Abstract
Background.
In contrast to pancreatic ductal adenocarcinoma (PDAC), neoadjuvant therapy (NAT) for periampullary adenocarcinomas is not well studied, with data limited to single-institution retrospective reviews with small cohorts. We sought to compare outcomes of NAT versus upfront resection (UR) for non-PDAC periampullary adenocarcinomas.
Patients and Methods.
Using the National Cancer Database (NCDB), we identified patients who underwent surgery for extrahepatic cholangiocarcinoma, ampullary adenocarcinoma, or duodenal adenocarcinoma from 2006 to 2016. We compared outcomes between NAT versus UR groups for each tumor subtype with 1:3 propensity score matching. Cox regression was used to identify predictors of survival.
Results.
Among 7656 patients who underwent resection for non-PDAC periampullary adenocarcinoma, the proportion of patients who received NAT increased from 6 to 11% for cholangiocarcinoma (p < 0.01), 1 to 4% for ampullary adenocarcinoma (p = 0.01), and 5 to 8% for duodenal adenocarcinoma (p = 0.08). Length of stay, readmission, and 30-day mortality were comparable between NAT and UR. All tumor subtypes were down-staged following NAT (p < 0.01). The R0 resection rate was significantly higher in patients with extrahepatic cholangiocarcinoma who received NAT, and these patients had improved median overall survival (38 vs 26 months, p < 0.001). After adjustment for clinicopathologic factors and adjuvant chemotherapy, use of NAT was associated with improved survival in patients with cholangiocarcinoma [hazard ratio (HR) 0.69, 95% confidence interval (CI) 0.54–0.89, p = 0.004] but not duodenal or ampullary adenocarcinoma. The survival advantage for cholangiocarcinoma persisted after propensity matching.
Conclusion.
This national cohort analysis suggests, for the first time, that neoadjuvant therapy is associated with improved survival in patients with extrahepatic cholangiocarcinoma.
Nonpancreatic periampullary adenocarcinomas, including ampullary adenocarcinoma, duodenal adenocarcinoma, and extrahepatic cholangiocarcinoma, are aggressive malignancies that are managed with surgical resection, mainly pancreaticoduodenectomy (PD). Despite the increased resectability rate of these tumors compared with PDAC,1,2 5-year survival following resection remains poor, ranging from 30 to 50%,2–6 suggesting a potential role for systemic therapy in the management of these tumors.
In contrast to pancreatic ductal adenocarcinoma (PDAC), for which systemic therapy represents a corner-stone of multimodality treatment,7–10 the role of chemotherapy in the management of operable nonpancreatic periampullary adenocarcinomas remains less defined. Although some prospective evidence supports the role of adjuvant chemotherapy in the treatment of these malignancies, a survival advantage is observed only when adjusting for prespecified risk factors, and not in the intent-to-treat cohort as seen in the ESPAC-3 trial for periampullary cancers and the BILCAP study for biliary cancers.11–13
Similar to PDAC, neoadjuvant therapy for other periampullary cancers confers a variety of theoretical advantages, including earlier systemic treatment for micrometastatic disease, direct assessment of tumor biology by evaluation for pathologic response, improved locoregional control through downstaging before surgical resection, and the ability to deliver all intended therapy before potentially morbid resections.8,14 Owing to these tumors’ rarity, limited data exist regarding the role of NAT for nonpancreatic periampullary adenocarcinomas, and reports are limited to single-institution retrospective studies with relatively small cohorts.
Therefore, the objectives of this study were to describe patterns of use of neoadjuvant chemotherapy (NAT) for nonpancreatic periampullary adenocarcinomas and to compare outcomes between patients who received NAT followed by resection versus upfront resection (UR) for extrahepatic cholangiocarcinoma, ampullary adenocarcinoma, and duodenal adenocarcinoma.
PATIENTS AND METHODS
Study Design, Data Source, and Definitions
The NCDB was queried for all adult patients (age ≥ 18 years) who underwent a curative resection for extrahepatic cholangiocarcinoma, ampullary adenocarcinoma, or duodenal adenocarcinoma between 2006 and 2016. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) topography and histology codes were used to identify patients with the respective cancer site diagnosis. The NCDB is a national clinical cancer registry that receives information from over 1500 Commission on Cancer (CoC)-accredited cancer programs in the USA and captures approximately 70% of incident cancer cases in the USA. Data were coded according to the Commission on Cancer Registry Operations and Data Standards Manual, the 7th edition of the American Joint Committee for Cancer (AJCC) Manual for Staging of Cancer, and the International Classification of Disease for Oncology (ICD-O-3). To reduce data errors and maintain the integrity of the database, all data were extracted from medical records by trained and certified tumor registrars. Data were validated both locally and at the NCDB level. Data were deidentified and submitted to the NCDB in compliance with the Health Insurance Portability and Accountability Act (HIPAA). The University of Pittsburgh (Pittsburgh, PA) Medical Center institutional review board approved this study as an exempt status.
The study was limited to those who underwent a formal resection for nonmetastatic, resectable periampullary cancers. Patients were excluded if they had a clinical stage 0, unknown clinical stage, arterial involvement, unknown chemotherapy status, no surgery, local excision, or unknown extent of surgical resection. Patients who had venous involvement but underwent pancreatic and/or duodenal resection for curative intent were included. Patient variables included age at diagnosis, race, gender, insurance status, year of diagnosis, and comorbidities (represented by the modified Charlson–Deyo scoring system). Clinical and pathologic variables of interest included tumor stage and grade, surgical margin status, extent of resection, length of hospital stay, readmission within 30 days, and mortality within 30 days. Hospital characteristics such as location and type were provided in the dataset. Tumor downstaging in patients who received NAT was analyzed on the basis of differences between the clinical and pathologic stage (combined T and N stages). Receipt of adjuvant chemotherapy was analyzed between groups.
