Abstract
Introduction
The objective of this study was to investigate the prevalence, incidence, and treatment patterns (treatment regimens, switches, duration) for diffuse large B-cell lymphoma (DLBCL) in a real-world setting.
Methods
This was a retrospective German claims data analysis of patients with DLBCL diagnosed between January 1, 2012, and December 31, 2020. The prevalence and cumulative incidence of DLBCL were found for 2019/2020. Line of treatment (LOT) and treatment setting from first DLBCL diagnosis to end of follow-up were described. Kaplan–Meier overall survival (OS) estimates since DLBCL diagnosis and start of treatment lines were calculated.
Results
Overall, 2633 incident DLBCL cases were identified (median age 75 years, 51% male). Of these, 2119 patients received at least one DLBCL-related treatment (LOT1), and 1567 patients died during follow-up. In 2019/2020, the prevalence and cumulative incidence of DLBCL was 34.8/36.7 per 100,000 patients and 14.0/12.7 per 100,000 patients, respectively. For LOT1, 1922 patients were given a chemotherapy-based regimen (1530 with CD20 antibodies). A total of 403 patients were administered a second line (LOT2), of which 183 patients received a CD20 antibody-containing chemotherapy regimen and 100 patients received stem cell transplantation or chimeric antigen receptor (CAR)-T therapy. Of the 136 LOT3+ treatments, 74 were chemotherapy regimens (54 with CD20 antibodies) and 18 were kinase inhibitors. The median time between treatment lines was less than 6 months. Among patients with at least LOT2, approximately 50% received more than one LOT during the first year after diagnosis. Approximately 25% of treated patients died within 6 months of treatment initiation. Of the 2633 included patients, the median OS from diagnosis was 31.0 months (treated patients: 46.8 months, untreated patients: 3.0 months).
Conclusions
Despite advances in the field, high unmet medical need in DLBCL remains. The treatment landscape is very heterogeneous, particularly in second- or later-line treatments, with few patients receiving potentially curative treatment beyond the first line. Treatment for DLBCL, particularly for transplant-ineligible patients, remains challenging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40487-024-00265-8.
Keywords: Diffuse large B-cell lymphoma, DLBCL, Overall survival, Treatment lines, Treatment patterns
Key Summary Points
| Why carry out this study? |
| Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma and accounts for approximately one third of all non-Hodgkin lymphoma cases. |
| Approximately 30–40% of patients with DLBCL are either refractory to initial treatment or relapse, and prognosis after treatment failure is usually poor. |
| The objective of this study was to investigate the prevalence, incidence, and treatment patterns (treatment regimens, switches, duration) for DLBCL in a real-world setting. |
| What was learned from the study? |
| Of the 2633 incident cases of DLBCL identified, 2119 patients received at least one DLBCL-related line of treatment (LOT1), and 25% of patients died within 6 months of treatment initiation. |
| The treatment landscape is very heterogeneous, particularly in second- or later-line treatments, with few patients receiving potentially curative treatment beyond the first line. |
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma and accounts for approximately one third of all non-Hodgkin lymphoma cases [1–3]. Worldwide incidence of DLBCL ranges from 2.3 to 13.8 cases per 100,000 person-years [4], with the incidence in Germany estimated at approximately 7 cases per 100,000 person-years [5]. Recent German prevalence data are unavailable, and the 10-year prevalence in Western Europe is roughly estimated at 45 per 100,000 patients [6]. With an average age at diagnosis of 65 to 70 years [3], the prevalence and incidence of DLBCL are expected to increase in the coming years due to the underlying aging of the population in this region [6].
Due to its aggressive nature, characterized by fast-growing tumors in lymph nodes or extranodal sites [7], the median survival rate of DLBCL is less than 1 year if untreated [2, 8]. Despite this, DLBCL is a potentially curable disease for patients who receive timely and appropriate treatment [9]. The majority of patients are treated with chemotherapy regimens [10], with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) as the current standard of care [11]. Most patients respond well to frontline R-CHOP treatment; long-term follow-up after R-CHOP administration has shown a median overall survival of approximately 8–10 years [12, 13].
However, results on the effectiveness of R-CHOP are often obtained from selected clinical trial populations that may have limited generalizability for the targeted population [14]. Approximately 30–40% of all patients are either refractory to initial treatment or relapse [15], most within 2 years of initial symptoms [16]. Prognosis after treatment failure is usually poor, with only a minority of patients being cured and a median overall survival (OS) of 6–9 months [17]. OS continues to decrease with later treatment lines [18]. The landscape for later-line treatments is heterogeneous, with no real standard of care for patients unable to receive potentially curative stem cell transplantation (SCT) [19]. As SCT is recommended only for selected patients who are eligible for high-dose chemotherapy [20], approximately 60% of patients are transplant ineligible [21]. Similarly, not all patients are eligible for potentially curative chimeric antigen receptor (CAR)-T cell therapy [22], presenting a therapeutic challenge for remaining patients with DLBCL. The treatment landscape for DLBCL is rapidly evolving, marked by the emergence of novel therapeutic options such as kinase inhibitors [23] and bispecific antibodies [24].
Information on the epidemiology of the disease [4, 6] and treatment regimens used in real-world settings [17, 25, 26] are limited. Therefore, this study complements the existing body of real-world evidence and investigates the prevalence, incidence, and current treatment patterns (treatment regimens, switches, and duration) for DLBCL in a real-world setting based on a patient population identified in a large German claims dataset.
Methods
Data Source and Study Population
This retrospective claims data analysis used an anonymized dataset provided by the regional German statutory health insurance fund AOK PLUS, which covers 3.6 million people insured in Germany (regions of Saxony and Thuringia) [27]. In Germany, statutory health insurance is mandatory for individuals whose annual income is below a fixed limit, which applies to around 90% of the German population [28, 29]. Individuals with higher incomes may be privately insured.
The dataset included demographics (age, sex, date of death), Charlson Comorbidity Index score (CCI; adapted for International Classification of Diseases, 10th Revision [ICD-10] codes) [30], outpatient treatment (visits to general practitioners and specialists, diagnosis codes), inpatient treatment (hospitalizations, operational and procedure [OPS] codes, diagnosis codes), and outpatient medication prescriptions (anatomic therapeutic classification [ATC] codes for medication identification, date of prescription, daily defined dose). Clinical trial data were not included in the dataset. Due to the non-interventional, retrospective nature of the analyzed data and because our analysis involved an anonymized dataset, neither ethical review nor informed consent of the patients was required according to German national legislation (§ 75 SGB X). As the responsible authority, the involved sickness fund (AOK PLUS) approved the use of the data for the purpose of this study.
The study period covered January 1, 2010, to December 31, 2021, with an inclusion period from January 1, 2012, to December 31, 2020. Patients with DLBCL with at least one inpatient and/or two confirmed consecutive outpatient specialist codes for DLBCL (ICD-10 German Modification [GM]: C83.3) and minimum age of 18 years at first confirmed DLBCL diagnosis (index date) were included. To limit the analysis to incident cases of DLBCL, patients who were either not continuously insured in the 2 years before the inclusion period or had any diagnosis code for DLBCL in this time were excluded. End of follow-up was defined as end of the study, loss to follow-up due to end of insurance, or death, whichever came first.
