Abstract
Human CD81 has been previously identified as the putative receptor for the hepatitis C virus envelope glycoprotein E2. The large extracellular loop (LEL) of human CD81 differs in four amino acid residues from that of the African green monkey (AGM), which does not bind E2. We mutated each of the four positions in human CD81 to the corresponding AGM residues and expressed them as soluble fusion LEL proteins in bacteria or as complete membrane proteins in mammalian cells. We found human amino acid 186 to be critical for the interaction with the viral envelope glycoprotein. This residue was also important for binding of certain anti-CD81 monoclonal antibodies. Mutating residues 188 and 196 did not affect E2 or antibody binding. Interestingly, mutation of residue 163 increased both E2 and antibody binding, suggesting that this amino acid contributes to the tertiary structure of CD81 and its ligand-binding ability. These observations have implications for the design of soluble high-affinity molecules that could target the CD81-E2 interaction site(s).
CD81 is a member of the tetraspanin family. Tetraspanins are membrane proteins containing four transmembrane domains, short intracellular domains and two extracellular loops (8). Tetraspanins have been shown to be involved in cell activation, proliferation, motility, and metastasis, as well as in cell fusion (14). Although CD81 is widely expressed, its level of expression varies in specific cell lineages and during differentiation. Moreover, its association with cell surface proteins differs in cell types of various lineages. In B cells CD81 is a component of the CD19–CD21–CD81–Leu-13 molecular complex, which plays a role in B-cell activation (2), whereas in T cells the molecule is associated with T-cell-specific molecules, including CD4 and CD8 (5, 15). In addition to its association with lineage-specific proteins, CD81 is associated with integrins and other tetraspanins (8). As a consequence of such protein associations, it is possible to activate multiple adhesion/signaling pathways in different cell types by engaging CD81 at their surface. For example, treatment of B-cell lines with a monoclonal antibody (MAb) specific for CD81 induces changes in cell adhesion and inhibits proliferation, whereas treatment of T-cell lines affects cell adhesion but not proliferation (10).
CD81 was recently reported to interact with the hepatitis C virus (HCV) envelope glycoprotein (gp) E2 and hypothesized to act as a putative viral receptor (12). We have confirmed this observation and have shown that cell surface-expressed human CD81, but not murine or monkey (Chlorocebus aethiops, African green monkey [AGM]) CD81, binds E2661, a truncated soluble version of the E2 gp. Furthermore, interaction of E2661 with the B-cell line Daudi had effects on cell adhesion and proliferation similar to the effects induced by anti-CD81 antibodies (3). This observation is of particular interest since in addition to causing acute and chronic liver disease, HCV is the major cause for mixed cryoglobulinemia (MC), a B-lymphocyte proliferative disorder. Recent reports suggest that most patients (up to 98%) with MC types II and III are infected with HCV (9). Conversely, approximately a third of patients with chronic HCV infections have type II and III MC (11).
A previous study by members of our group examined the interaction between E2661 and CD81 and demonstrated that the second large extracellular loop (LEL) of human CD81 bound E2661. (Prior to the identification of CD81 as the receptor for the HCV E2 ligand, the second extracellular domain was coined EC2; however, to prevent confusion between E2 and EC2, we have opted to refer to this region as the LEL.) A number of MAbs specific for linear epitopes within E2661 were able to define two discontinuous regions (amino acids 480 to 493 and 544 to 551) involved in CD81 binding (3). Our present study was aimed at identifying the amino acid residues in human CD81 critical for E2661 binding. Human CD81 differs from AGM CD81 at only four amino acids within the LEL, which may directly affect the ability of E2661 to bind COS cells. Alternatively, since CD81 is known to associate with multiple proteins at the cell surface, differential presentation of CD81 on COS cells may also affect E2661 binding. However, it should be noted that E2661 interacts with human CD81 expressed on rat KM3 cells and various human cell types with similar avidities, suggesting that additional human-specific cells factors are not required for the initial interaction at the cell surface (3). Since E2661 can interact with a recombinant glutathione S-transferase (GST) fusion protein expressing the human CD81 LEL, we investigated the interaction of the AGM CD81 LEL with the viral envelope protein. Recombinant CD81 proteins derived from AGM CD81 and from human CD81 and variants containing amino acid changes at residues shown to be polymorphic between the human and AGM proteins were expressed both as recombinant fusion proteins and at the surfaces of KM3 cells. These CD81 proteins were tested for reactivity with E2661 and with a panel of anti-human CD81 MAbs. These experiments identified a single amino acid residue in human CD81 that is critical for binding E2661 (and some anti-CD81 antibodies). Mutating human CD81 residue 186 (F186L) abolished its ability to bind E2661. Unexpectedly, mutation T163A of human CD81 enhanced E2661 binding. This observation has implications for the design of molecular inhibitors with high affinity that would target the CD81-E2 interaction sites.
MATERIALS AND METHODS
Cloning and expression of CD81 LEL fusion proteins.
