Abstract
The Internet Data Center (IDC) is one of the most important infrastructures in the field of information technology. The cooling system for heat dissipation of IDC is indispensable due to it generates a large amount of heat during its calculation process, which may potentially harm its normal operation. Electronic fluorinated fluids have been widely used in cooling systems of IDC with stable physical and chemical properties. However, the biological toxicity of electronic fluorinated fluids has not been fully evaluated and there is a lack of unified safety standards, which may pose potential risks to the environment and human health. Here, hexafluoropropylene terpolymer (HFPT) as an example has been systematically studied, fully considering the application scenarios of data centers. Also, the emergency effects of fluorinated coolants in mammalian models from the perspectives of inhalation, skin contact, accidental entry into eyes, accidental ingestion, and chronic toxicity, are evaluated. Multiple in vivo experiments have proven that HFPT not only has stable physical and chemical properties, that can maintain the safe operation of IDC, but also has low physiological toxicity to mammals and can provide health benefits to data center staff and the assurance of surrounding environment. This study proves the good biological safety of electronic fluorinated fluids and provides a reference for environmental assessment and risk management of liquid cooling technology in IDC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43188-024-00234-3.
Keywords: IDC, Cooling liquid, Toxicity, Hexafluoropropylene trimer, Biosafety
Introduction
Internet Data Center (IDC) is a network of specific equipment that collaborates globally and is used to transmit, accelerate, display, calculate, and store data information on the Internet [1–4]. Due to the rapid development of cyber-physical systems as part of the fourth industrial revolution, the demand for data processing, storage, communication, and other services has increased dramatically, as well as the amount of digital data generated [5, 6]. In response, the IDCs have gained a rapidly increasing strategic status as strategic facilities [7]. However, the rapid growth of data center computing volume and computational complexity is accompanied by high overall operating temperature [8]. Information and communication equipment's operation is largely influenced by the operating temperature, which affects its performance [9, 10]. Hence, heat dissipation of large data center equipment faces a huge challenge, which requires appropriate heat dissipation technology [11]. In order to promote industrial application prospects, the cooling technology of the data center must not only match the heat production and heat dissipation rate, but also have green energy-saving heat dissipation technology [12–14].
In IDCs, cooling technology must solve two major problems. The first step is to achieve a match between heat production and heat dissipation. Second, In addition, it is crucial to develop an energy-efficient and green heat dissipation technology that can be applied in industrial settings [15–17]. Cooling technologies currently available can be divided into two types: air cooling technology and liquid cooling technology [18]. It is generally considered that liquid cooling is a more efficient choice than air cooling [19], since air cooling has poor heat dissipation behavior and high energy consumption [20–22]. Using liquid cooling can increase the IDC’s power density, improve its operating performance, reduce noise pollution, and improve its energy efficiency [23, 24]. Depending on the application scenes, there are three types of coolant used in data centers: immersion coolant, cold plate coolant [25], and spray coolant. Fluorinated liquids are good immersion coolants because they are chemically inert, electrically insulating, thermally conductive, and have low corrosion rates [26]. Nevertheless, immersed coolants require frequent contact with relevant workers, so it is important to evaluate the biological safety of fluorinated electronic coolants.
In this study, we simulated the possible effects of fluorine-containing coolant on human health by using hexafluoropropylene terpolymer (HFPT) as a representative of fluorine-containing coolant. In Scheme 1, we constructed in vivo models for oral, transocular, inhaled, and transdermal absorption of HFPT using mice and rabbits, respectively, and assessed acute and chronic toxicology by physiological monitoring and histology. According to multiple in vivo experiments, HFPT has stable physical and chemical properties that enable the IDC to operate safely, as well as low physiological toxicity. As a result of our study, fluorine-containing coolants can not only maintain the security of IDC, but also protect physical and mental health of relevant operators, and provide a reference for environmental assessment and risk management of liquid cooling technologies.
Scheme 1.
Systematic illustration of the toxicological experiments that carried out in this study.In order to test the health effects of HFPT on IDC operators during practical application, we conducted experiments on animals from four different approaches
Materials and methods
Synthesis of HFPT
Ethylene glycol dimethyl ether is used as solvent, perfluoro-2-methyl-2-pentene and hexafluoropropylene are used as raw materials (molar ratio is 1:1), the main catalyst is potassium fluoride, and the co-catalyst is diethyl ether. Add 1.5 g of anhydrous potassium fluoride and 1.95 g of 18-crown-6 into a 500 mL polytetrafluoroethylene reactor, seal it, vacuumize the reactor to below 0.09 Mpa, and add 200 g of perfluoro-2-methyl-2-pentene and 100 mL of acetonitrile, continue to add 102 g of hexafluoropropylene under stirring. Put the reaction kettle into an oven, heat to 45 °C, react for 1.5 h, then cool to room temperature, and use a separatory funnel to separate the solvent from the fluorinated liquid to obtain the required HFPT fluorinated liquid.
