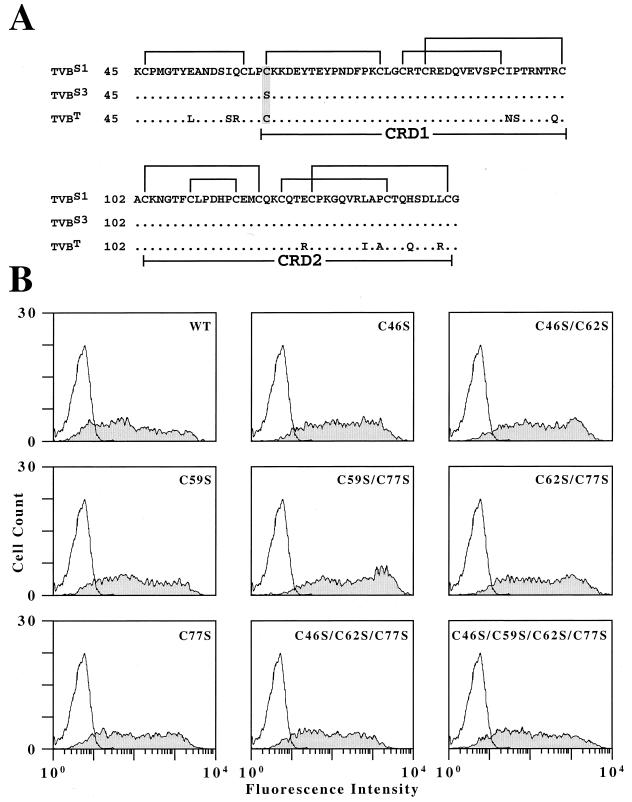
The pBK3-1 cDNA clone was sequenced and its open reading frame was found to differ by only a single nucleotide substitution (a T instead of an A at position 184) from that of the TVBS3 cDNA clone (6). Therefore, the extracellular domain of TVBS1 has the amino acid cysteine at position 62 and thus has an even number of cysteine residues (16) (Fig. 3A). By contrast, this residue in TVBS3 is a serine and therefore the extracellular domain of this protein has an odd number of cysteines (15) (Fig. 3A). This finding, coupled with the fact that TVBT also contains a cysteine at position 62 (1) that is important for subgroup E virus receptor function (data not shown), indicates that residue Cys-62 of TVB proteins is important for ALV-E entry. The fact that residue Cys-62 is important for the ALV-E receptor function of TVBS1 indicates that specific intrachain disulfide bonds might be necessary for this activity.
FIG. 3.
Mutational analysis of the first four cysteine residues of TVBS1. (A) TVBS3 and TVBS1 differ by a serine-to-cysteine substitution at residue 62 (shaded). The regions of TVBS1, TVBS3, and TVBT that encompass all extracellular cysteine residues (amino acids 45 to 144) are shown aligned, and the predicted intrachain disulfide bonds in TVBS1 are indicated. (B) All of the mutant receptors bearing cysteine mutations were expressed at the cell surface and bind to SUB-rIgG. Human 293 cells transfected with no DNA or with plasmids encoding wild-type TVBS1(ΔDD) (WT) or with mutant forms of this receptor were incubated with extracellular supernatant containing SUB-rIgG and with a fluorescein isothiocyanate-conjugated antibody specific for rabbit Igs. The cells were then analyzed by flow cytometry; the results shown were obtained with nonreceptor-expressing cells (open histograms) and receptor-expressing cells (shaded histograms).
Based on a sequence alignment of TVBS3 with other TNFR-like proteins, we initially predicted that residue Cys-59 might define the beginning of the first TNFR-related cysteine-rich domain (CRD1) in TVBS3 and that it would form an intrachain disulfide bond with Cys-77, leaving Cys-46 unpaired (Fig. 3A) (6). However, in light of the sequence information for TVBS1, we reasoned that Cys-46 could potentially pair with either Cys-59 or Cys-62 in TVBS1, and therefore if Cys-46 and Cys-59 form the favored disulfide, Cys-77 would be left unpaired in TVBS3 (Fig. 3A). In order to test this hypothesis, we made a series of mutations in TVBS1 which changed cysteines at positions 46, 59, 62, and 77 to serines and tested the ability of these altered proteins to function as receptors for subgroup B or E viruses.