Treatment Algorithm
To identify lines of treatment (LOTs) [25, 26, 31], a treatment algorithm was developed based on recent guidelines for the treatment of DLBCL [32] and input from a clinical expert. The start of the first-line therapy (LOT1) was the first date on which the patient received a DLBCL-related treatment in the inpatient or outpatient setting. The Supplementary Data lists all treatments of interest, the corresponding ATC/OPS codes (Supplementary Table S1), and the treatment algorithm in detail (Supplementary Table S2).
Treatment patterns were observed from index date to end of follow-up. All ATC or OPS codes within 30 days were assigned to one LOT. Treatment regimens consisting of only supportive care agents (prednisone, prednisolone, dexamethasone, mesna, and/or darbepoetin) were not considered a separate LOT. The end of an outpatient LOT was defined as the prescription of a new ATC code not included in the previous regimen or as a treatment discontinuation (a gap of at least 60 days after end of supply; end of the LOT was defined as the end of supply). Days of supply were based on the daily defined dose of the agent [27]. For treatment discontinuation, all agents of the regimen had to be discontinued, except for supportive care agents and monotherapy rituximab or lenalidomide. The end of an inpatient LOT was defined as a new OPS code (except for radiation) or treatment discontinuation of more than 6 months (the date of the last OPS code was considered the end of the LOT). Treatments received after the second-line treatment (LOT2) were combined and reported as LOT3+. Considerations for SCT and CAR-T-cell therapies are described in Supplementary Table S2.
Treatment Categories
Both inpatient and outpatient treatment regimens were categorized into eight treatment categories which fall into three broader categories: chemotherapy regimens (chemotherapy + CD20 antibodies, chemotherapy), non-chemotherapy regimens (CD20 antibodies, immunomodulators, kinase inhibitors, mechanistic target of rapamycin [mTOR]/proteasome inhibitors), and other treatments (SCT/CAR-T, radiation). Treatment categories were reported according to treatment setting: outpatient (outpatient only or inpatient/outpatient) and inpatient only.
All regimens under each treatment category are described in Supplementary Table S3. The category “chemotherapy + CD20 antibodies” included R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone), BR (bendamustine, rituximab), Pola-BR (polatuzumab vedotin, bendamustine, rituximab), R-GemOx (rituximab, gemcitabine, oxaliplatin), inpatient chemotherapy with rituximab, or similar combinations. The category “chemotherapy” included regimens without CD20 antibodies, including CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and outpatient prescriptions of chemotherapy agents. Non-chemotherapy regimens included regimens with and without CD20 antibodies. The radiation category referred to radiation alone, although patients may have received radiation in addition to treatments in other categories.
Statistical Analysis
Baseline characteristics were calculated for the entire cohort and compared between patients who did or did not receive any DLBCL treatment using nonparametric tests (chi-square, Mann–Whitney U). Treatment and treatment duration were reported using descriptive statistics. Prevalence (number of patients with DLBCL diagnosis divided by number of patients aged 18+ and continuously insured) and cumulative incidence (number of new DLBCL diagnoses divided by number of patients aged 18+ and continuously insured) were calculated for 2019/2020. Sex- and age-adjusted prevalence and cumulative incidence were derived by calculating the sex- and age-specific number of DLBCL cases per 100,000 in the AOK PLUS dataset, then standardizing these figures using demographic weights from the German Federal Office of Statistics (DESTATIS) to reflect the national population structure. OS was calculated using Kaplan–Meier methodology, including the proportion of patients alive after 6/12/36 months following the index date and median OS (95% confidence interval [CI]), censoring at end of follow-up. OS after the index date was also determined for patients who did or did not receive any DLBCL treatment. For patients who received DLBCL treatment, OS was calculated after start of LOT1 and LOT2.
Results for treatment categories with < 10 patients were not reported to preserve the anonymity of patients. Treatment duration could only be calculated for LOTs in the outpatient setting (date of first prescription/admission date to run-out date of last prescription/admission date).
Software used for data analysis included Microsoft Office Excel for Microsoft 365, RStudio version 4.2, and Stata Statistical Software version 17.
Results
Baseline Characteristics and Follow-Up
A total of 2633 patients with incident cases of DLBCL meeting the inclusion/exclusion criteria were identified between January 1, 2012, and December 31, 2020. The number of incident cases per year was consistent throughout the inclusion period (293 ± 13 patients per calendar year, Supplementary Table S4). The median age was 75 years, median Charlson Comorbidity Index (CCI) score was 8, and 51% of patients were male (Table 1). The median follow-up duration was 21 months, with 59.5% of patients dying during follow-up.
Table 1.
Baseline characteristics and follow-up duration
| All patients | Patients with DLBCL treatment | Patients with no DLBCL treatment | p value | |
|---|---|---|---|---|
| (n = 2633) | (n = 2119) | (n = 514) | ||
| Baseline characteristics | ||||
| Age, median (mean|SD) at index date | 75 (72.1|13.1) | 74 (71.1|12.9) | 80 (76.5|13.2) | < 0.001 |
| < 65 years, n (%) | 639 (24.3) | 556 (26.2) | 83 (16.1) | < 0.001 |
| 65 to 74, n (%) | 627 (23.8) | 546 (25.8) | 81 (15.8) | – |
| 75 to 84, n (%) | 1015 (38.5) | 809 (38.2) | 206 (40.1) | – |
| 85+ | 352 (13.4) | 208 (9.8) | 144 (28.0) | – |
| Sex, male, n (%) | 1343 (51.0) | 1082 (51.1) | 261 (50.8) | 0.908 |
| CCI, median (mean|SD) at index date n (%) | 8 (8.3 | 4.1) | 8 (8.6 | 4.1) | 7 (7.1 | 3.7) | < 0.001 |
| Top comorbidities at index date (ICD-10-GM), n (%) | ||||
| Primary hypertension (I10) | 1332 (50.6) | 1068 (50.4) | 264 (51.36) | 0.696 |
| Diabetes mellitus type 2 (E11) | 624 (23.7) | 486 (22.9) | 138 (26.8) | 0.061 |
| Fluid/electrolyte/acid–base imbalance (E87) | 604 (22.9) | 473 (22.3) | 131 (25.4) | 0.126 |
| Chronic kidney disease (N18) | 393 (14.9) | 281 (13.3) | 112 (21.7) | < 0.001 |
| Atrial fibrillation/flutter (I48) | 374 (14.2) | 274 (12.9) | 100 (19.5) | < 0.001 |
| Follow-up | ||||
| Duration of follow-up in months, median (mean|SD) | 21.0 (33.9 | 33.8) | 26.6 (37.2 | 33.5) | 2.9 (20.3 | 31.5) | < 0.001 |
| < 6 months, n (%) | 726 (27.6) | 422 (19.9) | 304 (59.1) | < 0.001 |
| 6 to < 12 months, n (%) | 277 (10.5) | 237 (11.2) | 40 (7.8) | – |
| 12 to < 24 months, n (%) | 396 (15.0) | 361 (17.0) | 35 (6.8) | – |
| 24 to < 48 months, n %) | 445 (16.9) | 400 (18.9) | 45 (8.8) | – |
| 48+ months, n (%) | 789 (30.0) | 699 (33.0) | 90 (17.5) | – |
| Reason for loss to follow-up, n (%) | ||||
| Death | 1567 (59.5) | 1163 (54.9) | 404 (78.6) | < 0.001 |
| End of study | 1051 (40.0) | 943 (44.5) | 108 (21.0) | – |
| End of insurance | 15 (0.5) | 13 (0.6) | 2 (0.3) | – |
CCI Charlson Comorbidity Index, DLBCL diffuse large B-cell lymphoma, ICD-10-GM International Classification of Diseases 10th Revision German Modification, SD standard deviation
At least one DLBCL-related treatment was received by 2119 (80.5%) of patients. Compared to patients without treatment, patients who received treatments were younger (median age 74 years vs. 80 years, p < 0.001), had longer follow-up duration (median duration 26.6 months vs. 2.9 months, p < 0.001), and had a lower percentage of deaths during follow-up (54.9% vs 78.6%, p < 0.001). At index, untreated patients more commonly had chronic kidney disease (p < 0.001) and atrial fibrillation/flutter (p < 0.001).
