Abstract
Introduction
Fluorescence in situ hybridization (FISH) is an essential ancillary study used to identify clinically aggressive subsets of large B-cell lymphomas that have MYC, BCL2, or BCL6 rearrangements. Small-volume biopsies such as fine needle aspiration biopsy (FNAB) and core needle biopsy (CNB) are increasingly used to diagnose lymphoma and obtain material for ancillary studies such as FISH. However, the performance of FISH in small biopsies has not been thoroughly evaluated or compared to surgical biopsies.
Methods
We describe the results of MYC, BCL2, and BCL6 FISH in a series of 222 biopsy specimens, including FNAB with cell blocks, CNBs, and surgical excisional or incisional biopsies from 208 unique patients aggregated from 6 academic medical centers. A subset of patients had FNAB followed by a surgical biopsy (either CNB or excisional biopsy) obtained from the same or contiguous anatomic site as part of the same clinical workup; FISH results were compared for these paired specimens.
Results
FISH had a low hybridization failure rate of around 1% across all specimen types. FISH identified concurrent MYC and BCL2 rearrangements in 20 of 197 (10%) specimens and concurrent MYC and BCL6 rearrangements in 3 of 182 (1.6%) specimens. The paired FNAB and surgical biopsy specimens did not show any discrepancies for MYC or BCL2 FISH; of the 17 patients with 34 paired cytology and surgical specimens, only 2 of the 49 FISH probes compared (4% of all comparisons) showed any discrepancy and both were at the BCL6 locus. One discrepancy was due to necrosis of the CNB specimen causing a false negative BCL6 FISH result when compared to the FNAB cell block that demonstrated a BCL6 rearrangement.
Discussion
FISH showed a similar hybridization failure rate in all biopsy types. Ultimately, MYC, BCL2, or BCL6 FISH showed 96% concordance when compared across paired cytology and surgical specimens, suggesting FNAB with cell block is equivalent to other biopsy alternatives for evaluation of DLBCL or HGBCL FISH testing.
Keywords: diffuse large B-cell lymphoma, high-grade B-cell lymphoma, double-hit lymphoma, FISH, BCL2 rearrangement, MYC rearrangement
1. Introduction
An important subset of diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBCL) have MYC and BCL2 rearrangements Large B-cell lymphoma or high-grade B-cell lymphoma with MYC and BCL2 rearrangements are commonly referred to as "double-hit" lymphoma (DHL), which portends more aggressive clinical behavior and inferior progression-free survival compared to other cases of diffuse large B-cell lymphoma, germinal center B-cell subtype, or other high-grade B-cell lymphoma (1–5). Both the 5th Edition of the World Health Organization Classification of Haematolymphoid tumors (WHO5) (6) and the International Consensus Classification of Lymphoid Neoplasms (ICC) (7) now classify diffuse large B-cell lymphoma and high-grade B-cell lymphoma with concurrent MYC and BCL6 “double-hit” lymphomas separately due to the unclear prognostic significance of this combination, with some studies not showing distinct biology for these cases (1, 2), but other studies demonstrating an association with a poor outcome (3, 8–11). Fluorescence in situ hybridization (FISH) is a frequently used technique to detect MYC, BCL2, and BCL6 rearrangements. Because diffuse large B-cell lymphoma and other high-grade B-cell lymphoma can look identical morphologically to “double-hit” lymphoma, at a minimum MYC FISH must be obtained in every case (see Figure 1 for an example of a case that has similar morphology to diffuse large B-cell lymphoma, NOS on various slide stain preparations, but ended up having both MYC and BCL2 rearrangements). In one survey of cytogenetics laboratories, the most common test strategy (67%) was upfront testing of MYC, BCL2, and BCL6 FISH on every case while fewer labs (26%) performed BCL2 and BCL6 FISH testing only if MYC is rearranged (12). In the same survey study, 56% of laboratories performed MYC break-apart probe (BAP) in combination with IGH/MYC dual fusion probe while 43% of laboratories performed only MYC BAP. MYC and BCL2 IHC are known to be poor predictors of MYC rearrangements at the gene level and polymorphisms have even been shown to create false negative MYC IHC results (13). Data are less clear regarding MYC and BCL6 rearrangements, but many institutions continue to test for BCL6 rearrangements given some data indicating these cases may have an inferior outcome (3, 8–11).
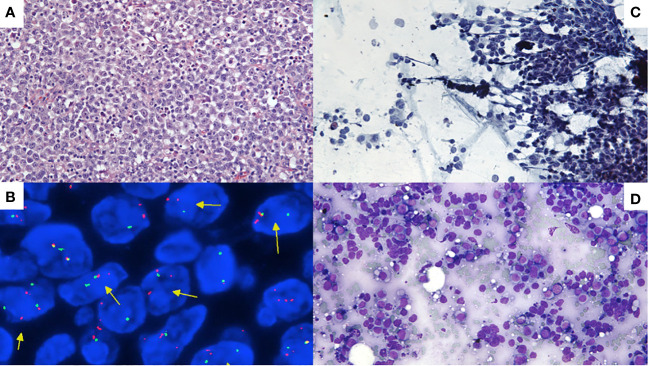
Figure 1.
(A) H&E-stained section of lymph node cell block demonstrating effacement by high-grade B-cell lymphoma with MYC and BCL2 rearrangements. (B) MYC interphase FISH showing separation of orange and green signals indicating a MYC rearrangement (see arrows). (C) Pap-stained smear slide showing aggregates of large atypical lymphoid cells. (D) May-Grünwald Giemsa (MGG)-stained smear slide of cytology smear slide similarly showing large cells. In general, Pap stains and MGG stains are complementary and helpful to obtain in all lymph node FNAB: Pap-stained slides demonstrate greater nuclear detail but make cells appear smaller and have poor cytoplasmic detail while May-Grünwald Giemsa stains show greater cytoplasmic detail and larger cell size but poor nuclear detail.
Fine needle aspiration biopsy (FNAB) is increasingly used for triage of lymphadenopathy and diagnosis of lymphoma. Additionally, cell blocks (CB) and smear slides from FNAB can effectively be used for FISH in various neoplasms (14–17). However, only a few, small single institutional studies have described FISH (such as MYC) in FNAB smears and cell block for lymphoma (18–21) and no comparisons to core biopsy or the gold standard excisional biopsy exist to our knowledge. We have two principal aims in this multi-institutional study of FISH performance in diffuse large B-cell lymphoma and high-grade B-cell lymphoma: 1) compare the success rate of MYC, BCL2, and BCL6 FISH hybridization across FNAB with cell block, core biopsy, and excisional biopsy, 2) compare FNAB to either core biopsy or excisional biopsy for FISH from the same patient.