Each of these mutant receptors was expressed on the surface of transfected cells at levels that were essentially equivalent to that obtained with the wild-type TVBS1(ΔDD) protein, as judged by flow cytometry with SUB-rIgG and a fluoresceinated secondary antibody (Fig. 3B). Indeed, all of the mutant receptors tested supported subgroup B viral entry at levels similar to that seen with the wild-type receptor (Table 1). By contrast, the only mutant receptor with any appreciable subgroup E receptor activity was TVBS1(ΔDD)-C62S/C77S that has cysteine residues at positions 46 and 59 (Table 1). This mutant permitted infection of MLV-lacZ (EnvE) at a level that was 2 to 3 orders of magnitude higher than those obtained with the other mutant receptors but was 2 orders of magnitude lower than that seen with the wild-type receptor (Table 1).
These data suggest that Cys-46 and Cys-59 may form a disulfide bond that is needed for subgroup E receptor function. Consistent with this interpretation, mutant receptors bearing substitutions of either of these two cysteines did not support MLV-lacZ (EnvE) entry (Table 1). The only mutant receptor that had both Cys-46 and Cys-59 present but did not support subgroup E viral entry at a significant level was TVBS1(ΔDD)-C77S (Table 1).
DISCUSSION
We have identified a cDNA clone from CEFs which encodes a cellular receptor specific for subgroup B, D, and E ALV and that can account for the nonreciprocal receptor interference seen between these three viral subgroups in chicken cells. Several lines of evidence demonstrate that the cDNA clone is derived from the s1 allele of tvb, including the evidence that its protein product has the activity expected of TVBS1 and that it differs at only one amino acid position from the product of the s3 allele of tvb, previously shown to be an ALV B- and D-specific receptor (6). Given this high degree of similarity to tvbs3 and the lack of evidence for other highly related genes in chickens (21), these data demonstrate that there is a single ALV-B, -D, and -E receptor gene encoded at tvbs1.
TVBS1 differs from the previously identified TVBS3 receptor by a single amino acid substitution, a cysteine in place of a serine at position 62. Based on previous alignments of TVBS3 with TNFR-I (6), we predicted that Cys-62 might form an intrachain disulfide bond with Cys-46 that is important for ALV-E infection. However, the mutational analysis of cysteine residues in this region of TVBS1 suggests that a disulfide bond between Cys-46 and Cys-59 is required for ALV-E entry. In addition, mutation of any of the first four cysteines of TVBS1, either singly or in multiple combinations, had no effect on ALV-B entry, including simultaneous substitution of the first four cysteines to serines. These results indicate that although ALV-B and ALV-E use a common receptor, they interact with this receptor in fundamentally different ways. ALV-E appears to be sensitive to structural constraints imposed by disulfide bonds in this region of TVBS1, whereas ALV-B is not. Based on the known crystal structure of TNFR-I (3), we assume that the putative disulfide bond between cysteines 46 and 59 of TVBS1 forms an extra loop in the membrane distal region of the receptor at the beginning of CRD1, possibly bringing two or more interacting regions of the protein together to form a site needed for subgroup E viral receptor function.
Based on our mutational data, Cys-46 and Cys-59 of TVBS3 may pair, leaving Cys-77 unpaired and probably buried within the structure. However, it is formally possible that Cys-46 is the unpaired cysteine residue in TVBS3, as we originally hypothesized (6). Therefore, TVBS3 might not serve as a receptor for ALV-E either because it lacks the putative Cys46-Cys59 disulfide bond or because a free cysteine at residue 77 might impose an inhibitory effect on subgroup E viral entry. Indeed, a similar inhibitory effect of a free cysteine at residue 62 in the mutant TVBS1(ΔDD)-C77S receptor might account for the inability of this protein to support ALV-E entry (Table 1). We are currently addressing these predictions using biochemical approaches to map the precise disulfide bonds in this region of TVBS1 and TVBS3.
Given that endogenous ALV proviruses are subgroup E specific (23), it is especially intriguing that there is only a single amino acid difference distinguishing TVBS3 from TVBS1 and that this single amino acid difference abrogates ALV-E entry. The only other known proteins which are structurally similar to the TVB proteins are the human TRAIL receptors, TRAIL-R1 (also known as DR4 or APO-2) and TRAIL-R2 (also known as DR5), and these receptors contain cysteines at positions equivalent to Cys-46, Cys-59, Cys-62, and Cys-77 in TVBS1 (7, 15–17, 19, 20, 22). Thus, the prototype of this class of TNFR-related receptors has cysteine residues represented at all four positions. Therefore, we would argue that selective pressure on the chicken population gave rise to the substitution of a serine for a cysteine at residue 62 in TVBS3, the direct consequence of which is the loss of binding to endogenous subgroup E viral glycoproteins. In support of this hypothesis, the turkey TVBT receptor, which is a subgroup E-specific receptor, contains a cysteine at residue 62 (1), and presumably turkeys are under no selective pressure to lose binding to ALV-E Env, since they lack endogenous ALV-E proviruses (23).