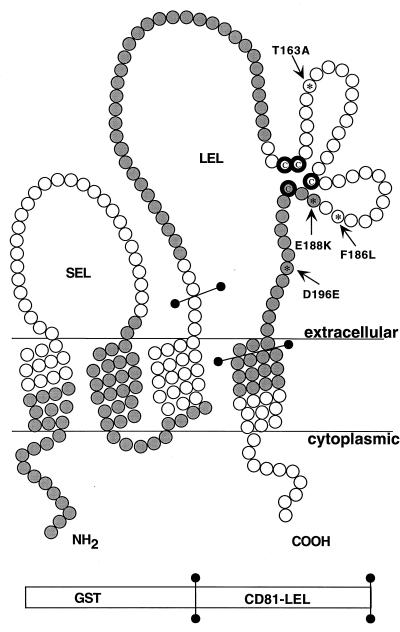
A diagram of the topological structure of CD81 is shown in Fig. 1. A fragment encoding amino acids 116 to 202 (LEL) of CD81 derived from the full-length human CD81 cDNA was inserted into the pGEX-2T vector (Stratagene, La Jolla, Calif.), which encodes a carboxyl-terminal fusion protein with GST as described previously (3). Similarly, a fragment encoding amino acids 109 to 201 of mouse CD81 was inserted into the same vector. Using the human LEL as a template, site-directed mutagenesis (QuikChange site-directed mutagenesis kit; Stratagene) was performed to generate three additional soluble GST fusion proteins containing the human LEL with the following mutations: T163A, F186L plus E188K, and D196E (codons underlined below).
FIG. 1.
Structural model of CD81 and soluble GST-CD81 LEL fusion proteins. Amino acid residues are shown as circles. Shaded circles indicate coding by odd-numbered exons, and bolded circles mark the positions of LEL cysteine residues evolutionarily conserved among tetraspanins. Bars delineate amino acid residues fused to GST. Amino acids that differ between human and AGM CD81 are numbered, with the specific human residue on the left and the AGM residue on the right of the number. SEL, small extracellular loop.
Primers used for the ACT→GCC change corresponding to position 163 were 5′GGC TCC AGC ACA CTG GCC GCT TTG ACC ACC TCA G3′ (sense) and 5′C TGA GGT GGT CAA AGC GGC CAG TGT GCT GGA GCC3′ (antisense).
Primers for the 186-plus-188 double mutation, TTC→CTC plus GAG→AAA, were 5′C ATC AGC AAC CTC CTC AAG AAA GAC TGC CAC CAG3′ (sense) and 5′CTG GTG GCA GTC TTT CTT GAG GAG GTT GCT GAT G3′ (antisense).
Primers used for the GAC→GAG change corresponding to position 196 were 5′CAC CAG AAG ATC GAT GAG CTC TTC TCC GGG AAG C3′ (sense) and 5′G CTT CCC GGA GAA GAG CTC ATC GAT CTT CTG GTG3′ (antisense).
PCR was performed for 16 to 18 cycles, and products were incubated with DpnI to digest parental methylated plasmid DNA, as recommended by the manufacturer.
The AGM CD81 LEL fusion protein was constructed by “sticky-end” PCR (17) using sense primers 5′GATCCAACAAGGACCAGATTGCCAAGGAT3′ and 5′CAACAAGGACCAGATTGCCAAGGAT3′ and antisense primers 5′CACAGCTTCCCGGAGAAGAGCTC3′ and 5′AATTCACAGCTTCCCGGAGAAGAGCTC3′. These primers created a BamHI restriction site at the 5′ end and an EcoRI restriction site at the 3′ end of the PCR product (restriction sites are underlined). The PCR product was cloned into the BamHI and EcoRI sites of expression vector pGEX-2T. Plasmids were first transformed into Escherichia coli strain XL1-Blue. All constructs were sequenced using the Big Dye terminator method and analyzed on an ABI Prism 373 DNA sequencer. Subsequently, E. coli strains SURE and BL2 were transfected with these plasmids for protein production. Bacteria transformed with the various CD81 LEL fusion constructs were induced for 3 h at 32°C by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and lysed by sonication, and fusion proteins were recovered by affinity chromatography on a glutathione-Sepharose 4B column according to the manufacturer's protocols (Pharmacia Biotech, Uppsala, Sweden). Purified fusion proteins were analyzed by Western blotting and by immunoassays as described below.
Cloning and expression of cell surface-expressed CD81 mutants.
The human CD81 cDNA insert (10) was transferred from the pCDM8 vector by XhoI digestion, end filling, and blunt ligation into the Sureclone vector (Pharmacia Biotech, St. Albans, United Kingdom). Correct orientation was ascertained by HindIII (vector site) and PstI (CD81 restriction site) digestion. Correct clones were transferred via the Sureclone restriction sites, HindIII and EcoRI, into the expression vector pEE6hCMV.neo. Primers used for cloning of the human CD81 open reading frame (bolded below) into pEE6 HindIII and EcoRI sites (underlined below) were 5′TCT AGA AAG CTT GCC ACC ATG GGA GTG GAG GGC T3′ (sense) and 5′TCT AGA GAA TTC TCA GTA CAC GGA GCT GTT3′ (antisense).
The four point mutations (underlined below), T163A, F186L, E188K, and D196E, were generated by overlap extension PCR (4). The following primers were used for mutagenesis: for T163A, 5′C ACA CTG GCT GCT TT3′ (sense) and 5′AA AGC AGC CAG TGT G3′ (antisense); for F186L, 5′AC CTC TTA AAG GAG G3′ (sense) and 5′C CTC CTT TAA GAG GT3′ (antisense); for E188K, 5′TC TTC AAG AAG GAC TGC3′ (sense) and 5′GCA GTC CTT CTT GAA GA3′ (antisense); and for D196E, 5′ATC GAT GAA CTC TTC TC3′ (sense) and 5′GA GAA GAG TTC ATC GAT3′ (antisense).
All constructs were sequenced using the Big Dye terminator method and analyzed on an ABI Prism 373 sequencer.
Generation of stable transfected cell lines.
The rat cell line KM3 was routinely subcultured in RPMI 1640 containing 5% fetal calf serum (FCS). The mutant constructs were transfected into the KM3 cell line as described previously (3). Briefly, 20 μg of column-purified DNA (Nucleobond AX100; BioGene Ltd., Kimbolton, United Kingdom) was electroporated by a single pulse at 250 V and 500 μF. Selection was achieved by supplementing the medium with 125 μg of G418 (Geneticin; Gibco BRL) per ml. CD81-expressing clones were isolated using a fluorescein isothiocyanate (FITC)-labeled anti-human CD81 MAb, 1.3.3.22 (Ancell, Bayport, Mich.), and the top 10% were sorted twice on a Vantage FACsort (Becton Dickinson, Oxford, United Kingdom).