2. Materials and methods
Six academic medical centers participated in this study. The medical centers were assigned data access groups in REDCap (see section below on REDCap) and they consisted of Massachusetts General Hospital (MGH), Memorial Sloan Kettering Cancer Center (MSKCC), San Francisco Veterans Administration Health Care System (SFVAHCS), Stanford, University of California San Francisco (UCSF), and University of Virginia (UVA).
A retrospective search was conducted of the pathology informatics systems with keywords “FISH” AND “diffuse large B-cell lymphoma” OR “high-grade B-cell lymphoma” over the 10-year period from 1/1/2010 to 12/31/2019. A complementary search was performed of cytogenetics lab data for all MYC FISH studies performed on cytology samples to catch cases missed by the pathology data system and then identify paired surgical samples through the pathology archive. Only specimens that had MYC FISH performed were included (n=222); of these cases, 200 had BCL2 FISH performed (90%) and 186 (84%) BCL6 FISH performed. Exclusion criteria included bone marrow biopsy specimens (due to alternative fixation used in some of these specimens and decalcification that could cause false negatives), and body fluid specimens (due to the numerous pre-analytic variables such as fixative type that might influence FISH performance in cell blocks). The age of the patient was recorded for each biopsy, but for Tables 1 , 2 , the age of the patient was determined as the age at the first biopsy specimen. The WHO4R classification terminology “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements” was originally used for “double hit” or “triple hit” cases in this patient biopsy cohort with an endpoint in 2019 irrespective of whether the morphology was more in keeping with diffuse large B-cell lymphoma or high-grade B-cell lymphoma (22). In keeping with the WHO5 and ICC, we subsequently distinguished MYC and BCL2 rearranged cases from MYC and BCL6 rearranged cases. This manuscript uses the terms high-grade B-cell lymphoma-MYC/BCL2 or high-grade B-cell lymphoma-MYC/BCL6 to distinguish these two groups regardless of whether the morphology was originally interpreted as diffuse large B-cell lymphoma or high-grade B-cell lymphoma.
Table 1.
Clinical characteristics of all 208 patients included in study.
| Number of patients | 208 |
|---|---|
| Age (mean (SD)) | 63.65 (16.55) |
| Gender = Male (%) | 123 (59.1) |
| Gender = Female (%) | 83 (40.3) |
| Data Access Group (%) | |
| MGH | 25 (12.0) |
| MSKCC | 13 (6.2) |
| SFVAHCS | 13 (6.2) |
| Stanford | 134 (64.4) |
| UCSF | 21 (10.1) |
| UVA | 2 (1.0) |
| History of B-cell non Hodgkin lymphoma? = Yes (%) | 81 (39.3) |
| Prior large B cell lymphoma diagnosis (%) | 23 (29.1) |
| DLBCL, NOS | 22 (95.6) |
| Prior follicular lymphoma diagnosis (%) | 52 (64.2) |
| Classic follicular lymphoma (grade 1–2) | 42 (80.8) |
| Classic follicular lymphoma (grade 3A) | 6 (11.5) |
| other or unknown | 2 (3.8) |
| Primary cutaneous follicle center lymphoma | 2 (3.8) |
| Prior diagnosis of low grade B cell lymphoma (%) | 13 (16.5) |
| CLL/SLL | 4 (30.8) |
| EMZL | 2 (15.4) |
| LPL | 2 (15.4) |
| Mantle cell lymphoma | 1 (7.7) |
| NMZL | 1 (7.7) |
| other or unknown | 1 (7.7) |
| Primary cutaneous MZL | 1 (7.7) |
| SMZL | 1 (7.7) |
| High-grade B-cell lymphoma diagnosis (%) |
4 (5.1) |
| Burkitt lymphoma | 1 (25.0) |
| High grade B-cell lymphoma, with MYC and BCL2 rearrangements | 1 (25.0) |
| High grade B-cell lymphoma, NOS | 2 (50.0) |
| Prior solid organ or stem cell transplant (%) |
16 (7.7) |
| Stem cell transplant | 9 (56.2) |
| Solid organ transplant | 7 (43.8) |
| Prior chemotherapy (%) | 52 (26.0) |
| Reason for chemotherapy (%) | |
| carcinoma | 4 (7.7) |
| carcinoma and lymphoma | 1 (1.9) |
| lymphoma | 46 (88.5) |
| other or unknown | 1 (1.9) |
The classification used for all diagnoses was the WHOR4, which is equivalent to WHO5 and ICC for these diagnoses. Abbreviations used: CLL/SLL (chronic lymphocytic leukemia/small lymphocytic lymphoma), EMZL (extranodal marginal zone lymphoma), LPL (lymphoplasmacytic lymphoma), NMZL (nodal marginal zone lymphoma), MZL (marginal zone lymphoma), SMZL (splenic marginal zone lymphoma), MGH (Massachusetts General Hospital), MSKCC (Memorial Sloan Kettering Cancer Center), SFVAHCS (San Francisco Veterans Administration Health Care System), UCSF (University of California San Francisco), UVA (University of Virginia).
Table 2.
Pathologic characteristics and FISH results of all 222 specimens compared across different biopsy types (fine needle aspiration biopsy, core needle biopsy, and excisional biopsy) with p values.