HFPT is a mixture of several perfluoroalkane, and the purity we synthesized reached 99.6%. When conducting animal experiments, undiluted stock solution is used for administration, and the toxicity under different circumstances is evaluated by controlling the time and volume of contact with HFPT.
Experimental instruments
AUY120 electronic balance (Shimadzu Corporation of Japan); RM2016 slicer (Shanghai Lycra Instrument Co., Ltd.); upright optical microscope (Nikon Corporation of Japan); dragonlab pipette (Dalong Xingchuang Experimental Instrument Co., Ltd.).
Acute oral toxicity of HFPT
Following the OECD test guideline No. 420, we prepared 14 healthy KM mice (five weeks old) and 18 healthy KM mice (four weeks old), half male and half female, weighing about (18 ± 2) g, were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The mice were adaptively fed for 4 days in an environment with a relative humidity of 50–70% and a temperature of 20–22°C. Ensure that animals are healthy and ready for experimentation.
Given that the low toxicity of HFPT we measured before, we wanted to figure out if the exposure time could cause any differences in the acute oral toxicity. In the acute oral toxicity test, we set different exposure time from 0 to 13 days. 14 mice were divided into 7 groups with different administration time, which were blank control group and observed 1, 3, 5, 7, 10, 13 days post administration respectively. Two mice in each group, one male and one female. Before administration, the tested mice were fasted for 6 h without restriction of drinking water, so as to achieve the purpose of emptying the food residue in their stomach. The route of administration of the test substance: Oral gavage once, with a volume of 20 mL/kg. The mice in the control group were not treated by gavage, kept normal diet and water supplement.
After the mice were exposed to the test sample, their body weight and poisoning reaction were observed and recorded every day, including appearance characteristics, behavioral activities, diet and mental status, whether they died, the severity, duration, and reversibility of the poisoning reaction, etc.
After the observation period, the mice were sacrificed uniformly and dissected immediately, their blood samples were collected for blood biochemical and blood routine analysis. During the dissection, the volume, color, and texture of each organ were observed for abnormalities, and their stomach, small intestine, heart, lung, kidney, liver, and spleen were collected and fixed with 4% paraformaldehyde solution, followed by different gradient ethanol solution was dehydrated step by step, embedded in paraffin, sectioned, stained with Hematoxylin and Eosin (H&E), and the morphological changes of each tissue were observed under microscope.
Furthermore, to ensure the reproducibility of the statistics, we set an additional experiment. We divided the mice of each gender into three different dosage groups of 0, 10 and 20 mg/mL, three mice for each group, then recorded the body weight changes in the subsequent 14 days after oral administration.
Acute inhalation toxicity of HFPT
Following the OECD test guideline No.403, we prepared twenty healthy Balb/c mice (Four weeks old), half male and half female, weighing about (16 ± 2) g, were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The mice were adaptively fed for 4 days in an environment with a relative humidity of 50–70% and a temperature of 20–22°C. Ensure that animals are healthy and ready for experimentation.
There was no control group in this experiment. The tested mice were not restricted from eating and drinking and their body weights were recorded within 24 h before administration. Test substance administration route: static exposure, one-time limited method. That is, in a closed container of a certain volume (a sealed box with a volume of 60 L was used in this experiment), add a certain amount of test sample (about 1.2 g), drop it into a medical cotton ball, and hang it inside the airtight container with a thin thread. Let it volatilize in the box in order to create an air environment with a concentration of 2000 mg/m3 of the test sample. At last, put the experimental animals into the container, make them inhale the sample for 4 h once at a time.
After the mice were exposed to the test sample, observe and record their toxic reactions daily, including appearance characteristics, behavioral activities, diet and mental status, whether they died, the severity, duration, and reversibility of the toxic reactions, etc. Furthermore, the body weight of the experiment animals were recorded every other day for 14 days.
Acute eye irritation/corrosion of HFPT
Following the OECD test guideline No.405, we prepared two healthy white rabbits, female, weighing (2.2 ± 0.2) kg, were provided by Wuhan Rabbit Accord Biotechnology Co., Ltd. The rabbits were adaptively fed for 4 days in an environment with a relative humidity of 50–70% and a temperature of 20–22°C. Ensure that animals are healthy and ready for experimentation.