Expression of soluble truncated HCV E2661.
Expression of soluble secreted recombinant E2661 by transient expression in HEK (293) cells has been described previously (3). Briefly, a eukaryotic expression vector encoding E2661 (p14.tE2.661.hiv) was transfected into 293 cells cultured in 100-mm-diameter dishes using the calcium phosphate precipitation method. After 72 h of incubation at 37°C in medium containing a low concentration (2%) of FCS, the medium was removed, clarified by centrifugation, and used as a source of secreted E2661. Supernatants from cells transfected with the vector alone were utilized as a mock antigen in subsequent assays.
Enzyme immunoassays (EIAs).
CD81 LEL fusion proteins were used to coat 96-well dishes at 5 μg/ml in phosphate-buffered saline (PBS) overnight at 4°C, and plates were blocked for 1 h at room temperature (RT) with 4% milk powder. E2661 (50 μl of 1 μg/ml) was diluted in eight wells in twofold dilutions and allowed to bind overnight at 4°C. Bound E2661 was detected using the E2-specific and conformation-dependent MAb H53 (mouse immunoglobulin G1 [IgG1]) (1) and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Southern Biotechnology Associates, Birmingham, Ala.). Likewise, anti-CD81 MAbs were diluted in eight wells twofold, allowed to bind for 2 h at RT, and visualized using HRP-conjugated goat anti-mouse IgG antibody (Southern Biotechnology Associates).
Affinity elution EIAs with ammonium thiocyanate.
Assays were performed as described above, with the following modification (16). After binding of E2661 (100 μl of 1 μg/ml), anti-CD81 MAbs, or anti-GST MAbs, the plates were washed to remove unbound protein and 100 μl of 0 to 3 M ammonium thiocyanate (NH4SCN) in PBS was added. The plates were incubated for 15 min at RT, washed, and visualized as described above. The absorbance reading in the absence of NH4SCN was defined as 100%. The affinity index was defined as the molar concentration of thiocyanate required to reduce the initial absorbance reading to 50% (7).
Flow cytometric analysis of E2661 binding to cells.
The interaction of E2661 gp with cells was quantified using a fluorescence-activated cell sorter-based assay. In brief, cells were washed twice in PBS–1% FCS–0.05% sodium azide (WB) and resuspended at 2 × 106/ml.
A total of 2 × 105 cells were incubated with E2661 at RT for 1 h; unbound antigen was removed by washing the cells twice in WB. Cells were incubated with MAb H53 (1 μg/ml) for 1 h at RT. Finally, cell-bound MAb was visualized with an anti-mouse IgG-phycoerythrin (PE) conjugate (Harlan Seralab, Bicester, United Kingdom) and analyzed with a fluorescence-activated cell sorter (Becton Dickinson). Median fluorescence intensities were determined using Cellquest software (Becton Dickinson).
Determination of intracellular CD81 levels.
Cell permeabilization was performed using a cell permeabilization kit according to the manufacturer's instructions (Harlan Sera-lab, Bicester, United Kingdom). Briefly, subconfluent adherent cells were harvested using nonenzymatic solution, washed in balanced salt solution–0.2% bovine serum albumin, resuspended at 106/ml, and plated at 100 μl/well in a 96-well plate. The cells were then fixed with 10 μl of reagent A for 15 min at RT, followed by two washes in PBS (pH 7.2). Surface expression was measured by adding saturating amounts of FITC-labeled anti-CD81 MAb (1.3.3.22), or an isotype control, in a volume of 20 μl (balanced salt solution-bovine serum albumin). Total expression was measured by adding FITC-labeled anti-CD81 or control MAb and 10 μl of lysis reagent B. Cells were mixed and incubated in the dark for 15 min at RT. Samples were washed then fixed in 1% paraformaldehyde and analyzed on a Coulter EPICS flow cytometer. Surface expression and total expression were first corrected for nonspecific binding (isotype control), and intracellular expression levels were calculated by subtracting extracellular from total expression levels.
MAbs.
Anti-CD81 antibodies used in these studies have been previously described (6). MAb 1.3.3.22 and anti-GST MAb were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.); MAb 1.3.3.22 was also obtained from Ancell. H53, a conformation-dependent anti-E2 MAb (1), was a generous gift from Jean Dubuisson.
Western blotting.
The CD81 LEL was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing and nonreducing conditions. After separation, the proteins were transferred to nitrocellulose and visualized directly with anti-CD81 MAb 5A6 or indirectly using E2661 protein and rat anti-E2 MAbs (7/16, 3/11, and 6/53). Bound IgG was detected via anti-mouse or anti-rat HRP-conjugated secondary antibodies, ECL detection reagents (Amersham Life Sciences), and exposure to photographic film.
Peptide synthesis.
