Abstract
During lytic infection, the virion host shutoff (vhs) protein of herpes simplex virus (HSV) mediates the rapid degradation of RNA and shutoff of host protein synthesis. In mice, HSV type 1 (HSV-1) mutants lacking vhs activity are profoundly attenuated. HSV-2 has significantly higher vhs activity than HSV-1, eliciting a faster and more complete shutoff. To examine further the role of vhs activity in pathogenesis, we generated an intertypic recombinant virus (KOSV2) in which the vhs open reading frame of HSV-1 strain KOS was replaced with that of HSV-2 strain 333. KOSV2 and a marker-rescued virus, KOSV2R, were characterized in cell culture and tested in an in vivo mouse eye model of latency and pathogenesis. The RNA degradation kinetics of KOSV2 was identical to that of HSV-2 333, and both showed vhs activity significantly higher than that of KOS. This demonstrated that the fast vhs-mediated degradation phenotype of 333 had been conferred upon KOS. The growth of KOSV2 was comparable to that of KOS, 333, and KOSV2R in cell culture, murine corneas, and trigeminal ganglia and had a reactivation frequency similar to those of KOS and KOSV2R from explanted latently infected trigeminal ganglia. There was, however, significantly reduced blepharitis and viral replication within the periocular skin of KOSV2-infected mice compared to mice infected with either KOS or KOSV2R. Taken together, these data demonstrate that heightened vhs activity, in the context of HSV-1 infection, leads to increased viral clearance from the skin of mice and that the replication of virus in the skin is a determining factor for blepharitis. These data also suggest a role for vhs in modulating host responses to HSV infection.
Herpes simplex virus (HSV) causes the rapid shutoff of macromolecular synthesis in infected cells. The factor responsible for this shutoff is the product of the UL41 gene, known as the virion host shutoff (vhs) protein (21, 33, 38). Vhs is a 58-kDa phosphoprotein, and approximately 200 copies are packaged within the tegument of the virus, allowing it to exert its effects immediately upon infection prior to de novo viral gene expression (11, 36). vhs-induced shutoff leads to polysomal disaggregation and nonspecific cytoplasmic degradation of cellular mRNAs (23–25, 35) and viral mRNAs belonging to all three kinetic classes (32). Although the exact mechanism remains to be determined, recent studies demonstrate that vhs-dependent endoribonucleolytic activity can be selectively targeted by specific cis-acting elements in the RNA (7, 8, 47). Two advantages to the virus may result from vhs-induced shutoff. First, it depletes the pool of translatable host mRNA such that viral mRNA can be preferentially translated; second, it prevents the overexpression of immediate-early and early viral genes, allowing efficient transition between the sequential kinetic classes. Despite these putative advantages, the effect of vhs deletion on the efficiency of viral replication in cell culture is minimal (35, 36, 42).
Primary sequence analysis of five of the neurotropic alphaherpesvirus genomes (HSV type 1 [HSV-1], HSV-2, varicella-zoster virus, equine herpesvirus 1, and pseudorabies virus) revealed that each has a homolog of vhs with 89% amino acid identity within four conserved domains (3). This conservation of vhs in the neurotropic viruses and its apparent absence in lymphotropic herpesviruses suggested that vhs must play an important role in neuropathogenesis. Supporting this idea, HSV-1 and HSV-2 mutant viruses lacking vhs function have a reduced ability to replicate in the cornea, vagina, trigeminal ganglia, dorsal root ganglia, and brain of the mouse and show an impaired ability to establish and reactivate from latency in a murine model 40–42; T. J. Smith and D. A. Leib, unpublished data). Despite this impaired ability to replicate in vivo, an HSV mutant lacking vhs activity remained highly immunogenic and served as both a prophylactic and therapeutic vaccine in mice (45, 46).
HSVs are causative agents of cold sores, keratitis, blepharitis, genital sores, and encephalitis (39). HSV-1 and HSV-2 are closely related, and their vhs genes are 87% identical at the amino acid level (9). Their vhs activities, however, differ significantly, with the shutoff activity of HSV-2 vhs being more than 40 times faster than that of HSV-1 (10, 12, 13, 20). HSV-1 and HSV-2 also show significant differences in virulence. HSV-2 is more efficient at causing necrotizing stromal keratitis and encephalitis and grows to higher titers than HSV-1 in animal models (39). In humans, although both are capable of infecting both genital and facial mucosae, HSV-2 genital infection is twice as likely to recur, and the recurrences are 8 to 10 times more frequent than in an HSV-1 infection (43). Additionally, HSV-2 is more neurovirulent than HSV-1, causing more neurological injury in infants surviving neonatal infection, and is more likely to cause meningitis in patients with primary genital infections (39). Although the role of HSV-2 vhs in pathogenesis was not directly addressed, HSV-2 vhs and ICP47 act synergistically to block antigen presentation by major histocompatibility complex class I, suggesting an immunomodulatory role for vhs (19, 44).