At a point in time 24 h before the experiment, the eyes of the two rabbits were checked visually with the aid of auxiliary light sources, and then further fluorescein tests were performed. Add 1 drop of 2% sodium fluorescein saline solution into the conjunctival sac of the test animal, rinse the eyes with warm saline after 15 s, and check under a 365 nm ultraviolet light source to ensure that the animals' eyes can be used for the experiment. 0.1 and 1 mL of the original sample were dropped on the cornea of the left eye of two rabbits respectively, and the right eye was used as a normal control of each rabbit. The eyes should not be washed within 24 h after exposure. At 1 h, 24 h, 48 h, 72 h, 4 d and 7 d after exposure, observing that if there appears damage to the cornea, iris and conjunctival membrane, and whether the pupillary response to light is normal. After the observation period, the rabbits were sacrificed and eyes on the both sides were taken, fixed with 4% paraformaldehyde solution, dehydrated step by step with different gradient ethanol solutions, embedded in paraffin, sectioned, stained with H&E, and observed their morphological changes under the microscope.
Acute skin irritation/corrosion of HFPT
Following the OECD test guideline No.402, we prepared two healthy SD rats, female, with a body weight of about (200 ± 20) g, provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The tested rats were adaptively fed for 4 days in an environment with a relative humidity of 50–70% and a temperature of 20–22°C. Ensure that animals are healthy and ready for experimentation.
At a point in time 24 h before the experiment, the hair of the same area on both sides of the back spine of the rats was carefully shaved, make sure that the skin was not damaged. 1 and 1.5 g of the original sample were applied to the skin on the right side of each rat's spine respectively, and the skin on the left side was used as the control group without treatment. Irritant medical waterproof stickers were used to prevent the rats from licking and destroying their skin. After 4 h, remove the gauze and clean the test substance.
After removing the test substance, observe the dermal reaction of the test site every other day, take pictures and record the phenomenon. According to the skin irritation reaction scoring table, we can have an intuitive perception of the damage caused to the skin. Calculate the highest weighted score at each observation point, and judge the skin toxicity caused by the sample to experimental animals. The observation should last for 14 days.
All animal experiments were approved by the Animal Experiment Ethics Committee of Huazhong University of Science and Technology (IACUC Number: S904).
Statistical analysis
Data are expressed as mean ± S.D. Significance between two groups was assessed by unpaired two-tailed Student's t-test and between each of the multiple groups was calculated using one-way ANOVA. ****p < 0.001, ***p < 0.001, **p < 0.01, *p < 0.05.
Results
Characterization and analysis of HFPT
The HFPT synthesized is a colorless and transparent liquid. Due to the extremely strong electronegativity of the fluorine atom and the extremely high bond energy of the carbon–fluorine bond, the stability and resistance to decomposition of the fluorinated solution are better than other liquid substances. We tested the basic physical properties of the synthesized HFPT, and the test results are shown in Table S1. Among them, it can be seen that HFPT has no flash point, and has better safety in use than other insulating cooling media. Especially in low-boiling point phase change applications, the flash point of hydrocarbon and silicon-based substances will decrease with the decrease of boiling point, and even be classified as flammable substances. Although the boiling point of fluorinated liquid is not high, at 120–125°C, there will be no such safety limit. In addition, as a heat transfer medium, although its thermal conductivity is lower than that of oils, due to its lower kinematic viscosity, the overall heat transfer capability of single-phase media is similar or even preferable. Moreover, HFPT also has many excellent physical and chemical properties: its dielectric constant has little effect on the integrity of high-speed signals, which can perfectly adapt to the application scenarios of data centers, and its ozone destruction potential (ODP) is 0, which means it will not cause damage to the ozone layer, can be regard as a green product.
Furthermore, the synthesized HFPT was tested by gas chromatograph-mass spectrometer (GC–MS, Fig. S1) and enthalpy of vaporization and specific heat capacitywere shown in Fig. S2 and Fig. S3 in supporting information. According to the above test results, this fluorinated fluid showed high insulation performance, high heat capacity and other performance advantages. However, before being put into practical application scenarios, as a chemical that will be in close contact with relevant workers, it must be tested whether it may cause physical damage to the human body. Therefore, we then conducted experiments on acute oral toxicity, acute inhalation toxicity, acute eye irritation/corrosion and acute dermal irritation/corrosion on HPFT to ensure that this substance can be applied safely.