Machine-assisted peptide synthesis was performed on a Perseptive Perkin-Elmer Pioneer synthesizer by 9-fluorenylmethoxy carbonyl/t-Bu chemistry. The resin used was Novasyn TGR; activation was achieved by HBTU-DIEA (1:2) or HATU-DIEA (1:2) using a fivefold excess of acylants over the resin amino groups and a coupling time of 30 min to 2 h. The peptides were cleaved with 88% trifluoroacetic acid (TFA)–5% phenol–2% triisopropylsilane–5% water. Crude peptides were purified by reversed-phase high-performance liquid chromatography on either a Waters Delta-Pack C18 column (200 by 25 mm, 100Å, 15 μm) or a Waters Delta-Pack C4 column (200 by 25 mm, 300Å, 15 μm), depending on size. The eluent system was H2O, 0.1% TFA, and acetonitrile–0.1% TFA. Analytical high-performance liquid chromatography was performed on an Ultrasphere C18 column (250 by 4.6 mm, 80Å, 5 μm [Beckman]) or a Vydac C4 column (150 by 4.6 mm, 300Å, 5 μm). Purified (≥90%) peptides were characterized by mass spectrometry, 1H nuclear magnetic resonance, and amino acid analysis.
RESULTS
Specificity of soluble-CD81–E2661 interactions.
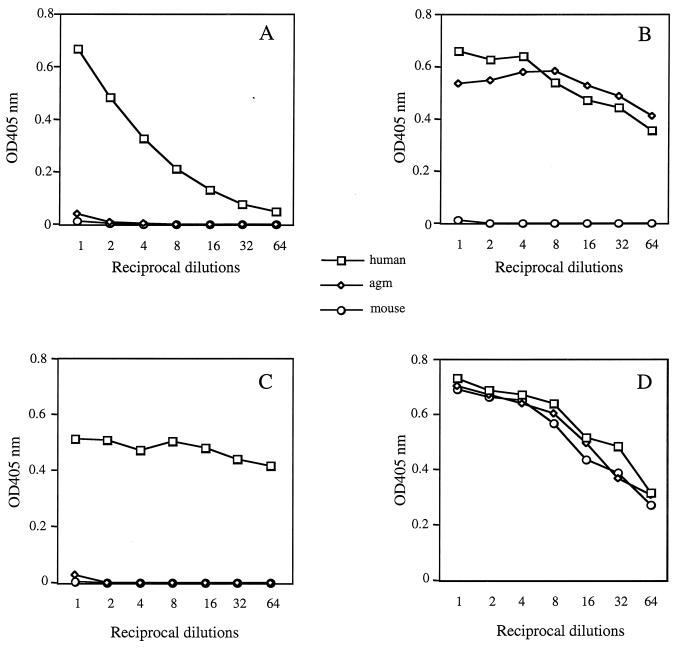
To determine whether E2661 could interact with soluble AGM CD81, a recombinant GST fusion protein containing the AGM CD81 LEL (Fig. 1) was constructed, expressed in bacteria, and purified as detailed in Materials and Methods. This recombinant protein was able to bind a number of conformation-dependent CD81 MAbs, suggesting that it was folded in a manner comparable to that of the native molecule (Fig. 2B and data not shown). Purified AGM CD81 LEL protein was compared for its ability to bind E2661 with the homologous soluble proteins derived from human and mouse CD81. The soluble AGM CD81 LEL protein was unable to bind E2661 (Fig. 2A). This result is consistent with our cell binding data (3), confirming that the block to cell binding was at the protein-protein level. The coating of the AGM CD81 LEL protein on the plate was equal to that of the human protein, as seen by their similar patterns of reactivity with the anti-CD81 MAb JS64 (Fig. 2B) and with anti-GST MAb (Fig. 2D). Interestingly, some anti-CD81 MAbs discriminated between the human and the AGM soluble CD81 proteins. Whereas JS64 bound human and AGM proteins similarly, other anti-human CD81 MAbs, such as 4TM-1 (Fig. 2C and data not shown), did not interact with the AGM protein. It is worth noting that all anti-CD81 MAbs tested to date are able to block E2 binding to cell surface-expressed CD81. MAbs able to discriminate between human and AGM CD81 may interact with an epitope that overlaps the E2661 binding site.
FIG. 2.
Specificity of HCV E2661 and anti-CD81 MAb interactions with CD81 LEL fusion proteins. EIA plates were coated with recombinant CD81 LEL fusion proteins (5 μg/ml), and binding of 50 μl of the following proteins at their starting dilutions to soluble HCV E2661 (1 μg/ml) (A), anti-human CD81 MAb JS64 (0.1 μg/ml) (B), anti-human CD81 MAb 4TM-1 (1 μg/ml) (C), and anti-GST MAb B-14 (1 μg/ml) (D) was detected as described in Materials and Methods. OD, optical density.
Conformation of the E2661 binding region within CD81.
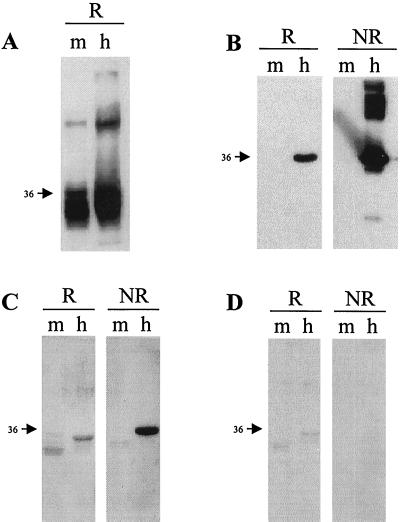
Both soluble human and mouse GST-CD81 LEL proteins reacted with an anti-GST MAb (Fig. 3A). This antibody recognized the full-length protein and lower-molecular-weight products. The highest 36-kDa molecular band of the human, but not the mouse, fusion protein reacted with anti-CD81 MAb (Fig. 3B). This antibody also reacted with nonreduced aggregated forms of the protein, which collapsed to a single band upon reduction. It should be noted that the reactivity of the antibody with the reduced soluble CD81 was much weaker than that seen with the nonreduced product. Indeed, recognition of CD81 by the majority of anti-CD81 MAbs is dependent upon correct folding of S-S bonds, since these MAbs recognize the native, cellular, nonreduced form of the protein. These results suggest that the native conformation of CD81 depends on S-S bonds (6). Similarly, E2661 was found to interact only with the nonreduced form of CD81 by Western blotting (Fig. 3C). It is interesting that anti-CD81 MAb 5A6 recognizes reduced and higher-molecular-weight forms of human CD81 LEL proteins seen under nonreducing conditions (Fig. 3B), whereas E2 recognizes only the monomeric nonreduced form of CD81 (Fig. 3C). Likewise, E2661 reacted in EIA with native human CD81 LEL proteins, but not with a reduced form of the soluble molecule or with reduced synthetic peptides corresponding to defined regions within the human CD81 LEL (Tables 1 and 2). Similarly, all of the anti-human MAbs tested, with the exception of 1D6, failed to bind the CD81 peptides (Table 1 and data not shown). Since MAb 1D6 can inhibit E2 interaction with CD81, we were interested in defining its minimal epitope. A series of peptides were synthesized and tested for the ability to bind 1D6 and E2661. 1D6 bound to all of the peptides tested, with the exception of peptides 1077 and 1084, with various affinities. The minimal region recognized by this antibody comprised amino acids 179 to 193, corresponding to peptide 1108 (Table 1). In contrast, E2 failed to interact with the peptides, with optical density signals of <0.2, despite showing good binding in the same assay to GST-human CD81 (Table 1).