Statistical Analysis
The study cohort was analyzed separately by tumor site and categorized into two treatment groups: (1) upfront resection (UR) and (2) neoadjuvant therapy (NAT). Neoadjuvant therapy included preoperative chemotherapy regardless of use of radiation. Baseline characteristics and unadjusted outcomes were compared using the Wilcoxon rank-sum test for continuous variables and the Pearson’s χ2 test or Fisher’s exact test for categorical variables. Overall survival was defined as the time from diagnosis to death or date of the last follow-up for patients who did not die. Median survival was estimated using the Kaplan–Meier method, and the log-rank test was used to compare survival between groups. Multivariable Cox proportional hazards modeling was constructed to examine the association of adjusted survival with receipt of NAT stratified by cancer site. For all multivariable survival analyses, the following variables were adjusted for: patient age, gender, race, hospital type, comorbidity score, year of diagnosis, clinical stage, and receipt of adjuvant chemotherapy. The multivariable model included only patients with complete data for all the variables included. Since NAT can potentially impact pathologic variables (T stage, N stage, lymphovascular and perineural invasion, and margin status), those variables were not used in modeling.
Propensity matching was then employed to confirm differences in survival. After stratification by receipt of NAT, a 1:3 (NAT:UR) propensity match was performed using a nearest-neighbor algorithm. The cohorts were matched by cancer site and the following variables: patient age, gender, race, insurance status, comorbidity score, year of diagnosis, clinical T stage and N stage, receipt of adjuvant chemotherapy, and hospital type. A p value of less than 0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC) and R version 3.6.3 (Vienna, Austria).
RESULTS
Overall Cohort Characteristics
The study included a total of 7656 patients who underwent surgery for nonpancreatic periampullary adenocarcinoma. Among these patients, 33% (N = 2514) had extrahepatic cholangiocarcinoma, 49% (N = 3762) had ampullary adenocarcinoma, and 18% (N = 1380) had duodenal adenocarcinoma. Within these subgroups, 157 patients with extrahepatic cholangiocarcinoma (6.6%), 94 with ampullary adenocarcinoma (2.6%), and 67 with duodenal adenocarcinoma received NAT (5.1%). The proportion of patients who received NAT increased over the study period (2006–2016) for all tumor sites from 6 to 11% for cholangiocarcinoma (p < 0.01), 1 to 4% for ampullary adenocarcinoma (p = 0.01), and 5 to 8% for duodenal adenocarcinoma (p = 0.08). Table 1 details demographic and clinical disease characteristics of the cohort by receipt of NAT and anatomic site. Across groups, patients who received NAT were slightly younger compared with those who underwent UR. There were no differences in gender, race, comorbidity scores, or hospital type by receipt of NAT among all three anatomic sites (Table 1).
TABLE 1.
Demographic and clinical disease characteristics of patients with resected nonpancreatic periampullary adenocarcinoma by receipt of neoadjuvant therapy (NAT) or upfront resection (UR) in the NCDB (2006–2016)
| Extrahepatic cholangiocarcinoma |
Ampullary adenocarcinoma |
Duodenal adenocarcinoma |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| UR (N = 2357) | NAT (N = 157) | p | UR (N = 3668) | NAT (N = 94) | p | UR (N = 1765) | NAT (N = 123) | p | |
|
| |||||||||
| Age, years | 68 (60–75) | 64 (53–72) | <0.001 | 68 (59–76) | 67 (59–72) | 0.167 | 67 (58–76) | 60 (50–68) | <0.001 |
| Female gender | 852 (36.1%) | 55 (35.0%) | 0.798 | 1565 (42.7%) | 35 (37.2%) | 0.342 | 791 (44.8%) | 58 (47.2%) | 0.640 |
| Race | |||||||||
| White | 1994 (84.6%) | 138 (87.9%) | 0.456 | 3091 (84.3%) | 82 (87.2%) | 0.271 | 1397 (79.2%) | 92 (74.8%) | 0.517 |
| Black | 183 (7.8%) | 11 (7.0%) | 254 (6.9%) | 8 (8.5%) | 282 (16.0%) | 24 (19.5%) | |||