Acute oral toxicity test results of HFPT
Acute oral toxicity refers to the short-term health damage effect of animals after administration of test samples to experimental animals once or several times within 24 h, and the schematic illustration of the oral toxicity experiment is shown in Fig. 1a. Experimental animals may show symptoms of poisoning after being given samples, such as losing weight in a short period of time, vomiting, diarrhea and so on, and severe cases may even die. Therefore, during the observation period of the experiment, we focused on recording the body weight changes, eating and defecation of the experimental animals. During the observation period, the mice given test samples acted normally and did not show obvious toxic reactions in terms of appearance, eating, excretion and behavior. The body weight changes of the mice during the observation are shown in Fig. 1b, c. The body weight of the tested mice shows a gradual upward trend in the period of experiment, only a small number of female mice showed signs of losing weight during the observation period. And in the additional experiment which explored the effects of different sample doses on mice, from Fig. 1d, e we could tell that the body weight mice in different dose group exhibited a daily upward trend. Compared with the control group, the weight trends of the mice in the low-dose (10 mg/mL) and high-dose (20 mg/mL) groups didn’t show any difference, and from which we can indicated that HFPT has no significant impact on the health of the mice.
Fig. 1.
Acute oral toxicity experiment of HFPT. a Schematic illustration of the acute oral toxicity experiment. After the mice were gavaged, their weights were recorded and observed within 14 days, and then they were euthanized and their blood and organs were examined. b Body weight changes of female and c male mice during the initial experiment. d Body weight changes of female and e male mice during the additional experiment
We also conducted further tests on the blood indicators of the experimental animals. The detection of blood routine and blood biochemical indicators can also reflect the health status of experimental animals. Experimental animals with toxic reactions may have abnormal indicators such as increased white blood cell index, decreased platelet index and so on. The specific data of blood routine and blood biochemical tests can be found in Table 1 and Table S2. Except for the high hematocrit of the female mice, all the other indicators were within the reference range. From Fig. 2a, it can be clearly seen that the number of the white blood cells and neutrophils in male mice is significantly higher, but the rest of the indicators have no obvious abnormalities. There were relevant researches indicating that mice may exhibit changes in the number of immune cells under acute stress [27]. It can be inferred that the experimental animals were in a state of stress caused by the emotional agitation because of improper operation state, which in turn caused the white blood cells and neutrophils to be higher than normal. The blood biochemical indicators focused on the examination of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) which related to liver function are available in Fig. 2b. The number of platelet and monocyte in blood are shown in Fig. 2c, d. Both indicators were within the reference range, which can further tell that the liver function of the experimental animals no abnormality occurred. The H&E staining histopathological sections of the major organs of the tested mice are shown in Fig. 2e, f. H&E staining can display the general structure of normal and diseased tissues based on retaining the original morphological structure, special components, and material colors of cells. It can be seen that at different administration times, the myocardial cells of the mice are arranged evenly and neatly, the liver cells are arranged radially with the central vein as the axis, and the cell cord structure is clear, the white pulp of the spleen is distributed between the red pulp, showing blue dots distribution, the structure of the lung tissue was complete, and the morphology of the alveolar epithelial cells was uniform, the morphology of the glomeruli and renal tubules was normal, and the staining was uniform, the villi of the small intestine were neatly arranged and intact. Such observing results suggest that the test substance did not cause morphological and functional damage to the main organs of mice.
Table 1.
Blood routine test results of acute oral toxicity in mice
| Parameters | Reference range | Unit | Average (female) | Average (male) |
|---|---|---|---|---|
| WBC | 0.8–6.8 | 109/L | 4.90 ± 2.20 | 7.56 ± 2.94 |
| Lymph# | 0.7–5.7 | 109/L | 3.66 ± 1.76 | 5.32 ± 1.92 |
| Mon# | 0.0–0.3 | 109/L | 0.12 ± 0.08 | 0.20 ± 0.10 |
| Gran# | 0.1–1.8 | 109/L | 1.12 ± 0.58 | 2.04 ± 2.66 |
| Lymph% | 55.8–90.6 | % | 73.62 ± 21.32 | 71.37 ± 18.47 |
| Mon% | 1.8–6.0 | % | 2.75 ± 0.65 | 2.97 ± 0.63 |
| Gran% | 8.6–38.9 | % | 23.63 ± 20.97 | 25.66 ± 18.64 |
| RBC | 6.36–9.42 | 1012/L | 9.17 ± 1.21 | 8.80 ± 3.33 |
| HGB | 110–143 | g/L | 142.30 ± 19.30 | 135.90 ± 50.90 |
| HCT | 34.6–44.6 | % | 45.77 ± 6.37 | 42.82 ± 18.32 |
| MCV | 48.2–58.3 | fL | 49.99 ± 2.61 | 48.49 ± 3.59 |
| MCH | 15.8–19 | pg | 15.48 ± 0.72 | 15.42 ± 0.52 |
| MCHC | 302–353 | g/L | 310.40 ± 9.40 | 318.70 ± 27.30 |
| RDW | 13–17 | % | 13.81 ± 2.41 | 13.39 ± 1.79 |
| PLT | 4.5–15.9 | 1011/L | 8.79 ± 5.23 | 9.38 ± 5.55 |
| MPV | 3.8–6.0 | fL | 5.40 ± 0.60 | 5.08 ± 0.38 |
WBC (white blood cell), Lymph# (the number of Lymphocyte in blood), Mon# (the number of monocyte in blood), Gran# (the number of granulocyte in blood), Lymph% (the percentage of lymphocyte in blood), Mon% (the percentage of monocyte in blood), Gran% (the percentage of granulocyte in blood), RBC (red blood cell), HGB (hemoglobin), HCT (hematocrit), MCV (mean corpuscular volume), MCH (mean corpuscular hemoglobin), MCHC (mean corpuscular hemoglobin concentration), RDW (red blood cell distribution width), PLT (platelet), MPV (mean platelet volume)
Fig. 2.