| Fine needle aspiration biopsy |
Core needle biopsy | Excisional biopsy | P value | |
|---|---|---|---|---|
| Number | 46 | 112 | 64 | |
| Age (mean (SD)) | 64.47 (15.70) | 65.00 (16.66) | 60.38 (15.81) | 0.178 |
| Gender (%) | 0.209 | |||
| Female | 19 (41.3) | 42 (37.5) | 31 (48.4) | |
| Male | 27 (58.7) | 70 (62.5) | 33 (51.6) | |
| Data Access Group (%) | <0.001 | |||
| MGH | 2 (4.3) | 19 (17.0) | 6 (9.4) | |
| MSKCC | 4 (8.7) | 12 (10.7) | 0 (0.0) | |
| SFVAHCS | 6 (13.0) | 6 (5.4) | 1 (1.6) | |
| Stanford | 20 (43.5) | 66 (58.9) | 54 (84.4) | |
| UCSF | 14 (30.4) | 7 (6.2) | 3 (4.7) | |
| UVA | 0 (0.0) | 2 (1.8) | 0 (0.0) | |
| Indication (%) | <0.001 | |||
| Additional diagnostic tissue | 1 (2.2) | 15 (13.4) | 27 (42.2) | |
| Additional tissue for ancillary studies |
0 (0.0) | 1 (0.9) | 2 (3.1) | |
| Clinical trial | 0 (0.0) | 1 (0.9) | 0 (0.0) | |
| Initial diagnosis | 27 (58.7) | 38 (33.9) | 11 (17.2) | |
| Other/unknown | 0 (0.0) | 2 (1.8) | 0 (0.0) | |
| R/o recurrence | 10 (21.7) | 19 (17.0) | 7 (10.9) | |
| R/o transformation | 7 (15.2) | 36 (32.1) | 15 (23.4) | |
| Staging | 1 (2.2) | 0 (0.0) | 2 (3.1) | |
| Tissue site (%) | 0.832 | |||
| Bone | 1 (2.2) | 6 (5.4) | 2 (3.1) | |
| Lymph node or related | 29 (63.0) | 60 (53.6) | 41 (64.1) | |
| Mediastinum | 1 (2.2) | 6 (5.4) | 2 (3.1) | |
| Organ | 7 (15.2) | 23 (20.5) | 7 (10.9) | |
| Other or unknown | 1 (2.2) | 2 (1.8) | 2 (3.1) | |
| Soft tissue | 7 (15.2) | 15 (13.4) | 10 (15.6) | |
| Final WHO diagnosis (%) | 0.206 | |||
| Burkitt | 1 (2.2) | 3 (2.7) | 1 (1.6) | |
| DLBCL | 31 (67.4) | 85 (75.9) | 57 (89.1) | |
| HGBCL | 4 (8.7) | 3 (2.7) | 2 (3.1) | |
| HGBCL-MYC/BCL2 | 7 (15.2) | 10 (8.9) | 2 (3.1) | |
| Other/unknown | 3 (6.5) | 8 (7.1) | 0 (0.0) | |
| PMLBCL | 0 (0.0) | 2 (1.8) | 1 (1.6) | |
| TCHRLBCL | 0 (0.0) | 1 (0.9) | 1 (1.6) | |
| MYC FISH (%) | 0.527 | |||
| negative | 33 (71.7) | 86 (76.8) | 55 (85.9) | |
| not done | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| positive | 11 (23.9) | 21 (18.8) | 7 (10.9) | |
| unsuccessful | 1 (2.2) | 3 (2.7) | 0 (0.0) | |
| variant 3’ signal loss | 1 (2.2) | 1 (0.9) | 1 (1.6) | |
| variant extra 5’ signaling | 0 (0.0) | 1 (0.9) | 0 (0.0) | |
| BCL2 FISH (%) | 0.274 | |||
| extra BCL2 signal on add(3) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| negative | 28 (60.9) | 60 (53.6) | 41 (64.1) | |
| not done | 6 (13.0) | 14 (12.5) | 2 (3.1) | |
| positive | 12 (26.1) | 36 (32.1) | 20 (31.2) | |
| unsuccessful | 0 (0.0) | 2 (1.8) | 0 (0.0) | |
| BCL6 FISH (%) | 0.718 | |||
| negative | 31 (67.4) | 70 (63.1) | 40 (62.5) | |
| not done | 7 (15.2) | 20 (18.0) | 9 (14.1) | |
| positive | 8 (17.4) | 18 (16.2) | 13 (20.3) | |
| unsuccessful | 0 (0.0) | 2 (1.8) | 0 (0.0) | |
| variant 5’ signal loss | 0 (0.0) | 1 (0.9) | 2 (3.2) |
CNB (core needle biopsy), DLBCL (diffuse large B-cell lymphoma), EB (excisional biopsy), FNAB (fine needle aspiration biopsy), HGBCL (high-grade B-cell lymphoma, NOS), HGBCL-MYC/BCL2 (high-grade B-cell lymphoma with MYC and BCL2 rearrangements), PMLBCL (primary mediastinal large B-cell lymphoma), TCHRLBCL (T-cell, histiocytic-rich large B-cell lymphoma), MGH (Massachusetts General Hospital), MSKCC (Memorial Sloan Kettering Cancer Center), SFVAHCS (San Francisco Veterans Administration Health Care System), UCSF (University of California San Francisco, UVA (University of Virginia).
All data extracted from pathology reports was entered into REDCap (Research Electronic Data Capture), a secure, encrypted online database. Study data were collected and managed using REDCap electronic data capture tools hosted at Stanford (23, 24). REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
At Stanford Cytogenetics Laboratory, FISH was performed on formalin-fixed, paraffin-embedded (FFPE) sections; the area of interest was circled on the corresponding H&E stained slide by the ordering pathologist. ZytoLight (ZytoVision Gmbh, Bremerhaven, Germany) break-apart probe sets were used for MYC, BCL2, and BCL6 FISH. The MYC probe set was used with orange probe 5’ to MYC and green probe 3’ to MYC on 8q24.21. BCL2 (18q21) probe set included 3’BCL2 in green and 5’BCL2 in orange. The same probe configuration was used for BCL6 (3q27): 3’BCL6 is green, 5’BCL6 is orange. All FISH results were scored in 100 interphase cells. A rearrangement was reported if 10% or more cells showed a split signal. The results of these FISH tests were compared to existing FISH results on a different specimen when available. Paired specimens were either obtained from the same anatomic site or from contiguous sites e.g. neck lymph node draining the thyroid or CNS lymphoma spreading to the eye.
At the MSKCC, FISH analyses for MYC, BCL2, and BCL6 (Abbott Molecular, Des Plaines, IL) were performed following a standard protocol, as previously described (25). The area of interest was usually circled on the slide, and the cytogenetics lab staff reviewed the slide to confirm a tumor-rich area was tested in all cases. For each probe set, 100 interphase cells were analyzed. A rearrangement was reported if 10% or more cells showed a split signal.
At UCSF, FISH analysis was performed on formalin-fixed paraffin-embedded (FFPE) tissue sections. The area of interest was circled by the ordering pathologist. Abbott Vysis Dual Color Break Apart probe sets (Des Plaines, IL) were used and FISH was set up according to the probe manufacturer’s instructions (https://www.molecular.abbott/int/en/vysis-fish-knowledge-center/fish-on-isolated-nuclei-from-paraffin). FISH signals were imaged and analyzed using MetaSystems software (Medford, MA). For each probe set, 50 interphase cells were analyzed. A MYC, BCL2, or BCL6 rearrangement was reported if 6.0% or more of cells showed split signals.
At SFVAHCS, MYC, BCL2, and BCL6 FISH were performed at Quest Diagnostics in San Juan Capistrano, CA. Because this was an external lab, the area to perform FISH was not specified or circled. FISH was performed using the probes specific for 3q27 (BCL6), 8q24.1 (MYC), 14q32.3 (IGH), and 18q21.1 (BCL2) [Abbott Molecular and SureFISH, Agilent DAKO]. The cutoff values for BCL6 rearrangement, MYC rearrangement, and t(14;18) in the paraffin-embedded tumor tissue are 11%, 15%, and 3%. For each probe set, 100 cells were analyzed.
At MGH, FISH was performed on 5-micron sections of FFPE tissue; an H&E section was reviewed to select regions for hybridization that contained a majority of tumor cells. Break-apart probes (MYC: Vysis LSI MYC Dual Color, Break Apart Rearrangement Probe; BCL2: Leica Kreatech BCL2 Proximal Green [18Q001B495] and BCL2 Distal Red [18Q002B550) probes]; BCL6: Leica Kreatech BCL6 Proximal Green [03Q008B495] and BCL6 Distal Red [03Q007B550] probes) were hybridized and used to calculate the number of cells out of 50 scored containing a rearrangement. A rearrangement was reported if more than 15% of cells showed split signals.