| Other | 180 (7.6%) | 8 (5.1%) | 323 (8.8%) | 4 (4.3%) | 86 (4.9%) | 7 (5.7%) | |||
| Insured | 2272 (97.9%) | 151 (98.7%) | 0.767 | 3507 (96.6%) | 89 (95.7%) | 0.559 | 1685 (97.0%) | 115 (99.1%) | 0.253 |
| Comorbidity score* | |||||||||
| 0 | 1645 (69.8%) | 122 (77.7%) | 0.099 | 2583 (70.4%) | 65 (69.1%) | 0.455 | 1222 (69.2%) | 90 (73.2%) | 0.330 |
| 1 | 537 (22.8%) | 25 (15.9%) | 832 (22.7%) | 25 (26.6%) | 390 (22.1%) | 27 (22.0%) | |||
| ≥ 2 | 175 (7.4%) | 10 (6.4%) | 253 (6.9%) | 4 (4.3%) | 153 (8.7%) | 6 (4.9%) | |||
| Hospital location | |||||||||
| Midwest | 583 (25.2%) | 52 (36.4%) | 0.001 | 894 (24.8%) | 28 (31.1%) | 0.175 | 438 (25.6%) | 33 (28.2%) | 0.693 |
| South | 846 (36.5%) | 39 (27.3%) | 1345 (37.2%) | 37 (41.1%) | 654 (38.2%) | 47 (40.2%) | |||
| Northeast | 546 (23.6%) | 22 (15.4%) | 741 (20.5%) | 16 (17.8%) | 387 (22.6%) | 25 (21.4%) | |||
| West | 342 (14.8%) | 30 (21.0%) | 632 (17.5%) | 9 (10.0%) | 235 (13.7%) | 12 (10.3%) | |||
| Hospital type | |||||||||
| Academic | 1479 (63.8%) | 98 (68.5%) | 0.247 | 2085 (57.7%) | 61 (67.8%) | 0.127 | 962 (56.1%) | 72 (61.5%) | 0.396 |
| Comprehensive community | 786 (33.9%) | 40 (28.0%) | 1415 (39.2%) | 28 (31.1%) | 693 (40.4%) | 40 (34.2%) | |||
| Community | 52 (2.2%) | 5 (3.5%) | 112 (3.1%) | 1 (1.1%) | 59 (3.4%) | 5 (4.3%) | |||
| Year of diagnosis** | |||||||||
| 2006 | 93 (93.9%) | 6 (6.1%) | <0.001 | 153 (98.7%) | 2 (1.3%) | 0.013 | 88 (93.6%) | 6 (6.4%) | 0.008 |
| 2007 | 113 (93.4%) | 8 (6.6%) | 167 (98.2%) | 3 (1.8%) | 95 (97.9%) | 2 (2.1%) | |||
| 2008 | 199 (97.5%) | 5 (2.5%) | 293 (98.3%) | 5 (1.7%) | 152 (93.3%) | 11 (6.7%) | |||
| 2009 | 231 (97.5%) | 6 (2.5%) | 352 (98.6%) | 5 (1.4%) | 170 (96.0%) | 7 (4.0%) | |||
| 2010 | 251 (93.0%) | 19 (7.0%) | 377 (97.7%) | 9 (2.3%) | 183 (94.8%) | 10 (5.2%) | |||
| 2011 | 248 (96.5%) | 9 (3.5%) | 342 (97.4%) | 9 (2.6%) | 177 (94.1%) | 11 (5.9%) | |||
| 2012 | 239 (92.3%) | 20 (7.7%) | 373 (96.9%) | 12 (3.1%) | 184 (93.4%) | 13 (6.6%) | |||
| 2013 | 258 (94.5%) | 15 (5.5%) | 454 (97.2%) | 13 (2.8%) | 182 (92.9%) | 14 (7.1%) | |||
| 2014 | 268 (93.7%) | 18 (6.3%) | 434 (98.0%) | 9 (2.0%) | 205 (94.0%) | 13 (6.0%) | |||
| 2015 | 254 (90.7%) | 26 (9.3%) | 384 (97.0%) | 12 (3.0%) | 173 (90.6%) | 18 (9.4%) | |||
| 2016 | 203 (89.0%) | 25 (11.0%) | 339 (95.8%) | 15 (4.2%) | 156 (89.7%) | 18 (10.3%) | |||
| Clinical stage | |||||||||
| I | 1101 (46.7%) | 64 (40.8%) | 0.148 | 2185 (59.6%) | 30 (31.9%) | 0.001 | 580 (32.9%) | 10 (8.1%) | <0.001 |
| II | 1256 (53.3%) | 93 (59.2%) | 1483 (40.4%) | 64 (68.1%) | 646 (36.6%) | 52 (42.3%) | |||
| III | 539 (30.5%) | 61 (49.6%) | |||||||
| Neoadjuvant RT | 0 | 76 (48.4%) | – | 0 | 35 (37.2%) | – | 0 | 59 (48.8%) | |
Data are presented as n (%) or median (IQR); data for all categorical variables are presented as column percentages, except for the variable
Charlson–Deyo comorbidity score
Year of diagnosis where data are presented as row percentages to reflect temporal trends per year; percentages may not add up to 100% owing to missing data NCDB National Cancer Data Base, UR upfront resection, NAT neoadjuvant therapy, IQR interquartile range, MIS minimally invasive resection
Pathologic Characteristics and Outcomes by Anatomic Site
Extrahepatic Cholangiocarcinoma
Clinical tumor stage was similar between those who received NAT and UR for extrahepatic cholangiocarcinoma (Table 1). A total of 24% of patients who received NAT experienced T tumor downstaging, and 9% experienced N tumor downstaging. The rate of complete tumor (R0) resection was higher in the NAT group compared with the UR group (83% vs 76%, p = 0.04). Length of index hospitalization, readmissions, and 30-day mortality were not different. Patients who received NAT were less likely to receive chemotherapy in the adjuvant setting (24% vs 48%, p < 0.001) than those who underwent UR (Table 2). A total of 37% (361 of 969) of patients who underwent UR and had pathologically confirmed nodal disease did not receive adjuvant chemotherapy (Supplementary Table 1).
TABLE 2.