Prevalence and Cumulative Incidence
The prevalence of DLBCL was 34.8 per 100,000 patients in 2019 and 36.7 per 100,000 patients in 2020. The cumulative incidence was 14.0 and 12.7 per 100,000 patients in 2019 and 2020, respectively. Age- and sex-adjusted prevalence and cumulative incidence in 2019/2020 were lower than crude estimates (prevalence: 30.9/33.3 per 100,000 patients, cumulative incidence: 12.3/11.3 per 100,000 patients).
Treatment Patterns
First Treatment Line
A total of 2119 patients were administered at least one line of treatment (Table 2). Most patients were given a chemotherapy-based regimen (n = 1922; 90.7%), with 1530 (79.6%) and 392 (20.4%) patients receiving chemotherapy with/without CD20 antibodies, respectively. Detailed prescription data could only be observed for patients receiving treatment in the outpatient setting (n = 1099). Of the patients with treatment in the outpatient setting, 509 (46.3%) and 90 (8.2%) patients were given R-CHOP (or similar) and BR, respectively. All other CD20 antibody-containing chemotherapy regimens were received by < 10 patients (Supplementary Table S5). Among non-chemotherapy regimens, 116 patients were given CD20 antibodies and 51 patients received only radiotherapy.
Table 2.
Treatment categories and treatment duration (LOT1-LOT3+) in patients with DLBCL
| Treatment categories, n (%) | LOT1 | LOT2 | LOT3+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All n = 2119 |
Outpatienta n = 1099 |
Inpatient only n = 1020 |
All n = 403 |
Outpatienta n = 196 |
Inpatient only n = 207 |
All n = 136 |
Outpatienta n = 87 |
Inpatient only n = 49 |
|
| Chemotherapy regimens | 1922 (90.7) | 1012 (92.1) | 910 (89.2) | 225 (55.8) | 124 (63.3) | 101 (48.8) | 74 (54.4) | 48 (55.2) | (≈55.0)e |
| Chemotherapy + CD20 antibodiesb | 1530 (72.2) | 966 (87.9) | 564 (55.3) | 183 (45.4) | 99 (50.5) | 84 (40.6) | 54 (39.7) | 35 (40.2) | (≈40.0)e |
| Chemotherapyc | 392 (18.5) | 46 (4.2) | 346 (33.9) | 42 (10.4) | 25 (12.8) | (≈8.0)e | 20 (14.7) | 13 (14.9) | < 10 |
| Non-chemotherapy regimens | 137 (6.5) | 86 (7.8) | 51 (5.0) | 69 (17.1) | 59 (30.1) | 10 (4.8) | 45 (33.1) | 36 (41.4) | < 10 |
| CD20 antibodies | 116 (5.5) | 76 (6.9) | 40 (3.9) | 42 (10.4) | (≈18.0)5 | < 10 | 14 (10.3) | < 10 | < 10 |
| Immunomodulatorsd | 12 (0.6) | < 10 | (≈1.0)e | < 10 | < 10 | < 10 | 12 (8.8) | < 10 | < 10 |
| Kinase inhibitorsd | < 10 | < 10 | – | 18 (4.5) | 18 (9.2) | – | 18 (13.2) | 18 (20.7) | – |
| mTOR/proteasome inhibitord | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| SCT or CAR-T | < 10 | – | < 10 | 100 (24.8) | 13 (6.6) | 87 (42.0) | < 10 | < 10 | < 10 |
| Radiation | 51 (2.4) | – | 51 (5.0) | < 10 | < 10 | < 10 | < 10 | – | < 10 |
| Treatment duration, median (IQR) | – | 183.0 (142.3–235.0) | – | – | 132.0 (60.0–273.3) | – | – | 115.0 (57.5–227.0) | – |
CAR-T chimeric antigen receptor T cells, IQR interquartile range, LOT line of treatment, mTOR mechanistic target of rapamycin, SCT stem cell transplantation
aTreatment lines in outpatient settings (outpatient only, inpatient/outpatient) were combined, as detailed prescription data could only be observed for patients receiving treatment in the outpatient setting. Treatment categories separated by outpatient only and inpatient/outpatient data are shown in Supplementary Table S5
bIncludes regimens of CD20 antibodies + inpatient chemotherapy, CD20 antibodies + chemotherapy agents, R-CHOP, BR, Pola-BR, R-GemOx, or similar treatment
cIncludes regimens of inpatient chemotherapy, chemotherapy agents, demethylation agents, alkylating agents, CHOP, or similar treatment
dIncludes regimens with and without CD20 antibodies
eValues approximated to preserve patient anonymity
Second Treatment Line
Of the 2119 patients with LOT1, a second-line treatment was initiated in 403 (19.0%) patients (Table 2). Most patients received a chemotherapy-based regimen (n = 225, 55.8%), of which the vast majority (n = 183 patients) received a CD20 antibody-containing chemotherapy regimen. Among patients receiving treatment in the outpatient setting (n = 196), BR was given to 42 (21.4%), R-CHOP to 23 (11.7%), Pola-BR to 12 (6.1%), and R-GemOx to 10 (5.1%) patients.
Out of 69 (17.1%) patients given non-chemotherapy regimens, 42 patients received only CD20 antibodies and 18 received a kinase inhibitor. SCT or CAR-T was performed for 100 (24.8%) patients (82% autologous SCT; 18% allogeneic SCT, unspecified SCT, or CAR-T).
Further Treatment Lines
Among the 403 patients with LOT2, 106 (26.3%) patients were given at least a third-line treatment. There were 30 LOT4-LOT7 treatment lines for a total of 136 LOT3+ treatments (Table 2).
A chemotherapy regimen was used for 74 (54.4%) treatments (54 [39.7%] with and only 20 [14.7%] without CD20 antibodies). Regarding treatments in the outpatient setting (n = 87), 16 (18.3%) of the prescribed chemotherapy regimens were BR or R-GemOx. Due to the small sample size, individual chemotherapy regimens without CD20 could not be evaluated. Of the 45 (33.1%) non-chemotherapy treatment lines, 18 were a kinase inhibitor.