FIG. 3.
Interaction of E2661 with recombinant CD81. Purified recombinant human CD81 LEL (h) and mouse CD81 LEL (m) proteins were analyzed by reducing (R) or nonreducing (NR) sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected with anti-GST MAb (A); anti-CD81 MAb 5A6 (B); E2661, followed by anti-E2 MAbs (C); and supernatant from mock-transfected cells, followed by anti-E2 MAbs (D). Numbers at the left indicate molecular mass, in kilodaltons.
TABLE 1.
Native but not reduced human CD81 LEL protein binds E2661, while anti-CD81 MAb 1D6 binds a defined reduced peptide within the LEL
| Peptide | Human CD81 amino acids | DTTa | Binding of E2661 | Binding of 1D6 (μg/ml)
|
|||
|---|---|---|---|---|---|---|---|
| 1.0 | 0.2 | 0.04 | 0.008 | ||||
| 1041 | 133–201 | + | 1.35 | 0.72 | 0.26 | 0.17 | |
| 1095 | 155–201 | + | 0.03 | 1.42 | 1.34 | 1.04 | 0.60 |
| 1101 | 164–201 | + | 0.21 | 1.45 | 1.30 | 0.87 | 0.41 |
| 1143 | 164–192 | + | 0.03 | 1.07 | 0.41 | 0.10 | 0.05 |
| 1144 | 173–192 | + | 0.02 | 1.25 | 0.48 | 0.30 | 0.05 |
| 1108 | 179–193 | + | 0.02 | 0.51 | 0.08 | 0.06 | 0.03 |
| 1077 | 146–155 | + | 0.03 | 0.05 | 0.05 | 0.02 | 0.03 |
| 1084 | 156–164 | + | 0.04 | 0.03 | 0.04 | 0.04 | 0.02 |
| GST-human CD81 | LEL | − | 2.48 | 1.58 | 1.29 | 0.97 | 0.41 |
| + | 0.22 | 1.74 | 1.21 | 0.94 | 0.61 | ||
| GST-mouse CD81 | LEL | − | 0.05 | 0.03 | 0.05 | 0.05 | 0.04 |
| + | 0.03 | 0.03 | 0.01 | 0.03 | 0.03 | ||
Peptides (10 mM) and proteins were incubated in PBS-dithiothreitol (DTT) (0.1 M) for 30 min at RT prior to coating of the ELISA plate.
TABLE 2.
Human peptide sequences
| Peptide | Sequencea |
|---|---|
| 1095 | LDCCGSSTLTALTTSVLKNNLCPSGSNIISNLFKEDCHQKIDDLFSGK |
| 1101 | ALTTSVLKNNLCPSGSNIISNLFKEDCHQKIDDLFSGK |
| 1143 | ALTTSVLKNNLCPSGSNIISNLFKEDCHQ |
| 1144 | NLCPSGSNIISNLFKEDCHQ |
| 1108 | SNIISNLFKEDCHQK |
| 1077 | VVKTFHETLD |
| 1084 | CCGSSTLTA |
Changes between human and AGM CD81 are bolded.
Identification of CD81 residues critical for E2661 binding.
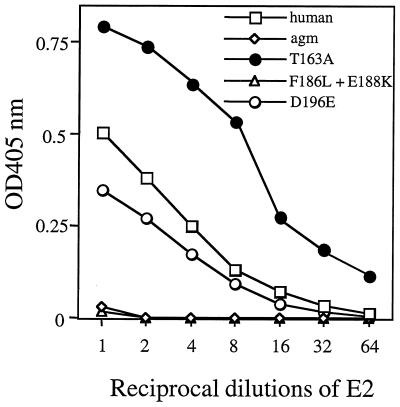
To define amino acid residues in CD81 critical for E2661 binding, human CD81 LEL mutants were constructed, expressed in bacteria, immobilized on EIA plates, and tested for the ability to bind E2661. The CD81 variants were tested for binding serially diluted E2661 and detected by MAb H53. This is a conformation-dependent anti-E2661 antibody that binds the E2661-CD81 complex (3). As seen earlier (Fig. 2A), human but not AGM CD81 LEL protein bound E2661. The D196E mutation of human CD81 reduced E2661 binding, while human CD81 LEL protein containing both mutations F186L and E188K was unable to bind E2661, suggesting that either or both of these residues are critical for interaction with E2661 (Fig. 4). Surprisingly, human CD81 LEL protein expressing the T163A mutation demonstrated enhanced binding to E2661 (Fig. 4). All these GST-CD81 LEL fusion proteins were equally coated on the plates, as determined by their reactivities with the nondiscriminating antibody JS64 (Fig. 2B) and with an anti-GST MAb (Fig. 2D). Human CD81 LEL protein and the variant proteins were tested for the ability to block E2661 binding to cell surface-expressed CD81 as described above. The human CD81-LEL T163A mutant completely blocked E2661 binding and the D196E mutant partially blocked the interaction, whereas neither AGM CD81 LEL protein nor the F186L-E188K double mutant competed with cellular CD81 for E2661 binding (data not shown).