Pathologic and postoperative outcomes by receipt of neoadjuvant therapy (NAT) versus upfront resection (UR)
| Extrahepatic cholangiocarcinoma |
Ampullary adenocarcinoma |
Duodenal adenocarcinoma |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| UR (N = 2357) | NAT (N = 157) | p | UR (N = 3668) | NAT (N = 94) | p | UR (N = 1765) | NAT (N = 123) | p | |
|
| |||||||||
| Pathologic stage | 0.153 | 0.639 | 0.006 | ||||||
| I | 517 (24.3%) | 35 (30.2%) | 1265 (37.9%) | 30 (40.5%) | 279 (17.3%) | 5 (5.1%) | |||
| II | 1610 (75.7%) | 81 (69.8%) | 2076 (62.1%) | 44 (59.5%) | 510 (31.7%) | 38 (38.4%) | |||
| III | 820 (51.0%) | 56 (56.6%) | |||||||
| T tumor downstaging | – | 26 (24.1%) | – | – | 19 (28.4%) | – | – | 29 (34.1%) | – |
| N tumor downstaging | – | 10 (8.5%) | – | – | 10 (15.2%) | – | – | 17 (20.2%) | – |
| Margin positivity (R1/R2) | 548 (24.1%) | 25 (16.7%) | 0.037 | 116 (3.2%) | 2 (2.3%) | 1.000 | 141 (8.2%) | 9 (7.7%) | 1.000 |
| Tumor grade | 0.834 | 0.364 | 0.204 | ||||||
| G1 | 276 (12.9%) | 13 (14.4%) | 455 (13.1%) | 13 (17.8%) | 151 (9.1%) | 8 (7.7%) | |||
| G2 | 1137 (53.0%) | 45 (50.0%) | 1913 (55.2%) | 35 (47.9%) | 919 (55.3%) | 50 (48.1%) | |||
| G3 | 733 (34.2%) | 32 (35.6%) | 1096 (31.6%) | 25 (34.2%) | 591 (35.6%) | 46 (44.2%) | |||
| Adjuvant chemotherapy | 1120 (47.5%) | 38 (24.2%) | 0.0001 | 1531 (41.7%) | 28 (29.8%) | 0.020 | 731 (41.4%) | 31 (25.2%) | <0.001 |
| Length of stay, days | 9 (7–14) | 9 (7–15) | 0.881 | 10 (7–15) | 11 (8–14) | 0.916 | 10 (7–16) | 10 (7–15) | 0.558 |
| 30-Day readmission | 191 (8.3%) | 11 (7.0%) | 0.654 | 300 (8.3%) | 12 (13.0%) | 0.125 | 139 (8.0%) | 14 (11.9%) | 0.164 |
| 30-Day mortality | 99 (4.6%) | 3 (2.3%) | 0.278 | 116 (3.5%) | 1 (1.3%) | 0.522 | 71 (4.4%) | 4 (3.8%) | 1.000 |
| Follow-up time, months | 22 (11–40) | 26 (16–47) | 0.003 | 31 (15–55) | 31 (17–50) | 0.766 | 28 (12–54) | 31 (18–58) | 0.114 |
| Median overall survival, months | 25.6 | 38.4 | <0.001 | 52.9 | 39.6 | 0.579 | 48.3 | 57.8 | 0.24 |
Data are presented as n (%), or median (IQR)
UR upfront resection, NAT neoadjuvant therapy, IQR interquartile range, RT radiation therapy
With a median follow-up of 22 months, patients with cholangiocarcinoma who received NAT had improved median overall survival compared with upfront resection (38.4 vs 25.6 months, p < 0.001) (Fig. 1A). After adjustment for patient, clinical disease factors, and adjuvant chemotherapy, NAT was associated with improved survival compared with UR [hazard ratio (HR) 0.69, 95% confidence interval (CI) 0.54–0.89, p = 0.004)] (Table 3).
FIG. 1.
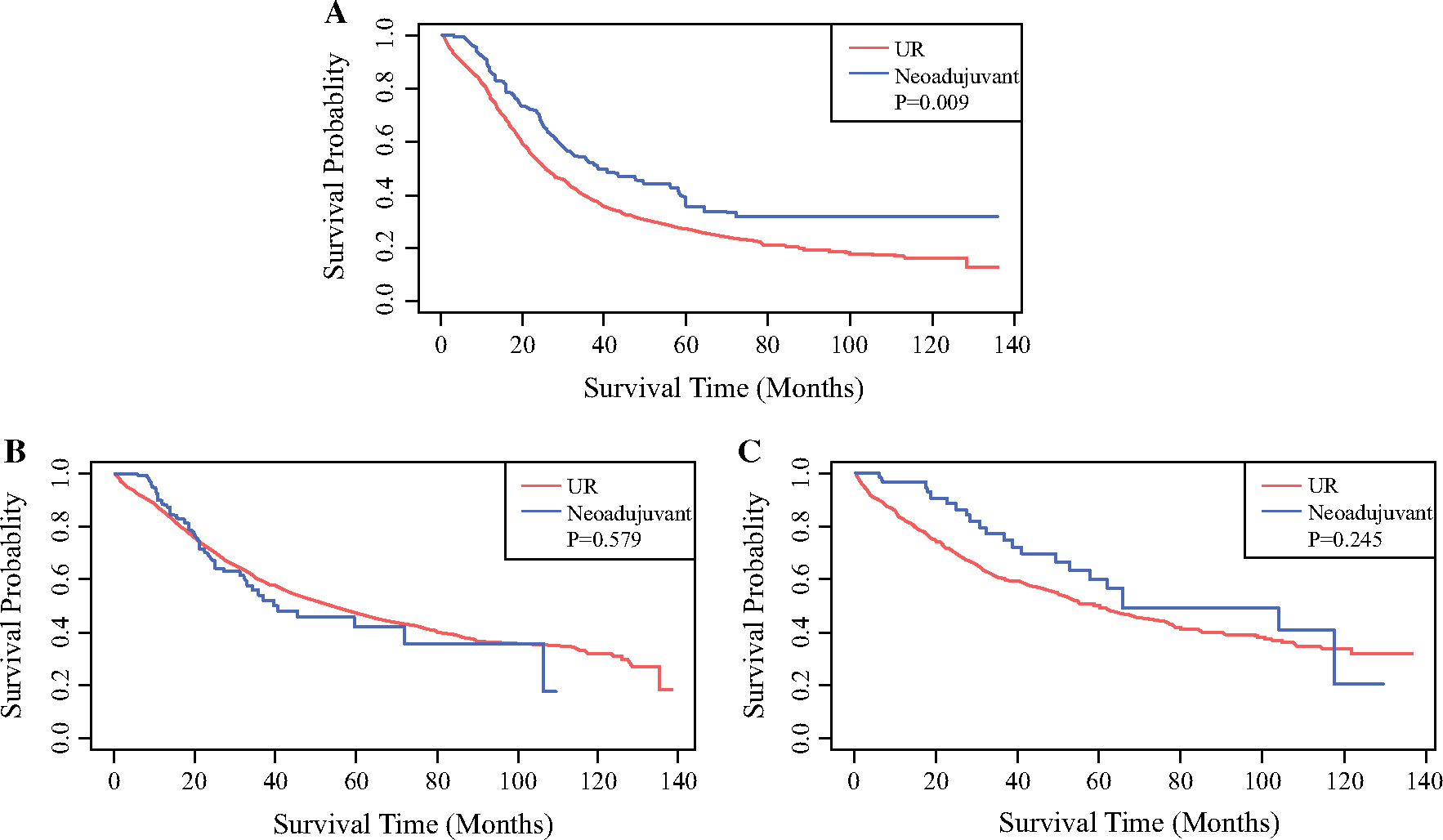