3. Results
A total of 222 specimens from 208 unique patients were identified with a large/high-grade B-cell lymphoma diagnosis and performance of either MYC, BCL2, or BCL6 FISH. The clinical characteristics of all patients are described in Table 1 . The pathologic characteristics of all specimens are compared across different specimen types and corresponding p values in Table 2 . The final large B-cell lymphoma diagnosis was established after FISH resulted e.g. high-grade B-cell lymphoma with MYC and BCL2 rearrangements or Burkitt lymphoma; the breakdown of different diagnoses can be seen under the header “Final WHO Diagnosis” in Table 2 . A breakdown of the diagnoses in our cohort is shown in Figure 2 .
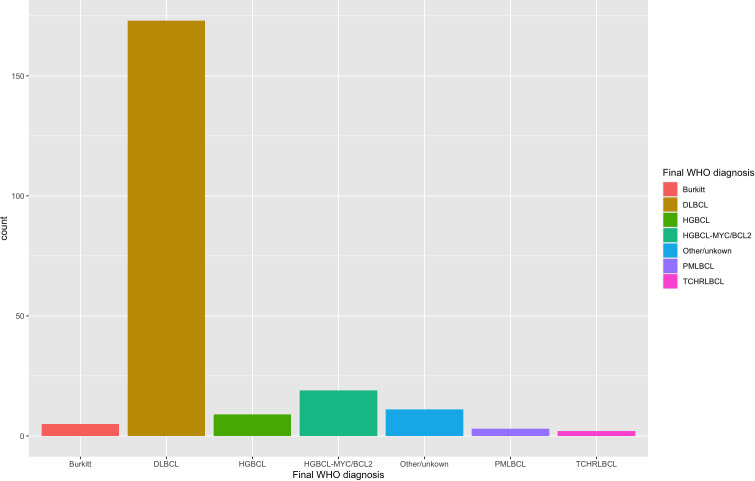
Figure 2.
Diagnosis of all large B-cell lymphoma specimens with MYC, BCL2, and/or BCL6 FISH performed. The diagnosis was retrieved retrospectively with FISH results already incorporated. Most cases were diffuse large B-cell lymphoma (DLBCL) (n=173). High-grade B-cell lymphoma with MYC and BCL2 rearrangements (HGBCL-MYC/BCL2) (n=19) and high-grade B-cell lymphoma, NOS (HGBCL) (n=9) were smaller subsets. Burkitt lymphoma (n=5), primary mediastinal large B-cell lymphoma (PMLBCL; n=3) and T-cell, histiocyte-rich large B-cell lymphoma (TCHRLBCL; n=2) were rare in this cohort.
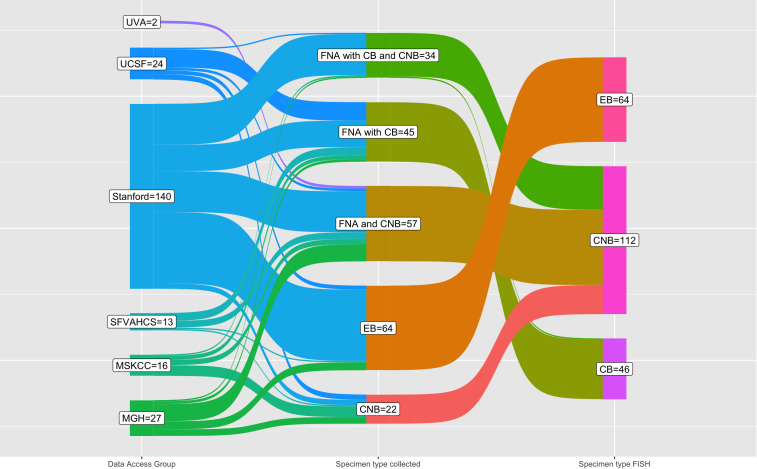
The breakdown of biopsy specimens by data access group (assigned to academic medical centers as previously described), specimen type collected, and specimen type that had FISH performed is shown by a Sankey diagram in Figure 3 . Despite the diversity and complexity of some specimen types, for instance, FNAB with cell block and core biopsy with varying degrees of imaging guidance, only three specimen types ultimately had FISH performed: cell block from FNAB, core biopsy, and surgical excisional or incisional biopsy. Most specimens with both a cell block from FNAB and core biopsy had FISH performed on the core biopsy with one specimen having FISH performed on the cell block. Most specimens undergoing FISH were lymph nodes (n=129), followed by non-nodal sites such as solid organs (n=37) or soft tissue (n=32), among others (see Table 2 ). Of the lymph nodes, most were cervical (n=37), retroperitoneal (n=21), inguinal (n=18), or axillary (n=15). All the indications for biopsy are shown in Table 2 ; the four most common indications were initial diagnosis (n=76), obtaining additional diagnostic tissue (n=43), evaluating for recurrence (n=36), and ruling out transformation (n=58).
Figure 3.
Sankey diagram of specimen type collected and specimen type undergoing FISH testing by institution (data access group). Of all the 222 specimens that underwent FISH testing in this cohort (right column), the majority are core biopsy, followed by excisional biopsy, and cell block. The core biopsy that have FISH performed include, of course, core biopsy only specimens but also FNAB with core biopsy and all FNA with cell block and core biopsy. One FNA with cell block and core biopsy had FISH performed on the cell block due to decalcification of the core biopsy (bone specimen). Abbreviations used: FNA (fine needle aspiration), CB (cell block from FNA), core biopsy (core needle biopsy), EB (excisional biopsy), MGH (Massachusetts General Hospital), MSKCC (Memorial Sloan Kettering Cancer Center), SFVAHCS (San Francisco Veterans Administration Health Care System), UCSF (University of California San Francisco), UVA (University of Virginia).