Treatment Switches
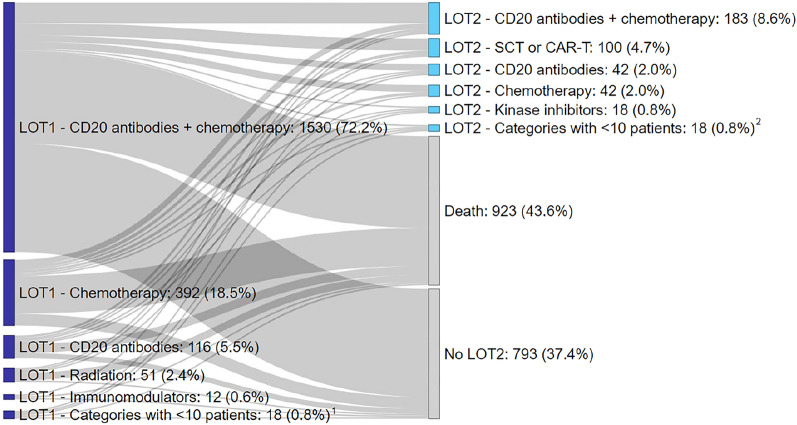
Treatment progression is depicted in Fig. 1. Patients that received a second-line treatment and were given chemotherapy containing CD20 antibodies in LOT1 (n = 277) commonly switched to SCT (n = 76; 27.4%), CD20 antibodies alone (n = 25; 9.0%), or chemotherapy without CD20 antibodies (n = 24; 8.7%). Patients who underwent LOT2 and were given chemotherapy without CD20 antibodies in LOT1 (n = 82) frequently switched to chemotherapy containing CD20 antibodies (n = 35; 42.7%) and SCT (n = 19; 23.2%). Of the patients administered CD20 antibodies alone in LOT1 and received a second line (n = 28), 15 (53.6%) patients were given chemotherapy containing CD20 antibodies in LOT2. Treatment switches from LOT2 to LOT3 could not be reported due to small sample size.
Fig. 1.
Sankey diagram of patient progression from LOT1 to LOT2 in DLBCL. 1Categories with < 10 patients in LOT1 were immunomodulators, kinase inhibitors, mTOR/proteasome inhibitors, SCT/CAR-T. 2Categories with < 10 patients in LOT2 were immunomodulators, mTOR/proteasome inhibitors, and radiation. Abbreviations: CAR-T chimeric antigen receptor T cells, LOT line of treatment, SCT stem cell transplantation
Treatment Duration and Number of Treatment Lines Within 1–5 Years of Follow-Up
The median treatment duration for outpatient treatments was longer for LOT1 (n = 1099; 183 days) than for LOT2 (n = 196; 132 days) and LOT3+ (n = 87; 115 days) (Table 2). Treatment durations by treatment category and including monotherapy rituximab/lenalidomide following treatment line are described in Supplementary Table S6.
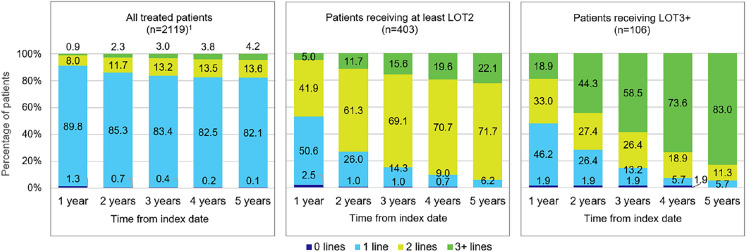
The median (IQR) time between treatment lines was less than 6 months (LOT1 to LOT2: 131 [1–466] days; LOT2 to LOT3: 127.5 [39–341] days). Out of all treated patients (n = 2119), the vast majority of patients (98.7%) received a treatment within 1 year of diagnosis (Fig. 2). Among patients with at least LOT2 or LOT3, approximately 50% of patients received more than one line of treatment during the first year after diagnosis (≥ LOT2: 46.9%; ≥ LOT3: 51.9%). For patients with at least three treatment lines (n = 106), 18.9% were already given a third line during the first year after diagnosis, and within 5 years of follow-up, the vast majority of patients (83.0%) received their third treatment line.
Fig. 2.
Number of treatment lines received by patients during follow-up. Figure shows the percentage of patients receiving 0, 1, 2, or 3+ treatment lines within 1–5 years after index date (first DLBCL diagnosis) among all treated patients, patients receiving at least LOT2, and patients receiving LOT3+ . 1Among all treated patients (n = 2119), 1716 (81.0%) received only 1 line, 403 (19.0%) received at least 2 lines, and 106 (5.0%) received 3+ lines. Abbreviations: LOT line of treatment
Survival of Patients with DLBCL
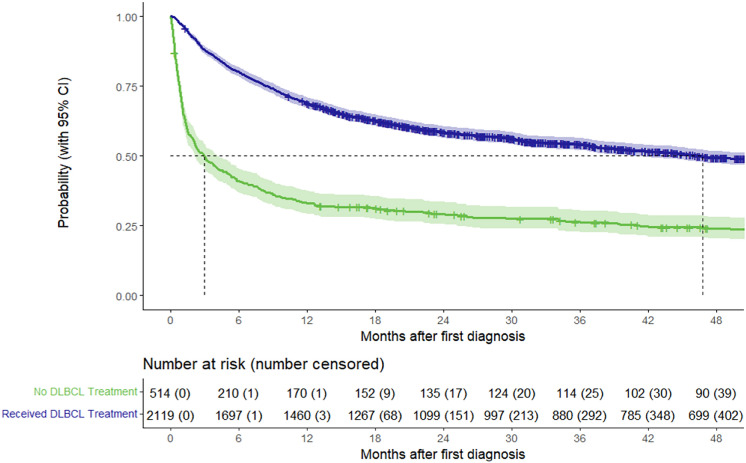
Of the 2633 patients with DLBCL included in the study, the percentage of patients alive at 6/12/36 months after diagnosis was 72.4%/61.9%/37.3%, respectively, and the median OS from index date was 31.0 months (95% CI 26.5–37.5). The median OS from the index date was considerably shorter among patients who did not receive treatment (3.0 months [95% CI 2.1–4.1], n = 514) than among treated patients (46.8 months [95% CI 40.6–54.7], n = 2119) (Fig. 3).
Fig. 3.
Kaplan–Meier OS analysis after index date of treated patients (n = 2119) and untreated patients (n = 514). Abbreviations: CI confidence interval, DLBCL diffuse large B-cell lymphoma
Based on Kaplan–Meier estimates of treated patients (n = 2119), 21% and 28% of treated patients died within 6 months of treatment initiation of LOT1 and LOT2, respectively. The percentage of patients alive at 6/12/36 months after start of LOT1 was 78.9%/67.8%/40.7%. The median OS from start of LOT1 (n = 2119) was 45.9 months (95% CI 40.0–54.1) (Supplementary Figure S1). Among patients who received a second-line treatment (n = 403), 72.0%/57.1%/24.6% were alive at 6/12/36 months, respectively, after start of LOT2, and median OS from start of LOT2 was 20.9 months (95% CI 17.9–26.3) (Supplementary Figure S2).