In this study, an intertypic recombinant virus, KOSV2, was generated in order to elucidate whether the type-specific differences in shutoff kinetics between HSV-1 and HSV-2 have consequences for viral pathogenesis in a murine model. The entire vhs open reading frame (ORF) of HSV-1 strain KOS was exchanged with that of HSV-2 strain 333 to create the recombinant KOSV2. KOSV2 was then tested for its ability to induce RNA degradation in cell culture and for its pathogenicity in vivo. Our intertypic mutant exhibited the same shutoff phenotype as HSV-2, and its growth in vitro was comparable to that of HSV-1 and HSV-2. In mice, growth of KOSV2 was indistinguishable in corneas and trigeminal ganglia from that of HSV-1 or HSV-2. There was, however, significantly reduced blepharitis and replication of KOSV2 in the skin compared to wild-type and marker-rescued viruses. This increased clearance in the skin and reduction of inflammatory disease suggests that heightened vhs activity in the context of HSV infection may modulate the inflammatory responses in a tissue-specific fashion.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (Vero) cells were maintained at 5% CO2 in a humidified incubator at 37°C and were propagated as described previously (34). Growth and plaque assays of all viruses were carried out as described previously (34). SB5, a plaque-purified stock of HSV-2 strain 333, was obtained from the American Type Culture Collection (VR-2546). Vero cell extracts used as controls were made as described for virus preparations and constituted mock lysates.
Generation of viral mutants.
The viral mutants used in this study were constructed from the parental HSV-1 strain KOS. The NsiI-HindIII fragment of p333 (provided by Sullivan Read, University of Missouri at Kansas City) (10) (Fig. 1) was cloned into the EcoRV-EcoNI sites of pUL41 (42) to generate plasmid pUL41V2 (not shown). To avoid intragenic recombination between HSV-1 vhs and HSV-2 vhs sequences, pUL41V2 was cotransfected with infectious HSV-1 DNA of a virus that had a complete deletion in the vhs ORF. This virus, dl41 (Fig. 1), was generated by cloning the human cytomegalovirus β-galactosidase (β-Gal) cassette of pHCMV-MP1-lacZ (provided by Paul Olivo, Washington University) into the MscI site of pΔUL41, a plasmid in which the EcoRV-EcoNI fragment of the HSV-1 vhs ORF is deleted, and cotransfecting it with infectious KOS DNA. Progeny from the pUL41V2 and infectious dl41 DNA cotransfection were screened by the selection of white plaques and analyzed by Southern blotting for an appropriately altered BamHI digestion pattern. Virus was plaque purified three times, grown to a high-titered stock, and designated KOSV2. Marker rescue to produce KOSV2R was accomplished in two steps to prevent the possibility of intragenic recombination between the vhs homologs of KOS and KOSV2. First, infectious KOSV2 DNA and pGALSCA-11 (42) were cotransfected into Vero cells. Blue-plaque progeny were analyzed by Southern blotting, and virus demonstrating an appropriately altered BamHI digestion pattern was plaque purified three times and grown to a high-titered stock designated KOSvhsBG (Fig. 1). KOSV2R was subsequently produced by cotransfection of infectious KOSvhsBG DNA with pUL41 in Vero cells and screened by Southern blotting as described for KOSvhsBG. All transfections were done by the calcium phosphate-DNA coprecipitation and glycerol shock method (37). Southern blot analyses of viral DNA was performed as described previously (34, 37). pUL41 was labeled with 32P by random priming and used as a probe in the plaque purification of KOSV2, KOSvhsBG, and KOSV2R.
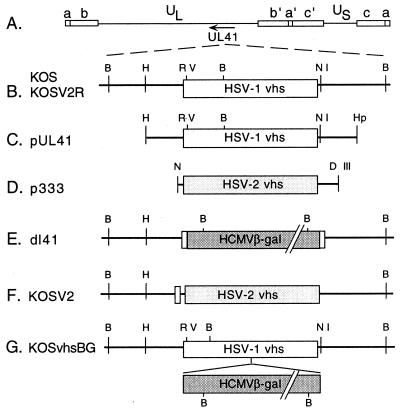
FIG. 1.
Maps of vhs (UL41) ORF, plasmids, and viral mutants used in this study. (A) Prototypical arrangement of the HSV-1 genome, showing unique long (UL) and unique short (US) segments flanked by internal (a′, b′, and c′) and terminal (a, b, and c) repeats. The direction of transcription of UL41 is indicated by the arrow. (B) Expanded view of the UL41 genomic region showing selected restriction enzyme sites for HSV-1 KOS and KOSV2R. (C) HindIII (H)-to-HpaI (Hp) limits of plasmid pUL41 containing the UL41 ORF of HSV-1 KOS. (D) NsiI (N) to HindIII limits of plasmid p333 containing the UL41 ORF of HSV-2 333. The NsiI-HindIII fragment of p333 was cloned into the EcoRV (RV)-EcoNI (NI) sites of pUL41 to generate plasmid pUL41V2 (not shown). pUL41V2 was cotransfected with dl41 infectious DNA (E) to make KOSV2 (F). (G) Intermediate viral mutant, KOSvhsBG, generated by cotransfecting pGALSCA-11 (42) and KOSV2 infectious DNA. KOSvhsBG infectious DNA was cotransfected with pUL41 to make the marker rescue virus KOSV2R.
Northern blot analysis and mRNA degradation assay.