Blood routine, blood biochemical indicators and the H&E staining results of the tested mice in acute oral toxicity experiment. a The content of white blood cells, lymphocytes, and neutrophils in the blood of mice. b The content of ALT, AST in the blood of mice. c The content of platelet in the blood of mice. d The content of monocyte in the blood of mice. It can be seen from the figure that most of the indicators are within the normal range. (*p < 0.05, **p < 0.01, ***p < 0.001 versus control.) e Pathological sections of heart, liver, spleen, lung, kidney, and small intestine of female and f male mice at different exposure times that observed by the microscope
Acute inhalation toxicity test results of HFPT
Acute inhalation toxicity refers to the short-term health damage effect after the experimental animals continuously inhale an inhalable test sample for a short period of time (within 24 h). It can be used to evaluate the toxic effects of gases, volatile substances or aerosols, particulate matter and other chemicals on the respiratory tract. Inhalation poisoning reactions that may occur in rodents include ataxia, dyspnea, irregular heartbeat, emaciation and so on, the severe cases may even cause death. The schematic illustration of the inhalation toxicity experiment is shown in Fig. 3a. Therefore, during the period of experiment, we focused on recording the weight changes of the experimental animals and other possible toxic reactions. During the observation period, the experimental animals did not show obvious toxic reactions in terms of appearance, eating, excretion and behavior. The body weight changes of the mice during the observation period are shown in Fig. 3b, c, which show a stable trend after exposure, and there was no significant fluctuation. We conducted regular behavioral observations on mice based on common toxic reactions in rodents. In the observation of the central nervous system and body, respiratory system, cardiovascular system, gastrointestinal system, and genitourinary system of mice, no obvious abnormalities were found, and mice can respond obviously to external stimuli. From this, it can be judged that the test substance did not cause health damage to the mice, and the toxicity was graded as slightly toxic or non-toxic.
Fig. 3.
Acute inhalation toxicity test results of HFPT. a Schematic illustration of the inhalation toxicity experiment. The mice were placed into a enclosed environment containing a certain concentration of HFPT, and their weight were recorded over the next 14 days. b Body weight changes of female and c male mice during the experiment
Acute eye irritation/corrosion test results of HFPT
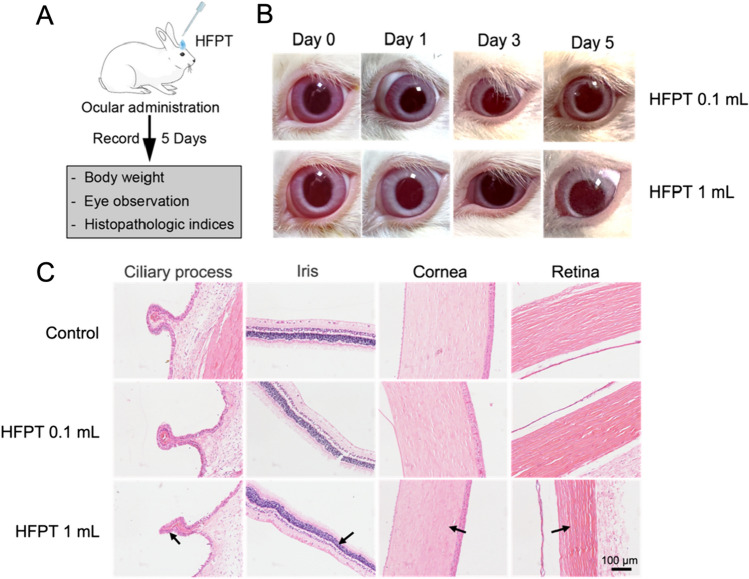
Eye irritation and corrosion refers to the reversible inflammatory changes and irreversible tissue damage of the eye after the surface of the eyeball contacts the test substance. The eyes of white rabbits are more sensitive to irritating or corrosive substances than humans in most cases, so they are often used as experimental animals for testing eye irritation. Eye damage is primarily observed from three sites: the cornea, iris, and conjunctiva. The test substance may cause pathological changes such as corneal ulcer, loss of light response, conjunctival hyperemia and edema in the tested animals. Therefore, during the experiment, we first directly observed the eyes of the tested animals, and later made slices and H&E staining of the eye tissues for further observing whether there were tissue damages under the microscope.