FIG. 4.
HCV E2661 binding to CD81 LEL fusion proteins. E2661 starting at 1 μg/ml was serially diluted and allowed to bind to EIA plates coated with 5 μg of each of the recombinant CD81 LEL fusion proteins per ml as described in the legend to Fig. 2A. Bound E2661 was detected using the E2-specific MAb H53 and goat anti-mouse IgG-HRP. OD, optical density.
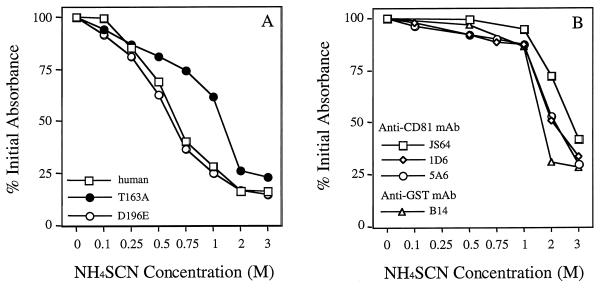
To compare the relative avidities of soluble mutant CD81 proteins with E2661, the complexes were exposed to increasing salt concentrations using previously described methods (7, 16). CD81-E2661 complexes were formed as described above, exposed to increasing concentrations of ammonium thiocyanate, and washed; the bound proteins were then detected. The avidity of interaction of the various mutant CD81 LEL proteins for E2661 is shown in Fig. 5A. This analysis revealed that 50% of E2661 was bound to the parental human CD81 LEL protein at 0.7 M salt and that mutation of residue 196 mildly affected the binding avidity, since 50% of the initially bound material remained bound at a slightly lower salt concentration, 0.65 M. However, mutation at residue 163 increased the binding avidity of E2661 such that 50% of E2661 remained bound after the salt concentration was doubled to 1.4 M. Furthermore, the avidity of the binding of the human CD81 LEL T163A mutant to E2661 was in the range of antibody binding. This can be seen in Fig. 5B, which shows the results of testing for the avidity of antibody binding to human CD81 LEL protein. The MAbs displayed different avidities for the protein; for example, 50% of the initial bound anti-GST antibody remained bound after treatment with 1.5 M salt, whereas the more avid anti-CD81 MAbs 5A6 and 1D6 required 2.0 M salt and the most avid anti-CD81 MAb, JS64, was eluted at 2.75 M ammonium thiocyanate (Fig. 5B).
FIG. 5.
Affinity elution analysis of mutant CD81-LEL proteins with NH4SCN. (A) EIA plates were coated with 5 μg of recombinant human CD81-LEL fusion protein, CD81-LEL mutant T163A protein, and CD81-LEL mutant D196E protein per ml, and E2661 was allowed to bind as described in the Materials and Methods. (B) EIA plates were coated with recombinant human CD81-LEL fusion protein (5 μg/ml). Binding of anti-CD81 MAbs JS64 (0.5 μg/ml), 1D6, and 5A6 and anti-GST MAb B-14 (all at 1 μg/ml) was detected as described in Materials and Methods. After binding of E2661 or anti-CD81 MAbs, 100 μl of 1 to 3 M ammonium thiocyanate was added. Plates were incubated for 15 min at RT, and results were visualized as described in Materials and Methods. The absorbance reading in the absence of NH4SCN was defined as 100%. The affinity index was defined as the molar concentration of thiocyanate required to reduce the initial absorbance reading to 50%.
Identification of CD81 residues critical for E2661 binding to the cell surface.
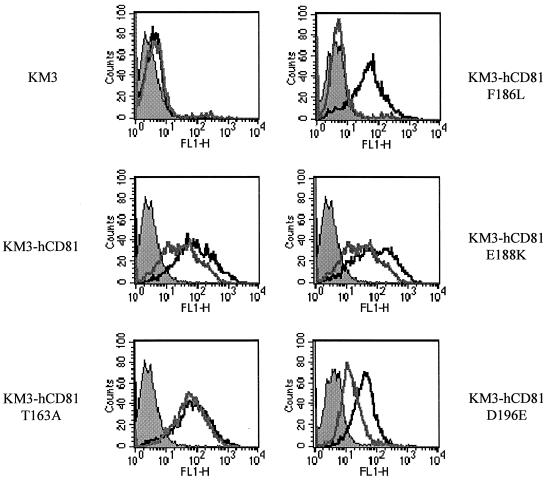
We have shown that mutation of residues known to be polymorphic between the human and AGM CD81 sequences affected E2661 binding to soluble CD81 (Fig. 4). To investigate whether cell surface-expressed CD81 behaves in a similar manner, proteins with single amino acid mutations were constructed and stably transfected into rat KM3 cells. Cells expressing each of the individual CD81 mutant proteins were tested for expression at the cell surface by reactivity with anti-CD81 MAb 1.3.3.22 and E2661. All of the CD81 proteins were expressed to different extents at the cell surface, as follows: human CD81 > T163A mutant > F186L mutant > E188K mutant = D196E mutant (Fig. 6). Using a cell permeabilization assay, we analyzed intracellular expression of the various CD81 mutants in transfected KM3 cells. Negligible amounts of human and T163A mutant CD81 were observed within cells; the F186L mutant was mostly expressed at the cell surface, with less than 20% of the total protein being located within the cell. However, considerable amounts (44 and 35%, respectively) of the E188K and D196E mutants were found within cells, suggesting that these mutations may affect intracellular trafficking of CD81.