Total cytoplasmic RNA was prepared from monolayer cultures of infected or mock-infected Vero cells as described previously (36). Monolayer cultures of 5 × 105 to 5 × 106 cells were mock infected or infected at a multiplicity of infection (MOI) of 20 with KOS, UL41NHB, SB5, KOSV2, or KOSV2R in the presence of actinomycin D (10 μg/ml). Mock-infected plates received Vero cell lysate only. Cytoplasmic RNAs were harvested at 2 and 8 h postinfection and analyzed for mRNA degradation by Northern blot analysis probing for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (14, 42). Filters were first probed for GAPDH, stripped, and then reprobed for the 28S ribosomal subunit. Phosphorimages were scanned on a Molecular Dynamics Storm 860 PhosphorImager and quantified. The level of GAPDH for mock-infected cells was set at 100% and compared with the 28S-normalized GAPDH values of virus-infected cells.
Animal procedures.
Outbred CD-1 female mice (weight, 21 to 25 g; Charles River Breeding Laboratories, Inc., Kingston, N.Y.) were anesthetized intraperitoneally with ketamine (87 mg/kg) and xylazine (13 mg/kg). Corneas were bilaterally scarified and inoculated with 5 μl of 2 × 106 PFU of virus per eye. Trigeminal ganglion homogenates were made by shaking in 1 ml of medium containing 100 μl of 1-mm-diameter glass beads in a Mini-Beadbeater (Biospec Products, Bartlesville, Okla.) twice for 30 s at high speed and then sonicating twice for 30 s. Eye swab material and trigeminal ganglion homogenates were assayed for virus as previously described (26).
Reactivation of virus from latency was assayed by removing trigeminal ganglia 28 days postinfection, cutting them into two pieces, and explanting them onto Vero cell monolayers. After 5 days in culture, explants were frozen, thawed, homogenized as described above, and assayed for infectious virus on new Vero cell monolayers.
Blepharitis and body weights of infected mice were scored in a masked fashion on days 1 to 7, 9, 16, 23, and 30 postinfection. Blepharitis was measured as follows: 0, no lesions; 1, minimal eyelid swelling; 2, moderate swelling and crusty ocular discharge; 3, severe swelling, moderate periocular hair loss, and skin lesions; and 4, severe swelling with eyes crusted shut, severe periocular hair loss, and skin lesions. Acute periocular conjunctival and skin viral titers were performed by cutting approximately a 1-cm area of skin around each eye and placing in 1 ml of preweighed medium containing 1-mm-diameter glass beads. Skin samples were reweighed, and results of assays of skin homogenates shaken and sonicated as described above were reported as PFU/gram (wet weight) of skin. All mice were housed at the Washington University School of Medicine biohazard facility and euthanized when necessary in accordance with all federal and university policies.
Histology.
Seven days postinfection, a 1-cm area of skin around each eye from mock- and virus-infected mice was removed and immediately fixed in 10% buffered neutral formalin. Paraffin-embedded sections of 5 μm were stained with hematoxylin and eosin and examined for inflammatory infiltrates.
RESULTS
Construction and marker rescue of KOSV2.
To examine the role of vhs kinetics in pathogenesis, an intertypic virus was constructed in which the β-Gal cassette within the HSV-1 KOS vhs-deleted virus, dl41, was replaced with the entire vhs ORF of HSV-2 strain 333. dl41 was constructed by cloning the HCMV β-Gal cassette into pΔUL41, a plasmid in which the entire vhs ORF is deleted, and cotransfecting it with infectious KOS DNA (Fig. 1). Southern blot analysis confirmed the predicted genotype for dl41 (data not shown). Intertypic progeny were screened by selection of white plaques and Southern blotting to generate KOSV2 (Fig. 1 and 2). Marker rescue to produce KOSV2R was accomplished in two steps in order to prevent the possibility of intragenic recombination between the vhs homologs of KOS and KOSV2. Probing with random-primed pUL41 yielded 6.6- and 3.9-kb BamHI fragments for wild-type KOS and marker-rescued KOSV2R viruses, 6.9-, 3.9-, 3.0-, and 1.0-kb fragments for KOSvhsBG, and a 10.5-kb fragment for KOSV2 (Fig. 2). The sizes of these fragments coincided precisely with calculated values, indicating that the genotypes of KOSV2, KOSvhsBG, and KOSV2R were as predicted by experimental design.
FIG. 2.