The schematic illustration of the eye irritation experiment is shown in Fig. 4a. Animals exposed to the test sample did not wash their eyes in 24 h and their eyes responded normally to light. The results of the naked eye observation are shown in Fig. 4b. During the observation period, the score was performed according to the extraocular scoring standard table, and the scoring results are shown in Table S3. Depending on the grade of eye irritation response, the intensity of eye irritation of the unwashed test substance within 24 h of exposure is virtually non-irritating. The H&E staining histopathological sections of rabbit eyes are shown in Fig. 4c. The ciliary body, iris, cornea and retina were mainly selected for observation. Under the microscope, the apigmented epithelium and pigmented epithelium of the ciliary process were neatly arranged, and there was no edema or loss of cells, the inner and outer layers of the cornea, iris, and retina were intact and clearly visible, and the cell morphology was normal, and there was no inflammatory cell infiltration. It suggested that the test substance did not cause damage to the eyes of rabbits, and the irritating intensity can be seen as negligible.
Fig. 4.
Acute eye irritation/corrosion test results of HFPT. a Schematic illustration of the eye irritation/corrosion experiment. Drop the sample into one eye of the rabbit, observe the eye condition within the next 5 days. b Visual observation images of rabbit eyes. c Observation images of H&E staining rabbit eye slices under the microscope
Acute dermal irritation/corrosion test results of HFPT
Dermal corrosion and irritation refer to the local irreversible damage and reversible inflammatory changes after the test sample is applied to the skin. After administration of the test sample, the skin of the experimental animals may appear erythema, eschar or edema, and the skin irritation of the test sample can be judged intuitively according to the severity of the skin damage of the test animal. In addition, it is also necessary to observe whether the dermal damage that occurs is reversible, and whether the toxic reaction will recover on its own during the observation period.
The observation results of the skin of the rats exposed to the test sample during the observation period are shown in Fig. 5a, and the schematic illustration of the dermal irritation experiment is shown in Fig. 5b. The rats exposed to the sample in the two dose groups all had slight skin irritation, but they could recover and disappear within a short time. Stimulation intensity can be regard as mildly irritating. The H&E staining histopathological sections of rat back skin is shown in Fig. 5c. Curly, loosely arranged and relatively thick collagen fiber bundles can be observed in different dose groups, and the overall cell content is less. Individual hair follicles, or follicles connected to sebaceous glands, may also be seen. The dermal tissue of the experimental group did not show obvious morphological abnormalities, suggesting that the test substance did not cause obvious damage to the skin of rats.
Fig. 5.

Acute dermal irritation/corrosion test results of HFPT. a Visual observation image of rat back skin. b Schematic illustration of the dermal irritation/corrosion experiment. We applied the sample to the shaved skin on one side of the rats’ spine, kept it for 4 h, and observed the changes of the skin within the following 14 days. c Observation images of H&E staining rat back skin slices under the microscope
Discussion
In response to the rapid growth of production and life in human society, some chemicals have become more widely used in daily life and at work. Therefore, before putting those chemical products into application, it is crucial to investigate their safety and biological toxicity beforehand, which has far-reaching social significance and important economic value, so that their application advantages can be maximized while ensuring their safety and environmental friendly characteristics are maintained. During the past few years, the IDC has been one of the most important infrastructures in information technology. Since fluorinated coolants are both chemically and physically stable, they have been widely used in IDCs. Fluorinated coolants lack harmonized safety standards, and biotoxicity studies have not been conducted. The working environment may present potential health risks associated with fluorinated coolants.
In this study, based on the application requirements, comprehensive consideration of reliability, sensitivity, cost-effectiveness and other factors, in vivo biological toxicity detection methods were adopted. Mammals are mainly used as research objects to explore the possible damage caused by HFPT to human beings in different contact modes, which lays a safe foundation for the practical application of this type of electronic fluorinated coolant. Acute toxicity tests of HFPT were conducted on mice, rats and rabbits using mouth, respiratory tract, eyes and skin exposure routes. The test process did not cause any death of experimental animals. As a result of rough naked eye observation, blood index examination, and microscopic observations of pathological sections of the experimental animals, it can be concluded that such electron fluoride is low-toxic or non-toxic to the above experimental animals. These experiments provide a reference value for the application and promotion of cooling fluids represented by electronic fluorinated fluids, as well as protecting the physical and mental health of relevant employees.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All animal experiments were approved by the Animal Experiment Ethics Committee of Huazhong University of Science and Technology. We also thank the Analytical and Testing Centre (HUST), the Research Core Facilities for Life Science (HUST) and the Centre for Nanoscale Characterization & Devices (CNCD) at WNLO of HUST for the help of measurement.