FISH detected a MYC rearrangement in 39 of 217 specimens successfully tested (18%), no rearrangement in 174 specimens, 4 variant MYC FISH results (three with 3’signal loss and one with extra 5’ signaling), and 4 unsuccessful hybridizations ( Figure 4 ). One specimen was not evaluated for MYC FISH but was evaluated for BCL2 and BCL6 FISH; this specimen was included in this study because the patient had a prior biopsy in this cohort that was evaluated for a MYC rearrangement, and the additional biopsy was to obtain more tissue for ancillary testing. FISH detected BCL2 rearrangements in 68 of 198 specimens successfully tested (34%), did not show a rearrangement in 129 specimens, 1 extra signal BCL2 FISH result on an add(3), 2 unsuccessful hybridizations and 22 specimens without any BCL2 FISH performed ( Figure 5 ). BCL2 rearrangements were detected in 20 of 37 MYC rearranged cases (54%), and these “double-hit” lymphomas are described in the next paragraph. FISH detected BCL6 rearrangements in 39 of 183 specimens successfully tested (21%), did not show a rearrangement in 141 specimens, 3 specimens with variant 5’ BCL6 signal loss patterns, 2 unsuccessful hybridizations, and 36 specimens without any BCL6 FISH performed. The hybridization failure rate was low for MYC (4 of 221 or 1.8%), BCL2 (2 of 200 or 1.0%), and BCL6 (2 of 185 or 1.1%) probes. Of the 8 unsuccessful hybridizations, 5 (63%) showed tissue limitations such as crush artifact, fibrosis, necrosis, and paucicellularity. FISH results across different biopsy types are shown in Table 2 .
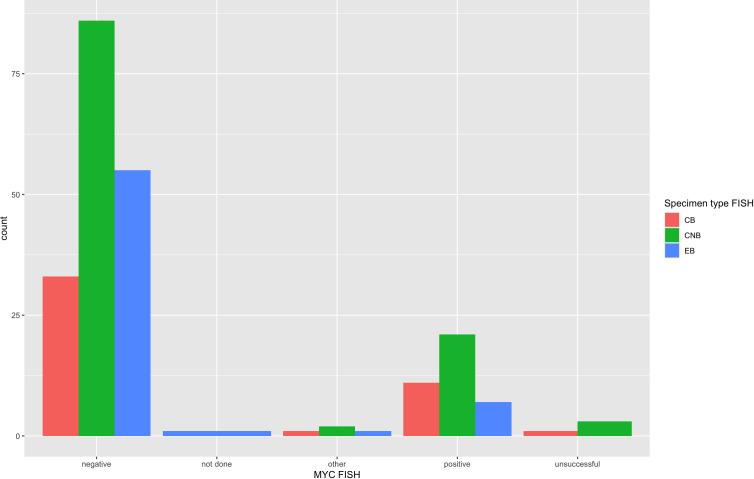
Figure 4.
MYC FISH results across specimen types. MYC FISH results from all 222 specimens are arranged per specimen type that had FISH performed. MYC was rearranged in 18% of specimens and showed 4 “other” variant MYC FISH results, which included 3 specimens with 3’ signal loss and 1 with extra 5’ signaling. The unsuccessful or hybridization failure rate was overall low at 1.8% and similar across specimen types.
Figure 5.
BCL2 FISH results across specimen types. BCL2 FISH results from all 222 specimens are arranged per specimen type that had FISH performed. BCL2 was rearranged in 34% of cases by FISH and showed 1 “other” variant result with 1 extra signal BCL2 FISH result on an add(3). The unsuccessful or hybridization failure rate was low at 1.0% and similar across specimen types.
Concurrent MYC and BCL2 rearrangements were detected in 20 of the 197 specimens (10%) that had FISH successfully performed at both MYC and BCL2 loci; for 19 of these 20 specimens, this result established the diagnosis as high-grade B-cell lymphoma with MYC and BCL2 rearrangements (high-grade B-cell lymphoma-MYC/BCL2). One case was indeterminate due to the morphologic differential diagnosis of high-grade B-cell lymphoma-MYC/BCL2 versus follicular lymphoma with MYC and BCL2 rearrangements, which is not regarded as equivalent to “double-hit” lymphoma according to WHO5/ICC. Of note, 4 additional cases (4 of 197 or 2%) had variant MYC FISH results, including 3 with 3’ signal loss and 1 with extra 5’ signaling. All four of these cases with variant MYC FISH results also had BCL2 rearrangements, raising the possibility of high-grade B-cell lymphoma with MYC and BCL2 rearrangements, without a definitive diagnosis. Of the “double-hit” lymphoma cases, 7 had FISH performed on FNA cell blocks, 10 had FISH on core biopsies, and 2 had FISH on the excisional biopsy. The clinical indications for these biopsy specimens were as follows: 7 for ruling out transformation, 7 for initial diagnosis, 4 for ruling out recurrence, and 1 for additional diagnostic tissue. Of note, one of the 19 “double-hit” lymphoma cases showed rearrangements with all three FISH probes MYC, BCL2, and BCL6, which falls in the same diagnostic category as cases with MYC and BCL2 rearrangements only but has been called “triple-hit” lymphoma in the literature. Three specimens of 182 (1.6%) showed MYC and BCL6 rearrangements, which according to the ICC, is diagnostic of the provisional entity high-grade B-cell lymphoma with MYC and BCL6 rearrangements. The WHO5 classification does not recognize this provisional entity but addresses the need for additional data in this patient group.
Of the 222 specimens with FISH performed, 16 paired cytology and surgical specimens were identified from 8 different patients (each patient had exactly one set of paired specimens). An additional 9 patients with paired cytology and surgical biopsy specimens were identified at one institution (Stanford) that had FISH performed on only one set of the pair; completion FISH was performed at Stanford on a research basis on the specimen missing FISH to enlarge the paired specimen cohort. In total, 34 paired specimens were analyzed from 17 patients ( Table 3 ). The 17 cytology cases included 13 FNAB with cell block and 4 FNAB with cell block and core biopsy (listed as core biopsy in Table 3 , because FISH was performed on the core biopsy). The 17 surgical cases were 10 excisional biopsies, 6 core biopsies, and 1 small bowel resection. Of the 102 possible FISH tests (3 different FISH loci across 34 paired specimens), MYC, BCL2, or BCL6 FISH was performed in 100 instances (98%). Patient 13 only had BCL2 FISH performed in both specimens and thus MYC and BCL6 FISH results were not comparable ( Table 3 ). Out of the 49 comparisons drawn between these 98 paired FISH tests, 2 comparisons (4%) were discrepant: both showed a BCL6 rearrangement in the FNA-cell block specimen but not in the paired excisional biopsy from patient 3 or core biopsy from patient 10 (see Table 3 ). No MYC or BCL2 rearrangements were present in any of the discrepant samples. The date of specimens was closely matched for the discrepant pairs, including differences of 5 days and 10 days between the acquisition of the cytology specimen and the surgical specimen. Both patients had the same lymph node sampled by FNA and excisional biopsy or core biopsy. For patient 10, necrosis was noted in the core biopsy with the negative BCL6 FISH.
Table 3.