There are several possible explanations for the selective pressure which gave rise to this substitution in TVBS3. First, we have already shown that ALV-B SU-receptor interactions can lead to the death of cultured avian fibroblasts (6). Therefore, this mutation may have arisen in order to prevent ALV-E Env-receptor-mediated apoptosis of certain cell types in vivo. Although ALV-E infections generally do not lead to cell death in CEFs (8), this fact does not preclude the possibility that ALV-E Env-receptor interactions may cause the death of certain cell types in vivo. An alternative explanation to account for the existence of this mutation is that TVB receptors might play an important role in immune responses against microbial pathogens, as has been seen for other TNFR-related proteins such as TNFR-I, Fas, and TRAIL (9, 11–13, 18). If so, binding to endogenous retroviral glycoproteins might interfere with this natural function, thus explaining the selective basis for this mutation. In accordance with this hypothesis, ALV-E may have lost the ability to completely interfere with TVBS1, so that at least a subpopulation of receptors coexpressed in cells with subgroup E viral proteins would retain their normal function.
We can conclude from the mutagenesis evidence that ALV-B and ALV-E have distinct disulfide bond requirements, and therefore these viruses interact differently with TVBS1. This result may account for the nonreciprocal receptor interference seen between these viral subgroups and may help to explain why ALV-B infections are generally cytopathic in CEFs, whereas ALV-E infections are not (24, 25). Because our data is most consistent with a putative disulfide bond between Cys-46 and Cys-59 in TVBS1 being important for ALV-E receptor function, the question arises why residue Cys-62 became altered in TVBS3 to prevent subgroup E Env binding. Possibly, the putative bond between Cys-46/59 is also important for ligand binding and must be preserved in the structure. The answer to this question and the biological significance of receptor interference in this system awaits molecular identification of the endogenous TVB ligand.
ACKNOWLEDGMENTS
We are grateful to Steve Blacklow, in whose laboratory the final experiments were performed, for his generosity and critical reading of the manuscript. We thank members of the Young laboratory for many helpful discussions and in particular John Naughton, who prepared the final figures for the paper, and Adrienne Boerger and Sara Klucking for providing reagents. We also thank John Daly for assistance with the flow cytometry, the Department of Microbiology and Molecular Genetics core sequencing facility, at Harvard Medical School, for help with DNA sequencing, and Richard Mulligan for providing unpublished reagents.
This work was supported by NIH grant CA70810.
REFERENCES
- 1.Adkins H B, Brojatsch J, Naughton J, Rolls M M, Pesola J M, Young J A. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon L D, Smith E J, Fadly A M, Crittenden L B. Development of an alloantiserum (R2) that detects susceptibility of chickens to subgroup E endogenous avian leukosis virus. Avian Pathol. 1996;25:551–568. doi: 10.1080/03079459608419161. [DOI] [PubMed] [Google Scholar]
- 3.Banner D W, D'Arcy A, Janes W, Gentz R, Schoenfeld H J, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 4.Bates P, Young J A, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 5.Boerger A L, Snitkovsky S, Young J A T. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc Natl Acad Sci USA. 1999;96:9867–9872. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary P M, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 8.Dorner A J, Coffin J M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 9.Granville D J, Carthy C M, Yang D, Hunt D W, McManus B M. Interaction of viral proteins with host cell death machinery. Cell Death Differ. 1998;5:653–659. doi: 10.1038/sj.cdd.4400388. [DOI] [PubMed] [Google Scholar]
- 10.Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1997. pp. 71–120. [PubMed] [Google Scholar]
- 11.Jeremias I, Herr I, Boehler T, Debatin K M. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–152. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Tijoe I M, Smith C A, Herzenberg L A. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau N R, Littman D R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacFarlane M, Ahmad M, Srinivasula S M, Fernandes-Alnemri T, Cohen G M, Alnemri E S. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–25420. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- 16.Pan G, Ni J, Wei Y F, Yu G, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 17.Pan G, O'Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 19.Schneider P, Bodmer J L, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, Goddard A D, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 21.Smith E J, Brojatsch J, Naughton J, Young J A T. The CAR1 gene encoding a cellular receptor specific for subgroup B and D avian leukosis viruses maps to the chicken tvb locus. J Virol. 1998;72:3501–3503. doi: 10.1128/jvi.72.4.3501-3503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, Goodwin R G, Rauch C T. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss R A. The Retroviridae. New York, N.Y: Plenum; 1993. [Google Scholar]
- 24.Weller S K, Joy A E, Temin H M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980;33:494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller S K, Temin H M. Cell killing by avian leukosis viruses. J Virol. 1981;39:713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young J A, Bates P, Varmus H E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zingler K, Young J A. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]