Discussion
German claims data were analyzed to estimate the prevalence and cumulative incidence of DLBCL and characterize real-world treatment patterns, including identification of treatment lines, different regimens, and treatment duration. The included patient population (n = 2633) was older (median age 75 years), highly comorbid (median CCI 8), and included a large proportion of untreated patients (19.5%). This is comparable to other real-world studies of DLBCL incidence in patient populations [26, 31], although this population differs greatly from patients included in clinical trials, which tend to exclude patients with a poor prognosis [14].
The prevalence and cumulative incidence of DLBCL were calculated for the most recently available year within the inclusion period (2020) and the previous year (2019). Prevalence in 2019/2020 was 34.8/36.7 per 100,000 patients (age- and sex-adjusted: 30.9/33.3 per 100,000), and the annual cumulative incidence of DLBCL was 14.0/12.7 per 100,000 patients in 2019/2020 (age- and sex-adjusted: 12.3/11.3 per 100,000).
Limited data on DLBCL prevalence are currently available, with only rough 10-year prevalence estimates provided by Kanas et al. (≈45 per 100,000 patients) [6]. Our findings for annual prevalence were in line with these data and contribute to the sparse knowledge in this field.
Cumulative incidence of DLBCL in our study was higher than the incidence rate estimates in Germany (7 cases per 100,000 person-years) [5], but in line with worldwide reports of incidence rates (2.3 to 13.8 cases per 100,000 person-years) [4]. This larger estimate was observed despite our conservative approach of including only patients with inpatient diagnoses or two confirmed consecutive outpatient specialist codes. The higher cumulative incidence found in our dataset could reflect an aging population, as the incidence of DLBCL is estimated to increase in the upcoming years [6]. The decline observed in cumulative incidence in 2020 may be a result of fewer diagnoses due to the COVID-19 pandemic [33]. However, a previous study using the AOK PLUS dataset found that the number of cancer diagnoses in 2020 did not differ significantly from 2019 [34].
The study revealed a large proportion of patient mortality (≈60%), highlighting the high medical need among patients with DLBCL. This is in part due to the approximately 20% of the included population who did not receive any DLBCL treatment during follow-up. This finding is in line with Borchmann et al., a study that used a different German claims dataset to examine survival outcomes of patients with DLBCL and which also found that 20% of included patients did not receive a DLBCL treatment regimen [25]. A study using US insurance data also described that approximately 25% of patients with DLBCL receive no treatment [35].
In this study, untreated patients had poor prognosis, with median OS of 3 months, which may be due to the significantly older population among untreated patients. Untreated patients may have had more advanced-stage disease [36] or poor performance status [37], which could not be ascertained in the data. Approximately 25% of untreated patients were still alive over 4 years after initial diagnosis, although the median survival rate of untreated DLBCL is reported as less than 1 year [2, 8]. Some patients categorized as untreated may have been treated through clinical trials, which could not be captured in our data. However, clinical trial recruitment in Germany is low compared to other European countries—only 500 patients per million inhabitants (0.5%) take part in clinical trials [38]. Due to our methodology for including patients, we also cannot rule out the possibility of false DLBCL diagnoses. Our Kaplan–Meier survival curves shown in Fig. 2 are comparable to Daneels et al., a study using Belgian health insurance data to describe first- and second-line treatments for DLBCL, which also showed poor survival outcomes for patients who did not receive chemo/radiotherapy in the first line [26].
Patient mortality was also common among treated patients, with approximately 25% of patients dying within 6 months of initiation of therapy (21.0% and 28.0% after start of LOT1 and LOT2). Patient mortality within 12 months after start of LOT1 or LOT2 was 32.2% and 42.9%, respectively. This underscores the aggressive course of the disease, despite the array of currently available treatment regimens for DLBCL. Median survival after the start of therapy among patients who received a second line or further (20.9 months, n = 403) was shorter compared to all treated patients (45.9 months, n = 2119). This is consistent with literature stating that prognosis after treatment failure is usually poor [17].
Among the 2119 patients who did receive DLBCL treatment, the vast majority (90.7%) were administered a chemotherapy regimen as a first-line therapy, mostly containing CD20 antibodies. The general guidelines for front-line DLBCL treatment recommend treatment with R-CHOP [39], and this regimen was also the most frequently identified chemotherapy combination (46.3% of outpatient treatments). Approximately 20% (n = 392) of chemotherapy patients did not receive CD20 antibodies. However, most of these patients (n = 346; 88.2%) received first-line treatment in the inpatient setting only, where chemotherapy treatments are identified only by OPS code. OPS codes for chemotherapy do not contain information about respective agents. We cannot rule out the possibility that in some cases, combination treatments such as R-CHOP were coded as chemotherapy in the inpatient setting, without the separate OPS code for rituximab (OPS 6-001.h, 6-001.j). Additionally, our results are consistent with Borchmann et al., which also found that approximately 20% of patients received regimens without rituximab in the first line [25]. Approximately 5% of all treated patients were given CD20 antibodies as monotherapy in the first-line treatment. As incident DLBCL is more commonly treated in combination with chemotherapy, patients who received monotherapy may have been older and presented with more comorbidities and therefore were unable to tolerate a full chemotherapy regimen [40]. Few patients (2.4%) received radiation therapy alone, likely as a treatment for localized disease [41].
We identified 403 patients (19%) that were administered a second-line treatment. This result was slightly higher than that of Daneels et al., which found that 16% of patients had refractory/relapsed disease, defined as second-line regimens within 2 years of diagnosis [26]. However, our study was able to capture second lines of treatment beyond 2 years of diagnosis, which may explain the higher percentages of identified patients. Reviews of DLBCL indicate that refractory/relapsed disease can occur in 30–40% of patients after initial treatment [15]. However, only 19% of patients in our study received LOT2 treatment, suggesting that a large proportion of refractory/relapsed patients get no second-line treatment due to death or ineligibility. Therefore, the actual proportion of refractory/relapsed patients in our sample may be higher than our findings.
Treatments received by patients in the second line were varied. In our study, SCT or CAR-T was performed as a second line for approximately one quarter of patients (n = 100; 24.8%), of which 82 patients were given autologous SCT. This is consistent with Harrysson et al., a Swedish medical chart review study, which found that approximately 22% of patients with a second line underwent SCT [17]. The overall low number of SCT treatments in our study also aligns with the study by Borchmann et al., which reported a very similar proportion of patients who underwent SCT. This implies that although SCT is a standard of care among transplant-eligible refractory/relapsed patients [20], many patients are unfit for SCT due to older age or a high number of comorbidities [19].
Additionally, despite CAR-T cell therapy being touted as an alternative to SCT for older and less fit patients [22], we observed very few patients receiving this treatment. CAR-T therapies axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) were first approved in Europe in 2018, which may explain the sparsity of this treatment in our dataset [42]. Lisocabtagene maraleucel (liso-cel) was not approved until 2022 [43], which is beyond the time frame of our analysis. Additionally, access to CAR-T after initial approval may have been constrained due to restrictive patient selection and limited numbers of CAR-T centers [42]. Our findings are in line with the number of CAR-T treatments performed in Germany [44] and the low numbers observed in Harrysson et al. [17].