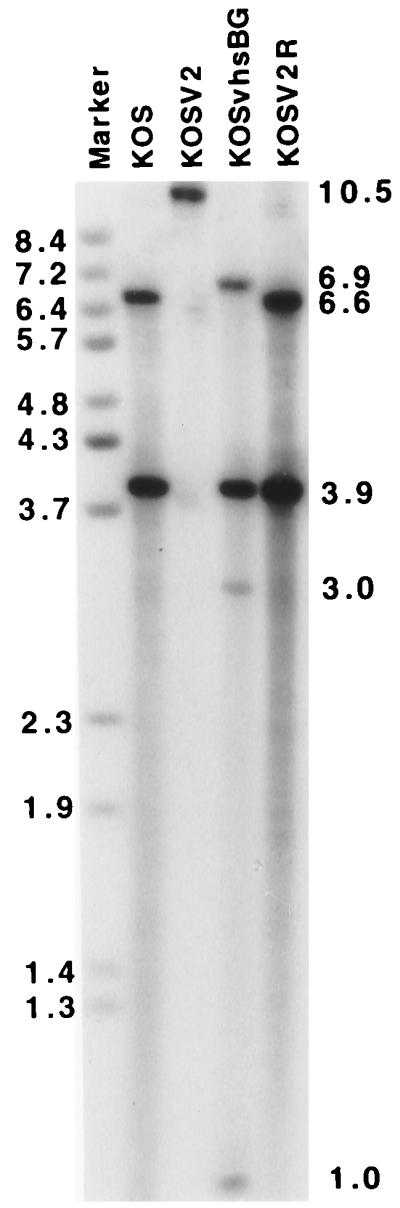
Southern blot analysis of KOS, KOSV2, KOSvhsBG, and KOSV2R, using pUL41 as a probe. Positions of the fragments with the expected sizes resulting from a BamHI digestion are indicated on the right: 6.6- and 3.9-kb fragments for KOS and KOSV2R (lanes 2 and 5, respectively), 10.5-kb fragments for KOSV2 (lane 3), and 6.9-, 3.9-, 3.0-, and 1.0-kb fragments for KOSvhsBG (lane 4). Sizes of BstEII digested bacteriophage lambda are indicated on the left in kilobases.
Measurement of vhs activity of KOSV2.
KOSV2 was examined for its ability to degrade GAPDH in parallel with wild-type HSV-1 KOS, HSV-2 333 (SB5), and the vhs null mutant UL41NHB (42). Cells were infected in the presence of actinomycin D to evaluate degradation of finite pools of RNA induced by preformed tegument-derived vhs. The kinetics of RNA degradation induced by KOSV2 were identical to that induced by wild-type SB5 and significantly faster than for KOS or KOSV2R (Fig. 3). At 2 h postinfection, when the level of GAPDH for mock-infected cells is set at 100% and compared with the 28S-normalized GAPDH values of virus-infected cells, KOS and KOSV2R levels were 75 and 82%, respectively, SB5 and KOSV2 levels were 13 and 17%, respectively, and the KOSvhsBG level was 120%. At 8 h postinfection, KOS and KOSV2R levels were 38 and 62%, respectively, SB5 and KOSV2 levels were both 4%, and the KOSvhsBG level was 125%. As expected, neither KOSvhsBG nor UL41NHB (not shown) showed any detectable vhs activity. Three independent experiments gave similar results. These data demonstrate that the vhs phenotype of HSV-2 had been conferred upon KOS in KOSV2 and restored to KOS in KOSV2R.
FIG. 3.
RNA degradation assay by Northern blot analysis. Cytoplasmic RNA was extracted from Mock-, KOS-, KOSvhsBG- (vhs null mutant), SB5 (HSV-2)-, KOSV2-, and KOSV2R-infected Vero cells at 2 and 8 h postinfection. Autoradiographic images show a Northern blot probed for GAPDH mRNA (top) and the same blot stripped and reprobed for 28S ribosomal subunit (bottom).
Replication kinetics and reactivation efficiency of KOSV2.
The growth kinetics of KOS, KOSV2, SB5, and KOSV2R were examined in Vero cells in a one-step growth curve. The viral yields and growth kinetics in vitro for KOSV2 and KOSV2R were similar to values for both wild-type HSV-1 and HSV-2 at a low MOI (Fig. 4). The growth of KOSV2 was also examined in vivo in mouse corneas and trigeminal ganglia. Acute viral replication in the mouse cornea was analyzed from days 1 to 5 postinfection following scarification and inoculation with 2 × 106 PFU per eye (Fig. 5A). The replications of KOS, SB5, KOSV2, and KOSV2R were indistinguishable at all times. Acute replication in the trigeminal ganglia was also determined following corneal scarification and inoculation with 2 × 106 PFU per eye. The replication of KOSV2 in trigeminal ganglia did not significantly differ from that of KOS or KOSV2R (P > 0.05 by Student's t test) (Fig. 5B). Reactivation frequencies were determined by explant cocultivation performed on day 28 postinfection. All viruses tested (10 of 10 for KOS, 12 of 12 for KOSV2, and 8 of 8 for KOSV2R) reactivated with an efficiency of 100%. SB5 could not be evaluated for reactivation because all mice infected with this virus developed fatal encephalitis by day 8 postinfection.
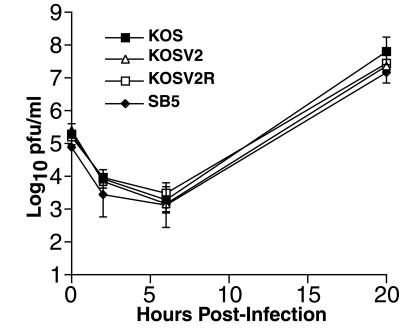
FIG. 4.
One-step growth kinetics of KOS, KOSV2, KOSV2R, and SB5 in Vero cells infected at an MOI of 0.1. Data represent three independent experiments in duplicate. The limit of detection is 10 PFU/ml.