Overall survival of patients who underwent resection with or without neoadjuvant chemotherapy for extrahepatic cholangiocarcinoma (A), ampullary adenocarcinoma (B), or duodenal adenocarcinoma (C)
TABLE 3.
Adjusted overall survival estimates for patients undergoing resection by receipt of neoadjuvant therapy and cancer site; extrahepatic cholangiocarcinoma
| Variable | HR (95% CI) | p |
|---|---|---|
|
| ||
| Neoadjuvant therapy (ref. = upfront resection) | ||
| Yes | 0.69 (0.54–0.89) | 0.004 |
| Patient age (ref. = age < 45 years) | ||
| 45–65 years | 1.15 (0.73–1.8) | 0.557 |
| >65 years | 1.59 (1.01–2.49) | 0.045 |
| Gender (ref. = male) | ||
| Female | 0.98 (0.88–1.09) | 0.714 |
| Race (ref. = white) | ||
| Black | 0.93 (0.76–1.14) | 0.466 |
| Other | 0.92 (0.75–1.13) | 0.447 |
| Insurance (ref. = no) | ||
| Insured | 0.98 (0.67–1.43) | 0.918 |
| Comorbidity score (ref. = 0)* | ||
| Comorbidity score 1 | 0.97 (0.85–1.1) | 0.612 |
| Comorbidity score ≥ 2 | 1.25 (1.03–1.52) | 0.026 |
| Hospital type (ref. = community) | ||
| Academic | 0.76 (0.55–1.05) | 0.091 |
| Comprehensive community | 0.82 (0.59–1.14) | 0.233 |
| Clinical tumor stage (ref. = stage I) | ||
| Stage II | 1.46 (1.31–1.62) | <.0001 |
| Neoadjuvant radiation (ref. = no) | ||
| Yes | 0.86 (0.46–1.63) | 0.648 |
| Adjuvant chemotherapy (ref. = no) | ||
| Yes | 0.83 (0.74–0.92) | 0.001 |
Charlson–Deyo comorbidity score
HR hazard ratio, CI confidence interval, p p-value, ref. reference
Ampullary Adenocarcinoma
Neoadjuvant therapy in ampullary adenocarcinoma was more often associated with advanced clinical tumor stage (p < 0.001) (Table 1). However, 28% of patients who received NAT achieved T tumor downstaging, and 15% achieved N tumor downstaging. The margin-negative (R0) resection rates were similar between the NAT and UR groups (98% vs 97%, p = 1.00). Patients who received NAT were less likely to receive chemotherapy in the adjuvant setting (30% vs 42%, p = 0.020) (Table 2). Thirty-seven percent (592/1607) of patients who underwent UR and had pathologically confirmed nodal disease did not receive adjuvant chemotherapy (Supplementary Table 1). Length of hospitalization, readmissions, and 30-day mortality were not different among those who received NAT versus UR.
With a median follow-up of 31 months, no significant difference was observed in overall survival between patients who received NAT and those who underwent UR (39.6 vs 52.9 months, p = 0.58) (Fig. 1B). After adjustment for preoperative factors and adjuvant chemotherapy, use of NAT was not associated with improved survival (HR 0.96, 95% CI 0.69–1.33, p = 0.78) in ampullary adenocarcinoma (Table 4).
TABLE 4.