The majority of patients (55.8%) were given chemotherapy regimens in the second line, with most patients in this category receiving chemotherapy containing CD20 antibodies. Daneels et al. also found that the majority of second-line regimens contained rituximab and chemotherapy, predominantly platinum-based [26]. Transplant-ineligible patients have historically been given chemoimmunotherapy regimens such as R-GemOx and BR with palliative intent, although these therapies often do not result in long-lasting remission [45]. Among LOT2 outpatient treatments (n = 196), only approximately one quarter (26.5%) of the regimens consisted of R-GemOx or BR, highlighting the heterogeneous treatment landscape and lack of a standardized approach among patients unfit for transplantation. With the addition of more market-authorized regimens such as Pola-BR (2020) and Tafa-Len (tafasitamab and Lenalidomide 2021) [46, 47], the variety of treatment options for DLBCL continues to grow.
Third-line or further treatments were also highly heterogeneous. The most frequent type of LOT3+ treatment was chemotherapy (54.4%), with a very low proportion of patients treated with chemotherapy without CD20 antibodies. This is in line with current guidelines, which recommend combination therapies, usually including a CD20 antibody [48]. Non-chemotherapy regimens comprising one third of the LOT3+ treatments and kinase inhibitors such as ibrutinib were administered in 13% of treatments. There is recent evidence that ibrutinib monotherapy may be used to effectively treat refractory/relapsed DLBCL with low toxicity [23]. The relatively frequent usage of non-chemotherapy regimens and newer treatment options in a real-world setting reflects the rapidly evolving treatment landscape of DLBCL and underlines the need to address treatment challenges among transplant-ineligible refractory/relapsed patients.
We observed that a large proportion of patients received treatment in inpatient settings only, although DLBCL is described as more commonly administered in the outpatient setting [49]. A study using US inpatient data found that chemotherapy was administered in approximately 30% of hospitalizations for DLBCL and more commonly for younger patients with fewer chronic conditions [49]. To our knowledge, no existing studies address the proportion of inpatient treatment for DLBCL within the context of Germany or Europe. Additional research is necessary to investigate reasons for the high proportion of inpatient treatment among these patients. We found that the proportion of patients receiving inpatient-only treatment decreased for LOT3+ treatments (36.0%) compared to LOT1 (48.1%) and LOT2 (51.4%). This may be because our study could not capture treatment in clinical trials, which occur more frequently in the hospital setting. However, as claims data do not contain information on the medical records of patients, it is difficult to interpret this finding.
Consistent with the literature, our study found that patients that were refractory or relapsed did so within a short period of time after DLBCL diagnosis [16, 17]. Although the dataset contained no information about symptom onset, we found that among patients with at least a second line, approximately 50% of patients received LOT2 treatment within 1 year after diagnosis. For patients with at least three treatment lines, 18.9% were already given a third line during the first year of follow-up. This number increased to 48.5% by 3 years of follow-up and 83.0% by 5 years of follow-up. This rapid progression between lines of therapy highlights the unmet need among refractory/relapsed patients. It is possible that the relatively short median time between treatment lines is due to our treatment line algorithm, which was used due to the lack of clinical information in claims data. However, our algorithm is consistent with methods used for other real-world studies [25, 26]. Short times between treatment lines may be indicative of refractory rather than relapsed disease. For example, Daneels et al. define refractory disease as the initiation of a second-line regimen within 12 weeks from the end of first-line therapy, whereas relapsed disease is defined as a second-line regimen beginning beyond 12 weeks of the end of the first-line therapy [26].
The main strength of this claims data analysis is the ability to capture incident cases irrespective of patient characteristics, such as age and comorbidities, and willingness to participate in clinical or observational studies, therefore contributing to high external validity. Additionally, we included patients who did not receive any DLBCL treatment, to avoid selection bias in excluding untreated patients. Our study also allowed for a long observation period, with up to 10 years of follow-up for patients with a first DLBCL diagnosis in 2012. The dataset covered both inpatient and outpatient treatment, and subsequently provides full coverage over all sectors of healthcare in Germany and information about the treatment setting of patients with DLBCL. Lastly, information from real-world clinical practice is limited (to our knowledge, there is no registry for patients with DLBCL in Germany), and therefore claims data analysis provides insight into this patient population. Our study contributes to the current body of real-world evidence on the epidemiology and treatment approaches for DLBCL.
We also acknowledge some intrinsic limitations, particularly related to the use of a retrospective anonymous claims dataset. The dataset did not include clinical details, such as stage of DLBCL at diagnosis or disease progression, as data are primarily used for reimbursement purposes. Treatment lines were identified indirectly via the specified algorithm, as we could not confirm the refractory/relapsed status of patients. Formally, we cannot rule out the possibility of false diagnosis, although we implemented a conservative approach to only include patients with inpatient diagnoses or two confirmed consecutive outpatient specialist codes. Our analysis also does not differentiate between subtypes of DLBCL, such as primary central nervous system lymphoma or post-transplant lymphoproliferative disease, which may be captured under the same ICD-10-GM code as DLBCL. Regional bias may be present, as AOK PLUS only insures patients from two German states (Saxony/Thuringia). However, in accordance with the German legal framework, health service reimbursement rules are identical across Germany, and previous studies have found no major regional differences in health care structures between Saxony/Thuringia and other German states [27].
A large number of treatment regimens were observed, and to protect patient anonymity, treatment categories were necessary to summarize the data. However, this subsequently led to loss of information. Additionally, detailed prescription data were only observed for treatments in the outpatient setting (outpatient only or inpatient/outpatient treatments). In the inpatient sector, chemotherapy treatments could be identified by OPS code, but the data did not contain information about the respective agents. Therefore, we could not ascertain the specific type of chemotherapy regimens received during inpatient treatment.
Our study also does not reflect all potential medical interventions applied to patients, such as participation in clinical trials which do not qualify for medical claims. Finally, due to the long study period, innovative therapies from recent years may be underrepresented compared to treatments that have been established for a longer time.
Conclusion
Despite advances in the field, high unmet medical need in DLBCL remains. A large proportion of patients do not receive systemic treatment for DLBCL and subsequently have exceptionally poor survival outcomes. Patient mortality over the course of the analysis was high, even among treated patients. Approximately 25% of patients died within 6 months of start of therapy, independent of treatment line, highlighting the aggressive disease course despite currently available treatment options. Additionally, rapid progression between lines of therapies was observed among refractory/relapsed patients, with approximately 50% of patients receiving at least a second line within 1 year after diagnosis.