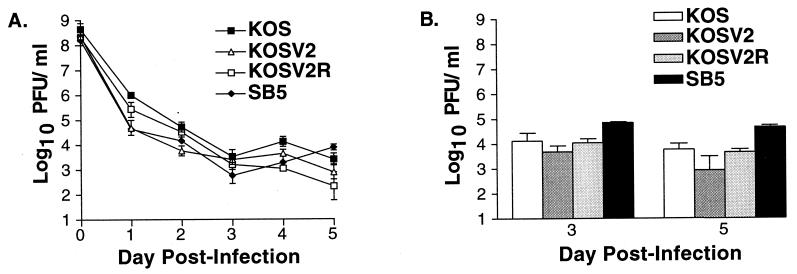
FIG. 5.
Acute viral replication of KOS, KOSV2, KOSV2R, and SB5 in mouse corneas (A) and trigeminal ganglia (B). Mice were infected via corneal scarification and inoculation with 2 × 106 PFU virus per eye. Each data point reflects the logarithmic mean number of PFU per milliliter of virus. Data represent at least three independent experiments with combined and averaged titers of at least eight mice per virus per day. The limit of detection is 10 PFU/ml.
Clinical disease in KOSV2-infected mice.
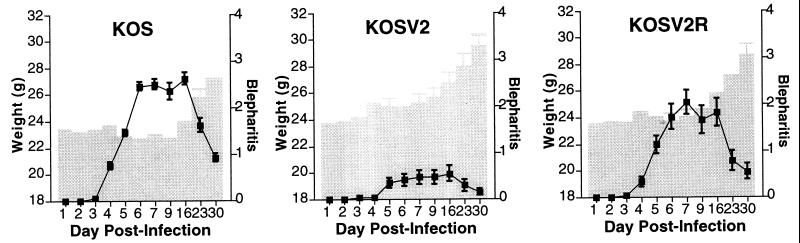
Blepharitis caused by KOS, KOSV2, and KOSV2R was examined days 1 to 7, 9, 16, 23, and 30 days postinfection (Fig. 6). Blepharitis and surrounding periocular disease peak between days 7 and 9 postinfection. Weight was plotted with blepharitis against time for each virus to provide an additional indicator of disease. The progression and severity of blepharitis in KOSV2-infected mice were significantly reduced compared to KOS- and KOSV2R-infected mice. Furthermore, the weights of KOS- and KOSV2R-infected mice remained stable or decreased slightly until 16 days postinfection, after which significant weight gain was seen. The timing of this increase in weight coincided with the resolution of periocular disease. In contrast, the weight of KOSV2-infected mice increased more or less throughout the entire infection period. SB5-infected mice showed the most severe disease, which progressed until encephalitis developed (data not shown). As previously demonstrated, mice infected with the vhs null virus, UL41NHB, appeared essentially uninfected (42). In addition, during the 30-day infection period, no significant differences were observed in the severity of stromal keratitis induced by KOS or KOSV2 (data not shown). Both viruses induced similar corneal opacity with moderate obscuring of the iris, scoring no greater than a 2 on a semiquantitative scale from 0 to 4 (15). Peak stromal keratitis for both viruses occurred between days 10 and 15 postinfection. These data demonstrate that increasing the vhs kinetics of KOS to that of HSV-2 significantly ameliorates blepharitis but not stromal keratitis in mice.
FIG. 6.
Weight and combined blepharitis and periocular disease scores in mice for 30 days following corneal scarification and infection with 2 × 106 PFU per eye of KOS, KOSV2, KOSV2R, or SB5. Blepharitis is shown as a line graph with weight as a superimposed bar graph. Animals were scored on days 1 to 7, 9, 16, 23, and 30 postinfection as follows: 0, no lesions; 1, minimal eyelid swelling; 2, moderate swelling and crusty ocular discharge; 3, severe swelling, moderate periocular hair loss, and skin lesions; and 4, severe swelling with eyes crusted shut, severe periocular hair loss, and skin lesions. Data represent two independent experiments with combined and averaged scores of at least 13 mice per virus per day.
Viral replication and histology in the skin.