FISH results from 17 paired FNAB or core biopsy and surgical samples are displayed.
| Patient | Biopsy 1 | Biopsy 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| site | Biopsy type | MYC | BCL2 | BCL6 | site | Biopsy type | MYC | BCL2 | BCL6 | |
| 1 | Left axillary | FNA-CB | 1 | 0 | 0 | Lymph nodes (Floor of mouth) | EB | 1 | 0 | 0 |
| 2 | Lymph node, left supraclavicular | FNA-CB | 0 | 1 | 0 | Left supraclavicular mass | EB | 0 | 1 | 0 |
| 3 | Lymph node, left neck | FNA-CB | 0 | 0 | 1 | Left neck node | EB | 0 | 0 | 0 |
| 4 | Lymph node, left neck level 2 | FNA-CB | 0 | 0 | 0 | Neck level 2 lymph nodes | EB | 0 | 0 | 0 |
| 5 | Right cervical level 2 lymph node | FNA-CB | 0 | 0 | 0 | Lymph node, right neck | EB | 0 | 0 | 0 |
| 6 | Left neck lymph node | FNA-CB | 0 | 0 | 0 | Lymph node, left neck | EB | 0 | 0 | 0 |
| 7 | Left thyroid | FNA-CB | 1 | 0 | 1 | Left level 5 lymph nodes | EB | 1 | 0 | 1 |
| 8 | Lymph node, axillary | FNA-CB | 1 | 0 | 0 | Lymph node, cervical | EB | 1 | 0 | 0 |
| 9 | Vitreous | FNA-CB | 0 | 0 | 0 | Brain | EB | 0 | 0 | 0 |
| 10 | Lymph node, axillary | FNA-CB | 0 | 0 | 1 | Lymph node, axillary | CNB | 0 | 0 | 0 |
| 11 | Lymph node, inguinal | FNA-CB | 0 | 0 | 0 | Lymph node, inguinal | CNB | 0 | 0 | 0 |
| 12 | Bone | FNA-CB | 1 | 1 | 0 | Bone | CNB | 1 | 1 | 0 |
| 13 | Soft tissue, other or unknown | FNA-CB | 0 | 1 | 0 | Chest wall | CNB | ND | 1 | ND |
| 14 | Right lower quadrant abdomen | CNB | 1 | 0 | 0 | Small Bowel | Resection | 1 | 0 | 0 |
| 15 | Pelvis | CNB | 0 | 0 | 0 | Iliac lymph node biopsy | EB | 0 | 0 | 0 |
| 16 | Mesentery | CNB | 0 | 1 | 0 | Lymph node, left mesenteric | CNB | 0 | 1 | 0 |
| 17 | Left abdominal wall | CNB | 0 | 0 | 0 | Left abdominal mass | CNB | 0 | 0 | 0 |
FISH results are reported qualitatively as 0 (negative), 1 (positive), or ND (not done). FISH results are colored green for concordance between small volume biopsy and surgical, red for discordance, and yellow for indeterminate because both specimens were not tested for the same probe. All cases are matched for anatomic site. Two discrepancies were noted between an FNA with cell block (FNA-CB) and surgical case (see BCL6 FISH result for patient 3 and 10); the discrepancy for patient 10 is likely attributable to necrosis noted on the core biopsy specimen causing a false negative FISH result.
FNAB-CB (fine needle aspiration biopsy with cell block), CNB (core needle biopsy), EB (excisional biopsy).
4. Discussion
4.1. MYC, BCL2, and BCL6 FISH demonstrate highly successful hybridization rates across all specimen types with no statistically significant difference noted between FNAB, core biopsy, and surgical excisions. Tissue limitations may explain the rare failures
The FISH failure rate was low for all three FISH probes: 1.8% for MYC (4 of 220), 1.0% for BCL2 (2 of 208), and 1.1% for BCL6 (2 of 187). The hybridization rate is essentially the same across small-volume specimens such as FNAB cell block and core biopsy and larger-volume specimens such as an excisional biopsy with a statistically non-significant p-value across all three specimen types (see Table 2 ). Figures 4 , 5 also demonstrate the breakdown of MYC and BCL2 FISH results across various specimen types and graphically show that hybridization failure rates are low and similar across specimen types. The 8 FISH probe failures were from 6 specimens (one case had failure at all three FISH probes). These 6 specimens consisted of 5 core biopsies (all of which also had FNABs and one of which also had a cell block) and 1 cell block from FNAB. Five of the 6 specimens with failed FISH attempts were noted to have tissue limitations such as crush artifact, fibrosis, necrosis, and paucicellularity, any or all of which may partially explain why these cases had probe hybridization failure.
4.2. High-grade B-cell lymphoma with MYC and BCL2 rearrangements was identified across all specimen types
MYC FISH detected a rearrangement in 18% of specimens, which is similar to prior series (1, 8, 9, 26, 27). High-grade B-cell lymphoma with MYC and BCL2 rearrangements comprised 9% of all large B-cell lymphoma specimens in our cohort, typical of the 8–10% rate reported in the literature (10, 28). Identification of this subset is critical due to the more aggressive clinical behavior that prompts more aggressive therapy. Figure 1 illustrates images from a case that was called diffuse large B-cell lymphoma at diagnosis but was later refined to HGBL-MYC/BCL2 based on FISH results; the images demonstrate three preparations routinely made for FNAB samples of lymph nodes in many cytopathology practices—H&E-stained section of cell block, Pap-stained smear slide (alcohol fixed), and May-Grünwald Giemsa-stained slide (air dried). MYC break-apart FISH was performed on the cell block of this FNAB specimen and is depicted. Morphology and even immunohistochemistry are poor predictors of high-grade B-cell lymphoma-MYC/BCL2, and FISH or other comparable fusion detection assay such as targeted or whole genome next generation sequencing (12, 29, 30), RNA based sequencing (31), or integrated DNA/RNA sequencing (32) must be performed to identify this important subset of large B-cell lymphoma (28).
Rare variant signal patterns were found with both MYC and BCL2 FISH probes, including signal loss and gain. Our cohort includes 3 patients with variant 3’MYC loss and 1 patient with 5’MYC poly-signaling; all the patients in our cohort with variant MYC signaling had concurrent BCL2 rearrangements, raising the possibility of whether these were “double-hit” lymphoma. In general, a scarcity of literature and clinical outcomes about these rare cases exists (33). Copy number variations of MYC and BCL2 have been previously shown to have different biology than structural rearrangements of both genes (13). Another study of variant MYC translocations in aggressive B-cell lymphomas found patients with 5’MYC gain were more refractory to chemotherapy or had an early relapse with a median event-free survival of only 6 months compared to patients with 3’MYC deletion who often responded to chemotherapy and had an event-free survival of 24 months (34). This study suggested based on survival data and the presence of IGH/MYC fusions or other IGK, IGL rearrangements in a subset that 5’MYC gain likely represents an unbalanced MYC rearrangement whereas the 3’MYC deletions were likely unrelated to MYC rearrangement. Based on this data, our cases with 3’MYC signal loss should be excluded from the “double-hit” lymphoma category. This data also suggests our 5’MYC gain case could be included in “double-hit” lymphoma, but given the overall lack of data and consensus in the literature at this point, the 5’MYC gain case was not included in the “double-hit” lymphoma category for our study.