Adjusted overall survival estimates for patients undergoing resection by receipt of neoadjuvant therapy and cancer site; ampullary adenocarcinoma
| Variable | HR (95% CI) | p |
|---|---|---|
|
| ||
| Neoadjuvant therapy (ref. = upfront resection) | ||
| Yes | 0.96 (0.69–1.33) | 0.783 |
| Patient age (ref. = age < 45 years) | ||
| 45–65 years | 1.65 (1.03–2.65) | 0.038 |
| >65 years | 2.53 (1.58–4.06) | <0.001 |
| Gender (ref. = male) | ||
| Female | 0.78 (0.71–0.87) | <0.001 |
| Race (ref. = white) | ||
| Black | 1.07 (0.88–1.31) | 0.5 |
| Other | 0.94 (0.78–1.14) | 0.549 |
| Insurance (ref. = no) | ||
| Insured | 1 (0.73–1.36) | 0.981 |
| Comorbidity score (ref. = 0) | ||
| Comorbidity score 1 | 1.21 (1.08–1.36) | 0.001 |
| Comorbidity score ≥ 2 | 1.29 (1.07–1.56) | 0.007 |
| Hospital type (ref. = community) | ||
| Academic | 1.02 (0.76–1.37) | 0.911 |
| Comprehensive community | 1.12 (0.83–1.5) | 0.475 |
| Clinical tumor stage (ref. = stage I) | ||
| Stage II | 1.59 (1.43–1.76) | <0.001 |
| Neoadjuvant radiation (ref. = no) | ||
| Yes | 0.99 (0.52–1.91) | 0.985 |
| Adjuvant chemotherapy (ref. = no) | ||
| Yes | 0.89 (0.8–0.99) | 0.036 |
HR hazard ratio, CI confidence interval, p p-value, ref. reference
Duodenal Adenocarcinoma
Similar to ampullary cancers, NAT in duodenal adenocarcinoma was more often associated with advanced clinical stage tumors (p < 0.001) (Table 1). However, 34% of patients who received NAT for duodenal adenocarcinoma achieved T tumor downstaging, and 20% achieved N tumor downstaging. The rate of margin-negative (R0) resection was similar (92%) between the NAT and UR groups. Length of hospitalization, readmissions, and 30-day mortality were comparable in both groups. Compared with those who received NAT, those who underwent UR were more likely to receive adjuvant chemotherapy (25% NAT vs 41% UR, p < 0.001) (Table 2). Thirty-eight percent (330/866) of patients who underwent UR and had pathologically confirmed nodal disease did not receive adjuvant chemotherapy (Supplementary Table 1).
With a median follow-up of 28 months, unadjusted overall survival was not significantly different between patients who received NAT and those who underwent UR (58 vs 48 months, p = 0.24) (Fig. 1C), and this did not change after adjustment for preoperative factors and adjuvant chemotherapy (HR 0.98, 95% CI 0.67–1.45, p = 0.93) (Table 5).
TABLE 5.
Adjusted overall survival estimates for patients undergoing resection by receipt of neoadjuvant therapy and cancer site; duodenal adenocarcinoma
| Variable | HR (95% CI) | p |
|---|---|---|
|
| ||
| Neoadjuvant therapy (ref. = upfront resection) | ||
| Yes | 0.98 (0.67–1.45) | 0.928 |
| Patient age (ref. = age < 45 years) | ||
| 45–65 years | 1.29 (0.77–2.15) | 0.330 |
| >65 years | 2.28 (1.38–3.78) | 0.001 |
| Gender (ref. = male) | ||
| Female | 0.91 (0.79–1.04) | 0.159 |
| Race (ref. = white) Black | 0.99 (0.82–1.20) | 0.902 |
| Other | 0.75 (0.52–1.08) | 0.124 |
| Insurance (ref. = no) | ||
| Insured | 1.39 (0.78–2.48) | 0.263 |
| Comorbidity score (ref. = 0) | ||
| Comorbidity score 1 | 1.18 (1.00–1.39) | 0.048 |
| Comorbidity score ≥ 2 | 1.15 (0.90–1.48) | 0.263 |
| Hospital type (ref. = community) | ||
| Academic | 0.61 (0.43–0.86) | 0.005 |
| Comprehensive community | 0.82 (0.58–1.15) | 0.250 |
| Clinical tumor stage (ref. = stage I) | ||
| Stage II | 1.46 (1.22–1.75) | <0.001 |
| Stage III | 2.30 (1.91–2.77) | <0.001 |
| Neoadjuvant radiation (ref. = no) | ||
| Yes | 0.60 (0.32–1.09) | 0.091 |
| Adjuvant chemotherapy (ref. = no) | ||
| Yes | 0.69 (0.59–0.80) | <0.001 |
HR hazard ratio, CI confidence interval, p p-value, ref. reference
Propensity-Matched Analysis
To confirm the findings identified by the Cox regression models, we performed a 3:1 propensity-matched analysis by cancer site. The adequacy of the matching process is presented in Supplementary Table 2. The survival difference results were qualitatively similar to the regression models (Fig. 2). Compared with upfront surgery, neoadjuvant therapy was associated with improved survival in extrahepatic cholangiocarcinoma (38 vs 25 months, p = 0.007) but not in ampullary adenocarcinoma (46 vs 42 months, p = 0.42) or duodenal adenocarcinoma (58 vs 37 months, p = 0.13).
FIG. 2.

Overall survival of matched* patients who underwent resection with or without neoadjuvant chemotherapy for extrahepatic cholangiocarcinoma (A), ampullary adenocarcinoma (B), or duodenal adenocarcinoma (C)
DISCUSSION
This is the first large national cohort study examining outcomes of neoadjuvant therapy versus upfront resection in the treatment of nonpancreatic periampullary adenocarcinoma. Neoadjuvant therapy was associated with significant tumor downstaging for all non-PDAC periampullary adenocarcinoma types and increased R0 resections for extrahepatic cholangiocarcinoma. After adjustment, NAT use was associated with a survival advantage for extrahepatic cholangiocarcinoma. There does not appear to be a survival benefit for the use of NAT for duodenal adenocarcinoma or ampullary adenocarcinoma in this national dataset.