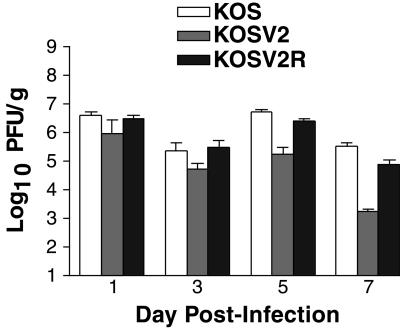
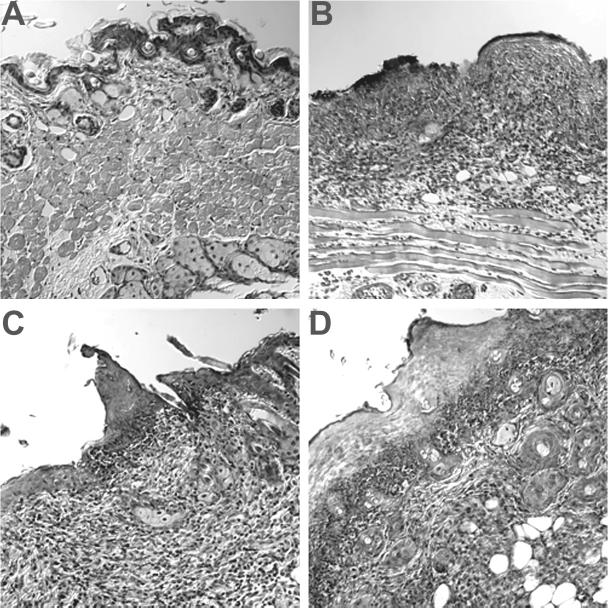
To determine if altered viral growth was responsible for the reduced clinical disease in KOSV2-infected mice, viral replication was determined in the periocular skin (Fig. 7). The three viruses replicated indistinguishably on days 1 and 3 postinfection. On day 5, however, KOSV2-infected mice had significantly reduced viral titers than either KOS- or KOSV2R-infected mice (P < 0.001 by Student's t test). This difference in titer is increased on day 7 and suggests enhanced viral clearance within the skin by KOSV2-infected mice. The peak titers of KOS and KOSV2R immediately precede the peak of clinical disease. Thus, the reduced blepharitis seen for KOSV2 parallels the reduced viral replication. To examine this further, a histological study of the infected tissues was undertaken. On day 7, during peak clinical disease, inflammation was observed in the periocular skin of all mice in the epidermal, dermal, and subjacent tissues (Fig. 8). Inflammatory infiltrates were comprised predominantly of polymorphonuclear cells, but mononuclear cells were also present. Although the presence of epithelial erosions, ulcers, necrosis, and edema was greater in KOS- and KOSV2R-infected mice, the presence of inflammatory infiltrates remained largely unchanged in all virus-infected groups.
FIG. 7.
Acute replication of KOS, KOSV2, KOSV2R, and SB5 in the periocular conjunctiva and skin of mice infected with 2 × 106 PFU per eye via corneal scarification and inoculation. Data are reported as PFU per gram (wet weight) of skin. Each data point reflects the combined and averaged titers of at least four mice. The limit of detection is 10 PFU/ml.
FIG. 8.
Representative hematoxylin-and-eosin-stained histology sections of the periocular skin of mice 7 days following corneal scarification and either mock infected (A) or infected with 2 × 106 PFU per eye of KOS (B), KOSV2 (C), or KOSV2R (D). Magnification, ×200.
DISCUSSION
A number of previous studies have demonstrated that the vhs-dependent RNA degradation activities of HSV-1 and HSV-2 differ significantly, with the activity of HSV-2 vhs being at least 40-fold higher (10, 12). The profound differences in pathogenesis between HSV-1 and HSV-2 coupled with our observations that HSV-1 vhs null mutants are attenuated in vivo led us to speculate that raising the vhs activity of HSV-1 may increase its pathogenicity. Our previous loss-of-function studies had shown decreased virulence for vhs mutants, so it was of interest to attempt a gain-of-function study to shed light on possible mechanisms of vhs function. In this study, KOSV2 and KOSV2R exhibited mRNA degradation activities that were indistinguishable from those of HSV-2 333 and HSV-1 KOS, respectively. This demonstrated that the vhs degradation phenotype of HSV-2 had been conferred upon HSV-1. Furthermore, the ability of the HSV-2 333 vhs gene alone to confer a higher shutoff activity on KOS confirms and extends the idea that vhs is both necessary and sufficient for induction of mRNA degradation (21, 33).
In this study, a marked and unexpected decrease in blepharitis and periocular disease was noted for KOSV2, a virus with increased vhs activity. We considered the hypothesis that this reduced periocular disease induced by KOSV2 was due to an inability of the recombinant to express vhs in vivo. This hypothesis is, however, considered unlikely because five independent vhs null mutants all exhibit a 100- to 1,000-fold reduction in corneal and ganglion titers and fail to induce RNA degradation in cell culture (40–42). In contrast, KOSV2 rapidly induces RNA degradation in cell culture and replicates normally in corneas and ganglia, strongly suggesting that KOSV2 expresses vhs in cell culture and in vivo. Previous work has shown that the loss of vhs activity is not associated with any significant changes in the ability of the virus to replicate in vitro in a variety of culture conditions and cells (35, 36, 42). This study has additionally shown that enhancement of vhs activity is also not detrimental to viral replication in vitro. This underscores the idea that the major function of vhs is concerned not with replication per se but rather with a modulation of viral growth and virulence in vivo, suggesting that vhs is in some way involved in overcoming host resistance. This inference is supported by the previous observation that a vhs null mutant remains highly immunogenic and functions efficiently both as a prophylactic and therapeutic vaccine, despite its very poor growth in vivo (45, 46).