High-grade B-cell lymphoma with MYC and BCL6 rearrangements was much less common in our cohort with 3 cases out of 182 specimens rearranged at both loci (1.6%). As previously mentioned, the significance of these cases is currently controversial. Some studies have not shown distinct biology for these cases (1, 2), but other studies have found an association with a poor outcome (3, 8–11). An additional 3 specimens show variant BCL6 rearrangements, all 5’BCL6 signal loss, but these specimens were MYC FISH negative. Additional studies are needed to further clarify the biology, clinical outcomes, and significance of these cases.
FISH may fail to identify a subset of diffuse large B-cell lymphoma and high-grade B-cell lymphoma that have inferior clinical outcomes. Gene expression profiling of germinal center B-cell diffuse large B-cell lymphoma can identify a double hit-like signature and inferior outcomes, but only half of these cases have structural rearrangements that can be detected by routine MYC and BCL2 break-apart FISH (5). Whole genome sequencing of these cases revealed cryptic MYC and BCL2 rearrangements, copy number gains and amplifications of MYC and MIR17HG, and focal deletions of the PVT1 promoter (5). While other technologies in the future may more effectively detect biologically equivalent “double-hit” lymphoma, FISH currently remains the current clinical gold standard for detecting “double-hit” lymphoma.
4.3. Paired specimens demonstrate 96% concordance with MYC, BCL2, and BCL6 FISH results across FNABs, core biopsies, and excisional biopsies
When matched for anatomic site and tissue limitations, paired cytology and surgical specimens in our study showed 96% concordance for FISH results ( Table 3 ). Two FISH discrepancies were found and both showed the following pattern: BCL6 rearrangement was detected in the FNAB while no BCL6 rearrangement was detected in the paired surgical specimen. Necrosis was noted in the core biopsy from patient 10, and this core biopsy yielded a negative BCL6 result, suggesting that this may be a false negative result. Because no MYC rearrangements were present in any of the discrepant samples, the diagnosis would not have changed whether a BCL6 rearrangement was or was not present.
Overall, this paired data suggests that FNAB cell block is a reasonable alternative to core biopsy or even excisional biopsy for diffuse large B-cell lymphoma and high-grade B-cell lymphoma FISH testing. No MYC or BCL2 FISH discrepancies were found in any pair and, therefore, assessment for “double-hit” lymphoma would not have changed. The only discrepancies between paired samples were at the BCL6 FISH locus; one of these discrepancies ultimately was attributed to a confounding variable described above.
A major limitation of this study is the heterogeneity of this retrospective and multi-institutional data set, which may limit applicability to some cytogenetic labs and pathology practice settings. Each institution used a different FISH lab with different probe sets and acquisition systems, different split signal thresholds for establishing the presence of a rearrangement, different numbers of interphase cells analyzed, and so on; these differences are reflected in the methods section. The biopsy specimens also have variable indications, which range from initial diagnosis to recurrence or transformation in the post-therapy setting. The data was collected from academic medical centers with highly specialized proceduralists and pathologists subspecializing in cytopathology and hematopathology, which may limit applicability to the community practice setting.
5. Conclusions
MYC, BCL2, and BCL6 FISH have highly successful hybridization rates that are similar across different specimen types in this cohort, including FNAB, core biopsy, and excisional biopsy. High-grade B-cell lymphoma with MYC and BCL2 rearrangements was detected in 9% of all large B-cell lymphoma specimens, including one case with rearrangements at all three loci MYC, BCL2, and BCL6; MYC and BCL6 rearrangements were found in 1.6% of specimens. No significant difference was found across biopsy types. Paired cytology and surgical specimens demonstrated 96% concordance at all three MYC, BCL2, and BCL6 FISH loci. FNAB with cell block is an equally effective alternative to core biopsy and excisional biopsy for assessment of MYC, BCL2, and BCL6 FISH, which is required for identification of the clinically aggressive subset of large B-cell lymphomas that carry both MYC and BCL2 rearrangements.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Stanford University IRB protocol 38992. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JM: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. UA: Writing – review & editing, Writing – original draft, Methodology, Data curation. CB: Writing – review & editing, Writing – original draft, Methodology, Data curation. SC: Data curation, Methodology, Writing – original draft, Writing – review & editing. SG: Data curation, Project administration, Writing – original draft, Writing – review & editing. RH: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. CK: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. OL: Data curation, Supervision, Writing – original draft, Writing – review & editing, Conceptualization. SL: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. AL: Writing – review & editing, Writing – original draft, Supervision, Data curation, Conceptualization. JM: Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. YN: Supervision, Writing – original draft, Writing – review & editing. RR: Writing – original draft, Writing – review & editing. ES: Writing – review & editing, Writing – original draft, Methodology, Investigation. JY: Data curation, Writing – original draft, Writing – review & editing. SZ: Data curation, Writing – original draft, Writing – review & editing. DG: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Acknowledgments
The R code for statistical analysis was built on several packages such as R-package ggsankey by David Sjoberg, ggplot2 by Hadley Wickham, table one by Kazuki Yoshida. These R packages were invaluable for statistical analysis of this data set.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. REDCap was funded by Stanford CTSA award number UL1 TR001085 from NIH/NCRR. Value-Based Award in Pathology from Stanford Healthcare supported the additional FISH testing and data entry at Stanford. The work at Memorial Sloan Kettering Cancer Center was funded by Grant/Award Number P30CA008748.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Cucco F, Barrans S, Sha C, Clipson A, Crouch S, Dobson R, et al. Distinct genetic changes reveal evolutionary history and heterogeneous molecular grade of DLBCL with MYC/BCL2 double-hit. Leukemia. (2020) 34:1329–41. doi: 10.1038/s41375-019-0691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evrard SM, Péricart S, Grand D. Targeted next generation sequencing reveals high mutation frequency of CREBBP, BCL2 and KMT2D in high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements. Haematologica. (2019) 104(4):e154–7. doi: 10.1016/j.morpho.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ennishi D, Jiang A, Boyle M, Collinge B, Grande BM, Ben-Neriah S, et al. Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. JCO. (2019) 37:190–201. doi: 10.1200/JCO.18.01583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sha C, Barrans S, Cucco F, Bentley MA, Care MA, Cummin T, et al. Molecular high-grade B-cell lymphoma: defining a poor-risk group that requires different approaches to therapy. JCO. (2019) 37:202–12. doi: 10.1200/JCO.18.01314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilton LK, Tang J, Ben-Neriah S, Alcaide M, Jiang A, Grande BM, et al. The double-hit signature identifies double-hit diffuse large B-cell lymphoma with genetic events cryptic to FISH. Blood. (2019) 134:1528–32. doi: 10.1182/blood.2019002600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBDO, Berti E, et al. The 5th edition of the world health organization classification of hematolymphoid tumors: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. (2022) 140:1229–53. doi: 10.1182/blood.2022015851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S, Desai P, Lin P, Yin CC, Tang G, Wang XJ, et al. MYC / BCL 6 double-hit lymphoma ( DHL ): a tumor associated with an aggressive clinical course and poor prognosis. Histopathology. (2016) 68:1090–8. doi: 10.1111/his.12884 [DOI] [PubMed] [Google Scholar]
- 9. Ye Q, Xu-Monette ZY, Tzankov A, Deng L, Wang X, Manyam GC, et al. Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B-cell lymphoma. Oncotarget. (2016) 7:2401–16. doi: 10.18632/oncotarget.6262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenwald A, Bens S, Advani R, Barrans S, Copie-Bergman C, Elsensohn MH, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: A study by the lunenburg lymphoma biomarker consortium. JCO. (2019) 37:3359–68. doi: 10.1200/JCO.19.00743 [DOI] [PubMed] [Google Scholar]
- 11. Pillai RK, Sathanoori M, Van Oss SB, Swerdlow SH. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. Am J Surg Pathol. (2013) 37:323–32. doi: 10.1097/PAS.0b013e31826cebad [DOI] [PubMed] [Google Scholar]
- 12. Gagnon MF, Penheiter AR, Harris F, Sadeghian D, Johnson SH, Karagouga G, et al. Unraveling the genomic underpinnings of unbalanced MYC break-apart FISH results using whole genome sequencing analysis. Blood Cancer J. (2023) 13:190. doi: 10.1038/s41408-023-00967-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collinge B, Ben-Neriah S, Chong L, Boyle M, Jiang A, Miyata-Takata T, et al. The impact of MYC and BCL2 structural variants in tumors of DLBCL morphology and mechanisms of false-negative MYC IHC. Blood. (2021) 137(16):2196–208. doi: 10.1182/blood.2020007193 [DOI] [PubMed] [Google Scholar]
- 14. Aisner DL, Sams SB. The role of cytology specimens in molecular testing of solid tumors: Techniques, limitations, and opportunities. Diagn Cytopathol. (2012) 40:511–24. doi: 10.1002/dc.22820 [DOI] [PubMed] [Google Scholar]
- 15. Aisner DL, Rumery MD, Merrick DT, Kondo KL, Nijmeh H, Linderman DJ, et al. Do more with less: tips and techniques for maximizing small biopsy and cytology specimens for molecular and ancillary testing: the university of colorado experience. Arch Pathol Lab Med. (2016) 140:1206–20. doi: 10.5858/arpa.2016-0156-RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. (2014) 25:356–71. doi: 10.1111/cyt.12174 [DOI] [PubMed] [Google Scholar]
- 17. Darras N, Mooney KL, Long SR. Diagnostic utility of fluorescence in situ hybridization testing on cytology cell blocks for the definitive classification of salivary gland neoplasms. J Am Soc Cytopathol. (2019) 8:157–64. doi: 10.1016/j.jasc.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 18. Mayall F. Fine needle aspiration cytology in the diagnosis of uncommon types of lymphoma. J Clin Pathol. (2003) 56:821–5. doi: 10.1136/jcp.56.11.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monaco SE, Teot LA, Felgar RE, Surti U, Cai G. Fluorescence in situ hybridization studies on direct smears: An approach to enhance the fine-needle aspiration biopsy diagnosis of B-cell non-Hodgkin lymphomas. Cancer Cytopathol. (2009) 117:338–48. doi: 10.1002/cncy.20040 [DOI] [PubMed] [Google Scholar]
- 20. Zhang S, Abreo F, Lowery-Nordberg M, Veillon DM, Cotelingam JD. The role of fluorescence in situ hybridization and polymerase chain reaction in the diagnosis and classification of lymphoproliferative disorders on fine-needle aspiration. Cancer Cytopathol. (2010) 118:105–12. doi: 10.1002/cncy.20070 [DOI] [PubMed] [Google Scholar]
- 21. Karabachev AD, Brundage WJ, Sajisevi MB, Ciolino AL. Feasibility of fine needle aspiration for diagnosis of b-cell lymphoma of the thyroid: a case series and review of the literature. Diagn Pathol. (2023) 18:69. doi: 10.1186/s13000-023-01346-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Informatics. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang SR, Aypar U, Rosen EY, Mata DA, Benayed R, Mullaney K, et al. A performance comparison of commonly used assays to detect RET fusions. Clin Cancer Res. (2021) 27:1316–28. doi: 10.1158/1078-0432.CCR-20-3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ennishi D, Mottok A, Ben-Neriah S, Shulha HP, Farinha P, Chan FC, et al. Genetic profiling of MYC and BCL2 in diffuse large B-cell lymphoma determines cell-of-origin–specific clinical impact. Blood. (2017) 129:2760–70. doi: 10.1182/blood-2016-11-747022 [DOI] [PubMed] [Google Scholar]
- 27. Künstner A, Witte HM, Riedl J, Bernard V, Stölting S, Merz H, et al. Mutational landscape of high-grade B-cell lymphoma with MYC-, BCL2 and/or BCL6 rearrangements characterized by whole-exome sequencing. haematol. (2021) 107:1850–63. doi: 10.3324/haematol.2021.279631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. (2018) 131:2060–4. doi: 10.1182/blood-2017-12-820605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chong LC, Ben-Neriah S, Slack GW, Freeman C, Ennishi D, Mottok A, et al. High-resolution architecture and partner genes of MYC rearrangements in lymphoma with DLBCL morphology. Blood Adv. (2018) 2:2755–65. doi: 10.1182/bloodadvances.2018023572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. (2018) 24:679–90. doi: 10.1038/s41591-018-0016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X, Johnson V, Johnson L, Cook JR. RNA-Based next generation sequencing complements but does not replace fluorescence in situ hybridization studies for the classification of aggressive B-Cell lymphomas. Cancer Genet. (2021) 252–253:43–7. doi: 10.1016/j.cancergen.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 32. Cassidy DP, Chapman JR, Lopez R, White K, Fan YS, Casas C, et al. Comparison between integrated genomic DNA/ RNA profiling and fluorescence in situ hybridization in the detection of MYC, BCL-2, and BCL-6 gene rearrangements in large B-cell lymphomas. Am J Clin Pathol. (2020) 153(3):353–9. doi: 10.1093/ajcp/aqz172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gagnon MF. MYC break-apart FISH probe set reveals frequent unbalanced patterns of uncertain significance when evaluating aggressive B-cell lymphoma. Blood Cancer J. (2021) 11(11):184. doi: 10.1038/s41408-021-00578-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang G, Li S, Toruner GA, Jain P, Tang Z, Hu S, et al. Clinical impact of 5 MYC or 3 MYC gain/loss detected by FISH in patients with aggressive B-cell lymphomas. Cancer Genet. (2023) 272-273:1–8. doi: 10.1016/j.cancergen.2022.12.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.