In this study, patients who received NAT for extrahepatic cholangiocarcinoma had higher R0 resection rates and improved survival than those who underwent upfront resection. These findings remained after adjustment and propensity matching. In a review of 40 patients treated for extrahepatic cholangiocarcinoma, McMasters et al. observed that all patients who received neoadjuvant chemoradiation achieved R0 resection compared with only 54% treated with upfront surgery. This finding was observed even though 66% of the neoadjuvant group had unresectable tumors at presentation.15 Their study did not show a survival difference by timing of systemic therapy, but the study may have been limited by the small number of patients who received neoadjuvant therapy (n = 9) and short follow-up time (median 12 months). In another study from Duke University (Durham, NC) of 45 patients with longer follow-up, the role of neoadjuvant versus adjuvant chemoradiation for cholangiocarcinoma was compared. The vast majority (91%) of patients who received neoadjuvant chemoradiation achieved R0 resections. Despite having more advanced disease at diagnosis, those patients had improved 5-year survival (53% vs 23%), though this difference did not reach statistical significance. The authors concluded that neoadjuvant chemoradiotherapy conferred local control and resectability benefits with the potential for improved survival.16
Important differences between the current study and the previous two reports are the significantly larger sample size and the contemporary nature of our report. The larger sample size allowed adjustment for important clinicopathologic and treatment factors, including the receipt of adjuvant chemotherapy. Like PDAC, the association between NAT and improved survival in extrahepatic cholangiocarcinoma could be explained by early treatment of occult systemic disease and selection of patients most likely to benefit from surgical resection. Additionally, NAT for extrahepatic cholangiocarcinoma was associated with significant tumor downstaging and higher R0 resections, factors previously demonstrated to be prognostic in cholangiocarcinoma.14,17 Notably, our findings of tumor downstaging, higher R0, and improved survival with NAT were demonstrated in operable/resectable disease, lending further support for its potential benefit in patients with locally advanced biliary cancers.18,19
Similar to cholangiocarcinoma, the use of NAT for duodenal adenocarcinoma was associated with tumor downstaging; however, it was not associated with improved survival either in the unadjusted analysis or after adjusting for clinicopathologic factors and receipt of adjuvant chemotherapy. Previous reports examining the role of NAT in these tumors are limited to small cohorts with locally advanced disease. These studies suggested duodenal cancers respond well to preoperative therapy,20,21 and outcomes are dramatically different for R0 versus R1 resection (5-year overall survival 60% versus 0%, respectively).22,23 In a small phase II trial examining the impact of preoperative 5-fluorouracil (5-FU), mitomycin C, and radiation therapy, NAT resulted in a pathologic complete response (pCR) and R0 resection in 80% of patients.20 In another study of 11 patients with localized duodenal cancer receiving neoadjuvant chemoradiation, pCR was achieved in only two patients, and overall survival did not differ between those who received neoadjuvant chemoradiation compared with those who underwent upfront surgery; however, those who had R0 resections following neoadjuvant therapy had improved survival compared with those with R0 resections at the time of upfront surgery (5-year survival 83% vs 53%, respectively; p = 0.07).22 A recent report from the NCDB comparing neoadjuvant versus adjuvant chemotherapy for duodenal adenocarcinoma (2004–2014) found no difference in overall survival.24 Our study examined neoadjuvant chemotherapy’s effect on survival while accounting for the impact of adjuvant chemotherapy, but we did not compare neoadjuvant chemotherapy with adjuvant chemotherapy.
Our study did not demonstrate a significant benefit in R0 resection rate or overall survival among patients with ampullary adenocarcinoma who received neoadjuvant therapy compared with those who underwent upfront surgery. A single-institution study of 142 patients who underwent pancreaticoduodenectomy for ampullary adenocarcinoma compared outcomes of neoadjuvant therapy to upfront surgery. Although 21% of the NAT patients achieved pCR, there was no significant difference in local recurrence and survival. The authors concluded that the routine use of NAT for ampullary adenocarcinoma could not be recommended but that it may represent an appropriate selection strategy for patients with high-risk clinical features that would render them most likely to benefit from tumor downstaging before surgery or the avoidance of postoperative complications that could delay or prevent receipt of adjuvant therapy.25 Similarly, another single-center study of 137 patients demonstrated that the addition of either neoadjuvant or adjuvant chemoradiation resulted in an improvement in 3-year local control but no significant survival difference.1 Taken together, the routine use of preoperative chemotherapy for ampullary adenocarcinoma may not be justified; however, some data suggest a benefit of adjuvant chemotherapy for patients with nodal metastasis and locally advanced disease.25–29 Owing to the limitation of sample size, we could not examine the effect of neoadjuvant chemotherapy for the subset of patients with high-risk ampullary tumors.
Despite the novel findings, our study is limited by selection bias inherent to retrospective studies. Since NAT is not commonly administered for non-PDAC periampullary cancers outside the setting of a clinical protocol, it is likely that some (if not a majority) of the neoadjuvant cholangiocarcinoma cases in this study were deemed to be PDAC preoperatively but found to be non-PDAC pathology following resection. It is less likely that this was the case for the ampullary or duodenal adenocarcinoma cohorts. This is a possible explanation for the more frequent use of neoadjuvant chemotherapy for lower-stage cholangiocarcinoma cases compared with ampullary or duodenal adenocarcinoma cases. Thus, an important limitation of this study is the heterogeneity of the cohort in terms of the relative contributions of resectable versus borderline resectable cases within each tumor type cohort with different implications for the role of neoadjuvant chemotherapy. An argument can be made that the true survival advantage for NAT may, in fact, be somewhat underestimated. Another limitation is that patients who failed NAT and transitioned to palliative therapy were not captured in this study. Finally, details on the type, duration, and completion of neoadjuvant chemotherapy were not available for analysis. Given the likelihood that patients with extrahepatic cholangiocarcinoma treated with neoadjuvant chemotherapy were initially misdiagnosed as PDAC, it is reasonable to conclude that these patients were most likely to receive standard of care neoadjuvant regimens for PDAC, namely FOLFIRINOX or gemcitabine/abraxane. Therefore, these are the regimens that we would favor evaluating in a prospective trial.