Author contributions
YTZ, PJZ and SPW contributed to conceptualization, methodology, experiments, data analysis, and draft preparation. JXF, YCC and YDZ contributed to funding, conceptualization, data analysis, review, and editing. WXZ and CHL contributed to data analysis, review, and editing. JQZ and JXF contributed to data analysis, review, and editing.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 52103319), Headquarters Technology Project of State Grid Corporation of China (5500202220118A-1-1-ZN).
Data availability
All data generated or analyzed during this study are included in this published article. Requests for data and materials should be addressed to Jin-xuan Fan (jxfan@hust.edu.cn) or Yuan-cheng Cao (yccao@hust.edu.cn).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Yuan-Cheng Cao, Email: yccao@hust.edu.cn.
Jin-Xuan Fan, Email: jxfan@hust.edu.cn.
References
- 1.Geng H. Data center handbook. Hoboken: Wiley; 2014. Data centers—strategic planning, design, construction, and operations; pp. 1–14. [Google Scholar]
- 2.Kant K. Data center evolution. A tutorial on state of the art, issues, and challenges. Comput Netw. 2009;53:2939–2965. doi: 10.1016/j.comnet.2009.10.004. [DOI] [Google Scholar]
- 3.Muhammad S, Pan Y, Magazzino C, Luo Y, Waqas M. The fourth industrial revolution and environmental efficiency: the role of fintech industry. J Clean Prod. 2022;381:135–196. doi: 10.1016/j.jclepro.2022.135196. [DOI] [Google Scholar]
- 4.Rong H, Zhang H, Xiao S, Li C, Hu C. Optimizing energy consumption for data centers. Renew Sust Energ Rev. 2016;58:674–691. doi: 10.1016/j.rser.2015.12.283. [DOI] [Google Scholar]
- 5.Cui DE, Zhou C, Luo Y, Lei Q, Tian Z, Zhang S, Fan J, Zhang L. Multi-scale modeling and fast inference for thermal environment analysis of air-cooled data center. J Build Eng. 2023;78:107722. doi: 10.1016/j.jobe.2023.107722. [DOI] [Google Scholar]
- 6.Shao X, Zhang Z, Song P, Feng Y, Wang X. A review of energy efficiency evaluation metrics for data centers. Energ Buildings. 2022;271:112308. doi: 10.1016/j.enbuild.2022.112308. [DOI] [Google Scholar]
- 7.Ma X, Shi W, Yang H. Improving the performance of indirect evaporative cooler for energy recovery from the perspective of nozzle configuration: a CFD model analysis. J Build Eng. 2023;76:107195. doi: 10.1016/j.jobe.2023.107195. [DOI] [Google Scholar]
- 8.Zhang Y, Shan K, Li X, Li H, Wang S. Research and Technologies for next-generation high-temperature data centers – State-of-the-arts and future perspectives. Renew Sust Energ Rev. 2023;171:112991. doi: 10.1016/j.rser.2022.112991. [DOI] [Google Scholar]
- 9.Han X, Tian W, VanGilder J, Zuo W, Faulkner C. An open source fast fluid dynamics model for data center thermal management. Energ Build. 2021;230:110599. doi: 10.1016/j.enbuild.2020.110599. [DOI] [Google Scholar]
- 10.Zhang Q, Meng Z, Hong X, Zhan Y, Liu J, Dong J, Bai T, Niu J, Deen MJ. A survey on data center cooling systems: technology, power consumption modeling and control strategy optimization. J Syst Archit. 2021;119:102253. doi: 10.1016/j.sysarc.2021.102253. [DOI] [Google Scholar]
- 11.Haghshenas K, Setz B, Blosch Y, Aiello M. Enough hot air: the role of immersion cooling. Energy Informatics. 2023;6:14. doi: 10.1186/s42162-023-00269-0. [DOI] [Google Scholar]
- 12.Deymi-Dashtebayaz M, Valipour Namanlo S, Arabkoohsar A. Simultaneous use of air-side and water-side economizers with the air source heat pump in a data center for cooling and heating production. Appl Therm Eng. 2019;161:114–133. doi: 10.1016/j.applthermaleng.2019.114133. [DOI] [Google Scholar]
- 13.Nada SA, Said MA. Comprehensive study on the effects of plenum depths on air flow and thermal managements in data centers. Int J Therm Sci. 2017;122:302–312. doi: 10.1016/j.ijthermalsci.2017.09.001. [DOI] [Google Scholar]