Both viral and host factors play a role in the development of blepharitis. Previous studies have shown that high input viral titer and persistence of HSV DNA in the conjunctiva and eyelids are associated with blepharitis (22, 28). T-cell responses have also been shown to be pivotal in controlling the extent and incidence of periocular disease (5, 6, 17, 18). Little is known about the potential impact of increased or decreased vhs activity upon such viral and host factors. One study, however, demonstrated that HSV-2 vhs in concert with ICP47 reduces major histocompatibility class I expression and blocks antigen presentation, consistent with the idea that vhs may serve to modulate the immune response (44). Other possible targets for immunomodulation by vhs during infection include the interferons (IFNs). IFNs are key mediators of innate resistance, which control early acute HSV infection. They have also been shown to inhibit the onset of immediate-early HSV gene expression and viral replication and to limit the progress of infection from peripheral tissues to the nervous system (1, 4, 16, 29, 31). Recent data have shown their importance in defining the phenotypes of several HSV mutants, including a vhs null mutant, in vivo, underscoring the importance of characterizing viral mutants in the context of the immune response (27). vhs may therefore cause altered T-cell activation or changes in the production of, or responses to, IFNs. Consistent with this idea, if blepharitis and periocular disease have an immunopathological component, alterations in the immune response caused by heightened vhs activity in KOSV2 may explain its reduced capacity to cause disease. Alternatively, heightened vhs activity in KOSV2 may lead to lower levels of certain immunogenic proteins (e.g., ICP27) than KOS (2), leading to a reduced immune response.
Taken together, these data show that enhanced vhs activity results in ameliorated blepharitis in a mouse model. They demonstrate that the degree of periocular disease is dependent on the amount of virus present in the skin. The effects of vhs are likely to be multifactorial and may affect, for example, T-cell activation and the IFN pathway, and such effects should be considered in the design of live-attenuated HSV vaccines (30). Further studies are under way to understand the precise mechanism by which vhs affects host responses to infection.
ACKNOWLEDGMENTS
We thank Sully Read for providing p333 and acknowledge assistance from Belinda McMahan for the histology. We also thank Skip Virgin, Sam Speck, Lynda Morrison, Peggy MacDonald, and their laboratories for helpful discussions.
This study was supported by NIH grants RO1 EY10707 to David A. Leib and P30-EY02687 to the Department of Ophthalmology and Visual Sciences. Support from Research to Prevent Blindness to the Department and a Robert E. McCormick Scholarship to David A. Leib are gratefully acknowledged.
REFERENCES
- 1.Balish M J, Abrams M E, Pumfery A M, Brandt C R. Enhanced inhibition of herpes simplex virus type 1 growth in human corneal fibroblasts by combinations of interferon-alpha and -gamma. J Infect Dis. 1992;166:1401–1403. doi: 10.1093/infdis/166.6.1401. [DOI] [PubMed] [Google Scholar]
- 2.Banks T A, Allen E M, Dasgupta S, Sandri-Goldin R, Rouse B T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize immediate-early protein ICP27. J Virol. 1991;65:3185–3191. doi: 10.1128/jvi.65.6.3185-3191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthomme H, Jacquemont B, Epstein A. The pseudorabies virus host-shutoff homolog gene: nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology. 1993;193:1028–1032. doi: 10.1006/viro.1993.1221. [DOI] [PubMed] [Google Scholar]
- 4.Bouley D M, Kanangat S, Wire W, Rouse B T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 5.Brandt C R. Susceptibility of +/+, +/nu and nu/nu BALB/c mice to ocular herpes simplex virus infection. Ophthalmic Res. 1992;24:332–337. doi: 10.1159/000267189. [DOI] [PubMed] [Google Scholar]
- 6.Dennis R F, Siemasko K F, Tang Q, Hendricks R L, Finnegan A. Involvement of LFA-1 and ICAM-1 in the herpetic disease resulting from HSV-1 corneal infection. Curr Eye Res. 1994;14:55–62. doi: 10.3109/02713689508999914. [DOI] [PubMed] [Google Scholar]
- 7.Elgadi M M, Hayes C E, Smiley J R. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J Virol. 1999;73:7153–7164. doi: 10.1128/jvi.73.9.7153-7164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgadi M M, Smiley J R. Picornavirus internal ribosome entry site elements target RNA cleavage events induced by the herpes simplex virus virion host shutoff protein. J Virol. 1999;73:9222–9231. doi: 10.1128/jvi.73.11.9222-9231.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett R D, Fenwick M L. Comparative DNA sequence analysis of the host shutoff genes of different strains of herpes simplex virus: type 2 strain HG52 encodes a truncated UL41 product. J Gen Virol. 1990;71:1387–1390. doi: 10.1099/0022-1317-71-6-1387. [DOI] [PubMed] [Google Scholar]
- 10.Everly D N J, Read G S. Mutational analysis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): characterization of HSV type 1 (HSV-1)/HSV-2 chimeras. J Virol. 1997;71:7157–7166. doi: 10.1128/jvi.71.10.7157-7166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenwick M L, Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J Gen Virol. 1982;61:121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick M L, Everett R D. Transfer of UL41, the gene controlling virion-associated host cell shutoff, between different strains of herpes simplex virus. J Gen Virol. 1990;71:411–418. doi: 10.1099/0022-1317-71-2-411. [DOI] [PubMed] [Google Scholar]