The treatment landscape is very heterogeneous, particularly in second- or later-line treatments, with few patients receiving potentially curative treatment beyond the first line. SCT or CAR-T was performed for only one quarter of refractory/relapsed patients, and the number of observations of CAR-T was especially limited. In later-line treatments, a larger proportion of non-chemotherapy regimens and newer treatment options was observed. Treatment for DLBCL, particularly for transplant-ineligible patients, remains a challenge for medical practitioners. However, the treatment landscape in DLBCL is evolving quickly, and our study provides timely insights into the current state of DLBCL management and patient outcomes. It can help to identify areas that need further exploration in DLBCL treatment strategies. As the field continues to advance, it will be interesting to observe how novel therapies such as CAR-T cells or bispecific antibodies will impact clinical practice in DLBCL in the coming years.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship and have given their approval for this version to be published. Scarlette Pacis, Anna Bolzani, Alexander Heuck, Klaus Gossens, Mathias Kruse, Ulf Maywald, Thomas Wilke, and Chistian Kunz were involved in the conceptualization and the design of the study and interpretation of the data. Scarlette Pacis and Anna Bolzani analyzed the data and drafted the manuscript. All authors critically reviewed the article and made substantial contributions to the finalization of the manuscript, especially including the discussion section, and have given their final approval of the version to be submitted.
Funding
Data collection, statistical analysis and medical writing support were provided by Cytel, Inc. (Berlin, Germany), which was funded by AbbVie Deutschland GmbH & Co. KG (Wiesbaden, Germany). The journal’s Rapid Service fee was also funded by AbbVie Deutschland GmbH & Co. KG.
Data Availability
The data used in this study are abstracted from anonymized, individual patient records and are not publicly available due to contractual agreements with the sickness fund (AOK PLUS).
Declarations
Conflict of Interest
Scarlette Pacis and Anna Bolzani are employees of Cytel. Alexander Heuck, Klaus Gossens, Mathias Kruse and Björn Fritz are AbbVie employees and may own AbbVie stock. Ulf Maywald has no potential conflict of interest, except those potentially related to his employer, AOK PLUS. Thomas Wilke is an employee of IPAM and he has received honoraria from several pharmaceutical/consultancy companies including Abbvie. Christian Kunz works at the Westpfalz-Klinikum GmbH, MVZ Kaiserslautern and declared no potential conflict of interest.
Ethical Approval
Due to the non-interventional, retrospective nature of the analyzed data and because our analysis involved an anonymized dataset, neither ethical review nor informed consent of the patients was required according to German national legislation (§ 75 SGB X). As the responsible authority, the involved sickness fund (AOK PLUS) approved the use of the data for the purpose of this study.
References
- 1.Danese MD, Griffiths RI, Gleeson ML, Dalvi T, Li J, Mikhael JR, et al. Second-line therapy in diffuse large B-cell lymphoma (DLBCL): treatment patterns and outcomes in older patients receiving outpatient chemotherapy. Leuk Lymphoma. 2017;58(5):1094–1104. doi: 10.1080/10428194.2016.1228924. [DOI] [PubMed] [Google Scholar]
- 2.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60(6):393–408. doi: 10.3322/caac.20087. [DOI] [PubMed] [Google Scholar]
- 3.Mayerhoff L, Lehne M, Hickstein L, Salimullah T, Prieur S, Thomas SK, et al. Cost associated with hematopoietic stem cell transplantation: a retrospective claims data analysis in Germany. J Comp Eff Res. 2019;8(2):121–131. doi: 10.2217/cer-2018-0100. [DOI] [PubMed] [Google Scholar]
- 4.Garg M, Takyar J, Dhawan A, Saggu G, Agrawal N, Hall A, et al. Diffuse large B-cell lymphoma (DLBCL): a structured literature review of the epidemiology, treatment guidelines, and real-world treatment patterns. Abstract Blood. 2022;140(Supplement 1):12106–12107. doi: 10.1182/blood-2022-169045. [DOI] [Google Scholar]
- 5.Skalt D, Moertl B, von Bergwelt-Baildon M, Schmidt C, Schoel W, Bücklein V, et al. Budget impact analysis of CAR T-cell therapy for adult patients with relapsed or refractory diffuse large b-cell lymphoma in Germany. Hemasphere. 2022;6(7):e736. doi: 10.1097/HS9.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanas G, Ge W, Quek RG, Keeven K, Nersesyan K, Arnason JE. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020–2025. Leuk Lymphoma. 2022;63(1):54–63. doi: 10.1080/10428194.2021.1975188. [DOI] [PubMed] [Google Scholar]
- 7.Kubuschok B, Held G, Pfreundschuh M. Management of diffuse large B-cell lymphoma (DLBCL) Cancer Treat Res. 2015;165:271–288. doi: 10.1007/978-3-319-13150-4_11. [DOI] [PubMed] [Google Scholar]
- 8.Adıyaman SC, Alacacıoğlu İ, Danyeli AE, Türkyılmaz D, Sevindik ÖG, Demirkan F, et al. Prognostic factors in elderly patients with diffuse large B-cell lymphoma and their treatment results. Turk J Haematol. 2019;36(2):81–87. doi: 10.4274/tjh.galenos.2019.0218.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poletto S, Novo M, Paruzzo L, Frascione PMM, Vitolo U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat Rev. 2022;110:102443. doi: 10.1016/j.ctrv.2022.102443. [DOI] [PubMed] [Google Scholar]
- 10.Major A, Smith SM. DA-R-EPOCH vs R-CHOP in DLBCL: how do we choose? Clin Adv Hematol Oncol. 2021;19(11):698–709. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Li L-r, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. 2020;13(1):175. doi: 10.1186/s13045-020-01011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–205. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvat M, Zadnik V, Južnič Šetina T, Boltežar L, Pahole Goličnik J, Novaković S, et al. Diffuse large B-cell lymphoma: 10 years' real-world clinical experience with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone. Oncol Lett. 2018;15(3):3602–3609. doi: 10.3892/ol.2018.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkins RA, Patel SP, Lee MJ, Switchenko JM, Ansell SM, Bartlett NL, et al. Improving eligibility criteria for first-line trials for patients with DLBCL using a US-based Delphi-method survey. Blood Adv. 2022;6(9):2745–2756. doi: 10.1182/bloodadvances.2021006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawalha Y. Relapsed/refractory diffuse large B-cell lymphoma: a look at the approved and emerging therapies. J Pers Med. 2021;11(12):1345. doi: 10.3390/jpm11121345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. 2018;182(5):633–643. doi: 10.1111/bjh.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrysson S, Eloranta S, Ekberg S, Enblad G, El-Galaly TC, Sander B, et al. Outcomes of relapsed/refractory diffuse large B-cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line—a population-based study of 736 patients. Br J Haematol. 2022;198(2):267–277. doi: 10.1111/bjh.18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klink AJ, Nabhan C, Lee CH, Laney J, Yang Y, Purdum AG, et al. Real-world management and outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma treated in the United States. J Clin Pathways. 2020;6(1):44–53. doi: 10.25270/jcp.2020.2.00112. [DOI] [Google Scholar]