- 14.Tradat MI, Manaserh YMA, Sammakia BG, Hoang CH, Alissa HA. An experimental and numerical investigation of novel solution for energy management enhancement in data centers using underfloor plenum porous obstructions. Appl Energ. 2021;289:116663. doi: 10.1016/j.apenergy.2021.116663. [DOI] [Google Scholar]
- 15.Deymi-Dashtebayaz M, Farahnak M, Abadi RNB. Energy saving and environmental impact of optimizing the number of condenser fans in centrifugal chillers under partial load operation. Int J Refrig. 2019;103:163–179. doi: 10.1016/j.ijrefrig.2019.03.020. [DOI] [Google Scholar]
- 16.Deymi-Dashtebayaz M, Farahnak M, Moraffa M, Ghalami A, Mohammadi N. Experimental evaluation of refrigerant mass charge and ambient air temperature effects on performance of air-conditioning systems. Heat Mass Transfer. 2018;54:803–812. doi: 10.1007/s00231-017-2173-6. [DOI] [Google Scholar]
- 17.Zhang K, Zhang Y, Liu J, Niu X. Recent advancements on thermal management and evaluation for data centers. Appl Therm Eng. 2018;142:215–231. doi: 10.1016/j.applthermaleng.2018.07.004. [DOI] [Google Scholar]
- 18.Mi R, Bai X, Xu X, Ren F. Energy performance evaluation in a data center with water-side free cooling. Energ Build. 2023;295:113278. doi: 10.1016/j.enbuild.2023.113278. [DOI] [Google Scholar]
- 19.Jin C, Bai X, Yang C. Effects of airflow on the thermal environment and energy efficiency in raised-floor data centers: a review. Sci Total Environ. 2019;695:133801. doi: 10.1016/j.scitotenv.2019.133801. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Zhang Y, Song J, Liu H, Jiang J. Energy saving with zero hot spots: a novel power control approach for sustainable and stable data centers. Sustainability. 2022;14:9005. doi: 10.3390/su14159005. [DOI] [Google Scholar]
- 21.Li Z, Kandlikar SG. Current status and future trends in data-center cooling technologies. Heat Transf Eng. 2015;36:523–538. doi: 10.1080/01457632.2014.939032. [DOI] [Google Scholar]
- 22.Luo Q, Wang C, Wu C. Study on heat transfer performance of immersion system based on SiC/white mineral oil composite nanofluids. Int J Therm Sci. 2023;187:108–203. doi: 10.1016/j.ijthermalsci.2023.108203. [DOI] [Google Scholar]
- 23.Kuncoro IW, Pambudi NA, Biddinika MK, Widiastuti I, Hijriawan M, Wibowo KM. Immersion cooling as the next technology for data center cooling: a review. J Phys: Conf Ser. 2019;1402:044–057. doi: 10.1088/1742-6596/1402/4/044057. [DOI] [Google Scholar]
- 24.Tong Z, Han Z, Fang C, Wen X. Two-phase thermosyphon loop with different working fluids used in data centers. Int J Heat Mass Transf. 2023;214:124393. doi: 10.1016/j.ijheatmasstransfer.2023.124393. [DOI] [Google Scholar]
- 25.Ji X, Yang X, Zhang Y, Zhang Y, Wei J. Experimental study of ultralow flow resistance fractal microchannel heat sinks for electronics cooling. Int J Therm Sci. 2022;179:107723. doi: 10.1016/j.ijthermalsci.2022.107723. [DOI] [Google Scholar]
- 26.Zhang H, Shao S, Xu H, Zou H, Tian C. Free cooling of data centers: a review. Renew Sust Energ Rev. 2014;35:171–182. doi: 10.1016/j.rser.2014.04.017. [DOI] [Google Scholar]
- 27.Poller WC, Downey J, Mooslechner AA, Khan N, Li L, Chan CT, McAlpine CS, Xu C, Kahles F, He S, Janssen H, Mindur JE, Singh S, Kiss MG, Alonso-Herranz L, Iwamoto Y, Kohler RH, Wong LP, Chetal K, Russo SJ, Sadreyev RI, Weissleder R, Nahrendorf M, Frenette PS, Divangahi M, Swirski FK. Brain motor and fear circuits regulate leukocytes during acute stress. Nature. 2022;607:578–584. doi: 10.1038/s41586-022-04890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Requests for data and materials should be addressed to Jin-xuan Fan (jxfan@hust.edu.cn) or Yuan-cheng Cao (yccao@hust.edu.cn).