- 13.Fenwick M L, Morse L S, Roizman B. Anatomy of herpes simplex virus DNA. XI. Apparent clustering of functions effecting rapid inhibition of host DNA and protein synthesis. J Virol. 1979;29:825–827. doi: 10.1128/jvi.29.2.825-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau D R, Visalli R J, Brandt C R. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Investig Ophthalmol Vis Sci. 1989;30:2474–2480. [PubMed] [Google Scholar]
- 16.Halford W P, Veress L A, Gebhardt B M, Carr D J. Innate and acquired immunity to herpes simplex virus type 1. Virology. 1997;236:328–337. doi: 10.1006/viro.1997.8738. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks R L, Tumpey T M. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr Eye Res. 1991;10:47–53. doi: 10.3109/02713689109020357. [DOI] [PubMed] [Google Scholar]
- 18.Hendricks R L, Tumpey T M, Finnegan A. IFN-γ and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 19.Hill A B, Barnett B C, McMichael A J, McGeoch D J. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J Immunol. 1994;152:2736–2741. [PubMed] [Google Scholar]
- 20.Hill T M, Sinden R K, Sadler J R. Herpes simplex virus types 1 and 2 induce shutoff of host protein synthesis in Friend erythroleukemia cells. J Virol. 1983;45:241–250. doi: 10.1128/jvi.45.1.241-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones F E, Smibert C A, Smiley J R. Mutational analysis of the herpes simplex virus virion host shutoff protein: evidence that vhs functions in the absence of other viral proteins. J Virol. 1995;69:4863–4871. doi: 10.1128/jvi.69.8.4863-4871.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kintner R L, Brandt C R. The effect of viral inoculum level and host age on disease incidence, disease severity, and mortality in a murine model of ocular HSV-1 infection. Curr Eye Res. 1994;14:145–152. doi: 10.3109/02713689508999926. [DOI] [PubMed] [Google Scholar]
- 23.Kwong A D, Frenkel N. The herpes simplex virus virion host shutoff function. J Virol. 1989;63:4834–4839. doi: 10.1128/jvi.63.11.4834-4839.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong A D, Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong A D, Kruper J A, Frenkel N. Herpes simplex virus virion host shutoff function. J Virol. 1988;62:912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Taylor K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maggs D J, Chang E, Nasisse M P, Mitchell W J. Persistence of herpes simplex virus type 1 DNA in chronic conjunctival and eyelid lesions of mice. J Virol. 1998;72:9166–9172. doi: 10.1128/jvi.72.11.9166-9172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittnacht S, Straub P, Kirchner H, Jacobsen H. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology. 1988;164:201–210. doi: 10.1016/0042-6822(88)90637-x. [DOI] [PubMed] [Google Scholar]
- 30.Morrison L A, Knipe D M. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberman F, Panet A. Inhibition of transcription of herpes simplex virus immediate early genes in interferon-treated human cells. J Gen Virol. 1988;69:1167–1177. doi: 10.1099/0022-1317-69-6-1167. [DOI] [PubMed] [Google Scholar]
- 32.Oroskar A A, Read G S. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989;63:1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pak A S, Everly D N, Knight K, Read G S. The virion host shutoff protein of herpes simplex virus inhibits reporter gene expression in the absence of other viral gene products. Virology. 1995;211:491–506. doi: 10.1006/viro.1995.1431. [DOI] [PubMed] [Google Scholar]
- 34.Rader K A, Ackland-Berglund C E, Miller J K, Pepose J S, Leib D A. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 35.Read G S, Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate-early) viral polypeptides. J Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Read G S, Karr B M, Knight K. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J Virol. 1993;67:7149–7160. doi: 10.1128/jvi.67.12.7149-7160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Smibert C A, Johnson D C, Smiley J R. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J Gen Virol. 1992;73:467–470. doi: 10.1099/0022-1317-73-2-467. [DOI] [PubMed] [Google Scholar]
- 39.Stanberry L R, Jorgenson D M, Nahmias A J. Herpes simplex viruses 1 and 2. In: Evans A S, Kaslow R A, editors. Viral infections of humans. 4th ed. New York, N.Y: Plenum Medical Book Co.; 1997. pp. 419–454. [Google Scholar]
- 40.Strelow L, Smith T, Leib D. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology. 1997;231:28–34. doi: 10.1006/viro.1997.8497. [DOI] [PubMed] [Google Scholar]
- 41.Strelow L I, Leib D A. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J Virol. 1996;70:5665–5667. doi: 10.1128/jvi.70.8.5665-5667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strelow L I, Leib D A. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sucato G, Wald A, Wakabayashi E, Vieira J, Corey L. Evidence of latency and reactivation of both herpes simplex virus (HSV)-1 and HSV-2 in the genital region. J Infect Dis. 1998;177:1069–1072. doi: 10.1086/515261. [DOI] [PubMed] [Google Scholar]
- 44.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-γ or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 45.Walker J, Laycock K A, Pepose J S, Leib D A. Postexposure vaccination with a virion host shutoff defective mutant reduces UV-B radiation-induced ocular herpes simplex virus shedding in mice. Vaccine. 1998;16:6–8. doi: 10.1016/s0264-410x(97)00177-1. [DOI] [PubMed] [Google Scholar]
- 46.Walker J, Leib D A. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine. 1998;16:1–5. doi: 10.1016/s0264-410x(97)00164-3. [DOI] [PubMed] [Google Scholar]
- 47.Zelus B D, Stewart R S, Ross J. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J Virol. 1996;70:2411–2419. doi: 10.1128/jvi.70.4.2411-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]