FIG. 6.
Affinity of E2 for cell surface-expressed CD81 variants. KM3 parental cells (solid graph) and transfectants expressing heterologous human CD81 (hCD81) and various mutants were tested for the ability to bind the anti-CD81 MAb 1.3.3.22 (5.0 μg/ml) (solid line) and a subsaturating concentration of E2661 (0.5 μg/ml) (shaded line). Bound E2 was visualized with MAb H53 and anti-mouse IgG-PE. The results are from a single experiment, which are representative of comparable data obtained in two independent experiments.
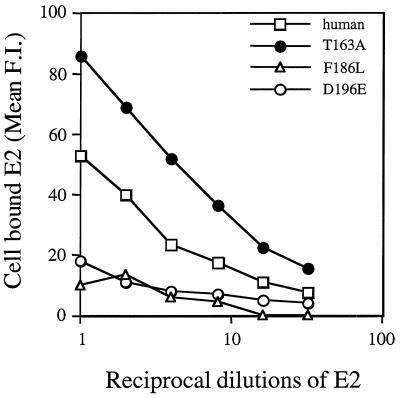
To overcome differential expression levels of the CD81 mutants, a subsaturating level of E2661 (0.5 μg/ml) was used to evaluate the interaction, whereby E2661 binding was not dependent upon the CD81 expression level(s). E2661 bound equivalently to parental and E188K proteins, showed no interaction with the F186L protein, bound with an intermediate phenotype to the D196E protein, and showed enhanced interaction with the T163A protein (Fig. 6). Thus, the results are comparable to those obtained with the soluble GST-CD81 fusion proteins and identify residue 186 as being critical for the interaction with E2661. The observations seen with a single concentration of E2661 were dose dependent and titrated in a linear fashion dependent upon E2661 concentration (Fig. 7). The similar patterns of binding of E2 to soluble recombinant and cell surface-expressed CD81 (Fig. 4 and 7) suggest comparable folding of the recombinant and cell surface-expressed proteins. Moreover, the T163A mutation in either form of the protein enhanced the interaction with E2661.
FIG. 7.
Dose-dependent binding of E2 to cellular CD81. Increasing dilutions of 1 μg of E2661 per ml were tested for the ability to bind parental KM3 and cells expressing wild-type and mutant human CD81 sequences. Bound E2 was visualized with MAb H53 and anti-mouse IgG-PE. The results are from a single experiment and are representative of data obtained in two independent experiments. F.I., fluorescence intensity.
Interestingly, anti-human CD81 MAbs produced against the native CD81 protein exhibited differential patterns of binding to the various mutant proteins. Like E2661, all MAbs showed increased binding to the T163A mutant and reduced binding to the F186L mutant (data not shown). As observed with the soluble CD81 (Fig. 2C and data not shown), there was no binding of the F186L mutant to the 4TM-1 MAb. The E188K and D196E mutants displayed reduced binding to most of the antibodies. The increased binding of the anti-human MAbs and E2661 to the T163A mutant suggests that amino acid 163 contributes to the tertiary structure of CD81, which enables increased binding to its natural ligands.
DISCUSSION
The evolutionary divergence of CD81 is most pronounced in exon 6 of the molecule. Yet, this exon encodes three of the cysteine residues that are conserved among tetraspanin molecules (8). Moreover, two of the cysteines encoded by exon 6 are adjacent and embedded in the CCG motif conserved in all tetraspanins of schistosome, nematode, Drosophila, and mammalian origins (8). Although the tertiary structure of tetraspanins has yet to be solved, it is highly likely that the three conserved cysteine residues encoded by exon 6 and the conserved cysteine encoded by exon 7 form S-S bridges (Fig. 1). Such bridges would restrain the tertiary structure of the protein and expose regions flanked by the bridging cysteines as diagramed in Fig. 1.
Human CD81 and AGM CD81 differ in four amino residues, all encoded within exons 6 and 7 (Fig. 1) and contained in the carboxyl-terminal region of the LEL, implicating one or more of these residues as important for HCV E2 binding (3, 12). To define which of these amino acid residues is most important for CD81-E2 interactions, we created both soluble and cell surface-expressed mutant CD81 proteins and tested their interactions with the viral protein and with a panel of anti-human CD81 MAbs. Soluble AGM CD81 LEL protein failed to react with the HCV E2661 (Fig. 2A). This recombinant protein was able to bind several anti-human CD81 MAbs, suggesting that the protein was folded correctly and bound to the EIA plates as efficiently as the human protein (Fig. 2B). The inability of E2661 to bind soluble AGM protein was mirrored by several MAbs raised against native human CD81. Thus, E2661 and a number of MAbs can discriminate between human and AGM CD81 and may therefore bind overlapping epitopes (Fig. 2C and data not shown).