Despite these limitations, this large national cohort study contributes to an improved understanding of the impact of NAT in nonpancreatic periampullary adenocarcinomas, particularly given the relative rarity of these tumors and the inherent difficulty in assessing various treatment options through randomized clinical trials. We demonstrate that NAT was associated with significant tumor downstaging, improved R0 resection rates, and a survival benefit in patients with extrahepatic cholangiocarcinoma. While we also identified associations between NAT and tumor downstaging for duodenal and ampullary adenocarcinomas, there was no discernable survival impact for either disease. This analysis supports further evaluation of the impact of neoadjuvant therapy in these aggressive malignancies.
Supplementary Material
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434-022-12257-x.
DISCLOSURE None to declare.
REFERENCES
- 1.Palta M, et al. Carcinoma of the ampulla of Vater: patterns of failure following resection and benefit of chemoradiotherapy. Ann Surg Oncol. 2012;19(5):1535–40. 10.1245/s10434-011-2117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakaeen FG, et al. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135(6):635–42. [DOI] [PubMed] [Google Scholar]
- 3.Veillette G, Castillo CF. Distal biliary malignancy. Surg Clin N Am. 2008;88(6):1429–47. [DOI] [PubMed] [Google Scholar]
- 4.Doepker MP, et al. Clinicopathologic and survival analysis of resected ampullary adenocarcinoma. J Surg Oncol. 2016;114(2):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albores-Saavedra J, et al. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100(7):598–605. [DOI] [PubMed] [Google Scholar]
- 6.Hester CA, et al. Incidence and comparative outcomes of periampullary cancer: a population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J Surg Oncol. 2019;119(3):303–17. [DOI] [PubMed] [Google Scholar]
- 7.Chawla A, et al. Neoadjuvant therapy is associated with improved survival in borderline-resectable pancreatic cancer. Ann Surg Oncol. 2020;27(4):1191–200. 10.1245/s10434-019-08087-z. [DOI] [PubMed] [Google Scholar]
- 8.Oba A, et al. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khorana AA, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(20):2324–8. [DOI] [PubMed] [Google Scholar]
- 10.Adam M, et al. Neoadjuvant chemotherapy for pancreatic adenocarcinoms lessens the deleterious effect of omission of adjuvant chemotherapy. Ann Surg Oncol. 2021;28:3800–7. 10.1245/s10434-020-09446-x. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147–56. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24. [DOI] [PubMed] [Google Scholar]
- 13.Primrose JN, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–73. [DOI] [PubMed] [Google Scholar]
- 14.Cloyd JM, et al. The role of preoperative therapy prior to pancreatoduodenectomy for distal cholangiocarcinoma. Am J Surg. 2019;218(1):145–50. [DOI] [PubMed] [Google Scholar]
- 15.McMasters KM, et al. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174(6):605–8 (discussion 608–9). [DOI] [PubMed] [Google Scholar]
- 16.Nelson JW, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73(1):148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijer LL, et al. Outcomes and treatment options for duodenal adenocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2018;25(9):2681–92. 10.1245/s10434-018-6567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi SW, et al. Efficacy of concurrent chemoradiotherapy with 5-fluorouracil or gemcitabine in locally advanced biliary tract cancer. Cancer Chemother Pharmacol. 2014;73(1):191–8. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20(1):318–24. 10.1245/s10434-012-2312-8. [DOI] [PubMed] [Google Scholar]
- 20.Yeung RS, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A phase II study. Cancer. 1993;72(7):2124–33. [DOI] [PubMed] [Google Scholar]
- 21.Onkendi EO, et al. Neoadjuvant treatment of duodenal adenocarcinoma: a rescue strategy. J Gastrointest Surg. 2012;16(2):320–4. [DOI] [PubMed] [Google Scholar]
- 22.Kelsey CR, et al. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(5):1436–41. [DOI] [PubMed] [Google Scholar]
- 23.Rose DM, et al. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg. 1996;183(2):89–96. [PubMed] [Google Scholar]
- 24.Linden K, et al. The role of neoadjuvant versus adjuvant therapy for duodenal adenocarcinoma: a national cancer database propensity score matched analysis. Am Surg. 2020;87:1066–73. [DOI] [PubMed] [Google Scholar]
- 25.Cloyd JM, et al. Influence of preoperative therapy on short- and long-term outcomes of patients with adenocarcinoma of the ampulla of Vater. Ann Surg Oncol. 2017;24(7):2031–9. 10.1245/s10434-017-5777-7. [DOI] [PubMed] [Google Scholar]
- 26.Hatzaras I, et al. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17(4):991–7. 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta VK, et al. Adjuvant chemoradiotherapy for “unfavorable” carcinoma of the ampulla of Vater: preliminary report. Arch Surg. 2001;136(1):65–9. [DOI] [PubMed] [Google Scholar]
- 28.Kwon J, et al. Survival benefit of adjuvant chemoradiotherapy in patients with ampulla of vater cancer: a systematic review and meta-analysis. Ann Surg. 2015;262(1):47–52. [DOI] [PubMed] [Google Scholar]
- 29.Xia BT, et al. Early recurrence and omission of adjuvant therapy after pancreaticoduodenectomy argue against a surgery-first approach. Ann Surg Oncol. 2016;23(13):4156–64. 10.1245/s10434-016-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