- 19.Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014;3(1):66–70. doi: 10.4103/2278-330X.126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lekakis LJ, Moskowitz CH. The role of autologous stem cell transplantation in the treatment of diffuse large B-cell lymphoma in the era of CAR-T cell therapy. Hemasphere. 2019;3(6):e295. doi: 10.1097/HS9.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. 2018;31(3):209–216. doi: 10.1016/j.beha.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Vic S, Lemoine J, Armand P, Lemonnier F, Houot R. Transplant-ineligible but chimeric antigen receptor T-cells eligible: a real and relevant population. Eur J Cancer. 2022;175:246–253. doi: 10.1016/j.ejca.2022.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Graf SA, Cassaday RD, Morris K, Voutsinas JM, Wu QV, Behnia S, et al. Ibrutinib monotherapy in relapsed or refractory, transformed diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(3):176–181. doi: 10.1016/j.clml.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchings M. The evolving therapy of DLBCL: bispecific antibodies. Hematol Oncol. 2023;41(S1):107–111. doi: 10.1002/hon.3154. [DOI] [PubMed] [Google Scholar]
- 25.Borchmann P, Heger JM, Mahlich J, Papadimitrious MS, Riou S, Werner B. Survival outcomes of patients newly diagnosed with diffuse large B-cell lymphoma: real-world evidence from a German claims database. J Cancer Res Clin Oncol. 2023;149(10):7091–7101. doi: 10.1007/s00432-023-04660-y. [DOI] [PubMed] [Google Scholar]
- 26.Daneels W, Rosskamp M, Macq G, Saadoon EI, De Geyndt A, Offner F, et al. Real-world estimation of first- and second-line treatments for diffuse large B-cell lymphoma using health insurance data: a Belgian population-based study. Front Oncol. 2022;12:824704. doi: 10.3389/fonc.2022.824704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller S, Brandes A, Knierim J, Novakovic M, Wilke T, Maywald U, et al. Epidemiology, diagnostics, and treatment of narcolepsy in Germany: the DORMIO study. J Sleep Med. 2021;18(2):88–99. doi: 10.13078/jsm.210007. [DOI] [Google Scholar]
- 28.Pflichtversicherungsgrenze Versicherungspflichtgrenze GKV PKV. https://www.versicherungspflichtgrenzen.de/html/pflichtversicherungsgrenzen.html. Accessed 09 Jan 2024
- 29.GKV Spitzenverband. Statutory health insurance. https://www.gkv-spitzenverband.de/english/statutory_health_insurance/statutory_health_insurance.jsp. Accessed 09 Jan 2024
- 30.Chae JW, Song CS, Kim H, Lee KB, Seo BS, Kim DI. Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson Comorbidity Index using ICD-10 database. Nephron Clin Pract. 2011;117(4):c379–c384. doi: 10.1159/000321525. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Laliberté F, Germain G, Raut M, Duh MS, Sen SS, et al. Real-world characteristics, treatment patterns, health care resource use, and costs of patients with diffuse large B-cell lymphoma in the U.S. Oncologist. 2021;26(5):e817–e26. doi: 10.1002/onco.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenz G, Chapuy B, Glaß B, Keil F, Klapper W, Nickelsen M, et al (2021). Diffuses großzelliges B-Zell-Lymphom. https://www.onkopedia.com/de/onkopedia/guidelines/diffuses-grosszelliges-b-zell-lymphom/@@guideline/html/index.html. Accessed 13 Feb 2023
- 33.Jacob L, Kalder M, Kostev K. Decrease in the number of patients diagnosed with cancer during the COVID-19 pandemic in Germany. J Cancer Res Clin Oncol. 2022;148(11):3117–3123. doi: 10.1007/s00432-022-03922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mevius A, Müller S, Wilke T, Maywald U. The impact of COVID-19 pandemic on the detection and management of cancer: a German claims data analysis abstract EPH144. Value Health. 2022 doi: 10.1016/j.jval.2022.09.1065. [DOI] [Google Scholar]
- 35.Diamond A, Bensken WP, Vu L, Koroukian SM, Caimi PF. Outcomes and characteristics of untreated diffuse large B-cell lymphoma (DLBCL) patients: a SEER-medicare database analysis. Blood. 2022;140(Supplement 1):10979–10980. doi: 10.1182/blood-2022-170446. [DOI] [Google Scholar]
- 36.Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am J Hematol. 2021;96(5):617–629. doi: 10.1002/ajh.26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith A, Crouch S, Howell D, Burton C, Patmore R, Roman E. Impact of age and socioeconomic status on treatment and survival from aggressive lymphoma: a UK population-based study of diffuse large B-cell lymphoma. Cancer Epidemiol. 2015;39(6):1103–1112. doi: 10.1016/j.canep.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VFA. Klinische Studien: Langsam, bürokratisch. https://www.vfa.de/de/arzneimittel-forschung/klinische-studien/klinische-studien-langsam-buerokratisch. Accessed 09 Feb 2024
- 39.Pfreundschuh M, Trümper L, Österborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 40.Morrison VA, Shou Y, Bell JA, Hamilton L, Ogbonnaya A, Raju A, et al. Evaluation of treatment patterns and survival among patients with diffuse large B-cell lymphoma in the USA. Future Oncol. 2019;15(9):1021–1034. doi: 10.2217/fon-2018-0788. [DOI] [PubMed] [Google Scholar]
- 41.Jones G, Plastaras JP, Ng AK, Kelsey CR. The evolving role of radiation therapy in DLBCL: from early-stage to refractory disease. Oncology (Williston Park) 2022;36(12):718–727. doi: 10.46883/2022.25920980. [DOI] [PubMed] [Google Scholar]
- 42.Hopfinger G, Rupp B, Greil R. Barriers to patient access of CAR T cell therapies in Austria. memo Magaz Eur Med Oncol. 2023;16(1):79–90. [Google Scholar]
- 43.EMA (2022) Breyanzi. https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi. Accessed 13 Mar 2023
- 44.DRST (2022) Deutsches Register für Stammzelltransplantationen Jahresberichte. https://www.drst.de/drst/download.html. Accessed 13 Mar 2023
- 45.Frontzek F, Karsten I, Schmitz N, Lenz G. Current options and future perspectives in the treatment of patients with relapsed/refractory diffuse large B-cell lymphoma. Ther Adv Hematol. 2022;13:20406207221103321. doi: 10.1177/20406207221103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.EMA (2020) Polivy. https://www.ema.europa.eu/en/medicines/human/EPAR/polivy. Accessed 13 Apr 2023
- 47.EMA (2021) Minjuvi. https://www.ema.europa.eu/en/medicines/human/EPAR/minjuvi. Accessed 13 Apr 2023
- 48.Onkologie L (2022) Diagnostik, Therapie und Nachsorge für erwachsene Patient*innen mit einem diffusen großzelligen BZell-Lymphom und verwandten Entitäten. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/DLBCL/Version_1/LL_DLBCL_Langversion_1.0.pdf. Accssed 25 Apr 2023
- 49.Kumar AJ, Henzer T, Rodday AM, Parsons SK. Chemotherapy is administered to a minority of hospitalized patients with diffuse large B-cell lymphoma and is associated with less likelihood of death during hospitalization. Cancer Epidemiol. 2018;53:137–140. doi: 10.1016/j.canep.2018.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are abstracted from anonymized, individual patient records and are not publicly available due to contractual agreements with the sickness fund (AOK PLUS).