Soluble, bacterially produced, human CD81 has previously been shown to interact with recombinant E2 and with the virus (3, 12). The soluble human CD81 LEL protein reacted with a panel of anti-human CD81 MAbs that were produced against native cell surface-expressed CD81 (data not shown). Thus, binding of all available anti-CD81 antibodies to the soluble protein is indicative of its correct folding. Furthermore, the majority of these antibodies react preferably with unreduced native and soluble CD81 in EIA and Western analysis (data not shown), indicating that appropriate S-S bridges are formed during bacterial expression. Similarly, E2661 protein bound only the full-length nonreduced form of soluble CD81 (Fig. 3 and Table 1), suggesting that the E2 binding site on CD81 is conformation dependent. In support of this conclusion, synthetic peptides containing the human CD81 LEL sequence were unable to bind the viral protein (Table 1). However, MAb 1D6 was able to bind several of the peptides, enabling us to identify amino acids 179 to 193 as the minimal epitope (Table 1). This particular antibody was derived by immunization with B cells that were pretreated with an anti-CD81 MAb (13). Preengagement of CD81 may have altered the tertiary structure of the protein, exposing the 1D6-reactive epitope. Recognition of a linear epitope by this antibody enables its reactivity with fixed and paraffin-embedded tissues (S. Levy and R. Wranke, unpublished observations).
To define the region within the CD81 LEL important for E2661 binding, mutant soluble proteins were expressed in bacteria and tested by EIA (Fig. 4). The F186L-E188K double mutant failed to react with E2661, whereas the T163A mutant demonstrated enhanced binding compared to that of the parental protein (Fig. 4). The avidity of E2661 binding was further tested by exposing the interacting proteins to increasing concentrations of the chaotropic agent ammonium thiocyanate (7, 16). This analysis confirmed that the increased binding of the T163A mutant to E2661 was attributable to an increase in the avidity of the interaction, whereby 50% of the protein remained bound to E2661 after exposure to 1.4 M salt, compared with 0.7 M for parental human CD81 LEL protein. The avidity of binding of the T163A mutant to E2661 is very similar to that seen for the interaction of the human CD81 LEL protein with the anti-GST MAb, of which 50% remained bound at 1.5 M salt (Fig. 5B). This analysis also demonstrated that monovalent E2661 does not bind the native human CD81 LEL protein as avidly as bivalent anti-CD81 MAbs. It is not surprising that the avidity of binding of the monovalent soluble E2661 to GST-CD81 is lower than that of antibody binding. However, it is possible to envisage that multiple E2 molecules expressed within the context of E1E2 oligomers on the virion surface may bind cellular CD81 with increased avidity.
Finally, it was important to determine whether mutations in CD81 could indeed influence binding of soluble E2661 to the cell surface. We therefore constructed plasmids that encoded each of the four amino acid changes and selected stable transfected KM3 cells expressing these mutants. This experiment identified residue 186 as being critical for the interaction with E2 since the protein with a mutation of this residue did not bind E2661. The E188K mutant bound E2661 with an affinity comparable to that of the parental human protein (Fig. 6 and 7). The enhanced binding of the cell surface-expressed T163A mutant to E2661 confirmed the protein-protein interaction data. Moreover, the binding of E2 to the cell surface-expressed CD81 variants (Fig. 7) was identical to that observed with immobilized soluble CD81 mutants (Fig. 5). The increased binding of the E2661 protein to the T163A mutant was not dependent on increased cell surface expression, as its level of expression was lower than that of parental human CD81. Nevertheless, the mutational analysis resulted in reduced expression of some mutant CD81 proteins. It will be interesting to determine if these mutant proteins demonstrate reduced association with cell surface proteins needed for trafficking of the molecule to the cell surface. Thus, we demonstrate that a single amino acid change, F186L, can abrogate CD81 interaction with E2, whereas the T163A mutation enhances the interaction.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant CA-34233 (S.L.), by The Wellcome Trust and The Lister Institute for Preventive Medicine (J.A.M.), and by British Heart Foundation grant PG/98163 and Arthritis Research Campaign fellowship M0543 (P.N.M.).
We thank Jean Dubuisson for his generous gift of MAb H53.
REFERENCES
- 1.Cocquerel L, Meunier J C, Pillez A, Wychowski C, Dubuisson J. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol. 1998;72:2183–2191. doi: 10.1128/jvi.72.3.2183-2191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon D T, Carter R H. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 3.Flint M, Maidens C, Loomis-Price L D, Shotton C, Dubuisson J, Monk P, Higginbottom A, Levy S, McKeating J A. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73:6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, Kakizaki M, Nishimura M, Yoshie O. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J Immunol. 1995;15:1229–1239. [PubMed] [Google Scholar]
- 6.Levy S, Todd S C, Maecker H T. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald R A, Hosking C S, Jones C L. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988;106:191–194. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- 8.Maecker H T, Todd S C, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 9.Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E, Scudeller G, et al. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 10.Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlotsky J M, Roudot-Thoraval F, Simmonds P, Mellor J, Ben Yahia M B, Andre C, Voisin M C, Intrator L, Zafrani E S, Duval J, et al. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med. 1995;122:169–173. doi: 10.7326/0003-4819-122-3-199502010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 13.Schick M R, Levy S. The TAPA-1 molecule is associated on the surface of B cells with HLA-DR molecules. J Immunol. 1993;151:4090–4097. [PubMed] [Google Scholar]
- 14.Tachibana I, Hemler M E. Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J Cell Biol. 1999;146:893–904. doi: 10.1083/jcb.146.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todd S C, Lipps S G, Crisa L, Salomon D R, Tsoukas C D. CD81 expressed on human thymocytes mediates integrin activation and IL-2-dependent proliferation. J Exp Med. 1996;184:2055–2060. doi: 10.1084/jem.184.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong C P, Okada C Y, Levy R. TCR vaccines against T cell lymphoma: QS-21 and IL-12 adjuvants induce a protective CD8+ T cell response. J Immunol. 1999;162:2251–2258. [PubMed] [Google Scholar]
- 17.Zeng G. Sticky-end PCR: new method for subcloning. BioTechniques. 1998;25:206–208. doi: 10.2144/98252bm05. [DOI] [PubMed] [Google Scholar]